Abstract
This study reports the development of a bioprocess involving the valorization of biodiesel-derived glycerol as the main carbon source for cell proliferation of Yarrowia lipolytica strains and production of metabolic compounds, i.e., citric acid (Cit), polyols, and other bio-metabolites, the substitution of process tap water with olive mill wastewater (OMW) in batch fermentations, and partial detoxification of OMW (up to 31.1% decolorization). Increasing initial phenolics (Phen) of OMW-glycerol blends led to substantial Cit secretion. Maximum Cit values, varying between 64.1–65.1 g/L, combined with high yield (YCit/S = 0.682–0.690 g Cit/g carbon sources) and productivity (0.335–0.344 g/L/h) were achieved in the presence of Phen = 3 g/L. The notable accumulation of endopolysaccharides (EPs) on the produced biomass was determined when Y. lipolytica LMBF Y-46 (51.9%) and ACA-YC 5033 (61.5%) were cultivated on glycerol-based media. Blending with various amounts of OMW negatively affected EPs and polyols biosynthesis. The ratio of mannitol:arabitol:erythritol was significantly affected (p < 0.05) by the fermentation media. Erythritol was the major polyol in the absence of OMW (53.5–62.32%), while blends of OMW-glycerol (with Phen = 1–3 g/L) promoted mannitol production (54.5–76.6%). Nitrogen-limited conditions did not favor the production of cellular lipids (up to 16.6%). This study addressed sustainable management and resource efficiency enabling the bioconversion of high-organic-load and toxic waste streams into valuable products within a circular bioeconomy approach.
1. Introduction
Sustainable development is a top priority for the United Nations, while shared blueprints, including the 2030 Agenda for Sustainable Development for human and environmental propensity [1,2] and the ‘Fit for 55’ EU policy for climate neutrality [3], have already been introduced. The long-term targets include decoupling economic growth from newly extracted resources, zero net emissions, and decarbonization of production processes. Industries of food-, edible oil- and lignocellulosic biomass processing, as well as biodiesel production, generate substantial volumes of carbon-laden water waste streams that must be properly managed to deviate from severe environmental and economic effects [4]. The efficient management of such feedstock via green manufacturing and microbial bioconversion to obtain value-added products is of utmost importance to facilitate the world’s transition towards sustainability, renewability, and circular bioeconomy concepts [5].
Olive oil processing produces around 30 million m3/per year of olive mill wastewater (OMW) globally [6]. These high quantities of OMW are toxic since they are conventionally deposited directly in aquatic ecosystems, leading to the increased organic charge and phytotoxicity of air and water. It has been reported that OWM could create 200–400 times more pollution than urban wastewater [4]. Crude glycerol (mostly from the biodiesel industry) production is estimated at around 41.9 billion, with 66% of it being generated from the biodiesel industry [7]. It constitutes the most severe obstacle to the effective development of biodiesel as an alternative biofuel. Several strategies have been explored for its value-added valorization [8], with microbial bioconversion being among the most promising.
Organic acids constitute significant building blocks that have a prominent contribution to the biotechnological production of commodity chemicals. The accumulation of most of the organic acids, i.e., citric acid (Cit), occurs via both catabolic and anabolic activities in several microbial strains [9]. The global Cit market is anticipated to reach USD 3.83 billion by 2025, at a CAGR of 4.9%, while the food and beverages applications of Cit are the main factors that drive its increasing market [10]. Cit is a “generally recognized as safe” tricarboxylic acid, and it is widely applied as a flavoring, emulsifying, stabilizing and texturing agent in food-related applications [11]. Numerous feedstocks have been explored for Cit production via fermentation with yeasts such as Yarrowia lipolytica. Y. lipolytica can utilize several hydrophilic and hydrophobic fermentation substrates, including commercial C5 and C6 sugars, cellulose- and hemicellulose-based hydrolysates, glycerol, and fatty compounds [12,13].
Natural sweeteners, i.e., mannitol, xylitol, arabitol and erythritol, are gaining a tremendous market share as alternatives of added sugars (sucrose, fructose, glucose, syrups) in novel formulas as they have been approved as food additives in the EU and US [14]. The worldwide market of polyols was US$26 billion in 2019, while it is projected to reach US$34 billion by 2024 [15]. This increase is mainly attributed to the increasing need for polyols in sectors of food, pharmaceuticals, polymers (building blocks for polyurethane production), and chemicals. Polyols are low-metabolizable sugar alcohols with a strong sweetening capacity, low caloric and glycemic profile, and several health-promoting properties related mostly to diabetes (i.e., reduced insulin response), obesity and non-cariogenic activity. Diabetes was related to 4 million deaths and costs of USD$727 billion for health care in 2017 [16]. Industrial production of polyols is carried out mostly by the catalytic reduction of sugars with hydrogen under elevated pressure and temperature, while the whole process requires highly pure sugars as the initiating material and high-cost chromatographic purification steps. Recently scientific research has focused on biotechnological approaches to improve the production efficiency (yield, productivity, cost) of polyols valorizing unconventional carbohydrate-rich feedstock that is, hemicellulosic hydrolysate derived from corn cob and rapeseed straw, fruit juices, oilseed meals, sugarcane bagasse, molasses, straws of wheat and rapeseed, residue of soybean extract, and glycerol, employing lactic acid bacteria or yeast strains i.e., Debaryomyces hansenii, Candida tropicalis, Kluyveromyces marxianus, and Yarrowia lipolytica etc. [14,16,17]
In the current study, two non-conventional yeast strains, namely Y. lipolytica LMBF Y-46 and Y. lipolytica ACA-YC 5033, were investigated for their potential to grow and to produce Cit in various blends of crude glycerol-OMW in shake flask batch fermentations. Their metabolic profile, including the co-production of EPs, microbial oil and polyols, was also monitored. The inhibitory effect of initial phenolic compounds (Phen) found in OMW was thoroughly investigated. The detoxification potential of both strains, as far as the decolorization of OMW was considered, was determined. The fatty acid profile of cellular lipids was reported under all the performed fermentation conditions.
The main novelty of this study is the fact that principles of circular bioeconomy were implemented, including microbial bioprocessing, to produce bioproducts of remarkable commercial interest, such as Cit and polyols. Microbial biomass rich in EPs and unsaturated microbial oil could also be considered as a co-product of high nutritional value with targeted end-uses. The whole fermentation process resulted in a substantial bioremediation of OMW via decolorization. In this way, a sustainable and circular process could be developed for the efficient valorization of major pollutants from the biodiesel industry and olive mills.
2. Materials and Methods
2.1. Microorganisms and Raw Materials
The yeast strains Y. lipolytica ACA-YC 5033 (isolated from traditional Greek wheat sourdough) [18] and Y. lipolytica LMBF Y-46 (isolated from gilt-head (sea) bream, Sparus aurata, fish) [19] were used to produce Cit. The strains were maintained on potato dextrose agar (39 g/L) at 6 °C, and they were regularly sub-cultured to ensure cells’ viability.
The OMWs were obtained from an olive mill in the Perichora region (Corinthia, Greece), equipped with a three-phase decanter. OMWs were centrifuged (9000 rpm, 15 min, 4 °C; Hettich-Universal 320R, Germany centrifuge) to remove any solids while the supernatant was collected and stored at −20 °C for further use. Crude glycerol (purity of 85%, 6% potassium and sodium salts, 1% lipids, 5% water, <0.1% methanol; w/w) was kindly provided by Elin Verd SA (Volos, Greece).
2.2. Formulation of Fermentation Media and Batch Fermentations for Cit Production
The OMW supernatant was properly diluted with water to achieve initial phenolics concentrations of around 1 g/L, 2 g/L and 3 g/L, and it was further enriched with crude glycerol at a final concentration of ca. 77.9 ± 0.52 g/L to formulate different growth media of OMW-glycerol blends (in terms of phenolic compounds concentration). A control experiment consisting of crude glycerol at a final concentration of ca. 77.9 ± 0.52 g/L was also performed for comparison. All growth media were supplemented with nitrogen sources (1 g/L yeast extract and 1 g/L peptone) and mineral salts at final concentration (in g/L) of: KH2PO4 7.0; Na2HPO4 2.5; MgSO4·7H2O 1.5; FeCl3·6H2O 0.15; CaCl2·2H2O 0.15; ZnSO4·7H2O 0.02; MnSO4·H2O 0.06.
Cit production was carried out in 250 mL Erlenmeyer flasks containing 50 mL of sterilized (at 121 °C for 20 min) growth media (blends of OMW-glycerol and synthetic media) and incubated for up to 244 h (28 °C, 180 rpm). The pH of the fermentation media was maintained within the range of 5–6 using appropriate volumes of 5 Μ ΝaOH under aseptic conditions. Prior to incubation, all flasks were inoculated with 2% v/v of an exponential phase preculture that was carried out using a synthetic medium consisting of 10 g/L glucose, 10 g/L yeast extract and 10 g/L peptone. The preculture-containing flasks were incubated in an orbital shaker (New Brunswick Scientific, Edison, NJ, USA) at 28 °C and agitation of 180 rpm for 24 h.
2.3. Analytical Methods
The fermentation broth was centrifuged (7690× g, 4 °C, 10 min), and the supernatant was collected for Cit, polyols and carbon sources analysis. The yeast biomass was collected and repeatedly washed with distilled water, followed by centrifugation (7690× g, 4 °C, 10 min). Total dry biomass (TDW, g/L) was determined gravimetrically after drying at 90 °C until constant weight. Total intracellular lipids were extracted from the dried biomass according to Folch et al. [20] using a 2:1 (v/v) chloroform:methanol solution and it was gravimetrically quantified in pre-weighed round bottom flasks after the solvent’s removal under reduced pressure (Büchi Rotavapor R-114).
The concentration of phenolic compounds was determined using the Folin–Ciocalteau method [21]. The absorbance was measured at a wavelength of 750 nm, and it was expressed as gallic acid equivalents (GAE). The decolorization of the fermented media was performed according to Xenopoulos et al. [22] with slight modifications. Briefly, the samples were diluted 30-fold, the pH value was adjusted to around 6, and the absorbance was measured at 395 nm.
The determination of total EPs was performed based on the method described by Xenopoulos et al. [22] with slight modifications. Specifically, 0.05 g of dry biomass was immersed in 10 mL of 2 M HCl, followed by a 30-min heat treatment (100 °C). The liquid phase was separated by filtration and neutralized with 10 mL of 2 M NaOH, and the carbon sources were determined via a 3,5-dinitrosalicylic acid assay as described by Miller [23]. The absorbance was measured at 540 nm, and the EPs were expressed as glucose (g/L) equivalents.
The free amino nitrogen (FAN) concentration of the fermentation broth was determined according to the ninhydrin colorimetric method [24], and the absorbance was measured at 570 nm.
Glycerol, mannitol, arabitol, erythritol, and Cit were determined by high-performance liquid chromatography analysis (Waters Association 600E) equipped with a UV (Waters 486) and RI detector (Waters 410). The column was Aminex HPX-87H (Biorad; 300 mm length × 7.8 mm internal diameter). The mobile phase was 5 mM H2SO4 aqueous solution with 0.6 mL/min flow rate at 65 °C.
Fatty acids were transformed into fatty acid methyl esters (FAMEs) using sodium methoxide followed by methanol with HCl as a catalyst. FAMEs were determined via gas chromatography Fisons 8060 equipped with a Chrompack column (60 m × 0.32 mm, Chrompack; CP-Wax 52 CB GC column-Agilent) and a flame ionization detector (FID) using He as the carrier gas (2 mL/min). The injection volume was equal to 1 μL while the GC was run in a splitless mode. The oven program was initiated at 50 °C, heated to 200 °C with a ratio of 25 °C/min (1 min), then increased with a ratio of 3 °C/min up to 240 °C, and increased to 250 °C with a ratio of 25 °C/min and maintained for 3 min. The detector temperature was 250 °C. FAMEs were identified by reference to a standard (Supelco® 37 Component FAME Mix, 10 mg/mL in CH2Cl2, 47885-U, Merck, Rahway, NJ, USA) [25].
2.4. Nomenclature
X (TDW; g/L): total yeast dry biomass; S (g/L): crude glycerol; EPs: total endo-polysaccharides (g/L); Phen (g/L): total initial phenolic compounds; Cit (g/L): citric acid; Man (g/L): mannitol; Ara (g/L): arabitol; Ery (g/L): erythritol; ΣPol (g/L): total polyols; FAN (mg/L): free amino nitrogen; YX/S (g/g): yield of biomass on initial glycerol (g of produced total dry weight per g of initial glycerol); YCit/S (g/g): yield of citric acid on available carbon sources (g of produced citric acid per g of initial glycerol plus the amount of consumed polyols); YΣPol/S (g/g): yield of total polyols on initial glycerol (g of total polyols produced per g of initial glycerol); YL/X (%, g/g): yield of cellular lipids on the biomass produced (g of lipids per g of produced total dry weight); Productivity Cit (g/L/h): citric acid productivity. All yields were calculated when the maximum values of each target product were obtained throughout fermentations.
2.5. Statistical Analysis
Statgraphics was used for statistical analysis. The data were compared using analysis of variance (ANOVA) and Pearson’s linear correlation at a 5% significance level. Significant differences between means were determined by the Honest Significant Difference (HSD-Tukey test) method at a level of p < 0.05. Data were reported as mean values ± standard deviation of three independent replicates (p < 0.05, 95%).
3. Results
The yeast strains Y. lipolytica LMBF Y-46 and ACA-YC 5033 were investigated on OMW-glycerol blends of different initial phenolic concentrations (Phen) for cell proliferation and production of metabolic compounds (Table 1). Kinetics studies were also performed in fermentation media in which OMW was not involved while glycerol was used as the sole carbon source (control experiment). The total dry weight (TDW, X), EPs and total polyols (ΣPol) produced by both Y. lipolytica strains were decreased at statistically significant levels in the presence of OMW, compared to the control experiments (p < 0.05). The maximum TDW of 9.5 g/L was observed at Phen = 0 g/L with a YX/S = 0.124 g/g initial carbon sources and a YEPs/X of 51.9% w/w in the case of Y. lipolytica LMBF Y-46, while respective values for Y. lipolytica ACA-YC 5033 were 10.7 g/L, YX/S = 0.139 g/g initial carbon sources and YEPs/X =61.5% w/w.

Table 1.
Fermentation efficiency of Y. lipolytica LMBF Y-46 and Y. lipolytica ACA-YC 5033 cultivated on blends of olive mill wastewater (OMW) and crude glycerol under nitrogen-limited conditions in shake-flask fermentations. The blends of OMW-glycerol were properly formulated to have different initial phenolics concentrations (Phen) of ca. 1 g/L, 2 g/L and 3 g/L. Different superscript letters within the same column (with respect to the same yeast strain) indicate statistically significant differences (p < 0.05).
Appreciable TDW (8.1–8.2 g/L) without statistically significant differences were attained at increasing Phen, while the EPs accumulation showed a substantial decrease (1.6 g/L) only at the highest Phen = 3 g/L when Y. lipolytica LMBF Y-46 was applied. In the case of Y. lipolytica ACA-YC 5033, TDW values were higher (9.1–9.8 g/L) with a very similar production pattern to the aforementioned. The EPs concentration (2.2–2.4 g/L) was not affected by increasing Phen.
The secretion of ΣPol by Y. lipolytica ACA-YC 5033 showed great differences at every level of comparison (revealed by Tukey’s test) when the Phen of OMW-glycerol blends eventually reached from 1 g/L to 3 g/L. Similar ΣPol production (22.0–22.6 g/L) was observed when Y. lipolytica LMBF Y-46 was grown on 1 g/L and 2 g/L of Phen while accumulation was negatively affected at 3 g/L of Phen. The ratio of mannitol:arabitol:erythritol (Man:Ara:Ery), based on ΣPol, was 4.2:1:8.5 and 3.5:1:5.2 when Y. lipolytica LMBF Y-46 and ACA-YC 5033 were respectively cultivated on the control media. The great alterations of these ratios in the presence of OMW were mainly attributed to mannitol and erythritol fluctuations. Specifically, mannitol was favored by increasing Phen (up to 2 g/L) while erythritol decreased. Indicatively, at 1 g/L of Phen, the Man:Ara:Ery ratios changed as 10.8:1:6.9 and 13:1:3 for Y. lipolytica LMBF Y-46 and ACA-YC 5033, respectively.
A statistically significant increase in Cit secretion (p < 0.05) was observed in the presence of OMW as well as with increasing Phen. Maximum Cit concentrations varying within 64.1–65.1 g/L were achieved at Phen of 3 g/L for both yeast strains. Y. lipolytica ACA-YC 5033 showed slightly higher values of productivity (0.344 g/L/h) and YCit/S (0.690 g/g carbon sources) compared to Y. lipolytica LMBF Y-46 (Table 1).
The production of microbial oil was quite moderate in all the examined cases (0.54–1.44 g/L). YL/X values were negligible, varying within 5.8–9.6% w/w even though nitrogen-limited conditions prevailed in the growth media (initial FAN concentration ≈85.1 ± 5.1 mg/L, after 48–72 h of fermentation FAN < 9.5 ± 0.75 mg/L) and glycerol was consumed at satisfying levels, suggesting a non-typical oleaginous behavior for the yeasts. When Y. lipolytica LMBF Y-46 was cultivated on OMW-glycerol blends containing 2 g/L, YL/X reached up to 16.6% (after 121 h of fermentation), while a YL/X =14.6% (after 165 h of fermentation) was determined on blends containing 3 g/L when Y. lipolytica ACA-YC 5033 was used. The fatty acid profile of microbial oil produced by both Y. lipolytica strains was analyzed. In all the examined cases, the primary fatty acid was oleic acid (C18:1; >50%), followed by palmitic acid (C16:0; >15%) and linoleic acid (C18:2; Table 2). As can be observed in Table 2, there is not any particular fluctuation pattern of fatty acids when different fermentation media (control and blends of OMW-glycerol) were applied.

Table 2.
Intercellular lipid accumulation (YL/X, % w/w) and the fatty acid composition of lipids (%, w/w, when the maximum lipids were produced in terms of both absolute and relative values) produced by Y. lipolytica LMBF Y-46 and ACA-YC 5033 on blends of olive mill wastewater (OMW) and crude glycerol under nitrogen-limited conditions in shake-flask fermentations. The blends of OMW-glycerol were properly formulated to have different initial phenolics concentrations (Phen) of ca. 1 g/L, 2 g/L and 3 g/L.
Figure 1 and Figure 2 present, respectively, the fermentation efficiency of Y. lipolytica LMBF Y-46 and ACA-YC 5033 during shake flask experiments when 0–3 g/L of Phen were applied. In fermentations performed with Y. lipolytica LMBF Y46 on blends of OMW-glycerol, X was fastly produced up to 72 h of fermentation reaching 91.9%, 88.1% and 86.7% of its maximum value (that was achieved after 121 h, see Table 1), when Phen of 1 g/L, 2 g/L and 3 g/L were respectively applied (Figure 1(Ba–Da)). At this early stage of fermentation, the so-called tropho-phase, the catabolism of glycerol, led mainly to biomass formation. In the case of the control fermentation, the maximum X concentration was determined after 240 h, while almost 92.6% of it was produced after 96 h (Figure 1(Aa)). A similar pattern was observed when Y. lipolytica ACA-YC 5033 was applied, with X production reaching up to 77.9%, 85.6%, 75.9%, and 80.1% of its maximum values when Phen of 0 g/L, 1 g/L, 2 g/L and 3 g/L were respectively employed (Figure 2(Aa–Da)). Maximum X values, in this case, were achieved in more prolonged fermentation times (141–165 h) compared to the Y46 stain. The EPs were rather fastly produced within 24–72 h of fermentation in all cases, while their accumulation plateaued thereafter (Figure 1(Aa–Da) and Figure 2(Aa–Da)). The maximum specific growth rate (μmax, h−1) of the batch processes for both strains was calculated by the formula where X1 was the first experimental point after inoculation and t1 was the respective time; X0 was the initial TDW concentration and t0 = 0 h according to the equation. For trials including both blank and OMW-glycerol-based media, μmax values were found to be in the range of 0.06–0.08 h−1 (data not shown). Therefore, suggesting that potential microbial inhibitory phenomena due to the presence of phenolic compounds did not occur.
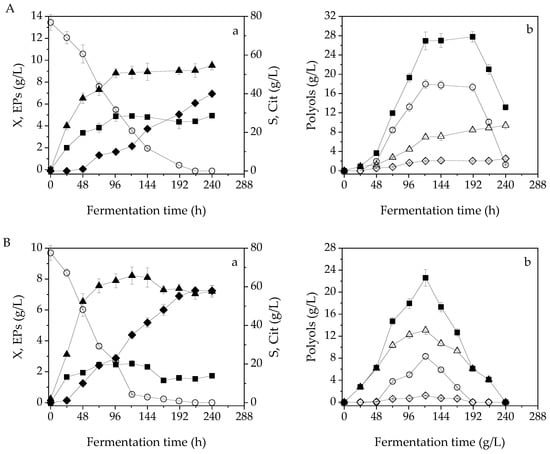

Figure 1.
Kinetics of (a) biomass (X, g/L, ▲) and endopolysaccharides (EPs, g/L, ■) and citric acid (Cit, g/L, ◆) production, as well as glycerol (S, g/L, ○) consumption and (b) total polyols (g/L, ◼), mannitol (g/L, △), arabitol (g/L, ◇), erythritol (g/L, ○) production during shake-flask fermentations of Y. lipolytica LMBF Y-46 when 0 g/L (A), 1 g/L (B), 2 g/L (C), 3 g/L (D) of phenolic compounds were applied in shake-flask nitrogen-limited media. The culture conditions were: 250 mL Erlenmeyer flasks filled up to 50 mL at 180 rpm, initial pH = 6.0, pH ranging between 4.8 and 6.0, incubation temperature T = 28 °C, initial glycerol concentration of 77.9 ± 0.52 g/L.
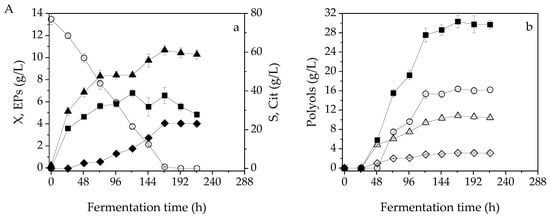

Figure 2.
Kinetics of (a) biomass (X, g/L, ▲) and endopolysaccharides (EPs, g/L, ■) and citric acid (Cit, g/L, ◆) production, as well as glycerol (S, g/L, ○) consumption and (b) total polyols (g/L, ◼), mannitol (g/L, △), arabitol (g/L, ◇), erythritol (g/L, ○) production during shake-flask fermentations of Y. lipolytica ACA-YC 5033 when 0 g/L (A), 1 g/L (B), 2 g/L (C), 3 g/L (D) g/L of phenolic compounds were applied in shake-flask nitrogen-limited media. The culture conditions were: 250 mL Erlenmeyer flasks filled up to 50 mL at 180 rpm, initial pH = 6.0, pH ranging between 4.8 and 6.0, incubation temperature T = 28 °C, initial glycerol concentration of 77.9 ± 0.52 g/L.
The secondary anabolic activity of Cit synthesis occurred when the yeast strains entered the so-called idiophase, which is related to nitrogen exhaustion from the culture media. More specifically, FAN was almost depleted from the media after 48 h of fermentation, being lower than 12.5 ± 0.91 mg/L (initial FAN concentration ≈85.1 ± 5.1 mg/L) in blends of OMW-glycerol in Y46 (1–3 g/L Phen) and ACA-YC 5033 (1–2 g/L Phen). This event directly triggered Cit production at 48 h of fermentation apart from the experiment carried out on OMW-glycerol blends with Phen = 3 g/L using Y. lipolytica ACA-YC 5033, that Cit production initiated at 72 h (in this case, FAN was almost depleted at 72 h, reaching 17.5 ± 1.32 mg/L). Cit showed a considerable and prolonged production rate, up to 165–215 h (Figure 1(Ba–Da) and Figure 2(Ba–Da)). Surprisingly, the FAN uptake during the control experiments was slower, as it was almost consumed (13.7 ± 1.93 mg/L) after 96 h. In each fermentation, after reaching its maximum value, Cit secretion plateaued, with slight variations thereafter (Figure 1 and Figure 2).
Glycerol was fastly catabolized by both strains during all fermentations. More specifically, when Y. lipolytica Y-46 was used, glycerol was consumed by (w/w) 96.1% after 191 h in the control experiment, and by 94.4%, 77.7%, and 89.5% on OMW-glycerol blends with Phen of 1 g/L, 2 g/L, and 3 g/L, respectively, after 121 h of fermentation (Figure 1(Aa–Da)). Thereafter, since glycerol was available in negligible quantities in the fermentation media, Cit biosynthesis was further prolonged since the yeast strains started to metabolize the so-far-produced polyols, valorizing them as the carbon source (Figure 1(Ab–Db)). The polyols consumption reached 27.7 g/L–13.1 g/L when the maxim Cit production was observed in the control fermentation. Similarly, ΣPol was consumed by 81.8%, 90.9% and 97.6% when maximum values of Cit were obtained in blends of OMW-glycerol with respective Phen of 1 g/L, 2 g/L and 3 g/L. When ACA-YC 5033 was cultivated on the control media, the Cit production rationally passed to a stationary phase after 169 h, while the polyols consumption for its synthesis was not observed. In the OMW-glycerol blends, a very similar pattern to the aforementioned was monitored (Figure 2). ΣPol were entirely consumed when maximum values of Cit were obtained in blends of OMW-glycerol with 2 g/L and 3 g/L of Phen, while when 1 g/L was applied, ΣPol depletion was determined equal to 60.1%. The yeast strain LMBF Y-46 mostly catabolized erythritol in the absence of OMW, while in blends of OMW-glycerol with 2 g/L and 3 g/L of Phen, mannitol, arabitol and erythritol were the most consumable polyols. At Phen = 1 g/L, the ΣPol decrease was mainly attributed to the consumption of both mannitol and erythritol, with similar observations made for ACA-YC 5033 at Phen = 1 g/L and 2 g/L (Figure 1(Ab–Db) and Figure 2(Ab–Db)).
Table 3 depicts the evaluation of color removal that was attained by both yeast strains on blends of OMW-crude glycerol. In all fermentations, maximum decolorization values were achieved at a more prolonged fermentation time by LMBF Y-46, compared to ACA-YC 5033. In the case of LMBF Y-46, its ability to decolorize OMW-glycerol blends was significantly reduced (p < 0.05) with increasing Phen concentration while the maximum value of 31.1% (after 121 h) was observed at Phen = 1 g/L. In the case of ACA-YC 5033, the highest color removal of 25.9% was monitored at Phen = 3 g/L after 96 h of fermentation. This strain’s capacity to decolorize OMW-glycerol blends was not affected by the different Phen.

Table 3.
Decolorization (%) of the fermentation media achieved by Y. lipolytica LMBF Y-46 and ACA-YC 5033 on blends of olive mill wastewater (OMW) and crude glycerol under nitrogen-limited conditions in shake-flask fermentations. The blends of OMW-glycerol were properly formulated to have different initial phenolics concentrations (Phen) of ca. 1 g/L, 2 g/L, and 3 g/L. Different letters within the same column (with respect to the same yeast strain) indicate statistically significant differences (p < 0.05).
4. Discussion
The current practice for olive oil production is the three-phase milling method that generates solid and liquid wastes in significant quantities. The liquid effluent, which is called OMW, is toxic for terrestrial and water bodies due to its high chemical and biochemical oxygen demand, heavy metals, strong acidity, dark color and phenolic components [26]. Indicatively, it has been reported that 1 m3 of OMW can cause similar environmental damage as 200 m3 of domestic sewage. The efficient management of OMW in industrial facilities is quite challenging due to large amounts being produced annually. Several lab-scale methods, including biological, chemical, and physical approaches, have been reported while physicochemical approaches have failed due to their high cost and generation of large slush effluents [27]. This study reported a sustainable strategy to valorize OMW, including microbial bioprocessing to produce biobased products of exceptional commercial interest, such as Cit and polyols. Microbial biomass rich in EPs and unsaturated microbial oil could also be considered a co-product of high nutritional value with targeted applications. Basic principles of circular bioeconomy were implemented while the whole fermentation process resulted in a substantial bioremediation of OMW via decolorization.
The formulation of the fermentation media included OMW blended with crude glycerol derived from the biodiesel industry. The OMW was dually considered as the fermentation media as well as the process water. This strategy is directly in line with the sixth sustainable development goal that ‘requires availability and sustainable management of water and sanitation for all. More specifically, it addresses Target 6.6, which sets bindings for the protection and restoration of water-related ecosystems, including mountains, forests, wetlands, rivers, aquifers, and lakes [1,2]. The economic viability of the whole approach could be increased since crude glycerol was utilized as the sole carbon source. Glycerol is mostly generated from the biodiesel production process, and it contains several impurities such as methanol, soap, free fatty acids, salts, di- and tri-glycerol and water. A potential stockpile of over 40 billion liters of crude glycerol could be available annually as the starting material for value-added end-uses [7]. The valorization of crude glycerol in fields of pharmaceuticals, food and cosmetics requires combined purification and refining methods that are cost and energy intensive. The price of reformed glycerol is five- to 10-fold higher than that of crude glycerol (3–20 cents/lb.), but still, many biodiesel manufacturers treat crude glycerol as a waste rather than purifying it for commercial applications [28,29]. High-added value alternatives, integrated within concepts of environmental circularity and bio-economy, could provide long-term and sustainable valorization models of crude glycerol. Especially the development of a crude-glycerol-based biorefinery could lead to the production of a spectrum of marketable products paving the way towards economically viable industrial bioprocesses, simultaneously increasing revenues for the existing biodiesel industry. The bioconversion of crude glycerol using robust microorganisms such as Y. lipolytica, which are tolerant to impurities, is of utmost importance [28,29].
The impurities contained in crude glycerol, i.e., potassium and sodium salts and methanol, could affect microbial proliferation and the biosynthesis of the biobased products. However, several studies have reported that these impurities have not shown any significant impact on the metabolism of certain Y. lipolytica strains, with similar results obtained when crude and commercial-grade glycerol were used [30,31]. In the study by Papanikolaou et al. [32], the maximum biomass formation was slightly higher in the case of pure glycerol compared to crude glycerol, while polyols production was not affected by glycerol purity. Additionally, the consumption pattern of both crude and pure glycerol was very similar. TDW formation was substantial in all fermentations (8.1–10.7 g/L), and it compares well with literature cited publications (Table 3). In most cases, the onset of new X production, after nitrogen-limited conditions prevailed in the fermentation media, did not arise concurrently with the production of storage lipids. In fact, insignificant amounts of lipids were produced inside the yeast cells without exceeding 20% w/w of cellular lipids in X, despite the fact that fermentations were performed under nitrogen limitation. This rise of biomass synthesis that was monitored at the late growth stages (Figure 1(Aa–Da) and Figure 2(Aa–Da)) suggests EPs accumulation in TDW, in accordance with other scientific reports [33,34]. The fermentation media that did not contain any phenolic compounds (control) favored EPs production both in absolute (g/L) and relative (%, w/w) values.
Glycerol has been extensively reported as an efficient and very promising carbon source for microbial oil production [35]. Additionally, Y. lipolytica is able to accumulate cellular lipids of more than 30% w/w in dry X when cultivated on glycerol alone or blended with OMW [36]. OMW has also been reported as a natural inducer of lipogenesis in several yeast strains [37]. Despite the aforementioned, in this study, the quite moderate microbial oil production by both Y. lipolytica strains could be attributed to the shift of metabolism towards the production of EPs and enhanced secretion of Cit into the medium. The Cit concentration increased with fermentation time, verifying the aforementioned assumption that the yeast metabolism was shifted towards Cit formation in detriment to lipids biosynthesis (Figure 1 and Figure 2). When glycerol is used as the fermentation media, Cit production is triggered by nitrogen-limited conditions. The latter conditions cause a rapid decrease of intracellular AMP, followed by the deactivation of NAD+- isocitrate dehydrogenase. Eventually, Cit is secreted inside the cytosol when critical values are reached. Yeast strains with non-typical oleaginous behavior (non-capable of accumulating microbial oil higher than 20% on TDW), such as these used in this study, secrete Cit into the fermentation environment contrary to oleaginous ones that convert it into cellular lipids [30]. Despite the low lipid production, blends of OMW-crude glycerol seemed to somehow enhance the intracellular lipid content of the yeast biomass compared to the control fermentations, indicating that some compounds contained in OMW might have created a positive effect on lipids accumulation. The opposite phenomenon was described by Dourou et al. [38] when Y. lipolytica A6 was cultivated on OMW-based media (with around 2 g/L Phen) supplemented with 50 g/L glycerol. In this case, YL/X reached 14.9% while the X production was much lower (5.6 g/L) compared to the yeasts evaluated in the current study (see Table 1). The fatty acid composition of cellular lipids produced by both strains was typical of Y. lipolytica lipids, with C18:1 being the predominant fatty acid, followed by C16:0, C18:0 and C18:2 [31,38]. The highest Phen of 3 g/L seemed to favor the synthesis of C18:2. Increasing Phen led to a decreasing tendency of C18:0 in the case of Y. lipolytica LMBF Y-46, while the opposite phenomenon was observed in the case of Y. lipolytica ACA-YC 5033. The fatty acid composition of lipids produced by Y. lipolytica is affected by the presence of OMW in the growth media since, in the study by Dourou et al. [38], the C16:1 and C18:0 content of microbial oil produced on OMW-glycerol media increased during fermentation while C18:1 remained almost stable. On the other hand, glucose-based fermentation seemed to result in a decreasing tendency of C16:0 and C18:0 with time [38].
So far, the most commercially efficient Cit producer is Aspergillus niger, while Y. lipolytica strains have been lately investigated as potential candidates [30,38]. The production of Cit employing A. niger strains is continuously growing on an annual basis, while concentrations between 150–200 g/L have been reported when fermentation is optimized or patents are developed [39,40]. Circularity approaches, valorizing green olive processing wastewaters enriched with sugars from white grape pomace, have reported the production of 85 g/L with a yield of 0.56 g/g when A. niger B60 was used [41]. In this study, Cit was effectively produced with both Y. lipolytica strains and valorizing blends of OMW-glycerol, especially when the highest Phen was applied (64.1–65.1 g/L). The positive effect of OMW supplementation on Cit production has been previously demonstrated when Y. lipolytica ACA-DC 5029 was grown in crude glycerol and OMW blends under submerged shake-flask fermentation [37]. The presence of other organic acids, such as pyruvic, a-ketoglutaric, or fumaric acid, was not detected in our investigation, while other studies [42] report the aforementioned acid in traces, <0.5% of the total acids. These quite high quantities of Cit achieved in the current investigation compare very well with literature cited publications (see Table 4). Indicatively, Y. lipolytica LGAM S (7) grown on OMW diluted in water (Phen = 2 g/L) and enriched with 50 g/L glycerol was able to produce 30.3 g/L of Cit, with a YCit/S = 0.62 g/g in shake flasks while bioreactor trials resulted in lower Cit production (21 g/L) but a slightly higher YCit/S = 0.68 g/g [38]. To add, the productivity values that are reported in this study (viz. c. 0.335–0.344 g/L/h) rank among the highest when compared to similarly performed experiments in terms of feedstock, microbial strain, and fermentation mode (Table 4). Commercial-based fermentation media, fed-batch configurations and genetic modification of strains could significantly improve the fermentation efficiency for Cit production. For instance, industrially sufficient amounts of Cit, within 80–85 g/L with YCit/S = 0.70–0.75 g/g, have been achieved on commercial glucose-containing media with the natural strain Y. lipolytica VKM Y-2373 [42]. Recombinant strains of Y. lipolytica, super-expressing the pyruvate carboxylase gene, were able to produce substantial Cit amounts of 95–111 g/L with enhanced yields (0.75–0.93 g/g) [43,44]. Fed-batch bioreactor fermentations led to a Cit production of ≈102 g/L, while this metabolic transition was attributed to higher oxygen saturation into the medium that occurred in the bioreactor experiments compared to flasks [32].

Table 4.
Comparative data on citric acid (Cit) production valorizing renewable glycerol when various Y. lipolytica strains were employed.
Nitrogen-limited conditions that prevailed in the fermentative environment at the very early stages of microbial growth, combined with pH values higher than 4.8, seemed to primarily favor the Cit biosynthesis while mannitol, arabitol and erythritol were present as co-products. The aforementioned conditions have also been verified by Papanikolaou et al. [48] that lead to Cit production as the major metabolic compound, besides polyols, when Y. lipolytica strains are employed. Additionally, the limitation of Y. lipolytica growth by biogenic macro-elements, that is, N, P, or S, has already been proven as an efficient strategy to regulate Cit production [42]. In other studies, the aforementioned conditions have directed the carbon catabolism mainly towards the polyols biosynthesis, in detriment to organic acids production, i.e., low concentrations of Cit <3.0 g/L were detected [32,49,50]. It is worth noting that after a significant reduction in glycerol concentration or after its total consumption by the yeast strains (Figure 1 and Figure 2), the re-consumption of metabolic products, i.e., mannitol, arabitol or erythritol, provided extra carbon source for prolonged Cit production [51]. The re-utilization of polyols at late culture stages has also been monitored during the growth of Y. lipolytica LMBF Y-46 on glycerol when the glycerol concentration in the medium reached essentially low concentrations [32].
The production of polyols via fermentation strategies is superior to chemical modification of pure sugars due to facile recovery of the product and removal of the by-products, low energy requirements and utilization of abundant renewable resources as the initiating material instead of pure sugars that are of very high cost [6,8]. Polyols constitute secondary anabolic compounds, while their biosynthesis is triggered by deficiencies of biogenic macro-elements, i.e., N [52]. Thus, this study, in agreement with other studies [32], demonstrated that the total X formation might occur at the same time with the production of polyols into the medium, indicating a non-growth associated profile of mannitol, arabitol and erythritol synthesis [48] (Figure 1(Ab–Db) and Figure 2(Ab–Db)). In the current investigation, very satisfying mannitol concentrations (13.0–13.1 g/L) were achieved with Y. lipolytica LMBF Y-46 on blends of OMW-glycerol at 1 g/L and 2 g/L Phen. The highest mannitol production (15.9 g/L) was attained when Y. lipolytica ACA-YC 5033 was cultivated on OMW-glycerol blends at 1 g/L Phen. Despite the relatively high production of individual polyols, it should be pointed out that growth on glycerol-based media under nitrogen limitation and pH > 4.8 (maintained constantly) shifted the carbon flow towards the Cit formation while individual polyols were constantly much lower than Cit concentrations. The fermentation media and the pH values have been reported to affect the ΣPol concentration as well as the ratio of Man:Ara:Ery [51]. Values of pH lower than 3 lead to enhanced polyols production. More specifically, ΣPol of 21–38.3 g/L has been achieved in pure glycerol-based growth media buffered at pH from 2.5 to 2.8 when nine strains of Y. lipolytica were investigated under batch shake flasks experiments, with erythritol accounting for more than 82–95%, besides mannitol and arabitol [51]. These results (ΣPol production) are similar or slightly higher compared to data obtained in our study with pure-glycerol-based cultures (ΣPol = 27.8–30.3 g/L) with erythritol representing around 53–62% (see Table 1, control experiment). Thus, YΣPol/S was somehow higher in our study, ranging within 0.360–0.394 g/g compared to the study of Tomaszewska et al. [51] (YΣPol/S = 0.21–0.38 g/g). Bioreactor batch cultures of Y. lipolytica Wratislavia K1 on crude glycerol have reached up to ΣPol = 80.2 g/L with a ratio of Man:Ara:Ery of 0.14:0.01:1 [51]. YΣPol/S values of 0.30–0.40 g/g [53], 0.48–0.67 g/g [54,55], 0.67–0.69 g/g (repeated-batch bioreactor) [56], and 0.80–0.82 g/g [57] have been attained under optimized conditions that favor their synthesis (including bioreactor trials, pH values, C/N ratio etc.), glycerol as the fermentation feedstock (pure or biodiesel-derived) and/or combined with mutants or genetically engineered strains of Y. lipolytica. The yeast strains that were evaluated in the current study, are wild-type isolates, originated from traditional Greek wheat sourdough (ACA-YC5033) and fish-origin products (LMBF Y-46), that require further optimization of the fermentative parameters for either enhanced Cit production or targeted polyols accumulation. Based on Table 1, it can be observed that when OMW was not involved in the formulation of the fermentation media, erythritol was the major polyol, while the carbon polyols’ flow shifted towards mannitol production (around 54–76%) when blends of OMW-crude glycerol were implemented. The presence of phenolic compounds found in OMW might have a major impact on the metabolic processes of Y. lipolytica strains promoting the production of particular polyols i.e mannitol, as observed in our study. The carbon sources contained in the growth media can also affect the overproduction of a particular polyol. For example, growth media supplemented with glucose led to the production of a erythritol to mannitol ratio of 3.25 while enhanced mannitol production combined with lack of erythritol were reported when fructose and glycerol were applied using Candida magnoliae [58].
Natural yeasts do not possess the enzymatic complexes (i.e., laccases, lignin peroxidases and manganese peroxidases) [59] to oxidize phenolic compounds that are contained in OMW or other relative feedstock [60]. Nevertheless, this study reported the partial decolorization of OMW-crude glycerol blends by both yeast strains, which is very promising since OMW remediation difficulties are interlinked with the breakdown of phenolic compounds and, therefore, the removal of color from this dark and toxic effluent [60,61]. More specifically, the color removal that was achieved by LMBF Y-46 reached up to 31.1%, while slightly lower values (25.9%) were determined when ACA-YC 5033 was employed (Table 3). Similar results have been reported in trials with Y. lipolytica ACA-DC 5029, which could remove the color of OMW- based media up to ~30% w/w [37]. The potential of Yarrowia strains to reduce the color of OMW is well documented. Still, it should be mentioned that color intensity is dependent on several physicochemical parameters and, thus, is not proportional to phenolics concentration [62,63,64]. Papanikolaou et al. [62] reported a remarkable potential of several Y. lipolytica strains to decolorize OMW, indicating that the color reduction of dark waste effluents may be a strain-dependent process. Although the bioremediation mechanism employing yeast strains is not clear, it could be assumed that yeast cells adsorb phenolics by van der Waals interactions which are weak and reversible [65], or they use phenolic compounds for energy and maintenance [66].
5. Conclusions
Blends of OMW-crude glycerol were efficiently valorized as feedstock to produce primary Cit, polyols with elevated mannitol concentration, cellular lipids and EPs by the non-conventional Y. lipolytica LMBF Y-46 and ACA-YC 5033. The utilization of OMW to substitute (partially or totally) tap water in bioprocesses could facilitate large-scale process transferability. This study also addressed the bioremediation of waste effluents via satisfying media decolorization that was achieved by both yeast strains. This approach suggests that Y. lipolytica strains could be considered as a very attractive biorefinery-oriented cell factory that may yield a spectrum of potentially marketable products of high added value leading simultaneously to the reduced environmental impact of the olive milling industries.
Author Contributions
Formal analysis, D.S. and E.T.; investigation, M.A., E.K. and D.S.; data curation, D.S. and E.T.; writing—original draft preparation, E.T., M.K. and D.S.; writing—review and editing, E.T., M.K. and D.S.; visualization, D.S. and S.P.; supervision, S.P. and D.S.; project administration D.S. and S.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Infrastructure of Microbiome Applications in Food Systems-FOODBIOMES” (MIS 5047291), which is implemented under the Action “Regional Excellence in R&D Infrastructures,” funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 14 December 2022).
- United Nations, Department of Economica and Social Affairs. Sustainable Development, the 17 SDGs. Available online: https://sdgs.un.org/goals (accessed on 11 December 2022).
- European Council. Fit for 55. Available online: https://www.consilium.europa.eu/en/policies/green-deal/fit-for-55-the-eu-plan-for-a-green-transition/ (accessed on 15 November 2022).
- Rocha, C.; Soria, M.A.; Madeira, L.M. Olive Mill Wastewater Valorization through Steam Reforming Using Multifunctional Reactors: Challenges of the Process Intensification. Energies 2022, 15, 920. [Google Scholar] [CrossRef]
- Tsouko, E.; Papadaki, A.; Papapostolou, H.; Ladakis, D.; Natsia, A.; Koutinas, A.; Kampioti, A.; Eriotou, E.; Kopsahelis, N. Valorization of Zante Currant Side-streams for the Production of Phenolic-rich Extract and Bacterial Cellulose: A Novel Biorefinery Concept. J. Chem. Technol. Biotechnol. 2020, 95, 427–438. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kourkoutas, Y. Exploitation of Olive Oil Mill Wastewaters and Molasses for Ethanol Production Using Immobilized Cells of Saccharomyces Cerevisiae. Environ. Sci. Pollut. Res. 2018, 25, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of Crude Glycerol to Value-Added Products: Perspectives of Process Technology, Economics and Environmental Issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous Phase Reforming Process for the Valorization of Wastewater Streams: Application to Different Industrial Scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Hu, W.; Li, W.; Yang, H.; Chen, J. Current Strategies and Future Prospects for Enhancing Microbial Production of Citric Acid. Appl. Microbiol. Biotechnol. 2019, 103, 201–209. [Google Scholar] [CrossRef]
- Grand View Research. Citric Acid Market Size. Available online: https://www.grandviewresearch.com/press-release/global-citric-acid-market (accessed on 28 November 2022).
- Cavallo, E.; Nobile, M.; Cerrutti, P.; Foresti, M.L. Exploring the Production of Citric Acid with Yarrowia Lipolytica Using Corn Wet Milling Products as Alternative Low-Cost Fermentation Media. Biochem. Eng. J. 2020, 155, 107463. [Google Scholar] [CrossRef]
- Morgunov, I.; Kamzolova, S.; Lunina, J. Citric Acid Production by Yarrowia Lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Soong, Y.V.; Liu, N.; Yoon, S.; Lawton, C.; Xie, D. Cellular and Metabolic Engineering of Oleaginous Yeast Yarrowia Lipolytica for Bioconversion of Hydrophobic Substrates into High-value Products. Eng. Life Sci. 2019, 19, 423–443. [Google Scholar] [CrossRef]
- Erian, A.M.; Sauer, M. Utilizing Yeasts for the Conversion of Renewable Feedstocks to Sugar Alcohols—A Review. Bioresour. Technol. 2022, 346, 126296. [Google Scholar] [CrossRef]
- Sardon, H.; Mecerreyes, D.; Basterretxea, A.; Avérous, L.; Jehanno, C. From Lab to Market: Current Strategies for the Production of Biobased Polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664–10677. [Google Scholar] [CrossRef]
- Martău, G.A.; Coman, V.; Vodnar, D.C. Recent Advances in the Biotechnological Production of Erythritol and Mannitol. Crit. Rev. Biotechnol. 2020, 40, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Paulino, B.N.; Molina, G.; Pastore, G.M.; Bicas, J.L. Current Perspectives in the Biotechnological Production of Sweetening Syrups and Polyols. Curr. Opin. Food Sci. 2021, 41, 36–43. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Müller, M.R.A.; Ehrmann, M.A.; Tsakalidou, E.; Seiler, H.; Vogel, R.; Kalantzopoulos, G. Polyphasic Identification of Wild Yeast Strains Isolated from Greek Sourdoughs. Syst. Appl. Microbiol. 2000, 23, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Tryfinopoulou, P.; Tsakalidou, E.; Nychas, G.-J. Characterization of Pseudomonas Spp. Associated with Spoilage of Gilt-Head Sea Bream Stored under Various Conditions. Appl. Environ. Microbiol. 2002, 68, 65–72. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of to-tal lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteau, V. On tyrosine and tryptophane in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.; Papanikolaou, S. Lipid Production by Yeasts Growing on Commercial Xylose in Submerged Cultures with Process Water Being Partially Replaced by Olive Mill Wastewaters. Processes 2020, 8, 819. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lie, S. The Ebc-Ninhydrin Method for Determination of Free Alpha Amino Nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- Tsouko, E.; Papadaki, A.; Papanikolaou, S.; Danezis, G.P.; Georgiou, C.A.; Freire, D.M.G.; Koutinas, A. Enzymatic Production of Isopropyl and 2-Ethylhexyl Esters Using γ-Linolenic Acid Rich Fungal Oil Produced from Spent Sulphite Liquor. Biochem. Eng. J. 2021, 169, 107956. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jeguirim, M.; Kinigopoulou, V.; Doulgeris, C.; Goddard, M.-L.; Jellali, S.; Matei Ghimbeu, C. Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural and Water Source and Bio-Fertilizer—Hydrothermal Carbonization. Sci. Total Environ. 2020, 733, 139314. [Google Scholar] [CrossRef] [PubMed]
- Zahi, M.R.; Zam, W.; El Hattab, M. State of Knowledge on Chemical, Biological and Nutritional Properties of Olive Mill Wastewater. Food Chem. 2022, 381, 132238. [Google Scholar] [CrossRef] [PubMed]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.-L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483. [Google Scholar] [CrossRef]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological Valorization of Biodiesel Derived Glycerol Waste through Production of Single Cell Oil and Citric Acid by Yarrowia Lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipid Production by Yarrowia Lipolytica Growing on Industrial Glycerol in a Single-Stage Continuous Culture. Bioresour. Technol. 2002, 82, 43–49. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological Characterization of a Novel Wild-Type Yarrowia Lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Daskalaki, A.; Perdikouli, N.; Aggeli, D.; Aggelis, G. Laboratory Evolution Strategies for Improving Lipid Accumulation in Yarrowia Lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of Added-Value Microbial Metabolites during Growth of Yeast Strains on Media Composed of Biodiesel-Derived Crude Glycerol and Glycerol/Xylose Blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef]
- Tsouko, E.; Papanikolaou, S.; Koutinas, A.A. Production of Fuels from Microbial Oil Using Oleaginous Microorganisms. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 201–236. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part II: Technology and Potential Applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia Lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.-E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of Olive Mill Wastewater into High-Added Value Products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Cairns, T.C.; Barthel, L.; Meyer, V. Something Old, Something New: Challenges and Developments in Aspergillus Niger Biotechnology. Essays Biochem. 2021, 65, 213–224. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Beolchini, F. Citric Acid Bioproduction: The Technological Innovation Change. Crit. Rev. Biotechnol. 2020, 40, 199–212. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Citric Acid Production from the Integration of Spanish-Style Green Olive Processing Wastewaters with White Grape Pomace by Aspergillus Niger. Bioresour. Technol. 2019, 280, 59–69. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic Peculiarities of the Citric Acid Overproduction from Glucose in Yeasts Yarrowia Lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef]
- Tan, M.-J.; Chen, X.; Wang, Y.-K.; Liu, G.-L.; Chi, Z.-M. Enhanced Citric Acid Production by a Yeast Yarrowia Lipolytica Over-Expressing a Pyruvate Carboxylase Gene. Bioprocess Biosyst. Eng. 2016, 39, 1289–1296. [Google Scholar] [CrossRef]
- Fu, G.-Y.; Lu, Y.; Chi, Z.; Liu, G.-L.; Zhao, S.-F.; Jiang, H.; Chi, Z.-M. Cloning and Characterization of a Pyruvate Carboxylase Gene from Penicillium Rubens and Overexpression of the Genein the Yeast Yarrowia Lipolytica for Enhanced Citric Acid Production. Mar. Biotechnol. 2016, 18, 1–14. [Google Scholar] [CrossRef]
- Tzirita, M.; Kremmyda, M.; Sarris, D.; Koutinas, A.A.; Papanikolaou, S. Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia Lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters. Energies 2019, 12, 3649. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.-P.; et al. Conversion of Biodiesel-Derived Glycerol into Biotechnological Products of Industrial Significance by Yeast and Fungal Strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.R.; Yellapu, S.K.; Yan, S.; Tyagi, R.; Drogui, P. Elucidating the Effect of Impurities Present in Different Crude Glycerol Sources on Lipid and Citric Acid Production by Yarrowia lipolytica SKY7. J. Chem. Technol. Biotechnol. 2021, 96, 227–240. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of Secondary Metabolites through Glycerol Fermentation under Carbon-excess Conditions by the Yeasts Yarrowia lipolytica and Rhodosporidium Toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Egermeier, M.; Russmayer, H.; Sauer, M.; Marx, H. Metabolic Flexibility of Yarrowia Lipolytica Growing on Glycerol. Front. Microbiol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Egermeier, M.; Sauer, M.; Marx, H. Golden Gate-Based Metabolic Engineering Strategy for Wild-Type Strains of Yarrowia lipolytica. FEMS Microbiol. Lett. 2019, 366, fnz022. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of Erythritol and Mannitol by Yarrowia lipolytica Yeast in Media Containing Glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Fickers, P.; Cheng, H.; Sze Ki Lin, C. Sugar Alcohols and Organic Acids Synthesis in Yarrowia Lipolytica: Where Are We? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A Comparative Study on Glycerol Metabolism to Erythritol and Citric Acid in Yarrowia lipolytica Yeast Cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef]
- Carly, F.; Vandermies, M.; Telek, S.; Steels, S.; Thomas, S.; Nicaud, J.-M.; Fickers, P. Enhancing Erythritol Productivity in Yarrowia Lipolytica Using Metabolic Engineering. Metab. Eng. 2017, 42, 19–24. [Google Scholar] [CrossRef]
- Rakicka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A.; Mirończuk, A.M. Polyol Production from Waste Materials by Genetically Modified Yarrowia Lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Dobrowolski, A.; Rakicka, M.; Rywińska, A.; Rymowicz, W. Newly Isolated Mutant of Yarrowia Lipolytica MK1 as a Proper Host for Efficient Erythritol Biosynthesis from Glycerol. Process Biochem. 2015, 50, 61–68. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Wang, Z.; Xia, J.; Yan, Y.; Hu, L.; Wang, X.; Xu, J.; He, A.; Zhao, P. Enhanced Erythritol Production by a Snf1-Deficient Yarrowia Lipolytica Strain under Nitrogen-Enriched Fermentation Condition. Food Bioprod. Process. 2020, 119, 306–316. [Google Scholar] [CrossRef]
- Khan, A.; Bhide, A.; Gadre, R. Mannitol Production from Glycerol by Resting Cells of Candida Magnoliae. Bioresour. Technol. 2009, 100, 4911–4913. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, M.S.; Dokianakis, S.N.; Kornaros, M.E.; Aggelis, G.G.; Lyberatos, G. Removal of Phenolics in Olive Mill Wastewaters Using the White-Rot Fungus Pleurotus Ostreatus. Water Res. 2002, 36, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive Oil Mill Wastewater Valorisation by Fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Lanciotti, R. Use of Yarrowia Lipolytica Strains for the Treatment of Olive Mill Wastewater. Bioresour. Technol. 2005, 96, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric Acid Production by Yarrowia Lipolytica Cultivated on Olive-Mill Wastewater-Based Media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs Non-Aerated Conversions of Molasses and Olive Mill Wastewaters Blends into Bioethanol by Saccharomyces Cerevisiae under Non-Aseptic Conditions. Ind. Crop. Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric Acid, Biomass and Cellular Lipid Production by Yarrowia Lipolytica Strains Cultivated on Olive Mill Wastewater-Based Media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Rizzo, M.; Ventrice, D.; Varone, M.A.; Sidari, R.; Caridi, A. HPLC Determination of Phenolics Adsorbed on Yeasts. J. Pharm. Biomed. Anal. 2006, 42, 46–55. [Google Scholar] [CrossRef]
- Chtourou, M.; Ammar, E.; Nasri, M.; Medhioub, K. Isolation of a Yeast, Trichosporon Cutaneum, Able to Use Low Molecular Weight Phenolic Compounds: Application to Olive Mill Waste Water Treatment. J. Chem. Technol. Biotechnol. 2004, 79, 869–878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).