Construction of Eicosatetraenoic Acid Producing Cell Factory by Genetic Engineering of Mucor circinelloides
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Transformation, and Fermentation Conditions
2.2. Plasmids Construction
2.3. Cell Dry Weight, Lipid Extraction and Analysis
2.4. Determination of Glucose and Ammonium Concentrations during the Fermentation
2.5. Genomic DNA Extraction, RNA Isolation and RT-qPCR Analysis
2.6. Statistical Analysis
3. Results
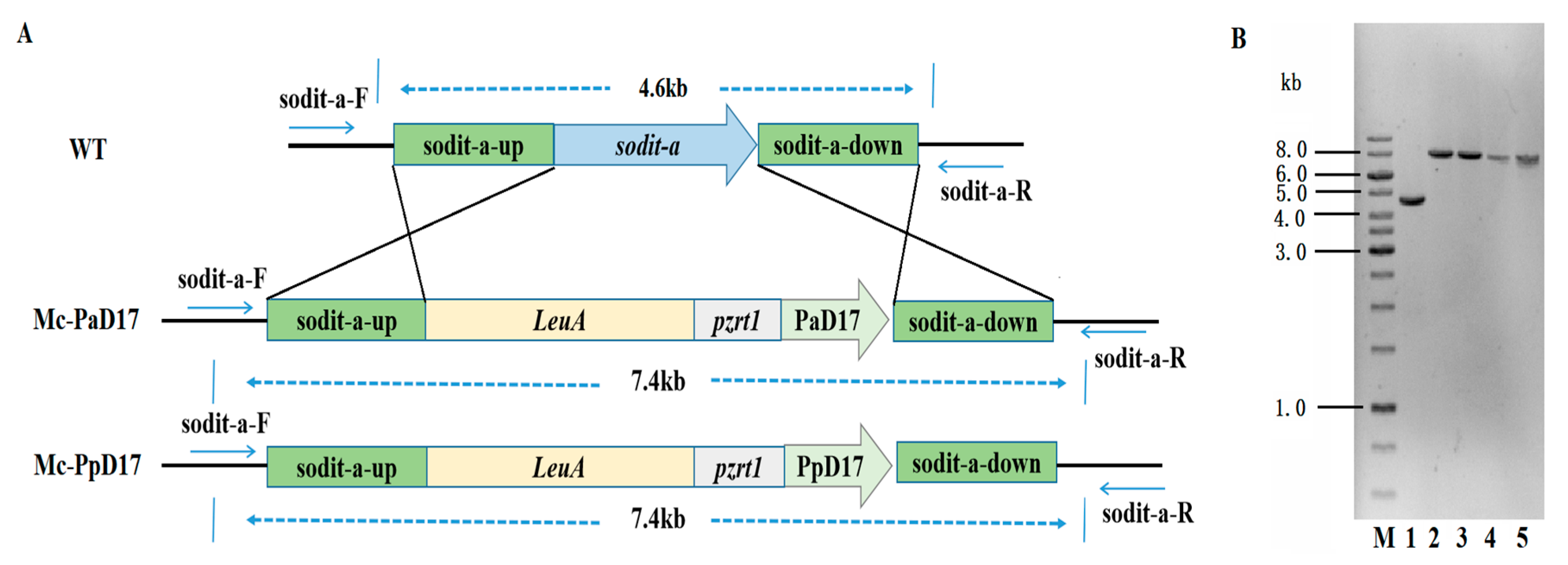
3.1. Generation of Delta-17 Overexpressing Strains of M. circinelloides by Genetic Engineering
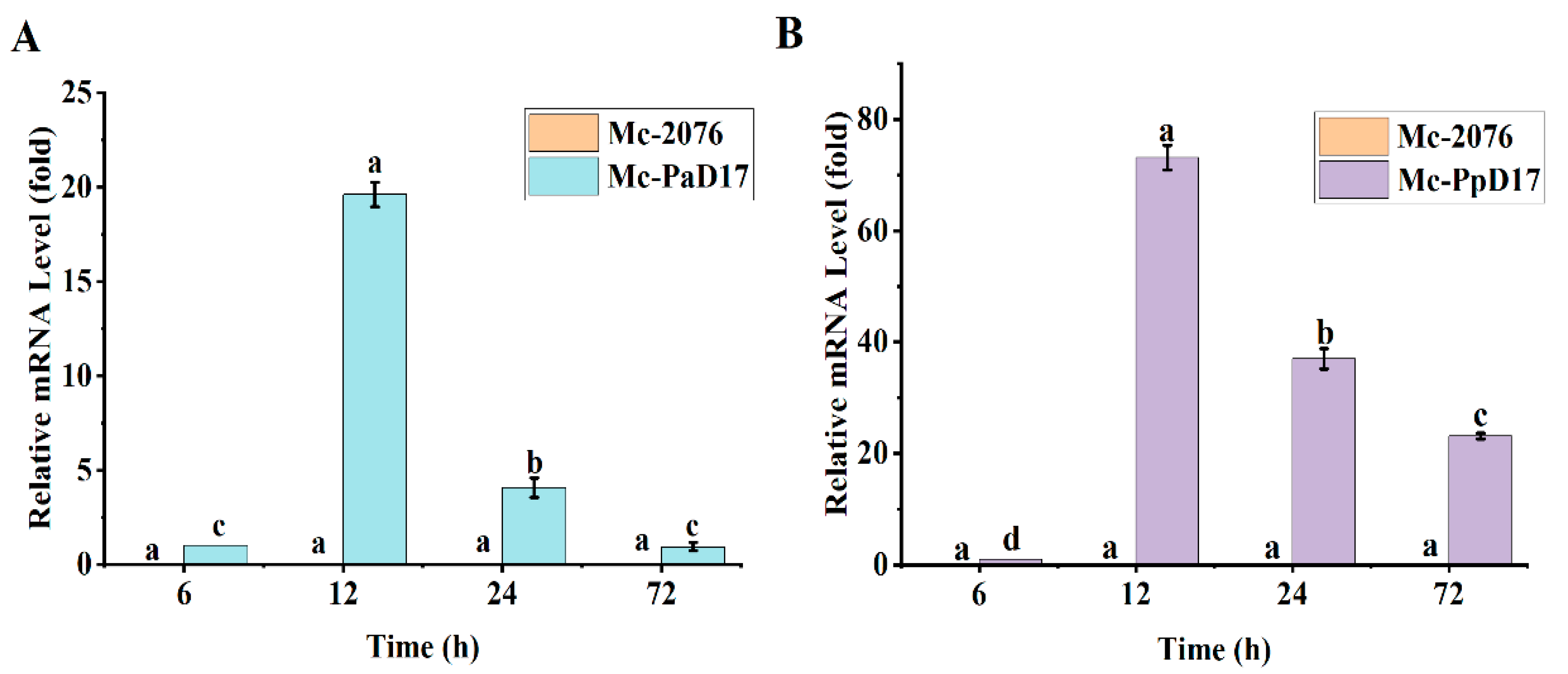
3.2. Expression Levels of Delta-17 Desaturase Gene in Strains
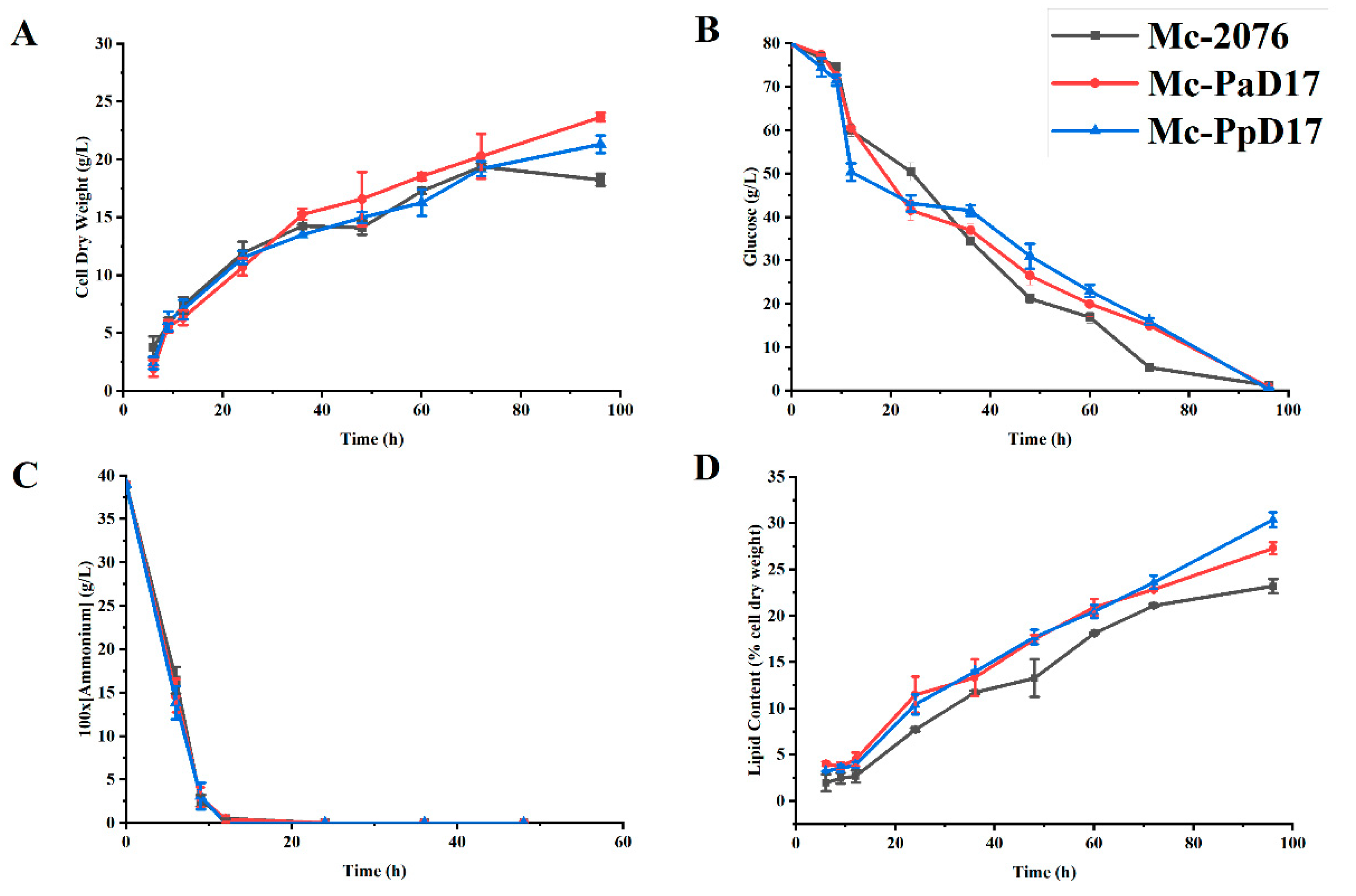
3.3. Cell Growth and Lipid Accumulation in Delta-17 Desaturase Gene Overexpressing Strains
3.4. ETA Accumulation in Delta-17 Desaturase Gene Overexpressing Strains
3.5. Effect of Overexpression of Delta-17 Desaturase Genes on the Transcription Level of Key Genes for Fatty Acid Biosynthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ETA | Eicosatetraenoic acid |
| DGLA | Dihomo-gamma linolenic acid |
| ARA | Arachidonic acid |
| EPA | Eicosapentaenoic acid |
| DPA | Docosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| SDA | Stearidonic acid |
| GLA | γ-Linolenic acid |
| ALA | α-Linolenic acid |
| LA | Linoleic acid |
| OA | Oleic acid |
| SA | Stearic acid |
| TFAs | Total fatty acids |
| CDW | Cell dry weight |
| GC | Gas chromatography |
| PUFAs | Polyunsaturated fatty acids |
| VLCPUFAs | Very-long-chain polyunsaturated fatty acids |
References
- Ghioni, C.; Porter, A.E.A.; Taylor, G.W.; Tocher, D.R. Metabolism of 18:4n-3 (stearidonic acid) and 20:4n-3 in salmonid cells in culture and inhibition of the production of prostaglandin F2α (PGF2α) from 20:4n-6 (arachidonic acid). Fish Physiol. Biochem. 2003, 27, 81–96. [Google Scholar] [CrossRef]
- Xue, Z.; He, H.; Hollerbach, D.; Macool, D.J.; Yadav, N.S.; Zhang, H.; Szostek, B.; Zhu, Q. Identification and characterization of new Δ-17 fatty acid desaturases. Appl. Microbiol. Biotechnol. 2013, 97, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lopez, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ge, J.; Zhang, Y.; Ling, H.; Yan, X.; Ping, W. Monoraphidium sp. HDMA-20 is a new potential source of α-linolenic acid and eicosatetraenoic acid. Lipids Health Dis. 2019, 18, 56. [Google Scholar] [CrossRef]
- Yang, J.; Khan, M.A.K.; López-García, S.; Nosheen, S.; Nazir, Y.; Zhang, H.; Garre, V.; Song, Y. Improved SDA production in high lipid accumulating strain of Mucor circinelloides WJ11 by genetic modification. Am. J. Biochem. Biotechnol. 2020, 16, 138–147. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Yang, J.; Hussain, S.A.; Zhang, H.; Garre, V.; Song, Y. Genetic Modification of Mucor circinelloides to Construct Stearidonic Acid Producing Cell Factory. Int. J. Mol. Sci. 2019, 20, 1683. [Google Scholar] [CrossRef]
- Guo, X.F.; Tong, W.F.; Ruan, Y.; Sinclair, A.J.; Li, D. Different metabolism of EPA, DPA and DHA in humans: A double-blind cross-over study. Prostaglandins Leukot. Essent. Fatty Acids 2020, 158, 102033–102040. [Google Scholar] [CrossRef]
- Conquer, A.J.; Cheryk, L.A.; Chan, E.; Gentry, P.A.; Holub, B.J. Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thromb. Res. 1999, 96, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Croset, M.; Bordet, J.C.; Lagard, M. Inhibition of prostaglandin H synthase and activation of 12-lipoxygenase by 8, 11,14, 17 eicosatetraenoic acid in human endothelial cells and platelets. Biochem. Pharmacol. 1999, 57, 631–638. [Google Scholar] [CrossRef]
- Oie, E.; Ueland, T.; Dahl, C.P.; Bobov, P.; Berge, C.; Yndestad, A.; Gullestad, L.; Aukrust, P.; Berge, R.K. Fatty acid composition in chronic heart failure: Low circulating levels of eicosatetraenoic acid and high levels of vaccenic acid are associated with disease severity and mortality. J. Intern. Med. 2011, 270, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Caleron, M.; Muro-Pastor, A.M.; Garces, R.; Martínez-Force, E. Functional characterization of a plastidial omega-3 desaturase from sunflower (Helianthus annuus) in cyanobacteria. Plant Physiol. Biochem. 2006, 44, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Guo, L.; Zhao, M.; Luo, T.; Zhang, R.; Zhang, F.; Hou, P.; Zhang, Y.; Xu, Y.; Wang, S.; et al. Molecular cloning, characterization, and expression of an omega-3 fatty acid desaturase gene from Sapium sebiferum. J. Biosci. Bioeng. 2008, 106, 375–380. [Google Scholar] [CrossRef]
- Okuda, T.; Ando, A.; Negoro, H.; Muratsubaki, T.; Kikukawa, H.; Sakamoto, T.; Sakuradani, E.; Shimizu, S.; Ogawa, J. Eicosapentaenoic acid (EPA) production by an oleaginous fungus Mortierella alpina expressing heterologous the Δ17-desaturase gene under ordinary temperature. Eur. J. Lipid Sci. Technol. 2015, 117, 1919–1927. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, X.; Li, X.; Wang, H.; Chen, H. The desaturase OPIN17 from Phytophthora infestans converts arachidonic acid to eicosapentaenoic acid in CHO cells. Appl. Biochem. Biotechnol. 2013, 171, 975–988.16. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Chen, H.; Mei, T.; Mei, T.; Tang, X.; Chang, L.; Gu, Z.; Zhang, H.; Chen, W.; Chen, Y.Q. Application of a omega-3 desaturase with an arachidonic acid preference to eicosapentaenoic acid production in Mortierella alpina. Front. Bioeng. Biotechnol. 2017, 5, 89. [Google Scholar] [CrossRef]
- Tang, X.; Chen, H.; Mei, T.; Ge, C.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Characterization of an omega-3 desaturase from phytophthora parasitica and application for eicosapentaenoic acid production in Mortierella alpina. Front. Microbiol. 2018, 9, 1878. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, X.; Zhang, H.; Garre, V.; Song, Y.; Ratledge, C. Improved γ-linolenic acid production in Mucor circinelloides by homologous overexpressing of delta-12 and delta-6 desaturases. Microb. Cell Fact. 2017, 16, 113. [Google Scholar] [CrossRef]
- Miyake, J.A.; Gomes, R.N.; Colquhoun, A. Gamma-Linolenic acid alters migration, proliferation and apoptosis in human and rat glioblastoma cells. Prostaglandins Other Lipid Mediat. 2020, 150, 106452. [Google Scholar] [CrossRef]
- Mishra, D.S.; Singh, S. Microbial Production of γ-Linolenic Acid: An Overview. J. Pharm. Res. 2011, 4, 1–5. [Google Scholar]
- Tang, X.; Zhao, L.; Chen, H.; Chen, Y. Complete genome sequence of a high lipid-producing strain of Mucor circinelloides WJ11 and comparative genome analysis with a low lipid-producing strain CBS277.49. PLoS ONE 2015, 10, e0137543. [Google Scholar] [CrossRef]
- Morin-Sardin, S.; Nodet, P.; Coton, E. Mucor: A Janus-faced fungal genus with human health impact and industrial applications. Fungal Biol. Rev. 2017, 31, 12–32. [Google Scholar] [CrossRef]
- Tang, X.; Chen, H.; Chen, Y.Q.; Chen, W.; Garre, V.; Song, Y.; Ratledge, C. Comparison of biochemical activities between high and low lipid-producing strains of Mucor circinelloides: An explanation for the high oleaginicity of strain WJ11. PLoS ONE 2015, 10, e0128396. [Google Scholar] [CrossRef] [PubMed]
- Ana, M.; Amélie, A.K.; Harrie-van, E.; Eve, S.; Stephen, J.P.; Smita, K.; Peter, J.E. bZIP67 regulates the omega-3 fatty acid content of Arabidopsis seed oil by activating FATTY ACID DESATURASE3. Plant Cell 2013, 25, 3104–3116. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Yang, J.; Hussain, S.A.; Zhang, H.; Liang, L.; Garre, V.; Song, Y. Construction of DGLA producing cell factory by genetic modification of Mucor circinelloides. Microb. Cell Fact. 2019, 18, 64–71. [Google Scholar] [CrossRef]
- Okuda, T.; Ando, A.; Negoro, H.; Kikukawa, H.; Sakamoto, T.; Sakuradani, E.; Shimizu, S.; Ogawa, J. Omega-3 eicosatetraenoic acid production by molecular breeding of the mutant strain S14 derived from Mortierella alpina 1S-4. J. Biosci. Bioeng. 2015, 120, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Stinson, E.E.; Kwoczak, R.; Kurantz, M.J. Effect of cultural conditions on production of eicosapentaenoic acid by Pythium irregulare. J. Ind. Microbiol. 1991, 8, 171–178. [Google Scholar] [CrossRef]
- Stredansky, M.; Conti, E.; Salaris, A. Production of polyunsaturated fatty acids by Pythium ultimum in solid-state cultivation. Enzyme Microb. Technol. 2000, 26, 304–307. [Google Scholar] [CrossRef]
- Wang, X.; Mohamed, H.; Bao, Y.; Wu, C.; Shi, W.; Song, Y.; Yang, J. Heterologous Expression of Two Malate Transporters from an Oleaginous Fungus Mucor circinelloides Improved the Lipid Accumulation in Mucor lusitanicus. Front. Microbiol. 2021, 12, 774825. [Google Scholar] [CrossRef]
- Zan, X.; Tang, X.; Chu, L.; Song, Y. Dual Functions of Lip6 and Its Regulation of Lipid Metabolism in the Oleaginous Fungus Mucor circinelloides. J. Agric. Food Chem. 2018, 66, 2796–2804. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S.; Nickerson, J.W. Induction of yeastlike development in mucor by carbon dioxide. J. Bacteriol. 1962, 84, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, S.; Xiong, X. Metabolic engineering of non-carotenoid-producing yeast Yarrowia lipolytica for the biosynthesis of zeaxanthin. Front. Microbiol. 2021, 12, 699235. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Torres-Martinez, S.; Garre, V. Distinct white collar-1 genes control specific light responses in Mucor circinelloides. Mol. Microbiol. 2006, 61, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Yang, J.; Yang, W.; Wang, X.; Wu, C.; Song, Y. Improved γ-linolenic acid production from cellulose in Mucor circinelloides via coexpression of cellobiohydrolase and delta-6 desaturase. J. Agric. Food Chem. 2022, 70, 4373–4381. [Google Scholar] [CrossRef]
- Gutierrez, A.; Lopez-Garcia, S.; Garre, V. High reliability transformation of the basal fungus Mucor circinelloides by electroporation. J. Microbiol. Methods 2011, 84, 442–446. [Google Scholar] [CrossRef]
- Kendrick, A.; Ratledge, C. Desaturation of polyunsaturated fatty acids in Mucor circinelloides and the involvement of a novel membrane-bound malic enzyme. Eur. J. Biochem. 1992, 209, 667–673. [Google Scholar] [CrossRef]
- Yang, J.; Canovas-Marquez, J.T.; Li, P.; Li, S.; Niu, J.; Wang, X.; Nazir, Y.; Lopea-Garcia, S.; Garre, V.; Song, Y. Deletion of plasma membrane malate transporters increased lipid accumulation in the oleaginous fungus Mucor circinelloides WJ11. J. Agric. Food Chem. 2021, 69, 9632–9641. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Mohamed, H.; Shah, A.M.; Li, S.; Pang, S.; Wu, C.; Xue, F.; Shi, W.; Sadaqat, B.; et al. Simultaneous overexpression of ∆6-, ∆12- and ∆9-desaturases enhanced the production of γ-linolenic acid in Mucor circinelloides WJ11. Front. Microbiol. 2022, 13, 1078157. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Jiang, Z.; Cui, Z.; Zhu, Z.; Liu, Y.; Tang, Y.J.; Hou, J.; Qi, Q. Engineering of Yarrowia lipolytica transporters for high-efficient production of biobased succinic acid from glucose. Biotechnol. Biofuels 2021, 14, 145. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1961, 8, 130–132. [Google Scholar] [CrossRef]
- Shi, H.; Chen, H.; Gu, Z.; Zhang, H.; Chen, W.; Chen, Y.Q. Application of a delta-6 desaturase with alpha-linolenic acid preference on eicosapentaenoic acid production in Mortierella alpina. Microb. Cell Fact. 2016, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wynn, J.P.; Li, Y.; Grantham, D.; Ratledge, C. A pre-genetic study of the isoforms of malic enzyme associated with lipid accumulation in Mucor circinelloides. Microbiology 2001, 147, 1507–1515. [Google Scholar] [CrossRef]
- Hao, G.; Chen, H.; Du, K.; Huang, X.; Song, Y.; Gu, Z.; Wang, L.; Zhang, H.; Chen, W.; Chen, Y.Q. Increased fatty acid unsaturation and production of arachidonic acid by homologous over-expression of the mitochondrial malic enzyme in Mortierella alpina. Biotechnol. Lett. 2014, 36, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, J.; Mohamed, H.; Wang, X.; Pang, S.; Wu, C.; Lopez-Garcia, S.; Song, Y. Identification and functional characterization of adenosine deaminase in Mucor circinelloides: A novel potential regulator of nitrogen utilization and lipid biosynthesis. J. Fungi 2022, 8, 774. [Google Scholar] [CrossRef]
- Takeno, S.; Sakuradani, E.; Murata, S.; Inohara-Ochiai, M.; Kawashima, H.; Ashikari, T.; Shimizua, S. Molecular evidence that the rate-limiting step for the biosynthesis of arachidonic acid in Mortierella alpina is at the level of an elongase. Lipids 2005, 40, 25–30. [Google Scholar] [CrossRef]
- Qiu, X.; Xie, X.; Meesapyodsuk, D. Molecular mechanisms for biosynthesis and assembly of nutritionally important very long chain polyunsaturated fatty acids in microorganisms. Prog. Lipid Res. 2020, 79, 101047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meesapyodsuk, D.; Qiu, X. Transgenic production of omega-3 very long chain polyunsaturated fatty acids in plants: Accomplishment and challenge. Biocatal. Agric. Biotechnol. 2014, 3, 38–43. [Google Scholar] [CrossRef]
- Yazawa, H.; Iwahashi, H.; Kamisaka, Y.; Kimura, K.; Aki, T.; Ono, K.; Uemura, H. Heterologous production of dihomo-gamma-linolenic acid in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 6965–6971. [Google Scholar] [CrossRef]
- Li, Y.T.; Li, M.T.; Fu, C.H.; Zhou, P.P.; Liu, J.M.; Yu, L.J. Improvement of arachidonic acid and eicosapentaenoic acid production by increasing the copy number of the genes encoding fatty acid desaturase and elongase into Pichia pastoris. Biotachnol. Lett. 2009, 31, 1011–1017. [Google Scholar] [CrossRef]
- Sakuradani, E.; Abe, T.; Iguchi, K.; Shimizu, S. A novel fungal ω3-desaturase with wide substrate specificity from arachidonic acid-producing Mortierella alpina 1S-4. Appl. Microbiol. Biotechnol. 2005, 66, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Chen, H.; Tang, X.; Gu, Z.; Zhao, J.; Zhang, H.; Chen, W.; Chen, Y.Q. Characterization and molecular docking of new Delta17 fatty acid desaturase genes from Rhizophagus irregularis and Octopus bimaculoides. RSC Adv. 2019, 9, 6871–6880. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, G.; Meng, Z.; Lin, L.; Sui, N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Adams, I.P.; Ratledge, C. Malic enzyme: The controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiology 2007, 153, 2013–2025. [Google Scholar] [CrossRef]
- Li, Z.; Sun, H.; Mo, X.; Li, X.; Xu, B.; Tian, P. Overexpression of malic enzyme (ME) of Mucor circinelloides improved lipid accumulation in engineered Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 4927–4936. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Wang, R.; Haque, M.E.; Liu, A. Overexpression of ACC gene from oleaginous yeast lipomyces starkeyi enhanced the lipid accumulation in Saccharomyces cerevisiae with increased levels of glycerol 3-phosphate substrates. Biosci. Biotechnol. Biochem. 2016, 80, 1214–1222. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Roskams, T.; Joniau, S.; Van Poppel, H.; Oyen, R.; Baert, L.; Heyns, W.; Verhoeven, G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int. J. Cancer 2002, 98, 19–22. [Google Scholar] [CrossRef]
- Kajiwara, S.; Oura, T.; Shishido, K. Cloning of a fatty acid synthase component FAS1 gene from Saccharomyces kluyveri and its functional complementation of S. cerevisiae fas1 mutant. Yeast Seq. Rep. 2001, 18, 1339–1345. [Google Scholar] [CrossRef]


| Time (Hours) | Fatty Acid Composition (Relative %, w/w) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C 16:0 | C 18:0 SA (%) | C 18:1 OA (%) | C 18:2 LA (%) | C 18:3 GLA (%) | C 18:3 ALA (%) | C 18:4 SDA (%) | C 20:3 DGLA (%) | C 20:4 ETA (%) | C (20:4) ETA mg/L | |
| Mc-2076 | ||||||||||
| 24 | 13.07 ± 0.24 | 6.17 ± 0.67 | 36.59 ± 0.37 | 12.85 ± 0.58 | 10.60 ± 1.10 | 1.26 ± 0.11 | 0.76 ± 0.01 | 9.44 ± 0.21 | - | - |
| 36 | 14.80 ± 0.31 | 7.01 ± 0.79 | 38.56 ± 0.51 | 12.91 ± 0.08 | 8.03 ± 0.03 | 1.33 ± 0.25 | 0.73 ± 0.05 | 7.36 ± 0.91 | - | - |
| 48 | 15.14 ± 0.01 | 6.55 ± 0.74 | 37.78 ± 0.05 | 13.66 ± 0.18 | 8.03 ± 0.34 | 1.08 ± 0.15 | 0.58 ± 0.05 | 8.27 ± 0.60 | - | - |
| 72 | 16.26 ± 0.15 | 6.45 ± 0.39 | 38.23 ± 0.33 | 13.84 ± 0.01 | 7.47 ± 0.45 | 1.28 ± 0.03 | 0.48 ± 0.76 | 7.74 ± 0.19 | - | - |
| 96 | 17.46 ± 0.23 | 6.52 ± 0.34 | 38.79 ± 0.52 | 14.36 ± 0.24 | 7.24 ± 0.39 | 1.34 ± 0.03 | 0.49 ± 0.36 | 7.05 ± 0.23 | - | - |
| Mc-PaD17 | ||||||||||
| 24 | 14.14 ± 1.01 | 7.89 ± 0.74 | 35.74 ± 2.20 | 15.23 ± 1.40 | 15.31 ± 2.91 | 1.02 ± 0.13 | 0.88 ± 0.12 | 5.30 ± 0.04 | - | - |
| 36 | 15.79 ± 0.35 | 8.68 ± 0.62 | 38.35 ± 1.75 | 14.40 ± 1.00 | 11.77 ± 1.60 | 1.18 ± 0.07 | 0.8 ± 0.07 | 7.16 ± 0.61 | - | - |
| 48 | 17.39 ± 0.40 | 8.46 ± 0.88 | 40.35 ± 0.27 | 13.32 ± 0.07 | 8.90 ± 0.14 | 1.35 ± 0.16 | 0.67 ± 0.16 | 6.98 ± 0.27 | - | - |
| 72 | 18.13 ± 1.14 | 8.94 ± 0.28 | 39.16 ± 0.96 | 14.16 ± 0.33 | 9.03 ± 0.30 | 1.69± 0.23 | 0.54 ± 0.06 | 5.29± 0.04 | - | - |
| 96 | 18.69 ± 0.26 | 8.14 ± 0.09 | 41.37 ± 0.52 | 13.47 ± 0.26 | 8.29 ± 0.44 | 1.47 ± 0.70 | 0.50 ± 0.03 | 5.12± 0.07 | - | - |
| Mc-PpD17 | ||||||||||
| 24 | 11.89 ± 0.35 | 4.69 ± 0.72 | 40.10 ± 0.72 | 14.44 ± 0.55 | 9.41 ± 0.74 | 2.68 ± 0.07 | 1.87 ± 0.17 | 5.36 ± 0.26 | 5.11 ± 0.65 | 68.49 ± 0.55 |
| 36 | 13.69 ± 0.40 | 5.36 ± 0.88 | 41.35 ± 0.27 | 15.90 ± 0.07 | 7.89 ± 0.14 | 2.35 ± 0.16 | 1.49 ± 0.16 | 5.32 ± 0.27 | 4.44 ± 0.37 | 55.72 ± 2.03 |
| 48 | 14.98 ± 0.22 | 5.68 ± 0.81 | 43.28 ± 0.98 | 15.22 ± 0.31 | 6.22 ± 0.90 | 2.03 ± 0.03 | 1.15 ± 0.03 | 5.42 ± 0.23 | 3.80 ± 0.23 | 86.60 ± 0.21 |
| 72 | 15.30 ± 0.37 | 4.82 ± 0.07 | 43.24 ± 0.73 | 15.17 ± 0.30 | 5.50 ± 0.70 | 1.47 ± 0.70 | 0.96 ± 0.03 | 6.09 ± 0.07 | 3.56 ± 0.18 | 105.19 ± 3.33 |
| 96 | 17.27 ± 0.29 | 5.64 ± 0.15 | 45.33± 1.29 | 14.91 ± 0.57 | 5.37 ± 0.75 | 1.20 ± 0.09 | 0.69 ± 0.16 | 4.98 ± 0.11 | 1.93 ± 0.03 | 110.27 ± 6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Yang, J.; Li, S.; Shi, W.; Xue, F.; Liu, Q.; Naz, T.; Mohamed, H.; Song, Y. Construction of Eicosatetraenoic Acid Producing Cell Factory by Genetic Engineering of Mucor circinelloides. Fermentation 2023, 9, 653. https://doi.org/10.3390/fermentation9070653
Wu C, Yang J, Li S, Shi W, Xue F, Liu Q, Naz T, Mohamed H, Song Y. Construction of Eicosatetraenoic Acid Producing Cell Factory by Genetic Engineering of Mucor circinelloides. Fermentation. 2023; 9(7):653. https://doi.org/10.3390/fermentation9070653
Chicago/Turabian StyleWu, Chen, Junhuan Yang, Shaoqi Li, Wenyue Shi, Futing Xue, Qing Liu, Tahira Naz, Hassan Mohamed, and Yuanda Song. 2023. "Construction of Eicosatetraenoic Acid Producing Cell Factory by Genetic Engineering of Mucor circinelloides" Fermentation 9, no. 7: 653. https://doi.org/10.3390/fermentation9070653
APA StyleWu, C., Yang, J., Li, S., Shi, W., Xue, F., Liu, Q., Naz, T., Mohamed, H., & Song, Y. (2023). Construction of Eicosatetraenoic Acid Producing Cell Factory by Genetic Engineering of Mucor circinelloides. Fermentation, 9(7), 653. https://doi.org/10.3390/fermentation9070653







