Exploring Lactobacillus plantarum on Fermentation Quality, Gas Emissions, and In Vitro Digestibility of Different Varieties of Litchi Leaves Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Analysis of Microbial Population, Organic Acid and Chemical Composition
2.3. In Vitro Dry Matter Digestibility and Gas Production
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Fresh Litchi Leaves Prior to Ensiling
3.2. Fermentation Quality
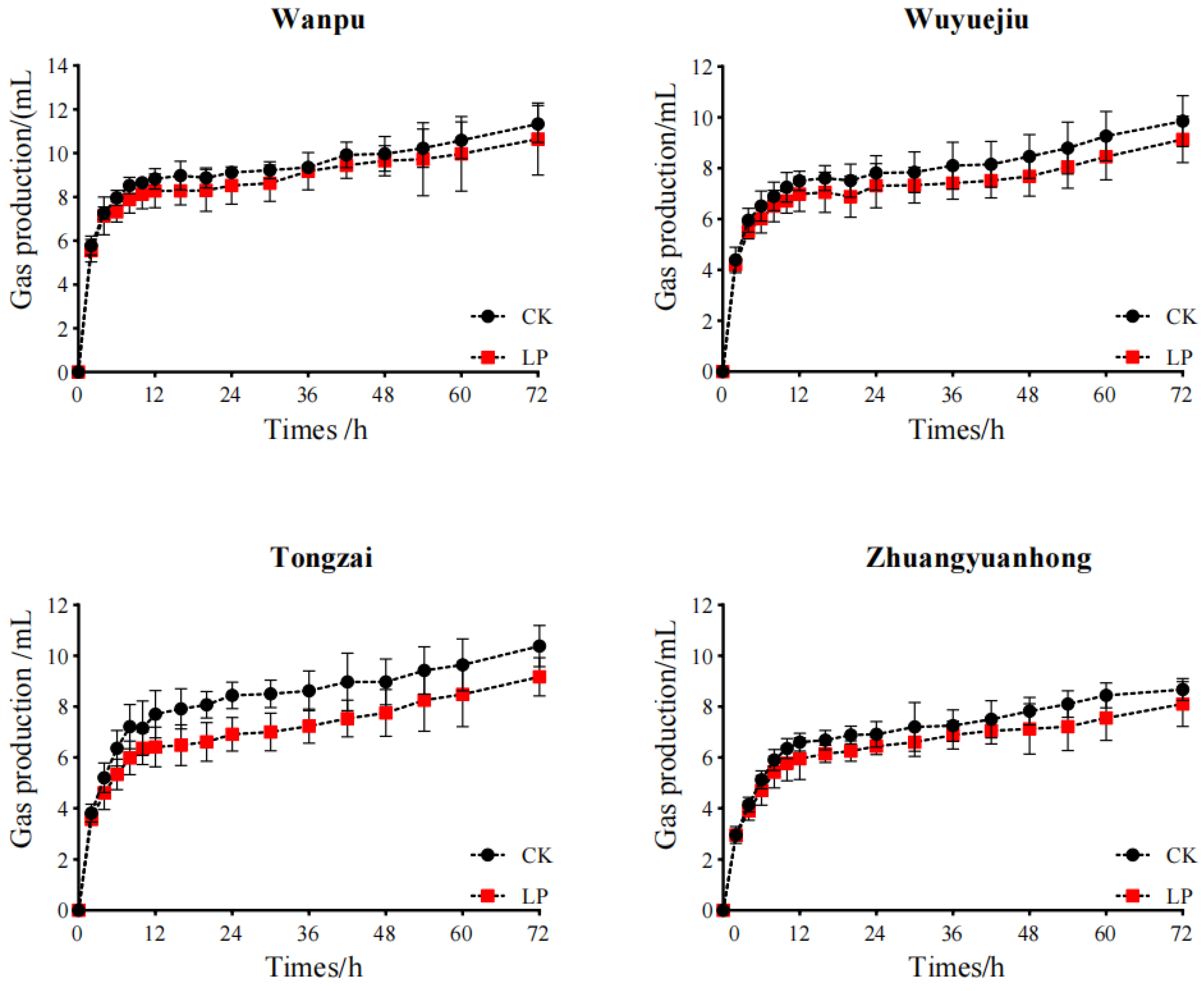
3.3. In Vitro Dry Matter Digestibility and Gas Production
4. Discussion
4.1. Characteristics of Fresh Litchi Leaves
4.2. Fermentation Quality
4.3. In Vitro Dry Matter Digestibility and Gas Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, J.; Ding, Z.; Su, R.; Wang, M.; Cheng, M.; Xie, D.; Guo, X. Storage temperature is more effective than lactic acid bacteria inoculations in manipulating fermentation and bacterial community diversity, co-occurrence and functionality of the whole-plant corn silage. Microbiol. Spectr. 2022, 10, e00101-22. [Google Scholar] [CrossRef] [PubMed]
- Besra, S.E.; Sharma, R.M.; Gomes, A. Antiinflammatory effect of petroleum ether extract of leaves of Litchi chinensis gaertn. (Sapindaceae). J. Ethnopharmacol. 1996, 54, 1–6. [Google Scholar] [CrossRef]
- Thiesen, L.C.; Nunes, M.L.D.O.; Meyre-Silva, C.; Pastor, V.D.; de Andrade, S.F.; Couto, A.G.; da Silva, L.M.; Bresolin, T.M.B.; Santin, J.R. The hydroethanolic Litchi chinensis leaf extract alleviate hepatic injury induced by carbon tetrachloride (CCl4) through inhibition of hepatic inflammation. Biomed. Pharmacother. 2018, 107, 929–936. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Sales, G.; Schwan, R.F.; Avila, C. Criteria for lactic acid bacteria screening to enhance silage quality. Appl. Microbiol. Int. 2021, 130, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Castellain, R.C.; Gesser, M.; Tonini, F.; Schulte, R.V.; Demessiano, K.Z.; Wolff, F.R.; Delle-Monache, F.; Netz, D.J.; Cechinel-Filho, V.; de Freitas, R.A.; et al. Chemical composition, antioxidant and antinociceptive properties of Litchi chinensis leaves. J. Pharm. Pharmacol. 2014, 66, 1796–1807. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, M.; Guo, X.; Chen, X.; Zhang, Q. Altering bacterial community: A possible way of lactic acid bacteria inoculants reducing CO2 production and nutrient loss during fermentation. Bioresour. Technol. 2021, 329, 124915. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, M.; Zhou, Y.; Gao, L.; Zhou, W.; Wang, M.; Zhu, Y.; Xu, W. Improving the quality of Napier grass silage with pyroligneous acid: Fermentation, aerobic stability, and microbial communities. Front. Microbiol. 2022, 13, 1034198. [Google Scholar] [CrossRef]
- Chen, L.; Guo, G.; Yuan, X.; Zhang, J.; Li, J.; Shao, T. Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 2016, 95, 1678–1685. [Google Scholar] [CrossRef]
- Du, Z.; Lin, Y.; Sun, L.; Yang, F.; Cai, Y. Microbial community structure, co-occurrence network and fermentation characteristics of woody plant silage. J. Sci. Food Agric. 2022, 102, 1193–1204. [Google Scholar] [CrossRef]
- Fang, D.; Dong, Z.; Wang, D.; Li, B.; Shi, P.; Yan, J.; Zhuang, D.; Shao, T.; Wang, W.; Gu, M. Evaluating the fermentation quality and bacterial community of high-moisture whole-plant quinoa silage ensiled with different additives. J. Appl. Microbiol. 2022, 132, 3578–3589. [Google Scholar] [CrossRef]
- Fant, P.; Ramin, M.; Jaakkola, S.; Grimberg, Å.; Carlsson, A.S.; Huhtanen, P. Effects of different barley and oat varieties on methane production, digestibility, and fermentation pattern in vitro. J. Dairy Sci. 2019, 103, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Alfonso, M.; Depino, S.; Alessandri, E. Effect of planting density on nutritional quality of green-chopped corn for silage. J. Dairy Sci. 2014, 97, 5918–5921. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Guo, X.; Wu, S.; Chen, D.; Ge, L.; Zhou, W.; Zhang, Q.; Pian, R. Tannin tolerance lactic acid bacteria screening and their effects on fermentation quality of stylo and soybean silages. Front. Microbiol. 2022, 13, 991387. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, T.; Fliegerová, K.; Zebeli, Q. Suitability of anaerobic fungi culture supernatant or mixed ruminal fluid as novel silage additives. J. Appl. Microbiol. 2022, 106, 6819–6832. [Google Scholar] [CrossRef]
- He, L.; Zhou, W.; Wang, C.; Yang, F.; Chen, X.; Zhang, Q. Effect of cellulase and Lactobacillus casei on ensiling characteristics, chemical composition, antioxidant activity, and digestibility of mulberry leaf silage. J. Dairy Sci. 2019, 102, 9919–9931. [Google Scholar] [CrossRef] [PubMed]
- Hisham, M.B.; Hashim, A.M.; Hanafi, N.M.; Rahman, N.A.; Mutalib, N.E.A.; Tan, C.K.; Nazli, M.H.; Yusoff, N.F.M. Bacterial communities associated with silage of different forage crops in Malaysian climate analysed using 16S amplicon metagenomics. Sci. Rep. 2022, 12, 7107. [Google Scholar] [CrossRef]
- Killerby, M.A.; Almeida, S.T.R.; Hollandsworth, R.; Guimaraes, B.C.; Leon-Tinoco, A.; Perkins, L.B.; Henry, D.; Schwartz, T.J.; Romero, J.J. Effect of chemical and biological preservatives and ensiling stage on the dry matter loss, nutritional value, microbial counts, and ruminal in vitro gas production kinetics of wet brewer’s grain silage. J. Anim. Sci. 2022, 100, skac095. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chang, J.-C.; Cheng, S.-Y.; Wang, C.-M.; Jhan, Y.-L.; Lo, I.-W.; Hsu, Y.-M.; Liaw, C.-C.; Hwang, C.-C.; Chou, C.-H. New bioactive chromanes from Litchi chinensis. J. Agric. Food Chem. 2015, 63, 2472–2478. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, G.Q.; Wei, S.N.; Kim, H.J.; Li, Y.F.; Kim, J.G. Changes in fermentation pattern and quality of Italian ryegrass (Lolium multiflorum Lam.) silage by wilting and inoculant treatments. Anim. Biosci. 2021, 34, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Wu, H.; Meng, Q.; Khan, M.Z.; Zhou, Z. Effect of hybrid type on fermentation and nutritional parameters of whole plant corn silage. Animals 2021, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Mamun, F.; Rahman, M.; Zamila, M.; Subhan, N.; Hossain, H.; Hasan, S.R.; Alam, A.; Haque, A. Polyphenolic compounds of litchi leaf augment kidney and heart functions in 2K1C rats. J. Funct. Foods 2020, 64, 103662. [Google Scholar] [CrossRef]
- Morsy, T.A.; Gouda, G.A.; Kholif, A.E. In vitro fermentation and production of methane and carbon dioxide from rations containing Moringa oleifera leave silage as a replacement of soybean meal: In vitro assessment. Environ. Sci. Pollut. Res. R 2022, 29, 69743–69752. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Wang, X.; Lu, Y.; Guo, L.; Li, X.; Yang, F. Exploring the silage quality of alfalfa ensiled with the residues of astragalus and hawthorn. Bioresoure Technol. 2020, 297, 122249. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef]
- Pan, L.; Harper, K.; Queiroz, O.; Copani, G.; Cappellozza, B.I. Effects of a Bacillus-based direct-fed microbial on in vitro nutrient digestibility of forage and high-starch concentrate substrates. Transl. Anim. Sci. 2022, 6, txac067. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E. Dry matter level effects on alfalfa silage quality I. nitrogen transformations Trans. ASAE 1987, 30, 7–14. [Google Scholar] [CrossRef]
- Thiesen, L.C.; Zonta, S.L.; Sobral, C.R.F.; Ferreira, R.A.; Sant’ana, R.; Meyre-Silva, C.; Santin, J.R.; Cruz, A.B.; Bresolin, T.M.B.; Couto, A.G. Quality control of litchi chinensis leaf: A potential raw material for cosmetic industry. Rev. Bras. Farmacogn. 2020, 30, 139–144. [Google Scholar] [CrossRef]
- Tian, J.; Yin, X.; Zhang, J. Effects of wilting during a cloudy day and storage temperature on the fermentation quality and microbial community of Napier grass silage. J. Sci. Food Agric. 2022, 102, 4384–4391. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of mixing Neolamarckia cadamba leaves on fermentation quality, microbial community of high moisture alfalfa and stylo silage. Microb. Biotechnol. 2019, 12, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pian, R.; Chen, X.; Zhang, Q. Effects of polyphenol oxidases on proteolysis and lipolysis during ensiling of Moringa oleifera leaves with or without pyrocatechol. Anim. Feed Sci. Technol. 2021, 275, 114870. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Z.; Li, J.; Chen, L.; Shao, T. Pediococcus acidilactici strains as silage inoculants for improving the fermentation quality, nutritive value and in vitro ruminal digestibility in different forages. J. Appl. Microbiol. 2019, 126, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Shi, W.; Sun, J.; Xia, T.; Huang, F.; Liu, Y.; Li, H.; Teng, K.; Zhong, J. Dynamic changes in fermentation quality and structure and function of the microbiome during mixed silage of sesbania cannabina and sweet sorghum grown on saline-alkaline land. Microbiol. Spectr. 2022, 10, e02483-22. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Xie, Y.; Zhang, Y.; Lin, Y.; Zheng, Y.; Yang, X.; Wang, N.; Ni, K.; Yang, F. Effect of sucrose and lactic acid bacteria additives on fermentation quality, chemical composition and protein fractions of two typical woody forage silages. Agriculture 2021, 11, 256. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, C.; He, L.; Zhou, W.; Yang, F.; Zhang, Q. The bacterial community and fermentation quality of mulberry (Morus alba) leaf silage with or without Lactobacillus casei and sucrose. Bioresoure Technol. 2019, 293, 122059. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; He, J.; Wu, D.; Jiang, Y.; Prasad, K.N.; Zhao, M.; Lin, S.; Jiang, G.; Luo, W.; Yang, B. Identification of sesquilignans in litchi (Litchi chinensis Sonn.) leaf and their anticancer activities. J. Funct. Foods 2014, 8, 26–34. [Google Scholar] [CrossRef]
- Wen, L.; Wu, D.; Jiang, Y.; Prasad, K.N.; Lin, S.; Jiang, G.; He, J.; Zhao, M.; Luo, W.; Yang, B. Identification of flavonoids in litchi (Litchi chinensis Sonn.) leaf and evaluation of anticancer activities. J. Funct. Foods 2014, 6, 555–563. [Google Scholar] [CrossRef]
- Wen, L.; You, L.; Yang, X.; Yang, J.; Chen, F.; Jiang, Y.; Yang, B. Identification of phenolics in litchi and evaluation of anticancer cell proliferation activity and intracellular antioxidant activity. Free. Radic. Biol. Med. 2015, 84, 171–184. [Google Scholar] [CrossRef]
- Wen, L. Identification of Bioactive Compounds in Litchi (Litchi chinensis sonn) Leaf and their Bioactivities; South China University of Technology: Guangzhou, China, 2013. [Google Scholar]
- Yi, Q.; Wang, P.; Tang, H.; Yu, M.; Zhao, T.; Sheng, Z.; Luo, H. Fermentation quality, in vitro digestibility, and aerobic stability of ensiling spent mushroom substrate with microbial additives. Animals 2023, 13, 920. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, X.; Wu, S.; Wu, N.; Chen, X.; Zhou, W. Effects of pyroligneous acid on diversity and dynamics of antibiotic resistance genes in alfalfa silage. Microbiol. Spectr. 2022, 10, e01554-22. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, F.; Wang, Y.; Fan, X.; Feng, C.; Wang, Y. Dynamics of fermentation parameters and bacterial community in high-moisture alfalfa silage with or without lactic acid bacteria. Microorganisms 2021, 9, 1225. [Google Scholar] [CrossRef]
- Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. The sustainable mitigation of ruminal methane and carbon dioxide emissions by co-ensiling corn stalk with Neolamarckia cadamba leaves for cleaner livestock production. J. Clean. Prod. 2021, 311, 127680. [Google Scholar] [CrossRef]

| Item | Wanpu | Wuyejiu | Tongzai | Zhuangyuanhong |
|---|---|---|---|---|
| Dry matter (%FM) | 49.21 ± 0.27 | 49.29 ± 0.19 | 49.87 ± 0.12 | 44.96 ± 0.25 |
| Crude protein ( % DM) | 9.92 ± 0.25 | 9.73 ± 0.23 | 8.79 ± 1.14 | 9.29 ± 0.40 |
| Neutral detergent fiber (% DM) | 64.89 ± 1.60 | 63.36 ± 0.64 | 66.14 ± 3.63 | 59.54 ± 0.12 |
| Acid detergent fiber (% DM) | 51.21 ± 2.41 | 51.27 ± 2.71 | 52.24 ± 2.35 | 46.36 ± 1.19 |
| Water soluble carbohydrate (% DM) | 1.62 ± 0.15 | 1.72 ± 0.12 | 1.67 ± 0.04 | 1.86 ± 0.06 |
| Lactic acid bacteria (LAB, log10 CFU·g−1 FM) | 4.69 ± 0.24 | 4.67 ± 0.42 | 5.11 ± 0.74 | 4.88 ± 0.37 |
| Yeasts (log10 CFU·g−1 FM) | 2.92 ± 0.26 | 2.15 ± 0.21 | 2.49 ± 0.20 | 2.42 ± 0.10 |
| Molds (log10 CFU·g−1 FM) | 2.63 ± 0.31 | 2.30 ± 0.30 | 2.36 ± 0.39 | 2.46 ± 0.41 |
| Coliform bacteria (CB, log10 CFU·g−1 FM) | 4.53 ± 0.63 | 4.26 ± 0.94 | 4.75 ± 0.16 | 4.44 ± 0.11 |
| Items | Treatment | Day | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| D3 | D7 | D14 | D30 | T | D | T*D | |||
| pH value | CK | 5.71 a | 5.60 abA | 5.47 abA | 5.39 bA | 0.05 | ** | ** | NS |
| LP | 5.18 a | 4.99 bB | 4.97 bB | 4.93 bB | 0.03 | ||||
| DM(% FM) | CK | 48.4 ab | 48.0 ab | 47.8 b | 49.0 a | 0.20 | * | * | NS |
| LP | 48.3 b | 48.9 ab | 48.6 ab | 49.3 a | 0.14 | ||||
| LAB (log10 CFU·g−1 FM) | CK | 7.87 bB | 8.42 aB | 7.49 cB | 7.88 b | 0.10 | ** | ** | ** |
| LP | 8.57 bA | 9.06 aA | 8.54 bA | 8.08 c | 0.11 | ||||
| Yeasts (log10 CFU·g−1 FM) | CK | 2.60 | 2.50 | 2.41 | 2.15 | 0.55 | ND | ND | ND |
| LP | 2.45 | 2.15 | ND | ND | 0.12 | ||||
| Molds (log10 CFU·g−1 FM) | CK | 2.50 | 2.40 | ND | ND | 0.13 | ND | ND | ND |
| LP | 2.57 | 2.26 | ND | ND | 0.14 | ||||
| Coliform bacteria (log10 CFU·g−1 FM) | CK | 7.38 abA | 7.72 aA | 7.20 bA | 7.18 bA | 0.09 | ** | * | NS |
| LP | 6.66 B | 6.58 B | 6.08 B | 6.22 B | 0.11 | ||||
| Lactic acid (%DM) | CK | 1.57 | 0.71 | 0.51 | 0.39 | 0.21 | NS | NS | NS |
| LP | 1.84 | 1.87 | 1.40 | 1.00 | 0.30 | ||||
| Acetic acid (%DM) | CK | ND | ND | ND | 0.11 | ND | ND | ND | ND |
| LP | 0.11 | 0.09 | 0.10 | 0.15 | 0.01 | ||||
| Crude protein ( %DM) | CK | 10.00 ab | 10.00 ab | 10.70 a | 9.55 b | 0.18 | * | NS | NS |
| LP | 10.10 | 10.00 | 10.30 | 10.00 | 0.12 | ||||
| Ammonia-N (%TN) | CK | 0.29 b | 0.55 b | 0.74 b | 1.99 aA | 0.20 | ** | ** | ** |
| LP | 0.25 c | 0.40 c | 0.69 b | 0.95 aB | 0.09 | ||||
| Items | Treatment | Day | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| D3 | D7 | D14 | D30 | T | D | T*D | |||
| pH value | CK | 5.75 bA | 5.75 bA | 5.89 aA | 5.69 bA | 0.02 | ** | ** | ** |
| LP | 5.20 aB | 5.03 bB | 5.00 bB | 4.82 cB | 0.04 | ||||
| DM (%FM) | CK | 48.3 | 47.6 | 47.4 | 48.1 | 0.15 | * | NS | NS |
| LP | 48.7 | 48.9 | 48.0 | 48.5 | 0.21 | ||||
| LAB (log10 CFU·g−1 FM) | CK | 7.48 bB | 8.10 aB | 7.96 a | 7.40 b | 0.10 | ** | ** | ** |
| LP | 8.47 aA | 8.71 aA | 7.87 b | 7.85 b | 0.13 | ||||
| Yeasts (log10 CFU·g−1 FM) | CK | 2.44 | ND | ND | ND | ND | ND | ND | ND |
| LP | ND | ND | ND | ND | ND | ||||
| Molds (log10 CFU·g−1 FM) | CK | ND | ND | ND | ND | ND | ND | ND | ND |
| LP | ND | ND | ND | ND | ND | ||||
| Coliform bacteria (CB, log10 CFU·g−1 FM) | CK | 7.45 bA | 7.81 aA | 7.50 bA | 7.49 bA | 0.05 | ** | ** | ** |
| LP | 6.54 aB | 6.10 abB | 5.79 bB | 5.07 cB | 0.17 | ||||
| Lactic acid (LA, %DM) | CK | 1.16 | 0.59 a | 0.60 | 0.52 B | 0.11 | ** | NS | ** |
| LP | 0.65 b | 1.57 ab | 1.23 ab | 2.12 aA | 0.20 | ||||
| Acetic acid (AA,%DM) | CK | 0.08 | ND | ND | ND | ND | ND | ND | ND |
| LP | ND | 0.09 ab | 0.06 b | 0.12 a | 0.01 | ||||
| Crude protein (CP, %DM) | CK | 9.51 | 10.10 | 9.74 | 9.31 | 0.17 | NS | NS | NS |
| LP | 9.65 | 10.00 | 10.30 | 9.21 | 0.18 | ||||
| Ammonia-N (%TN) | CK | 0.34 c | 0.36 cB | 0.70 b | 1.50 aA | 0.14 | ** | ** | ** |
| LP | 0.41 b | 0.47 bB | 0.54 ab | 0.71 aB | |||||
| Items | Treatment | Day | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| D3 | D7 | D14 | D30 | T | D | T*D | |||
| pH value | CK | 5.54 bA | 5.64 a | 5.57 abA | 5.42 cA | 0.03 | ** | ** | * |
| LP | 5.24 bB | 5.55 a | 5.00 bB | 4.96 bB | 0.08 | ||||
| DM (%FM) | CK | 49.1 | 48.4 | 48.6 | 49.2 | 0.18 | NS | * | NS |
| LP | 49.6 a | 48.5 b | 48.8 b | 49.0 ab | 0.16 | ||||
| LAB (log10 CFU·g−1 FM) | CK | 7.68 bB | 8.15 aB | 7.80 bB | 7.24 cB | 0.11 | ** | ** | NS |
| LP | 8.45 bA | 8.88 aA | 8.33 bA | 7.80 cA | 0.12 | ||||
| Yeasts (log10 CFU·g−1 FM) | CK | ND | ND | ND | ND | ND | ND | ND | ND |
| LP | ND | ND | ND | ND | ND | ||||
| Molds (log10 CFU·g−1 FM) | CK | ND | ND | ND | 3.15 | ND | ND | ND | ND |
| LP | ND | ND | ND | ND | ND | ||||
| Coliform bacteria (CB, log10 CFU·g−1 FM) | CK | 7.82 a | 7.73 a | 7.64 aA | 7.09 bA | 0.10 | ** | ** | * |
| LP | 7.46 a | 7.59 a | 6.65 bB | 5.91 bB | 0.23 | ||||
| Lactic acid (LA, %DM) | CK | 1.58 a | 1.29 abB | 0.69 ab | 0.37 b | 0.20 | ** | NS | NS |
| LP | 3.07 | 4.70 A | 2.32 | 2.11 | 0.59 | ||||
| Acetic acid (AA,%DM) | CK | ND | ND | ND | 0.06 | ND | NS | NS | NS |
| LP | 0.13 | 0.11 | 0.10 | 0.07 | 0.02 | ||||
| Crude protein (CP, %DM) | CK | 9.02 | 8.68 | 8.45 | 9.00 | 0.16 | NS | NS | NS |
| LP | 9.04 | 8.45 | 9.21 | 9.29 | 0.15 | ||||
| Ammonia-N (%TN) | CK | 0.34 c | 0.42 c | 0.73 b | 1.16 aA | 0.10 | NS | ** | * |
| LP | 0.32 b | 0.66 a | 0.73 a | 0.80 aB | 0.07 | ||||
| Items | Treatment | Day | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| D3 | D7 | D14 | D30 | T | D | T*D | |||
| pH value | CK | 5.82 bA | 6.64 aA | 5.65 cA | 5.53 dA | 0.13 | ** | ** | NS |
| LP | 5.05 bB | 5.66 aB | 4.88 bB | 4.87 bB | 0.10 | ||||
| DM (% FM) | CK | 44.4 a | 43.8 b | 44.1 ab | 44.7 a | 0.12 | ** | ** | NS |
| LP | 44.6 ab | 44.4 b | 44.3 b | 45.0 a | 0.11 | ||||
| LAB (log10 CFU·g−1 FM) | CK | 7.72 bB | 8.24 aB | 7.68 bB | 7.08 cB | 0.13 | ** | ** | NS |
| LP | 8.64 bA | 8.99 aA | 8.51 bA | 7.88 cA | 0.13 | ||||
| Yeasts (log10 CFU·g−1 FM) | CK | 3.13 | 3.06 | 2.38 | 2.66 | 0.14 | ND | ND | ND |
| LP | 2.64 | ND | ND | ND | ND | ||||
| Molds (log10 CFU·g−1 FM) | CK | ND | ND | ND | 2.15 | ND | ND | ND | ND |
| LP | ND | ND | ND | ND | ND | ||||
| Coliform bacteria (CB, log10 CFU·g−1 FM) | CK | 7.75 aA | 7.95 aA | 7.69 aA | 6.76 bA | 0.15 | ** | ** | * |
| LP | 6.81 aB | 6.60 aB | 5.76 bB | 5.21 bB | 0.21 | ||||
| Lactic acid (LA, %DM) | CK | 0.86 | 0.13 cB | 0.58 b | 0.49 | 0.15 | NS | * | NS |
| LP | 0.27 c | 0.65 A | 0.82 | 1.32 a | 0.15 | ||||
| Acetic acid (AA,%DM) | CK | 0.06 | 0.10 A | 0.09 | 0.08 | 0.01 | NS | NS | NS |
| LP | ND | 0.04 bB | 0.12 a | 0.09 ab | 0.01 | ||||
| Crude protein (CP, %DM) | CK | 8.26 | 9.12 | 8.65 | 8.83 | 0.27 | NS | NS | NS |
| LP | 9.03 | 8.99 | 9.16 | 9.09 | 0.14 | ||||
| Ammonia-N (%TN) | CK | 0.29 c | 0.57 b | 0.59 b | 1.12 aA | 0.34 | * | ** | NS |
| LP | 0.33 b | 0.49 ab | 0.38 ab | 0.69 aB | 0.06 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Zhou, Y.; Yang, D.; Zhou, W.; Chen, X.; Zhang, Q. Exploring Lactobacillus plantarum on Fermentation Quality, Gas Emissions, and In Vitro Digestibility of Different Varieties of Litchi Leaves Silage. Fermentation 2023, 9, 651. https://doi.org/10.3390/fermentation9070651
Chen D, Zhou Y, Yang D, Zhou W, Chen X, Zhang Q. Exploring Lactobacillus plantarum on Fermentation Quality, Gas Emissions, and In Vitro Digestibility of Different Varieties of Litchi Leaves Silage. Fermentation. 2023; 9(7):651. https://doi.org/10.3390/fermentation9070651
Chicago/Turabian StyleChen, Dandan, Yuxin Zhou, Dan Yang, Wei Zhou, Xiaoyang Chen, and Qing Zhang. 2023. "Exploring Lactobacillus plantarum on Fermentation Quality, Gas Emissions, and In Vitro Digestibility of Different Varieties of Litchi Leaves Silage" Fermentation 9, no. 7: 651. https://doi.org/10.3390/fermentation9070651
APA StyleChen, D., Zhou, Y., Yang, D., Zhou, W., Chen, X., & Zhang, Q. (2023). Exploring Lactobacillus plantarum on Fermentation Quality, Gas Emissions, and In Vitro Digestibility of Different Varieties of Litchi Leaves Silage. Fermentation, 9(7), 651. https://doi.org/10.3390/fermentation9070651






