Abstract
Seaweeds have a variety of biological activities, and their aromatic characteristics could play an important role in consumer acceptance. Here, changes in aroma compounds were monitored during microbial fermentation, and those most likely to affect sensory perception were identified. Ulva sp. and Laminaria sp. were fermented and generally recognized as safe microorganisms, and the profile of volatile compounds in the fermented seaweeds was investigated using headspace solid-phase microextraction with gas chromatography–mass spectrometry. Volatile compounds, including ketones, aldehydes, alcohols, and acids, were identified during seaweed fermentation. Compared with lactic acid bacteria fermentation, Bacillus subtilis fermentation could enhance the total ketone amount in seaweeds. Saccharomyces cerevisiae fermentation could also enhance the alcohol content in seaweeds. Principal component analysis of volatile compounds revealed that fermenting seaweeds with B. subtilis or S. cerevisiae could reduce aldehyde contents and boost ketone and alcohol contents, respectively, as expected. The odor of the fermented seaweeds was described by using GC–olfactometry, and B. subtilis and S. cerevisiae fermentations could enhance pleasant odors and reduce unpleasant odors. These results can support the capability of fermentation to improve the aromatic profile of seaweeds.
1. Introduction
Food aroma compounds are volatile molecules that can be released during eating to reach the olfactory receptors. The aroma compounds in seaweeds serve as sex pheromones and chemical defense compounds against herbivores and pathogens [1]. Seaweeds and their aroma compounds have significant application potential in processing food products as seasonings because of their unique and strong aroma and flavors, such as marine, green, and umami aromas [2]. The aroma compounds in seaweeds can differ among species, including the most abundant ones, such as hydrocarbons, ketones, aldehydes, alcohols, esters, halogenated compounds, carboxylic acids, furans, phenols, sulfur compounds, pyrazines, pyridines, and amines [3,4].
Seaweeds have a variety of biological activities, such as antiviral, anti-oxidant, anti-inflammation, and anti-cancer activities, because of their availability, diversity, and productivity [5], and they have been used as food and medicine for centuries. In Asian countries, seaweeds are frequently consumed fresh and dried; however, seaweeds are not normally ingested in the unprocessed form in Western societies, where seaweeds remain of minor importance in spite of their nutritional benefits [6]. For example, the ingestion of red seaweed Bangia fuscopurpurea potentially reduces the risk of cardiovascular and chronic metabolic diseases, but its fishy malodor may limit consumers’ acceptance [7]. Food sensory properties can play an important role in consumers’ preferences and acceptance, of which the aroma compound is of prime importance [8]. Consequently, the identification of seaweed volatile compounds has drawn increasing attention for enhancing their application potential in food [3,9].
Microbial fermentation is often used to improve/enhance the sensory quality of food, such as cereals, meats, vegetables, and dairy foods, by removing undesirable off-flavors and/or generating new aroma compounds [10]. Recently, the application of microbial fermentation in removing undesirable odors in seaweeds has drawn increasing attention. Seo et al. [11] indicated that the inoculation of the fungus Aspergillus oryzae could decrease isovaleric acid and allyl isothiocyanate and reduce the peculiar smell of seaweed kelp extracts. In addition, the co-fermentation of Bacillus subtilis (BS) and Lactobacillus plantarum of the microalga Spirulina could reduce off-odors and produce creamy flavor compounds, such as acetoin and ethyl lactate [12]. However, research on sensory profiles of seaweeds during fermentation remains limited, not to mention that there are thousands of seaweed species.
Therefore, the aroma compound profiles of seaweeds should be investigated during microbial fermentation to promote the development of new products and to widen the application of seaweeds in food or beverage sectors. This study aimed to qualitatively and quantitatively characterize the volatiles and odor-active compounds in the green seaweed Ulva sp. and brown seaweed Laminaria sp. fermented with various microorganisms using headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–mass spectrometry (GC–MS) and GC–olfactometry (GC–O). Consequently, the changes in aroma compounds were monitored during microbial fermentation, and those most likely to impact sensory perception were identified.
2. Materials and Methods
2.1. Seaweeds
Dried green seaweed Ulva sp. was provided by Taiwan Yes (Taiwan Fertilizer Co., Ltd.) in Hualien County, Taiwan, and dried brown seaweed Laminaria sp. was purchased in Penghu County, Taiwan. The dried seaweed powders were prepared according to Lu et al. [13]. In summary, the seaweeds were washed, air-dried (40 °C), ground, and sieved through 0.25 mm pores and stored in a freezer until use.
2.2. Fermentation Strains
Five microorganisms were used in this study. Bacillus subtilis BCRC 10255, Saccharomyces cerevisiae BCRC 21685, Lactobacillus delbrueckii subsp. bulgaricus BCRC 10696 and Lactobacillus casei BCRC 10697 were purchased from Bioresource Collection and Research Center (BCRC) in Hsinchu City, Taiwan. Bacillus subtilis was cultured in Luria-Bertani medium (LB) at 30 °C at 150 rpm, Saccharomyces cerevisiae was cultured in Yeast Extract Peptone Dextrose medium (YPD) (Formedium, Norfolk, UK) at 24 °C at 150 rpm, and three lactic acid bacteria were cultured at Lactobacilli MRS medium (BD Difco, Franklin Lakes, NJ, USA) at 37 °C. The microbial cells were cultured to OD 0.6–0.8 (during the exponential phase) prior to the inoculation for seaweed fermentations.
2.3. Fermentation on Seaweeds
The seaweed suspension preparation and microbial fermentation were performed according to Hung et al. [14] with modifications. Seaweed powder (Ulva or Laminaria sp.) was mixed with distilled water in a 500 mL flask to make seaweed suspension (5%, w/v). The seaweed suspension was sterilized by autoclaving (121 °C/20 min). The microbial cultures were inoculated into the sterile seaweed suspension with a 0.1% (v/v) inoculation for fermentations. The fermentation for Bacillus subtilis, Saccharomyces cerevisiae, and the lactic acid bacteria was conducted at 30 °C /150 rpm, 24 °C/150 rpm, and 37 °C/static, respectively. The samples were taken at 0, 12, 24, 48, and 72 h during fermentation for the analyses of microbial counts by using aerobic plate counts. The volatile compounds were extracted by using headspace-solid phase microextraction after fermentation and analyzed by using gas chromatography-mass spectrometry. The control groups were the seaweed suspensions (Ulva or Laminaria sp.) without any microbial inoculation.
2.4. Headspace-Solid Phase Microextraction (HS-SPME)
The HS-SPME analysis was performed according to López-Pérez, Picon, and Nuñez [9] with some modifications. The volatile compounds were extracted by the 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber method (Supelco Inc., Bellefonte, PA, USA), and has been used previously for extracting volatile compounds from seaweeds [9,15]. First, 4 mL of fermentation broth was mixed with 4 mL distilled water, and 1.5 g sodium chloride (NaCl) was added to improve extractive efficiency in HS-SPME. Zhang et al. [16] indicate that adding NaCl can improve ionic strength in the solution and further impact the extractive efficiency in HS-SPME. Five μL of 100 ppm ethyl cinnamate (Sigma-Aldrich, St. Louis, MO, USA) was added as the internal standard. The prepared sample was placed in a 60 °C water bath for 20 min with stirring for balancing, and then the SPME fiber was exposed to the headspace of the sample at the same temperature for 30 min. Finally, the SPME fiber was inserted into the GC injector for desorption at 250 °C for 5 min under non-splitting mode.
2.5. Gas Chromatography–Mass Spectrometry (GC–MS) and Gas Chromatography Olfactometry (GC–O)
The GC–MS analysis was performed on an Agilent Technologies-6890N GC coupled with Agilent 5973I Mass Selective Detector (Agilent Technologies, Santa Clara, CA, USA). The GC–MS system was equipped with a DB-WAX capillary column (30 m × 0.25 mm × 0.15 μm) (Agilent Technologies, Santa Clara, CA, USA). Helium was used as a carrier gas at a constant flow rate of 1.0 mL/min. The oven temperature was initially set at 40 °C for 2 min, then heated to 160 °C by 6 °C/min, and finally to 225 °C at 10 °C/min, maintained for 10 min. The mass spectrometry was operated in the electron impact (EI) mode at 70 eV and screened from 33 to 450 m/z. Library WILEY 275L was used to identify the volatile compounds for fermentation samples. The concentration of the identified volatile compounds was calculated by the relative peak area between the internal standard and the analyte [17].
The equation is followed by
C2 (μg/L) = C1 (μg/L) × (A2/A1)
C1 (A1): concentration (peak area) of the internal standard, C2 (A2): concentration (peak area) of the identified volatile compound
The GC–O system shared the same equipment and analysis condition on the GC with the GC–MS. An olfactory detection port was equipped (OPD-3, Gerstel, Linthicum, MD, USA) on the GC. Once the analyte was separated by the GC, the column fluent was split by 1:1 to the mass spectrometry and the sniffing port individually. The odor descriptions of volatile compounds in the GC–O were followed by Ning et al. [18] with modifications. Three trained panelists evaluated the odor intensity by indicating strong (S), medium (M), and weak (W), and the sniffed retention indices and the notes of the odor were recorded as well.
2.6. Statistical Analysis
All experiments and analyses were conducted in triplicate. Data were expressed as mean ± SD and statistically analyzed by using IBM SPSS Statistics 23.0 (IBM, Armonk, NY, USA). The statistically significant differences between the samples were determined by one-way analysis of variance (ANOVA), and Scheffe’s test was used to indicate the significant differences where p < 0.05. Principle component analysis (PCA) and hierarchical clustering heatmaps were carried out by using MetaboAnalyst 5.0. These two analyses were applied to understand the changes in volatile compounds between fermented and unfermented seaweeds.
3. Results and Discussion
3.1. Seaweed Fermentation by the GRAS Microorganisms
In this study, five GRAS microorganisms were selected, i.e., B. subtilis (BS), Saccharomyces cerevisiae (SC), and Lb. acidophilus (LA), Lb. delbrueckii subsp. bulgaricus (LB), and Lb. casei (LC) for seaweed fermentations. The cell growth of these five strains in Ulva and Laminaria suspensions is shown in Figure S1. In Ulva and Laminaria suspensions, the cell count of BS and SC increased at 0–24 h, and the growth of lactic acid bacteria was observed during 0–12 h. In addition, the yeast maintained their viable cell number at 5–6 log CFU/mL in both seaweed suspensions during fermentation, and the bacteria maintained their viable cell number at 7–9 log CFU/mL, indicating that seaweed suspension could serve as the sole biomass for microbial fermentations.
3.2. Monitoring the Volatile Compound Profiles in Seaweed Fermentation
3.2.1. Ulva sp. Fermentation
A total of 51 volatile compounds were identified in the unfermented and fermented Ulva sp. suspension (Table 1) using HS-SPME–GC–MS. The profile of volatile compounds varies among the Ulva sp. suspensions fermented with five microorganisms. Twelve ketones were detected in the unfermented Ulva sp., and 20, 13, 11, 11, and 10 ketones were determined in the Ulva sp. suspension fermented with BS, SC, LA, LB, and LC, respectively. In the unfermented Ulva sp., aliphatic ketones with long chains (≥C7), such as 2,2,6-Trimethylcyclohexanone (floral), 6-Methyl-5-hepten-2-one (also known as sulcatone; citral and musty), and β-Ionone (violet, floral) [19], were identified. β-ionone is a potent odorant in seafood, and sulcatone has been identified in various foods as a metabolite of lycopene [20]. These ketone compounds could be detected in the commonly dehydrated Ulva lactuca [9]. The total amount of ketones was significantly increased in the Ulva suspension after BS fermentation, particularly short-chain aliphatic ketones (C ≤ 7), such as pentanone (fruity and pungent) and heptanone (cheesy, fruity, and spicy). In general, aliphatic ketones with shorter chains have a strong aroma, and they can be generated through the lipoxygenation of fatty acids during fermentation [21]. On the contrary, lactic acid bacteria fermentations could reduce the total amount of ketones

Table 1.
Changes of the aromatic profile in the green seaweed Ulva sp. fermented by various microorganisms.
Ten aldehydes were detected in the unfermented Ulva sp., including linear, branched, or unsaturated aldehydes (Table 1). Most aldehydes have short chains (C ≤ 7), such as furfural, hexanal, and heptanal. According to Peinado et al. [22], the notes of hexanal and heptanal were described as fishy odors. Aldehydes such as hexanals and heptanals were reduced after all microbial fermentations, in which BS fermentation was of the most significance. Moreover, the amount of safranal, a compound that contributes a spicy saffron-like note with herbaceous, tobacco facets, and floral undertones, was doubled after SC fermentation.
As for alcohols, the aroma compound 1-penten-3-ol (green) [20] was identified in the unfermented Ulva sp., and it was decreased in each microbial fermentation. The total alcohol content decreased after microbial fermentations except for SC (Table 1). Five additional alcohols were generated in the Ulva sp. suspension after SC fermentation, such as 1-pentanol (fermented, oily, sweet) and 1-hexanol (fruity, floral aromatic) [20,23]. In the metabolism of brewing yeast, the Ehrlich pathway is a metabolic route to produce high alcohol content from amino acids, involving transamination, decarboxylation, and reduction [24]. This finding might explain the alcohol production during the SC fermentation of Ulva sp.
Benzaldehyde (bitter almond and burnt sugar) was identified in the unfermented Ulva sp., and it was converted into benzyl alcohol (floral and rose) [20] by alcohol dehydrogenase after BS or SC fermentation [25]. Our results were similar to those reported by Wang et al. [26]; that is, benzaldehyde and benzyl alcohol were decreased and increased, respectively, through SC fermentation. On the contrary, benzaldehyde was oxidized into benzoic acid (sweet and pleasant odor) [27] during lactic acid fermentation, which was also observed in lactic acid bacteria-fermented dairy products [28]. In addition, the number of other aroma compounds, such as furan 2-ethyfuran (coca), was reduced after microbial fermentations, but BS fermentation increased the amount of 2,5-dimethylpyrazine (roasted, nutty) and dimethyl sulfide (boiled cabbage). The overall changes in volatile compounds for the unfermented and fermented Ulva sp. were summarized in the heat map (Figure 1). Compared with the unfermented Ulva sp., BS or SC fermentation could reduce aldehyde contents, which might mitigate potential unpleasant odors from seaweed. BS fermentation improved ketone production, whereas SC increased alcohol production during fermentation. By contrast, volatile acids were found in the Ulva sp. suspension fermented with lactic acid bacteria (LA, LB, and LC).
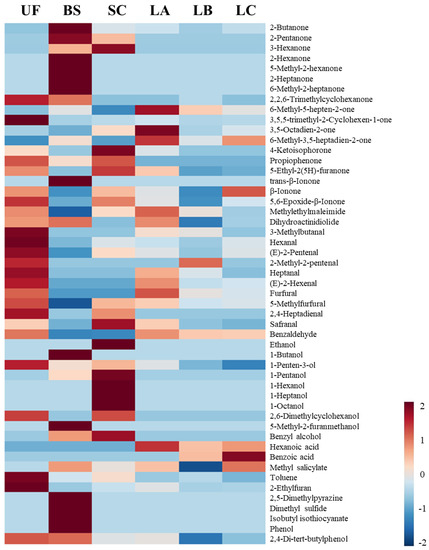
Figure 1.
Heat map of the volatile compounds in the green seaweed Ulva sp. fermented by various microorganisms. Colors indicate the Z-score distances to the mean levels of the observations. A Z-score greater than 1.645 (red color) or lower than −1.645 (blue color) is significant at the 0.05 level. UF: unfermented; BS: B. subtilis; SC: S. cerevisiae; LA: Lb. acidophilus; LB: Lb. delbrueckii subsp. bulgaricus; LC: Lb. casei.
3.2.2. Laminaria sp. Fermentation
A total of 55 volatile compounds were identified in the unfermented and fermented Laminaria sp. (Table 2). In addition, a total of 31 volatile compounds were identified in the unfermented Laminaria sp., and 36, 40, 41, 40, and 40 volatile compounds were found in the Laminaria sp. suspension fermented with BS, SC, LA, LB, and LC, respectively.

Table 2.
Changes of the aromatic profile in the brown seaweed Laminaria sp. fermented by various microorganisms.
Total ketones were increased after fermentation with BS, SC, LB, or LC (Table 2), with BS showing the most increases. 1-Octen-3-one (metallic and mushroom) [29] and β-ionone were identified in the fermented and unfermented groups. Notably, 3-octanone, described as herb, butter, and resin notes [30], was generated in the fermented Laminaria sp. suspension, and 3-hydroxy-2-butanone (sweet), also known as acetoin, was generated in the BS-fermented group. β-ionone, which was responsible for the rose aroma [31] and sweet note in green tea [32], was enhanced during SC, LA, LB, or LC fermentation.
Linear, branched, or volatile unsaturated aldehydes, which were identified in brown seaweeds, may contribute to seaweed-like or seafood-like odors [11,22]. Our data indicated that the total aldehyde content was decreased in the Laminaria sp. suspension fermented with BS, SC, and LA (Table 2). Pan et al. [33] have reported that yeast fermentation can improve the odor of tiger puffer skin gelatin and reduce its volatile aldehydes. Heptanal (dry fish notes), hexanal (fishy notes) [22,34], and furfural (burnt note) were reduced in the fermented Laminaria sp. suspension, which is consistent with the observation in the fermented Ulva sp. (Table 1), indicating the potential application of fermentation in removing off-odors from seaweeds.
The major alcohol compound detected in the unfermented Laminaria sp. is 1-octen-3-ol (529.91 μg/L, Table 2), which is described as fishy and grassy notes [34]. Based on the report of López-Pérez, Picon, and Nuñez [9], 1-octen-3-ol has a high odor activity value and low perception threshold, and it is considered the major alcohol compound in brown seaweeds (Himanthalia elongata and Laminaria ochroleuca). SC fermentation could significantly reduce 1-Octen-3-ol. In addition, a high content of alcohols was produced via fermentation, such as 1-pentanol (fermented, oily, sweet) and 1-hexanol (fruity, floral aromatic), which is consistent with our observations in the fermented Ulva sp. (Table 1). The EMP pathway and Ehrlich pathway are two possible metabolic routes for converting carbohydrates and amino acids into branched alcohols during yeast fermentation, respectively [34]. Thus, more alcoholic compounds were identified in the Laminaria sp. fermented with SC compared with the unfermented group.
With regard to volatile acids, tetradecanoic acid was identified in the unfermented Laminaria sp. and acid varieties, and total acid contents increased after microbial fermentation (Table 2). Saturated fatty acids, such as hexanoic acid (fatty, cheesy), octanoic acid (fatty, waxy) [20], and decanoic acid, were generated during microbial fermentation. In addition, a branched carboxylic acid (2-methylbutanoic acid) was identified only in Laminaria fermented with BS. Based on the report of Leejeerajumnean et al. [35], 2-methylbutanoic acid was identified in soybean products fermented with Bacillus sp.
Therefore, changes in volatile compounds in the unfermented and fermented Laminaria sp. were demonstrated in the heat map (Figure 2). Compared with the unfermented Laminaria sp., BS and SC seemed to significantly decrease aldehyde content during fermentation. In addition, BS fermentation enhanced ketone production, and SC fermentation boosted alcohol formation in fermented Laminaria sp. suspensions, which were also observed in Ulva sp. fermentation (Figure 1; Table 1). On the contrary, lactic acid bacteria (LA, LB, and LC) might slightly reduce aldehyde production when fermenting Laminaria sp., compared with BS and SC. Based on changes in the profile of volatile compounds among fermentations, BS and SC have shown application potential in mitigating off-odors from seaweeds (Ulva sp. and Laminaria sp.) and modifying their aromatic perceptions after fermentation. Thus, principal component analysis (PCA) and odor analysis of fermented seaweeds were performed.
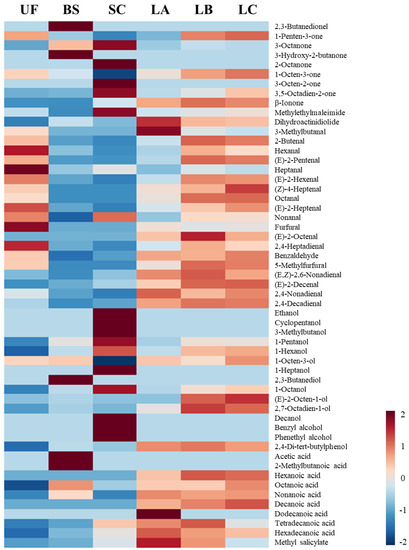
Figure 2.
Heat map of the volatile compounds in the brown seaweed Laminaria sp. fermented by various microorganisms. Colors indicate the Z-score distances to the mean levels of the observations. A Z-score greater than 1.645 (red color) or lower than −1.645 (blue color) is significant at the 0.05 level. UF: unfermented; BS: B. subtilis; SC: S. cerevisiae; LA: Lb. acidophilus; LB: Lb. delbrueckii subsp. bulgaricus; LC: Lb. casei.
3.3. Principal Component Analysis (PCA) of the Volatile Compounds in the Fermented Seaweeds
3.3.1. Ulva sp.
The PCA of chemical groups of the volatile compounds in the unfermented and fermented Ulva sp. is shown in Figure 3. The PCA can explain 84.1% of the total variances in the dataset (59.5% and 24.6% by principal component 1 [PC1] and principal component 2 [PC2], respectively). Based on the score plot (Figure 3A), the BS is in the first quadrant of the plot, whereas the unfermented Ulva sp. is in the second quadrant. This result shows the difference in the composition of volatile compounds between the BS and the unfermented Ulva sp. Moreover, in the loading plot (Figure 3B), the ketone family is observed in the positive axis of the PC1, whereas the aldehyde family is observed in the negative axis of the PC1. This negative correlation between the ketone and aldehyde family indicates the difference in volatile compounds between the BS and the unfermented Ulva. The aforementioned statement is supported by Table 1, i.e., ketones are increased, and aldehydes are reduced after BS fermentation. On the contrary, alcohol formation is associated with SC fermentation, which demonstrates the highest alcohol content among other fermentations.
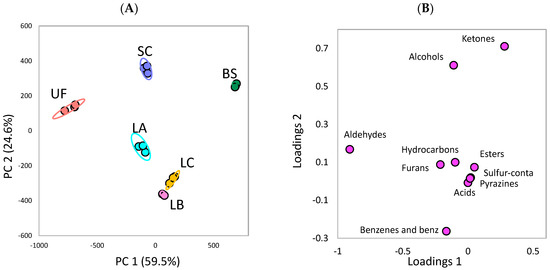
Figure 3.
Principal component analysis plots of chemical groups of the volatile compounds in the green seaweed Ulva sp. fermented by various microorganisms: (A) score plot (B) loading plot. UF: unfermented; BS: B. subtilis; SC: S. cerevisiae; LA: Lb. acidophilus; LB: Lb. delbrueckii subsp. bulgaricus; LC: Lb. casei. Sulfur-conta, sulfur containing compounds; benz, benzene derivatives.
In evaluating the specific compound affecting the difference among fermentations, the PCA of a single volatile compound is shown in Figure 4. The PC1 and PC2 can explain 57.1% and 20.5% of the total variances in the dataset, respectively (total: 77.6%). Based on the score plot (Figure 4A), the BS and unfermented group are in a different quadrant of PC1, which correlates to Figure 3A. In addition, 2-heptanone is identified in the positive axis of PC1 in the loading plot (Figure 4B), which indicates that this compound likely affects the difference between the BS and the unfermented Ulva.
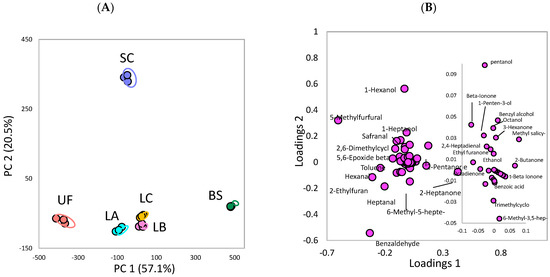
Figure 4.
Principal component analysis plots of the volatile compounds in the green seaweed Ulva sp. fermented by various microorganisms: (A) score plot (B) loading plot. UF: unfermented; BS: B. subtilis; SC: S. cerevisiae; LA: Lb. acidophilus; LB: Lb. delbrueckii subsp. bulgaricus; LC: Lb. casei.
Moreover, 2-heptanone (436.59 μg/L) was formed after fermentation with BS, and its content was dominant at around 33% of total ketones (1308.42 μg/L) in the fermented Ulva (Table 1). The difference in volatile compounds between the SC and the unfermented group based on PC2 is shown in Figure 4A. Based on the loading plot (Figure 4B), 1-hexanol is in the positive axis of PC2, which indicates its contribution to the difference in volatile compounds between the SC and the unfermented Ulva. This result might correspond to the formation of 1-hexanol (289.18 μg/L) after SC fermentation (Table 1). Based on our GC–O analysis, the odor intensity of 1-hexanol, described as floral and sweet, is enhanced by SC fermentation. Therefore, 2-heptanone might contribute to the difference between Ulva fermented with BS and unfermented Ulva. Furthermore, 1-hexanol plays an important role in the fermentation of Ulva with SC compared with the unfermented Ulva (Figure 4). These results are consistent with the PCA of chemical groups shown in Figure 3.
3.3.2. Laminaria sp.
The PCA of chemical groups of volatile compounds in the unfermented and fermented Laminaria sp. is shown in Figure 5. The results showed that these variables could explain 91.7% of the total variances among the samples. BS and SC are located in the positive axis of PC1, whereas the unfermented group is in the negative axis of PC1 (Figure 5A). Therefore, fermenting Laminaria sp. by BS or SC can change the composition of chemical groups in volatile compounds. In addition, the chemical groups of volatile compounds are distinct in BS and SC because these two groups are in the positive and negative axes of PC2, respectively. Based on the loading plot (Figure 5B), ketones in BS and alcohols in SC primarily cause the difference. As shown in Table 2, after fermentation, BS acquired higher ketone content than SC, whereas SC obtained higher alcohol content than BS.
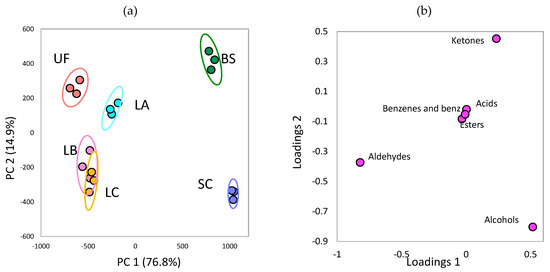
Figure 5.
Principal component analysis plots of chemical groups of the volatile compounds in the brown seaweed Laminaria sp. fermented by various microorganisms: (A) score plot (B) loading plot. UF: unfermented; BS: B. subtilis; SC: S. cerevisiae; LA: Lb. acidophilus; LB: Lb. delbrueckii subsp. bulgaricus; LC: Lb. casei. Sulfur-conta, sulfur containing compounds; benz, benzene derivatives.
Another PCA was conducted to evaluate the correlation between a single volatile compound and the type of fermentation (Figure 6). PC1 and PC2 explained 55.6% and 29.9%, respectively, of the total variance in the dataset (Total: 85.5%). Consistent with Figure 5, the volatile compound compositions of BS and SC are differentiated from the unfermented Laminaria sp. (Figure 6A). Moreover, based on the loading plot (Figure 6B), acetoin (i.e., 3-hydroxy-2-butanone) and 2,3-butanediol are positively correlated with BS, and 1-heptanol and 1-octanol are positively correlated with SC, thereby causing the difference in volatile compounds between the two fermented Laminaria. This finding is consistent with that shown in Figure 5, i.e., the difference between BS and SC is associated with the ketone and alcohol family.
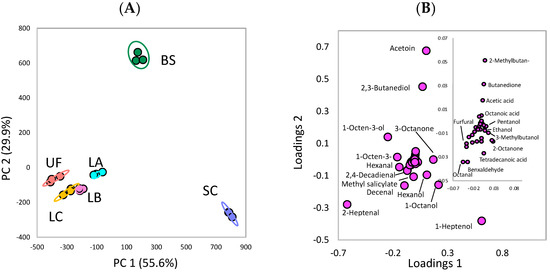
Figure 6.
Principal component analysis plots of the volatile compounds in the brown seaweed Laminaria sp. fermented by various microorganisms: (A) score plot (B) loading plot. UF: unfermented; BS: B. subtilis; SC: S. cerevisiae; LA: Lb. acidophilus; LB: Lb. delbrueckii subsp. bulgaricus; LC: Lb. casei.
As shown in Table 2, acetoin accounts for the highest content (63.4%) in the ketone family from BS fermentation, followed by 1-heptanol (41.1%) and 1-octanol (20.5%) in the alcohol family from SC fermentation. On the contrary, in the third quadrant of the score plot (Figure 6A), the unfermented group and fermentation with lactic acid bacteria (LA, LB, and LC) are in the negative axis of PC1 and PC2. This finding indicates that lactic acid bacteria may not significantly modify the composition of volatile compounds as compared with the unfermented group. Based on the loading plot (Figure 6B), (E)-2-heptenal shows a higher correlation with the unfermented group and fermentation with lactic acid bacteria. Furthermore, (E)-2-heptenal was significantly reduced from 1179.00 to 538.71 and 429.48 μg/L by fermenting with BS and SC, respectively (Table 2). In addition, (E)-2-heptenal contents were significantly lower in BS and SC fermentation than in lactic acid bacteria fermentation (Table 2). This finding may support the result that the groups of lactic acid bacteria and BS/SC are positioned on different quadrants in PCA.
Therefore, according to the PCA (Figure 3, Figure 4, Figure 5 and Figure 6), fermenting Ulva sp. or Laminaria sp. with BS or SC can modify the composition of volatile compounds compared with unfermented seaweeds. These results can be supported by Table 1 and Table 2. Volatile and non-volatile compounds can contribute to the sensory characteristics of foods, which may further affect consumer preference [36,37,38]. Hence, GC–O was applied to evaluate the odor description and intensity of the fermented seaweeds.
3.4. Sensory Evaluation of the Intensity of the Odor-Active Compounds in the Fermented Seaweeds
GC–O is considered as a major technique for analyzing the effect of volatile compounds on sensory characteristics, which has been applied to seaweeds [3]. The results of GC–O analysis for the unfermented and fermented Ulva sp. are summarized in Table 3.

Table 3.
Odor description and intensity of the odor-active compounds in the green seaweed Ulva sp. fermentations.
A total of 22 odor-active compounds were detected, including six ketones, four aldehydes, four alcohols, one ester, one pyrazine, and six unidentified compounds. Among the pleasant odors, floral, sweet, and fruity were described. After fermentation with BS and SC, aromatic notes were enhanced, compared with the unfermented Ulva. For example, benzyl alcohol could contribute to floral and fruity aromas after BS and SC fermentation. Benzyl alcohol is described as flower, apple peel, and sweet aromas in Chinese bayberry [39], and it has been reported in many fruits [40]. In addition, 1-hexanol and 1-heptanol could provide extra floral/sweet and sweet aromas in the SC group, and the floral aroma of 6-methyl-5-hepten-2-one was enhanced in the BS group (Table 3). Notably, after fermentation with BS, 2,5-dimethylpyrazine is formed (Table 1), and it contributes to the nutty and roasted aromas in the fermented Ulva sp. (Table 3).
This odor description is consistent with the report of Guo et al. [41], indicating that 2,5-dimethylpyrazine is the critical contributor to roasted peanut flavor in Oolong tea. According to Leejeerajumnean, Duckham, Owens, and Ames [35], 2,5-dimethylpyrazine was found in several Bacillus-fermented soybean products. With regard to strong odor intensity, β-ionone (violet, floral) is the only compound that is described as having a strong and pleasant odor in all groups. Based on the report of Buttery et al. [42], β-ionone has a lower odor threshold (0.007 ppb) than 6-methyl-5-hepten-2-one (50 ppb) in water. This finding might indicate that the odor intensity of β-ionone is strong and greater than 6-methyl-5-hepten-2-one. In strengthening the pleasant odor of Ulva sp. by fermenting with BS and SC, both fermentations can mitigate the unpleasant odors (Table 3). For example, the odor intensity of hexanal (fishy, grassy) was reduced in the BS- and SC-fermented Ulva sp. suspensions. Described as caramelly, fried, and toasty, 5-Methylfurfural was not detected in the BS-fermented Ulva sp. suspension by GC–O. These reductions are consistent with that shown in Table 1 in this study. BS and SC have demonstrated their application potential in improving pleasant odors and lightening unpleasant odors of Ulva by fermentation.
The results of the GC–O analysis for the unfermented and fermented Laminaria sp. are outlined in Table 4. A total of 36 odor-active compounds were detected, including 7 ketones, 11 aldehydes, 8 alcohols, 1 acid, 1 ester, and eight unidentified compounds. Only three compounds, namely, β-ionone (violet, floral), benzaldehyde (fruity, sweet), and 1-octanol (waxy, citrus), were detected as pleasant odors in the unfermented Laminaria sp. suspension. In addition, the pleasant odors could be strengthened in the Laminaria suspension fermented with BS and SC. For example, 2,3-butanedione, described as yogurt and creamy, has shown a medium odor intensity in BS, whereas 1-heptanol, described as sweet, has shown a strong odor intensity in SC. Compared with BS, SC can provide more fruity and floral notes in the fermented Laminaria sp. suspension.

Table 4.
Odor description and intensity of the odor-active compounds in the brown seaweed Laminaria sp. fermented by various microorganisms.
With regard to the unpleasant odors, fishy, fatty, and oily notes were described the most in the Laminaria sp. suspension (Table 4). The compounds that contributed to the strong unpleasant odor intensity included 1-octen-3-one, (E)-2-heptenal, and (E)-2-decenal. The note of 1-octen-3-one was described as fishy and metallic in this study, and Peinado, Girón, Koutsidis, and Ames [22] described the odors of 1-octen-3-one as metallic, dirty, and dusty in brown seaweeds. As shown in Table 4, BS and SC fermentations could relieve the intensity of the unpleasant odors in the fermented Laminaria sp. suspensions. The note intensity of octanal, (E)-2-heptadienal, and 2,4-heptadienal, which contributed to fatty notes, were reduced or eliminated by BS and SC. Notably, the note of 1-penten-3-one (pungent and fresh) [43], categorized in the neutral odors, was weakened during fermentation with BS and SC, and a similar result was observed in Table 2. Modifying the aroma profile for food ingredients could be investigated and applied in the food industry. For example, in mitigating the beany aroma in the legume-based beverage, fermentation with lactic acid bacteria is applied [44]. In this study, based on our GC–O analysis, BS and SC have shown application potential in enhancing pleasant odors and reducing unpleasant odors for seaweeds Ulva sp. and Laminaria sp.
4. Conclusions
In this study, volatile compounds in the green seaweed Ulva sp. and the brown seaweed Laminaria sp. fermented by GRAS microorganisms were presented by using HS-SPME and GC–MS. The aromatic profiles of the seaweeds were successfully modified by fermentation, particularly with BS and SC. Categories and total contents of aldehydes were significantly reduced in fermented seaweeds by BS and SC, respectively. Moreover, fermentation with BS could improve ketone content, while fermentation with SC could enhance alcohol content in Ulva and Laminaria. These results are supported by the PCA results, revealing that the ketones in BS fermentation and alcohols in SC fermentation play critical roles in causing differences in the volatile compound profile compared with the unfermented seaweeds. Finally, through GC–O analysis, BS and SC could enhance pleasant odors (e.g., floral, fruity, and sweet) and weaken unpleasant odors (e.g., grassy, fatty, and fishy) from the unfermented seaweeds. This study exhibits the potential of modifying the aromatic profiles of Ulva and Laminaria by fermentation, including improving pleasant odors and diminishing unpleasant odors. These achievements can expand the potential of utilizing seaweeds for agricultural and food industries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9020135/s1, Figure S1: The viable cell counts of various microbial strains in (A) green seaweed Ulva sp. and (B) brown seaweed Laminaria sp. during fermentation.
Author Contributions
H.-T.V.L. and M.-C.F. conceived and designed the experiments; C.-Y.P., H.-J.L., M.-Y.H., W.-J.L. and C.-L.H. performed the experiments; Y.-H.R.H. and C.-Y.P. analyzed the data; Y.-H.R.H. and H.-T.V.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Center of Excellence for the Oceans, National Taiwan Ocean University from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (NTOU-RD-AA-2021-1-02018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Chorng-Lan Pan from National Taiwan Ocean University for providing the lactic acid bacteria strains.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2005, 43, 1–91. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. The rise of seaweed gastronomy: Phycogastronomy. Bot. Mar. 2019, 62, 195–209. [Google Scholar] [CrossRef]
- Vilar, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Volatile compounds of six species of edible seaweed: A review. Algal Res. 2020, 45, 101740. [Google Scholar] [CrossRef]
- Gressler, V.; Stein, É.M.; Dörr, F.; Fujii, M.T.; Colepicolo, P.; Pinto, E. Sesquiterpenes from the essential oil of Laurencia dendroidea (Ceramiales, Rhodophyta): Isolation, biological activities and distribution among seaweeds. Rev. Bras. De Farmacogn. 2011, 21, 248–254. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Qin, Y. Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Du, X.; Xu, Y.; Jiang, Z.; Zhu, Y.; Li, Z.; Ni, H.; Chen, F. Removal of the fishy malodor from Bangia fusco-purpurea via fermentation of Saccharomyces cerevisiae, Acetobacter pasteurianus, and Lactobacillus plantarum. J. Food Biochem. 2021, 45, e13728. [Google Scholar] [CrossRef]
- Francezon, N.; Tremblay, A.; Mouget, J.-L.; Pasetto, P.; Beaulieu, L. Algae as a source of natural flavors in innovative foods. J. Agric. Food Chem. 2021, 69, 11753–11772. [Google Scholar] [CrossRef]
- López-Pérez, O.; Picon, A.; Nuñez, M. Volatile compounds and odour characteristics of seven species of dehydrated edible seaweeds. Food Res. Int. 2017, 99, 1002–1010. [Google Scholar] [CrossRef]
- Hernández, T.; Estrella, I.; Pérez-Gordo, M.; Alegría, E.G.; Tenorio, C.; Ruiz-Larrrea, F.; Moreno-Arribas, M. Contribution of malolactic fermentation by Oenococcus oeni and Lactobacillus plantarum to the changes in the nonanthocyanin polyphenolic composition of red wine. J. Agric. Food Chem. 2007, 55, 5260–5266. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Bae, H.-N.; Eom, S.-H.; Lim, K.-S.; Yun, I.-H.; Chung, Y.-H.; Jeon, J.-M.; Kim, H.-W.; Lee, M.-S.; Lee, Y.-B. Removal of off-flavors from sea tangle (Laminaria japonica) extract by fermentation with Aspergillus oryzae. Bioresour. Technol. 2012, 121, 475–479. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.-H.; Ren, D.-F.; Lu, J. Mixed fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-J.; Lin, H.-J.; Hsu, P.-H.; Lai, M.; Chiu, J.-Y.; Lin, H.-T.V. Brown and red seaweeds serve as potential efflux pump inhibitors for drug-resistant Escherichia coli. Evid.-Based Complement Altern. Med. 2019, 2019, 1836982. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of ulvan oligosaccharides with antioxidant and angiotensin-converting enzyme-inhibitory activities by microbial enzymatic hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Vilar, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. A chemometric approach to characterize the aroma of selected brown and red edible seaweeds/extracts. J. Sci. Food Agric. 2021, 101, 1228–1238. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Wang, D.; Zhang, L.; Chen, G. Study on the volatile profile characteristics of oyster Crassostrea gigas during storage by a combination sampling method coupled with GC/MS. Food Chem. 2009, 115, 1150–1157. [Google Scholar] [CrossRef]
- Hosoglu, M.I. Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem. 2018, 240, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, F.-p.; Chen, H.-t.; Liu, S.-y.; Gu, C.; Song, Z.-y.; Sun, B.-g. Identification of volatile components in Chinese Sinkiang fermented camel milk using SAFE, SDE, and HS-SPME-GC/MS. Food Chem. 2011, 129, 1242–1252. [Google Scholar] [CrossRef]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its occurrence and biological function and metabolic engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Neta, M.; Narain, N. Volatile components in seaweeds. Examines Mar. Biol. Oceanogr. 2018, 2, 195–201. [Google Scholar]
- Park, M.K.; Kim, Y.-S. Comparative metabolic expressions of fermented soybeans according to different microbial starters. Food Chem. 2020, 305, 125461. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Kiritsakis, A. Flavor components of olive oil—A review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; Van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; James, P.; Ward, O. Aromatic aldehydes as substrates for yeast and yeast alcohol dehydrogenase. Biotechnol. Bioeng. 1989, 33, 657–660. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Biotransformation of green tea (Camellia sinensis) by wine yeast Saccharomyces cerevisiae. J. Food Sci. 2020, 85, 306–315. [Google Scholar] [CrossRef]
- Moreira, R.; Trugo, L.; Pietroluongo, M.; De Maria, C. Flavor composition of cashew (Anacardium occidentale) and marmeleiro (Croton species) honeys. J. Agric. Food Chem. 2002, 50, 7616–7621. [Google Scholar] [CrossRef]
- Sieber, R.; Bütikofer, U.; Bosset, J. Benzoic acid as a natural compound in cultured dairy products and cheese. Int. Dairy J. 1995, 5, 227–246. [Google Scholar] [CrossRef]
- Lubran, M.B.; Lawless, H.T.; Lavin, E.; Acree, T.E. Identification of metallic-smelling 1-octen-3-one and 1-nonen-3-one from solutions of ferrous sulfate. J. Agric. Food Chem. 2005, 53, 8325–8327. [Google Scholar] [CrossRef]
- Synos, K.; Reynolds, A.; Bowen, A. Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT-Food Sci. Technol. 2015, 64, 227–235. [Google Scholar] [CrossRef]
- Brunke, E.J.; Hammerschmidt, F.J.; Schmaus, G. Scent of roses–recent results. Flavour Fragr. J. 1992, 7, 195–198. [Google Scholar] [CrossRef]
- Hattori, S.; Takagaki, H.; Fujimori, T. Identification of volatile compounds which enhance odor notes in Japanese green tea using the OASIS (Original aroma simultaneously input to the sniffing port) method. Food Sci. Technol. Res. 2005, 11, 171–174. [Google Scholar] [CrossRef]
- Pan, J.; Jia, H.; Shang, M.; Li, Q.; Xu, C.; Wang, Y.; Wu, H.; Dong, X. Effects of deodorization by powdered activated carbon, β-cyclodextrin and yeast on odor and functional properties of tiger puffer (Takifugu rubripes) skin gelatin. Int. J. Biol. Macromol. 2018, 118, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Leejeerajumnean, A.; Duckham, S.C.; Owens, J.D.; Ames, J.M. Volatile compounds in Bacillus-fermented soybeans. J. Sci. Food Agric. 2001, 81, 525–529. [Google Scholar] [CrossRef]
- King, E.S.; Kievit, R.L.; Curtin, C.; Swiegers, J.H.; Pretorius, I.S.; Bastian, S.E.; Francis, I.L. The effect of multiple yeasts co-inoculations on Sauvignon Blanc wine aroma composition, sensory properties and consumer preference. Food Chem. 2010, 122, 618–626. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.-G.; Kim, M.K. Volatile and non-volatile compounds in green tea affected in harvesting time and their correlation to consumer preference. J. Food Sci. Technol. 2016, 53, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Lee, D.-J.; Kim, J.-Y.; Lim, S.-T. Volatile composition and sensory characteristics of onion powders prepared by convective drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Li, Y.; Xu, Y.; Jiang, W.; Tao, Y. Characterization of aroma compounds in Chinese bayberry (Myrica rubra Sieb. et Zucc.) by gas chromatography mass spectrometry (GC-MS) and olfactometry (GC-O). J. Food Sci. 2012, 77, C1030–C1035. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Guo, X.; Song, C.; Ho, C.-T.; Wan, X. Contribution of L-theanine to the formation of 2, 5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018, 263, 18–28. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Mall, V.; Sellami, I.; Schieberle, P. New degradation pathways of the key aroma compound 1-penten-3-one during storage of not-from-concentrate orange juice. J. Agric. Food Chem. 2018, 66, 11083–11091. [Google Scholar] [CrossRef] [PubMed]
- Ritter, S.W.; Gastl, M.I.; Becker, T.M. The modification of volatile and nonvolatile compounds in lupines and faba beans by substrate modulation and lactic acid fermentation to facilitate their use for legume-based beverages—A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4018–4055. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).