Abstract
High-amylose starch has unique functional properties and nutritional values in food applications. This type of starch is generally resistant to enzymatic digestion in the gastrointestinal tract, and contains an increased fraction of resistant starch (RS), which is a type of dietary fiber. The digestion and fermentation of high-amylose starch in the gut are of current research interest, as the processes are related to its nutritional functionality. This review summarizes recent in vitro and in vivo studies on the digestion and fermentation of high-amylose starches from different botanical sources and those that have been obtained by modifications. The RS content and fermentation properties are compared among high-amylose starches. This review aims to provide a current understanding of the relationship between high-amylose starch structures and fermentation-related nutritional properties. The results of these studies suggest that both modifications and food processing of high-amylose starch result in distinct fermentation products and nutritional properties. The review provides insight into the potential future applications of diverse high-amylose starches as bioactive compounds to modulate colonic fermentation.
1. Introduction
Cereal grains contain a unique blend of bioactive components, including nondigestible carbohydrates, vitamins, minerals, phytochemicals, and antioxidants. Nondigestible carbohydrates have been shown to impact the host by altering the composition of the gut microbiota and modulating cellular differentiation and apoptosis in the colon [1]. There is growing evidence that nondigestible carbohydrates could potentially prevent or manage chronic health conditions such as diabetes and obesity [2,3,4]. Starch is the most common type of carbohydrate in the diet and is found in grain-based staple foods, such as white bread, noodles, pasta and tortillas, etc. However, these foods often contain a large fraction of highly digestible starch [5,6,7], and can lead to a rapid increase in blood sugar levels. In the management of diabetes, maintaining blood glucose levels within a safe range is crucial. Therefore, there has been an increasing interest in starches that are resistant to the action of digestive enzymes.
High-amylose starches have gained popularity because of their high resistance to enzymatic hydrolysis and their contribution to dietary fiber intake. A wide range of high-amylose types of major starch-containing food crops is now available, including wheat, maize, barley, potatoes, peas, etc. [8,9,10]. High-amylose starches are being intensively studied to evaluate their nutritional functionality. These starches are generally more resistant to enzymatic digestion in the gastrointestinal tract and contain a higher fraction of resistant starch (RS). Their nutritional effects are not limited to reducing glycaemic responses, but also depend on colonic fermentation, which produces important metabolites, in particular short-chain fatty acids (SCFAs). SCFAs play a crucial role in improving physical and mental health by reducing precursors of colorectal cancer, regulating macronutrient metabolism, and altering hormone secretion [11].
Currently, the structural features of high-amylose starch can be manipulated through biological, chemical, and physical approaches. These approaches have expanded the range of available varieties for targeted nutritional functions. Recent studies have shown that high-amylose starch from food crops is a potential platform for delivering health benefits and addressing non-communicable diseases such as type 2 diabetes and obesity [9,12]. However, high-amylose starch with different structural features may have different nutritional properties, such as butyrate and gas production, due to individual variations in microbiota composition. Therefore, it is timely to review these advances and evaluate how the structural features of high-amylose starch impact its fermentation products. This mini-review critically evaluates (1) the structural features of high-amylose starch that confer resistance to enzymes, and (2) the effect of high-amylose starch structures on fermentation products and nutritional properties.
2. Structure and Unique Functional Properties of High-Amylose Starch
2.1. Multilevel Structure
Starch is a complex polymer made up of glucose molecules, which can be divided into two types: amylose and amylopectin. Amylose is mostly linear with rare branches, while amylopectin has a higher percentage of branch points (approximately 5%) [13]. In wild-type starch, amylose typically makes up about 25% of the starch, but the typical amylose contents of starches are dependent on botanical sources, varying from 6 to 33% in seeds of cereals [8]. Starch with a higher proportion of amylose than the wild type is referred to as high-amylose starch. Starch has multiple levels of structure, from the lowest level, individual chains, to the highest level, starch deposition in grains. The structural features that are responsible for the unique properties of high-amylose starch have been widely studied at multiple length scales (such as the lower proportion of short chains, the appearance of intermediate amylopectin with elongated chains, the lower degree of branching, and the increased fraction of V-type polymorph, etc.) However, the effects of these structural features on digestion resistance, fermentation products, and nutritional values need to be carefully evaluated in order to fully exploit their potential applications.
At the level of individual chains, high-amylose starches obtained through biological approaches, such as the suppression of starch branching enzymes and soluble starch synthases, generally have an increased proportion of relatively long chains with the degree of polymerization (DP) greater than ~30 [9,14,15]. Additionally, these starches may also show a decrease in relatively short chains, with DP between ~10 and ~20, and an increase in very short chains with DP less than ~10. These changes in chain length and distribution can affect the properties of high-amylose starch and its potential applications.
Individual chains are linked by α-(1–6) glycosidic bonds to form the whole starch molecules. In high-amylose starch, whole starch molecules typically have a lower degree of branching. Furthermore, high-amylose starch may contain less-branched amylopectin with extra-long chains and branched amylose. Such whole starch molecules are usually referred to as intermediate materials. The intermediate amylopectin or amylose cannot be unambiguously classified using the ratio of large-to-small whole starch molecules, or long-to-short individual chains. These structural features cannot be easily characterized using traditional methods, such as iodine colorimetry. The method tends to overestimate the amylose content of high-amylose starch due to interference from amylopectin–iodine complexes. The interference can be significant for high-amylose starches in which amylopectin has extra-long chains. However, an experimental two-dimensional (2D) distribution has been developed to better understand the complex structure of high-amylose starch [16].
High-amylose starch exhibits unique structural features at higher levels, including semicrystalline lamellae and granules. The location of amylose in starch granules is still a topic of debate, but it is generally accepted that amylose is located tangentially to the radial orientation of amylopectin chains. The distribution of amylose is not even within the starch granules, and the periphery of the granules was proposed to contain a higher amylose content than the core of the granules [17]. Increased amylose content in high-amylose content impacts the interactions among starch molecules. For example, the glucan chains in high-amylose wheat starches (HAWS) are organized differently and are relatively more mobile compared to high-amylose maize starch (HAMS) with the same apparent amylose content [18]. The difference was proposed to be due to the greater proportion of very long chains/branches in HAWS compared to HAMS. The finding emphasizes the important role of molecular structure in determining the higher-level structure of high-amylose starch.
High-amylose starches tend to have amylopectin double helices packed into hexagonal unit cells, resulting in B-type crystallinity in the X-ray diffraction pattern. B-type crystallinity has a diffraction singlet at 17° and peaks at approximately 5.5° Bragg angles (2θ). In contrast, wild-type cereal starch typically exhibits A-type crystallinity, which is characterized by a main diffraction doublet at 17° and 18° 2θ. Monoclinic unit cells in the A-type starch are relatively more compact and bind less water as compared to B-type unit cells. Additionally, lower granular crystallinity and increased fraction of V-type polymorph were generally found in high-amylose starches, compared to wild-type starch. The long chains of amylopectin and chains near branching points are less likely to form double helices, but along with amylose, they can constitute the amorphous region of granules [19]. The increased fractions of amylose and long chains of amylopectin lead to reduced crystallinity [20,21,22,23,24]. The reduction could be accompanied by the conversion of A-type into B-type crystallinity, which is dependent on the extent of amylose content elevation. The less regular order in high-amylose starches can also be observed by small-angle X-ray scattering patterns, in which the characteristic 9 to 10 nm repeat structure becomes weaker [18,22,25]. Similarly, when viewed under polarized light, the Maltese cross pattern tends to diminish in high-amylose starches [18,26], likely due to a less orderly organization on the light wavelength scale of several hundred nanometers.
The granular shape of high-amylose starch tends to be asymmetrical and deformed, while native wild-type starch generally has spherical and angular shapes. Wheat starch granules with increased amylose content become crescent-shaped and shrunken [23,24,26,27]. Elongated and filamentous granules were reported in wheat starch with very high apparent amylose content (>90%), whereas such granules were not found in wheat starch with lower levels of amylose content. Additionally, the size A-type granules (large lenticular granules) of wheat starch decrease with increasing amylose content levels [23]. Elongated and filamentous granules were also observed in high-amylose starch from maize and rice, possibly due to increased amylose interaction on the granular surface that causes granule agglomeration [28,29].
2.2. Unique Functional Properties
High-amylose starch has a range of unique properties that make it valuable in food and nutrition applications. Compared to regular starch, it has reduced water-holding capacity (in native granular form), increased melting temperature, limited granular swelling, reduced leachate during gelatinization, and improved gelling capacity of gelatinized starch. These properties make high-amylose starch useful for a variety of applications in food processing, medicine, and other industries.
In particular, high-amylose starch has low enzymatic digestibility, which confers various nutritional or physiological effects. These include increased intake of dietary fiber, improved glycemic control, reduced caloric value, and increased production of colonic SCFAs. High-amylose starch can also be used to encapsulate drugs and probiotics and formulate oral rehydration solutions, etc. The mechanisms and structural basis underlying the digestive resistance of high-amylose starch are discussed in detail in Section 3.
3. Enzymatic Resistance of High-Amylose Starch
High-amylose starch, also known as resistant starch type 2, generally fulfills the definition of dietary fiber by food regulatory agencies. Potential physiological benefits of resistant starch have been recognized by agencies such as the European Food Safety Authority (EFSA), Food Standards Australia New Zealand (FSANZ), and the U.S. Food and Drug Administration (FDA). The multilevel structural features of high-amylose starches linked to digestive resistance have been extensively investigated. Previous (mostly in vitro) studies have generally found that amylose content is correlated with resistant starch content in the native granular form of starch as well as in foods (Table 1). Biological approaches appear to be the most effective at elevating the level of amylose content and, thus, digestive resistance, while physical and chemical techniques can only modify the starch properties to a limited extent (Table 1). The fundamental but rate-determining steps of the conversion of high-amylose starch into absorbable products (small sugars) have been reviewed elsewhere to link the multilevel structural features to digestibility [9]. The two fundamental steps are the diffusion or absorption of the enzyme onto the substrate and the catalytic event once digestive enzymes attach to the starch substrate.
Several structural features of high-amylose starches in their native or food-related forms can limit the enzyme attachment to the starch substrate. High-amylose starches often have an increased content of granular surface-bound proteins, such as high-amylose wheat starches [30] and high-amylose rice starches [31]. These proteins can have barrier effects that prevent or slow down enzyme binding to the substrate and reduce the digestion rate. High-amylose cereal starches have also been reported to have reduced interior surface area due to a lack of channels or pores [29,32]. The features of the interior surface play an important role in starch digestibility and the structure of its digestion residue [33,34,35]. Moreover, food processing of high-amylose cereal ingredients can result in the formation of a relatively integral food structure. This improved food structure integrity (a dense and less porous starch–protein matrix) is associated with the thermal stability of granule structures, and was found to limit the action of digestive enzymes [5].
After the adsorption of the enzymes onto the substrate, the glucan chains must fit into the active sites of enzymes before the breakdown of glycosidic linkages can occur. Multiple structural features have been proposed to limit the catalytic process. At the molecular level, long glucan chains in high-amylose starch are postulated to maintain the crystalline structure by extending through multiple crystals, contributing to the digestive resistance of high-amylose starches [36,37]. Furthermore, the amorphous packing of starch polymers with elongated branches has been shown to contribute to the digestive resistance of granular high-amylose wheat starch [38] and high-amylose maize starch [39]. At the submolecular level (<10 nm), the helical structure does not fit into the active site of α-amylases. The glucan chains of high-amylose starch tend to retain their helical structures during thermal processing [40]. Even if the helical structure is disrupted during thermal processing, the higher the proportion of long glucan chains, the faster and to the greater extent it retrogrades into complex structures [41]. During heating and chilling, more amylose or longer branch chains can complex with lipids to form amylose–lipid complexes [42]. These processes that limit the catalytic event are associated with increased amylose content levels.

Table 1.
Resistant starch content of native and modified high-amylose starches.
Table 1.
Resistant starch content of native and modified high-amylose starches.
| Approach to Improve Amylose Content | Botanical Sources (Amylose Content, %) | Reference | Approach to Improve Amylose Content | Digestion Model Used | Resistant Starch Content (%) |
|---|---|---|---|---|---|
| Biological approach | Wheat (downregulated SS iIa: 34.0%→43.5%/ downregulated SBE iIa: 33.3%→57.8%); food processing into pasta | [43] | Downregulated SSIIa or SBE lla | In vitro model (pepsin, amyloglucosidase) | ↑1.33% (downregulated SS iIa)/↑7.15% (downregulated SBE iIa) |
| Wheat (bread wheat: 22.9%→55.7%/durum wheat: 24.4%→47.4%); milled | [26] | Downregulated SBE iIa | In vitro model (pancreatic alpha-amylase, amyloglucosidase) | ↑10.38% (bread wheat)/↑4.63% (durum wheat) | |
| Wheat (27.2%→84.2%) | [44] | Downregulated SBEs (IIa, IIb) | NA | ↑36.00% | |
| Wheat (27.9%→44.9%) | [45] | Downregulated SBEs (IIa, IIb) | Resistant starch kit | ↑5.50% | |
| Wheat (30.7%→50.0%) | [46] | Downregulated SBEs (IIa, IIb) | Resistant starch kit | ↑2.43% | |
| Wheat (23.0%→31.4%); cooked | [15] | Downregulated SBE (IIa) | In vitro model (pancreatic alpha-amylase, glucoamylase) | ↑ | |
| Wheat (32.3%→61.8%) | [47] | Targeted mutagenesis of SBEIIa | In vitro model (pancreatic alpha-amylase, amyloglucosidase) | ↑13.30% | |
| Rice (19.6%→41.2%); cooked | [20] | Downregulated SBE IIb | In vitro model (simulated oral phase, gastric phase, and intestinal phase) | ↑4.60% | |
| Rice (27.2%→64.8%) | [48] | Downregulated SBE I, SBE IIb | In vitro model (pancreatic alpha-amylase, amyloglucosidase) | ↑15.10% | |
| Chemical approach | Rice (30.6%) | [49] | Acid and heat-moisture treatments (citric acid/lactic acid/acetic acid) | In vitro model (alpha-amylase, amyloglucosidase) | ↑32.70% (citric acid) /↑28.80% (lactic acid) /↑26.70% (acetic acid) |
| Waxy rice (0.0%→30.3%); cooked (before pullulanase debranching) | [50] | Enzymatic modification (pullulanase) | In vitro model (pancreatic alpha-amylase, amyloglucosidase) | ↑28.28% | |
| Rice (enzymatic modification: 42.7%→49.2%/heat-moisture treatment: 42.7%→43.2%/acid treatment: 42.7%→43.9%) | [51] | Enzymatic modification (pullulanase)/heat-moisture treatment/acid treatment (citric acid) | In vitro model (pepsin, alpha-amylase, amyloglucosidase) | ↑6.26% (enzymatic modification)/↑2.52% (heat-moisture treatment)/↑10.77% (acid treatment) | |
| Maize (58.6%→58.5%) | [52] | Enzymatic modification (maltogenic alpha-amylase) | In vitro model (pancreatin, amyloglucosidase) | ↑1.00% | |
| Wheat (26.2%→35.6%) | [53] | Annealing (50 °C, 96 h, 1:3 w/v) | In vitro model (saccharifying enzyme, pancreatic alpha-amylase) | ↑4.61% | |
| Physical approach | Maize (85.0%→83.7%) | [54] | Annealing (45 °C, 72 h, 1:2 w/v) | In vitro model (pancreatic alpha-amylase, amyloglucosidase) | ↑0.60% |
| Maize (51.3%→49.1%) | [55] | Microwave (2450 MHz, 1.2 kW, 1 min) | In vitro model (pancreatin, amyloglucosidase) | ↑14.10% | |
| Barley (23.1%→23.4%) | [56] | Heat-moisture treatment (110 °C, 2 h, 30% moisture content) | In vitro model (pancreatin, amyloglucosidase) | ↑11.35% | |
| Quinoa (9.1%→16.4%) | [57] | Electron beam irradiation (8 kGy, 2 kGy/h) | In vitro model (pancreatin, amyloglucosidase) | ↑23.50% | |
| Potato (25.0%→28.8%) | [58] | Sonication (28 kHz, 300 W, 30 min, 25 °C) | In vitro model (pancreatic alpha-amylase, amyloglucosidase) | ↑ | |
| Lotus stem (41.9%→49.1%) | [59] | Sonication (20 kHz, 400 W, 35 min, 0 °C) | In vitro model (pancreatic alpha-amylase, amyloglucosidase) | ↑7.99% |
SBE: starch-branching enzyme; SS: starch synthases.
4. Colonic Fermentation of High-Amylose Starch and Nutritional Properties
The fermentation of high-amylose starch in the colon produces short-chain fatty acids, consisting primarily of acetate, propionate, and butyrate, which are absorbed by the colonic mucosa and can have various beneficial effects on the host [60,61]. This fermentation process has the potential to increase glucose tolerance and insulin sensitivity, decrease inflammation, and improve intestinal barrier integrity [62,63,64]. The nutritional properties of high-amylose starch could be affected by its specific structural changes (e.g., the degree of cross-linking, the degree of substitution, and the degree of association with other molecules) due to chemical modifications [65,66] and its interactions with other food components [67]. The resistance of high-amylose starch against human digestive enzymes may be relevant to its resistance against microbial degradative enzymes, which may influence its fermentation rate and extent, as well as shifts in the microbiota and fermentation products (Figure 1). However, there is limited information on the role of multilevel structural features of high-amylose starches in determining their fermentation properties. In this section, previous results from in vitro fermentation models, in vivo studies, and dietary intervention studies are summarized to provide an overview of the fermentation properties and nutritional functionality of high-amylose starches with different structural features.
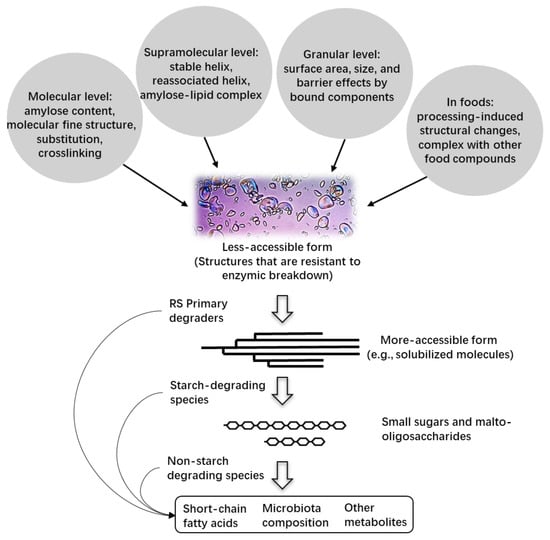
Figure 1.
Schematic illustration of high-amylose starch (a type of resistant starch) fermented by the colonic microbiota. Primary degraders break down resistant starch (RS), releasing soluble starch molecules that can be used by starch-degrading species. This process may also increase the surface area of RS granules, allowing other starch-degrading species to access and utilize this energy source. Smaller sugars and malto-oligosaccharides released during the processes could support the growth of non-starch degrading species. The figure was adapted from [63].
4.1. In Vitro Studies and Animal Studies
Fermentation is a complex metabolic process by which organisms convert carbohydrates into various biochemicals. Two major groups of biochemicals that are produced by fermentation are short-chain fatty acids (SCFAs) and gases, while the most common volatile fatty acids produced during the fermentation of carbohydrates are acetate, propionate, and butyrate [68,69]. The relationship between starch structure and SCFA production is often a focus of the in vitro fermentation studies of high-amylose starches (Table 2). Starch with elevated amylose content leads to increased production of SCFAs [70,71,72,73] and significant changes in the relative abundance of specific bacterial populations [61,67,71,72,73,74,75]. Animal studies have consistently demonstrated that the levels and total amount of SCFAs produced during fermentation are higher when high-amylose starch is used compared to regular starch [76,77,78,79,80,81,82,83,84,85,86]. As shown in Table 2, there is also evidence that feeding animals high-amylose starch can increase the relative abundance of specific bacterial populations, such as Bifidobacterium and Lactobacillus [78,85]. This effect may be due to the indigestible fraction of high-amylose starch favoring the production of SCFAs, which in turn lowers the colonic pH. The resulting changes in pH create an environment conducive to the growth and reproduction of specific bacterial populations, such as RS primary degraders and starch-degrading species. High-amylose starch, especially with an amylose content of 93%, has been observed to exhibit a notable reduction in cumulative gas production at 72 h and the fastest rate of gas production during in vitro fermentation [61]. The result is consistent with the previous research on chemically modified high-amylose starch by cross-linking [74]. However, no significant effects on gas production were observed in propionylated starch [72,75]. Furthermore, the presence of ammonium, which is generally used as an indicator for protein fermentation, was also recorded in in vitro fermentation of high-amylose starch. As Bui et al. (2020) reported, there was no discernible difference in end-point NH4+ production among wheat starches varying in amylose content.
The fermentation properties of high-amylose starches also depend on the plant sources [79]. This may be due to differences in structural features controlled by starch biosynthesis in different plants, including those noted in Figure 1. Additionally, modifying high-amylose starch through chemical methods can further increase the production of specific SCFAs, with the production depending on the degree of substitution [72,82]. This may be because acetyl groups from acetylated starch were released by gut microbiota [87,88]. Lower pH created by acetylated starch is more conducive to the growth of SCFA-producing species. Another possible explanation is that improved starch resistance against the host enzymes can result in a greater proportion of undigested starch residues reaching the colon. Changes in in vitro fermentation properties were also reported in cross-linked high-amylose maize starches [74] and processed forms (cooked and cooked–cooled) [71].
The changing patterns of molecular structure in the in vitro fermentation by microbial enzymes were found to differ from those of the remnant starch obtained from in vitro digestion by pancreatic α-amylase [89]. This observation implies that molecular structural features that contribute to slow degradation during small intestinal digestion are not the same as those that affect colonic fermentation, likely due to the difference in enzyme types and combinations. However, the structural basis for resistant starch fermentability by gut microbiota was not fully understood. While there is a wealth of knowledge on the relationship between starch structural features and enzymatic breakdown, there are still gaps in our understanding of how these features impact the complex process of fermentation in the lower gut, which involves multiple amylolytic enzymes and other fiber-degrading mechanisms. More in-depth structural characterization throughout the digestion and fermentation processes of starches in relation to various RS-forming mechanisms will help to bridge the gaps [90,91].
Animal studies (Table 2) also showed changes in SCFA production and microbiota in the fecal matter of animals fed with high-amylose starch. Moreover, rats fed high-amylose starch have higher levels of fasting plasma glucagon-like peptide-1 (GLP-1) than those fed ordinary starch [84]. This hormone stimulates insulin secretion and regulates food intake, which are crucial in the management of diabetes and obesity. High-amylose starch was also shown to inhibit colon cell proliferation in rats, potentially lowering the risk of colon cancer [92].

Table 2.
Short-chain fatty acid (SCFA) and gas production and microbiota shift of high-amylose starches from in vitro fermentation and animal studies.
Table 2.
Short-chain fatty acid (SCFA) and gas production and microbiota shift of high-amylose starches from in vitro fermentation and animal studies.
| Fermentation Studies | Botanical Sources (Amylose Content, %) | Reference | Approach to Improve Amylose Content | Fermentation Models Used | Fermentation Properties (SCFA, Gas Production, and Microbiota Composition Changes) |
|---|---|---|---|---|---|
| Animal models | Maize (70%) | [84] | NA | In vivo fermentation model (7-week-old male rats) | Butyrate: ↑9.85 µmol per cecal content Acetate: ↑109.0 µmol per cecal content Propionate: ↑15.3 µmol per cecal content Total: ↑135.0 µmol per cecal content (cecal content, than low-amylose group) |
| Maize (63.3%) | [86] | NA | In vivo fermentation model (4-week-old male rats) | Butyrate: ↑10.1 µmol/g wet matter Acetate: ↑40.0 µmol/g wet matter Propionate: ↑10.3 µmol/g wet matter Total: ↑58.9 µmol/g wet matter Bacteroidetes ↑ (cecal content, than low-amylose group) | |
| Maize (85%) | [76] | NA | In vivo fermentation model (adult male rats) | Butyrate: ↑26.0 µmol Acetate: ↑225.0 µmol Propionate: ↑21.0 µmol Total: ↑276.0 µmol (cecal content, than low-amylose group) | |
| Maize (85%) | [78] | NA | In vivo fermentation model (4-week-old pigs) | Butyrate: ↑ Acetate: ↑ Propionate: ↑ Total: ↑ Total anaerobes↑, Lactobacillus ↑ (colonic digesta, than low-amylose group) | |
| Maize (80%) | [85] | NA | In vivo fermentation model (ileal-cannulated pigs) | Butyrate: ↑0.52 µmol/g wet matter Acetate: ↓0.7 µmol/g wet matter Propionate: ↑1.49 µmol/g wet matter Total: ↑8.5 µmol/g wet matter Bifidobacterium ↑ (feces, than low-amylose group) | |
| High-amylose maize | [82] | NA | In vivo fermentation model (male rats) | Butyrate: ↑ Acetate: ↑ Propionate: ↑ Total: ↑ Proteobacteria↓, Bacteroidetes↑ (feces, than low-amylose group) | |
| High-amylose maize | [93] | NA | In vivo fermentation model (adult male rats) | Butyrate: ↑ Acetate: ↑ Propionate: ↑ Total: ↑ (feces, than low-amylose group) | |
| High-amylose maize | [81] | NA | In vivo fermentation model (5-week-old male rats) | Butyrate: ↑3.0 μmol/g feces Acetate: ↑17.7 μmol/g feces Propionate: ↑7.0 μmol/g feces Total: ↑28.9 μmol/g feces (than low-amylose group) | |
| High-amylose maize | [83] | NA | In vivo fermentation model (adult male rats) | Butyrate: ↑24.9 µmol Acetate: ↑120.2 µmol Propionate: ↑38.9 µmol Total: ↑184.8 µmol (cecal content, than low-amylose group) | |
| High-amylose wheat/high-amylose maize | [79] | NA | In vivo fermentation model (male rats) | Butyrate: ↑9.0 µmol (wheat)/↑4.0 µmol (maize) Acetate: ↑21.0 µmol (wheat)/↑8.0 µmol (maize) Propionate: ↑12.0 µmol (wheat)/↑3.0 µmol (maize) Total: ↑44.0 µmol (wheat)/↑17.0 µmol (maize) (colonic digesta, than low-amylose group) | |
| Wheat (25.5%→74.4%) | [27] | Downregulated SBEs (IIa, IIb) | In vivo fermentation model (4-week-old male rats) | Butyrate: ↓2.8 mmol/kg Acetate: ↑5.0 mmol/kg Propionate: ↑3.9 mmol/kg Total: ↑4.5 mmol/kg (cecal digesta, than low-amylose group) | |
| Wheat (27.9%→44.9%) | [45] | Downregulated SBEs (IIa, IIb) | In vivo fermentation model (8-week-old rats) | Butyrate: ↑11.7 µmol Acetate: ↑79.8 µmol Propionate: ↑28.2 µmol Total: ↑119.7 µmol (cecal content, than wild-type starch) | |
| Rice (27.2%→64.8%) | [48] | Downregulated SBEs (I, IIb) | In vivo fermentation model (feeding rats for four weeks) | Butyrate: ↑11.9 μmol/g dry feces Acetate: ↑60.5 μmol/g dry feces Propionate: ↑68.1 μmol/g dry feces Total: ↑140.6 μmol/g dry feces (feces, than wild-type starch) | |
| Barley (41%) | [80] | NA | In vivo fermentation model (4-week-old pigs) | Butyrate: ↓4% Acetate: ↓6.4% Propionate: ↑7.9% Total: ↑19 mMol/kg digesta sample NH3: ↑1 mMol/kg digesta sample (colonic digesta, than low-amylose group) | |
| High-amylose barley | [77] | NA | In vivo fermentation model (adult male rats) | Butyrate: ↑12.0 µmol Acetate: ↑142.0 µmol Propionate: ↑9.0 µmol Total: ↑161.0 µmol (feces, than low-amylose group) | |
| In vitro models | High-amylose maize | [72] | Propionylated | In vitro fermentation model (bacteroides-dominated enterotype inocula) | Butyrate: ↓1.0 mM/50 mg dry feces Acetate: ↑0.2 mM/50 mg dry feces Propionate: ↑0.7 mM/50 mg dry feces Total: ↑2.4 mM/50 mg dry feces Bacteroidetes ↑ Gas production → (than unmodified starch) |
| High-amylose maize; Cooked | [71] | NA | In vitro fermentation model (inoculum from human stool samples) | Butyrate: ↑ Acetate: ↑ Propionate: ↑ Total: ↑ (than low-amylose group) | |
| High-amylose maize | [70] | Debranched (pullulanase), recrystallization combined with HMT | In vitro fermentation model (inoculum from human stool samples) | Butyrate: ↑2.4 mM Acetate: ↑1.8 mM Propionate: ↑2.7 mM Total: ↑7.4 mM (fermentation for 24 h, than unmodified starch) | |
| High-amylose maize; cooked, recrystallized, and amylase digested | [75] | NA | In vitro fermentation model (inoculum from pig fecal samples) | Butyrate: → Acetate: → Propionate: → Total: ↓0.6 mmol/g dry matter DMCV: ↑14 mL, Rmax: ↓0.8 mL/h NH3 production: ↓ (fermentation for 146 h, than cooked and recrystallized group) | |
| Maize (69.8%) | [73] | Propionylated | In vitro fermentation model (inoculum from human stool samples) | Butyrate: ↓ Acetate: ↑ Propionate: ↑ Total: ↑ Firmicutes ↑, Bacteroidetes: ↓ Gas production → (fermentation for 24 h, than unmodified starch) | |
| Maize (65.0%) | [74] | Chemical cross-linking | In vitro fermentation model (inoculum from human stool samples) | Butyrate: ↓ Acetate: ↑ Propionate: → Total: ↓ Firmicutes/Bacteroidetes ratio: ↓ Gas production: ↓ (fermentation for 24 h, than unmodified starch) | |
| Wheat (32%→84%) | [67] | Downregulated SBEs (IIa, IIb) | In vitro fermentation model (using wheat-based foods and inoculum from human stool samples) | Butyrate: ↓ Acetate: ↓ Propionate: ↑ Total: ↓ DMCV: ↓10 mL/gDM, Rmax: ↓ (fermentation for 48 h, than wild-type starch) | |
| Wheat (37%→93%) | [61] | Downregulated SBEs (IIa, IIb) | In vitro fermentation model (inoculum from human stool samples) | Butyrate: ↓ Acetate: ↓ Propionate: ↓ Total: ↓ DMCV: ↓55 mL/g DM, Rmax: ↓6.3 mL/h, NH4+ production: no difference (fermentation for 72 h, than wild-type starch) |
DMCV: the total cumulative gas values per gram dry matter; DM: dry matter; HMT: heat–moisture treatment; SCFA: short-chain fatty acids; Rmax: the fastest rate of gas production. NA: not applicable.
4.2. Dietary Intervention Studies
Dietary intervention studies have shown the effects of high-amylose starch on glucose and insulin homeostasis and fecal composition (including SCFA content and microbial composition) (Table 3). High amylose from wheat, maize, barley, etc., has been shown to improve the production of SCFAs [94,95,96,97,98]. It is important to note that the SCFA levels present in feces do not necessarily reflect those found in the colon. This is due to the fact that SCFAs are produced through the fermentation process in the large intestine, and the composition of the gut microbiome can vary greatly between the upper and lower sections of the colon. Furthermore, SCFAs can be absorbed and metabolized by the colonic epithelium and gut microbiota before the excursion within the feces, and therefore measuring SCFA levels in feces alone may not provide a complete understanding of SCFA production and metabolism throughout the entire colon.
There is conflicting evidence regarding the effects of resistant starch type 2 from high-amylose starch on acute postprandial responses and insulin resistance and/or sensitivity. There are several studies indicating that resistant starch type 2 from high-amylose starch can attenuate acute postprandial responses [96,97,99,100,101,102,103,104,105,106,107,108], while no effects were also reported [109,110,111]. While several studies concluded that high-amylose starch has no effects on insulin resistance and/or sensitivity [106,112,113], a limited extent of changes was reported [96,97,100,101,104,107,108,111,114]. The effect of high-amylose starch on glucose and insulin homeostasis was also evaluated previously, with the conclusion that there is insufficient evidence to conclude that it can improve insulin resistance and/or sensitivity [115]. In addition, high-amylose starches have been shown to have positive effects on bowel movement, increasing stool frequency and volume [94,95,96,102,104]. In particular, an increased concentration of butyrate was reported in the stool, which supports the growth of intestinal epithelial cells and creates a protective environment in the colon [95,96]. Similarly, a high-amylose starch-rich diet increased the abundance of SCFA-producing bacteria in the stool [94]. It should be noted that the information provided in Table 3, which is related to the variations in glucose levels, insulin levels, and glycemic responses, primarily in healthy subjects, is inadequate to yield a comprehensive understanding of the clinical significance of these changes. Additionally, the possible health implications of these observations, such as enhanced diabetes management or modified weight loss outcomes, are not adequately explored in the current studies. Future research is needed to bridge these gaps in knowledge, in order to gain a holistic understanding of the nutritional implication of these findings.

Table 3.
Colonic fermentation of high-amylose starches and results from dietary intervention studies.
Table 3.
Colonic fermentation of high-amylose starches and results from dietary intervention studies.
| Botanical Sources | Reference | Modifications or Food Processing | Study Design | Results | |
|---|---|---|---|---|---|
| Wheat | High-amylose wheat | [94] | Bread/biscuits with low-amylose wheat or low-amylose wheat refined, high-amylose wheat or high-amylose wheat refined | Healthy subjects (n = 80), random, double-blinded, 4-arm parallel | Fecal butyrate excretion: ↑38% (p < 0.05, refined high-amylose wheat vs. refined low-amylose wheat) Abundance of fecal SCFA-producing bacteria: ↑Roseburia inulinivorans (p < 0.001) of refined high-amylose wheat than at baseline (week 0) |
| High-amylose maize flour (amylose content = 67%) | [105] | Cookies with high-amylose maize flour, low-amylose maize flour, or 100% wheat flour | Healthy men (n = 30), random, double-blinded | Cumulative postprandial glycemic AUC (0 to 200 min): ↓0.18 mmol/L (p < 0.05) Cumulative blood glucose AUC (0 to 200 min): ↓75.53 mmol×min/L (p < 0.05) (than 100% wheat flour) | |
| High-amylose wheat (amylose content = 74.3%) | [100] | Bread containing low-amylose wheat or low-amylose wheat refined, high-amylose wheat or high-amylose wheat refined | Healthy subjects (n = 20), random, double-blinded | Postprandial glycemic AUC: ↓25 mmol/L×3 h (p < 0.001) Plasma glucose concentration: ↓33% Glycemic AUC response: ↓39% (p < 0.0001) Insulinemic AUC response: ↓24–30% (p < 0.05) (than low-amylose group) Whole-meal or refined flour did not affect the glycemic, insulinemic, or incretin response | |
| Maize | High-amylose maize (amylose content = 33%) | [96] | Muffins with low or high (RS2 20 g) amylose maize starch | Hypertriglyceridemia subjects (n = 23), random | Postprandial glucose area: ↓0.25 mmol × min/L SCFA concentrations in fecal water: ↑32% (p < 0.001) Overall postprandial plasma insulin concentration: ↓17% Postprandial insulin area: ↓62 pmol × min/L (p < 0.01) Frequency of bowel actions: ↑0.2 actions/day (than low-amylose group) |
| High-amylose maize starch | [95] | Supplements containing HAMS (RS 30 g/day) or low-fiber content | Healthy subjects (n = 24), random | Fecal bulk: ↑22 g/day (p < 0.001) Fecal SCFA output: ↑2.32 mmol/day Fecal SCFA concentration: ↑5.3 mmol/L Fecal butyrate: SCFA ratio significantly 31% (p = 0.035) (than low-fiber supplement) | |
| High-amylose maize starch | [102] | Breakfast with amylopectin starch plus cellulose, amylopectin starch plus lactulose, or high-amylose starch plus cellulose | Healthy subjects (n = 10), random, single-blinded | PPGR: ↓ Glucose tolerance: ↑ Non-esterified fatty acids concentrations: ↑ (high-amylose starch plus cellulose vs. amylopectin starch plus cellulose group) | |
| High-amylose maize starch | [116] | Sachets containing waxy maize starch (Amioca) or HAMS (RS2 30 g/day) | Healthy subjects (n = 10), random, single-blinded | SCFA concentrations (acetate and propionate): ↑ (not colonic SCFAs) Plasma insulin response: ↓ No effect on Postprandial glycemic AUC, HOMA, and FBG than waxy maize starch | |
| High-RS maize starch | [111] | Bread with or without HAMS | Healthy subjects (n = 15), random | Blood glucose incremental AUC: ↓44.4 mmol × min/L Insulin incremental AUC: ↓4.8 pmol × min/L (p < 0.05) Colonic fermentation in the late postprandial phase: ↑23.3 ± 4.5 ppm (breath H2) No effect on PPGR (than white wheat flour bread) | |
| High-amylose maize starch (resistant starch content = 60%) | [112] | A test breakfast and lunch containing either 48 g RS or not | Healthy males (n = 10), random, single-blinded, cross-over | PPGR: ↓ AUC C-peptide: ↑26,457 nmol/L 300 min AUC Insulin: ↑40,714 pmol/L 300 min (than breakfast and lunch without RS) | |
| High-amylose maize starch | [103] | Chinese steamed bun formulation containing either HAMS or not | Healthy females (n = 15), random, single-blinded | PPGR: ↓80.9 mmol × min/L (p = 0.004) Mean GI: ↓30.07 Incremental postprandial glycemic AUC values: ↓ (than wheat flour without HAMS) | |
| High-amylose maize | [110] | Sachets containing waxy maize starch (Amioca) or HAMS (RS2 15 or 30 g/day) | Obese subjects (n = 33), random, double-blinded | FSIVGTT: ↑in men (n = 11) Fecal SCFA output: ↑ No effect on HOMA and FBG (than waxy maize starch) | |
| High-amylose maize starch | [97] | Bread with or without HAMS (RS2 12 g/day) | Obese subjects (n = 15), random, subject-blinded | Fecal acetate: ↓6.87 μmol/L Fecal propionate: ↑0.82 μmol/L Fecal butyrate: ↑0.08 μmol/L (than bread without HAMS) | |
| High-amylose maize | [108] | Sachets containing waxy maize starch or HAMS (RS2 40 g/day) | Insulin-resistant subjects (n = 15), random, controlled | HOMA for insulin resistance: ↓ (p = 0.029) Fasting insulin: ↓21 pmol/liter (p = 0.041) FBG: ↓(p = 0.017) Postprandial glucose disposal: ↑65% (than waxy maize starch) | |
| High-amylose maize | [113] | Cookies containing waxy maize starch or HAMS (RS2 15 or 30 g/day) | Healthy and non-diabetic women (n = 51; 23 women completed all 3 arms), random, placebo-controlled, double-blinded, cross-over | Insulin resistant group (n = 14): insulin sensitivity after consuming HAMS 30 g/day ↑(than HAMS 15 g/day); Insulin sensitive group (n = 9): no significant difference (p > 0.05) Both groups: no significant differences in fasting glucose or insulin after consuming HAMS 15 or 30 g/day (than waxy maize starch) | |
| High-amylose maize starch | [114] | Bagel containing only hard wheat flour or 60% substitution of HAMS (RS2 25 g/day) | Subjects at high risk of type 2 diabetes (n = 24), random, double-blinded | HOMA for insulin resistance: ↓ Insulin incremental AUC: ↓18.9% (p = 0.04) (than hard wheat flour) | |
| High-amylose maize | [106] | Muffins with or without HAM (RS2 30 or 0 g/day) | Overweight, healthy adults (n = 18), randomized-controlled, parallel-arm, double-blinded | Postprandial glycemic AUC: ↓ Glucose homeostasis: ↑ Insulin change not observed (than muffins without HAM) | |
| High-amylose maize starch | [107] | Muffin top containing modified HAMS (RS4) or not | Healthy adults (n = 12), random, double-blinded, controlled | Postprandial glycemic AUC: ↓33% (p = 0.037) Maximum glucose concentration: ↓8% Postprandial serum insulin incremental AUC: ↓38% (p < 0.001) (than control) | |
| High-amylose maize | [101] | Sachets containing waxy maize starch or HAMS (RS2 40 g/ day) | Insulin-resistant, obese subjects (n = 12), random, single-blinded | Insulin and C-peptide concentrations: ↑ Glucose effectiveness:↓0.01/min (p = 0.06) (than waxy maize starch) | |
| High-amylose maize starch | [99] | Soups with 50 g maltodextrin, whole grain (RS 27 g/day), high-amylose (RS 23 g/day), regular cornstarch, or no added starch | Healthy men (n = 17), random | Postprandial glycemic AUC: ↓ (than soups without added starch) | |
| Barley | High-amylose maize | [104] | Breakfast and lunch containing barley flour (more HAM) or white wheat flour (without HAM) | Healthy women (n = 14), random, single-blinded | Postprandial glycemic AUC: ↓22% AUC of insulin: ↓32% No effect on HOMA and FBG (than white wheat flour) |
| High-starch amylose (amylose content = 42%) | [109] | Barley tortillas varying in fiber and/or starch composition | Healthy adults (n = 12), random, double-blinded | No significant difference in GI between the low-amylose and high-amylose groups No difference in the glucose iAUC or percentage change at 30 min between the low-amylose and high-amylose groups Insulin concentrations at 15–60 min: ↑ all groups | |
AUC: area under the curve; GLP-1: glucagon-like peptide-1; GI: Glycemic index; HAMS: high-amylose maize starch; HOMA: homeostasis model assessment; FBG: fasting blood sugar level; FSIVGTT: frequently sampled intravenous glucose tolerance test for insulin sensitivity; PPGR: postprandial glucose response; SCFA: short chain fatty acids.
5. Conclusions
This mini-review highlights the fact that not all high-amylose starches (a type of resistant starch) are functionally similar, and the structural differences of high-amylose starch can impact its utilization by gut microbiota and, therefore, its fermentation properties and nutritional functionality. Because there are large inter-individual differences in the microbiome, it is unlikely that there is a single optimal structure and dosage of high-amylose starch that will be effective for all individuals. In addition, diet plays a significant role in shaping the composition of the gut microbiota [117], and changes in the microbiota can affect the fermentation of high-amylose starch. Therefore, future studies need a better understanding of how the structural features of high-amylose starch and those relevant to processing and modifications impact the gut microbiota, and the association between microbiota composition and fermentation products of high-amylose starch. This may allow the design of personalized resistant starch with desirable fermentability to promote certain microbiota groups for specific nutritional purposes. A better understanding of the link between starch structural features and beneficial nutritional functionality will also enable the selection of more nutritious grains at the stage of breeding and food processing.
Author Contributions
Conceptualization, H.-T.L.; investigation, W.Z. and H.Z.; writing—original draft preparation, H.-T.L., W.Z. and H.Z.; writing—review and editing, H.-T.L., C.C. and Q.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Open Project Program of the State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science and Technology (No. SKLFNS-KF-202110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Hai-Teng Li acknowledges the Natural Science Foundation of Jiangsu Province (BK20210749) and the Fellowship of China Postdoctoral Science Foundation (No.2021M701481).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of whole grain foods and risk of type 2 diabetes: Results from three prospective cohort studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Li, Z.; Fox, G.P.; Gidley, M.J.; Dhital, S. Protein-starch matrix plays a key role in enzymic digestion of high-amylose wheat noodle. Food Chem. 2021, 336, 127719. [Google Scholar] [CrossRef]
- Reyniers, S.; De Brier, N.; Ooms, N.; Matthijs, S.; Piovesan, A.; Verboven, P.; Brijs, K.; Gilbert, R.G.; Delcour, J.A. Amylose molecular fine structure dictates water–oil dynamics during deep-frying and the caloric density of potato crisps. Nat. Food 2020, 1, 736–745. [Google Scholar] [CrossRef]
- Li, C.; Dhital, S.; Gidley, M.J. High-amylose wheat bread with reduced in vitro digestion rate and enhanced resistant starch content. Food Hydrocoll. 2021, 123, 107181. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Li, H.-T.; Gidley, M.J.; Dhital, S. High-amylose starches to bridge the “fiber gap”: Development, structure, and nutritional functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef]
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.R.; Regina, A. High amylose wheat: A platform for delivering human health benefits. J. Cereal Sci. 2018, 82, 99–105. [Google Scholar] [CrossRef]
- Tizzotti, M.J.; Sweedman, M.C.; Tang, D.; Schaefer, C.; Gilbert, R.G. New 1H NMR Procedure for the Characterization of Native and Modified Food-Grade Starches. J. Agric. Food Chem. 2011, 59, 6913–6919. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-C.; Capitani, T.; Trzasko, P.; Jeffcoat, R. Molecular structure of a low-amylopectin starch and other high-amylose maize starches. J. Cereal Sci. 1998, 27, 289–299. [Google Scholar] [CrossRef]
- Shimbata, T.; Ai, Y.; Fujita, M.; Inokuma, T.; Vrinten, P.; Sunohara, A.; Saito, M.; Takiya, T.; Jane, J.-L.; Nakamura, T. Effects of homoeologous wheat starch synthase IIa genes on starch properties. J. Agric. Food Chem. 2012, 60, 12004–12010. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, F.; Meng, D.; Hasjim, J.; Gilbert, R.G. Two-dimensional macromolecular distributions reveal detailed architectural features in high-amylose starches. Carbohydr. Polym. 2014, 113, 539–551. [Google Scholar] [CrossRef]
- Jane, J.-L. Current understanding on starch granule structures. J. Appl. Glycosci. 2006, 53, 205–213. [Google Scholar] [CrossRef]
- Li, H.-T.; Dhital, S.; Flanagan, B.M.; Mata, J.; Gilbert, E.P.; Gidley, M.J. High-amylose wheat and maize starches have distinctly different granule organization and annealing behaviour: A key role for chain mobility. Food Hydrocoll. 2020, 105, 105820. [Google Scholar] [CrossRef]
- Wang, T.; Bogracheva, T.; Hedley, C. Starch: As simple as A, B, C? J. Exp. Bot. 1998, 49, 481–502. [Google Scholar] [CrossRef]
- Butardo, V.M.; Fitzgerald, M.A.; Bird, A.R.; Gidley, M.J.; Flanagan, B.M.; Larroque, O.; Resurreccion, A.P.; Laidlaw, H.K.C.; Jobling, S.A.; Morell, M.K.; et al. Impact of down-regulation of starch branching enzyme IIb in rice by artificial micro RNA- and hairpin RNA- mediated RNA silencing. J. Exp. Bot. 2011, 62, 4927–4941. [Google Scholar] [CrossRef]
- Huang, J.; Shang, Z.; Man, J.; Liu, Q.; Zhu, C.; Wei, C. Comparison of molecular structures and functional properties of high-amylose starches from rice transgenic line and commercial maize. Food Hydrocoll. 2015, 46, 172–179. [Google Scholar] [CrossRef]
- Regina, A.; Blazek, J.; Gilbert, E.; Flanagan, B.M.; Gidley, M.J.; Cavanagh, C.; Ral, J.-P.; Larroque, O.; Bird, A.R.; Li, Z.; et al. Differential effects of genetically distinct mechanisms of elevating amylose on barley starch characteristics. Carbohydr. Polym. 2012, 89, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Dhital, S.; Slade, A.J.; Yu, W.; Gilbert, R.G.; Gidley, M.J. Altering starch branching enzymes in wheat generates high-amylose starch with novel molecular structure and functional properties. Food Hydrocoll. 2019, 92, 51–59. [Google Scholar] [CrossRef]
- Yamamori, M.; Fujita, S.; Hayakawa, K.; Matsuki, J.; Yasui, T. Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Int. J. Plant Breed. Res. 2000, 101, 21–29. [Google Scholar] [CrossRef]
- Blazek, J.; Salman, H.; Rubio, A.L.; Gilbert, E.; Hanley, T.; Copeland, L. Structural characterization of wheat starch granules differing in amylose content and functional characteristics. Carbohydr. Polym. 2009, 75, 705–711. [Google Scholar] [CrossRef]
- Slade, A.J.; McGuire, C.; Loeffler, D.; Mullenberg, J.; Skinner, W.; Fazio, G.; Holm, A.; Brandt, K.M.; Steine, M.N.; Goodstal, J.F.; et al. Development of high amylose wheat through TILLING. BMC Plant Biol. 2012, 12, 69–85. [Google Scholar] [CrossRef]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef]
- Jiang, H.; Horner, H.T.; Pepper, T.M.; Blanco, M.; Campbell, M.; Jane, J.-L. Formation of elongated starch granules in high-amylose maize. Carbohydr. Polym. 2010, 80, 533–538. [Google Scholar] [CrossRef]
- Wei, C.; Qin, F.; Zhu, L.; Zhou, W.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q. Microstructure and ultrastructure of high-amylose rice resistant starch granules modified by antisense RNA inhibition of starch branching enzyme. J. Agric. Food Chem. 2010, 58, 1224–1232. [Google Scholar] [CrossRef]
- Li, H.-T.; Sartika, R.S.; Kerr, E.D.; Schulz, B.L.; Gidley, M.J.; Dhital, S. Starch granular protein of high-amylose wheat gives innate resistance to amylolysis. Food Chem. 2020, 330, 127328. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, Y.; Qiu, C.; Corke, H.; Sui, Z. Extraction and characterization of starch granule-associated proteins from rice that affect in vitro starch digestibility. Food Chem. 2019, 276, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.K.; Blazek, J.; Flanagan, B.M.; Dhital, S.; Larroque, O.; Morell, M.K.; Gilbert, E.P.; Gidley, M.J. Molecular, mesoscopic and microscopic structure evolution during amylase digestion of extruded maize and high amylose maize starches. Carbohydr. Polym. 2015, 118, 224–234. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Relationship between granule size and in vitro digestibility of maize and potato starches. Carbohydr. Polym. 2010, 82, 480–488. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Buléon, A.; Pérez, S. Physical characteristics of starch granules and susceptibility to enzymatic degradation. Eur. J. Clin. Nutr. 1992, 46, S3–S16. [Google Scholar]
- Zhang, G.; Ao, Z.; Hamaker, B. Slow digestion property of native cereal starches. Biomacromolecules 2006, 7, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Jane, J.-L.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Zhang, G.; Ao, Z.; Hamaker, B.R. Nutritional property of endosperm starches from maize mutants: A parabolic relationship between slowly digestible starch and amylopectin fine structure. J. Agric. Food Chem. 2008, 56, 4686–4694. [Google Scholar] [CrossRef]
- Li, H.-T.; Dhital, S.; Flanagan, B.M.; Mata, J.; Gilbert, E.P.; Gilbert, R.G.; Gidley, M.J. Amorphous packing of amylose and elongated branches linked to the enzymatic resistance of high-amylose wheat starch granules. Carbohydr. Polym. 2022, 295, 119871. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Blazek, J.; Flanagan, B.M.; Dhital, S.; Larroque, O.; Morell, M.K.; Gilbert, E.P.; Gidley, M.J. Molecular, mesoscopic and microscopic structure evolution during amylase digestion of maize starch granules. Carbohydr. Polym. 2012, 90, 23–33. [Google Scholar] [CrossRef]
- Tester, R.F.; Qi, X.; Karkalas, J. Hydrolysis of native starches with amylases. Anim. Feed Sci. Technol. 2006, 130, 39–54. [Google Scholar] [CrossRef]
- Gidley, M.J. Factors affecting the crystalline type (A-C) of native starches and model compounds: A rationalisation of observed effects in terms of polymorphic structures. Carbohydr. Res. 1987, 161, 301–304. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Sissons, M.; Sestili, F.; Botticella, E.; Masci, S.; Lafiandra, D. Can manipulation of Durum wheat amylose content reduce the glycaemic index of Spaghetti? Foods 2020, 9, 693. [Google Scholar] [CrossRef]
- Regina, A.; Berbezy, P.; Kosar-Hashemi, B.; Li, S.; Cmiel, M.; Larroque, O.; Bird, A.R.; Swain, S.M.; Cavanagh, C.; Jobling, S.A.; et al. A genetic strategy generating wheat with very high amylose content. Plant Biotechnol. J. 2015, 13, 1276–1286. [Google Scholar] [CrossRef]
- Hazard, B.; Zhang, X.; Naemeh, M.; Hamilton, M.K.; Rust, B.; Raybould, H.E.; Newman, J.W.; Martin, R.; Dubcovsky, J. Mutations in durum wheat genes affect grain yield components, quality, and fermentation responses in rats. Crop Sci. 2015, 55, 2813. [Google Scholar] [CrossRef]
- Schonhofen, A.; Hazard, B.; Zhang, X.; Dubcovsky, J. Registration of common wheat germplasm with mutations in SBEII genes conferring increased grain amylose and resistant starch content. J. Plant Regist. 2016, 10, 200–205. [Google Scholar] [CrossRef]
- Li, J.; Jiao, G.; Sun, Y.; Chen, J.; Zhong, Y.; Yan, L.; Jiang, D.; Ma, Y.; Xia, L. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol. 2020, 19, 937–951. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, M.; Meng, X.; Cheung, S.C.K.; Yu, H.; Huang, J.; Sun, Y.; Shi, Y.; Liu, Q. High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnol. J. 2012, 10, 353–362. [Google Scholar] [CrossRef]
- Hung, P.V.; Vien, N.L.; Lan Phi, N.T. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73. [Google Scholar] [CrossRef]
- Shi, M.-M.; Gao, Q.-Y. Physicochemical properties, structure and in vitro digestion of resistant starch from waxy rice starch. Carbohydr. Polym. 2011, 84, 1151–1157. [Google Scholar] [CrossRef]
- Bao, J.; Zhou, X.; Hu, Y.; Zhang, Z. Resistant starch content and physicochemical properties of non-waxy rice starches modified by pullulanase, heat-moisture treatment, and citric acid. J. Cereal Sci. 2022, 105, 103472. [Google Scholar] [CrossRef]
- Li, J.; Kong, X.; Ai, Y. Modification of granular waxy, normal and high-amylose maize starches by maltogenic α-amylase to improve functionality. Carbohydr. Polym. 2022, 290, 119503. [Google Scholar] [CrossRef]
- Su, C.; Saleh, A.S.; Zhang, B.; Zhao, K.; Ge, X.; Zhang, Q.; Li, W. Changes in structural, physicochemical, and digestive properties of normal and waxy wheat starch during repeated and continuous annealing. Carbohydr. Polym. 2020, 247, 116675. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yu, J.; Wang, S. A comparative study of annealing of waxy, normal and high-amylose maize starches: The role of amylose molecules. Food Chem. 2014, 164, 332–338. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, W.; Pu, H.; Blennow, A.; Liu, X.; Guo, D. Short-time microwave treatment affects the multi-scale structure and digestive properties of high-amylose maize starch. Int. J. Biol. Macromol. 2019, 137, 870–877. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Luo, X.; Xing, J.; Zhang, Q.; Wang, R.; Wang, L.; Chen, Z. Effects of electron beam irradiation on the physicochemical properties of quinoa and starch microstructure. Starch Stärke 2020, 72, 1900178. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Zhang, B.; Luo, F.; Lin, Q.; Ding, Y. Understanding the aggregation structure, digestive and rheological properties of corn, potato, and pea starches modified by ultrasonic frequency. Int. J. Biol. Macromol. 2021, 189, 1008–1019. [Google Scholar] [CrossRef]
- Noor, N.; Gani, A.; Jhan, F.; Jenno, J.; Dar, M.A. Resistant starch type 2 from lotus stem: Ultrasonic effect on physical and nutraceutical properties. Ultrason. Sonochem. 2021, 76, 105655. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Bui, A.T.; Williams, B.A.; Hoedt, E.C.; Morrison, M.; Mikkelsen, D.; Gidley, M.J. High amylose wheat starch structures display unique fermentability characteristics, microbial community shifts and enzyme degradation profiles. Food Funct. 2020, 11, 5635–5646. [Google Scholar] [CrossRef] [PubMed]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.M.; Photenhauer, A.L.; Pollet, R.M.; Brown, H.A.; Koropatkin, N.M. Starch digestion by gut bacteria: Crowdsourcing for carbs. Trends Microbiol. 2020, 28, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. New view on dietary fiber selection for predictable shifts in gut microbiota. mBio 2020, 11, 2179–2188. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Bui, A.T.; Williams, B.A.; Murtaza, N.; Lisle, A.; Mikkelsen, D.; Morrison, M.; Gidley, M.J. Wheat-based food form has a greater effect than amylose content on fermentation outcomes and microbial community shifts in an in vitro fermentation model. Food Hydrocoll. 2020, 114, 106560. [Google Scholar] [CrossRef]
- Williams, B.A.; Verstegen, M.W.A.; Tamminga, S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 2001, 14, 207–228. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Chang, R.; Jin, Z.; Lu, H.; Qiu, L.; Sun, C.; Tian, Y. Type III resistant starch prepared from debranched starch: Structural changes under simulated saliva, gastric, and intestinal conditions and the impact on short-chain fatty acid production. J. Agric. Food Chem. 2021, 69, 2595–2602. [Google Scholar] [CrossRef]
- Liu, Y.; Matheyambath, A.C.; Polic, I.I.; LaPointe, G. Differential fermentation of raw and processed high-amylose and waxy maize starches in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). J. Funct. Foods 2021, 86, 104735. [Google Scholar] [CrossRef]
- Xie, Z.; Ding, L.; Huang, Q.; Fu, X.; Liu, F.; Dhital, S.; Zhang, B. In vitro colonic fermentation profiles and microbial responses of propionylated high-amylose maize starch by individual Bacteroides-dominated enterotype inocula. Food Res. Int. 2021, 144, 110317. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, S.; Wang, Z.; Fu, X.; Huang, Q.; Yuan, Y.; Wang, K.; Zhang, B. In vitro fecal fermentation of propionylated high-amylose maize starch and its impact on gut microbiota. Carbohydr. Polym. 2019, 223, 115069. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, B.; Chen, T.; Li, C.; Fu, X.; Huang, Q. Chemical cross-linking controls in vitro fecal fermentation rate of high-amylose maize starches and regulates gut microbiota composition. J. Agric. Food Chem. 2019, 67, 13728–13736. [Google Scholar] [CrossRef]
- Warren, F.J.; Fukuma, N.M.; Mikkelsen, D.; Flanagan, B.M.; Williams, B.A.; Lisle, A.T.; Cuiv, P.O.; Morrison, M.; Gidley, M.J. Food starch structure impacts gut microbiome composition. Msphere 2018, 3, e00086-18. [Google Scholar] [CrossRef]
- Bird, A.R.; Brown, I.L.; Topping, D.L. Low and high amylose maize starches acetylated by a commercial or a laboratory process both deliver acetate to the large bowel of rats. Food Hydrocoll. 2006, 20, 1135–1140. [Google Scholar] [CrossRef]
- Bird, A.R.; Flory, C.; Davies, D.A.; Usher, S.; Topping, D.L. A novel barley cultivar (Himalaya 292) with a specific gene mutation in starch synthase lla raises large bowel starch and short-chain fatty acids in rats. J. Nutr. 2004, 134, 831–835. [Google Scholar] [CrossRef]
- Bird, A.R.; Vuaran, M.; Brown, I.; Topping, D.L. Two high-amylose maize starches with different amounts of resistant starch vary in their effects on fermentation, tissue and digesta mass accretion, and bacterial populations in the large bowel of pigs. Br. J. Nutr. 2007, 97, 134–144. [Google Scholar] [CrossRef]
- Conlon, M.A.; Kerr, C.A.; McSweeney, C.S.; Dunne, R.A.; Shaw, J.M.; Kang, S.; Bird, A.R.; Morell, M.K.; Lockett, T.J.; Molloy, P.L.; et al. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a western diet. J. Nutr. 2012, 142, 832–840. [Google Scholar] [CrossRef]
- Jha, R.; Rossnagel, B.; Pieper, R.; Van Kessel, A.; Leterme, P. Barley and oat cultivars with diverse carbohydrate composition alter ileal and total tract nutrient digestibility and fermentation metabolites in weaned piglets. Animal 2010, 4, 724–731. [Google Scholar] [CrossRef]
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Morita, T.; Esterman, A.; Young, G.P. Effect of dietary resistant starch and protein on colonic fermentation and intestinal tumourigenesis in rats. Carcinogenesis 2007, 28, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, F.; Wang, J.; Wang, A.; Yao, X.; Strappe, P.; Zhou, Z.; Wu, Q.; Guo, T. Starch acylation of different short-chain fatty acids and its corresponding influence on gut microbiome and diabetic indexes. Food Chem. 2022, 389, 133089. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Kasaoka, S.; Kiriyama, S.; Brown, I.L.; Topping, D.L. Comparative effects of acetylated and unmodified high-amylose maize starch in rats. Starch-Starke 2005, 57, 246–253. [Google Scholar] [CrossRef]
- Nagata, R.; Kamibayashi, R.; Bochimoto, H.; Fukuma, N.; Shimada, K.; Tachibe, M.; Takaishi, Y.; Han, K.-H.; Fukushima, M. Chemical modification of cornstarch by hydroxypropylation enhances cecal fermentation-mediated lipid metabolism in rats. Starch-Starke 2020, 72, 1900050. [Google Scholar] [CrossRef]
- Regmi, P.R.; Metzler-Zebeli, B.U.; Gaenzle, M.G.; van Kempen, T.A.T.G.; Zijlstra, R.T. Starch with high amylose content and low In vitro digestibility increases intestinal nutrient flow and microbial fermentation and selectively promotes bifidobacteria in pigs. J. Nutr. 2011, 141, 1273–1280. [Google Scholar] [CrossRef]
- Saito, K.; Ito, T.; Kuribayashi, T.; Mochida, K.; Nakakuki, T.; Shibata, M.; Sugawara, M. Effect of raw and heat-moisture treated high-amylose corn starch on fermentation by the rat cecal bacteria. Starch-Starke 2001, 53, 424–430. [Google Scholar] [CrossRef]
- Li, M.; Wang, F.; Wang, J.; Wang, R.; Strappe, P.; Zheng, B.; Zhou, Z.; Chen, L. Manipulation of the internal structure of starch by propionyl treatment and its diverse influence on digestion and in vitro fermentation characteristics. Carbohydr. Polym. 2021, 270, 118390. [Google Scholar] [CrossRef]
- Marino, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef]
- Li, H.-T.; Gilbert, R.G.; Gidley, M.J. Molecular-structure evolution during in vitro fermentation of granular high-amylose wheat starch is different to in vitro digestion. Food Chem. 2021, 362, 130188. [Google Scholar] [CrossRef]
- Petropoulou, K.; Salt, L.J.; Edwards, C.H.; Warren, F.J.; Garcia-Perez, I.; Chambers, E.S.; Alshaalan, R.; Khatib, M.; Perez-Moral, N.; Cross, K.L.; et al. A natural mutation in Pisum sativum L. (pea) alters starch assembly and improves glucose homeostasis in humans. Nat. Food 2020, 1, 693–704. [Google Scholar] [CrossRef]
- Gidley, M.J. Give peas a chance. Nat. Food 2020, 1, 663–664. [Google Scholar] [CrossRef]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. New Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Hu, Y.; Brown, I.L.; Young, G.P. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr. Metab. 2009, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Gondalia, S.V.; Wymond, B.; Benassi-Evans, B.; Berbezy, P.; Bird, A.R.; Belobrajdic, D.P. Substitution of refined conventional wheat flour with wheat high in resistant starch modulates the intestinal microbiota and fecal metabolites in healthy adults: A randomized, controlled trial. J. Nutr. 2022, 152, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Vuksan, V.; Kendall, C.W.; Würsch, P.; Jeffcoat, R.; Waring, S.; Mehling, C.C.; Vidgen, E.; Augustin, L.S.; Wong, E. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J. Am. Coll. Nutr. 1998, 17, 609–616. [Google Scholar] [CrossRef]
- Noakes, M.; Clifton, P.M.; Nestel, P.J.; Le Leu, R.; McIntosh, G. Effect of high-amylose starch and oat bran on metabolic variables and bowel function in subjects with hypertriglyceridemia. Am. J. Clin. Nutr. 1996, 64, 944–951. [Google Scholar] [CrossRef]
- Penn-Marshall, M.; Holtzman, G.I.; Barbeau, W.E. African Americans may have to consume more than 12 grams a day of resistant starch to lower their risk for type 2 diabetes. J. Med. Food 2010, 13, 999–1004. [Google Scholar] [CrossRef]
- Sobh, M.; Montroy, J.; Daham, Z.; Sibbald, S.; Lalu, M.; Stintzi, A.; Mack, D.; Fergusson, D.A. Tolerability and SCFA production after resistant starch supplementation in humans: A systematic review of randomized controlled studies. Am. J. Clin. Nutr. 2022, 115, 608–618. [Google Scholar] [CrossRef]
- Anderson, G.H.; Cho, C.E.; Akhavan, T.; Mollard, R.C.; Luhovyy, B.L.; Finocchiaro, E.T. Relation between estimates of cornstarch digestibility by the Englyst in vitro method and glycemic response, subjective appetite, and short-term food intake in young men. Am. J. Clin. Nutr. 2010, 91, 932–939. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Regina, A.; Klingner, B.; Zajac, I.; Chapron, S.; Berbezy, P.; Bird, A.R. High-amylose wheat lowers the postprandial glycemic response to bread in healthy adults: A randomized controlled crossover trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef]
- Bodinham, C.L.; Smith, L.; Wright, J.; Frost, G.S.; Robertson, M.D. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS ONE 2012, 7, e40834. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, F.; Benini, L.; Del Rio, D.; Casiraghi, C.; Pellegrini, N.; Scazzina, F.; Jenkins, D.J.A.; Vantini, I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 2006, 83, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Haini, N.; Jau-Shya, L.; Rosli, R.G.M.; Mamat, H. Effects of high-amylose maize starch on the glycemic index of Chinese steamed buns (CSB). Heliyon 2022, 8, e09375. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.B.; Lau, C.W.H.; Noakes, M.; Bowen, J.; Clifton, P.M. Effects of meals with high soluble fibre, high amylose barley variant on glucose, insulin, satiety and thermic effect of food in healthy lean women. Eur. J. Clin. Nutr. 2007, 61, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Luhovyy, B.L.; Mollard, R.C.; Yurchenko, S.; Nunez, M.F.; Berengut, S.; Liu, T.T.; Smith, C.E.; Pelkman, C.L.; Anderson, G.H. The effects of whole grain high-amylose maize flour as a source of resistant starch on blood glucose, satiety, and food intake in young men. J. Food Sci. 2014, 79, H2550–H2556. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, M.P.; Preisendanz, S.; Juma, S.; Imrhan, V.; Prasad, C.; Vijayagopal, P. Resistant starch lowers postprandial glucose and leptin in overweight adults consuming a moderate-to-high-fat diet: A randomized-controlled trial. Nutr. J. 2017, 16, 14. [Google Scholar] [CrossRef]
- Stewart, M.L.; Zimmer, J.P. Postprandial glucose and insulin response to a high-fiber muffin top containing resistant starch type 4 in healthy adults: A double-blind, randomized, controlled trial. Nutrition 2018, 53, 59–63. [Google Scholar] [CrossRef]
- Robertson, M.D.; Wright, J.W.; Loizon, E.; Debard, C.; Vidal, H.; Shojaee-Moradie, F.; Russell-Jones, D.; Umpleby, A.M. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 3326–3332. [Google Scholar] [CrossRef]
- Ames, N.; Blewett, H.; Storsley, J.; Thandapilly, S.J.; Zahradka, P.; Taylor, C. A double-blind randomised controlled trial testing the effect of a barley product containing varying amounts and types of fibre on the postprandial glucose response of healthy volunteers. Br. J. Nutr. 2015, 113, 1373–1383. [Google Scholar] [CrossRef]
- Maki, K.C.; Pelkman, C.L.; Finocchiaro, E.T.; Kelley, K.M.; Lawless, A.L.; Schild, A.L.; Rains, T.M. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J. Nutr. 2012, 142, 717–723. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Ostman, E.M.; Hoist, J.J.; Bjorck, I.M.E. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J. Nutr. 2008, 138, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Al-Mana, N.M.; Robertson, M.D. Acute effect of resistant starch on food intake, appetite and satiety in overweight/obese males. Nutrients 2018, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Gower, B.A.; Bergman, R.; Stefanovski, D.; Darnell, B.; Ovalle, F.; Fisher, G.; Sweatt, S.K.; Resuehr, H.S.; Pelkman, C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: A randomized, controlled trial. Nutr. Metab. 2016, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Dainty, S.A.; Klingel, S.L.; Pilkey, S.E.; McDonald, E.; McKeown, B.; Emes, M.J.; Duncan, A.M. Resistant starch bagels reduce fasting and postprandial insulin in adults at risk of type 2 diabetes. J. Nutr. 2016, 146, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.F. An introductory review of resistant starch type 2 from high-amylose cereal grains and its effect on glucose and insulin homeostasis. Nutr. Rev. 2019, 77, 748–764. [Google Scholar] [CrossRef]
- Robertson, M.D.; Bickerton, A.S.; Vidal, H.; Frayn, K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [CrossRef]
- Firth, J.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Mayer, E.A. Food and mood: How do diet and nutrition affect mental wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).