Abstract
Terpenes are compounds that include monoterpenes, sesquiterpenes, diterpenes, and terpenes, where isoprene is the basic structural unit of multiple oxygenated hydrocarbons. Terpenes are often found in plants, especially in some Zingiberaceae or tulips, and they have been obtained by direct extraction, chemical synthesis, and microorganisms. They exhibit anti-bacterial, anti-viral, anti-oxidant, and analgesic activity, aid digestion, and have other biological activities. Terpenes are widely used as factors in food health and as anti-cancer treatments. They are used especially as active substances in fermented Chinese baijiu products and gives Chinese baijiu its fruity aroma. To a certain extent, terpenes affect the quality of baijiu. In pharmaceutical products, terpenes, especially limonene and elemene, have strong biological activity that reduces the mitosis of tumor cells, induces tumor cell apoptosis, and inhibits tumor cell growth. However, the low yield of terpenes limits its application. Therefore, we review the sources of terpenes, focus on the biosynthesis pathway of sesquiterpenes, and explore the latest research progress on terpenes that play a biologically active and functional role in fermented products and pharmaceutical products, especially in the development and utilization of the Chinese baijiu industry. We provide new research ideas for the improvement and optimization of terpenes in various industries.
Keywords:
terpenes; elemene; limonene; sesquiterpene; health function factor; fermented food; pharmaceutical 1. Introduction
Terpenes are a class of hydrocarbons, and their oxygenated derivatives are found widely in plants, microorganisms, marine organisms, fungi, and insects. There are many kinds of terpene compounds, and their isomers exist in various forms. These can be divided into monoterpenes (limonene), sesquiterpenes (elemene), diterpenes (camphene), and polyterpenes (Figure 1) [1]. Most of these terpene compounds have strong lipophilic tendencies [2]. They are soluble in organic solvents, and they contain asymmetric carbon atoms with optical rotations. Low-molecular-weight terpenes, such as monoterpenes and sesquiterpenes, are volatile oily liquids; their boiling point increases with an increase in molecular weight and the number of double bonds. The high-molecular-weight terpenoids, such as diterpenoids and triterpenoids, are mostly solid crystals. In addition, the carbon skeleton double bond and functional group double bond of terpenes react with corresponding reagents, which can be used to separate, to extract, and to identify terpenes.
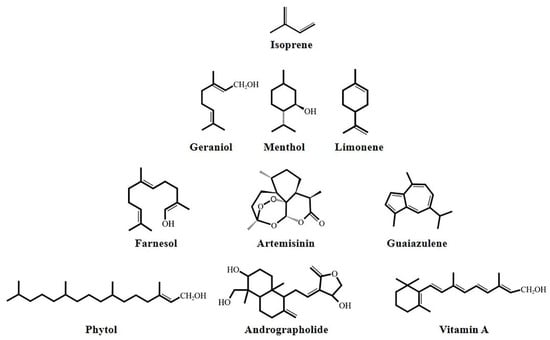
Figure 1.
Chemical structure of terpenoids.
Terpene compounds are rich in variety and exhibit various forms. With the continuous advancement in industrial processes, their applications are becoming more extensive. As a fermented food, Chinese baijiu is one of the six major distilled liquors in the world. There are a variety of flavor types, which mainly include 12 mainstream flavor types [3]. However, the current market is mainly dominated by strong flavor types represented by Wuliangye and Luzhou Laojiao (also known as ‘Luzhou-flavor liquor’) and Maotai and Langjiu (commonly known as ‘Maotai liquor’). In these many flavor types of Chinese baijiu, there are >300 trace components of main flavor substances, which include terpenes, alcohols, acids, esters, amino acids, hydroxyl compounds, acetals, nitrogen-containing compounds, sulfur-containing compounds, furan compounds, phenolic compounds, and ethers [4]. These terpenes are derived mainly from raw materials or Daqu.
Daqu is fermented at a high temperature, low pH, and with a high ethanol concentration, but the microorganisms in it can still reproduce in large quantities. After a series of metabolic processes, various flavor substances and functional factors are accumulated in large quantities, so that fermented grains begin to exhibit special physiological activity after fermentation [5,6,7,8,9,10,11]. These terpenes provide a unique flavor to distillers’ yeast fermentations that stimulates the taste buds and smell of consumers, The most important terpene in this process is β-elemene, which is a class of sesquiterpene terpenoids found in trace amounts in Chinese baijiu; it is considered a health function factor that not only gives baijiu a special aroma, but which exhibits anti-tumor, anti-cancer, anti-inflammatory, and other biological activities [12]. The content and type of these terpenes in baijiu affect the quality of Chinese baijiu directly.
In the pharmaceutical industry, terpenes are used mainly as new anti-cancer drugs. For example, elemene, which is a natural anti-cancer compound with minor toxic side effects, inhibits lung cancer, liver cancer, nasopharyngeal carcinoma, and brain tumors [1]. In addition, terpenes, such as pinene and paclitaxel, are used widely in clinical medicine. At the same time, terpenes can also be synthesized into adhesives, pesticides, curing agents, and other products, which are generally applicable in agriculture, chemistry, and other fields.
Research on terpenes in food and pharmaceutical products, which includes research on synthesis pathways and biological activity, affects the yield of terpenes and the quality of products directly. The production pathway of terpenes can be produced by microorganisms in the biosynthesis pathway of mevalonate (MVA). However, to control or to increase the yield of terpenes, it is necessary to improve the synthesis efficiency of the precursor substance isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) contained in terpenes, which comprises the substrate [13]. Therefore, the structure and function of the key enzymes that catalyze the formation of precursor substances can be analyzed, and the fermentation and production process of Daqu can be interrupted by humans to regulate the activity of protease. This makes it possible to control the content of terpenes [14]. IPP and DMAPP are important precursors of terpenoids. These two molecules are modified by downstream metabolic pathways to form a variety of known sesquiterpenes [15]. The expression level of isopentenyl diphosphate isomerase (IPI) affects the metabolic flow of precursor synthesis directly, which is the master switch for downstream metabolic pathways. In summary, the structure of IPI, which is a key enzyme in the synthesis of terpenoids, plays an important role in the synthesis of terpenoids by microorganisms (Figure 2) [16]. Therefore, it is difficult, yet crucial, to study the microbial synthesis pathway of terpenes, clarify the regulatory mechanism of terpenes, and master the application and main functional activity of terpenes in food and pharmaceutical products [17].
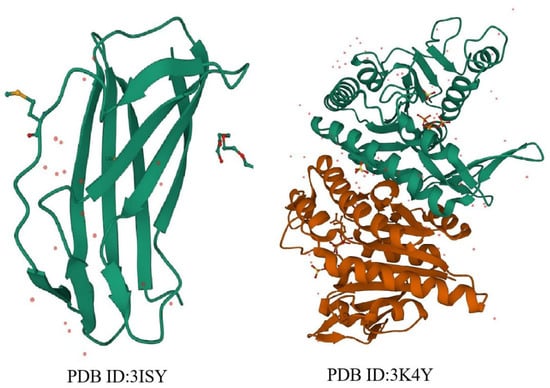
Figure 2.
Three-dimensional structure of isopentenyl pyrophosphate isomerase. (PDB ID: 3ISY) Crystal structure of an intracellular proteinase from bacillus subtilis. (PDB ID: 3K4Y) Crystal structure of Isopentenyl Phosphate Kinase from M. jannaschii in complex with IPP.
2. Synthesis Pathway of Terpenes
Terpene compounds have a wide range of synthetic pathways. From existing reports, most terpene compounds are obtained directly from plants, but the amount of terpene compounds in plants is small and their regulatory mechanism is not clear. Therefore, finding other sources of terpenes and exploring the synthesis pathways of different terpenes can solve the problem of low abundance of terpenes for the pharmaceutical, food, and especially liquor industries. This paper is guided by the sesquiterpene compound elemene in terpenes. By exploring the source of terpenes in liquor and the synthesis path of different isomers of elemene, the problems of low synthesis, low purity, and limited sources of terpenes could be solved. By analogy, other terpenes that can also be obtained by biological or genetic engineering will foster the promotion and use of terpenes.
2.1. Microbial Sources of Sesquiterpene Elemene
Elemene is a natural compound found in turmeric. Elemene contains three unsaturated double bonds; based on the position of the double bonds, they are divided into γ-elemene, β-elemene, and δ-elemene. Elemene sesquiterpenes are nitrogen-containing or oxygen-containing derivatives of elemene [18]. They all exhibit biological activity that includes anti-tumor, anti-cancer, and anti-inflammation properties, and they can also inhibit the rupture of tumor cells to a large extent [11]. Therefore, it is of great significance to study the synthesis of such compounds. Terpenoids are found widely in animals and plants in nature. At present, about 60,000 natural terpenoids have been isolated. The number of compounds and structural skeletal types of sesquiterpene natural products are the most common terpenoids. Most sesquiterpenes exhibit a wide range of biological activity, which includes antibacterial activity, neuroprotective activity, cytotoxic activity, anti-inflammatory activity, and anti-cancer activity. Therefore, studying the activity and application of these substances will help us understand the potential value of these compounds [19].
Terpenes in Chinese baijiu mainly come from two sources: one is from raw materials, that is, the raw materials of liquor production contain terpenes and include bound and free forms. Free terpenes enter fermented grains directly and are distilled and retained in liquor. Bound terpenes are broken due to enzymatic reactions or acid-heating, and the terpene–glycosidic bond is broken, so that the terpenes are free (Figure 3); this is the main source of terpenes in medicinal liquor [20]. Free terpenes contained in Xiaoqu and Daqu in medicinal baijiu are derived from the Chinese herbal medicine added during the production of Daqu and Xiaoqu [21].
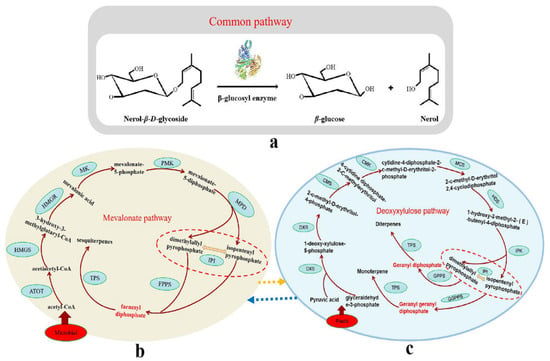
Figure 3.
Biosynthesis pathway of terpenes in Chinese Baijiu. (a) synthesis path of conjugated terpenes in Chinese baijiu. (b) microbial pathway of terpenes. (c) plastid pathway of terpenes. ATOT: Acetoacetyl CoA thiolase, HMGS: Hydroxymethylglutaryl CoA synthase, HMGR: Hydroxy methylglutaryl CoA reductase, MK: mevalonate kinase, PMK: Mevalonate dioxyphosphate kinase, MPD: diphosphomevalonate decarboxylase, IPI: isopentenyl diphosphate isomerase, FPPS: Farnesyl phosphate synthase, TPS: terpene synthase, DXS: Deoxyxylulose phosphate synthase, DXR: Deoxyxylulose phosphate reducing isomerase, CMS: Cytidine diphosphate methylerythritol synthase, CMK: Cytidine diphosphate methyl erythrose kinase, MCS: Methylerythritol diphosphate synthase, HDS: Hydroxymethyl butenyl diphosphate synthase, IPK: Isopentenyl monophosphate kinase, GPPS: Geranyl diphosphate synthase, GGPPS: Geranylgeranyl diphosphate synthase.
Another important source of terpenes is from the microbial pathway, that is, in the koji-making and wine-making process. Some microorganisms, such as Streptomyces, begin with acetyl-CoA and the methyl valonate pathway to generate terpenoids; this pathway is consistent with the plant terpene production pathways [22]. In this pathway, three acetyl-Co-A molecules form 3-hydroxy-3-methylglutaryl-Co-A (HMG-CoA). HMG-CoA is reduced to methylvalonate, which is then phosphorylated and decarbonylated to form IPP [22]. IPP can also be synthesized by a non-methylvalonic acid pathway. The next series of reactions, which include the binding of IPP units, form geranyl diphosphate, farnesyl diphosphate, and geranyl geranyl diphosphate. These compounds are the precursors of monoterpenes, sesquiterpenes, and diterpenes, respectively, and other terpenoids are derived from the cyclization or secondary modification of these three precursors (Figure 3) [23].
2.2. Synthesis of the Sesquiterpene Lemene and Its Isomers
Elemene is a pale yellow or yellow, clear liquid, and is an active anti-cancer ingredient extracted from the ginger plant turmeric. Its main biological activity is to reduce tumor cells that have mitotic ability, induce tumor cell apoptosis, inhibit tumor cell growth, and control malignant pleural effusion [24]. It is easily soluble in petroleum ether, chloroform, or ethanol, and it has poor compatibility with water. The toxicity and side effects are small, and it has little effect on normal cells and surrounding leukocytes and has no teratogenic and mutagenic effects. However, elemene is derived mainly from plants. The high cost, low purity, complicated chemical synthesis steps, and harsh requirements for the separation and extraction of elemene from plants limit its application. Therefore, it would be advantageous to find a feasible synthesis technology for elemene-type sesquiterpenes.
2.2.1. Total Synthesis of γ-Elemene Sesquiterpenes
γ-elemene sesquiterpenes are natural sesquiterpenoids isolated from the volatile oil of turmeric rhizomes. These compounds exhibit anti-inflammatory, anti-fungal, anti-cancer, anti-viral, and anti-microbial activity. Due to the application of natural products in the development of new drugs in recent years, natural products such as γ-elemene-type sesquiterpenes with anti-tumor activity have attracted the interest of researchers.
Wu (1995) extracted γ-elemene sesquiterpenes from the roots of crabapples, which have neuroprotective and anti-inflammatory properties [25]. Lou et al. (2010) isolated four new sesquiterpenes from turmeric; these compounds had a strong inhibitory effect on lipopolysaccharide-induced nitroso production. Inorganic free radical nitroso is involved in physiological and pathological processes, such as vasodilation, nonspecific host defense, and chronic or acute inflammation [26]. Subsequently, Qiu et al. (2013) extracted and isolated seven sesquiterpenes from the rhizomes of Curcuma wenyujin; γ-elemene sesquiterpenes showed strong inhibitory activity, which suggested that the presence of hydroxyl and nitrogen atoms affected its biological activity [27]. In the summer of 2015, 11 natural sesquiterpenoids, which exhibited inhibitory effects on macrophage-induced nitroso, were isolated from turmeric essential oil [28,29].
From the above synthesis, γ-elemene had good biological activity, such as neuroprotective and anti-inflammatory properties, and there have been an increasing number of reports on the synthesis of γ-elemene-type sesquiterpenes, which provides technical guidance for research on these compounds. γ-elemene-type sesquiterpenes and β-elemene-type sesquiterpenes are similar in structure, so the study of γ-elemene-type sesquiterpenes can help us to explore β-elemene-type sesquiterpenes and the synthetic pathway of fennel-type sesquiterpenes.
2.2.2. Total Synthesis of β-Elemene-Type Sesquiterpenes
The technology for synthesizing sesquiterpenes of β-elemene includes the construction of the main chiral center in the molecule, modifying it to construct the lactone ring structure and, finally, improving the synthesis of the entire compound. β-elemene is an organic compound that consists mainly of two basic elements, carbon and hydrogen. Its chemical name is 1-methyl-1-vinyl-2,4-diisopropylcyclohexane. Its molecular formula is C15H24, its molecular weight is 204.355, its density is 0.862 g/cm3, its boiling point is 252.1°, its flash point is 98.3 °C, and storage conditions to 2–8 °C are appropriate. Li et al. (2016) found that β-elemene contained only carbon and hydrogen, that it was almost insoluble in water, and that its bioavailability was not high. The introduction of water-soluble and biocompatible compounds into β-elemene is based on the premise that the carboxyl group is well-protected, and that it reacts with chlorinated β-elemene to form an alcohol ester compound to obtain β-elemene amino acid derivatives [30]. However, the synthesis conditions of this pathway are difficult to control, so β-elemene amino acid derivatives are synthesized directly without protecting the carboxyl group (Figure 4).
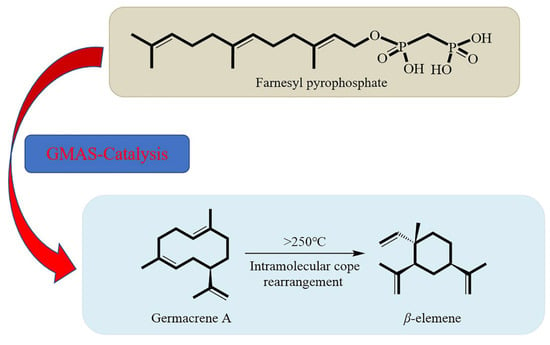
Figure 4.
Synthetic steps of β-elemene. GMAS: Germacrene A is a synthase catalyst.
If β-elemene is used as a raw material, many new substances can be formed after some chemical reactions, and derivatives of β-elemene are also formed. For example, β-elemene chloride can be synthesized by an oxo reaction at the allylic position. If it continues, nine compounds of β-elemene can be synthesized by introducing nitrogen-containing aromatic heterocycles into the β-elemene parent through a nucleophilic substitution reaction. β-elemene aromatic heterocyclic derivatives are then separated by preparative thin layer chromatography to obtain the single isomer of the target compound.
2.2.3. Biosynthesis of Elemene
β-elemene belongs to the group of sesquiolefins without oxygen, and is an oily monomer separated from the volatile oil of Wen Curcuma (Curcuma wenyujin). Its chemical name is 1-methyl-1-vinyl-2,4-diisopropylcyclohexane; it is a natural terpenoid compound with high efficiency, low toxicity, and broad-spectrum anti-microbial activity. However, the high cost and low purity of β-elemene isolated from plants, the cumbersome chemical synthesis steps, and the harsh conditions for β-elemene limit its wide application of. There are two main pathways for the synthesis of terpenes: the mevalonate (MVA) pathway and the deoxyxylulose-5-phosphate (DXP) pathway. The final product of the MVA pathway is sesquiterpenoids, and the final product of the DXP pathway is monoterpenes or diterpenes. Therefore, the search for a new biosynthetic pathway for β-elemene must start with the MVA pathway. The promotion and application of elemene is of great significance.
Gao (2021) [31] studied the microbial biosynthetic precursor of β-elemene, gemene A. The IPP gene, which is a key enzyme in the biosynthesis of terpenes, was cloned successfully. At the same time, in this paper, this gene was cloned from the Lactuca sativa cDNA as a template, and from cyanobacteria (GAS). This gene was codon-optimized and the whole-gene was connected to the pET-28a vector. The authors used E. coli BL21 (DE3) as the expression host for recombinant expression. The cloned LTC1 gene can be used as a source for microbial synthesis of β-elemene. Therefore, we successfully constructed a heterologous MVA pathway composed of eight genes that were expressed functionally in Escherichia coli by using overlap extension PCR technology and conventional molecular-cloning technology. These genes were co-expressed to achieve β-elemene biosynthesis.
However, by constructing the β-elemene biosynthesis pathway in microorganisms, this system has low biosynthetic efficiency for β-elemene and would not be suitable for industrial production. The experiment showed that the gemene A gene mainly existed in the form of inclusion bodies (low protein solubility) after being expressed in Escherichia coli, which was the limiting factor related to the production of gemene A. However, IPI of microbial origin was chosen as the starting point, and the biochemical enzymatic activity of IPI catalyzed the iso-differentiation between IPP and DMAPP. Through structural transformation, these two molecules were modified through downstream metabolic pathways to form more than 30,000 known terpenoids [14]. Therefore, the expression level of IPI directly affected the metabolic flow of the five-carbon precursor pool for terpenoid synthesis and was the master switch of the downstream metabolic pathway (Figure 4). The protein of this enzyme is conserved widely in the biological world (e.g., plants, animals, algae, fungi, and bacteria). The molecular mechanism of the terpenoid biosynthesis pathway provides a baseline for future work.
3. Types and Antibacterial Activity of Terpenes in Food and Pharmaceutical Products
3.1. Types of Terpenes in Chinese Baijiu and Their Antibacterial Activity
Chinese liquor is one of the six major distilled spirits in the world; about 98% of its components are water and ethanol, and about 2% are trace components with aroma and flavor [32]. Approximately 2% of the trace elements, which consist of thousands of species, determine the style and taste of Chinese baijiu, its health characteristics, and efficacy [33]. The most important functional substances of Chinese baijiu come from pyrazines and terpenes, and pyrazines are the unique flavor compounds in Chinese baijiu [34]. Twenty-six different pyrazine compounds have been detected in Chinese baijiu and, of those, pyrazines in Maotai-flavor and Jian-flavor baijiu have the highest concentration of 3000–6000 μg/L; the concentration range is 500–1500 μg/L, and the total amount of pyrazine compounds in individual Luzhou-flavor baijiu can also reach the level of Maotai-flavor baijiu. Terpenes are present in Luzhou-flavor, Maotai-flavor, Fen-flavor, and Yao-flavor baijiu. Sixty-eight terpenes have been detected in baijiu; 30 terpenes were detected in Luzhou-flavor liquors, which included β-elemene, limonene, caryophyllene, terpene ether, terpene alcohol, terpene ketone, terpene aldehyde, and terpene ester [35,36]. The sources of terpenes in liquor are mainly raw materials and Daqu. In addition, microbial transformation is also an important way to synthesize terpenes [37,38] (Table 1).
3.1.1. β-Elemene
Terpenoids are among the flavor compounds produced by microbial fermentation, which contain strong fragrances of flowers, fruits, roses, and tobacco, and especially β-elemene. β-elemene is an extremely important health ingredient and flavor substance. The production and enrichment of the terpene β-elemene, which is the main product of koji fermentation, not only gives the unique flavor to Chinese baijiu, but stimulates the taste buds and olfactory senses of consumers. The most important property of baijiu, however, is the molecule β-elemene itself as a health ingredient, with antitumor, anticancer, antiinflammation, antimicrobial, and other biological activities. In particular, β-elemene showed strong inhibitory activity against Escherichia coli, Micrococcus Tetragenus, and Bacillus subtilis, with minimum inhibitory concentrations of 31.25 µg/mL, and 500 µg/mL, respectively [39,40]. In addition, it also inhibited the rupture of tumor cells to a large extent. Currently, it is used widely in the treatment of lung cancer, gastrointestinal tumors, brain tumors, and other cancers, with strong efficacy in killing tumor cells and inhibiting the growth of tumor cells [41].
3.1.2. Limonene
Limonene, which is 1-methyl-4-isopropyl-cyclohexene, is a monocyclic terpene found widely in plants. It has a pleasant lemon fragrance and is found commonly in citrus fruits, vegetables, and spices. It has a wide range of functions, such as preservation, bacteriostasis, anti-inflammation, and anti-tumor activity, as well as acting as an expectorant, anti-asthmatic, and cholagogue. Dong et al. (2017) used gas chromatography to determine that the content of limonene in alcohol–water systems, such as pepper wine, was 0.8 ug/mL [42]. Limonene is a typical class of isoprenoid anti-cancer substances, and R-(+)-limonene is a therapeutic agent that can treat chemically-induced breast cancer and human gastric cancer metastasis [43]. Limonene in baijiu is used mainly as a health function factor; its content in baijiu is small, but it also can give wine a special flavor and, in the production of beer, it produces a bitter taste and a special hop aroma.
3.1.3. Caryophyllene
β-caryophyllene, also known as β-clove oil essence, is a kind of dicyclic sesquiterpene, which is generally derived from the metabolic processes in plants and fungi. The production of β-caryophyllene is based on plant extraction, but the content of β-caryophyllene in plants is low, and the concentration of the obtained product is low. The extraction is followed by chemical synthesis, but the process is complex, and the cost is high. However, caryophyllene synthesis by microbial fermentation has a fast growth rate and low cost [44]. In medicine, β-caryophyllene is mainly caryophyllene, and its oxidation products have anti-bacterial, lipid-lowering, anti-inflammatory, anti-tumor, and bactericidal activity, and it can also be used as a sedative. Caryophyllene has an inhibitory effect on bacteria that causes rot, even when the concentration of some Escherichia coli is only 62.5 µg/mL [45]. β-caryophyllane was also found in fermented wine, raw wine, fermented grains, and Daqu, but the content of β-caryophyllane is higher in medicinal baijiu [46].
3.1.4. Farnesol
Farnesol, which is also known as 7,11-trimethyl-2,6,10-dodecatrien-1-ol, is a non-cyclic sesquiterpene alcohol compound derived mainly from plants. There are four isomers, which are usually used directly as mixtures. It has a unique bell orchid aroma and is the main source of the aroma. It is used widely as a major aroma component in cosmetics and an anticancer drug in the pharmaceutical industry. It can also be used as an ideal aviation fuel [47]. It is mainly a functional compound in the liquor industry, which provides a special aroma in Chinese baijiu.

Table 1.
Common terpenes in Chinese baijiu and their antimicrobial activity.
Table 1.
Common terpenes in Chinese baijiu and their antimicrobial activity.
| Terpenes | Source | CAS | Anti-Microbial Activity | Function | Ref. |
|---|---|---|---|---|---|
| β-elemene | Zingiberaceae plants, microorganisms | 515-13-9 | Anti-fungal and anti-bacterial activity, especially for Escherichia coli, Bacillus subtilis, Tetralogy cocci, etc. | A national second-class anti-tumor new drug, Can inhibit lung cancer, liver cancer, esophageal cancer, nasopharyngeal carcinoma | [40,45] |
| Limonene | Plant, microbial transformation | 138-86-3 | Hydrocarbon monoterpenes, mainly for Gram-positive bacteria and pathogenic molds with strong inhibition | With anti-inflammatory, anti-oxidant, anti- high blood pressure, and other effects in the pharmaceutical industry, With anti-corrosion and fresh-keeping effects in food processing and imparts a lemon-like aroma to wine | [48,49] |
| β-caryophyllene | Microbial catalysis, endophytic fungal transformation | 87-44-5 | Antibacterial activity, Inhibition of pathogenic fungi, spoilage bacteria, etc. No anti-viral activity | Edible spice or as a raw material for synthetic spice, mainly used as a trace aroma substance in baijiu, giving baijiu a burnt sweet aroma | [44,50,51] |
| Farnesol | Animals, plants, microorganisms | 4602-84-0 | Inhibitory effect on non-albicans Candida, Paracoccus brasiliensis, and bacteria | Regulates bile acid, glucose, and lipid metabolism and inhibits non-alcoholic steatohepatitis (NASH), Used widely in cosmetics and food additives | [42,52] |
| Geraniol | Saccharomyces Cerevisiae engineering bacteria | 106-24-1 | Has strong antifungal effect | As a spice and sweetener in food, has anti-inflammatory properties, and prevents the occurrence of cardiovascular diseases | [53] |
| β-damascenone | Plants, Saccharomyces cerevisiae, Pichia | 23696-85-7 | None | With anti-viral, anti-cancer, anti-inflammatory, and other activity, and it is used mainly as a flavoring agent in food | [54,55] |
| Anisaldehyde | Chemical synthesis (p-hydroxybenzaldehyde salt) | 123-11-5 | Inhibits mycotoxins | Has strong antioxidant properties, and it is used widely in food, spices, medicine | [56,57] |
Note: Chemical Abstracts Service, CAS.
3.2. The Application and Function of Terpenes in Pharmaceutical Products
According to Global Cancer Data 2021, there were 192,900,000 new cancer cases and 99,600,000 cancer deaths worldwide in this year; today’s cancer rates are a major global public health problem. In the search for ways to treat malignant tumors, research into anti-cancer and cancer prevention active ingredients has been a major focus of the pharmaceutical industry. With increased research on the molecular mechanisms of tumorigenesis and malignant transformation, many anti-cancer drugs have been screened by targeting the related enzymes and receptors in the process of tumorigenesis and development [11]. For example, the natural diterpenoid taxol was isolated and purified from Taxus plants and represents a new generation of anti-tumor drugs. The new internationally recognized anti-cancer drug elemene, which is derived from Curcuma wenyujin, has strong medicinal activity for inhibiting tumors, lung cancer, and nasopharyngeal carcinoma. Therefore, the research and application of anticancer drugs is of great significance in the pharmaceutical industry (Table 2).

Table 2.
Common terpenes in medicine and their medicinal activity.
Terpene compounds are a kind of isoprene polymer, which is derived from mevalonate. It is an important chemical component of natural medicine with a variety of skeleton structures and a large number of biological activities. As an important medicinal compound, menthol, a monoterpenoid compound, has analgesic and antipruritic effects, and piperidone has the effects of relaxing smooth muscle and treating bronchial asthma. The sesquiterpene compound β-elemene can be used as an anti-cancer drug, which has a good effect on inhibiting cancerous pleural effusion [75]. In addition, some terpenoids have good inhibitory effects on platelet aggregation, dilation of the cardiovascular and cerebrovascular system, increased blood flow, heart rate regulation, tge reduction of blood pressure, reduction of blood lipids, and reduction of serum cholesterol. In terms of the impact on the digestive system, oleanolic acid has the effect of protecting liver reductase enzymes, and glycyrrhetinic acid is beneficial to the gallbladder and stomach, anti-gastric ulcers, etc. [76]; in terms of the role of the respiratory system, andrographolide has a certain anti-upper respiratory tract infection effect, and menthone has anti-asthmatic, expectorant, cough-suppressant and other effects; for the nervous system, some terpenoids have the effects of sedation, analgesia, local anesthesia, central excitation and neurolysis. In terms of anti-tumor effects, paclitaxel has a good effect on breast cancer and ovarian cancer, and some terpenoid derivatives attempt to treat liver cancer [77].
3.2.1. Biological Activity of Elemene and Its Application
β-elemene is a volatile sesquiterpene, which is a natural anticancer compound with high efficiency, low toxicity, and a broad spectrum. It is extracted mainly from Curcuma wenyujin and Taro. It is China’s first internationally recognized new drug for chest cancer, ascites, and solid tumors, and it comes with independent intellectual property rights. At the same time, elemene in oral emulsions and injections is a new national, second-class drug in clinical trials. Elemene induced tumor cell apoptosis and inhibited the growth of tumor cells. It can also be used for cancerous ascites, pleural effusion, and cerebral edema, can change and enhance immunogenic responses, and promote the body’s immune response. Elemene can also enhance the efficacy of treating solid tumors, and reduce the side effects of radiotherapy and chemotherapy. Elemene is an anti-cancer plant drug; it does not contain epoxy, nitro, onion ring, or benzene ring toxic groups [78], has a broad spectrum of anti-tumor activity, and presents minor side effects in the treatment of lung cancer, liver cancer, nasopharyngeal carcinoma, and brain tumors. It has a significant inhibitory effect on other cancer cells [79], and related reports have confirmed that β-elemene prevents lipid-induced, inflammatory pathways in heart failure [80].
Although elemene has many clinical applications, it is a volatile terpenoid oil, has poor water solubility, is not conducive to absorption, and exhibits low bioavailability, which limit its scope for clinical applications [81]. In addition, elemene is also accompanied by fever, local pain, bleeding, and other adverse reactions during clinical use.
3.2.2. Biological Activity of Limonene and Its Application
Limonene, which is also known as dipentene and 1,8-terpene diene, is a natural functional monoterpene with a chemical formula of C10H16. It is insoluble in water and can be mixed with ethanol and ether. It is a colorless clarified liquid with a typical orange aroma of light sweetness. It has been used widely in the pharmaceutical industry. It mainly comes from plants, bacteria, and fungi. Because of its availability, simple extraction, varied applications, and high practical value, research on limonene has increased gradually and deepened in recent years. The application scenarios have expanded gradually, and include food, medicine, and agriculture. Limonene has three isomers: L-limonene, D-limonene, and D,L-limonene. Among them, D-limonene is used more widely and has more prominent pharmacological activity. In addition to being used as a food additive, a detergent, and insect repellent, D-limonene is used in the field of medicine for the treatment of cholecystitis. In addition, it also inhibits gastroesophageal reflux and promotes gastrointestinal peristalsis, dissolves gallstones, and relieves angina pectoris [82]. Limonene has broad-spectrum antibacterial activity against foodborne pathogens (e.g., Escherichia coli, Staphylococcus aureus, Shigella). The limonene threshold has a scavenging effect on hydroxyl radicals at 0.12 g/L [58], and this increases with an increase in concentration. Its anti-aging effect can be applied to health care products and skin care products, and its antioxidant capacity increases with an increase in concentration. Second, limonene has anti-inflammatory and anti-tumor effects, and its mechanism mainly acts to inhibit the production of cancer by inhibiting pro-inflammatory factors that are associated with cancer [83]. Limonene can also act on the respiratory mucosa to generate the secretion of mucus and to relieve bronchial spasm, which relieves coughs and asthma.
As a natural monoterpene with various sources and with good antibacterial and clinical effect, limonene has great development potential and economic value. However, the low purity of limonene obtained in the natural way, the low yield, and the lack of security during use have limited the promotion and application of limonene. Limonene is still in the preliminary exploration stage, according to existing reports. Although it has been studied intensively in the food field as a preservative, anti-oxidant, and condiment, research in clinical medicine is lacking. Therefore, due to recent technical studies of the pharmacological mechanism of limonene and evaluations of its safety for use, it is believed that limonene and some limonene-rich substances can be used in the pharmaceutical industry.
3.2.3. Biological Activity and Application of Caryophyllene
Caryophyllene is a bicyclic sesquiterpene compound, which exists naturally in lemon, clove leaf oil, nutmeg, and other plants. Caryophyllene can also be synthesized by constructing Saccharomyces cerevisiae bacteria. Caryophyllene has three isomers, α-, β-, and γ-; β-caryophyllene has wide applications and strong biological activity. In the food industry, β-caryophyllene mainly serves as a food flavor, given its citrus, spice, and other aromas. β-caryophyllene is also used as a local anesthetic and in the treatment of colitis [60]. β-caryophyllene has low water solubility, high affinity for cell membranes, and it is a selective agonist. Its functions include regulating cytokine release inside and outside the central nervous system and migration of immune cells, and protection against heat-induced injury [59]. However, the β-caryophyllene double bond is oxidized easily to peroxide in air, and the unstable nature strongly irritates the skin, which can easily lead to a skin allergy [60]. This limits the clinical application of β-caryophyllene, therefore, a technical solution to inhibit the oxidation reaction of caryophyllene would provide theoretical guidance for the clinical application of caryophyllene.
3.3. Application and Function of Terpenes in Other Products
In addition to the role of terpene substances as a health functional factor in the food industry to enhance food quality, they serve as anti-cancer drugs to inhibit the spread of tumor cells, to solve the problem of malignant pleural effusion, and to improve the quality of life of patients. With the continuous advancement of industrialization, terpene compounds are also an important class of spices and raw materials for cosmetics. Some compounds are also important industrial raw materials, such as polyterpene compounds, which can be used in the automotive and aircraft industry.
3.3.1. Pinene
Pinene is the most important of the terpene compounds. There are two isomers: α-pinene and β-pinene. It is a natural terpene substance, a colorless and transparent liquid, insoluble in propylene glycol and glycerol, slightly soluble in water, and soluble in most organic solvents, such as ethanol, ether, chloroform, and glacial acetic acid. It is an important raw material for the synthesis of flavors. It is used mainly for the synthesis of terpineol, linalool, and some sandalwood flavors [66]. It can also be used for daily chemicals and other industrial products. Examples of the application of pinene polymer include α-pinene synthetic terpene resin, which is used in printing ink and paint [61]. Second, pinene can be used to synthesize plasticizers for a curing agent of epoxy resin, flea insecticide, optical glass adhesive, and wood preservative. There are also applications in rubber processing and adhesive bonding. However, β-pinene is mainly used to prepare other compounds through oxidation, reducing agent addition reactions, and so on. Using β-pinene as the starting material, a wide variety of compounds can be synthesized by chemical modification. These compounds can be used as repellents, antifeedants, fungicides, insecticides, pesticide synergists, and pharmacologically active substances [84].
3.3.2. Isoprene
Isoprene, which is 2-methyl-1,3-butadiene, is a common precursor of terpenes, and it is a colorless, volatile liquid. High-purity isoprene is also known as synthetic natural rubber’ because its molecular structure and properties are closest to natural rubber [85]. It is used widely in rubber-processing fields, such as tires, tapes, and hoses [86]. Secondly, styrene and isoprene, synthesized through anionic polymerization, are ideal materials for the modification of adhesives, coatings, and plastics. Graded rubber can also be synthesized with isobutylene [87]. In the fine chemical industry, pyrethroid insecticides, such as permethrin, cypermethrin, and deltamethrin, were prepared from isoprene using the vinylidene chloride addition method through ethyl dichloropyruvate.
Terpene compounds belong to the category of preface research in the pharmaceutical, health and diet fields because of their large number, wide coverage and com-plex synthesis. Terpenes are a group of hydrocarbons and oxygenated derivatives with a similar structure, which are widely found in plants. They have extremely important physiologically active functions, such as anti-oxidant, anti-viral, anti-inflammatory, cough suppressant and analgesic effects.
In addition to being an important health functional factor, terpenes are also natural aroma components [88]. Almost all volatile components of flower fragrance contain ter-penes, which are also characteristic aroma components of fruit fragrance [89]. Further-more, terpenes are volatile substances in almost all floral fragrances and are often used as some spices or special aroma substances. Additionally, as an effective anti-cancer sub-stance, caryophyllene and anisaldehyde are also approved as food spices by GB 2760-1996 [90]. The main volatile components in seasonings are hydrocarbons, aldehydes, ethers and sulfur compounds. Among them, the volatile components that have a signifi-cant contribution to the special odor of the seasoning are a-pinene, a-thujene, a-curcumene, β-elemene, β-sesquiphellandrene, β-bisabolene, D-limonene, 3-carene, myrcene, cycloal-kane, γ-terpinene, 4-allyl anisole and anethole [91]. Although the content of limonene and β-elemene is not high, it is the main source of olefins in pepper paste and contributes to the formation of pepper paste aroma [92]. Geraniol is the main component of the distinctive scent of peony flowers and is one of the flavorings of various roses. It is also an acyclic monoterpene compound in the food, perfume and cosmetic industries [93]. In addition, terpenes are also a source of wine aromas. β-damascenone compounds have a natural rose aroma and sweetness, the sweet aroma of grapes, and a persistent aroma. They are mainly used as to enhance the aroma of wines [94]. They have great value potential for both the wine and Chinese baijiu industry, as well as the pharmaceutical industry.
The research on the application and mechanism of terpene will provide new ideas for the improvement and optimization of terpene production and quality control.
4. Discussion
Terpenes are important functional factors in the food industry, especially where they play an important role in the quality of Chinese baijiu. In particular, β-elemene not only provides a special flavor to baijiu, but also improves its quality. Terpenes are used widely in clinical medicine as new anti-cancer drugs in the pharmaceutical industry. However, research on terpenes is mainly limited by the difficulty of sourcing the product, uncontrollable synthesis pathways, unclear biological and antibacterial activity in baijiu, medicine and other industrial products, and their small scope for application.
In terms of the source of terpenes, most of the terpenes in existing reports are extracted directly from plants, but the effective content of terpenes (e.g., elemene, limonene) in plants is low, the purity is low, and the steps are cumbersome, which is not conducive to the acquisition of a large quantity of terpenes. On the other hand, terpenes isolated from microbial sources have high purity and a controllable yield.
By analogy, other terpenes, if also obtained through biological means, will promote the promotion and use of terpenes.
Terpenes are important functional substances in baijiu and directly affect its quality. More than 68 terpenes have been detected in Chinese baijiu. Most of these terpenes are derived from liquor-making raw materials and Daqu, but microbial transformation is also an important method of producing terpenes. The study of the anti-bacterial activity and types of these terpene functional substances in baijiu aids our understanding of Chinese baijiu more comprehensively and objectively with terpenes as the starting point.
Moreover, terpenes have a place in the food, pharmaceutical and cosmetic industries as a natural new drug molecule. Especially in the pharmaceutical industry, terpenes provide many new active molecules for the development of new drugs, such as paclitaxel, selenodiene and limonene. These natural products exert their anti-tumor and anti-cancer activities through different or novel mechanisms. In this paper, terpenoids are connected by isoprene, containing five carbon atom units to form monoterpenes, sesquiterpenes, diterpenes, and triterpenoids, containing different amounts of isoprene units. These terpenoids exert different biological activities through different cellular mechanisms and structural modifications. As plant-signaling molecules, many triterpenoids also show a wide range of activities in the human body, such as anti-tumor, anti-bacterial, anti-inflammatory and anti-viral activities. The peroxide bridge in the sesquiterpene artemisinin molecule interacts with ferrous ions to produce free radicals that cause tumor cell death; paclitaxel is a natural anticancer drug extracted from purple shirt or yew tree. Some second-generation paclitaxel compounds obtained by the continuous modification of paclitaxel structure have unique anti-cancer mechanisms, and their chemical structure affects the biological activity of the drug to a certain extent.
Terpenes account for a significant proportion of the pharmaceutical industry today. Applications of these new terpene anticancer drugs have reduced the burden on the pharmaceutical industry to a certain extent, and this has also provided more choices for patients. However, the application of terpenes in the pharmaceutical industry faces the problems of complex sources, low yield, and low purity. However, if more biological pathways can be found to synthesize terpenes, the promotion and application of terpenes in the pharmaceutical industry can be better realized. Second, the safety and pharmacological activity of terpenes in medicine have always been a focus and a difficult problem to be solved. A further exploration of the mechanisms of action of terpenes in drugs should provide a better grasp of its pharmacological effects.
5. Future Perspectives
With the advancement of industrialization, the use of terpenes as raw materials with different molecular conformations will be used more frequently in the aerospace, automotive, and aircraft industries, which will promote economic and social development. However, the yield, purity, and mechanism of terpenes limit the promotion of terpenes. Therefore, it is an effective entry point to select appropriate microbial sources, to explore the optimal synthesis path, to analyze the structure of key enzymes in the synthesis path, to clarify the mechanism of action, and to explore the biological activity, functional activity, and application of terpenes in various industries. In particular, the synthesis of terpenes through microbial pathways is a hot topic in current research. Studying the metabolic pathways of different terpenes and the mechanisms of action will provide new ideas for the improvement and optimization of other terpenes.
In conclusion, terpenes are widely used as a health function factor in food, medicine and other industries. Terpenes in fermented foods can be both food flavor substances and health functional factors in a sense. For example, the content and type of terpenes in Chinese baijiu directly affect the flavor and quality of baijiu. For the pharmaceutical industry, terpenes, as a new anticancer drug, have the problem of improving the quality of life of cancer patients after cancer to a certain extent. In addition, terpenes are involved in both the use of spices and some industrial raw materials. For example, caryophyllene and anisaldehyde are approved as food spices by national standards. Limonene and pinene are the main sources of pepper flavor. Farnesol, as a fragrance component, can be used in decorative cosmetics, high-grade perfume, shampoo, soap and other toiletries, as well as non-cosmetic products, such as household cleaners and detergents. Based on the application of terpenes in food and medicine, this paper discusses the biological characteristics of terpenes, which provides a theoretical basis for the application of terpenes to a certain extent, and also provides new ideas for the research and development of terpenes in other industries. Terpene compounds obtained by biological methods have the advantages of clear genetic background, controllable yield and strong biocompatibility. The related methods should be the focus of future research.
Author Contributions
M.F., S.Y., L.L. and J.L.: Conceptualization, documentation and data curation. M.F., J.Z. and S.Y.: writing—original draft preparation. M.F., X.L., H.W. and S.Y.: writing—review and editing. D.Z. and J.L.: supervision. C.W., X.L. and J.L.: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Industry-University-Research Collaboration project of Wuliangye Co., Ltd. (grant no.CXY2020ZR02), Key Research and Development Program of Sichuan Province (grant no.2021YFS0341, 2020YJ0155), Key Program of Key Laboratory of Brewing Biotechnology and Application of Sichuan Province (grant no.NJ2017-01) and Talent introduction Program of SUSE (2017RCL25).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data sets used and analyzed are available on reasonable request.
Acknowledgments
We would like to thank Thomas A. Gavin, Cornell University, for help with editing this paper.
Conflicts of Interest
There are no conflict of interest.
References
- Zhang, J.H.; Liu, W.J.; Luo, H.M. Research Progress on the Activity of Terpenoids in Medicinal Plants. World Sci. Technol. -Mod. Tradit. Chin. Med. 2018, 20, 419–430. [Google Scholar]
- Tong, B.Q.; Maimone, T. Enlightening Terpene Synthesis. Chem 2019, 5, 1368–1370. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, H. China’s Baijiu Industry Current Situation and Development Trend. Liquor. Mak. 2022, 49, 39−41+46. [Google Scholar]
- Zheng, J.; Peng, Z.F.; Zhao, D. Research Progress in Flavor Chemistry of Traditional Daqu. Liquor. Mak. Sci. Technol. 2017, 3, 89–94. [Google Scholar]
- Xu, Z.J.; XU, Z.C.; Tang, Q.L. Research Progress on Flavor and Quality Characteristics of Chinese Traditional Solid Daqu Wine. Wine Sci. Technol. 2017, 1, 84–89. [Google Scholar]
- Mira, S.W.; Dominik, D.N.; Antonio, D.D.; Ulrich, F.C. Distribution of Yeast Cells, Temperature and Fermentation By-Products in White Wine Fermentations. Am. J. Enol. Vitic. 2019, 70, 339–350. [Google Scholar]
- Zhou, P.; Luo, H.B.; Huang, D.; Deng, B.; Wang, Q.; Feng, X.Y.; Wang, H. Preliminary Study of Tolerance and Enzyme Production Characteristics of Thermoduric Bacillus Licheniformis Strain in Medium/High Temperature Daqu. J. Food Sci. Technol. 2016, 41, 14–20. [Google Scholar]
- Ming, H.M.; Guo, Z.; Zhou, J.; Chen, M.E.; Xu, D.F.; Yao, X. Optimization of Flavor Components Fermentation Conditions of Bacillus Licheniformis from Daqu by Central Composite Design. Sci. Technol. Food Ind. 2015, 36, 182–186. [Google Scholar]
- Liang, C.; Du, H.; Xu, Y. Prokaryotic Microbial Community Structure and Flavor Component Succession during Daqu Storage. Acta Microbiol. Sin. 2017, 44, 384–393. [Google Scholar]
- Wang, Q.; Liu, K.Y.; Liu, L.L.; Zheng, J.; Chen, T.; Chen, F.; Li, P.P.; Zhang, M.; Shen, J. Correlation Analysis Between Aroma Components and Microbial Communities in Wuliangye-flavor Raw Liquor Based on HS-SPME/LLME-GC–MS and PLFA. Food Res. Int. 2021, 140, 0963–9969. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, C.C.; Wang, L.L.; Chen, S.; Yan, X. Optimization and Validation of a Head Space Solid-phase Microextraction-arrow Gas Chromatography-mass Spectrometry Method Using Central Composite Design for Determination of Aroma Compounds in Chinese Liquor (Baijiu). J. Chromatogr. A 2019, 10, 460–584. [Google Scholar] [CrossRef]
- Zhong, S.Y. Total Synthesis of Several γ-Elemene Sesquiterpenes. Ch.M. Thesis, North University of China, Shanxi, China, 2021; pp. 7843–7850. [Google Scholar]
- Liu, D.; Li, Y.; Li, L.Q.; Zhu, F. Bioinformatics Analysis of Isopentenyl Pyrophosphate (IPP) Isomerase. Anhui Agric. Sci. 2007, 20, 6018−6019+6023. [Google Scholar]
- Yang, Y.Z. Cloning and Functional Analysis of Isopentenyl Pyrophosphate Isomerase Gene from Patchouli. Bo.M. Thesis, Hainan University, Hainan, China, 2019. [Google Scholar]
- Luo, J.; Zhu, S.L.; Wang, L.; He, J.L.; Ouyang, L.H.; Zhou, J.Y. Research Progress on the Composition of Brewing Microorganisms and Flavor Substances in Strong-Flavor Baijiu. Chin. Inst. Food Sci. Technol. 2021, S1, 28–32. [Google Scholar]
- Huang, H.C.; Jiang, R.R.; Zhang, M.X. The Progress on Analytical Methods and Isolated Techniques on the Development of β-Elemene. Chin. J. Mod. Appl. Pharm. 2011, 28, 116−120+152. [Google Scholar]
- You, L.; Lv, Z.; Li, C.; Ye, W.; Zhou, Y.; Jin, J.; Han, Q. Worldwide Cancer Statistics of Adolescents and Young Adults in 2019: A Systematic Analysis of the Global Burden of Disease Study 2019. ESMO Open 2021, 6, 100255. [Google Scholar] [CrossRef]
- Fan, W.L.; Xu, Y.; Hu, G.G. Research Progress of Terpenes in Chinese Baijiu. In Proceedings of the First Symposium on Chinese Baijiu, Wuxi, China, 26–27 November 2011; pp. 44–55. [Google Scholar]
- Fan, W.L. “Analysis Technology of Flavor Substances and Microorganism Research of Key Flavor Substances in Chinese Old White Dry Flavor Liquor”Achievements through Technical Identification. Brewing 2010, 37, 97. [Google Scholar]
- Li, H.F. A β-Elemene Synthetase, Encoding Gene, Vector, Engineering Bacteria and Its Application. CHN. Patent CN201310280524.6, 27 November 2013. [Google Scholar]
- Zhu, L.F. Study on the Recombinant Expression and Catalysis of β-Elemene by Mevalvalonate Pathway in Vitro. Bio.M. Thesis, Hangzhou Normal University, Zhejiang, China, 2021. [Google Scholar]
- Wu, M.D.; Chen, J.; Chen, Y.J. Herbal Extract Elemene Intrathoracic Injection in the Treatment of Lung Cancer Patients with Malignant Pleural Effusion: A Meta-Anaylsis. J. Cancer Res. Ther. 2014, 10, 56–59. [Google Scholar] [CrossRef]
- Wang, Q.T.; Zhang, Z.L.; Xiong, H.; Zhou, D.S.; Li, J.; Liang, J.; Wang, Y.F. Evaluation of the Efficacy and Safety of Elemene in Treating Malignant Pleural Effusion Caused by Tumors. Am. J. Ind. Med. 2018, 97, 12542. [Google Scholar] [CrossRef]
- Wu, S.L.; Li, W.S. Chemical Constituents from the Roots of Neolitsea Hiiranensis. J. Chin. Chem. Soc. 1995, 42, 555–560. [Google Scholar] [CrossRef]
- Lou, Y.; Zhao, F.; He, H.; Peng, K.F.; Chen, L.X.; Qiu, F. Four New Sesquiterpenes from Curcuma wenyujin and Their Inhibitory Effects on Nitric-Oxide Production. Chem. Biodivers. 2010, 7, 1245–1253. [Google Scholar] [CrossRef]
- Qiu, G.G.; Yan, P.C.; Shao, W.W.; Zhou, J.; Lin, W.W.; Fang, L.L.; Zhao, X.W.; Dong, J.Y. Two New Sesquiterpenoids Including a Sesquiterpenoid Lactam from Curcuma Wenyujin. Chem. Pharm. Bull. 2013, 61, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Zhou, L.; Ma, J.H.; Wang, Y.; Ding, L.Q.; Zhao, F.; Chen, L.X.; Qiu, F. Sesquiterpenes from the Essential Oil of Curcuma Wenyujin and Their Inhibitory Effects on Nitric Oxide Production. Fitoterapia 2015, 103, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, J.; Zhao, B.j.; Jiang, Z.Y.; Feng, L.; Jia, X.b. Advance in Research on Anticancer Drug ß-elemene and Its Derivatives. Chin. Tradit. Herb. Drugs 2018, 49, 1184–1191. [Google Scholar]
- Li, S.; Wang, Y.C. Synthesis and Development of β-Elemene. Biotechnol. World 2016, 1, 239. [Google Scholar]
- Gao, Y.Y. Study on Microbial Biosynthesis of β-Elemene Pregene-Gimaene A. Bio.M. Thesis, Hangzhou Normal University, Zhejiang, China, 2021. [Google Scholar]
- Zheng, F.P.; Ma, Y.J.; Hou, M.; Sun, J.Y.; Sun, X.T.; Huang, M.Q.; Li, H.H.; Sun, B.G. Progress and Prospect in Aroma Components in Top Six Distilled Spirits. J. Food Sci. Technol. 2017, 35, 1–12. [Google Scholar]
- Zhao, Y.P.; Zheng, X.P.; Song, P.; Sun, Z.L.; Tian, T.T. Characterization of Volatiles in the Six Most Well-Known Distilled Spirits. J. Am. Soc. Brew. Chem. 2013, 71, 161–169. [Google Scholar] [CrossRef]
- Ding, H.L.; Ao, L.; Deng, B.; Ao, Z.H.; Song, C.; Liu, M.; Lin, F. Analysis of Trace Healthy Components of Chinese Baijiu. China Brew. 2018, 37, 11–14. [Google Scholar]
- Hu, G.L.; Fan, W.L.; Xu, Y.; Jia, Q.y.; Ran, X.H. Research on Terpenoids in Dongjiu. Baijiu-Mak. Sci. Technol. 2011, 7, 29–33. [Google Scholar]
- Fan, W.L.; Xu, Y. Review of Important Functional Compounds Terpenes in Baiiu (Chinese Liquor). Liquor. Mak. 2013, 40, 11–16. [Google Scholar]
- Zhang, Z.S.; Yang, R.G.; Zhu, L.Y.; Wu, X.M. Strategies for Improving the Yield of Microbial Terpenoids production. China Biotechnol. 2017, 37, 97–103. [Google Scholar]
- Wu, Y.Q.; Yu, J.X.; Cheng, Z.H. Research Progress on Microbial Transformations of Diterpenoids. Chin. J. Biochem. Pharm. 2016, 36, 9–15. [Google Scholar]
- Wang, J.; Xu, C.; Chen, Y.; Shao, L.; Li, T.; Fan, X.X.; Yu, L.L.; Zhang, R.N.; Chen, B.; Chen, X.B. β-elemene Enhances the Antitumor Activity of Erlotinib by Inducing Apoptosis through AMPK and MAPK Pathways in TKI-resistant H1975 Lung Cancer Cells. J. Cancer 2021, 12, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.W.; Zhang, Z.Z.; Ling, T.j.; Zhang, Y.G. Constituents in the Essential Oil of Vitex Negundo Linn. Var. Cannabifolia (Sieb.et Zucc.) Hand.-Mazz. and Their Antibacterial Activities. Sci. Technol. Food Ind. 2010, 31, 75–79. [Google Scholar]
- Cao, T.; Liu, X.Y.; Ding, X.; Bai, W.D.; Zeng, X.F.; Deng, Q.H.; Ren, J.P.; Gu, Z.D. Advances in Research and Application of Limonene. Acad. Period. Farm Prod. Process. 2017, 16, 51–54. [Google Scholar]
- Dong, S.Y.; Yuan, Y.J.; Zhang, Q.; Wang, Y.L.; Chen, H.F.; Dou, J.W.; Cheng, X.X. Determination of Limonene and Linalool in Zanthoxylum Bungeanum Liquor. China Condiment 2017, 42, 125–128. [Google Scholar]
- Lu, X.G.; Zhan, L.B.; Feng, B.A.; Qu, M.Y.; Yu, L.H.; Xie, J.H. Inhibition of Growth and Metastasis of Human Gastric Cancer Implanted in Nude Mice by d-Limonene. World J. Gastroenterol. 2004, 10, 2140–2144. [Google Scholar] [CrossRef]
- Cai, K.Y.; Chen, P.; Feng, X.D.; Mao, H.; Chen, X.L.; Wan, X.F.; Ming, D.; Guo, T.T.; Zheng, P. A β-Caryophyllene-Producing Fungus and Its Application. CHN. Patent CN113444646B, 2 September 2020. [Google Scholar]
- Xuan, Q.; Zhang, L.Q.; Yang, J. Study on β-Elemene Metabolites Produced by Endophytic Fungi of Curcuma Zedoary. Nat. Prod. Res. Dev. 2011, 3, 473–475. [Google Scholar]
- Fan, W.L.; Xu, Y. Review of Functional Factors and Quality Safety Factors of Baijiu (Chinese Liquor). Liquor. -Mak. Sci. Technol. 2012, 3, 17–22. [Google Scholar]
- Guo, J.Q.; Wang, Z.; Zhang, W.X.; Liu, W.F. Metabolic Engineering of Saccharomyces Cerevisiae to Improve Farnesol Production. Acta Microbiol. Sin. 2021, 21, 1257–1268. [Google Scholar]
- Baser, K.H.C.; Demirci, F. Chemistry of Essential Oils. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: New York City, NY, USA, 20 January 2007; pp. 43–86. [Google Scholar]
- Li, H.J.; Lan, W.J. Biotransformation of Limonene by Microorganisms. Adv. Chem. Ser. 2011, 23, 2318–2325. [Google Scholar]
- Cheng, A.X.; Xiang, C.Y.; Li, J.X. The Rice (E)-Beta-Caryophyllene Synthase Accounts for the Major Inducible Volatile Sesquiterpenes. Phytochem. Rev. 2007, 68, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Juan, B.; Hyeun, J. Biosynthesis of Beta-Caryophyllene, a Novel Terpene-Based High-Density Biofuel Precursor, Using Engineered. Escherichia coli. Renew. Energy 2016, 99, 216–223. [Google Scholar]
- Shen, Z.Y.; Cai, X.B.; Lu, L.G. Application of Farnesoid X Receptor Agonists in Treatment Steatohepatitis of Nona-Lcoholic. J. Clin. Hepatobiliary Dis. 2022, 38, 1402–1405. [Google Scholar]
- Xu, M.G.; Shi, J.; Li, Y. Research Progress of Geraniol in the Prevention and Treatment of Cardiovascular Diseases. Int. J. Cardiovasc. Dis. 2021, 48, 356–359. [Google Scholar]
- Shen, J.Y.; Zhang, B.Q.; Duan, C.Q. Selection of Indigenous Saccharomyces Cerevisiae Strains from Spontaneous Fermentation of Vidal Ice Wine in Huan Ren Region and Evaluation of Their Oenological Properties. J. Food Sci. 2020, 41, 148–157. [Google Scholar]
- Xu, Y.; Wu, Q.; Wang, W. Screening of β-Daphanone Producing Strains in Chinese Baijiu Brewing by Flavor Orientation Technology and Its Application. CHN. Patent 2,420,3, 12 October 2011. [Google Scholar]
- Mei, B.; Dai, W.; Yang, G. Experimental and Clinical Studies on the Action of Anisaldehyde Against Fungi. Chin. J. Dermatol. 1995, 6, 364−366+439. [Google Scholar]
- Han, J.C.; Sun, L.Y. The Preparation of P-Anisaldehyde by P-Hydroxylbenzaldehyde’salt. Fine Chem. Intermed. 2005, 2, 62–66. [Google Scholar]
- Huang, H.C. The Study on the Separation of β-Elemene and the Preparation of Its Liposomes. Bio.M. Thesis, Tianjin University, Tianjin, China, 2003. [Google Scholar]
- Sun, J. D-Limonene: Safety and Clinical Applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Chen, C.; Sheng, Y.; Tang, L. Pharmacology, Toxicology and Clinical Application of D-Limonene. Chin. J. Pharm. 2017, 48, 1698–1703. [Google Scholar]
- Kimura, J.; Takahashi, S.; Ogiso, T. Lack of Chemoprevention Effects of the Monoterpene D-Limonene in a Rat Multi-Organ Carcinogenesis Model. Cancer Sci. 1996, 87, 589–594. [Google Scholar]
- Ghelardini, C. Local Anaesthetic Activity of β-Caryophyllene. IL Farm. 2001, 7, 387–389. [Google Scholar] [CrossRef]
- Sun, M.Y.; Wang, H.; Jiang, L.Z. Research Progress of Microcapsules Prepared by Spray Drying as a Delivery System for Bioactive Components. Soybean Sci. Technol. 2022, 1, 11. [Google Scholar]
- Hao, Z.; Cui, J.; Yang, H. Research Progress of Geraniol in Tumor Therapy. Proc. Anticancer. Res. 2021, 5, 5. [Google Scholar]
- Li, X.Q.; Hui, H.Y.; Luo, Z.C. In Vitro Study on the Antifungal Activity of Geraniol, β-Citronellol and Eugenol. Mod. J. Lab. Med. 2016, 2, 87–89. [Google Scholar]
- Battilana, J.; Emanuelli, F.; Gambino, G. Functional Effect of Grapevine 1-Deoxy-D-Xylulose 5-Phosphate Synthase Substitution K284N on Muscat Flavour Formation. J. Exp. Bot. 2011, 62, 5497–5508. [Google Scholar] [CrossRef]
- Sun, L.H.; Sun, L.M. Research Progress of Geraniol. Northwest Pharm. J. 2009, 24, 428. [Google Scholar]
- Volzong, C.; Masini, O.; Comelli, N.A. α-Pinene Conversion by Modified-Kaolinitic Clay Mater. Chem. Phys. 2005, 93, 296–300. [Google Scholar]
- Luo, C.Q.; Duan, W.G.; Cen, B. Study on a New Technology of Synthesizing α-Pinene Maleic Anhydride Adduct. J. Guang Xi Univ. 2006, 1, 15–19. [Google Scholar]
- Yuan, X.M.; Zhang, P.H.; Zhao, Z.D.; Lu, Y.J.; Wang, M.X. Research Advances in the Catalysts for Synthesis of Borneol From α-Pinene by Esterification/Saponification Method. Ind. Catal. 2015, 23, 424–428. [Google Scholar]
- Hu, Z.X.; Deng, A.P.; Fu, X.D.; Li, D.Z. Optimization of Formulation and Preparation Process of Paclitaxel Polylactic Acid-Glycolic Acid Nanoparticles by Factor Design-Response Surface Method. China Pharm. 2012, 15, 626–629. [Google Scholar]
- Lin, H.M.; Chen, Y.Y.; Shi, A.H.; Zhu, G.Y. Phase 3 Randomized Low-Dose Paclitaxel Chemoradiotherapy Study for Locally Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2017, 6, 260. [Google Scholar] [CrossRef]
- Hu, Z.H. Research Progress in Pharmacological Mechanism and Clinical Application of Paclitaxel. J. Chongqing Coll. Educ. 2005, 6, 46–49. [Google Scholar]
- Zhu, L.; Chen, L. Progress in Research on Paclitaxel and Tumor Immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lei, J.Y.; Li, S.L.; Guo, L.Q.; Lin, J.F.; Wu, G.H.; Lu, J.; Ye, Z.W. Progress in Biological Activities and Biosynthesis of Edible Fungi Terpenoids. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent Progress in the Antiviral Activity and Mechanism Study of Pentacyclic Triterpenoids and their Derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Yang, L.L.; Chen, F.; Gao, C.; Chen, J.B.; Li, J.Y.; Liu, S.Y.; Zhang, Y.Y.; Wang, Z.Y.; Qian, S. Design and Synthesis of Tricyclic Terpenoid Derivatives as Novel PTP1B Inhibitors with Improved Pharmacological Property and in Vivo Antihyperglycaemic Efficacy. J. Enzym. Inhib. Med. Chem. 2020, 35, 152–164. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Q.; Hu, W. Research Progress of Mechanism in Antitumor Activity of Elemene and Its Derivatives. Nat. Sci. Ed. 2018, 17, 170–176. [Google Scholar]
- Chen, Y.; Zhen, Y.U.; Zhang, P. Effect of Elemene on Expression of PPAR γ-mRNA in Gastric Cancer SGC-7901 Cells. Zhejiang Med. J. 2008, 7, 689-690+693. [Google Scholar]
- Yang, H.; Wang, X.P.; Yu, L.L. The Antitumor Activity of Elemene is Associated with Apoptosis. Chin. J. Oncol. 1996, 18, 169–172. [Google Scholar] [CrossRef]
- Erika, P.; Watt, K.N.; Hergott, C.A.; Rahman, N.M.; Psallidas, I. Management of Malignant Pleural Effusion: Challenges and Solutions. Cancer Manag. Res. 2017, 9, 203–587. [Google Scholar]
- Mann, A.P.; Verma, A.; Sethi, G. Overexpression of Tissue Transglutaminase Leads to Constitutive Activation of Nuclear Factor-Kappa in Cancer Cells: Delineation of a Novel Pathway. Jpn. J. Cancer Res. 2006, 66, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- La, M.; Fu, L.H.; Su, T. α-Pinene as Copolymer Monomer for Preparation of White Latex. China Adhes. 2007, 16, 43–45. [Google Scholar]
- Su, H.L. Application of Steroidal Bio-Biotransformation in the Pharmaceutical Industry. In Proceedings of the 2019 4th International Conference on Life Sciences, Medicine, and Health(ICLSMH 2019), Xian, China, 23 August 2019; pp. 84–89. [Google Scholar]
- Sharkey, T.D.; Yeh, S.S. Isoprene Emission from Plants. Annu. Rev. Plant Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Z. Application Technology and Analysis on Development Outlook of Isoprene Rubber. CSRIA 2007, 19, 68–77. [Google Scholar]
- Ye, L.D.; Lv, X.M.; Yu, H.W. Engineering microbes for isoprene production. Metab. Eng. 2016, 38, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Sun, B.G. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Duan, M.Y.; Wang, F.Q.; Wu, G.P. Analysis of Flavor Characteristics of 4 Pepper Essential Oils. Food Sci. 2022, 43, 213–219. [Google Scholar]
- Shen, H.S. Development of Potato Instant Vermicelli Seasoning and Detection and Analysis of Flavor Substances. J. Northwest AF Univ. 2019, 8, 217. [Google Scholar]
- Xiao, L.; Wang, P.; Han, Y. Effects of Zanthoxylum Bungeanum on Volatile Substances in Pepper PasteP. Chin. Condiment 2022, 47, 161–165. [Google Scholar]
- Li, Y.; Zhao, H. Study on the Extraction and Separation Process of Peony Seasoning. Liquor. Mak. 2022, 49, 138–143. [Google Scholar]
- Tomasino, E.; Bolman, S. The Potential Effect of β-Ionone and β-Damascenone on Sensory Perception of Pinot Noir Wine Aroma. Molecules 2021, 26, 1288. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).