Abstract
The production of Volatile Fatty Acids (VFAs) from wastewater holds significant importance in the context of biorefinery concepts due to their potential as valuable precursors for various bio-based processes. Therefore, the primary objective of this research is to investigate the fermentation of Winery Wastewater (WW) in an Upflow Anaerobic Sludge Blanket (UASB) reactor to generate VFAs, with particular emphasis on Caproic Acid (HCa) production and the dynamics of the microbiota, under varying Hydraulic Retention Time (HRT) periods (8, 5, and 2.5 h). The change from an 8 h to a 5 h HRT period resulted in an approximately 20% increase in total VFA production. However, when the HRT was further reduced to 2.5 h, total VFA production decreased by approximately 50%. Concerning the specific production of HCa, expressed in grams of Chemical Oxygen Demand (gCOD), the maximum yield was observed at around 0.9 gCOD/L for a 5-h HRT. Microbial population analysis revealed that Eubacteria outnumbered Archaea across all HRTs. Population dynamics analysis indicated that the Firmicutes Phylum was predominant in all cases. Within this phylum, bacteria such as Clostridium kluyveri and Clostridium sp., known for their ability to produce HCa, were identified. Based on the results obtained, the application of the UASB reactor for WW treatment, within the biorefinery framework, has the potential to provide a practical alternative for HCa production when operated with a 5 h HRT.
1. Introduction
The circular economy represents the emerging socio-economic and environmental model emphasizing a production and consumption approach centred on sharing, lending, reusing, repairing, revitalising, and recycling existing materials and products, rather than disposing of them as waste [1,2]. Indeed, considering that the EU generates over 2 billion tonnes of waste each year, effectively addressing this challenge is critical for environmental security and combating climate change [2].
This substantial volume of materials originates from various sectors of the economy, including agriculture, industry, energy, services, and even households. Cow’s milk, grapes, and olives serve as the primary components in the creation of some of the world’s most prized food products, including cheese, wine, and olive oil. These foods items, particularly grapes and olives, are mainly found in the Mediterranean region, notably in France, Greece, Italy, Spain, and Tunisia. However, their production generates a significant amount of waste, with wine production in particular resulting in significant production of Winery Wastewater (WW) [3,4].
The application of circular economy principles to this WW can involve its utilization as secondary raw materials [3,4,5,6]. Incorporating WW and its derived products into the concept of a biorefinery is one way to achieve this. A biorefinery is a facility that integrates processes and equipment to convert biomass into fuels, energy, and chemicals, as defined by the National Renewable Energy Laboratory (NREL). Within this concept, we can identify the anaerobic biorefinery, which is based on Anaerobic Digestion (AD) of organic matter to produce a range of value-added products, including Volatile Fatty Acids (VFAs) and biogas (bio-hydrogen (H2) and/or methane (CH4)) [7]. Biogas, generated through AD, serves as a significant source of renewable energy, finding applications in electricity generation and heat supply. Additionally, VFAs are produced as a product of this process and can be used as a direct feedstock for the production of various bio-based products (e.g., bio-H2, CH4, solvents, or biopolymers). Therefore, both biogas (CH4 and bio-H2) and VFAs can be integrated into a biorefinery scenario [8]. Within this concept, the production and utilisation of biogas as bioenergy (renewable energy) represent a significant technological advancement, and AD from waste presents an appealing pathway to attain this goal [9].
AD is a multistage process involving hydrolysis, acidogenesis, acetogenesis, and methanogenesis, with biogas as the final product. In contrast, Acidogenic (dark) Fermentation (AF) encompasses only the hydrolysis and acidogenesis stages within AD, resulting in the production of VFAs and bio-H2 as the final product. The bacteria engaged in these initial stages belong to several phyla, including Firmicutes, Bacteriodetes, Spirochaetes, Actinobacteria, Chloroflexi, and Proteobacteria [10,11].
Microorganisms play a pivotal role in AD, as they are indispensable for the process. Analysing microbial communities can be accomplished through various techniques, including DNA sequencing, metagenomics, metatranscriptomics, and metaproteomics. These methods offer insights into the diversity, dynamics, and functional capabilities of microbial populations, thereby contributing to a comprehensive comprehension of microbial ecology and their interactions within the studied ecosystem, which is vital for achieving optimal performance.
In recent decades, the effectiveness of anaerobic technology in wastewater treatment has become increasingly evident [12]. Various technologies are available for AD of wastes and effluents, with options including suspended and adhered biomass reactors. Adhered biomass reactors (advanced reactors) offer several advantages compared to suspended biomass reactors. These advantages include a higher specific weight, enabling them to stay in the system even with upflow conditions, and the ability to handle higher feed rates without significantly increasing reactor volume [10]. Among these options, the Upflow Anaerobic Sludge Blanket (UASB) reactor emerges as an effective anaerobic process for treating wastewater with high organic loads, as evidenced by its successful applications [6,13]. The start-up of the UASB reactor requires a period of acclimatisation of the anaerobic sludge contained within the reactor (approximately one third of the reactor volume). This start-up phase holds critical importance for all types of anaerobic reactors [14]. In high-rate anaerobic technology reactors, like the UASB, the activation phase of anaerobic sludge must be carefully managed to preserve the existing structure (microbial communities present in different layers) and prevent the detachment of non-attached biomass. The integrity of granular sludge stands as the critical feature in the UASB reactor, ultimately shaping the success of processes within its reaction column [15]. Nevertheless, it is important to note that biomass does not always have to be in granular form or attached biomass. In cases where the UASB reactor is employed to treat domestic wastewaters/blackwaters. biomass can be in suspension form [16].
The Hydraulic Retention Time (HRT) exerts a significant influence on the performance of the AD process. In UASB reactors with supported biomass, it is important to distinguish between the HRT and the Solids Retention Time (SRT) of the biomass. As the HRT decreases, the Organic Loading Rate (OLR) increases [6,17]. It is important to investigate the impact of HRT for each operated AD system, considering the specific substrates or substrate mixtures used as feedstock. Gaining insight into the optimal HRT is vital for comprehending each process, establishing the best operating conditions, and ultimately achieving maximum yields. This knowledge also contributes to a broader perspective that is essential for successful future industrial scale-up [18].
The production of methane, and, thus, the possibility of energy recovery, is one of the advantages of the UASB reactor [19]. Additionally, the production and recovery of VFAs is of great interest due to their high potential as a renewable carbon source. VFAs have a wide range of applications in various industries (pharmaceutical, food, or chemical), and they can serve as valuable feedstock (biogas, biodiesel, or bioplastics). Consequently, VFA recovery through AD is becoming a prominent area of research [20]. While UASB reactors have been explored for VFA production, the emphasis has primarily been on Short Chain Fatty Acids (SCFAs) [19]. There is a growing focus on the production of valuable and versatile SCFAs such as Acetic Acid (HAc), Butyric Acid (HBu), and Propionic Acid (HPr) [21,22].
More recently, there has been a growing interest in the production of Medium-Chain Fatty Acids (MCFA), such as Caproic Acid (HCa), from organic waste and wastewater [23]. This interest arises from the potential to convert soluble SCFAs into less water soluble MCFAs through a process known as Chain Elongation (CE). In VFA extraction, the cost associated with acid solubility accounts for approximately 30% to 40% of the overall process cost, rendering the entire endeavour economically unfeasible. As an alternative, the production of less soluble acids could be a viable solution [8]. MCFAs can be produced through chemical processes that traditionally use fossil sources [11,24,25]. This process involves adding two carbons to the carbon chain length of SCFAs and utilising ethanol (EtOH), which is often present in winery effluent, as an Electron Donor (ED) to facilitate this conversion [22,23,26,27].
The primary advantage of utilizing EtOH to produce HCa lies in the energy gained during the formation of the product. According to stoichiometric reactions, one mole of HCa contains approximately 3452 kJ of energy [24]. Therefore, when aiming to produce HCa through fermentation, it is crucial to consider the requirements of the microbiota involved in the process. Specific anaerobic bacteria, such as Clostridium kluyveri and Clostridium sp. are capable of producing HCa from various carbon sources. C. kluyveri employs ethanol and acetate or succinate, whereas C. sp. utilizes galactitol or glucose for this purpose [25].
The current study aimed to investigate the effects of Hydraulic Retention Times (HRTs) in an Upflow Anaerobic Sludge Blanket (UASB) reactor with Winery Wastewater (WW), to produce a Volatile Fatty Acids (VFAs) effluent enriched in caproic acid (HCa), an important Medium-Chain Fatty Acid (MCFA). Furthermore, the influence of Hydraulic Retention Times (HRTs) changes on microbial communities was investigated.
2. Materials and Method
2.1. Substrate Characterization
The substrate used in this study was the synthetic WW (see Table 1), which was prepared by combining white and red table wines in a 50:50 ratio to achieve a concentration of 17.1 mL/L of wine in water.

Table 1.
Initial characterization of synthetic Winery Wastewater (WW).
Additionally, 0.5 g/L ammonium chloride was added to serve as a nitrogen source in the system, which is essential for microbial growth [28]. Given that the optimal Carbon/Nitrogen (C/N) ratio should be around 30:1 and above 8:1 to prevent inhibition of microbial activity due to ammonia accumulation [29,30].
Finally, the pH was adjusted using a 5 M sodium hydroxide solution until neutrality was achieved (approximately 0.25 mL/L).
Winery Wastewater (WW), Total Chemical Oxygen Demand (TCOD), Soluble Chemical Oxygen Demand (SCOD), Total Suspended Solids (TSS), Volatile Suspended Solids (VSS), Ethanol (EtOH), Phosphorus Phosphate (PO4-P), Total Ammonia Nitrogen (TAN), Total Volatile Fatty Acid (TVFA).
2.2. Operating Conditions
The design of the UASB reactor is relatively simple [31]. The experiments were conducted using a 5-litre capacity UASB digestion reactor, as shown in Figure 1, representing a design previously developed by the research group. In this case, the system had been acclimatised and adapted to the substrate. The initial anaerobic inoculum was sourced from an industrial UASB reactor with a well-developed granular structure. The inoculum consisted of anaerobic granular sludge from an industrial UASB reactor used for treating paper mill wastewater, maintained at a temperature of 30–32 °C. During the start-up phase, one-third of the lab-scale UASB reactor’s volume was inoculated with anaerobic granular sludge to achieve an initial concentration in the reactor of around 2%.
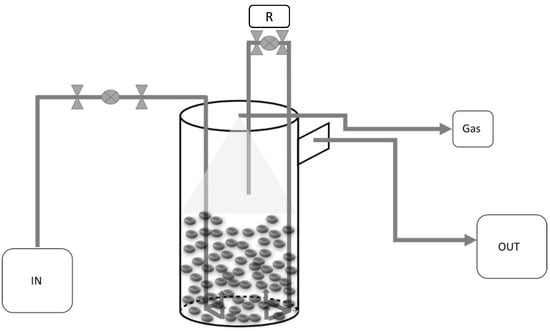
Figure 1.
Diagram of the reactor with labelled components: IN: for feeding the reactor from a tank using a pump; OUT: for the treated effluent overflow; Gas: for biogas output and collection in a Tedlar bag; R: for recirculation inside the reactor facilitated by a peristaltic pump.
The top of the reactor is equipped with five different ports, each serving a specific purpose. One port is designated for feeding the feedstock, while two other ports facilitate the recirculation of the effluent. The fourth port is utilised for the release of biogas. The treated effluent is collected from the overflow at the top of the UASB reactor and directed into a separate tank. Most of the solids accumulate at the bottom of the reactor, forming a sludge bed. A triphasic separator is located at the top of the reactor to prevent solids from leaving the system with the effluent.
Both the feed and recirculation processes operated continuously, with a peristaltic pump driving the operations. The pump speed for recirculation was carefully adjusted to maintain a total biomass uptake velocity (Vuptot) of approximately 0.6 m/h as a typical operating condition for this type of reactor [32].
The reactor had previously been operated under different HRTs for screening purposes. Using this as a reference, the experimental design for this study was proposed with three distinct HRTs; one optimal, one critical, and one in between. Additionally, it was considered that macroscopic changes in the control parameters could be observed between one HRT and the other.
A high OLR and a low HRT promote the growth of hydrolytic and acidogenic bacteria, facilitating the accumulation of VFAs [11]. Therefore, a descending HRT sequence of 8, 5, and 2.5 h was employed. These values corresponded to OLRs, expressed in grams of Chemical Oxygen Demand (gCOD), of 6.4, 10.3, and 20.6 gCOD/(L·d), respectively, as shown in Table 2. Each HRT was maintained for a minimum of one month to ensure stable performance during each operating period.

Table 2.
Variation of Organic Loading Rate (OLR) and feed flow at different Hydraulic Retention Times (HRTs).
Grams of Chemical Oxygen Demand (gCOD), Feed Volume (FV), and Flow Rate (FR). Organic Loading Rate (OLR) in gCOD/(L·d) and Hydraulic Retention Times (HRT) in h.
2.3. Analytical Methods
To start-up and monitor the reactor, an initial assessment of the substrate and routine evaluations of critical parameters throughout the process were performed. The following steps were carried out: pH was measured using a benchtop pH meter (Mettler Toledo, USA), and the Total Ammonia Nitrogen (TAN) was determined using the distillation method in adherence to standard guidelines [33].
Total Suspended Solids (TSS), Volatile Suspended Solids (VSS), Total Chemical Oxygen Demand (TCOD), and Soluble Chemical Oxygen Demand (SCOD) were assessed using the Standard Methods [33].
Ethanol (EtOH) and Phosphorus Phosphate (PO4-P) were analysed using a commercial kit. EtOH analysis was conducted with a Megazyme kit (AOAC Method 2019.08). For PO4-P quantification, the HACH TNT+ (TNT844-LM) reagent kit and a HACH® spectrophotometer were employed.
To determine the concentration of individual Acetic Acid (HAc), Propionic Acid (HPr), Butyric Acid (HBu), Valeric Acid (HVa), and Caproic Acid (HCa), and cumulative Volatile Fatty Acids (VFAs), the samples were diluted with distilled water at a ratio of 1:50 or 1:100. Subsequently, they were filtered through a 0.20 μm filter and 0.1 M hydrochloric acid was added. VFA concentrations were then determined using ionic chromatography (Dionex ICS-1100 with AS23 column, Thermo Fisher Scientific, Waltham, MA, USA).
To monitor the biogas produced in the Tedlar bags, the gas composition was measured daily using a methanimeter (Biogas 5000 gas analyser Landtec), and the daily gas volume was determined using an acid water displacement volumetric gasometer.
2.4. Microbial Community Analysis
Microbial community analysis involves the study and characterisation of the various microorganisms present in a specific environment or sample. These analyses typically encompass the identification of microorganism types, their relative abundance. and their potential roles within the ecosystem.
The microbial community analysis involved DNA sequencing of the biomass granules. A total of seven samples were collected for microbiological analysis: the first for pre-start-up characterization, followed by subsequent samples in the middle of each period and on the last day of each experimental period before the new HRT was set. DNA extraction was performed using a solid sample PowerSoil DNA Isolation extraction kit (Qiagen DNeasy PowerSoil Pro 2495), following the manufacturer’s instructions. The extracted samples were then sent to an external laboratory for sequencing. The samples were stored in a cold and aqueous solution until DNA extraction. To ensure the accuracy of the DNA extraction, a confirmation gel test was performed. The region of interest was the V3-V4 region of the 16S rRNA gene, and the extracted DNA was stored at −80 °C until being sent to BMR Genomics S.R.L. for sequencing.
Data analysis was carried out using BMR Genomics software (Qiime2 16S-V3V4 analysis Silva based).
3. Results and Discussion
Due to the strong influence of HRT and the OLR on process performance and acid profile, researchers are exploring alternatives to Continuous Stirred Tank Reactors (CSTR). Some of these being investigated are: the Sequence Batch Reactor (SBR), the Anaerobic Membrane Bioreactor (AnMBR), and the UASB. These reactors offer the advantage of separating SRT from HRT. This means that high feed rates can be achieved without significantly increasing the reactor volume, thereby increasing the cost-effectiveness of the process [11]. In this study, the UASB reactor was chosen, as it is well-suited for treating wastewater with low solids concentrations [4,15,34]. A wide range of industrial effluents have been successfully treated using the UASB reactor [31].
3.1. Influence of HRT on Effluent Characteristics
The stability threshold for a high-rate reactor, such as the UASB, in AD is usually identified as 8–6 h. Operating the reactor below this time interval can lead to destabilisation of the microbiota [14]. For this reason, the first HRT was chosen at the upper boundary of the specified range, set at 8 h. The second HRT was set just around the recommended optimum value, at 5 h. Finally, an HRT of 2.5 h, below the theoretical optimal interval, was chosen.
The analysis was carried out on the effluent of each HRT tested. Daily measurements of pH and VFA were performed. TCOD, SCOD, TSS, VSS, and TAN were measured twice a week. PO4-P and EtOH measurements were conducted once a week. The results presented below in Table 3 for each HRT represent the averages of the values obtained during each period after stabilization.

Table 3.
Medium values of the characterization of the feed (influent) and the effluent for each HRT.
Process pH affects not only raw material degradability, but also the acid profile [11]. The pH in the reactor is a critical factor for VFA production because most acidogenic microorganisms cannot survive in extreme environments (pH 3 or pH 12) [35]. Prior research has indicated that a slightly acidic to neutral pH range (5.5–7.0) favours higher VFA production during AF [36,37,38]. In order to produce VFAs from wastewater, the pH should be kept between 5 and 6 [39]. Throughout our experimental period, the pH remained close to 5 (5.29–4.71), which falls within the optimal range for this stage.
Most of the organic matter present in wastewater is soluble and easily biodegradable, but it is deficient in nitrogen and phosphorus (P) [4,40]. For this reason, ammonium chloride was added to compensate this nitrogen deficiency [40]. In particular, TAN can cause toxicity and inhibition of anaerobic systems at concentrations above 4000–5000 mg/L [17,41,42]. In our reactor, since the maximum concentration reached is 250 mg/L, this phenomenon was not observed. TAN concentration remained constant during the 8 h HRT but decreased by almost half for the other two HRTs due to net metabolic incorporation into the new biomass within the granules [15].
As discussed in several studies, the high concentration of COD in comparison to nutrients levels, underscores the significance of the COD:N:P ratio for biomass growth in AD [4,43,44]. Consequently, N and P concentrations are limiting compared to COD [4,40,43]. PO4-P concentration in WW fluctuates between 40 to 5 mg/L during the various annual stages of wine production, so, phosphorus is not a limiting factor for WW [4,40,45].
In the first two HRT, there is a net incorporation of PO4-P into the biomass, with an effluent concentration of approximately 2.3 mg/L compared to a feed concentration of about 6 mg/L [45]. This indicates net consumption of 60%. However, in the last HRT (2.5 h), the mean value of PO4-P in the effluent increases to 3.5 mg/L, with a variability percentage exceeding 60%. This suggests an unstable reactor performance and destabilisation, likely attributed to the degradation of the granules inside the digester. The same trend can be seen in the evolution of the solids (as discussed in Section 3.1.1), where they initially decrease and then increase with shorter HRT. This behaviour is associated with a certain degradation of the granule structure and, consequently, a destabilisation of the system.
The TCOD in WW is notably higher than that found in municipal wastewater, with peaks above 35 g/L during the harvesting period [4]. The usual concentrations are 2–10 g/L, during the harvest period the range is 6–8 g/L, while for the rest of the year the concentrations are 1–3 g/L [4,40]. In all tested HRTs, both TCOD and SCOD remained stable, with mean values of 2.47 g/L for TCOD and 2.34 g/L for SCOD. In general, waste materials commonly used to produce VFAs are rich in organic matter, with a TCOD above 4 g/L [17]. In our case, WW exhibited a TCOD concentration of around 2.14 g/L, a value that falls within the typical range for non-seasonal winery effluents [4].
Some studies suggest that EtOH can contribute up to 30% of the soluble COD in WW [4]. In this study, a net consumption of EtOH is observed in the first two HRTs, with efficiencies reaching up to 75%. These efficiencies are directly linked to the production of HCa, accounting for approximately 45–50% of the total VFA production (see Section 3.1.2). On the other hand, there is no EtOH consumption in the last HRT, which is another indicator of system destabilisation.
3.1.1. Removal Efficiencies of TSS and VSS with HRT
As the HRT decreased, there was an increase in the concentration of TSS and VSS, as indicated in Table 3. In general, it was found that the reduction of VSS in the system exceeded 60% during HRT of 8 h and 5 h for different OLR. The efficiency of AD treatment can be evaluated by examining the reduction of solids, as shown in Figure 2. According to the literature, the removal efficiency of TSS is reported to be around 70% for HRT of 8–6 h, which is consistent with the values observed in Figure 2 [34]. The removal efficiency of TSS decreased from 74.3% to 29.9% as the OLR increased from 6.4 to 10.3 gCOD/(L·d), and it even reached negative values when the OLR reached 20.5 gCOD/(L·d). The increase in the OLR, and consequently the higher upflow velocity, may be associated with a rise in the particles being dragged into the effluent due to the entrainment of the accumulated particles in the sludge bed. Therefore, an increase in OLR resulted in a decrease in TSS and VSS reduction, as reported in other studies [17], suggesting system instability, as previously discussed for other parameters. Furthermore, it can be observed that VSS/TSS in the effluent exhibits a linear correlation, increasing as HRT decreases, as per Equation (1) with a R2 = 0.97.
VSS/TSS = −0.39·HRT + 4.41
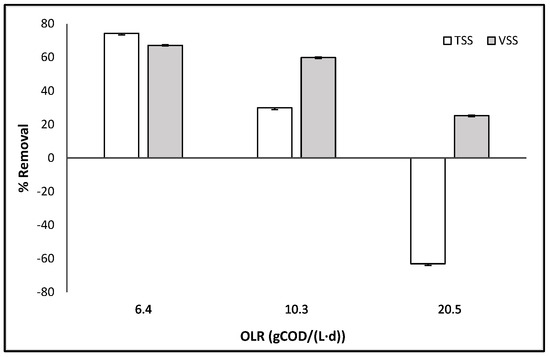
Figure 2.
Evolution of suspended solid removal, total and volatile, with OLR (gCOD/(L·d)).
This parameter and its evolution indicate a net release of active biomass as HRT decreases, which means that these microorganisms leave the system [15]. This phenomenon is directly related to the destabilisation observed in the system, as it indicates a significant loss of active microorganisms, which can negatively affect the efficiency and capacity of the AD process [16].
3.1.2. VFAs Production
In AF for VFA production, retention time and mixed microbial cultures in the anaerobic reactor are critical operational parameters [28]. The development of different VFAs shows variability based on these operational parameters. Previous research studies have highlighted that acidic pH conditions and higher OLR promote VFA production [46,47].
Finding the optimum HRT is essential for achieving the highest possible production yield. Working at higher HRTs could be advantageous for VFA production as microorganisms have more time to react and hydrolysis is favoured. However, prolonged HRT could lead to disruption of VFA production and growth of undesirable microorganisms in the AF, such as archaea, which degrade VFAs. Therefore, acetogenesis and methanogenesis must be prevented in order for VFAs to accumulate [11,17]. As can be seen in Figure 3, when the HRT is augmented from 2.5 h to 5 h, there is a 46% increase in TVFA production, but when HRT is extended from 5 h to 8 h, there is only a 26% increase. Regarding the amount of VFA produced relative to feed (OLR), the %COD to VFA increased with increasing HRT and was 5%, 17%, and 21% for 2.5 h, 5 h, and 8 h HRT, respectively.
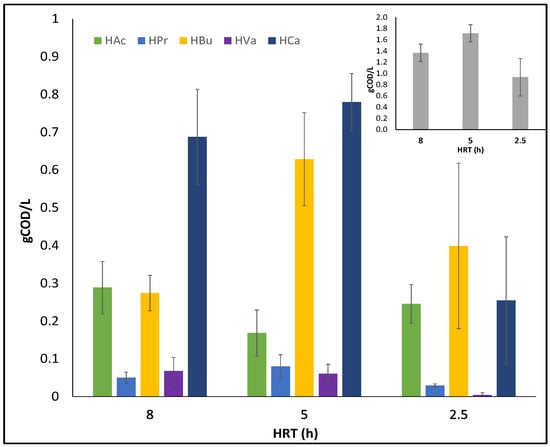
Figure 3.
Evolution of the concentration of VFAs produced in each HRT: Acetic Acid (HAc), Propionic Acid (HPr), Butyric Acid (HBu), Valeric Acid (HVa), and Caproic Acid (HCa). In the upper right corner of the graph, Total Volatile Fatty Acids (TVFAs) are represented.
HAc showed fluctuations throughout the process, initially increasing to values close to 300 mgCOD/L, decreasing during the medium HRT and then increasing again for the shorter HRT. HPr and HVa remained at low levels in all effluents. Finally, both HBu and HCa followed the same trend as TVFA, reaching maximum values of 630 mgCOD/L for HBu and 780 mgCOD/L for HCa with a 5 h HRT and decreasing by 36.5% and 68%, respectively, when the HRT was reduced to 2.5 h.
The three most common mixed VFAs produced by AD of waste streams are HAc, HPr, and HBu [17,36]. However, in our study, the predominant VFA obtained is HCa, as can be seen in Figure 3. This prevalence of HCa is attributed to the presence of EtOH in the WW.
VFAs containing more than four carbons (such as butyric, isobutyric, valeric, and isovaleric acids) are not directly utilised by methanogenic microorganisms. They require preliminary conversion into HAc, which serves as a precursor through direct metabolic conversion into CH4 and carbon dioxide (CO2) [48,49].
One of the ways in which HBu and HAc are produced is through the metabolic β-oxidation process of HCa and other VFAs [50]. This suggests that HCa is converted to HAc [51]. For an HRT of 8, the concentration of HCa was 50%, while those of HAc and HBu were 21% and 20%, respectively. In contrast, for HRTs of 5 and 2.5, the production of HCa was 45% and 27%, with an increase in HBu to 37% and 43%, respectively.
HAc is the most widely used organic acid and one of the most commercially important VFAs [36,51]. However, the global HCa market is undergoing rapid change and is projected to reach 358.8 million by 2030, growing at an average annual rate of 8.1% [36,52]. Therefore, achieving maximum efficiencies of this acid under the conditions described has a significant positive impact.
There are several studies suggesting that EtOH contributes as an ED, thereby promoting the production of MCFAs, including HCa. EtOH in the influent can promote hydrolysis, with small, degraded molecules undergoing further fermentation during acidogenesis, ultimately producing VFAs [21,22,26,27]. These studies suggest that EtOH can be used as an ED to facilitate CE [22,24,50]. In Figure 4, it can be observed that a higher presence of HCa in the medium corresponds to a decrease of EtOH in the effluents. According to the stoichiometry, if there is not enough EtOH to react with all the HAc (2EtOH:1HAc), the production of HBu and HCa is interrupted [24].
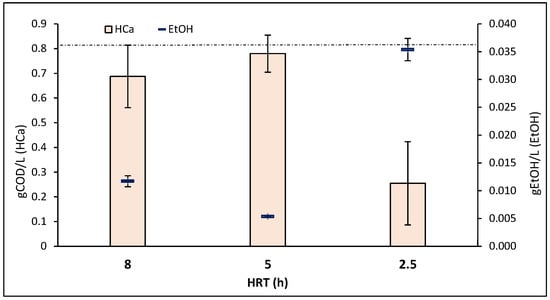
Figure 4.
Average concentrations of caproic acid and ethanol at each HRT. The dotted line indicates the medium ethanol concentration in the influent (0.036 gEtOH/L).
This suggests that the use of EtOH for CE, as proposed in previous studies, aligns with our results. For the maximum concentration of HCa at 5 h HRT, the presence of EtOH in the medium is negligible (Figure 4). It can be observed that the initial EtOH concentration has been reduced by approximately 68% for the 8 h HRT and 85% for the 5 h HRT. However, for the 2.5 h HRT, its reduction is negligible and the production of HCa for this time also decreases by 67% compared to the maximum obtained at 5 h HRT. EtOH acts as an ED and is assimilated by microbial consortia, which use it to produce VFAs, particularly favouring the production of MCFAs. Among the well-studied genera in this context, C. kluyveri stands out, as it can use EtOH as an ED to produce long-chain VFAs. Similarly, the genus Megasphaera elsdeni demonstrates the ability to employ lactate for the same purpose [20].
3.2. Biogas Production
Biogas production occurs in anaerobic environments through the action of microorganisms, using VFAs as precursors [17]. It is, therefore, challenging to establish optimal conditions for all groups of microorganisms in a digester [17,53].
AF is used to convert biodegradable biomass into VFAs, CO2, and H2 through the action of anaerobic acidogenic microorganisms. However, the production of H2 can be limited by the accumulation of VFAs in the system (end product inhibition) [54,55].
Regarding the biogas production (Table 4), daily analyses were carried out to control the stability of the reactor and to establish a relationship between the biogas and the microbial community inside the reactor.

Table 4.
Medium daily values of the percentages and volume of biogas for each HRT.
The results show that there was a 33% increase in total biogas production when transitioning from an 8 h HRT to a 5 h HRT. However, a significant decline of approximately 94% in biogas production was observed at the 2.5 h HRT, signifying a substantial change in reactor stability. It can be observed that for the first two HRTs, the CH4 generation expressed as % of the converted COD is around 24%, whereas for the 2.5 h HRT, this % decreases to around 1%.
The data indicate that although there were variations in the total volume of biogas produced, the proportions of gas composition remained relatively stable. The % CO2 content remained consistent, ranging from 6% to 9% across all HRTs. Volatile Fatty Acids (VFAs) are formed during AD, which also produces biogas as a product. Biogas is rich in CH4, with a minimum content of 65–70%. The CH4 levels obtained in this study were quite high, exceeding the upper boundary of 85% [31,36]. Values were around 91–94% in all HRTs studied. Such high CH4 levels, over 90%, have also been reported in other studies [56,57]. However, the variability of the 2.5 h HRT caused this value to fluctuate and to decrease to 85%. This fluctuation confirmed the destabilisation of the system.
The significant variability observed in all parameters related to biogas production at the lowest HRT was also indicative of system destabilisation.
3.3. Microbial Community Dynamics
AD is a multi-step process involving a wide variety of microorganisms, including fermentative bacteria (acidogens), hydrogen-producing, acetate-forming bacteria (acetogens), and archaea that convert acetate or hydrogen to methane (methanogens). The imbalance in any one step can lead to the collapse of the entire system [58,59].
Operating parameters such as pH, HRT, and OLR, among others, have a synergistic effect on the microbial communities involved in the fermentation processes, influencing cellular metabolism [60].
In UASB reactors, it has been observed that bacteria can naturally form aggregates in the form of flocs and granules [34]. By extracting DNA from the granules, it was possible to identify and analyse the microbial populations. It has also allowed the study and identification of the dominant groups of microorganisms, both their diversity and abundance present when environmental changes occur, as well as due to the variation of HRTs.
The microbiota was categorized into archaea and bacteria populations, resulting in a total of 240,413 high-quality archaeal sequences and 77,632 bacterial sequences. The relative abundance of bacterial and archaeal communities during fermentation is shown in Figure 5a.
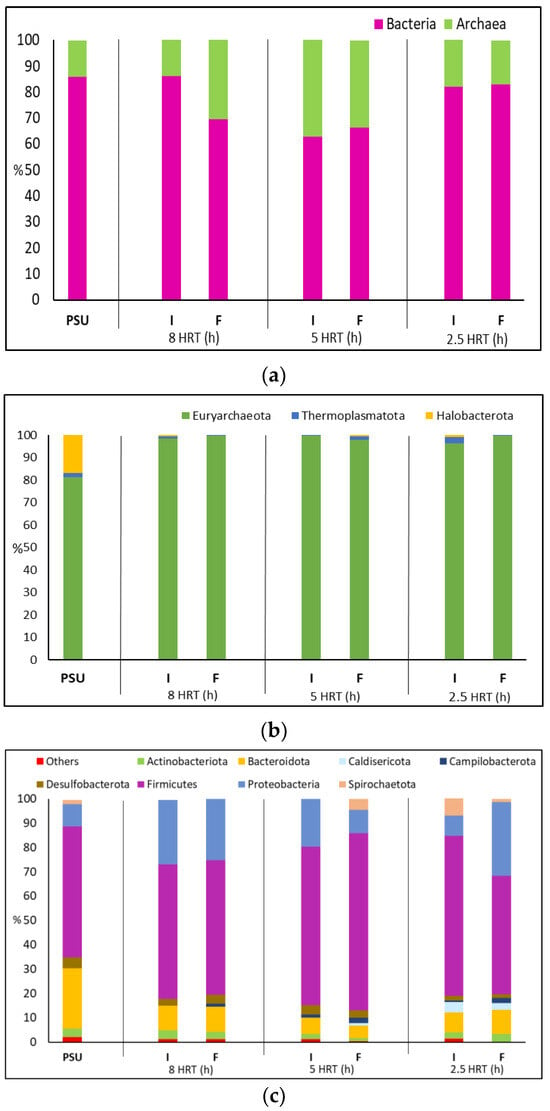
Figure 5.
(a) Evolution of the percentage abundance of Archaea versus Bacteria at each HRT; (b) Evolution of the percentage of abundance of the Phylum Bacterium in each HRT; (c) Evolution of the percentage of abundance of the Phylum Archaea in each HRT. (PSU (Pre-Start-Up), I (Intermediate), and F (Final)).
No significant differences were observed in the distribution of archaeal phyla among the various HRTs. Euryarchaeota was the predominant phylum, comprising over 96% of the archaeal population (Figure 5b). This dominance aligns with findings from other studies. The remaining two phyla each represented less than 3% of the total archaeal population across all HRTs [61]. The predominant genera with an abundance >15% detected in the Pre-Start-Up (PSU) were Methanosaeta (16%), Methanobacterium (18.5%), and Methanobrevibacter (63%). Methanosaeta is one of the major genera of acetoclastic methanogens when the microbial community is acclimated to HBu [62]. This genus belongs to the phylum Halobacterota, as shown in Figure 5b, where its abundance is approximately 17% in the PSU. However, in the different HRTs studied, its presence does not exceed 1%, and in some cases, it is absent. On the other hand, both Methanobacterium and Methanobrevibacter belong to the family Methanobacteriaceae (hydrogenotrophic methanogens), which reduce CO2 with H2 [63]. For the 8 h HRT, the most abundant genus is Methanobrevibacter, accounting for over 90%, followed by Methanobacterium at 4–8%. The remaining genera (Methanosaeta and Methanomethylophilus) have negligible abundances, sometimes even disappearing. In the case of 5 h HRT, we find that, as in the 8 h HRT, the two dominant genera are Methanobrevibacter (93–79%) and Methanobacterium (7–19%) for the intermediate and final samples of this HRT. Although these genera remain dominant, there is a decrease in the abundance Methanobrevibacter accompanied by an increase in Methanobacterium. The other two genera, as observed in other HRTs, have abundances of <2%. Finally, for the 2.5 h HRT, Methanobrevibacter initially decreases to 52% and then increases to 92%. In contrast, Methanobacterium increases to 45% and then decreases to 8%. This shift in the microbial community, as observed in previous sections, confirms the destabilization of the system at this HRT.
Figure 5c shows the main bacterial phyla found in the different HRTs studied. Over 11 bacterial phyla were detected, and only those with a significant abundance of more than 1% are shown, while the rest were grouped together. The most abundant bacterial phyla in all fermentation samples were Firmicutes (>48%), Proteobacteria (>8%), and Bacteroidota (<5%). Dominance of Firmicutes and Proteobacteria as dominant phyla has also been reported in other studies, and in particular bacteria belonging to phylum Firmicutes have been identified as important microorganisms for maximising VFA formation [11,61]. Bacteroidota is a phylum involved in the degradation of complex polymers and the hydrolysis of proteins into VFAs (HAc) and NH3 [61,64,65]. This phylum is present with an abundance of approximately 10% for the 8 h HRT, decreases to 6% for the 5 h HRT and then increases again to 9% when the HRT is reduced to 2.5 h. It is worth noting that in the sample taken for PSU, this phylum had a predominance of 25%, resulting in a reduction of about 33% compared to the initial relative abundance.
Firmicutes is a Gram-positive bacterium widely reported in AD and is known to be important in acid hydrolysis [61,64]. The high abundance of Firmicutes, approximately 50%, may be related to its adaptation to high VFAs and low pH conditions [66]. This phylum was found in all HRTs with the highest relative abundances, which were 55%, 69%, and 57% for 8 h, 5 h, and 2.5 h HRTs, respectively. This phylum usually has the highest relative abundance (65–83%) in AF [11]. Within this phylum, we can find some of the genera known to be VFAs producers, as shown in Table 5.
The Proteobacteria phylum consists of acidogenic bacteria involved in the degradation of organic matter and the consumption of VFAs. It was one of the dominant phyla in the AD of sewage sludge [65,67]. We found this phylum with an abundance of 9% in the PSU, which increased by over 50% for the 8 h and 5 h HRTs.
Spirochaetota is a phylum of syntrophic acetate-oxidising bacteria that are often present in bacterial communities in reactors. Treponema belongs to this phylum, possesses the capability to hydrolyse cellulose and hemicellulose to produce HAc from H2 and CO2 [64]. In the HRTs tested, the abundance of this phylum is low, never exceeding 8% and even disappearing in the 8 h HRT. In the specific case of the genus Treponema, it is below 2% in all cases.
Within the Desulfobacterota phylum, with an average abundance of about 3%, the metal-reducing genus can be found, such as the species Desulfovibrio, with almost complete dominance within its phylum. This species can grow syntrophically, producing H2, or act as an electron carrier between species [62,68].
The first reported case of carbon CE was in 1942, in an experiment using a pure culture of C. kluyveri, where EtOH was converted to HCa. EtOH was oxidised to HAc, then to HBu and finally to HCa [59,69]. C. kluyveri, belonging to the order Clostridiales and family Clostridiaceae. It was found in the reactor with a relative abundance of 0.1% at the beginning of the reactor, 1% for the intermediate 8 h HRT and 2.7% for the final of 8 h HRT, 1.2% for the intermediate 5 h HRT, 0.5% for the final 5 h HRT, 0.8% for the intermediate 2.5 h HRT, and 0.7% for the final 2.5 h HRT. However, if we calculate the specific percentage of this bacterium within the relative abundance of its genus (Table 5), we see a predominance of 36.89% for the 5 h HRT, 24.57% for the 8 h HRT, and 7.25% for the 2.5 h HRT, for the intermediate states of each HRT. An HRT of 5 h shows the highest predominance of these bacteria, which is consistent with the results obtained for HCa concentrations. The detection of Clostridium butyricum, known for its ability to produce HBu and CO2, was observed. However, its presence was consistently at or below 1% in all cases. In the class Clostridia, but belonging to another order, Oscillospirales, and family Ruminococcaceae, the genus Caproiciproducens was found (Table 5). It has been described that the strain Caproiciproducens galactitolivorans BS-1T produces H2, CO2, EtOH, HAc, HBu, and HCa as the final metabolic products of anaerobic fermentation, which corresponds to the production of HCa [50,59]. HCa producers, such as C. sp. (genus Caproiciproducens) [59] among others, were found in the following proportions: 3.5% for 8 h HRT, 2.3% for 5 h HRT, and 1.7% for 2.5 h HRT.
Table 5 shows the frequency of certain genera known to produce certain VFAs. In general, we can observe variations in abundance between the intermediate and final periods of the 2.5 h HRT, as was observed for the Archaea.

Table 5.
Percentage of abundance of bacteria producing the different VFAs for each HRT and authors with similar genus identification.
Table 5.
Percentage of abundance of bacteria producing the different VFAs for each HRT and authors with similar genus identification.
| VFAs | Genus | % Abundance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 8 HRT | 5 HRT | 2.5 HRT | |||||||
| PSU | I | F | I | F | I | F | |||
| HAc | Acetobacter | 0.21 | 4.08 | 5.77 | 2.02 | 3.36 | 2.93 | 2.24 | [36,50,51,53] |
| Clostridium | 2.11 | 4.07 | 9.13 | 4.88 | 3.65 | 11.03 | 1.01 | [36,50,51,53] | |
| HPr | Propionibacterium | 0.38 | 0.54 | 0.29 | 0.21 | 1.18 | 0.11 | [36,50] | |
| Acidaminococcus | 0.86 | 0.72 | 0.56 | 0.33 | 0.17 | 0.23 | 0.22 | [70] | |
| HBu | Butyricicoccus | 0 | 0.02 | 0.04 | 0.1 | 0.03 | 0.05 | 0.1 | [71] |
| Butyrivibrio | 0.02 | 0.02 | 0.01 | 0 | 0 | 0.01 | 0.05 | [36,50] | |
| Acidaminococcus | 0.86 | 0.72 | 0.56 | 0.33 | 0.17 | 0.23 | 0.22 | [70] | |
| Clostridium | 2.11 | 4.07 | 9.13 | 4.88 | 3.65 | 11.03 | 1.01 | [36,50] | |
| Eubacterium | 0.5 | 0.22 | 0.14 | 0.06 | 0.06 | 0 | 0.08 | [36,50] | |
| HCa | Caproiciproducens | 7.27 | 4.72 | 4.11 | 3.83 | 3.19 | 3.14 | 0.97 | [59] |
| Clostridium | 2.11 | 4.07 | 9.13 | 4.88 | 3.65 | 11.03 | 1.01 | [36,50,59] | |
4. Conclusions
The effect of Hydraulic Retention Time (HRT) on the acidogenic fermentation stage of a UASB technology for the treatment of synthetic Winery Wastewater was investigated, assessing various system-specific parameters and microbiota evolution.
A net generation of caproic acid was observed in all the studied HRTs. There is a direct correlation between the final concentration of this acid and the removal of ethanol in the effluent.
In all sceneries, the population of Eubacteria was higher than that of Archaea. The dominant phyla within their respective domains were Euryarchaeota and Firmicutes.
The optimal operational results were obtained at 5 h HRT, where the following observations were made.
- The maximum total production of Volatile Fatty Acids in the effluent was 1.7 gCOD/L, with a 45% of HCa (0.9 gCOD/L).
- Approximately 21% of feed COD is converted to VFA.
- The Archaeal population remained stable at over 35%, most of them (>96%) belonged to the phylum Euryarchaeota, within which the family Methanobacteriaceae (hydrogenotrophic methanogens) dominated with more than 98%.
- Within the Eubacteria population, we identified genera known for their HCa production capabilities, including Clostridium kluyveri and Clostridium sp. Both genera belong to the dominant phylum Firmicutes, which constituted 69% of the population.
- The biogas has a high methane content (>94%), with 24% of the fed COD converted into CH4.
When operating at the lowest HRT (2.5 h), system destabilization is observed, as evidenced by various parameters, such as the removal efficiencies of suspended solids and ethanol, a decrease in VFAs, and reduced biogas production.
Based on the results obtained, the application of a UASB reactor for the treatment of WW could potentially serve as a practical alternative within the context of bio-refinery and the circular economy for the production of caproic acid.
Author Contributions
M.E.I.-L.: software, formal analysis, investigation, visualization, and writing—original draft preparation. N.F.: conceptualization, methodology, resources, supervision, writing—review and editing. D.B.: conceptualization, methodology, resources, writing—review and editing, supervision, project administration and funding acquisition. J.L.G.-M.: supervision, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The stage of M. Eugenia Ibañez-López has been co-financed by the European Union within the framework of the ERDF Operational Program 2014–2020 and by the Ministry of Economic Transformation, Industry, Knowledge and Universities of the Junta de Andalucía. (Project reference: FEDER-UCA18-107460), Verinsur, S.A., ZonoSistem. Ingeniería del Ozono S.L. and the Own Research Plan—UCA 2022-2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors do not have permission to share data.
Acknowledgments
The stay of M. Eugenia Ibañez-López has been co-financed by the European Union within the framework of the ERDF Operational Program 2014–2020 and by the Ministry of Economic Trans-formation, Industry, Knowledge and Universities of the Junta de Andalucía. (Project reference: FEDER-UCA18-107460), Verinsur, S.A., ZonoSistem. Ingeniería del ozono S.L. and the Own Plan—UCA 2022–2023. Thanks to the laboratory of the University of Verona ‘LabICAB’ (Laboratory of Chemical Plants for the Environment and Bioprocesses) for allowing me to carry out my stay with them. To Davide Bertasini and Riccardo Lo Coco for showing me how to work in that laboratory.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kuci, A.; Fogarassy, C. European Green Deal Policy for the Circular Economy: Opportunities and Challenges. Hung. Agric. Eng. 2021, 39, 65–73. [Google Scholar] [CrossRef]
- European Parliament. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits?&at_campaign=20234-Economy&at_medium=Google_Ads&at_platform=Search&at_creation=RSA&at_goal=TR_G&at_audience=circular%20economy&at_topic=Circular_Economy&at_location=ES&gclid=CjwKCAjwxOymBhAFEiwAnodBLO_wVOkTIxnucxu-_fh1c-y_r_XMJibQvl1RBl8h7h7fK-GVP2W8iRoCMFkQAvD_BwE (accessed on 21 September 2023).
- Gottardo, M.; Bolzonella, D.; Tuci, G.A.; Valentino, F.; Majone, M.; Pavan, P.; Battista, F. Producing volatile fatty acids and polyhydroxyalkanoates from foods by-products and waste: A review. Bioresour. Technol. 2022, 361, 127716. [Google Scholar] [CrossRef]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Muthukumar, L.A.; Rosso, D. Winery wastewater treatment: A critical overview of advanced biological processes. Crit. Rev. Biotechnol. 2019, 39, 489–507. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. General Introduction. Gender and Land Rights Database, WWW Document. Available online: https://www.fao.org/home/en/ (accessed on 21 September 2023).
- Sivaprakasam, S.; Balaji, K. Effect of HRT on biogas yield in treating dairy industry wastewater using Upflow Anaerobic Sludge Fixed Film reactor. Mater. Today Proc. 2020, 43, 1443–1448. [Google Scholar] [CrossRef]
- Surendra, K.; Sawatdeenarunat, C.; Shrestha, S.; Sung, S.; Khanal, S.K. Anaerobic Digestion-Based Biorefinery for Bioenergy and Biobased Products. Ind. Biotechnol. 2015, 11, 103–112. [Google Scholar] [CrossRef]
- Patel, A.; Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Valorization of volatile fatty acids derived from low-cost organic waste for lipogenesis in oleaginous microorganisms—A review. Bioresour. Technol. 2021, 321, 124457. [Google Scholar] [CrossRef] [PubMed]
- Del Nery, V.; Alves, I.; Damianovic, M.H.R.Z.; Pires, E.C. Hydraulic and organic rates applied to pilot scale UASB reactor for sugar cane vinasse degradation and biogas generation. Biomass-Bioenergy 2018, 119, 411–417. [Google Scholar] [CrossRef]
- Morales-Palomo, S.; González-Fernández, C.; Tomás-Pejó, E. Prevailing acid determines the efficiency of oleaginous fermentation from volatile fatty acids. J. Environ. Chem. Eng. 2022, 10, 107354. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol. Biofuels Bioprod. 2023, 16, 1–17. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Ramasamy, E.V.; Gajalakshmi, S.; Sanjeevi, R.; Abbasi, S.A. Studies on treatment of low-strength effluents by UASB reactor and its application to dairy industry wash waters. Indian J. Biotechnol. 2007, 6, 234–238. [Google Scholar]
- Gao, Y.; Cai, T.; Yin, J.; Li, H.; Liu, X.; Lu, X.; Tang, H.; Hu, W.; Zhen, G. Insights into biodegradation behaviors of methanolic wastewater in up-flow anaerobic sludge bed (UASB) reactor coupled with in-situ bioelectrocatalysis. Bioresour. Technol. 2023, 376, 128835. [Google Scholar] [CrossRef] [PubMed]
- Mainardis, M.; Buttazzoni, M.; Goi, D. Up-Flow Anaerobic Sludge Blanket (UASB) Technology for Energy Recovery: A Review on State-of-the-Art and Recent Technological Advances. Bioengineering 2020, 7, 43. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Guha, S.; Harendranath, C.S.; Tripathi, S. Influence of extrinsic factors on granulation in UASB reactor. Appl. Microbiol. Biotechnol. 2006, 71, 145–154. [Google Scholar] [CrossRef]
- Boiocchi, R.; Zhang, Q.; Gao, M.; Liu, Y. Modeling and optimization of an upflow anaerobic sludge blanket (UASB) system treating blackwaters. J. Environ. Chem. Eng. 2022, 10, 107614. [Google Scholar] [CrossRef]
- Chuenchart, W.; Logan, M.; Leelayouthayotin, C.; Visvanathan, C. Enhancement of food waste thermophilic anaerobic digestion through synergistic effect with chicken manure. Biomass-Bioenergy 2020, 136, 105541. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Pérez, M. Thermophilic-mesophilic temperature phase anaerobic co-digestion of sewage sludge, wine vinasse and poultry manure: Effect of hydraulic retention time on mesophilic-methanogenic stage. Chem. Eng. J. 2023, 451, 138478. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Caccamo, F.M.; Calatroni, S.; Torretta, V.; Katsoyiannis, I.A.; Miino, M.C.; Rada, E.C. Applications of Up-Flow Anaerobic Sludge Blanket (UASB) and Characteristics of Its Microbial Community: A Review of Bibliometric Trend and Recent Findings. Int. J. Environ. Res. Public Health 2021, 18, 10326. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- He, J.; Shi, Z.; Luo, T.; Zhang, S.; Liu, Y.; Luo, G. Phenol promoted caproate production via two-stage batch anaerobic fermentation of organic substance with ethanol as electron donor for chain elongation. Water Res. 2021, 204, 117601. [Google Scholar] [CrossRef]
- Chen, W.-S.; Strik, D.P.; Buisman, C.J.; Kroeze, C. Production of Caproic Acid from Mixed Organic Waste: An Environmental Life Cycle Perspective. Environ. Sci. Technol. 2017, 51, 7159–7168. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, W.d.A.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic fermentation for n-caproic acid production: A review. Process. Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Choi, K.; Jeon, B.S.; Kim, B.-C.; Oh, M.-K.; Um, Y.; Sang, B.-I. In Situ Biphasic Extractive Fermentation for Hexanoic Acid Production from Sucrose by Megasphaera elsdenii NCIMB 702410. Appl. Biochem. Biotechnol. 2013, 171, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Wei, W.; Sun, J.; Xu, Q.; Dai, X.; Ni, B.-J. Medium-Chain fatty acids and long-chain alcohols production from waste activated sludge via two-stage anaerobic fermentation. Water Res. 2020, 186, 116381. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Guarino, G.; Carotenuto, C.; Di Cristofaro, F.; Papa, S.; Morrone, B.; Minale, M. Does the C/N ratio really affect the bio-methane yield? a three years investigation of buffalo manure digestion. Chem. Eng. Trans. 2016, 49, 463–468. [Google Scholar] [CrossRef]
- Melis, E.; Asquer, C.; Carboni, G.; Scano, E.A. Role of Cannabis sativa L. in energy production: Residues as a potential lignocellulosic biomass in anaerobic digestion plants. In Current Applications, Approaches, and Potential Perspectives for Hemp; Academic Press: Cambridge, MA, USA, 2023; pp. 111–199. [Google Scholar] [CrossRef]
- Poh, P.E.; Chong, M.F. Development of anaerobic digestion methods for palm oil mill effluent (POME) treatment. Bioresour. Technol. 2009, 100, 1–9. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, T.H. Applicability and trends of anaerobic granular sludge treatment processes. Biomass Bioenergy 2014, 60, 189–202. [Google Scholar] [CrossRef]
- Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Seghezzo, L.; Zeeman, G.; van Lier, J.B.; Hamelers, H.V.M.; Lettinga, G. A Review: The anaerobic treatment of sewage in uasb and EGSB reactors. Bioresour. Technol. 1998, 65, 175–190. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of Ph. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.A.D.M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the World of Volatile Fatty Acids Production and Application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile fatty acid production from organic waste with the emphasis on membrane-based recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Latessa, S.H.; Hanley, L.; Tao, W. Characteristics and practical treatment technologies of winery wastewater: A review for wastewater management at small wineries. J. Environ. Manag. 2023, 342, 118343. [Google Scholar] [CrossRef]
- Yu, H.Q.; Fang, H.H.P. Acidification of mid-and high-strength dairy wastewaters. Water Res. 2001, 35, 3697–3705. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Improvement of the anaerobic digestion of sewage sludge by co-digestion with wine vinasse and poultry manure: Effect of different hydraulic retention times. Fuel 2022, 321, 124104. [Google Scholar] [CrossRef]
- Fernández, B.; Seijo, I.; Ruiz-Filippi, G.; Roca, E.; Tarenzi, I.; Lema, J.M. Characterization, management and treatment of wastewater from white wine production. Water Sci. Technol. 2007, 56, 121–128. [Google Scholar] [CrossRef]
- Moletta, R. Winery and distillery wastewater treatment by anaerobic digestion. Water Sci. Technol. 2005, 51, 137–144. [Google Scholar] [CrossRef]
- Valderrama, C.; Ribera, G.; Bahí, N.; Rovira, M.; Giménez, T.; Nomen, R.; Lluch, S.; Yuste, M.; Martinez-Lladó, X. Winery wastewater treatment for water reuse purpose: Conventional activated sludge versus membrane bioreactor (MBR). A comparative case study. Desalination 2012, 306, 1–7. [Google Scholar] [CrossRef]
- Cheng, F.; Brewer, C.E. Conversion of protein-rich lignocellulosic wastes to bio-energy: Review and recommendations for hydrolysis + fermentation and anaerobic digestion. Renew. Sustain. Energy Rev. 2021, 146, 111167. [Google Scholar] [CrossRef]
- Tena, M.; Luque, B.; Perez, M.; Solera, R. Enhanced hydrogen production from sewage sludge by cofermentation with wine vinasse. Int. J. Hydrogen Energy 2020, 45, 15977–15984. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobu, M.; Ogawa, H.I.; Kato, Y. Degradation of Volatile Fatty Acids in Highly Efficient Anaerobic Digestion. Available online: www.elsevier.com/locate/biombioe (accessed on 21 September 2023).
- Malinowsky, C.; Nadaleti, W.; Debiasi, L.R.; Moreira, A.J.G.; Bayard, R.; Borges de Castilhos, A., Jr. Start-up phase optimization of two-phase anaerobic digestion of food waste: Effects of organic loading rate and hydraulic retention time. J. Environ. Manag. 2021, 296, 113064. [Google Scholar] [CrossRef]
- Kim, B.C.; Jeon, B.S.; Kim, S.; Kim, H.; Um, Y.; Sang, B.I. Caproiciproducens galactitolivorans gen. Nov., sp. nov., a bacterium capable of producing caproic acid from galactitol, isolated from a wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2015, 65, 4902–4908. [Google Scholar] [CrossRef]
- Mostafa, N.A. Production and recovery of volatile fatty acids from fermentation broth. Energy Convers. Manag. 1999, 40, 1543–1553. [Google Scholar] [CrossRef]
- Global Industry Analysts, Inc. Caproic Acid—Global Strategic Business Report; Global Industry Analysts, Inc.: San Jose, CA, USA, 2023. [Google Scholar]
- Lv, W.; Schanbacher, F.L.; Yu, Z. Putting microbes to work in sequence: Recent advances in temperature-phased anaerobic digestion processes. Bioresour. Technol. 2010, 101, 9409–9414. [Google Scholar] [CrossRef]
- Wang, D.; Duan, Y.; Yang, Q.; Liu, Y.; Ni, B.J.; Wang, Q.; Zeng, G.; Li, X.; Yuan, Z. Free ammonia enhances dark fermentative hydrogen production from waste activated sludge. Water Res. 2018, 133, 272–281. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Subramanian, S. Sequential production of hydrogen and methane by anaerobic digestion of organic wastes: A review. Environ. Chem. Lett. 2021, 19, 1043–1063. [Google Scholar] [CrossRef]
- Solera, R.; Romero, L.I.; Sales, D. Analysis of the methane production in thermophilic anaerobic reactors: Use of autofluorescence microscopy. Biotechnol. Lett. 2001, 23, 1889–1892. [Google Scholar] [CrossRef]
- García Morales, J.L. Dinámica de Colonización de la Biopelícula bacteriana en Reactores Anaerobios Termofílicos. Ph.D. Thesis, Universidad de Cádiz, Cádiz, Spain, 1998. [Google Scholar]
- Ramirez, I.; Volcke, E.I.P.; Rajinikanth, R.; Steyer, J.P. Modeling microbial diversity in anaerobic digestion through an extended ADM1 model. Water Res. 2009, 43, 2787–2800. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Y.; Liu, C.; Zhang, J.; Pan, J.; Luo, L.; Wu, G.; Awasthi, M.K.; Yan, B. Caproic acid production from anaerobic fermentation of organic waste—Pathways and microbial perspective. Renew. Sustain. Energy Rev. 2023, 175, 113181. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Zhou, H.; Brown, R.C.; Wen, Z. Anaerobic digestion of aqueous phase from pyrolysis of biomass: Reducing toxicity and improving microbial tolerance. Bioresour. Technol. 2019, 292, 121976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Yu, Q.; Dang, Y.; Li, Y.; Quan, X. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 2016, 102, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Eyice, Ö.; Cetecioglu, Z.; Plaza, E. The study of structure of anaerobic granules and methane producing pathways of pilot-scale UASB reactors treating municipal wastewater under sub-mesophilic conditions. Bioresour. Technol. 2019, 290, 121733. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Liu, J.; Yang, F.; Zhu, W.; Yuan, X.; Hu, Y.; Ciu, Z.; Wang, X. Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass. Bioresour. Technol. 2017, 241, 349–359. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Zhang, Y.; Si, D.; Chen, Q. Evolution of microbial community along with increasing solid concentration during high-solids anaerobic digestion of sewage sludge. Bioresour. Technol. 2016, 216, 87–94. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Z.; Li, Y. Impact of different ratios of feedstock to liquid anaerobic digestion effluent on the performance and microbiome of solid-state anaerobic digesters digesting corn stover. Bioresour. Technol. 2016, 200, 744–752. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Z.; Li, Y. Sequential batch thermophilic solid-state anaerobic digestion of lignocellulosic biomass via recirculating digestate as inoculum—Part II: Microbial diversity and succession. Bioresour. Technol. 2017, 241, 1027–1035. [Google Scholar] [CrossRef]
- Walker, C.B.; He, Z.; Yang, Z.K.; He, Q.; Zhou, J.; Voordouw, G.; Wall, J.D.; Arkin, A.P.; Hazen, T.C.; Stolyar, S.; et al. The electron transfer system of syntrophically grown Desulfovibrio vulgaris. J. Bacteriol. 2009, 191, 5793–5801. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.A.; Taha, S.M. Clostridium kluyverii, an Organism Concerned in the Formation of Caproic Acid from Ethyl Alcohol. J. Bacteriol. 1942, 43, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Böck, A. Fermentation. In Encyclopedia of Microbiology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 132–144. [Google Scholar] [CrossRef]
- Chang, S.C.; Shen, M.H.; Liu, C.Y.; Pu, C.M.; Hu, J.M.; Huang, C.J. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer. Oncol. Lett. 2020, 20, 327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).