Abstract
Application of cool temperatures were studied to encourage Metschnikowia pulcherrima P01A016 and Meyerozyma guilliermondii P40D002 prior inoculation of Saccharomyces cerevisiae D254 to lower ultimate ethanol concentrations achieved. Merlot grape must was distributed into 300 L temperature-controlled tanks and inoculated with non-Saccharomyces yeasts three days before S. cerevisiae. For control fermentations, S. cerevisiae was inoculated with maximum temperatures set to 25 °C (temperature regime I) while those with Mt. pulcherrima or My. guilliermondii were initially set to 15 °C (temperature regime II) or 17.5 °C (temperature regime III) before increasing to 25 °C after adding S. cerevisiae. Once fermentations achieved dryness (≤2 g/L residual sugar), wines were bottled and stored for six months at 7 °C before sensory analysis. Ethanol reduction by Mt. pulcherrima was not observed in wines fermented under II but was by III (0.8% v/v). In contrast, musts inoculated with My. guilliermondii yielded wines with ethanol concentrations lowered by 0.3% (II) or 0.4% v/v (III). Sensory panelists found wines with Mt. pulcherrima to express lower sensory scores for ‘hotness’, ‘bitterness’, and ‘ethanol’ flavor with fewer differences noted for My. guilliermondii. Reducing final ethanol concentrations of Merlot wines were achieved by Mt. pulcherrima or My. guilliermondii using cooler initial fermentation temperatures without adversely affecting final wine quality.
1. Introduction
Depending on regional legal regulations, wine processing methods such as “saignée” and/or “water-back” may be used by the industry to dilute the concentrations of sugars in grape musts as a means to lower the amounts of ethanol ultimately produced fermentation. As an alternative to dilution, Gonzalez et al. [1] proposed the use of non-Saccharomyces yeasts which partially consume fermentable sugars to metabolites other than ethanol before inoculation of S. cerevisiae. As noted by Skoniecznys et al. [2]), non-Saccharomyces yeasts like Metschnikowia pulcherrima tend to exhibit poor fermentation characteristics and musts are therefore sequentially inoculated with S. cerevisiae to complete fermentation. Using this approach, reported reductions in ethanol have ranged from 0.25% to 3.7% v/v depending on yeast species, conditions, and medium [3,4,5,6,7,8,9,10,11,12,13].
To date, much of the research involving non-Saccharomyces yeasts has utilized small-scale fermentations (≤5 L) and/or synthetic grape juice media which are not always representative of larger, industrial ferments of grape musts [12,14,15,16]. For instance, oxygen availability becomes less with increases in fermentation volume [17], a factor which would affect the oxidative metabolisms associated with many non-Saccharomyces yeasts [4,7,18]. Furthermore, temperature gradients formed under the grape skin caps during larger fermentations [19] would also influence ethanol tolerances of these yeasts given sensitivities to temperature [20,21].
Many studies have focused on factors affecting survival of non-Saccharomyces yeasts including lower temperatures than those used industrially to conduct fermentation [22,23,24,25,26]. However, few have investigated optimization of growth/metabolism under fermentative conditions. In one report, Maturano et al. [27] noted that of the factors studied, fermentation temperature and timing of inoculation had the largest impact on ethanol reduction of sterilized grape musts using Hanseniaspora uvarum and Candida membranaefaciens with S. cerevisiae. While Aplin et al. [13] utilized 300 L tanks to conduct fermentations of Merlot grape musts with non-Saccharomyces yeasts, temperatures were initially at 20 °C yet uncontrolled during vinification. Furthermore, Aplin et al. [13] observed reductions in ethanol in those fermentations inoculated with Mt. pulcherrima but not Meyerozyma guilliermondii, another yeast with commercial potential [12,16]. Conducting grape fermentations at 20 °C, Aplin et al. [13] reported no adverse quality impacts of Mt. pulcherrima or My. guilliermondii on wine quality but also very few sensory differences. Likewise, García et al. [10] observed decreased ethanol concentrations in a Spanish white wine applying sequential inoculations of My. guilliermondii with S. cerevisiae although information regarding impacts on quality were lacking. In this study, the objective was to examine the impact of lower grape must temperatures on the abilities of non-Saccharomyces yeasts (i.e., Mt. pulcherrima and My. guilliermondii) towards reducing final ethanol contents of Merlot wines without adversely affecting sensory quality. Here, pilot plant-scale tanks (300 L) will be utilized to produce the wines fermented under different temperature regimes prior to chemical and sensory analyses.
2. Materials and Methods
2.1. Yeast Strains and Starter Cultures
Metschnikowia pulcherrima P01A016 and Meyerozyma guilliermondii P40D002 were previously isolated from vineyards located at the Irrigated Research and Extension Center, Washington State University (Prosser, WA, USA) as described by Bourret et al. [28]. Saccharomyces cerevisiae D254 was acquired from Lallemand Inc. (Montréal, QC, Canada). All yeasts were maintained on yeast peptone dextrose (YPD) agar plates incubated at 28 °C.
Starter cultures of non-Saccharomyces were prepared in YPD broth (initially 10 mL followed by transfers to 1 L) from single colonies grown on YPD agar. Upon reaching late exponential phase, cells were harvested by centrifugation at 3000× g for 15 min, washed twice with 0.2 M phosphate buffer (pH 7.0), and resuspended in Merlot grape must diluted 1:1 with sterile water prior to inoculation. Active dry cultures of S. cerevisiae were rehydrated as per manufacturer’s instructions prior to inoculation.
2.2. Fermentations
Merlot grapes were obtained from a commercial vineyard located in south central Washington state in 2018. Vines were trained to a bilateral cordon system, spur pruned, irrigated using regulated deficit practices with 30 lbs of nitrogen per acre applied between bloom and veraison. After crushing/destemming harvested grapes, grape must (145 g/L glucose, 142 g/L fructose, pH 3.43, 2.59 g/L titratable acidity, and 61 mg N/L yeast assimilable nitrogen) was homogenously distributed into 300 L stainless steel, jacketed fermentation tanks (120 kg/tank). Continuous temperature control and monitoring via probe was achieved using TankNET software (Acrolon Technologies, Inc., Sonoma, CA, USA). Enough potassium metabisulfite (Sigma Aldrich, St. Louis, MO, USA) was added to each fermenter to achieve 25 mg/L total SO2.
On day 0, either S. cerevisiae (control) or non-Saccharomyces (treatment) were inoculated into duplicate tanks. For control fermentations, S. cerevisiae was inoculated as previously described (yielding initial populations of approximately 106 to 107 cfu/mL) and lids were attached while maximum temperatures were set to 25 ± 1 °C (temperature regime I). For treatment fermentations, either Mt. pulcherrima or My. guilliermondii were added at approximately 106 cfu/mL and tank lids were not attached while maximum temperatures were set at either 15 ± 1 °C (temperature regime II) or 17.5 ± 1 °C (temperature regime III). After three days, S. cerevisiae was inoculated as previously described, tanks were closed by reattaching the lids, and maximum temperatures raised to 25 °C. For all fermentations, cap management consisted of twice daily punch-downs and two additions of 0.25 g/L Fermaid-K (Lallemand), one 12 h after inoculation of S. cerevisiae and another on two days later.
At approximately 0° Brix, fermenting musts were pressed into 100 L stainless steel tanks and stored at ambient temperature (21° ± 2 °C). All wines underwent spontaneous malolactic fermentation prior to addition of potassium metabisulphite (30 mg/L total SO2) and storage at 9 °C. After adjustment to 0.4 mg/L molecular SO2, wines were sterile-filtered through 0.45 μm polyvinylidene fluoride cartridges (MilliporeSigma, Bellerica, MA, USA) housed in stainless-steel filter housings (Pall, Port Washington, NY, USA) into sterile 750 mL screw-capped bottles previously flushed with N2 gas. After bottling, the wines were stored at 7 °C for six months prior to chemical and sensory analyses.
2.3. Analytical Analyses
Yeast culturabilities were monitored throughout fermentation and storage by spiral plating (Autoplate 4000, Spiral Biotech, Bethesda, MD, USA) using Wallenstein Laboratory agar (WL, Becton, Dickinson, and Company, Franklin Lakes, NJ, USA) for total yeast populations and lysine agar (Oxoid, Hamphshire, England) for non-Saccharomyces yeasts. All plates were incubated for two to four days at 28 °C prior to counting. Populations of S. cerevisiae were estimated based on the difference between plate counts on those two media.
Glucose, fructose, ethanol, glycerol, and organic acids were quantified with an Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a refractive index detector according to Eyéghé-Bickong et al. [29] with some modifications. Samples were filtered through 0.45 μm polyethersulfone membranes (MilliporeSigma) into crimp-top vials prior to analysis. Separation was accomplished by an Aminex HPX-87H column (300 × 7.8 mm, BIO-RAD, Hercules, CA, USA) equilibrated to 60 °C using 0.005 M H2SO4 as the mobile phase at 0.6 mL/min. During fermentation, soluble solids (° Brix) were measured with a portable density meter (DMA35, Anton-Paar, Graz, Austria).
2.4. Sensory Analyses
To determine if there were perceivable differences between the treatment tank replicates (2), a difference from control test was completed. Experienced panelists (n = 8) were recruited to evaluate the samples and wines from replicate tanks showing no differences were pooled. To reduce fatigue, panelists were provided with water and unsalted crackers and were required to wait 2 min between samples.
Sensory analysis wines was conducted after six months of storage in bottles at 7 °C. Wines were then evaluated by a trained panel consisting of students and staff (n = 10, 5 females, 5 males, aged 22 to 36 with a mean of 29) recruited from Washington State University. Panelists received 12 h of training across six weeks using feedback calibration through Compusense Cloud 8.8 sensory acquisition software (Compusense, Guelph, Ontario, Canada). During the first training session, panelists were instructed to remove the lid from the wine glass and preform three short, sharp sniffs, allowing 30 s to pass in between evaluations. For tasting, panelists were instructed to take the sample into the mouth, swish for 10 to 15 s, expectorate, wait for 30 s, then start evaluating, reporting the highest intensity for each attribute experienced. Samples (40 mL) were presented to panelists in three-digit coded ISO standard, covered wine glasses (in triplicate) at room temperature in individual tasting booths under white light at the Washington State University Sensory Evaluation Facility. Responses were collected using a 15 cm, unstructured line scale with anchor points ‘low’ (10% of the scale) and ‘high’ (90% of the scale) using Compusense software.
2.5. Statistical Analyses
Statistical analyses for chemical analysis were performed by ANOVA while mean separations were accomplished by Fisher’s least significant difference (LSD) using XLSTAT (Addinsoft, New York, NY, USA). For sensory data, three-way ANOVA was used to analyze panelist, treatment, and replicate interactions while means were separated using Fisher’s LSD. Differences were considered significant when p < 0.05. Principal component analysis (PCA) of covariance for panel data was performed using XLSTAT.
3. Results
For control fermentations with only S. cerevisiae inoculated (Figure 1), populations were approximately one log higher than expected but peaked close to 109 cfu/mL by day 4 before entering a slow decline. In fact, viability of S. cerevisiae never decreased to <107 over 24 days. Must temperatures steadily increased from approximately 18 °C up to the maximum set temperature of 25 °C before slowly declining to 23 °C. As must temperatures initially increased, viabilities of indigenous non-Saccharomyces yeasts also declined from 105 cfu/mL to undetectable levels by day 8.
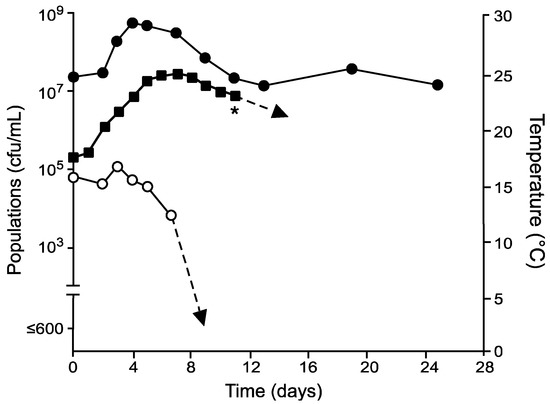
Figure 1.
Culturable populations of S. cerevisiae ( ), total non-Saccharomyces yeasts (
), total non-Saccharomyces yeasts ( ), and temperatures (
), and temperatures ( ) in grape musts inoculated on day 0 with S. cerevisiae following fermentation temperature regime I. * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
) in grape musts inoculated on day 0 with S. cerevisiae following fermentation temperature regime I. * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
 ), total non-Saccharomyces yeasts (
), total non-Saccharomyces yeasts ( ), and temperatures (
), and temperatures ( ) in grape musts inoculated on day 0 with S. cerevisiae following fermentation temperature regime I. * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
) in grape musts inoculated on day 0 with S. cerevisiae following fermentation temperature regime I. * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
Lowering initial fermentation temperatures greatly affected short- and long-term survival of non-Saccharomyces yeasts. In general, temperatures maintained at 15 °C or 17.5 °C for the first three days of fermentation resulted in increases of total non-Saccharomyces yeast populations (Figure 2 and Figure 3). Those inoculated with Mt. pulcherrima and conducted under temperature regime II maintained total populations of non-Saccharomyces yeasts ≈106 cfu/mL (Figure 2a). Raising initial fermentation temperatures to 17.5 °C (temperature regime III) resulted in a high number of cells (107 cfu/mL) with populations still detected by day 13 after the peak temperature of 25 °C had been reached (Figure 2b). Under the same conditions, My. guilliermondii behaved differently by exhibiting a slightly longer lag phase under temperature regime II (Figure 3a) than under III (Figure 3b) but still displayed increases in total populations of approximately one log. Like fermentations inoculated with Mt. pulcherrima, total non-Saccharomyces populations remained detectable longer than control fermentations (Figure 1), up to day 13. The presence of Mt. pulcherrima or My. guilliermondii did not affect the growth of S. cerevisiae where viabilities all reached 108 to 109 cfu/mL.
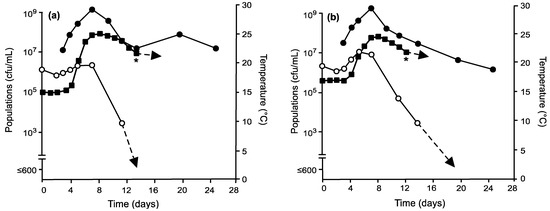
Figure 2.
Culturable populations of S. cerevisiae ( ), total non-Saccharomyces yeasts (
), total non-Saccharomyces yeasts ( ), and fermentation temperatures (
), and fermentation temperatures ( ) in grape musts inoculated on day 0 with Mt. pulcherrima prior to inoculation with S. cerevisiae on day 3 and following fermentation temperature regime II (a) or III (b). * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
) in grape musts inoculated on day 0 with Mt. pulcherrima prior to inoculation with S. cerevisiae on day 3 and following fermentation temperature regime II (a) or III (b). * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
 ), total non-Saccharomyces yeasts (
), total non-Saccharomyces yeasts ( ), and fermentation temperatures (
), and fermentation temperatures ( ) in grape musts inoculated on day 0 with Mt. pulcherrima prior to inoculation with S. cerevisiae on day 3 and following fermentation temperature regime II (a) or III (b). * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
) in grape musts inoculated on day 0 with Mt. pulcherrima prior to inoculation with S. cerevisiae on day 3 and following fermentation temperature regime II (a) or III (b). * Denotes time when the wine was pressed (approximately 0° Brix) prior to storage at 21 °C.
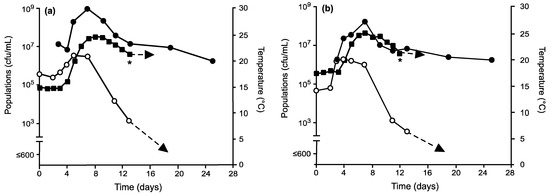
Figure 3.
Culturable populations of S.
cerevisiae ( ), total non-Saccharomyces
yeasts (
), total non-Saccharomyces
yeasts ( ), and temperatures
(
), and temperatures
( ) in grape musts
inoculated on day 0 with My. guilliermondii prior to inoculation with S.
cerevisiae on day 3 and following fermentation temperature regime II (a)
or III (b). * Denotes time when the wine was pressed (approximately 0°
Brix) prior to storage at 21 °C.
) in grape musts
inoculated on day 0 with My. guilliermondii prior to inoculation with S.
cerevisiae on day 3 and following fermentation temperature regime II (a)
or III (b). * Denotes time when the wine was pressed (approximately 0°
Brix) prior to storage at 21 °C.
 ), total non-Saccharomyces
yeasts (
), total non-Saccharomyces
yeasts ( ), and temperatures
(
), and temperatures
( ) in grape musts
inoculated on day 0 with My. guilliermondii prior to inoculation with S.
cerevisiae on day 3 and following fermentation temperature regime II (a)
or III (b). * Denotes time when the wine was pressed (approximately 0°
Brix) prior to storage at 21 °C.
) in grape musts
inoculated on day 0 with My. guilliermondii prior to inoculation with S.
cerevisiae on day 3 and following fermentation temperature regime II (a)
or III (b). * Denotes time when the wine was pressed (approximately 0°
Brix) prior to storage at 21 °C.
While initial sugar utilization was more rapid for those fermentations inoculated with S. cerevisiae alone, non-Saccharomyces yeasts still displayed sugar depletion during the first 3 days of fermentation (data not shown). As evidence, S. cerevisiae consumed 70 g/L glucose/fructose by day 3, while fermentations inoculated with non-Saccharomyces yeasts on day 0 displayed glucose/fructose depletion between 6 to 36 g/L. However, despite the three-day delay in inoculation of S. cerevisiae in fermentations inoculated with non-Saccharomyces yeasts, all fermentations were depleted of glucose and fructose by day 25 (Table 1).

Table 1.
Composition of finished wines fermented by S. cerevisiae alone (fermentation temperature regime I) or with selected non-Saccharomyces yeasts prior to inoculation of S. cerevisiae on day 3 (fermentation temperature regimes II or III).
Besides sugars, the presence of non-Saccharomyces yeasts affected production of other yeast metabolites (Table 1). Most wines inoculated with the non-Saccharomyces yeasts contained lower amounts of ethanol compared to those with only S. cerevisiae. While wines inoculated with S. cerevisiae alone displayed the highest ethanol concentrations (15% v/v), wines inoculated with Mt. pulcherrima following temperature regime II achieved concentrations lower by approximately 0.8% v/v. Reductions were also observed in fermentations inoculated with My. guilliermondii following temperature regimes II (0.3% v/v) or III (0.4% v/v). Differences in ethanol can be attributed to both yeast species and fermentation temperature with significant interactive effects (Table 2). Besides ethanol, differences were also noted for glycerol, succinic acid, and acetic acid (Table 1). For instance, all wines inoculated with non-Saccharomyces contained less amounts of glycerol and succinic acid than wines inoculated with S. cerevisiae alone. Additionally, all wines except those inoculated with Mt. pulcherrima following temperature regime II, contained less acetic acid than those inoculated with S. cerevisiae alone. Most differences were attributed to yeast species rather than fermentation temperature, but interactive affects were observed for glycerol (Table 2).

Table 2.
Significance and F ratios from analysis of variance of chemical composition of Merlot wines inoculated with or without non-Saccharomyces yeasts followed by S. cerevisiae.
Initial evaluation by experienced panelists (n = 8) had revealed no significant sensory differences between replicate fermentation tanks of inoculated with Mt. pulcherrima which allowed these wines to be pooled. Subsequent descriptive analysis of wines by the trained panel (n = 10) revealed significant differences between 26 of the 44 sensory attributes evaluated (Table 3). No significant differences were noted between wines produced with Mt. pulcherrima under temperature regime II compared to those inoculated only with S. cerevisiae. However, wines with the largest ethanol reduction (Mt. pulcherrima under temperature regime III) were rated significantly lower in undesirable aroma (‘herbaceous’), taste (‘bitter’), flavor (‘sweaty’, ‘ethanol’, ‘sulfur’, and ‘herbaceous’) and mouthfeel (‘hot/ethanol’ and ‘roughness’) while higher in desirable attributes (‘round’) compared to those wines inoculated with S. cerevisiae alone. These wines were also rated lower for other aroma (‘dried fruit’), mouthfeel (‘tingle’ and ‘sharp’), flavor (‘dried fruit’) as illustrated in Figure 4.

Table 3.
Mean scores for sensory attributes of wines inoculated without or with different non-Saccharomyces yeasts following fermentation temperature regimes I, II, or III.
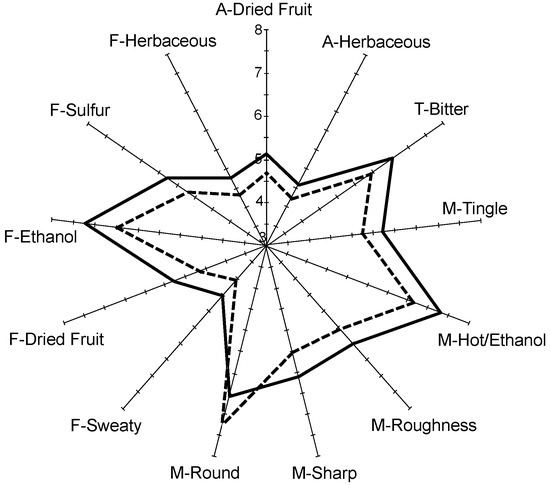
Figure 4.
Spider chart of mean values of sensory attributes found to be significantly different (p< 0.05) by trained panel (n = 10) between wines produced with S. cerevisiae alone under fermentation temperature regime I (solid line) or with sequential inoculation of Mt. pulcherrima/S. cerevisiae under fermentation temperature regime III (dashed line). ‘A’ denotes aroma attribute, ‘T’ denotes taste attribute, ‘M’ denotes mouthfeel attribute, and ‘F’ denotes flavor attribute.
Unlike fermentation with Mt. pulcherrima, replicate tanks of wines inoculated with My. guilliermondii following temperature regime II were noted to be significantly different by the experienced panel. As sensory differences appeared to exist between tank replicates, these wines were evaluated by the trained panel separately (Table 3). In fact, the tank replicates differed in aroma (‘spicy’) but primarily mouthfeel (‘roughness’, ‘drying’, ‘puckering’, and ‘sharp’) with one replicate tank possessing attributes not different from wines inoculated with only S cerevisiae.
To better visualize differences, principal component analysis (PCA) was performed including only significant (p < 0.05) sensory attributes as illustrated in Figure 5. Here, principal component 1 (F1) explained 70.78% of the variance between wines while principal component 2 (F2) explained 11.51%. Wines inoculated with S. cerevisiae alone (temperature regime I) or with My. guilliermondii (temperature regimes IIa and III) were described by having similar attributes of ‘solvent’, ‘herbaceous’, ‘sweaty’, ‘dried fruit’, and ‘ethanol’ but differed from My. guilliermondii (IIb). Wines inoculated with Mt. pulcherrima (temperature regime III) varied the most from the other wines produced.
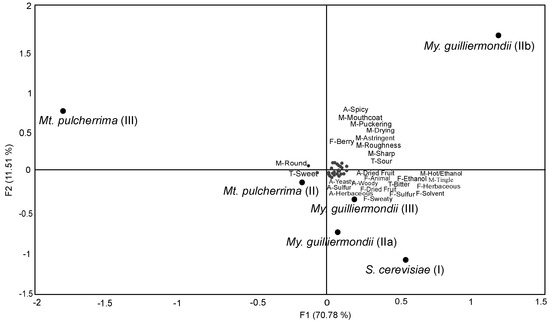
Figure 5.
Principal component analysis of sensory attributes found to be significantly different (p < 0.05) by trained panel (n = 10) between wines produced with S. cerevisiae alone (fermentation temperature regime I) or with sequential inoculation of Mt. pulcherrima or My. guilliermondii followed by S. cerevisiae (fermentation temperature regimes II or III).
4. Discussion
Cooler initial temperatures of grape musts affected subsequent growth and survival of both uninoculated (indigenous) and inoculated non-Saccharomyces yeasts during vinification. Musts with added Mt. pulcherrima or My. guilliermondii had higher total populations of non-Saccharomyces yeasts than those ferments containing only unidentified indigenous species. As evidence, viability of total non-Saccharomyces yeasts in musts with Mt. pulcherrima or My. guilliermondii peaked between 106 and 107 cfu/mL and remained detectable for 12 to 13 days in musts following temperature regimes II or III. In comparison, indigenous non-Saccharomyces populations in musts maintained under temperature regime I steadily declined to undetectable levels.
Non-Saccharomyces yeasts are generally thought to remain viable longer at temperatures lower than 25 °C but above 10 °C compared to S. cerevisiae [20,30]. As evidence, Zott et al. [23] reported better growth of non-Saccharomyces yeasts during cold maceration of musts at 15 °C compared to maceration at either 4 °C or 10 °C. Better growth at lower temperatures may be due to improved ethanol tolerances [31] as illustrated by Kloeckera apiculata (Hanseniaspora uvarum) and Candida stellata which exhibit increased ethanol tolerances at temperatures of 10 °C to 15 °C [20,21].
In addition to temperature, oxygen availability affects the metabolism of non-Saccharomyces yeasts. In the present study, tank lids were applied to fermentations three days after inoculation of non-Saccharomyces yeasts following the suggestions of Morales et al. [4]. Here, the authors recommended that fermentation under somewhat aerobic conditions during the first 48 h would encourage growth and survivability of Mt. pulcherrima yet limit production of acetic acid. In agreement, Aplin and Edwards [12] reported elevated concentrations of acetic acid formed under aerobic conditions by a number of non-Saccharomyces yeasts (e.g., C. californica, C. oleophila, C. railenensis, C. saitoana, Hanseniaspora uvarum, Issatchenkia orientalis, Metschnikowia chrysoperlae, Mt. pulcherrima, Meyerozyma caribbica, My. guilliermondii, Pichia fermentans, P. kluyveri, P. membranifaciens, Wickerhamomyces anomalus, and Yamadazyma mexicana) compared to non-aerated fermentations of a high sugar grape must (310 g fermentable sugar/L).
Though a significant difference was not observed between ethanol concentrations of wines fermented without Mt. pulcherrima (control) or with the yeast under temperature regime II, a reduction of 0.8% v/v was noted under temperature regime III. This finding suggested that more sugar was metabolized to by-products other than ethanol by this yeast at 17.5 °C compared to 15 °C, in agreement with previous reports regarding increased sugar metabolism with increasing temperatures [26,32,33,34]. However, García et al. [10] noted that not all strains of Mt. pulcherrima behave similarly where only two of six strains tested yielded wines with lower concentrations of ethanol than those produced by S. cerevisiae alone, in agreement with Contreras et al. [7].
Similar to previous findings [10,16], fermentations inoculated with My. guilliermondii also resulted in lower concentrations of ethanol. Unlike Mt. pulcherrima, however, My. guilliermondii impacted final ethanol concentrations for fermentations maintained under temperature regime II (15 °C) where a reduction of 0.3% v/v was observed. Earlier research by Aplin et al. [13] reported no ethanol reduction of Merlot wines produced using 300 L tanks when musts containing My. guilliermondii were fermented at >20 °C. Based on the current findings, lower temperatures of grape musts were preferred by My. guilliermondii whereas warmer temperatures favored Mt. pulcherrima.
Ethanol reductions using specific combinations of non-Saccharomyces/S. cerevisiae yeasts implied changes in metabolic carbon flux to by-products other than ethanol. Varela et al. [35,36] proposed that sugars were metabolized to metabolites from a partial operation citric acid cycle, namely succinic acid, as well as increased concentrations of glycerol. However, the present study revealed lower concentrations of these metabolites in wines with less ethanol compared to those inoculated with S. cerevisiae. Alternatively, other studies have suggested that non-Saccharomyces may respire grape must sugars to CO2 or form other by-products from the citric acid cycle such as fumaric acid [1,11,37]. More research is required to better understand the ultimate fate of carbon from sugar under different conditions such as composition, temperature, oxygen availability, and nutrient status of grape musts.
One concern relying upon non-Saccharomyces yeasts is the observation that these yeasts can adversely affect alcoholic fermentation and/or wine quality depending on species but also conditions. For instance, Ciani et al. [31] noted sluggish alcoholic fermentations in grape musts inoculated with Torulaspora delbrueckii or Kluyveromyces (Lachancea) thermotolerans while those with H. uvarum yielded unacceptable concentrations of ethyl acetate. Medina et al. [38] noted that non-Saccharomyces can affect nutrient availability towards primary fermentation and therefore recommended addition of not only a nitrogen source (e.g., diammonium phosphate) but also a vitamin mixture to limit risks of stuck fermentation. Oro et al. [39] noted that strains of Mt. pulcherrima were antagonistic towards other non-Saccharomyces yeasts, in contrast to Contreras et al. [6] who reported the reverse, inhibition of Mt. pulcherrima by yeasts commonly found in grape musts, H. uvarum, Pichia kluyveri, and T. delbrueckii. As a lack of nutrients does not necessarily explain inhibitory behavior by some non-Saccharomyces yeasts [40], Contreas et al. [6] suggested synthesis of a killer toxin. In the present research, neither strain of Mt. pulcherrima or My. guilliermondii undesirably influenced subsequent fermentation by S. cerevisiae.
Sensory analysis of the wines revealed that wines with Mt. pulcherrima fermented under temperature regime III yielded better panelist scores compared to those wines fermented with only S. cerevisiae (temperature regime I). While scores for adverse attributes including aroma (‘herbaceous’), taste, (‘bitter’), flavor (‘sweaty’, ‘ethanol’, and ‘sulfur’), and mouthfeel (‘hot/ethanol’, ‘roughness’, ‘sharp’) were lower with Mt. pulcherrima, other commonly deemed enhancing attributes were higher such mouthfeel (‘round’). Of importance was that these wines were rated lowest in ‘bitterness’ and ‘hotness/ethanol’, two attributes which tend to dominate the sensory profile when ethanol content is high [41,42]. Differences in sensory characteristics were due, in part to production of volatile aroma/flavor molecules as pointed out by Seguinot et al. [43]. However, fewer differences were noted between other treatments, in particular with My guilliermondii, compared to wines with only S. cerevisiae present even though ethanol reductions of 0.3% (temperature regime II) to 0.4% (temperature regime III) v/v were noted. In fact, sensory differences due to differing ethanol concentrations were probably not observed because the difference threshold has been reported to be approximately 1% v/v [44]. Limited judge training may have also affected results as illustrated by Chambers et al. [45] who noted that significant panelist effects could only be eliminated with extensive training (120 h).
In conclusion, reductions in ethanol concentrations in final wines were achieved using Mt. pulcherrima under fermentation temperature regime III without adversely affecting sensory characteristics. Furthermore, lower reductions in ethanol were observed for wines inoculated with My guilliermondii under either temperature regime II or III but without significant changes in sensory attributes. This research suggests that these non-Saccharomyces species may decrease ethanol concentrations without lowering wine quality when fermented in larger volumes under cooler conditions.
Author Contributions
Conceptualization, C.G.E.; methodology, J.J.A.; formal analysis, J.J.A.; investigation, C.G.E.; resources, C.G.E.; data curation, C.G.E.; writing—original draft preparation, C.G.E.; writing—review and editing, J.J.A.; visualization, C.G.E.; supervision, C.G.E.; project administration, C.G.E.; funding acquisition, C.G.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Washington State Grape and Wine Research Program with additional support from Lallemand (Montréal, QC, Canada) and Washington State University through the School of Food Science (Pullman, WA, USA). This work was also supported, in part, by the USDA National Institute of Food and Agriculture, Hatch project 1016366 and project No. 0846 of the Agricultural Research Center, Washington State University, Pullman, WA, USA, 99164-6376.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Washington State University (IRB#15370-004) on 16 August 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are not available from other forums.
Acknowledgments
The authors gratefully acknowledge the Washington State Grape and Wine Research Program, Lallemand Inc., and the School of Food Science at Washington State University for financial and material support. The research conducted was obtained from the thesis by Tara L. Barton as required for the Master of Science in Food Science degree.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gonzalez, R.; Quirós, M.; Morales, P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Technol. 2013, 29, 55–61. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Satora, P.; Skoneczny, S.; Pater, A. Determination of the oenological properties of yeast strains isolated from spontaneously fermented grape musts obtained from cool climate grape varieties. Eur. Food Res. Technol. 2020, 246, 2299–2307. [Google Scholar] [CrossRef]
- Gobbi, M.; De Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biotechnol. 2016, 100, 5515–5526. [Google Scholar] [CrossRef]
- Rodrigues, A.J.; Raimbourg, T.; Gonzalez, R.; Morales, P. Environmental factors influencing the efficacy of different yeast strains for alcohol level reduction in wine by respiration. LWT Food Sci. Technol. 2016, 65, 1038–1043. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Sequential non-Saccharomyces and Saccharomyces cerevisiae fermentations to reduce the alcohol content in wine. Fermentation 2020, 6, 60. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: The effect of sequential inoculation timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.J.; Edwards, C.G. Impacts of non-Saccharomyces species and aeration on sequential inoculation with Saccharomyces cerevisiae to produce lower alcohol Merlot wines. J. Sci. Food Agric. 2020, 101, 1715–1719. [Google Scholar] [CrossRef]
- Aplin, J.J.; Paup, V.D.; Ross, C.F.; Edwards, C.G. Chemical and sensory profiles of Merlot wines produced by sequential inoculation of Metschnikowia pulcherrima or Meyerozyma guilliermondii. Fermentation 2021, 7, 126. [Google Scholar] [CrossRef]
- Casalta, E.; Aguera, E.; Picou, C.; Rodriguez-Bencomo, J.J.; Salmon, J.M.; Sablayrolles, J.M. A comparison of laboratory and pilot-scale fermentations in winemaking conditions. Appl. Microbiol. Biotechnol. 2010, 87, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Viana, T.; Loureiro-Dias, M.C.; Prista, C. Efficient fermentation of an improved synthetic grape must by enological and laboratory strains of Saccharomyces cerevisiae. AMB Express 2014, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.J.; White, K.P.; Edwards, C.G. Growth and metabolism of non-Saccharomyces yeasts isolated from Washington state vineyards in media and high sugar grape musts. Food Microbiol. 2019, 77, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Jolly, N.; Jacobson, D.; Bauer, F.F. The effect of scale on gene expression: Commercial versus laboratory wine fermentations. Appl. Microbiol. Biotechnol. 2012, 93, 1207–1219. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima selected strain for ethanol reduction in wine: Influence of cell immobilization and aeration condition. Foods 2019, 8, 378. [Google Scholar] [CrossRef]
- Schmid, F.; Schadt, J.; Jiranek, V.; Block, D.E. Formation of temperature gradients in large- and small-scale red wine fermentations during cap management. Aust. J. Grape Wine Res. 2009, 15, 249–255. [Google Scholar] [CrossRef]
- Gao, C.; Fleet, G.H. The effects of temperature and pH on the ethanol tolerance of the wine yeasts, Saccharomyces cerevisiae, Candida stellata and Kloeckera apiculata. J. Appl. Bacteriol. 1988, 65, 405–409. [Google Scholar] [CrossRef]
- Erten, H. Relations between elevated temperatures and fermentation behaviour of Kloeckera apiculata and Saccharomyces cerevisiae associated with winemaking in mixed cultures. World J. Microbiol. Biotechnol. 2002, 18, 377–382. [Google Scholar] [CrossRef]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef]
- Andorrà, I.; Landi, S.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of fermentation temperature on microbial population evolution using culture-independent and dependent techniques. Food Res. Int. 2010, 43, 773–779. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Tanguler, H. Influence of temperatures and termentation behaviour of mixed cultures of Williopsis saturnus var. saturnus and Saccharomyces cerevisiae associated with winemaking. Food Sci. Technol. Res. 2013, 19, 781–793. [Google Scholar] [CrossRef][Green Version]
- Maturano, Y.P.; Mestre, M.V.; Kuchen, B.; Toro, M.E.; Mercado, L.A.; Vazquez, F.; Combina, M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019, 289, 40–48. [Google Scholar] [CrossRef]
- Bourret, T.B.; Grove, G.G.; Vandemark, G.J.; Henick-Kling, T.; Glawe, D.A. Diversity and molecular determination of wild yeasts in a central Washington State vineyard. N. Am. Fungi 2013, 8, 1–32. [Google Scholar] [CrossRef]
- Eyéghé-Bickong, H.A.; Alexandersson, E.O.; Gouws, L.M.; Young, P.R.; Vivier, M.A. Optimisation of an HPLC method for the simultaneous quantification of the major sugars and organic acids in grapevine berries. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 885–886, 43–49. [Google Scholar] [CrossRef]
- Heard, G.M.; Fleet, G.H. The effects of temperature and pH on the growth of yeast species during the fermentation of grape juice. J. Appl. Bacteriol. 1988, 65, 23–28. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Abeln, F.; Chuck, C.J. The role of temperature, pH and nutrition in process development of the unique oleaginous yeast Metschnikowia pulcherrima. J. Chem. Technol. Biotechnol. 2020, 95, 1163–1172. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effect of fermentation temperature and culture media on the yeast lipid composition and wine volatile compounds. Int. J. Food Microbiol. 2008, 121, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H.; Heard, G.M. Yeast: Growth During Fermentation. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of non-Saccharomyces yeasts affects nutrient availability for Saccharomyces cerevisiae during wine fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.K.; Castura, J.C.; Ross, C.F. Temporal check-all-that-apply characterization of Syrah wine. J. Food Sci. 2016, 81, 1521–1529. [Google Scholar] [CrossRef]
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. Ann. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of nutrient availability on the fermentation and production of aroma compounds under sequential inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Pickering, G.J. Ethanol difference thresholds in wine and the influence of mode of evaluation and wine style. Am. J. Enol. Vitic. 2008, 59, 146–152. [Google Scholar]
- Chambers, D.H.; Allison, A.-M.A.; Chambers, E. Training effects on performance of descriptive panelists. J. Sens. Stud. 2004, 19, 486–499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).