Solid Fungi Starters Using Aspergillus spp. under Different Manufacturing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Manufacturing Method of Solid-State Fungi Starter
2.2. Microbial Contamination and Spore Count
2.3. Enzyme Activity Analysis
2.4. Analysis of Free Amino Acid Content
2.5. Analysis of Volatile Flavor Compounds
2.6. Statistical Analysis
3. Results
3.1. Manufactured Solid Fungal Starter
3.2. Microbial Contamination and Spore Count
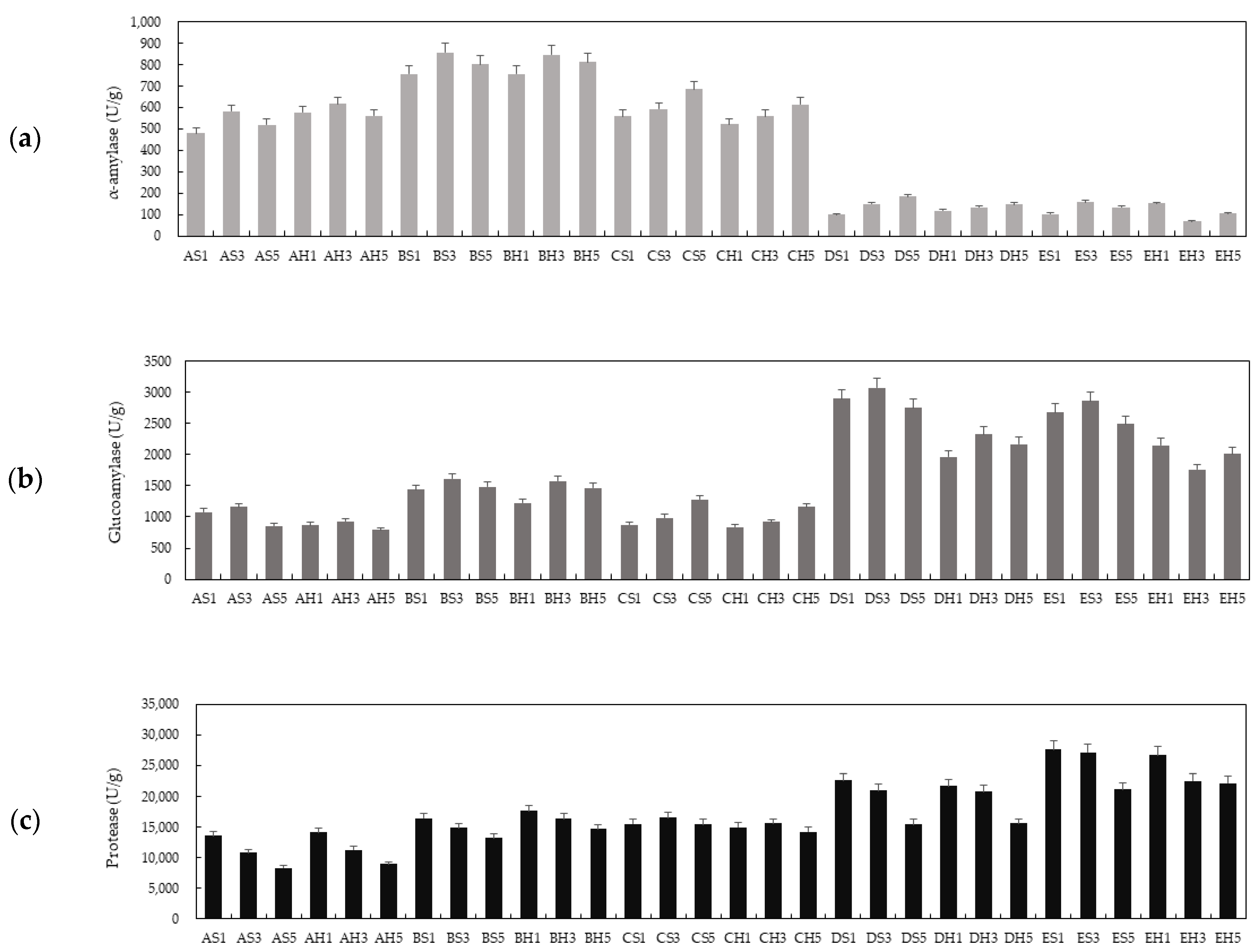
3.3. Enzyme Activity
3.4. Free Amino Acid Content
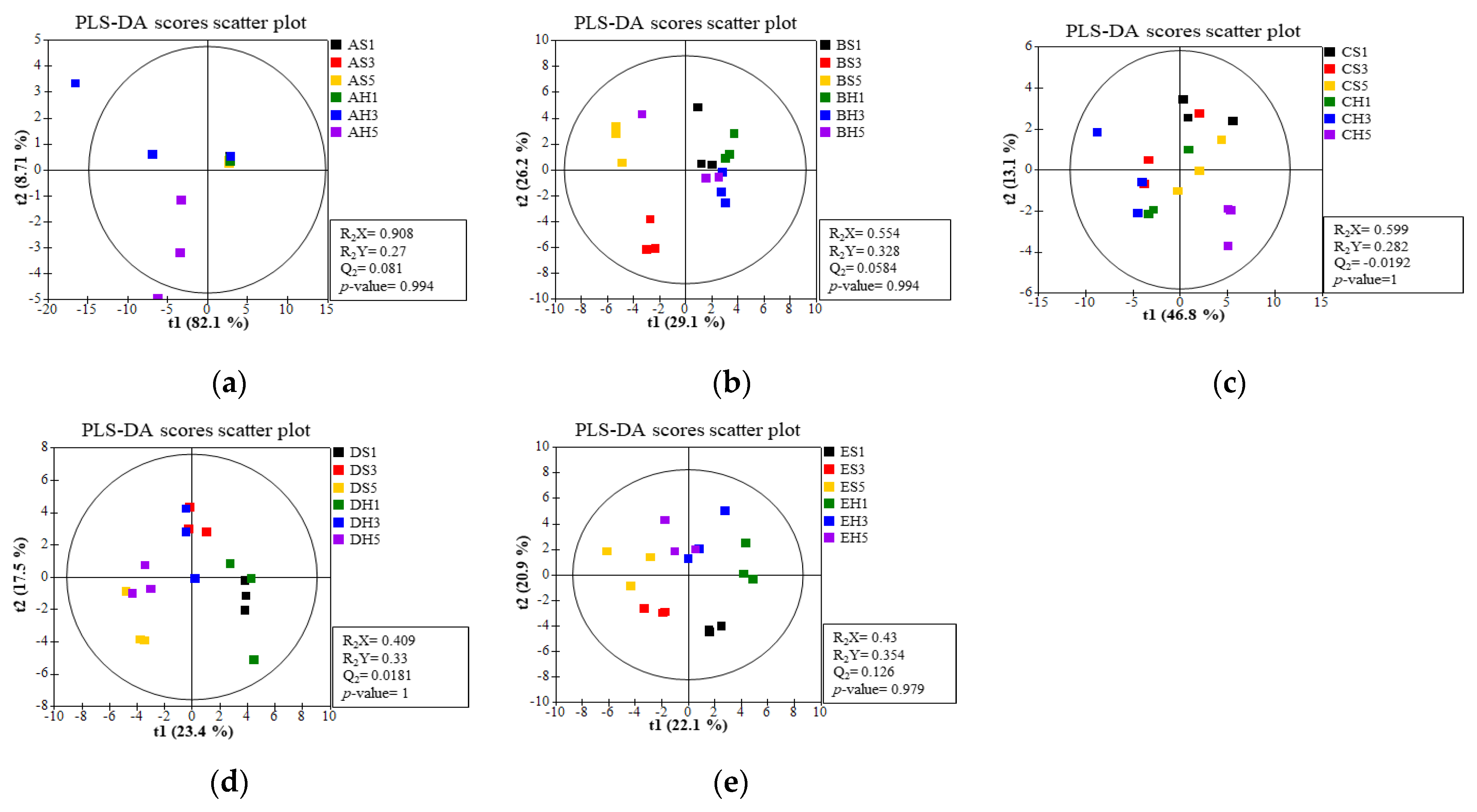
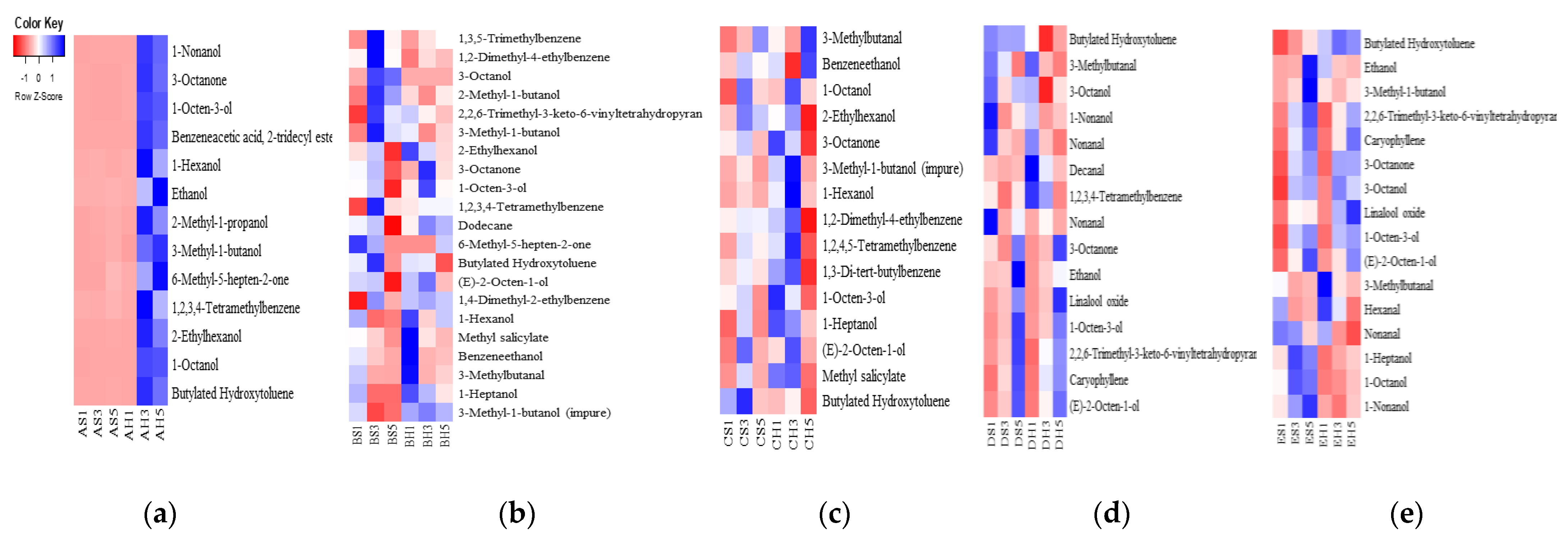
3.5. Volatile Flavor Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beak, C.H.; Beak, S.Y.; Mun, J.Y.; Choi, H.S.; Kang, J.E.; Jung, S.T.; Yeo, S.H. Quality characteristics and preparing of solid starter using fungal strains for Takju. Korea J. Food Preserv. 2016, 23, 797–803. [Google Scholar] [CrossRef]
- Lee, S.J.; Ahn, B.H. Sensory profiling of rice wines made with Nuruks using different ingredients. Korean J. Food Sci. Technol. 2010, 42, 119–123. [Google Scholar]
- Jeon, C.O. Industrial application and starter development for traditional fermentation food using lab evolution method. E-bioindustry 2014, 27, 1–4. [Google Scholar]
- Lee, D.H.; Kang, H.Y.; Lee, Y.S.; Cho, C.H.; Kim, S.J.; Lee, J.S. Effects of yeast and Nuruk on the quality of Korean Yakju. Korean J. Microbiol. Biotechnol. 2011, 39, 274–280. [Google Scholar] [CrossRef]
- Bal, J.; Yun, S.H.; Chun, J.S.; Kim, B.T.; Kim, D.H. Taxonomic characterization, evaluation of toxigenicity, and saccharification capability of Aspergillus section Flavi isolates from Korean traditional wheat-based fermentation starter Nuruk. Mycobiology 2016, 44, 155–161. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.A.; Jeenes, D.J.; Gou, X.; Archer, D.B. Molecular basis of glucoamylase overproduction by a mutagenised industrial strain of Aspergillus niger. Enzyme Microbial. Technol. 2000, 26, 193–200. [Google Scholar] [CrossRef]
- Thenmozhi, M.; Kannabiran, K.; Kumar, R.; Khanna, V.G. Antifungal activity of Streptomyces sp. VITSTK7 and its synthesized Ag2O/Ag nanoparticles against medically important Aspergillus pathogens. J. Mycol. Med. 2013, 23, 97–103. [Google Scholar] [CrossRef]
- Park, I.S.; Chung, Y.H. Some factors affecting glucoamylase production from Aspergillus sp. Korean J. Appl. Microbiol. Bioeng. 1989, 17, 519–523. [Google Scholar]
- Lee, S.H.; Jung, H.J.; Yeo, S.H.; Kim, H.S.; Yu, T.S. Characteristics of α-amylase of, a new species, Aspergillus coreanus NR 15-1. Korean J. Biotechnol. Bioeng. 2004, 19, 301–307. [Google Scholar]
- Kim, J.W.; Doo, H.S.; Kwon, T.H.; Kim, Y.S.; Shin, D.H. Quality characteristics of Doenjang Meju fermented with Aspergillus species and Bacillus subtilis during fermentation. Korean J. Food Preserv. 2011, 18, 397–406. [Google Scholar] [CrossRef]
- Kim, H.E.; Han, S.Y.; Kim, Y.S. Quality characteristics of Gochujang Meju prepared with different fermentation tools and inoculation time of Aspergillus oryzae. Food Sci. Biotechnol. 2010, 19, 1579–1585. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choi, S.J.; Kwak, J.K.; Seo, M.J.; Moon, T.W.; Lee, Y.W. Aspergillus oryzae strains isolated from traditional Korean Nuruk: Fermentation properties and influence on rice wine quality. Food Sci. Biotechnol. 2013, 22, 425–432. [Google Scholar] [CrossRef]
- Ghoson, M.D.; Faten, A.M.; Waill, A.E. The ancient koji mold (Aspergillus oryzae) as a modern biotechnological tool. Bioresour. Bioprocess. 2021, 8, 52. [Google Scholar]
- NTS Liquors Licence Aid Center. Coursebook on the Preparation of Takju and Yakju; NTS Liquors Licence aid Center: Seoul, Republic of Korea, 2010; pp. 89–90.
- Kim, B.M.; Lee, H.J.; Song, Y.H.; Kim, H.J. Effect of salt stress on the growth, mineral contents, and metabolite profiles of spinach. J. Sci. Food Agric. 2021, 101, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, B.M.; Lee, H.J.; Jang, G.J.; Song, S.H.; Lee, J.I.; Lee, S.B.; Shim, J.M.; Lee, K.W.; Kim, J.H.; et al. Effects of different salt treatments on the fermentation metabolites and bacterial profiles of Kimchi. J. Food Sci. 2017, 82, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.S.; Kim, J.; Kim, H.S.; Hyun, J.S.; Ha, H.P.; Park, M.G. Bibliographical study on microorganism of traditional Korean Nuruk (since 1945). J. Korean Soc. Food Sci. 1998, 27, 789–799. [Google Scholar]
- Lee, D.H.; Seo, J.S.; Ha, T.M.; Lee, Y.S.; Cho, C.H. Quality characteristics of Yakju fermented with pretreated Nuruk. J. Korean Soc. Food Sci. Nutr. 2020, 49, 1366–1376. [Google Scholar] [CrossRef]
- Donato, M.; Antonia, S.; Massimo, F.; Antonio, F.L.; Giancarlo, P. Penicillium species: Crossroad between quality and safety of cured meat production. Curr. Opin. Food Sci. 2017, 17, 36–40. [Google Scholar]
- Kim, J.Y.; Gwon, H.M.; Kim, S.Y.; Yeo, S.H. Quality characteristics of solid starters manufactured with Aspergillus oryzae OF5-20. Korean J. Food Preserv. 2020, 27, 915–924. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, W.S.; Kim, Y.J. Comparison of fermentation characteristics of Korean traditional alcoholic beverage with different input step and treatment of rice and Nuruk (Korean-style bran Koji). Korean J. Dietary Culture 1996, 11, 339–348. [Google Scholar]
- Kim, J.Y.; Mun, J.Y.; Kim, S.H.; Kim, S.Y.; Yeo, S.W. Quality characteristics of solid fermented Koji manufactured from aflatoxin-reduced Aspergillus sp. starter. J. East Asian So. Diet. Life 2018, 28, 384–390. [Google Scholar] [CrossRef]
- Hu, H.I.; Brink, J.V.D.; Gruben, B.S.; Wosten, H.A.B.; Gu, J.D.; Vries, R.P.D. Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int. Biodeterior. Biodegrad. 2011, 65, 248–252. [Google Scholar] [CrossRef]
- Gelver, I.B.; Gruntjes, T.; Vinck, A.; Veluw, J.G.V.; Wosten, H.A.B.; Boeren, S.; Voervoort, J.J.M.; Vries, R.P.D. Mixed colonies of Aspergillus niger and Aspergillus oryzae cooperatively degrading wheat bran. Fungal Genet. Biol. 2017, 102, 331–337. [Google Scholar]
- Laothanachareon, T.; Bunterngsook, B.; Champreda, V. Profiling multi-enzyme activities of Aspergillus niger strains growing on various agro-industrial residues. 3 Biotech. 2022, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Tasted; American Chemical Society: Washington, DC, USA, 1989; pp. 158–174. [Google Scholar]
- Ninomiya, K. Science of umami taste: Adaptation to gastronomic culture. Ninomiya Flavour. 2015, 4, 13. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, T.S.; Noh, B.S. Organic acid, free sugar and free amino acid of Takju prepared using rice Nuruks. J. Nat. Sci. SWINS 2004, 16, 75–83. [Google Scholar]
- Kang, J.E.; Choi, H.S.; Kim, J.W.; Kim, C.W.; Yeo, S.H.; Jung, S.T. Quality characteristics of Yakju with Nuruk extracts. Korean J. Food Sci. Technol. 2016, 48, 223–230. [Google Scholar] [CrossRef]
- Lee, S.W.; Yoon, S.R.; Kim, G.R.; Kyung, H.K.; Jeong, Y.J.; Yeo, S.H.; Kwon, J.H. Effect of Nuruks and crude amylolytic enzyme on free amino acid and volatile components of brown rice vinegar prepared by static culture. Korean J. Food Sci. 2011, 43, 570–576. [Google Scholar] [CrossRef]
- Kaminski, E.; Stawicki, S.; Wasowicz, E. Volatile flavor compounds produced by molds of Aspergillus, Penicillium, and fungi imperfecti. Appl. Microbiol. 1974, 27, 1001–1004. [Google Scholar] [CrossRef]
- Lee, S.J.; Kwon, Y.H.; Kim, H.J.; Ahn, B.H. Chemical and sensory characterization of Korean commercial rice wines (Yakju). Food Sci. Biotechnol. 2007, 16, 374–380. [Google Scholar]
- Jung, H.Y.; Lee, S.J.; Lim, J.H.; Kim, B.K.; Park, K.J. Chemical and sensory profiles of Makgeolli, Korean commercial rice wine, from descriptive, chemical, and volatile compound analyses. Food Chem. 2014, 152, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.D.; Yang, S.H.; Choi, M.R.; Kim, J.K. Volatile component of Korean soybean paste produced by Bacillus subtilis PM3. J. Microbiol. Biotechnol. 1995, 5, 143–148. [Google Scholar]
- Hong, Y.; Park, S.K.; Choi, E.H. Flavor characteristics of Korean traditional distilled liquors produced by the co-culture of Saccharomyces and Hansenula. Kor. J. Appl. Microbiol. Biotechnol. 1999, 27, 236–245. [Google Scholar]
- Koizumi, T.; Suzuki, M. Alcohol formation by Aspergillus oryzae. J. Ferment. Technol. 1998, 56, 222–226. [Google Scholar]
- Lee, S.M.; Shin, K.J.; Lee, S.J. Exploring Nuruk aroma; Identification of volatile compounds in commercial fermentation starters. Food Sci. Biotechnol. 2016, 25, 393–399. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Xie, Y.; Liang, J. Antifungal properties and mechanisms of three volatile aldehydes (octanal, nonanal and decanal) on Aspergillus flavus. Grain Oil Sci. Technol. 2021, 4, 131–140. [Google Scholar] [CrossRef]
- Nargesi, S.; Abastabar, M.; Valadan, R.; Mayahi, S.; Youn, J.H.; Hadayati, M.T.; Seyedmousavi, S. Differentiation of Aspergillus flavus from Aspergillus oryzae targeting the cyp51A gene. Pathogens 2021, 10, 1279. [Google Scholar] [CrossRef]
- Park, M.K.; Seo, J.A.; Kim, Y.S. Comparative study on metabolic changes of Aspergillus oryzae isolated from fermented foods according to culture conditions. Int. J. Food Microbiol. 2019, 307, 108270. [Google Scholar] [CrossRef]
- Bruna, J.M.; Hierro, E.M.; de la Hoz, L.; Mottram, D.S.; Fernandez, M.; Ordonez, J.A. The contribution of Penicillium aurantiogriseum to the volatile composition and sensory quality of dry fermented sausages. Meat Sci. 2001, 59, 97–107. [Google Scholar] [CrossRef]

| Strain 1 | Liquid Starter (v/w)(%) | ||
|---|---|---|---|
| 1 | 3 | 5 | |
| AO 81-2 |  |  |  |
| AO 82-3 |  |  |  |
| AO 2-6 |  |  |  |
| AN IF13-3 |  |  |  |
| AN 4-3-2 |  |  |  |
| Strain 1 | Drying Condition | Liquid Starter (v/w)(%) | Microbial Contamination | Spores of Aspergillus spp. (×108 Spores/g) | ||
|---|---|---|---|---|---|---|
| General Bacteria (CFU/g) | Penicillium spp. (-), (+) | |||||
| AO 81-2 | L.T. 2 | 35 °C 24 h | 1 | 9.0 × 102 | - | 2.7 ± 0.3 |
| 3 | 2.0 × 103 | - | 2.2 ± 0.2 | |||
| 5 | 2.0 × 103 | - | 2.3 ± 0.3 | |||
| H.T. | 45 °C 18 h | 1 | 1.0 × 103 | - | 2.4 ± 0.4 | |
| 3 | 3.6 × 103 | - | 2.0 ± 0.2 | |||
| 5 | 4.4 × 103 | - | 2.1 ± 0.2 | |||
| AO 82-3 | L.T. | 35 °C 24 h | 1 | 4.1 × 102 | - | 0.9 ± 0.2 |
| 3 | 3.8 × 102 | - | 1.7 ± 0.1 | |||
| 5 | 8.0 × 101 | - | 1.8 ± 0.3 | |||
| H.T. | 45 °C 18 h | 1 | 1.5 × 103 | - | 0.7 ± 0.2 | |
| 3 | 2.2 × 102 | - | 1.5 ± 0.2 | |||
| 5 | 1.2 × 102 | - | 1.9 ± 0.3 | |||
| AO 2-6 | L.T. | 35 °C 24 h | 1 | 2.3 × 102 | - | 2.7 ± 0.2 |
| 3 | 1.0 × 101 | - | 3.4 ± 0.4 | |||
| 5 | 1.0 × 101 | - | 4.2 ± 0.6 | |||
| H.T. | 45 °C 18 h | 1 | 9.3 × 102 | - | 2.6 ± 0.4 | |
| 3 | 3.5 × 102 | - | 3.1 ± 0.3 | |||
| 5 | 1.0 × 101 | - | 4.0 ± 0.6 | |||
| AN IF13-3 | L.T. | 35 °C 24 h | 1 | 8.8 × 102 | - | 1.3 ± 0.1 |
| 3 | 6.4 × 102 | - | 2.3 ± 0.1 | |||
| 5 | 1.7 × 102 | - | 2.8 ± 0.2 | |||
| H.T. | 45 °C 18 h | 1 | 1.1 × 103 | - | 1.2 ± 0.3 | |
| 3 | 9.7 × 102 | - | 2.1 ± 0.2 | |||
| 5 | 2.3 × 102 | - | 2.6 ± 0.2 | |||
| AN 4-3-2 | L.T. | 35 °C 24 h | 1 | 3.9 × 102 | - | 1.2 ± 0.0 |
| 3 | 1.0 × 101 | - | 2.1 ± 0.3 | |||
| 5 | N.D.3 | - | 2.3 ± 0.2 | |||
| H.T. | 45 °C 18 h | 1 | 4.2 × 102 | - | 0.8 ± 0.4 | |
| 3 | 4.0 × 102 | - | 1.8 ± 0.2 | |||
| 5 | N.D. | - | 2.1 ± 0.3 | |||
| Strain | AO 1 81-2 | AO 82-3 | AO 2-6 | AN IF 13-3 | AN 4-3-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | p-Value 2 | VIP 3 | p-Value | VIP | p-Value | VIP | p-Value | VIP | p-Value | VIP | |
| Esters | |||||||||||
| Benzeneacetic acid, 2-tridecyl ester | 3.5 × 10−1 | 0.91 | 1.5 × 10−1 | 0.79 | 2.6 × 10−1 | 0.78 | 1.8 × 10−1 | 0.65 | 3.5 × 10−1 | 0.91 | |
| Methyl salicylate | 9.0 × 10−1 | 0.49 | 1.2 × 10−7 | 0.97 | 6.0 × 10−3 | 1.09 | 7.4 × 10−2 | 0.54 | 9.0 × 10−1 | 0.49 | |
| Alcohols | |||||||||||
| Ethanol | 2.9 × 10−2 | 0.60 | 3.7 × 10−1 | 0.45 | 1.5 × 10−1 | 0.35 | 7.8 × 10−7 | 1.47 | 2.9 × 10−2 | 0.60 | |
| 2-Methyl-1-propanol | 1.1 × 10−1 | 0.50 | 4.7 × 10−1 | 0.62 | 4.1 × 10−1 | 1.10 | 3.6 × 10−1 | 0.46 | 1.1 × 10−1 | 0.50 | |
| 2-Methyl-1-butanol | 6.5 × 10−2 | 0.77 | 4.5 × 10−3 | 1.15 | 1.6 × 10−1 | 1.63 | 4.4 × 10−1 | 0.45 | 6.5 × 10−2 | 0.77 | |
| 3-Methyl-1-butanol | 1.7 × 10−2 | 0.94 | 4.3 × 10−2 | 0.93 | 1.7 × 10−1 | 1.61 | 8.3 × 10−2 | 0.93 | 1.7 × 10−2 | 0.94 | |
| 1-Hexanol | 6.4 × 10−1 | 0.71 | 1.2 × 10−2 | 1.09 | 1.1 × 10−4 | 1.18 | 6.2 × 10−2 | 1.18 | 6.4 × 10−1 | 0.71 | |
| 3-Octanol | - | - | 1.2 × 10−6 | 1.37 | 5.5 × 10−2 | 1.34 | 3.6 × 10−2 | 1.16 | 6.0 × 10−3 | 1.22 | |
| 1-Octen-3-ol | 2.3 × 10−6 | 1.52 | 1.1 × 10−3 | 1.26 | 3.9 × 10−2 | 0.62 | 7.6 × 10−8 | 1.62 | 2.3 × 10−6 | 1.52 | |
| 1-Heptanol | - | - | 6.0 × 10−5 | 1.34 | 2.1 × 10−2 | 1.25 | 6.7 × 10−1 | 0.78 | 3.9 × 10−2 | 1.19 | |
| 2-Ethylhexanol | 3.6 × 10−1 | 0.87 | 2.4 × 10−3 | 1.11 | 1.1 × 10−2 | 0.95 | 1.1 × 10−1 | 1.12 | 3.6 × 10−1 | 0.87 | |
| 1-Octanol | 5.0 × 10−3 | 1.34 | 1.6 × 10−1 | 0.94 | 1.4 × 10−2 | 0.89 | 5.1 × 10−1 | 1.00 | 5.0 × 10−3 | 1.34 | |
| (E)-2-Octen-1-ol | 1.0 × 10−3 | 1.42 | 1.1 × 10−2 | 1.19 | 1.4 × 10−1 | 0.77 | 1.0 × 10−3 | 1.45 | 1.0 × 10−3 | 1.42 | |
| 1-Nonanol | - | - | - | - | - | - | 9.0 × 10−3 | 1.29 | 4.8 × 10−2 | 1.10 | |
| Benzeneethanol | 4.8 × 10−2 | 0.40 | 3.6 × 10−3 | 0.78 | 1.6 × 10−2 | 1.13 | 6.2 × 10−2 | 1.30 | 1.5 × 10−1 | 0.40 | |
| Aldehydes | |||||||||||
| 3-Methylbutanal | - | - | 2.5 × 10−4 | 0.96 | 1.2 × 10−2 | 1.62 | 3.4 × 10−5 | 1.49 | 3.0 × 10−3 | 1.14 | |
| Hexanal | - | - | - | - | - | - | 7.9 × 10−2 | 1.16 | 6.0 × 10−3 | 1.18 | |
| Nonanal | - | - | - | - | - | - | 2.4 × 10−2 | 1.28 | 1.1 × 10−2 | 1.27 | |
| Decanal | - | - | - | - | - | - | 1.7 × 10−2 | 0.81 | 1.1 × 10−1 | 1.04 | |
| Ketones | |||||||||||
| 2,4-Dimethylheptane | 8.2 × 10−2 | 1.10 | 6.9 × 10−2 | 1.09 | 7.4 × 10−1 | 0.50 | 2.1 × 10−1 | 0.86 | 8.2 × 10−2 | 1.10 | |
| 3-Octanone | 2.8 × 10−6 | 1.49 | 2.5 × 10−3 | 1.10 | 2.9 × 10−2 | 0.78 | 1.1 × 10−4 | 1.45 | 2.8 × 10−6 | 1.49 | |
| 6-Methyl-5-hepten-2-one | 1.3 × 10−1 | 1.01 | 2.4 × 10−4 | 0.21 | 3.0 × 10−1 | 0.29 | 2.7 × 10−1 | 1.05 | 1.3 × 10−1 | 1.01 | |
| Miscellaneous | |||||||||||
| Dodecane | 1.5 × 10−1 | 1.02 | 3.0 × 10−2 | 1.07 | 1.3 × 10−1 | 0.87 | 4.3 × 10−1 | 0.45 | 1.5 × 10−1 | 1.02 | |
| 1,3,5-Trimethylbenzene | 8.2 × 10−1 | 0.11 | 1.4 × 10−2 | 1.22 | 4.4 × 10−1 | 0.72 | 3.4 × 10−1 | 0.99 | 8.2 × 10−1 | 0.11 | |
| 1,4-Dimethyl-2-ethylbenzene | 2.2 × 10−1 | 0.85 | 9.2 × 10−3 | 0.85 | 1.7 × 10−1 | 0.61 | 1.9 × 10−1 | 0.58 | 2.2 × 10−1 | 0.85 | |
| 1,2-Dimethyl-4-ethylbenzene | 3.0 × 10−1 | 0.52 | 7.0 × 10−3 | 1.20 | 2.6 × 10−2 | 1.04 | 1.5 × 10−1 | 0.67 | 3.0 × 10−1 | 0.52 | |
| 1,2,4,5-Tetramethylbenzene | 9.9 × 10−1 | 0.05 | 3.0 × 10−1 | 0.58 | 8.0 × 10−3 | 1.02 | 1.8 × 10−1 | 0.37 | 9.9 × 10−1 | 0.05 | |
| 1,3-Di-tert-butylbenzene | 4.4 × 10−1 | 0.73 | 6.6 × 10−2 | 1.06 | 3.2 × 10−2 | 0.95 | 6.8 × 10−2 | 0.28 | 4.4 × 10−1 | 0.73 | |
| 1,2,3,4-Tetramethylbenzene | 3.7 × 10−1 | 0.88 | 7.5 × 10−3 | 1.15 | 5.2 × 10−2 | 0.9 | 3.7 × 10−2 | 0.79 | 3.7 × 10−1 | 0.88 | |
| Linalool oxide | - | - | - | - | - | - | 5.3 × 10−9 | 1.5 | 1.6 × 10−2 | 1.06 | |
| 2,2,6-Trimethyl-3-keto-6-vinyltetrahydropyran | 1.0 × 10−3 | 1.43 | 3.4 × 10−3 | 0.99 | 2.4 × 10−2 | 0.99 | 1.7 × 10−5 | 1.52 | 1.0 × 10−3 | 1.43 | |
| Caryophyllene | - | - | - | - | - | - | 1.1 × 10−7 | 1.57 | 3.3 × 10−4 | 1.43 | |
| Butylated hydroxyltoluene | 1.0 × 10−3 | 1.48 | 1.3 × 10−4 | 1.12 | 5.0 × 10−3 | 1.51 | 1.0 × 10−2 | 0.67 | 1.0 × 10−3 | 1.48 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Kang, H.B.; Kim, S.H.; Jeong, W.S.; Kim, S.-Y.; Yeo, S.-H. Solid Fungi Starters Using Aspergillus spp. under Different Manufacturing Conditions. Fermentation 2023, 9, 487. https://doi.org/10.3390/fermentation9050487
Lee SJ, Kang HB, Kim SH, Jeong WS, Kim S-Y, Yeo S-H. Solid Fungi Starters Using Aspergillus spp. under Different Manufacturing Conditions. Fermentation. 2023; 9(5):487. https://doi.org/10.3390/fermentation9050487
Chicago/Turabian StyleLee, Su Jeong, Han Byul Kang, Sun Hee Kim, Woo Soo Jeong, So-Yeong Kim, and Soo-Hwan Yeo. 2023. "Solid Fungi Starters Using Aspergillus spp. under Different Manufacturing Conditions" Fermentation 9, no. 5: 487. https://doi.org/10.3390/fermentation9050487
APA StyleLee, S. J., Kang, H. B., Kim, S. H., Jeong, W. S., Kim, S.-Y., & Yeo, S.-H. (2023). Solid Fungi Starters Using Aspergillus spp. under Different Manufacturing Conditions. Fermentation, 9(5), 487. https://doi.org/10.3390/fermentation9050487





