Abstract
A positive effect of flaxseed mucilage (FSM) addition (at concentrations of 0.1, 0.2, and 0.4%) to MRS and milk whey nutrient medium on the survival, auto-aggregation, hydrophobicity, adhesive, and antioxidant properties of L. bulgaricus, L. fermentum AG8, and L. plantarum AG9 was shown. It was found that the AG 8 strain became less sensitive to 7% NaCl concentrations (the cell survival rate in the experiment with 0.4% flaxseed mucilage increased by 10% compared to the control). Cultivation in the presence of FSM led to an increase in auto-aggregation, especially in the case of AG8 (from 60 to 85%) and AG9 (from 50 to 80%) strains, and an increase in hydrophobicity was seen: for L. fermentum AG8, it was from 30% to 62–72%, for L. fermentum AG9 from 30% to 35–42%, and for L. bulgaricus from 20% to 30%. The adhesive properties of the L. fermentum AG8 and L. plantarum AG9 cells increased from 0.472 to 1 nN (nanonewton) and from 0.630 to 2.5 nN, respectively. The presence of flaxseed mucilage increased the total phenolic content in cell-free supernatants after 48 h of cultivation. The concentration of 0.1–0.2% FSM increased the OH-scavenging activity of milk whey nutrient medium cell-free supernatants of strains AG8 and AG9 by 7–10%. Flaxseed mucilage can serve as a promising bioactive additive that elevates antioxidant activity, increases the resistance and survival of Lactobacillus cells in the gastrointestinal tract, and leads to the synthesis of lipase and α-glucosidase inhibitors. The co-culture of these lactic acid bacteria in the presence of FSM and milk components in the form of whey leads to the synthesis of lipase and α-glucosidase inhibitors more than the culturing on de Man, Rogosa, and Sharpe broth.
1. Introduction
The popularity of lactic acid bacteria (LAB) and products containing these microorganisms has been growing progressively due to the expanding evidence of the health benefits associated with their use. Many strains with probiotic properties have been identified among Lactobacillus; these LABs improve the function of the gastrointestinal tract through a beneficial effect on the intestinal microflora, they normalize metabolism, and are characterized by antioxidant properties and resistance to antibiotic agents [1,2,3]. To impart their benefits, probiotics must survive food processing and storage, as well as withstand the stress conditions in the gastrointestinal tract environment to reach the small intestine and colonize the host. However, studies show that lactic acid bacteria are unstable to various unfavorable environmental conditions. The number of viable probiotic microorganisms decreases during storage; additionally, during the process of passage through the sgastrointestinal tract, a significant number of lactobacilli die due to nonresistance to bile and acid, which eventually leads to a decrease in the activity of microorganisms [4]. The use of different approaches to enhance probiotic survival through the gastrointestinal tract has been extensively studied [5,6]. However, few data are available to describe the effects of individual components and their underlying mechanisms of action for enhancing the survival of lactobacilli [7].
Recently, there has been a particular interest in determining the prebiotic potential (i.e., fermentability of the intestinal microflora) of plant seed mucilages. Although the current knowledge of the interaction between the gut microbiome and plant seed mucilage is rather limited, several studies have been conducted to determine the ability of plant seed mucus to stimulate the growth of probiotic cells (Lactobacillus and Bifidobacterium) and/or maintain their biological activity under normal and cytotoxic conditions [8,9,10,11,12,13].
In nature, there are various plants that produce mucus, e.g., seeds of a number of families of angiosperms (Asteraceae, Cabbage, Acanthaceae, Clearwort, Malva, Plantain, etc.), as well as some seeds of desert plants. Flax seeds hold the record for the production of easily extractable mucilages, producing a substance enriched in complex branched polysaccharides based on xylan and rhamnogalacturonan units [14,15,16,17,18]. In addition to polysaccharides, flaxseeds are also rich in lignans, bioactive peptides, minerals (predominantly phosphorus, magnesium, and calcium), and vitamins (mainly γ-tocopherol) [19,20]. Among bioactive molecules in flaxseed mucilage, phenolic compounds (lignans) are especially plentiful and valuable [21]. The flaxseed lignans contain secoisolaricirezinol (SECO) and the diglucoside secoisolaricirezinol (SDG), which are very strong antioxidants and phyto-estrogens [18]. SECO and SDG as well as gerbacin (8-hydroxycaempferol) are referred to as the natural plant flavonoids that have antidiabetic properties, protect the cardiovascular system, lower blood cholesterol, slow the progression of tumor growths that are associated with hormonal failure (breast cancer), and aid kidney function in patients with nephritis [22,23]. It is also known that carbohydrates contained in the flaxseed gum have a special biological activity. These components have a positive effect on the growth of probiotic Bifidobacterium microorganisms; they normalize the gastrointestinal tract, protect the liver (regulate cholesterol in the blood), have anti-tumor, anti-aging, and anti-oxidant properties, reduce the risk of cardiovascular diseases [24], have prebiotic properties, and are considered as promising probiotic encapsulating agents [13]. The encapsulation of Bifidobacterium infantis and Lactobacillus plantarum, by spray drying with mixtures of maltodextrin combined with mucilage and soluble protein extracted from chia seed and flaxseed, displayed high probiotic cell survival, and enhanced the microorganisms’ resistance to the simulated gastric juice and bile solution; thus, they exhibited high viability after 45 days of refrigerated storage [13]. The supplementation of kefir samples with pure flaxseed mucilage significantly enhanced the viability of LAB at the end of the storage period [23]. The addition of flaxseed mucilage and gum arabic has been shown to increase the survival of probiotic cultures Lactobacillus acidophilus and Bifidobacterium lactis in kefir and change the physicochemical properties of the product, in particular, increasing its viscosity [25,26]. In general, adding seed gum, mucilage, powder, or guar to fermented dairy foods, particularly yogurt, significantly improves the structure and consistency (thickens, emulsifies, and forms a gel), increases the moisture-holding power, and reduces syneresis [27,28,29,30,31,32,33].
It was shown that there are differences in the viability and probiotic properties between LAB species and strains [33,34]. In our previous studies, novel EPS-producing LAB strains (L. fermentum AG8 and L. plantarum AG9 [2,35]) with probiotic properties have been isolated from the clover silage. The strains have demonstrated a high acidification rate and pronounced antibacterial activity against bio-film-embedded pathogens as well as exhibited appropriate milk-fermenting properties. In this work, the effect of flaxseed mucilage on the antioxidant, probiotic, and structural-mechanical properties of Limosilactobacillus fermentum AG8, Lactiplantibacillus plantarum AG9, and Lactobacillus delbrueckii subs. bulgaricus, and the ability of flaxseed mucilage to support lactobacilli under aggressive conditions were investigated. In the perspective of using these strains in the food industry and in veterinary practice, we investigated changes in the probiotic properties of LABs cells, and the antioxidant properties of metabolic products when cultured on de Man, Rogosa, and Sharpe broth and milk whey nutrient medium (MWNM).
2. Materials and Methods
2.1. Strains, Mediums, Flaxseed Mucilage
Limosilactobacillus fermentum AG8 and Lactiplantibacillus plantarum AG9 have been isolated from the silage. These strains provide a high milk acidification rate and exhibit potential probiotic properties [2]. Lactobacillus delbrueckii subs. bulgaricus (“Lactosynthesis”, Moscow, Russia) served as a commercial strain for food products. LABs were stored in de Man, Rogosa, and Sharpe (MRS) broth (Himedia, India) with 50% glycerol at −80 °C. For culture activation, a 100 μL aliquot of each culture was individually transferred into MRS broth or milk whey broth and incubated at 37 °C for 24 h. The milk whey nutrient medium (MWNM) was prepared using whey powder (Evolution, Russia): 10% whey powder was dissolved in distilled water, boiled for 5 min, and filtered through a cloth filter. Then, 1% glucose was added to the filtered whey and autoclaved at 1 atm for 15 min.
Flaxseed mucilage was obtained with water extraction by mechanical shaking (180 rpm; flaxseed to deionized water ratio of 1:8 (w/v)) at room temperature for 18 h with subsequent vacuum filtration over a nylon mesh. The extracted flaxseed mucilage was freeze-dried by lyophilization and ground in a mortar to a powder. The obtained flaxseed mucilage was added to the MRS and MWNM at concentrations of 0.1, 0.2, and 0.4% before autoclaving.
2.2. Incubation of LAB with Flaxseed Mucilage, LAB Enumeration, and Preparation of the Cell-Free Supernatants
The preparation of the cell suspension was as follows: Lactobacillus were inoculated into MRS broth or MWNM with or without flaxseed mucilage (0.1, 0.2 and 0.4%) at 2% inoculum and cultured at 37 °C for 24 h. After centrifugation at 6000 r/min for 10 min, the obtained cell-free supernatants were prepared by directly filtering the supernatants using sterile 0.22 μM syringe filters (Minisart®, Sartorius, Gottingen, Germany). Cells were resuspended in a sterile 0.85% NaCl solution and suspensions were used for probiotic (resistance tests) property analysis. Cell-free supernatants (MRS and MWNM) were collected and stored frozen at −30 °C. Before antioxidant and enzyme inhibition assays, cell-free supernatants were unfrozen at +4 °C.
The number of LAB was determined using the streak plate method with MRS agar (Himedia, India). To measure the amount of LAB, each sample (100 μL) was serially diluted with 0.85% NaCl solution (900 μL). After spreading the diluted solution (100 μL) onto MRS agar plates, they were cultured at 37 °C for 48–72 h. The total number of viable cells was expressed as a log value.
2.3. General Probiotic Properties In Vitro
2.3.1. Cell Resistance to 3 and 7% NaCl, Acid, Bile Salts
Cell suspensions (described 2.2) were used for all resistance tests. Initial cell contentration was in the range of 107–108 CFU/mL Tested strains were inoculated in the tubes with the MRS broth, with 3 and 7% NaCl. MRS without NaCl was used as a control.
MRS broth for resistance to acid was acidified with hydrochloric acid to pH 2. The tested strains were inoculated in the tubes with the acidified MRS broth. MRS was used as a control.
The resistance to bile salts method proposed by Bao et al. (2010) [36] was used to estimate strain tolerance to bile salts. MRS broth tubes supplemented with 0.3% (w/v) bile salts (Russia) were inoculated with the tested strains. MRS without bile salts was used as a control.
The survival percentage was calculated using the following equation: Survival% = log CFU MRS (NaCl, pH 2 or bile salt)/log CFU MRS × 100, where log CFUMRS stress agents represents the total viable cells after treatment with stress agents, and log CFU MRS means the total number of viable cells in the control.
2.3.2. Cell Resistance to Simulated Gastrointestinal Tract (GIT) Conditions
The strain’s resistance to simulated gastric and intestinal juice was carried out according to Qin et al. (2022) [37]. Cell suspensions (described 2.2) were used for all resistance tests. Initial cell contentration was in the range of 107–108 CFU/mL. Briefly, pellets of active bacterial cultures were resuspended in artificial gastric juice (0.5% NaCl, 3 g/L pepsin, pH 3.0 was adjusted with 1 M HCl), and then in artificial intestinal fluid (0.5% NaCl, 1 g/L trypsin, 1 g/L bile salt, pH 8 was adjusted with 1 M NaOH) with incubations at 37 °C for 2 h every step. The survival percentage was calculated using the following equation: Survival% = log CFUf/log CFU0 × 100, where log CFUf represents the total viable cells after treatment and log CFU0 represents the starting number of microorganisms.
2.3.3. Antibiotic Resistance
The antibiotic susceptibility of the LAB isolates was determined by the disc diffusion method, as described earlier [38]. Briefly, bacteria were grown in MRS broth without or with flaxseed mucilage (0.1, 0.2 and 0.4%) for 24 h at 37 °C, and then 0.1 mL suspension was pour-plated on MRS agar plates. Then, antibiotic discs (Scientific Research Center of Pharmacotherapy, Russia) were placed onto the surface of inoculated plates. All isolates were screened for their susceptibility to ceftriaxone (30 μg), amoxicillin (25 μg), clindamycin (2 μg), erythromycin (15 μg), cefoxitin (30 μg), and streptomycin (300 μg). After 48 h of incubation in anaerobic conditions at 37 °C, the diameter of the inhibition zone was measured.
2.4. Bacterial Surface Properties
The bacterial surface properties were studied following the methodology described by Maldonado et al. (2012) [39]. The hydrophobicity and and auto-aggregation were tested only after MRS cultivation. MWNM was not used in the tests because it contains increased amounts of residual casein and whey proteins. These proteins make the nutrient medium turbid. In this case, there is no possibility of gentle separation of bacterial cells from protein particles; methodologically, it is impossible to perform a correct test for autoaggregation and hydrophobicity.
The hydrophobicity percentage (% H) was calculated using the following equation: % H = [(A0 –Af)/A0] × 100. A0 and Af are the optical density (OD) at 600 nm before (initial OD600 nm = 0.9 ± 0.1) and after extraction within hexadecane, respectively. For the bacterial autoaggregation, the results were expressed as a percentage of autoaggregation (%A) = [(A0 − Af)/A0] × 100. A0 and Af are the OD at 600 nm before and after 2 and 24 h. The scores of hydrophobicity and auto-aggregation were ranged as high (60–100%), medium (30–60%), and low (0–30%).
2.5. Titratable Acidity and Total Phenolic Compounds (TPCs)
Titratable acidity (TA) was determined for all groups (broths MRS and MWNM) without or with flaxseed mucilage (0.1, 0.2, and 0.4%) through titration until the pH reached 8.3 using distilled water (5 mL of broth in 10 mL of distilled water). Next, 0.1 N NaOH was used to estimate the amount of lactic acid (%) using the following equation:
where 10—dilution factor; W—weight of sample (g) for titration; VNaOH—volume of NaOH used to neutralize the lactic acid; 0.1—normality of NaOH.
LA% = (10 × VNaOH × 0.009 × 0.1)/W × 100,
Total phenolic compounds (TPCs) in cell-free supernatants were determined using Folin–Ciocalteu reagent. For that, 250 μL of the sample solution was added to 250 μL of Folin–Ciocalteu reagent; then, 1.25 mL of sodium carbonate solution (5%) and 0.75 mL of pure water were added after 10 min and mixed. Then, the mixture was incubated for 1 h in the dark and the absorbance was measured at 750 nm on a Spectrophotometer SF-2000 (OKB Spektr, St. Petersburg, Russia). The results were expressed in tyrosine equivalents, μg/mL.
2.6. Antioxidant Activity of Cell-Free Supernatants of MRS and MWNM
2.6.1. Evaluation of Radical-Scavenging Ability (RSA) by 2,2-Di-phenyl-1-picrylhydrazyl (DPPH) Assay
The radical scavenging capacity was analyzed according to [40], with modifications. Briefly, 1 mL of sample (5-fold diluted cell-free supernatant) (broths MRS and MWNM without or with flaxseed mucilage (0.1, 0.2 and 0.4%) was mixed with 1 mL of freshly prepared DPPH solution (0.12 mM ethanol) and incubated at 25 °C in the dark for 30 min. The reaction mixture was centrifuged for 2 min at 10,000 rpm, and the absorbance was measured at 517 nm using a Spectrophotometer SF-2000 (Russia). As a reference for the control absorbance DPPH solution, 0.12 mM ethanol has been used. The radical-scavenging activity was calculated as follows:
DPPH* scavenging activity % = [(control absorbance − extract absorbance)/(control absorbance)] × 100.
2.6.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
The ferric-reducing antioxidant power (FRAP) assay was carried out following the procedure described by [41], with modifications. Cell-free supernatants (broths MRS and MWNM without or with flaxseed mucilage (0.1, 0.2 and 0.4%) were 2-fold pre-diluted for analysis. Briefly, 1 mL of the sample was mixed with 1 mL of 0.2 M potassium sodium phosphate buffer (pH 6.5) and 1 mL of 1% potassium ferricyanide. The reaction mixture was incubated for 20 min at 50 °C, cooled, and 1 mL of 10% trichloroacetic acid was added. The mixture was centrifuged at 2000 rpm for 10 min at 25 °C; the supernatant was diluted twice with water (2 mL + 2 mL) and 400 μL of 0.1% FeCl3. For reference, a buffer was added instead of the potassium ferricyanide. The absorbance was measured at 700 nm (Spectrophotometer SF-2000) and expressed as reducing force, which was expressed as absorbance at 700 nm relative to the control.
2.6.3. HO Free Radical Scavenging Ability
HO free radical scavenging ability was carried out following the procedure described by Qin et al., 2022 [37], with modification. Cell-free supernatants (broths MRS and MWNM without or with flaxseed mucilage (0.1, 0.2 and 0.4%) were 5-fold pre-diluted for analysis. Then, we added 0.5 mL of a 5 mM/L ferrous sulfate (FeSO4) solution, 0.5 mL of a 5 mM/L salicylic acid ethanol solution, and 0.5 mL of a 3 mM/L hydrogen peroxide solution to 0.5 mL of cell-free extract, which was then mixed and incubated at 37 °C for 30 min at 9000 r/min, and centrifugated for 5 min. The supernatant OD measured at 510 nm was considered as B1, and OD at 510 nm as B0; ddH2O was used to replace the cell suspension in the case of cell-free extract as the control group.
OH* scavenging activity (inhibition)% = (B0 − B1)/B0 × 100.
2.6.4. Fe-Chelating Activity
The FIC activity assay was carried out following the procedure described by Muniandy et al., 2016 [42], with modifications. Cell-free supernatants were 2-fold pre-diluted for analysis (broths MRS and MWNM without or with flaxseed mucilage (0.1, 0.2 and 0.4%)). The iron(II) chloride (FeCl2) solution (2 mM) and ferrozine solution (5 mM) were both diluted 20 times prior to use in the assay. A diluted FeSO4 x H2O solution (1 mL) was added to cell-free supernatants and mixed well. A diluted ferrozine solution (1 mL) was then added to the mixture, followed by incubation for 10 min at room temperature. Absorbance at 562 nm was measured against distilled water as a blank (Spectrophotometer SF-2000). The control was a mixture of FeCl2 x H2O solution (1 mL), ferrozine solution (1 mL), and distilled water (1 mL). The FIC ability of samples was calculated using the above-mentioned equation.
FIC (inhibition)% = (B0 − B1)/B0 × 100.
2.7. In Vitro Lipase Inhibition Assay
The lipase inhibition activity in cell-free supernatants was determined by a method in Al-Yousef et al., 2021 [43]. In this method, the lipase activity was measured using p-nitrophenyl butyrate (NPB) as a substrate. Lipase solution (100 µg/mL) was prepared in a 0.1 mM potassium phosphate buffer (pH 6.0). The sample (20 µL) (MRS and MWNM with 0.1, 0.2, 0.4% and without FSM) and 580 µL potassium phosphate buffer (pH 6.0) were mixed, and the mixture was preincubated with lipase solution (0.6 mL) for 10 min at 37 °C. The reaction was then started by adding 100 µL NPB substrate. After incubation at 37 °C for 15 min, the amount of p-nitrophenol released in the reaction was measured at 405 nm (Spectrophotometer SF-2000). The results were expressed as percentage inhibition, which was calculated using the formula:
where (Ac) is the absorbance of the control and (As) is the absorbance in the presence of the test substance.
Lipase inhibition % = (Ac − As)/Ac × 100.
2.8. In Vitro α-Glucosidase Inhibition Assay
The in vitro α-glucosidase inhibitory activity was determined according to the method described by Tao et al. [44]. The α-glucosidase inhibitory effect of the cell-free supernatants (MRS and MWNM with 0.1, 0.2, 0.4% and without FSM) was evaluated by using p-nitro-phenyl-a-D-glucopyranoside (p-NPG) substrate solution (0.5 mg/mL in 0.1 M potassium phosphate buffer, pH 6.8). The sample (20 µL), 0.1 M potassium phosphate buffer (180 µL), and ⍺-glucosidase solution (400 µL) were mixed. After pre-incubation at 37 °C for 10 min, 1000 µL pNPG was added as the substrate, and an enzymatic reaction was performed at 37 °C for 60 min. ⍺-Glucosidase activity was determined by measuring the release of p-nitrophenol from pNPG at 410 nm (Spectrophotometer SF-2000). A solution without the cell-free supernatant was used as a control. A solution without the substrate was used as a blank. The inhibition percentage was calculated as follows:
where (Ac) is the absorbance of the control and (As) is the absorbance in the presence of the test substance.
Glucosidase inhibition % = (Ac − As)/Ac × 100,
2.9. Scanning Probe Microscopy (SPM)
SPM was used to study the effect of flaxseed mucilage on the cell surface, adhesion, and deformation. Images of cells were obtained by SPM using a Dimension FAstScan scanning probe microscope (Bruker Nano Surfaces, Santa Barbara, CA, USA). For microscopy, LABs cells were obtained by culturing with and without 0.4% FSM (described in 2.2). Cell samples were fixed with 2.5% glutaraldehyde for 4–5 h, subsequently washed three times with 0.2 M Na-K phosphate buffer (pH 7.0), and then dehydrated using 30%, 40%, 50%, 60%, 70%, and 80% (twice for each concentration) ethanol at 15 min, and 95% ethanol three times at 30 min. A drop of the bacterial suspension was deposited on the quartz glass and allowed to dry for 15–20 min. The set-up was placed on the sample stage of a Dimension FastScan SPM with a Nanoscope V controller (Bruker, Billerica, MA, USA). Bruker ScanAssyst probes with a radius of curvature of 2 nm were used for the nanomechanical mapping of the samples. Topographic and mechanical data were collected simultaneously.
2.10. Statistical Analysis
All experiments were carried out in triplicate. Significance was established at p < 0.05. The results were analyzed for statistical significance with a two-way ANOVA by GraphPad Prism software v.6.0 at a significance level of p < 0.05.
3. Results
3.1. Hydrophobicity and Auto-Aggregation of LABs Cells
The adhesion of LAB to epithelial and intestinal mucosal cells is considered to be an important factor for the modulation of the immune system [45], and for antagonistic activity against enteropathogens [46]. It is associated with the hydrophobicity of LABs and their ability to auto-aggregate. These properties are associated with the ability of LABs to colonize the mucosal surfaces of the gastrointestinal tract and attach to them. Therefore, the level of auto-aggregation can be used for the preliminary screening of potentially probiotic bacteria suitable for the colonization of the human intestinal tract.
This study revealed that the commercial strain L. bulgaricus has a low level of hy-drophobicity (approximately 10–20%), which decreases when exposed to low mucilage concentrations (up to 0.2%), but grows up 30% when the mucilage con-centration is increased to 0.4% (Figure 1A). L. fermentum AG8 and L. plantarum AG9 strains had 30% hydrophobicity in the control, and the addition of FMS increased hy-drophobicity to 62–72% for AG8 and 35–42% for AG9. According to the obtained data, the hydrophobicity of LABs does not correlate with their au-to-aggregation properties. This corresponds with the results reported for bifidobacte-ria, for which hydrophobicity levels showed considerable variability and did not cor-relate with the degree of cell adhesion to intestinal mucus [47,48].
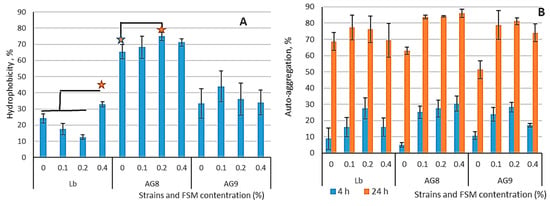
Figure 1.
Hydrophobicity (A) and auto-aggregation (B) of LABs cells after cultivation on MRS medium with addition of flaxseed mucilage in different concentrations. Asterisks show significant difference at p < 0.05.
High auto-aggregation of probiotic strains is a useful property because it leads to an increase in the number of probiotic organisms in the macroorganism. In the control groups, L. bulgaricus had high auto-aggregation (68.7%), while the AG8 (63%) and AG9 (51.3%) strains had significantly lower auto-aggregation (Figure 1B). Cultivation in the presence of flaxseed mucilage led to an increase in LAB’s ability to auto-aggregate, especially in the case of AG8 and AG9 strains.
3.2. Characteristics of Probiotic Properties of LAB at Cultuvation on a MRS
In light of the potential use of the FSM in fermented dairy products, it is crucial to assess the impact of its components on the survival of lactic acid bacteria. Figure 2 shows the cell survival of different LAB species after cultivation on MRS medium with FSM. All three strains tested became more sensitive to 3% NaCl after cultivation with FSM. The situation changed when tested with 7% NaCl. L. bulgaricus cells became even more sensitive; the survival rate decreased by 10% compared to the control. The survival rate of strain AG9 did not change regardless of the FSM concentration. On the contrary, the AG 8 strain became less sensitive to high salt concentrations; the cell survival rate in the experiment with 0.4% FSM increased by 10% compared to the control.
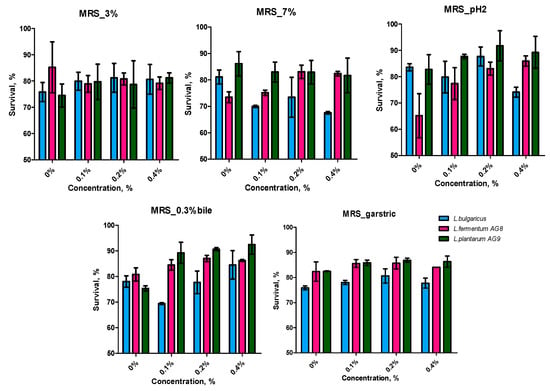
Figure 2.
Probiotic properties of LABs after cultivation on MRS medium with addition of flaxseed mucilage in different concentrations (3%—resistance to 3 NaCl; 7%—resistance to 7% NaCl; pH 2—resistance to acid conditions, 0.3% bile—resistance to 0.3% bile salts; gastric—resistance to simulated gastrointestinal tract (GIT) conditions). Means with standard deviation were shown.
A number of factors in the upper gastrointestinal tract (high acidity, digestive enzymes, and bile salts) can reduce the survival of probiotic lactic acid bacteria [49]. Acid resistance is important for the ability of LAB cells to survive in the stomach and further migrate to the intestine. The differentiation of the cell survival of commercial L. bulgaricus strains and potentially probiotic strains L. fermentum AG8 and L. plantarum AG9 from silage was detected when they were exposed to pH 2. The cultivation of AG8 and AG9 in the presence of FSM increased cell survival in an acidic medium, while L. bulgaricus became more sensitive to acid.
The results obtained in the bile acid test in the case of strains L. fermentum AG8 and L. plantarum AG9 repeat the pattern of acid resistance. L. bulgaricus cells became less sensitive to bile acids if they were grown in the presence of 0.4% FSM.
L. fermentum AG8 and L. plantarum AG9 cells, under gastrointestinal simulation conditions, had a higher survival rate when grown in the presence of FSM, whereas L. bulgaricus cells had a lower survival rate. These data correlate with the trend obtained when testing at pH 2.
3.3. Characteristics of Probiotic Properties of LAB during Cultuvation on a Milk Whey Broth
In addition to MRS, a milk whey-based nutrient medium with the addition of glucose was used to simulate fermented milk product conditions, and the joint effect of milk proteins and FMS on LABs cell survival. The use of MWNM for cultivation led to increased cell resistance to 3% NaCl, especially in combination with FSM, but the concentration of FSM with the highest survival in strains differed: L. bulgaricus—0.4%, L. fermentum AG8—0.4%, and L. plantarum AG9—0.1% (Figure 3). As expected, increasing the salt concentration to 7% resulted in a decrease in survival. However, FSM had a protective effect on L. fermentum AG8 and L. plantarum AG9 cells, especially at concentrations of 0.2 and 0.4%. As in the case of MRS on whey nutritional medium, L. bulgaricus cells became more sensitive to high concentrations of salt.
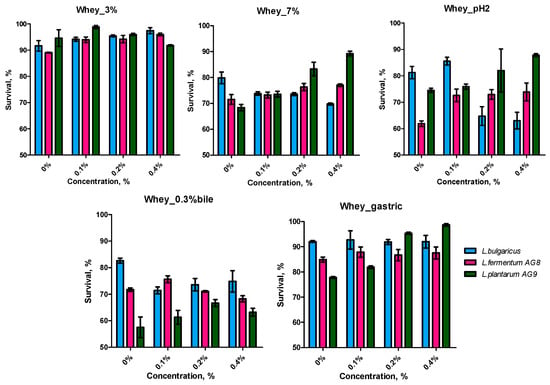
Figure 3.
Probiotic properties of LAB after cultivation on whey medium in the presence of different concentrations of flaxseed mucilage. (3%—resistance to 3 NaCl; 7%—resistance to 7% NaCl; pH 2—resistance to acid conditions, 0.3% bile—resistance to 0.3% bile salts; gastric—resistance to simulated gastrointestinal tract (GIT) conditions). Means with standard deviation were shown.
The survival of AG8 and AG9 at pH 2 after cultivation in whey medium increased when the FSM concentration rose. These results repeat the trend shown on MRS but at a lower absolute level. The survival of L. bulgaricus decreased to the control level when the strain was grown in medium with 0.2 and 0.4% FSM. Cultivation in the presence of FSM on milk whey led to a decrease in the resistance to the bile of L. bulgaricus, had no significant effect on L. fermentum AG8, and increased the bile resistance of L. plantarum AG9. Depending on the strain, FSM had different effects on survival under gastric juice conditions. FSM increased the survival of AG9 and had no effect on the survival of the L. bulgaricus and L. fermentum AG8 strains.
Thus, changing the MRS cultivation medium to milk whey medium led to an increase in the probiotic properties of the commercial L. bulgaricus strain. The probiotic properties of non-starter strains AG8 and AG9 practically did not change, or they deteriorated a little when the cultivation medium was changed. However, the addition of FSM to the cultivation medium with milk whey resulted in a significant increase in the cell resistance of non-starter strains AG8 and AG9. Different polysaccharides can increase the resistance of the probiotic bacteria Lactobacillus casei 01 strain to gastric juice [50]. Previously, prebiotic properties have been described for flaxseed mucilage, which contained a mixture of rhamnogalacturonan I and arabinoxylan [16,51,52].
The antibiotic resistance of lactic acid bacteria is a particularly important indicator of probiotic strains. The cultivation of L. bulgaricus with different concentrations of FSM resulted in no statistically significant changes in antibiotic resistance (Table 1). AG8 testing showed increased resistance to cefoxitin when the strain was grown with FSM at a concentration of 0.1–0.2%, while increasing the FSM concentration resulted in an increased sensitivity of cells to cefoxitin. The strain L. plantarum AG9 became resistant to cefoxitin after cultivation with FSM; no statistically significant changes were found in other cases. Thus, the co-cultivation of the studied lactic acid bacteria with FSM does not increase their sensitivity to antibiotics of different classes.

Table 1.
Antibiotic resistance of LABs cells after cultivation on MRS in the presence of different concentrations of flaxseed mucilage (mean values ± SD, n = 3).
3.4. Scanning Probe Microscopy (SPM)
The use of SPM made it possible to visualize the appearance of the cells as well as determine the adhesive (adhesion is the tendency of surfaces to cling to each other; in our case, surfaces are the tip and bacterial cell wall) and deformational properties of the lactobacilli cells (Figure 4). The adhesive properties of the different species differ greatly. L. bulgaricus cells showed an adhesion strength of 3.34 nN, while AG8 and AG9 cells showed only 0.472 nN and 0.630 nN. The adhesive properties of the cells changed in the presence of FSM, and the effects detected varied with the species of lactobacilli.
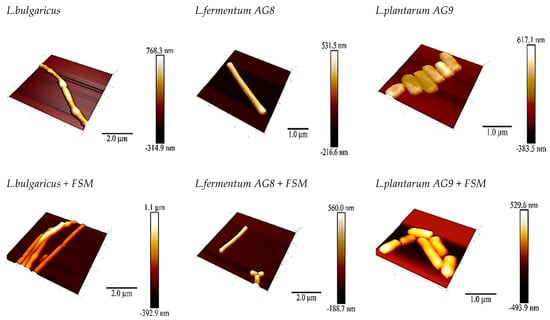
Figure 4.
SPM microscopy of Lactobacillus cells after cultivation on MRS or MRS in the presence of flaxseed mucilage (0.4%).
In the case of L. bulgaricus with FSM, the adhesion became 0.3 nN, which is 10 times less than in the control. In the case of L. fermentum AG8 and L. plantarum AG9, the adhesion increased to 1 and 2.5 nN, respectively. Thus, the cells of non-starter strains removed from silage under the influence of 0.4% FSM become more adhesive; probably, these changes allow AG8 and AG9 cells to be more hydrophobic in the presence of FSM (Supplementary File).
Deformation measurement showed that the bacterial cells became less hard in the presence of FSM: L. bulgarucus—5.4 nm (control) and 7.6 nm (FSM); L. fermentum AG8—0.36 nm (control) and 2.0 nm (FSM); and L. plantarum AG9—2.4 nm (control) and 2.76 nm (FSM). The greatest increase in hardness (5.5-fold) was detected for the strain L. fermentum AG8 (Supplementary File). The effect of FSM on the adhesive and deformational properties of lactic acid bacteria cells, which affect the probiotic properties, in particular hydrophobic autoaggregation, is undeniable.
3.5. Effect of FSM on Lactic Acid Synthesis and Antioxidant Properties
Lactic acid synthesis is an important indicator showing that the metabolic functions of lactic acid bacteria have been preserved. A significant decrease in lactic acid production will affect the health properties of the bacteria. FSM at concentrations of 0.1–0.2% did not inhibit lactic acid synthesis in any strains with MRS incubation. A decrease in lactic acid production in strains AG8 and AG9 with an FSM concentration of 0.4% was detected. The level of synthesized acids of strains AG8 and AG9 on the medium with whey was higher than that of L. bulgaricus. The addition of FSM to the whey medium intensified acid production by 0.15–0.17% relative to the control (Figure 5). The tendency to reduce acid production was revealed when the FSM level was increased to 0.4%. The optimal FSM concentration for lactic acid production in the whey medium is 0.2%.
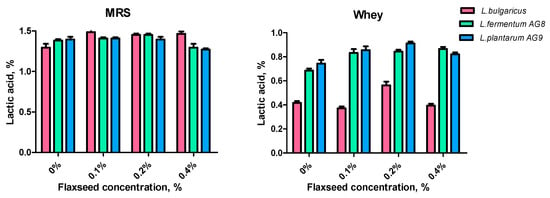
Figure 5.
Effect of flaxseed mucilage on the lactic acid concentration of cell-free supernatants after 48 h of LAB cultivation on MRS or MWB (whey).
Considering that flax mucus contains lignans, a source of phenolic compounds, the amount of total phenolic content (TPC) was determined in cell-free supernatants after 48 h of MRS or MWB (whey) cultivation (Figure 6). The amount of phenolic compounds released into cell-free supernatants during metabolism differed between strains. The AG9 strain in the MRS control accumulates the most low-molecular-weight phenolic compounds, while the AG8 strain accumulates the least. Adding 0.2% FSM to the MRS medium increased TPC, especially in the case of AG8. Interestingly, AG9 and AG8 accumulated less TPC when the MSF concentration was increased to 0.4%. L. bugaricus accumulated the greatest amount of TPC in the case of 0.4% FSM.
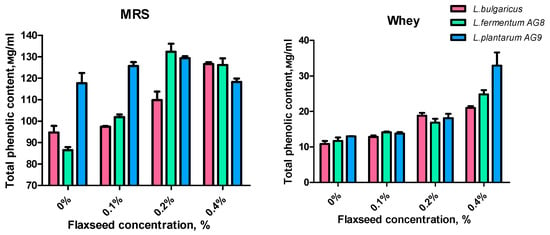
Figure 6.
Effect of flaxseed mucilage on total phenolic content (TPC) of cell-free supernatants after 48 h of LAB cultivation on MRS or MWB (whey).
When whey broth was used, the amount of TPC increased linearly for all strains as the FSM concentration increased. Interestingly, when 0.4% FSM was added, strain AG9 accumulated more TPC compared to the other strains. Considering that the initial concentration of the mucilage is the same, we can assume the effect of flaxseed mucilage on metabolic processes, and on the activation of proteolysis with the release of free aromatic amino acids from whey proteins. In addition, we can assume that FSM modifications are accompanied by the release of phenolic compounds.
Lactic acid bacteria have a great potential to synthesize a complex of agents with antioxidant properties [37,38,39,40]. Due to the co-culture of LAB with FSM, it is important to identify the effect of mucus on the antioxidant properties of the metabolic products. The antioxidant capacities (DPPH) of MRS or whey medium with FSM are shown in Figure 7. Flaxseed mucilage increased the DPPH values significantly in the case of MRS. DPPH in the case of AG8 and AG9 (with FSM) was approximately 75%, while for L. bugaricus it was lower (about 65%). The lower RSA was also observed when all strains were grown on MWNM, but, in this case, RSA also increased when FSM was added (especially at 0.4%).
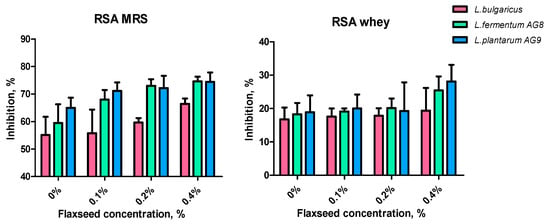
Figure 7.
Effect of flaxseed mucilage on radical scavenging activity (RSA) of cell-free supernatants after 24 h of LAB cultivation on MRS or MWB (whey).
The addition of FSM increased the OH scavenging activity of the tested strains (Figure 8). The concentration of 0.1–0.2% FSM increased the OH scavenging activity of MWNM cell-free supernatants of strains AG8 and AG9 by 7–10% (Figure 8, OH whey).
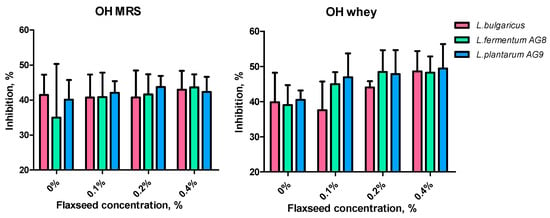
Figure 8.
Effect of flaxseed mucilage on hydroxyl scavenging activity of cell-free supernatants after 24 h of LAB cultivation on MRS or MWB (whey).
A statistically significant effect of FSM in different concentrations on the Fe-chelating activity of cell-free supernatants was not found (Figure 9).
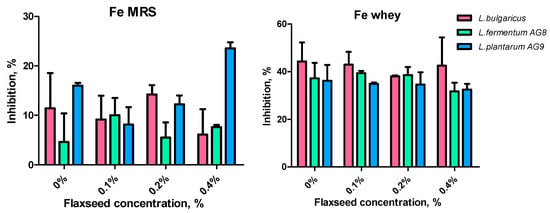
Figure 9.
Effect of flaxseed mucilage on Fe-chelating activity of cell-free supernatants after 24 h of LAB cultivation on MRS or MWB (whey).
3.6. Effect of FSM on Enzyme Activity
Lactic acid bacteria and the products of their metabolism can inhibit lipase in vitro [53]. We did not find an increase in lipase inhibiting capacity when LABs were grown on MRS medium (Figure 10). Lipase inhibition increased in the presence of FSM when the culture medium was changed to MWNM. The AG9 strain showed the greatest ability to inhibit lipase.
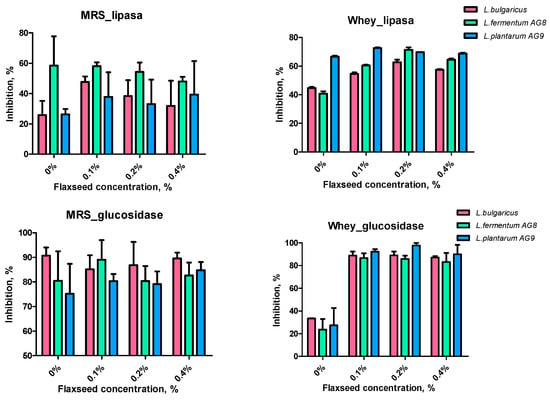
Figure 10.
Effect of cell-free supernatants on inhibition of lipase and glucosidase.
Thus, co-cultivation of the studied lactic acid bacteria in the presence of FSM and milk components in the form of whey leads to the synthesis of lipase and α-glucosidase inhibitors.
Previously, it was shown that inhibition of α-glucosidase by bacteria leads to a decrease in glucose production in the intestinal tract, and thus prevents type 2 diabetes mellitus [54]. The starter L. bulgaricus strain showed the highest ability to inhibit α-glucosidase when grown on MRS, including in the presence of FSM. As in the case of lipase, the addition of FSM did not affect the synthesis of α-glucosidase inhibitory components in the MRS cell-free supernatants. Additionally, in MWNM, the addition of FSM 3-fold increased the inhibition of α-glucosidase in vitro (Figure 10).
4. Discussion
Many factors, including polysaccharides, can influence the probiotic properties of lactic acid bacteria. A few authors have described the positive role of chia seed mucus, flaxseed mucilage [12,13], and alginate [55]. We found that changes in the viability of lactobacilli depend on the concentration of flaxseed mucilage, the species of lactobacilli, and the growing medium. The species- and strain-specificity of lactobacilli responses to various stresses are indicated by a number of authors: L. plantarum [56,57] and L. casei [58,59].
The cultivation of lactobacilli in the presence of milk protein (medium with whey permeate) and flaxseed mucilage leads to increased survival under conditions of simulated digestive juices, especially in L. plantarum AG9. Previously, Lavari et al., 2015 [60] showed that cells of Lactobacillus rhamnosus 64 grown in whey permeate were more resistant to mild heat stress. It can be assumed that the presence of albumin proteins in milk contributes to the formation of resistance, which once again proves the possibility of using fermented dairy products as carriers of probiotic bacteria. Under in vitro conditions, a good survival rate can be considered to be between 70% [53] and 80% [35]. The presence of flaxseed mucilage at a concentration of 0.1–0.2% in the cultivation medium increases the survival rate of Lactobacillus to 80% and higher under simulated gastrointestinal tract conditions. This is probably due to the fact that lactobacilli cells are encapsulated in their growth in the presence of FSM, which is confirmed by the changes in their structural and mechanical properties. It was previously revealed that the encapsulated cells in flaxseed mucilage have an increased survival rate in simulated gastric juice [12].
The effect of FSM on the antioxidant properties of the cell-free extract is due to the presence of phenolic compounds. A number of studies have shown that the total phenolic content can be used as an indicator of antioxidant activity [61,62,63], although the total phenolic content does not include all antioxidants. The composition of phenolic compounds in flaxseed gum includes caffeic acid, p-coumaric acid, epicatechin, ellagic acid, cinnamic acid, and vanillic acid [64]. These compounds have been extensively studied because they provide protection, for example, against the damaging effects of oxidative stress [65]. An increase in radical-scavenging activity was detected when the concentration of FSM was increased, which indicates the contribution of FSM to the formation of the antioxidant properties of the cell-free extract after the cultivation of LABs. In addition, FSM modification by chemical methods leads to an increase in antioxidant activity compared to the initial mucus [66]. Therefore, it can be assumed that modifications under the influence of lactic acid bacteria lead to changes in FSM antioxidant properties.
Certainly, the antioxidant properties of free-cell extract are also formed by LAB metabolites. LABs have various biologically active substances such as vitamins, amino acids, enzymes, exopolysaccharides (EPS), short-chain fatty acids, organic acids, phenolic compounds, bioactive peptides, etc., which have a positive effect on human health [67]. We previously revealed that exopolysaccharides and the protein-free fraction of fermented milk obtained after the fermentation of milk with L. fermentum AG8 and L. plantarum AG9 strains possess a spectrum of antioxidant properties [35]; they are capable of binding free radicals, hydroxyl radicals, superoxide radicals, and iron.
All the obtained results indicate the importance of using flaxseed mucilage for the formation of increased resistance of LAB cells. These data are consistent with the results of a study using polysaccharide-alginate to protect probiotic cells [68]. Providing an LAB survival of at least 106 CFU/mL during passage through the human gastrointestinal tract is desirable for health benefits [69]. Our results show that in an in vitro system, a high number of viable bacterial cells can successfully survive under gastrointestinal conditions. It was found that under conditions of nutrient media, including sweet whey, more bioactive substances are synthesized in the presence of FSM, which can play a positive role in the gut (e.g., antioxidant activity) as well as in bioavailability (adsorption in the blood). In addition, probiotics were found to increase their functionality, their antioxidant activity, and their ability to adhere to epithelial cells.
5. Conclusions
The results of this study contribute to the study of the interaction between lactic acid bacteria and plant polysaccharides, a promising bioactive additive. First of all, a positive effect of FSM on the survival of L. bulgaricus, L. fermentum AG8, and L. plantarum AG9 in simulated gastric juice was shown. This effect depended, in particular, on the bacterial species and culture medium, changes in LAB-cell auto-aggregation, and hydrophobicity after FSM exposure. It is important to note the species-specificity of the responses of different lactobacilli to the presence of flaxseed mucilage. This study also presented new results on the combination of flaxseed mucilage, sweet milk whey, and LABs during fermentation, leading to the formation of increased antioxidant activity. LAB in the presence of FSM and milk components in the whey leads to the synthesis of lipase and α-glucosidase inhibitors.
In view of this, a food product that combines high amounts of healthy bacteria and polyphenols, the source of which can be flaxseed mucilage, can be considered a functional product. At the same time, low concentrations of 0.1–0.4% flaxseed mucilage already result in healthy effects, while such low concentrations of FSM limit the effect on the sensory (taste) properties of the final products. Undoubtedly, further studies are needed, including (1) studying the effect of flaxseed mucilage on dairy products, including its antioxidant properties; and (2) revealing the effect of such a dairy product on the mammalian organism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9050486/s1, Supplementary File.
Author Contributions
This study was made possible through the collaboration of all authors. Conceptualization, E.N.; methodology, E.N.; software, E.N., P.M. and M.K.; validation, E.N.; formal analysis, A.S., T.P. and M.K.; investigation, A.S., T.P. and M.K.; resources, E.N. and P.M.; data curation, A.S., T.P. and M.K.; writing—original draft preparation, E.N. and P.M.; writing—review and editing, E.N. and P.M.; visualization, E.N. and P.M.; supervision, E.N.; project administration, E.N.; funding acquisition, E.N. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by RSF, project number 22-26-20022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available in the manuscript file.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Gänzle, M.G. Host-adapted lactobacilli in food fermentations: Impact of metabolic traits of host adapted lactobacilli on food quality and human health. Curr. Opin. Food Sci. 2020, 31, 71–80. [Google Scholar] [CrossRef]
- Gavrilova, E.; Anisimova, E.; Gabdelkhadieva, A.; Nikitina, E.; Vafina, A.; Yarullina, D.; Bogachev, M.; Kayumov, A. Newly isolated lactic acid bacteria from silage targeting biofilms of foodborne pathogens during milk fermentation. BMC Microbiol. 2019, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E.; Petrova, T.; Vafina, A.; Ezhkova, A.; Yahia, M.N.; Kayumov, A. Textural and functional properties of skimmed and whole milk fermented by novel Lactiplantibacillus plantarum AG10 strain isolated from silage. Fermentation 2022, 8, 290. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Shulinger, U. Introduction to pre- and probiotics. Food Res. Int. 2002, 35, 125–129. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Champomier Vergès, M.-C.; Zuñiga, M.; Morel-Deville, F.; Peréz-Martínez, G.; Zagorec, M.; Ehrlich, S.D. Relationships between arginine degradation, pH and survival in Lactobacillus sakei. FEMS Microbiol. Lett. 1999, 180, 297–304. [Google Scholar] [CrossRef]
- Capozzi, V.; Arena, M.P.; Russo, P.; Spano, G.; Fiocco, D. Stressors and food environment: Toward strategies to improve robustness and stress tolerance in probiotics. In Probiotics, Prebiotics, and Synbiotics; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; Chapter 16; pp. 245–256. [Google Scholar]
- Peredo, A.G.; Beristain, C.I.; Pascual, L.A.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. LWT-Food Sci. Technol. 2016, 73, 191–196. [Google Scholar] [CrossRef]
- Mueller, M.; Cavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic potential of neutral oligo- and polysaccharides from seed mucilage of Hyptis suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Mustafa, S.; Adzahan, N.M.; Muhammad, K. In vitro prebiotic activities of tamarillo (Solanum betaceum Cav.) hydrocolloids. J. Funct. Foods 2015, 19, 10–19. [Google Scholar] [CrossRef]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilus La-05 encapsulated by spray drying: Effect of mucilage and protein from flaxseed (Linum usitatissimum L.). LWT-Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Rubilar, M.; Shene, C. Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linum usitatissimum L.) mucilage and soluble protein by spray drying. Food Chem. 2017, 216, 97–105. [Google Scholar] [CrossRef]
- Muralikrishna, G.; Salimath, P.V.; Tharanathan, R.N. Structural features of an arabinoxylan and a rhamno-galacturonan derived from linseed mucilage. Carbohydr. Res. 1987, 161, 265–271. [Google Scholar] [CrossRef]
- Cui, W.; Mazza, G.; Biliaderis, C.G. Chemical structure, molecular size distribution and rheological properties of flaxseed gum. J. Agric. Food Chem. 1994, 42, 1891–1895. [Google Scholar] [CrossRef]
- Naran, R.; Chen, G.; Carpita, N.C. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol. 2008, 148, 132–141. [Google Scholar] [CrossRef]
- Ding, H.H.; Qian, K.Y.; Goff, H.D.; Wang, Q.; Cui, S.W. Structural and conformational characterization of arabinoxylans from flaxseed mucilage. Food Chem. 2018, 254, 266–271. [Google Scholar] [CrossRef]
- Western, T.L. The sticky tale of seed coat mucilages: Production, genetics, and role in seed germination and dis-persal. Seed Sci. Res. 2011, 22, 1–25. [Google Scholar] [CrossRef]
- Qian, K.Y.; Cui, S.W.; Wu, Y.; Goff, H.D. Flaxseed gum from flaxseed hulls: Extraction, fractionation, and characterization. Food Hydrocoll. 2012, 28, 275–283. [Google Scholar] [CrossRef]
- Čukelj, N.; Novotni, D.; Sarajlija, H.; Drakula, S.; Voučko, B.; Ćurić, D. Flaxseed and multigrain mixtures in the development of functional biscuits. LWT 2017, 86, 85–92. [Google Scholar] [CrossRef]
- Wang, Y.; Fofana, B.; Roy, M.; Ghose, K.; Yao, X.H.; Nixon, M.S.; Nair, S.; Nyomba, G.B.L. Flaxseed lignan secoisolariciresinol diglucoside improves insulin sensitivity through upregulation of GLUT4 expression in diet-induced obese mice. J. Funct. Foods 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Baba, W.N.; Jan, K.; Punoo, H.A.; Wani, T.A.; Dar, M.M.; Masoodi, F.A. Techno-functional properties of yoghurts fortified with walnut and flaxseed oil emulsions inguar gum. LWT 2018, 92, 242–249. [Google Scholar] [CrossRef]
- Veeramani, C.; Alsaif, M.A.; Al-Numair, K.S. Herbacetin, A flaxseed flavonoid, ameliorates high percent dietary fat induced insulin resistance and lipid accumulation through the regulation of hepatic lipid metabolizing and lipid-regulating enzymes. Chem. Biol. Interact. 2018, 288, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Courtois, J. Oligosaccharides from land plants and algae: Production and applications in therapeutics and biotechnology. Curr. Opin. Microbiol. 2009, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- HadiNezhad, M.; Duc, C.; Han, N.; Farah, H. Flaxseed soluble dietary fibre enhances lactic acid bacterial survival and growth in kefir and possesses high antioxidant capacity. J. Food Res. 2013, 2, 152–163. [Google Scholar] [CrossRef]
- Alhssan, E.; Ercan, S.S.; Bozkurt, H. Effect of flaxseed mucilage and gum arabic on probiotic survival and quality of kefir during cold storage. Foods 2023, 12, 662. [Google Scholar] [CrossRef]
- Bali, V.; Panesar, P.S.; Bera, M.B.; Panesar, R. Fructo-oligosaccharides: Production, purification and potential applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1475–1490. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Shen, J.; Wang, Y.; Ghosh, S.; Reaney, M.J.T. Variation of composition and functional properties of gum from six Canadian flaxseed (Linum usitatissimum L.) cultivars. Int. J. Food Sci. Technol. 2016, 51, 2313–2326. [Google Scholar] [CrossRef]
- Fedeniuk, R.W.; Biliaderis, C.G. Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. J. Agric. Food Chem. 1994, 42, 240–247. [Google Scholar] [CrossRef]
- Chen, H.H.; Xu, S.Y.; Wang, Z. Gelation properties of flaxseed gum. J. Food Eng. 2006, 77, 295–303. [Google Scholar] [CrossRef]
- Khalloufi, S.; Corredig, M.; Goff, H.D.; Alexander, M. Flaxseed gums and their adsorption on whey protein-stabilized oil-in-wateremulsions. Food Hydrocoll. 2009, 23, 611–618. [Google Scholar] [CrossRef]
- Basiri, S.; Haidary, N.; Shekarforoush, S.S.; Niakousari, M. Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydr. Polym. J. 2018, 187, 59–65. [Google Scholar] [CrossRef]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett. Appl. Microbiol. 1998, 27, 183–185. [Google Scholar] [CrossRef]
- Ronka, E.; Malinen, E.; Saarela, M.; Rinta-Koski, M.; Aarnikunnas, J.; Palva, A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003, 83, 63–70. [Google Scholar] [CrossRef]
- Nikitina, E.; Petrova, T.; Sungatullina, A.; Kharina, M.; Mikshina, P.; Gavrilova, E.; Kayumov, A. The profile of exopolysaccharides produced by various Lactobacillus species from silage during not-fat milk fermentation. Fermentation 2023, 9, 197. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, S.; Dong, X.; Wang, Y.; Zhang, H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control. 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Qin, S.; Huang, Z.; Wang, Y.; Pei, L.; Shen, Y. Probiotic potential of Lactobacillus isolated from horses and its therapeutic effect on DSS-induced colitis in mice. Microb. Pathog. 2022, 165, 105216. [Google Scholar] [CrossRef]
- Bruslik, N.L.; Akhatova, D.R.; Toimentseva, A.A.; Abdulkhakov, S.R.; Ilyinskaya, O.N.; Yarullina, D.R. Estimation of probiotic lactobacilli drug resistance. Antibiot. Khimioterapiia 2015, 60, 6–13. [Google Scholar]
- Maldonado, N.C.; de Ruiz, C.S.; Otero, M.C.; Sesma, F.; Nader-Macías, M.E. Lactic acid bacteria isolated from young calve—Characterization and potential as probiotics. Res. Vet. Sci. 2012, 92, 342–349. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Yurtaeva, T.A.; Tsyganov, M.S.; Ezhkova, G.O. Physico-chemical and antioxidant properties of skimmed varenets (Slavic baked milk yogurt) mixed with enzyme-modified potato starches. Curr. Res. Nutr. Food Sci. J. 2021, 9, 88–99. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Pack Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Alqahtani, A.S.; Hassan, W.H.B.; Alzoubi, A.; Abdelaziz, S. Chemical profile, in vitro antioxidant, pancreatic lipase, and alpha-amylase inhibition assays of the aqueous extract of elettaria cardamomum L. fruits. J. Chem. 2021, 2021, 5583001. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 2013, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Isolauri, E.; Salminen, S.J. The role of the intestinal microflora for the development of the immune system in early childhood. Eur. J. Nutr. 2002, 41, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Leahy, S.C.; Higgins, D.G.; Fitzgerald, G.F.; Van Sinderen, D. Getting better with bifidobacteria. J. Appl. Microbiol. 2005, 98, 1303–1315. [Google Scholar] [CrossRef]
- Gueimonde, M.; Noriega, L.; Margolles, A.; De Los Reyes-Gavilan, C.G.; Salminen, S. Ability of Bifidobacterium strains with acquired resistance to bile to adhere to human intestinal mucus. Internat. J. Food Microbiol. 2005, 101, 341–346. [Google Scholar] [CrossRef]
- Vlková, E.; Rada, V.; Smehilová, M.; Killer, J. Auto-aggregation and co-aggregation ability in bifidobacteria and clostridia. Folia Microbiol. 2008, 53, 263–269. [Google Scholar] [CrossRef]
- Qin, X.-S.; Gao, Q.-Y.; Luo, Z.-G. Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles. Food Hydrocoll. 2021, 116, 106658. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Pastell, H.; Westermann, P.; Meyer, A.S.; Tuomainen, P.; Tenkanen, M. In vitro fermentation of arabinoxylan-derived carbohydrates by Bifidobacteria and mixed fecal microbiota. J. Agric. Food Chem. 2009, 57, 8598–8606. [Google Scholar] [CrossRef]
- Sørensen, H.R.; Pedersen, S.; Meyer, A.S. Optimization of reaction conditions for enzymatic viscosity reduction and hydrolysis of wheat arabinoxylan in an industrial ethanol fermentation residue. Biotechnol. Prog. 2006, 22, 505–513. [Google Scholar] [CrossRef]
- Marquez, A.; Andrada, E.; Russo, M.; Bolondi, M.L.; Fabersani, E.; Medina, R.; Gauffin-Cano, P. Characterization of autochthonous lactobacilli from goat dairy products with probiotic potential for metabolic diseases. Heliyon 2022, 8, e10462. [Google Scholar] [CrossRef]
- Panwar, H.D.; Calderwood, I.R.; Grant, S.; Grover, B.D. Green Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur. J. Nutr. 2014, 53, 1465–1474. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioprocess Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
- Parente, E.; Ciocia, F.; Ricciardi, A.; Zotta, T.; Felis, G.E.; Torriani, S. Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: A multivariate screening study. Int. J. Food Microbiol. 2010, 144, 270–279. [Google Scholar] [CrossRef]
- Zotta, T.; Guidone, A.; RIanniello, G.; Ricciardi, A.; Parente, E. Aerobic metabolism and oxidative stress tolerance in the Lactobacillus plantarum group. World J. Microbiol. Biotechnol. 2013, 29, 1713–1722. [Google Scholar]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Parente, E.; Reale, A.; Rossi, F.; Iacumin, L.; Comi, G.; Coppola, R. Assessment of aerobic and respiratory growth in the Lactobacillus casei group. PLoS ONE 2014, 9, e99189. [Google Scholar] [CrossRef]
- Guilbaud, M.; Zagorec, M.; Chaillou, S.; Champomier-Vergès, M. Intraspecies diversity of Lactobacillus sakei response to oxidative stress and variability of strain performance in mixed strains challenges. Food Microbiol. 2012, 29, 197–204. [Google Scholar] [CrossRef]
- Lavari, L.; Ianniello, R.; Páez, R.; Zotta, T.; Cuatrin, A.; Reinheimer, J.; Parente, E.; Vinderola, G. Growth of Lactobacillus rhamnosus 64 in whey permeate and study of the effect of mild stresses on survival to spray drying. LWT-Food Sci. Technol. 2015, 63, 322–330. [Google Scholar] [CrossRef]
- Abozed, S.S.; El-kalyoubi, A.; Abdelrashid, A.; Salama, M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agric. Sci. 2014, 59, 63–67. [Google Scholar] [CrossRef]
- Oliveira, A.M.F.; Pinheiro, L.S.; Pereira, C.K.S.; Matias, W.N.; Gomes, R.A.; Chaves, O.S.; de Souza, M.D.F.V.; de Almeida, R.N.; de Assis, T.S. Total phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants 2012, 1, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.; Mantovani, R.A.; Raposo, M.F.J.; Coimbra, M.A.; Vicente, A.A.; Cunha, R.L. Effect of extraction temperature on rheological behavior and antioxidant capacity of flaxseed gum. Carbohydr. Polym. 2019, 213, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Bettaieb, A.; Haj, F.J.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch. Biochem. Biophys. 2016, 559, 13–21. [Google Scholar] [CrossRef]
- Liang, S.; Liao, W.; Ma, X.; Li, X.; Wang, Y. H2O2 oxidative preparation, characterization and antiradical activity of a novel oligosaccharide derived from flaxseed gum. Food Chem. 2017, 230, 135–144. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- Champagne, C.P.; Raymond, Y.; Guertin, N.; Martoni, C.J.; Jones, M.L.; Mainville, I.; Arcand, Y. Impact of a yogurt matrix and cell microencapsulation on the survival of Lactobacillus reuteri in three in vitro gastric digestion procedures. Benef. Microbes 2015, 6, 753–763. [Google Scholar] [CrossRef]
- Shinde, T.; Sun-Waterhouse, D.; Brooks, J. Co-extrusion encapsulation of probiotic Lactobacillus acidophilus alone or together with apple skin polyphenols: An aqueous and value-added delivery system using alginate. Food Bioprocess Technol. 2014, 7, 1581–1596. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).