Abstract
Biosurfactants are surface-active molecules, produced by several microorganisms, that possess unique properties such as low toxicity and biodegradability. Their application in various industries depends on their purity and their specific properties, such as emulsification and stability. Therefore, this study focuses on the production of biosurfactant from Bacillus atrophaeus in an air-lift bioreactor. It analyzes the effects of agitation rate and temperature on biosurfactant production, as well as the concurrent separation process using a foam fractionation column. Moreover, the ability of the produced biosurfactant to form emulsions in water with several substrates (vegetables oils, hydrocarbons, and fossil fuels) was determined, and the stability of the soybean oil–water emulsion (used as an example) at different temperatures and pH values was verified. The biosurfactant produced, tentatively identified as iturin, was only detected in the coalescent liquid after passing through the foam fractionation column, demonstrating the complete separation of the biosurfactant. The best operational conditions for production and separation were an air flow of 1.00 vvm and a temperature of 34 °C (emulsifier index (EI24) = 66.9%, and productivity (Pp) = 967.5% mL h−1). Vegetable oils, hydrocarbons, and fossil fuels were emulsified in water, highlighting the soybean oil, whose emulsion oil–water had the highest ES (3333.3 min) at a temperature of 50 °C and a pH value of 9.0.
1. Introduction
Surfactants are amphiphilic long-chain molecules formed by a hydrophilic head (water-soluble) and a hydrophobic tail (oil-soluble), which are able to reduce surface and interface tension and form oil-in-water microemulsions [1,2]. Traditionally, commercialized surfactants are chemically synthesized, and the global market for these surfactants was valued at $1.74 billion in 2013 [3]. However, more stringent regulations and recurrent global demand for eco-friendly products have led to the development of an equally efficient alternative, namely, the biosurfactants. The global biosurfactant market has an estimated global annual growth rate of 4.3%, growing from $2.21 billion (442 thousand tons) in 2018 to $2.6 billion in 2023 [4].
Usually, biosurfactants are produced by certain microorganisms, such as bacteria (Bacillus subtilis [5]; B. atrophaeus [6]; Pseudomonas aeruginosa [7]; and Acinetobacter calcoaceticus [8]), yeasts (Yarrowia lipolytica [9]), and filamentous fungi [10]. Biosurfactants possess properties such as relative non-toxicity; low critical micellar concentration (CMC); high biodegradability, selectivity, and foaming ability; better specific activity in extreme temperatures, pH, and salinity, and their synthesis is based on renewable feed stocks, facilitating their wide industrial application [11].
The development of the biosurfactant production process takes place in several stages, starting with the selection of the microorganism, which can be obtained from natural or genetically modified sources. Next, the composition of the culture medium and the type of bioreactor are defined, optimizing the culture and operating conditions. The appropriate purification process also needs to be evaluated, and finally there are additional considerations such as stability, quality, packaging, market accessibility, and distribution.
The appropriate selection of the type of bioreactor is a crucial step and depends on the type of microorganism. Normally, fermentations for biosurfactant production occur in submerged culture, in an aerobic manner, using a continuous stirred-tank reactor (CSTR). They can be operated in batch mode (single and fed), continuous, and semi-continuous. However, these bioreactors have disadvantages, such as operating costs associated with aeration and oxygenation, high cell concentration that makes oxygen limited, scale-up hampered by volume, and excessive foam formation that leads to reactor overflow [12].
To avoid the aforementioned drawbacks of the CSTR, alternative designs have been proposed, such as a membrane-based reactor [13], an immobilized cell reactor [14], rotating disc bioreactors [15], and an air-lift reactor [16]. The air-lift bioreactor is extensively used in wastewater treatment as well as biochemical and pharmaceutical processes. It is formed by an internal loop, where an insert (bag or draft tube) is used to separate the riser from the downcomer [17]. The bioreactor offers some advantages compared to CSTR [18], such as efficient mass and heat transfer, high fluid circulation rate, low pressure drops, and low shear stress.
The literature is extensive in presenting various applications of biosurfactants, which include restoring contaminated environments; serving as greener alternatives in commercial laundry detergent; microbial enhanced oil recovery; food processing industries; bio-nanotechnology; pharmaceutical industries; agriculture and agrochemical industries; and the cosmetics industry [19,20,21].
The required purity of the biosurfactant is closely correlated with its application. For instance, the medical, cosmetic, and pharmaceutical industries require a very pure biosurfactant. On the other hand, areas like environmental processes and petroleum do not necessitate high purity [22]. The downstream process is responsible for the majority of the costs (60–80%) of the final product, making it the economic bottleneck of biosurfactant manufacturing [23]. For this reason, several unit operations have been proposed for the effective separation of these biosurfactants. These include solvent extraction, precipitation [24], centrifugation, aqueous two-phase systems [25], and chromatographic techniques [26]. Moreover, the separation can be directly linked to the upstream process, as seen by foam fractionation.
During aerobic fermentation, oxygen (air) is continuously sprayed into the bioreactor due to the low solubility of oxygen in water, and particularly in the culture broth [27]. The introduction of air and the production of the biosurfactant, which is positioned on the air–liquid surface, decreases the surface tension and consequently generates bubbles. The film liquid of the foam entraps the gas at a volumetric fraction ranging from 0.50 to 0.97 [28]. According to Oraby et al. [29], the generated foam ascends from the liquid surface, filling the top space of the bioreactor. It then crosses the entire length of the separation column. After descending the curve of the column, the bubbles coalesce and are harvested in the collector. Thus, the produced biosurfactant is removed from the fermented broth in a small amount of liquid, which is then concentrated.
The aim of this work is to determine the best operational conditions (aeration rate and temperature) to produce biosurfactant from B. athrofaeus in an air-lift reactor, and concomitantly proceed with the separation using a semi-batch foam fractionation column coupled to the reactor. Additionally, the ability to emulsify vegetable oils, fuels, and hydrocarbons, along with the stability of the biosurfactant at various temperatures and pH values, was also studied.
2. Materials and Methods
2.1. Microorganism and Culture Conditions
The strain Bacillus atrophaeus ATCC-9372, used in the present work, was generously provided by the Universidad Nacional de Rosario (UNR) (Rosario—Santa Fe, Argentina). The microorganism was maintained on nutrient agar slants, stored at 4 °C, and transferred monthly to new tubes.
The culture medium (g L−1) consisted of the following components: glycerol (18.0), peptone (10.0), NaCl (5.0), Na2HPO4 (2.5), yeast extract (1.5), and sucrose (1.5). The broth was prepared using distilled water, and its initial pH value was adjusted to 7.0 with pHmeter Digimed®/DM-20 (São Paulo, SP, Brazil) using NaOH (0.1 M) or HCl (0.1 M). The broth was then sterilized by autoclaving at 121 °C for 20 min. The composition and operational condition were previously determined by the Instituto de Procesos Biotecnológicos y Químicos of the UNR. Therefore, we have established these conditions for carrying out the studies in this work.
2.2. Reactor and Separator Design
Figure 1 depicts the experimental apparatus used for the production and simultaneous separation of the biosurfactant. The biosurfactant production was carried out in a bioreactor airlift (Tecnal TecBio V) (Piracicaba, SP, Brazil) with 7.0 L of total capacity, containing 6.0 L of culture media as the working volume. In order to separate the produced biosurfactant simultaneously, a foam fractionation column with a height of 50 cm and an internal diameter of 25 mm was vertically integrated onto one of the outputs of the bioreactor. On the top of the column, there was a pronounced curved region of 180°, which facilitated the collection of the air-entrained foam. Foam bubbles spontaneously rupture, primarily due to the effects of gravity, allowing the liquid to flow towards the bottom of the container. The liquid is then collected as a sample for analysis.
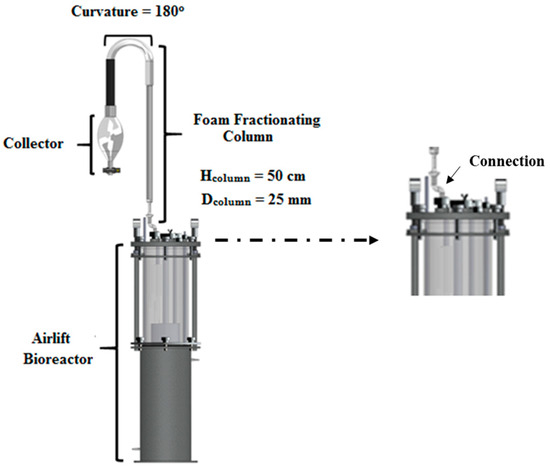
Figure 1.
Schematic representation of the experimental apparatus used for biosurfactant production in an airlift bioreactor, with simultaneous separation in a foam fractionation column.
2.3. Fermentation Conditions
The bioreactor was filled with 5.4 L of culture medium and inoculated with 0.6 L, 10% (v v−1), of a 24 h old inoculum. All fermentations were carried out in duplicate for 12 h at different air flows (0.25, 0.5, 0.75, and 1.00 vvm) and temperatures (31, 34, 37 and 40, °C) in the absence of chemical antifoam. Samples of fermented broth were collected in the bioreactor, while the sample of biosurfactant was harvested in the collector. During the process, the optical density, pH value, substrate (glycerol and sucrose) concentration, emulsification index, and volume of coalescent liquid after the foam fractionation column were measured every 1 h.
The substrate consumption was calculated according to Equation (1),
where S0 represents the initial substrate concentration (glycerol, sucrose, or both, represented by carbon source), and S corresponds to the substrate concentration at each time interval.
The productivity (P) was expressed as the ratio of the emulsion index (EI24, %)—PP, or cell concentration (X, g.L−1)—Px to the fermentation time, as described in Equations (2) and (3),
where the subscript P and X represent the product (biosurfactant) and the cell concentration, respectively, and t represents the fermentation time.
The yield of the product—biosurfactant (YP/X)—was determined using Equation (4), in which we assume that, at the initial time (t = 0 h), the IE24 and X were zero.
2.4. Analytical Determinations
The analyses were carried out using the cell-free fermented broth after centrifugation at 2000 rpm for 10 min. The experiments were conducted in duplicate at room temperature, and results were expressed as means with their standard deviations.
Optical density (OD): Biomass growth was analyzed by measuring the optical densities of samples, using a UV-spectroscophotometer (Bioteck Microplacas Epoch™ 2) (Kamakshipalya, India) at a wavelength of 600 nm. By dividing the OD by the correction factor (3.25)—determined previously using a calibration curve (OD versus dry mass)—we calculated the biomass concentration (g L−1)
pH value: The pH was determined potentiometrically using a pHmeter Digimed®/DM-20.
Glycerol concentration: The methodology was developed by Valdez et al. [30] using an enzymatic kit for the determination of triglycerides (Bioclin, Belo Horizonte, MG, Brazil). Glycerol is enzymatically (glycerokinase and glycerol-3-phosphate oxidase) converted using H2O2, which is then eliminated through the action of peroxidase, using an oxygen acceptor (p-chlorophenol) and 4-aminoantipyrine. This reaction produced water and a cherry-red compound that was analyzed. Next, 10 µL of cell-free fermented broth was added to an Eppendorf tube containing 1 mL of the enzymatic kit reagent and incubated at 37 °C for 10 min. The glycerol concentration was measured using UV-spectroscopy (Bioteck Microplacas Epoch™ 2) at a wavelength of 500 nm. The calibration curve was previously established by using glycerol at different concentrations, with water applied as a blank solution.
Sugar concentration: Glucose concentration was determined through enzymatic analysis (the glucose oxidase method) using an enzymatic kit provided by Bioclin (Belo Horizonte, MG, Brazil). The experiment was carried out at 37 °C, and the absorbance was read at 505 nm. To determine the sucrose concentration, sucrose hydrolysis was initially performed using 1 mL of cell-free fermented broth and 1 mL of 0.1 M HCl at 100 °C for 10 min. The solution was cooled in an ice bath, and 1 mL of 1.0 M NaOH was added. The glucose thus produced was determined enzymatically, as previously described. The sucrose concentration was measured by subtracting the glucose value after hydrolysis from the initial value, and applying a stoichiometric factor of 1.9.
Colorimetric detection of lipopeptide classes: A solution of bromothymol blue (BTB), at a concentration 0.2 mM, was prepared in phosphate-buffered saline (0.2 M Na2HPO4, 0.2 M NaH2PO4, and 1 M NaCl) to achieve a pH of 7.2 [31]. Equal volumes (0.5 mL) of cell-free fermented broth, harvested in the fermenter or in the collector, and BTB were mixed and left for 5 min at room temperature. The formed stains correspond to different classes of lipopeptides: iturin (yellow), fengycin (light green), and surfactin (green). It should be noted that the proposed methodology only allows for an inference about the class of biosurfactant produced.
Emulsification Index (EI24): This was measured according to the methodology proposed by Cooper and Goldenberg [32], with some modifications. Briefly, a 3 mL aliquot of hexane was added to 2 mL of cell-free fermented broth. The mixture was homogenized at a high speed with a vortex for 2 min and left at room temperature for 24 h. The EI24 was calculated as the percentage of the height of the emulsified layer (mm) divided by the total height of the liquid column (mm), according to Equation (5).
where H is the height of the layer.
Additionally, the study investigated the ability of the produced biosurfactant to form emulsions using various vegetable oils (soy, olive, canola, sunflower, corn, and coconut), fuels (kerosene and gasoline), and hydrocarbons (hexane and heptane).
Emulsifier Stability (ES): To analyze the thermal stability of the biosurfactant, the cell-free fermented broth was maintained at a constant temperature range of 10–70 °C, while the effect of pH was observed by adjusting the pH value using NaOH (1 M) or HCl (1 M) in the range of 5 to 10. According to Lima and Alegre [33], the rate of emulsion decay follows a first-order process kinetic (Equation (6)).
where EA represents the emulsification activity (Abs), t denotes the time (min), and kd represents the decay constant.
By integrating the equation between 0 and t, Equation (7) was obtained.
where EA0 was the index of emulsification in initial time (t = 0 min).
The slope of the ln (EA/EA0) versus t plot corresponds to the decay constant, which describes the stability of the emulsifier. To facilitate the understanding of stability, ES was defined as presented in Equation (8):
where ES corresponds to the emulsifier stability.
To determine the EA, a 0.1 mL sample of soybean oil (0.1 mL) was added to 5 mL of liquid collected from the foam fractionation column. The mixture was mixed for 2 min at 50 °C, in order to form the emulsion. The resulting uniform emulsion was allowed to stand for 60 min, and the absorbance was determined at 540 nm every 10 min. The vessel used contained the liquid collected in the collector after the foam fractionation column. One unit of EA was defined as the quantity of biosurfactant that caused an emulsion with an absorbance of 1.0 at 540 nm [34].
3. Results and Discussion
3.1. Biosurfactant Production
Biosurfactant production by B. atrophaeus and concomitant separation using a foam fractionation column were evaluated by determining the influence of aeration rate and temperature (Tables S1–S7 of the Supplementary Materials). The best operational conditions are as shown in Table 1 and Table 2.

Table 1.
Effects of aeration rate on the production of biosurfactant from Bacillus atrophaeus at 37 °C and initial pH 7.0.

Table 2.
Effects of temperature on the production of biosurfactant from Bacillus atrophaeus at 1.00 vvm and initial pH 7.0.
Biosurfactant production by B. atrophaeus, and the simultaneous separation using a foam fractionation column were evaluated by investigating the effects of aeration rate and temperature (Tables S1–S7 of the Supplementary Materials). The optimal operational conditions are presented in Table 1 and Table 2.
B. atrophaeus has been reported as a biological agent that produces biosurfactants in culture media containing different carbon sources, such as sucrose and glycerol [35,36]. Therefore, these carbon sources were used in the culture medium formulation for this work, as indicated by the research group at the National University of Rosario, Argentina. In both cases, the fermentations were carried out in a rotary shaker, with maximum biosurfactant production detected in the fermented broth (IE24 < 62%). Therefore, it is easy to understand that a downstream process must be implemented in order to perform biosurfactant separation. In our experiments, the presence of the biosurfactant was not detected (EI24 = 0%) in the samples collected in the bioreactor, being present only in the collector. The bacterial biosurfactant contributed to forming the foam, which ascended across the foam fractionation column, coalesced, and was detected in the collector. This observation allows us to state that the process of separation concomitant with the production of biosurfactants is efficient. In this way, we obtain an aqueous solution that concentrates the biosurfactant when compared to that present in the fermented broth.
First, the effects of different aeration rates (0.25, 0.50, 0.75, and 1.00 vvm) were studied at 37 °C and with an initial pH of 7.0. The aeration rate applied to fermentation was sufficient for a satisfactory supply of sucrose for the bacteria (∆Ssucrose > 91%); on the other hand, its increase from 0.25 to 1.00 vvm impaired glycerol consumption (41.9% > ∆Sglycerol > 19.6%). However, the increase in the aeration rate played an important role in the increase in biomass concentration (X), facilitating the removal of gases and by-products of catabolism, in addition to increasing the supply of oxygen to the aerobic cell of B. atrophaeus [37].
Increasing the air supply in the system leads to a rise in foaming. Thus, the disengagement of bubbles is also increased, causing a constant overflow of the fermented broth from the bioreactor [38]. Most of the liquid contained in the Plateau channels of the foam is drawn out of the bioreactor. These channels imprint a large pressure differential between the liquid and gaseous phases [39,40]. This phenomenon was observed for up to 10 h during fermentation, resulting in an increase in the volume collected in the collector after displacement through the foam fractionation and coalescence column. The biosurfactant is also drained with the liquid, as it plays an important role in foam formation. According to Sarachat et al. [41], large volumes of drained fermented broth result in a wet foam with low biosurfactant removal as 1.00 vvm (EI24 = 65.5%), and lower aeration rate increases the residence time of the bubbles, which move slowly up to the top to generate more dried foam and higher enrichment of biosurfactant (0.25 vvm − EI24 = 67.6%). Santos et al. [42] optimized the production of biosurfactant from B. subtilis in an orbital shaker using pineapple waste, and reported an EI24 of 58.60 below that found in this work. Productivity reached a maximum value of 623.6 % mL h−1 after 10 h of processing, and was followed, at an inversely proportional rate, by the conversion of biomass into the product, since biomass increases with aeration as previously discussed. Therefore, for the next experiments, an aeration rate of 1.00 vvm was chosen due to greater productivity (high volume and EI24). The effects of different temperatures (31, 34, 37, and 40 °C) on the production of biosurfactant from B. atrophaeus were studied at 1.00 vvm of aeration rate and an initial pH of 7.0, as shown in Table 2. According to Moshtag et al. [8], higher temperatures increase the enzymatic activity and, consequently, the metabolism and the bacterial reproduction; however, they can also decrease the oxygen solubility in the culture medium, along with biomass production and product formation. Lower temperatures allow for higher microbial growth, destabilize the foam, and reduce the volume obtained in the collector, corroborating with the foam formation time that progresses from 12 (31 °C) to 9 h (40 °C). Moreover, the temperature negatively influences the carbon source consumption rate for glycerol, which changes from 32% (34 °C) to 91.5% (37 °C). The optimum temperature for biosurfactant production was 34 °C, generating the highest EI24 (66.9%) and productivity (967.5 % mL h−1). The microorganisms present a clearly thermostable nature, as suggested by Khopade et al. [43].
Productivity (Pp) was the parameter chosen to determine the best biosurfactant production condition, as it takes into account the EI24 and the volume of coalesced biosurfactant collected. Monitoring the best conditions for the production of biosurfactant from B. atrophaeus (at 34 °C, initial pH 7.0, and 1.00 vvm of aeration rate for 12 h) is illustrated in Figure 2. The maximum production of biosurfactant occurred at 12 h of fermentation, and was determined by combining the results of the collected volume (347.1 mL) and the IE24 (66.9%), which generated a productivity of 967.5% mL h−1.
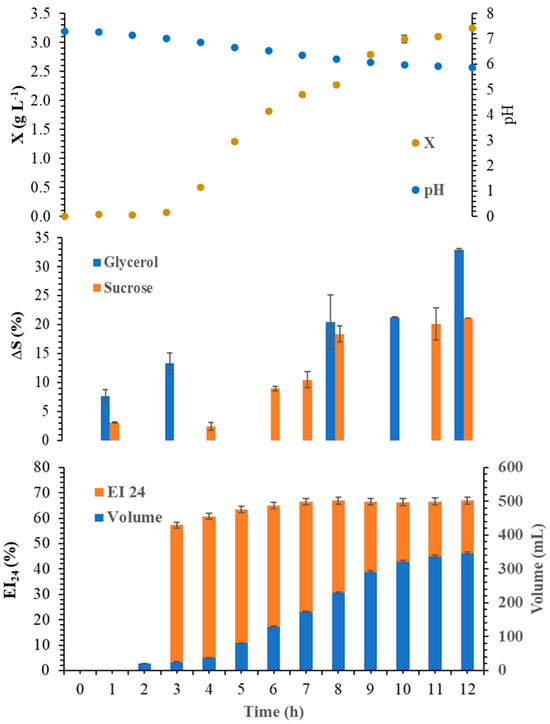
Figure 2.
Time course profile of the production of the biosurfactant formed from B. atrophaeus at 34 °C, initial pH 7.0, and 1.00 vvm of aeration rate for 12 h.
The pH drops as the fermentation progresses, probably due to the organic acids formed from the metabolism of the carbon source [44,45]. Carbohydrate consumption increases throughout fermentation, but does not exceed 31.9% of glycerol and 21.1% of sucrose. According to Das et al. [46], media containing sucrose are more suitable for microbial growth, while media formulated with glycerol are more suitable for producing biosurfactants. Therefore, the bacterium has an excessively long growth lag phase (3 h), after which the microorganism begins the exponential growth phase (3 to 10 h), then finally enters a stationary growth phase until the end of fermentation (12 h).
Obtaining coalesced foam volume and biosurfactant production starts in the exponential phase, with higher EI24 observed at 12 h (during the stationary phase of bacteria growth). Therefore, the biosurfactant production depends on the bacteria growth, as reported by Silva et al. [47] using a foam fractionation column to separate surfactin from Bacillus sp. The EI24 is reactively high and constant throughout the period of coalesced foam collection, demonstrating that the separation of the biosurfactant occurs constantly during the studied fermentation period.
3.2. Biosurfactant Properties
The methodology developed by Ong and Wu [31] was used to infer the probable type of the biosurfactant produced using B. atrophaeus. The identification is based on the spatial arrangement of amino acids present in the biosurfactant molecule and, consequently, on the different coloration caused by the presence of bromothymol blue. Using this protocol, the identity of surfactin is confirmed by a blue color, fengycin by green, and iturin by yellow. In all working conditions, the reaction presented staining ranging from the darkest to the lightest yellow hue, denoting the probable presence of iturin (Figure 3). Wan et al. [48] reported the iturin production from different species of Bacillus.
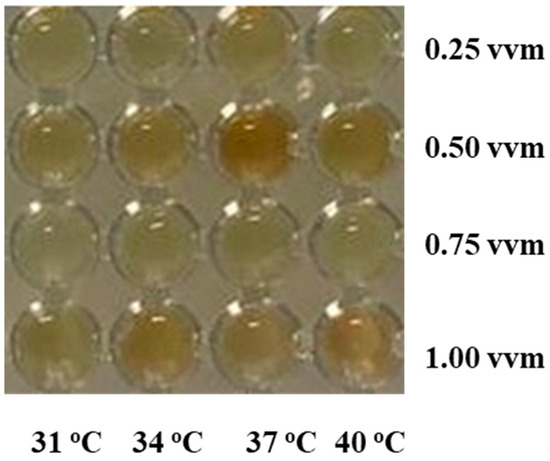
Figure 3.
Colorimetric response for the biosurfactant produced from B. atrophaeus in different operational conditions after mixture with bromophenol blue.
The importance and the use of a biosurfactant in different industries are based on its capability to form stable emulsions. The oily matrices were chosen in order to have examples of vegetable oils that allow the use of hydrocarbons in the cosmetic and food industries, hydrocarbons applied in the chemical industries, and fuel for possible application in the field of effluent treatment and tank cleaning. The measurement of EI24 was evaluated using vegetable oils (soybean, canola, corn, sunflower, olive, and coconut oil), hydrocarbons (hexane and heptane), and fuels (kerosene and gasoline) at pH 7.0 and 25 °C.
Figure 4 depicts the EI24 of iturin from B. atrophaeus against a panel of important vegetable oils, hydrocarbons, and fuels.
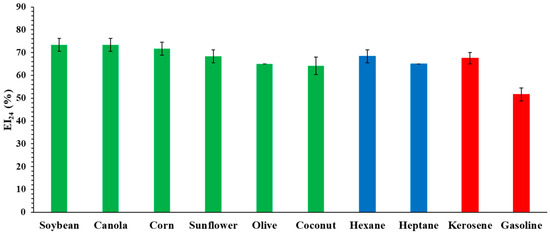
Figure 4.
Emulsification property of the biosurfactant produced from B. atrophaeus against different compounds, at pH 7.0 and 25 °C. Green (vegetable oil), blue (hydrocarbons) and red (fossil fuels).
The produced biosurfactant was able to emulsify all used vegetable oil, forming a stable emulsion. According to Sakthipriya et al. [49], the properties of biosurfactants are dependent on the type of substrate. Vegetable oils are easier to emulsify than hydrocarbons and fuels, as also observed by Sifour et al. [50] using biosurfactant from Pseudomonas aeruginosa. Biosurfactant from B. subtilis produced a lower EI24 (soybean—56%; canola—65.8%; and corn—64.5) [51] than in this work. For hydrocarbon, the EI24 reduced with an increase in carbon (Hexane—68.3% and Heptane—65.0%), as observed by Li et al. [52]. The EI24 for kerosene is higher than for gasoline, as also observed by Rahman et al. [53]. The profile presented in this article corroborates the observations of Deshmukh and Kathwate [54] regarding biosurfactants produced by Pseudomonas aeruginosas applied to kerosene and soybeans.
The industrial use of biosurfactants depends on their stability under different operational conditions, mainly temperature and pH [55]. Therefore, the stability study was carried out over a wide range of temperatures (10–70 °C) and pH (5–10), as shown in Figure 5 and Figure 6. For the ES measurement, the EA was evaluated every 10 min for 1 h (Figures S1 and S2 of the Supplementary Materials).
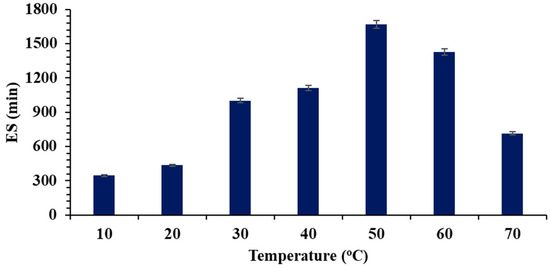
Figure 5.
Thermostability of the soybean oil–water emulsion, formed using biosurfactant from B. atrophaeus, against different compounds at pH 7.0.
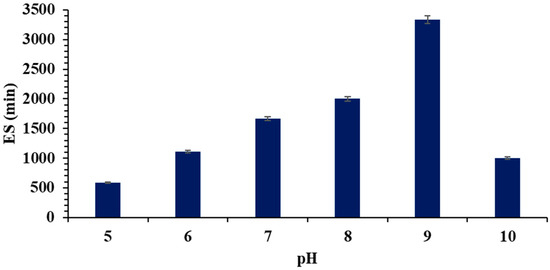
Figure 6.
pH stability of the soybean oil–water emulsion, formed using biosurfactant from B. atrophaeus, at 50 °C.
The stability of the biosurfactant increases up to medium temperatures (50 °C); afterwards, it becomes thermolabile. Elkhawaga [56] and Bouassida et al. [57]—using biosurfactants from Streptomyces griseoplanus and B. subtilis, respectively—also reported a loss of thermal stability like that observed in this work. However, other authors observed thermostability for biosurfactants up to 125 °C [58,59], demonstrating that it is fundamental to determine the stability of each biosurfactant.
The pH plays an important role in the stability of the biosurfactant. It is noted that at neutral pH, the ES values are low and increase with the alkalinity of the medium. According to Khopade et al. [43], pH value has a positive effect on the stability of the formed oil–water emulsions, as alkaline pH causes an improvement in the stability of the fatty acids in the biosurfactant, and the presence of NaOH precipitates secondary metabolites. On the other hand, in an acid medium, biosurfactants tend to coalesce and precipitate, decreasing the ES. However, at more alkaline pH values (pH = 10), a severe reduction in the stability of the biosurfactant is also observed.
4. Conclusions
This work explains that B. atrophaeus ATCC-9372 was able to produce biosurfactant—tentatively identified as iturin using the colorimetric method—which was isolated during fermentation in an air-lift bioreactor by coupling a foam fractionation column. All the biosurfactant produced was only detected in the coalesced foam obtained from the collector, indicating the concentration and separation of other metabolites from the fermented broth. The best operational condition was chosen through the combination of IE24 and collected volume expressed by Pp, and was observed using an aeration rate of 1.00 vvm and a temperature of 34 °C (EI24 = 66.9%, volume = 347.1 mL, and Pp = 967.5% mL h−1). In addition, the biosurfactant was able to form oil–water solutions in this order: vegetable oils > hydrocarbons > fossil fuels. The stability of the soybean oil–water emulsion in the presence of the biosurfactant produced here was dependent on the temperature and the pH value, with the best condition being 50 °C and pH 9.0 (ES = 3333.3 min).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9110959/s1, Figure S1: Effect of temperature on the decay of the emulsifying activity of the soybean oil-water system stabilized by Bacillus atrophaeus biosurfactant at pH 7.0. ●—10 °C, ●—20 °C, ●—30 °C, ●—40 °C, ●—50 °C, ●—60 °C, ●—70 °C. Figure S2: Effect of the pH value on the decay of the emulsifying activity of the soybean oil-water system stabilized by Bacillus atrophaeus biosurfactant at 50 °C. ●—5, ●—6, ●—7, ●—8, ●—9, ●—10. Table S1: Monitoring the fermentation process to produce biosurfactant from B. atrophaeus using 0.25 vvm at pH 7.0 and 37 °C.; Table S2: Monitoring the fermentation process to produce biosurfactant from B. atrophaeus using 0.50 vvm at pH 7.0 and 37 °C; Table S3: Monitoring the fermentation process to produce biosurfactant from B. atrophaeus using 0.75 vvm at pH 7.0 and 37 °C; Table S4: Monitoring the fermentation process to produce biosurfactant from B. atrophaeus using 1.00 vvm at pH 7.0 and 37 °C; Table S5: Monitoring the fermentation process to produce biosurfactant from B. atrophaeus using 1.00 vvm at pH 7.0 and 31 °C; Table S6: Monitoring the fermentation process to produce biosurfactant from B. atrophaeus using 1.00 vvm at pH 7.0 and 34 °C; Table S7: Monitoring the fermentation process to produce biosurfactant from B. atrophaeus using 1.00 vvm at pH 7.0 and 40 °C.
Author Contributions
Conceptualization, M.d.F.F.R. and P.S.S.J.; methodology, M.d.F.F.R. and M.S.L.; validation, M.M.P.; formal analysis, M.d.F.F.R. and M.S.L.; investigation, L.P.M.; resources, C.M.F.S.; data curation, M.M.P. and Á.S.L.; writing—original draft preparation, M.d.F.F.R. and P.S.S.J.; writing—review and editing, L.P.M., M.M.P. and Á.S.L.; visualization, M.M.P. and C.M.F.S.; supervision, Á.S.L. and C.M.F.S.; project administration, Á.S.L.; funding acquisition, Á.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), without grant number; and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 302988/2019-0. CIEPQPF is supported by the FCT through the projects UIDB/EQU/00102/2020 and UIDP/EQU/00102/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vanjani, S.J.; Upasani, V.N. Criticall review on biosurfactant analysis, purification and characterization using rhamnoolipid as a model biosurfactan. Bioresour. Technol. 2017, 232, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on surfactant flooding: Phase behavior, retention, IFT, and field applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2018, 126, 2–13. [Google Scholar] [CrossRef]
- Farias, B.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Silva, R.C.F.S.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–29. [Google Scholar] [CrossRef]
- Wu, B.; Xiu, J.; Yu, L.; Huang, L.; Yi, L.; Ma, Y. Biosurfactant production by Bacillus subtilis SL and its potential for enhanced oil recovery in low permeability reservoirs. Sci. Rep. 2022, 12, 7785. [Google Scholar] [CrossRef]
- Routhu, S.R.; Nagarjuna Chary, R.; Shaik, A.B.; Prabhakar, S.; Ganesh Kumar, C.; Kamal, A. Induction of apoptosis in lung carcinoma cells by antiproliferative cyclic lipopeptides from marine algicolous isolate Bacillus atrophaeus strain AKLSR1. Process Biochem. 2019, 79, 142–154. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Zang, T.; Wei, J.; Wu, H.; Wei, C.; Qiu, G.; Li, F. A biosurfactant-producing Pseudomonas aeruginos S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons. Bioresour. Technol. 2019, 281, 421–428. [Google Scholar] [CrossRef]
- Moshtagh, B.; Hawboldt, K.; Zhang, B. Biosurfactant production by native marine bacteria (Acinetobacter calcoaceticus P1-1A) using waste carbon sources: Impact of process conditions. Can. J. Chem. Eng. 2012, 9, 2386–2397. [Google Scholar] [CrossRef]
- Ferreira, T.F.; Martins, F.F.; Cayres, C.A.; Amaral, P.F.F.; Azevedo, D.A.; Coelho, M.A.Z. Biosurfactant production from the biodegradation of n-paraffins, isoprenoids and aromatic hydrocarbons from crude petroleum by Yarrowia lipolytica IMUFRJ 50682. Fermentation 2023, 9, 21. [Google Scholar] [CrossRef]
- Khana, A.H.A.; Tanveer, S.; Kiyani, A.; Barros, R.; Iqbal, M.; Yousaf, S. Biosurfactant-producing Aspergillus, Penicillium, and Candida performed higher biodegradation of diesel oil than a non-producing fungal strain. Appl. Biochem. Microbiol. 2023, 59, 282–289. [Google Scholar] [CrossRef]
- Rivera, A.D.; Urbina, M.A.M.; Lopez, V.E.L. Advances on research in the use of agro-industrial waste in biosurfactant production. World J. Microbiol. Biotechnol. 2019, 35, 155. [Google Scholar] [CrossRef]
- Pott, R.W.M.; Von Johannides, J. Process Development in Biosurfactant Production. In Biosurfactants for the Biobased Economy. Advances in Biochemical Engineering/Biotechnology; Hausmann, R., Henkel, M., Eds.; Springer: Cham, Switzerland; Basel, Switzerland, 2022; Volume 181, pp. 195–233. [Google Scholar] [CrossRef]
- Coutte, F.; Lecouturier, D.; Yahia, S.A.; Leclère, V.; Béchet, M.; Jacques, P.; Dhulster, P. Production of surfactin and fengycin by Bacillus subtilis in a bubbleless membrane bioreactor. Appl. Microbiol. Biotechnol. 2010, 87, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Chtioui, C.; Dimitrov, K.; Gancel, F.; Nikov, I. Biosurfactants production by immobilized cells of Bacillus subtilis ATCC 21332 and their recovery by pertraction. Process Biochem. 2010, 45, 1795–1799. [Google Scholar] [CrossRef]
- Chtioui, O.; Dimitrov, K.; Gancel, F.; Dhulster, P.; Nikov, I. Rotating discs bioreactor, a new tool for lipopeptides production. Process Biochem. 2012, 47, 2020–2024. [Google Scholar] [CrossRef]
- Vipulanandan, C.; Ghurye, G.L.; Willson, R.C. Biosurfactant production using mixed cultures under non-aseptic conditions. Mater. Res. Soc. Symp. Proc. 1994, 344, 315–322. [Google Scholar] [CrossRef]
- Ramonet, F.; Haddadi, B.; Harasek, M. Optimal design of double stage internal loop air-lift bioreactor. Energies 2023, 16, 3267. [Google Scholar] [CrossRef]
- Teli, S.M.; Mathpati, C.S. Experimental and numerical study of gas-liquid flow in a sectionalized external-loop airlift reactor. Chin. J. Chem. Eng. 2021, 32, 39–60. [Google Scholar] [CrossRef]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K.; Dayarathne, H.N.P.; Jamal, A.; Aftab, M.N.; Mainali, B.; Choi, Y. Current advances in the classification, production, properties and applications of microbial biosurfactants—A critical review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, characterization and application of biosurfactant in various industries: A critical review on progress, challenges and perspectives. Environ. Technol. Innov. 2021, 24, 102090. [Google Scholar] [CrossRef]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef]
- Venkataraman, S.; Rajendran, D.S.; Kumar, P.S.; Vo, D.V.N.; Vaidyanathan, V.K. Extraction, purifcation and applications of biosurfactants based on microbial-derived glycolipids and lipopeptides: A review. Environ. Chem. Lett. 2022, 20, 949–970. [Google Scholar] [CrossRef]
- Invally, K.; Sancheti, A.; Ju, L.K. A new approach for downstream purification of rhamnolipid biosurfactants. Food Bioprod. Process. 2019, 114, 122–131. [Google Scholar] [CrossRef]
- Joshi, S.J.; Geetha, S.J.; Desai, A.J. Characterization and application of biosurfactant produced by Bacillus licheniformis R2. Appl. Biochem. Biotechnol. 2015, 177, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Luo, L.; Jin, M.; Zhao, M.; Niu, J.; Deng, S.; Long, X. Efficient isolation of biosurfactant rhamnolipids from fermentation broth via aqueous two-phase extraction with 2-propanol/ammonium sulfate system. Biochem. Eng. J. 2022, 188, 108676. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, U. Production, purification and characterization of biosurfactant isolated from Bacillus oceanisediminis H2. Mater. Today Proc. 2023, 81, 1012–1016. [Google Scholar] [CrossRef]
- Schumpe, A.; Quicker, G.; Deckwer, W.D. Gas solubilities in microbial culture media. In Reaction Engineering. Advances in Biochemical Engineering; Fiechter, A., Ed.; Springer: Heidelberg, Germany, 1982; Volume 24, pp. 1–38. [Google Scholar]
- Junker, B. Foam and its mitigation in fermentation systems. Biotechnol. Prog. 2007, 23, 767–784. [Google Scholar] [CrossRef]
- Oraby, A.; Weickardt, I.; Zibek, S. Foam fractionation methods in aerobic fermentation processes. Biotechnol. Bioeng. 2022, 119, 1697–1711. [Google Scholar] [CrossRef]
- Valdez, H.C.; Amado, R.S.; Souza, F.C.; D’Elia, E.; Vieira, E.C. Determinação de glicerol livre e total em amostras de biodiesel por método enzimático com detecção colorimétrica. Quim. Nova 2012, 35, 601–607. [Google Scholar] [CrossRef]
- Ong, S.A.; Wu, J.C. A simple method for rapid screening of biosurfactant-producing strains using bromothymol blue alone. Biocatal. Agric. Biotechnol. 2018, 16, 121–125. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Lima, A.S.; Alegre, R.M. Evaluation of emulsifier stability of biosurfactant produced by Saccharomyces lipolytica CCT-0913. Braz. Arch. Biol. Technol. 2009, 52, 285–290. [Google Scholar] [CrossRef]
- Cirigliano, M.C.; Carman, G.M. Purification and characterization of liposan, a bioemulsifier from Candida lipolytica. Appl. Environ. Microbiol. 1985, 50, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Xue, Q.; Gao, H.; Lai, H.; Wang, P. Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery. Microb. Cell Factories 2016, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.C.M.; Oliveira, K.S.; Kobayashi, M.J.; Penna, T.C.V.; Converti, A. Biosurfactant production by cultivation of Bacillus atrophaeus ATCC 9372 in semidefined glucose/casein-based media. In Applied Biochemistry and Biotecnology; Mielenz, J.R., Klasson, K.T., Adney, W.S., McMillan, J.D., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 539–554. [Google Scholar] [CrossRef]
- Roukas, T.; Mantzouridou, F. Effect of the aeration rate on pullulan production and fermentation broth rheological properties in an airlift reactor. J. Chem. Technol. Biotechnol. 2001, 76, 371–376. [Google Scholar] [CrossRef]
- Al-Masry, W.A.; Dukkan, A.R. The role of gas disengagement and surface active agents on hydrodynamic and mass transfer characteristics of airlift reactors. Chem. Eng. J. 1997, 65, 263–271. [Google Scholar] [CrossRef]
- Anazadehsayed, A.; Rezaee, N.; Naser, J.; Nguyen, A.V. A review of aqueous foam in microscale. J. Colloid Interface Sci. 2018, 256, 203–229. [Google Scholar] [CrossRef]
- Zeng, Y.; Farajzadeh, R.; Eftekhari, A.A.; Vincent-Bonnieu, S.; Muthuswamy, A.; Rossen, W.R.; Hirasaki, G.J.; Biswal, S.L. Role of gas type on foam transport in porous media. Langmuir 2016, 32, 6239–6245. [Google Scholar] [CrossRef]
- Sarachat, T.; Pornsunthorntawee, O.; Chavadej, S.; Rujiravanit, R. Purification and concentration of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa SP4 using foam fractionation. Bioresour. Technol. 2010, 101, 324–330. [Google Scholar] [CrossRef]
- Santos, C.V.M.; Vieira, I.M.M.; Santos, B.L.P.; Souza, R.R.; Ruzene, D.S.; Silva, D.P. Biosurfactant production from pineapple waste and application of experimental design and statistical analysis. Appl. Biochem. Biotechnol. 2023, 195, 386–400. [Google Scholar] [CrossRef]
- Khopade, A.; Biao, R.; Liu, X.; Mahadik, K.; Zhang, L.; Kokare, C. Production and stability studies of the biosurfactant isolated from marine Nocardiopsis sp. B4. Desalination 2012, 285, 198–204. [Google Scholar] [CrossRef]
- Giro, M.E.A.; Martins, J.J.L.; Rocha, M.V.P.; Melo, V.M.M.; Gonçalves, L.R.B. Clarified cashew apple juice as alternative raw material for biosurfactant production by Bacillus subtilisin a batch bioreactor. Biotechnol. J. 2009, 4, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Czinkóczky, R.; Németh, A. The effect of pH on biosurfactant production by Bacillus subtilis DSM10. Hung. J. Ind. Chem. 2020, 48, 37–43. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sen, R. Substrate dependent production of extracellular biosurfactant by a marine bacterium. Bioresour. Technol. 2009, 100, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T.S.; Soares, C.M.F.; Lima, A.S.; Santana, C.S. Integral production and concentration of surfactin from Bacillus sp. ITP-001 by semi-batch foam fractionation. Biochem. Eng. J. 2015, 104, 91–97. [Google Scholar] [CrossRef]
- Wan, C.; Fan, X.; Lou, Z.; Wang, H.; Olatunde, A.; Rengasamy, K.R.R. Iturin: Cyclic lipopeptide with multifunction biological potential. Crit. Rev. Food Sci. Nutr. 2022, 62, 7976–7988. [Google Scholar] [CrossRef]
- Sakthipriya, N.; Doble, M.; Sangwai, J.S. Action of biosurfactant producing thermophilic Bacillus subtilis on waxy crude oil and long chain paraffins. Int. Biodeterior. Biodegrad. 2015, 105, 168–177. [Google Scholar] [CrossRef]
- Sifour, M.; Al-Jilawi, M.; Aziz, G.M. Emulsification properties of biosurfactant produced from Pseudomonas aeruginosa BR 28. Pak. J. Biol. Sci. 2007, 10, 1331–1335. [Google Scholar] [CrossRef]
- Durval, I.J.B.; Ribeiro, B.G.; Aguiar, J.S.; Rufino, R.D.; Converti, A.; Sarubbo, L.A. Application of a biosurfactant produced by Bacillus cereus UCP 1615 from waste frying oil as an emulsifier in a cookie formulation. Fermentation 2017, 7, 189. [Google Scholar] [CrossRef]
- Li, J.; Deng, M.; Wang, Y.; Chen, W. Production and characteristics of biosurfactant produced by Bacillus pseudomycoides BS6 utilizing soybean oil waste. Int. Biodeterior. Biodegrad. 2016, 112, 72–79. [Google Scholar] [CrossRef]
- Rahman, K.S.M.; Rahman, T.J.; Lakshmanaperumalsamy, P.; Marchant, R.; Banat, I.M. The potential of bacterial isolates for emulsificationwith a range of hydrocarbons. Acta Biotechnol. 2003, 23, 335–345. [Google Scholar] [CrossRef]
- Deshmukh, N.; Kathwate, G. Biosurfactant production by Pseudomonas aeruginosa Strain LTR1 and its application. Biointerface Res. Appl. Chem. 2023, 13, 10. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf. B 2010, 76, 298–304. [Google Scholar] [CrossRef]
- Elkhawaga, M.A. Optimization and characterization of biosurfactant from Streptomyces griseoplanus NRRL-ISP5009 (MS1). J. Appl. Microbiol. 2018, 124, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, M.; Ghazala, I.; Ellouze-Chaabouni, S.; Ghribi, D. Improved biosurfactant production by Bacillus subtilis SPB1 mutant obtained by random mutagenesis and its application in enhanced oil recovery in a sand system. J. Microbiol. Biotechnol. 2018, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bezza, F.A.; Chirwa, E.M.N. Production and applications of lipopeptide biosurfactant for bioremediation and oil recovery by Bacillus subtilis CN2. Biochem. Eng. J. 2015, 101, 168–178. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Murty, V.R. Production of a lipopeptide biosurfactant by a novel Bacillus sp. and its applicability to enhanced oil recovery. Microbiology 2013, 2013, 621519. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).