Abstract
One of the alternatives to SO2 as an antimicrobial is the use of bioprotection yeasts, which colonize the medium preventing the proliferation of undesirable microorganisms. In this work, the bioprotective effect of a mixed inoculum formed by Torulaspora delbrueckii/Lachancea thermotolerans during fermentation was evaluated. For this purpose, fermentations were carried out using this mixed inoculum and the populations of yeasts, lactic bacteria and acetic bacteria, and the physical–chemical parameters of the wines obtained were studied. The results were compared with those obtained in spontaneous fermentation with and without SO2. The different fermentation strategies caused a differentiation in the yeast species present during fermentation. Regarding populations of lactic acid bacteria, results showed that the effect of the addition of the mixed inoculum was comparable to that exerted by SO2. On the other hand, due to the high sensitivity of acetic acid bacteria to SO2, the sulfite vinifications showed a lower population of acetic acid bacteria in the early stages of fermentation, followed by the vinifications with the mixed inoculum.
1. Introduction
In the winemaking industry, SO2 is the most widely used additive, since it provides triple protection to the wine due to its antimicrobial, antioxidant and antioxidasic activity [1,2]. This compound inhibits the development of undesirable microorganisms in the wine, favoring the colonization of the medium by yeasts of the Saccharomyces cerevisiae species, which have a greater tolerance to SO2. As it is a compound with high reducing properties, it is the first to react with oxygen, thus protecting the must or wine from oxidation. In addition, it is capable of inhibiting enzymes such as tyrosinase or laccase, which are responsible for the enzymatic oxidation of polyphenols. Due to these three main characteristics, SO2 is regarded as an essential tool by many winemakers. However, there is currently a tendency for consumers to demand more natural products with a minimum content of additives. SO2 can cause negative health effects, especially in sensitive or asthmatic people, including headaches, respiratory tract irritation or abdominal pain and diarrhea [3,4]. For this reason, legislation has evolved over the last few years, reducing the concentrations of SO2 allowed in wines. In 2009, the EC Regulation 606/2009 established the maximum concentration of SO2 at 150 mg/L in dry red wines. Furthermore, European Directive 2003/89/EC requires the specification of the presence of sulfites on the product label when their concentration exceeds 10 mg/L of SO2. In line with the limits established in the European Union, the International Organization of Vine and Wine (OIV) published the maximum acceptable limits of sulfur content in 2012, setting the maximum concentration of SO2 in red wines at 150 mg/L.
In this context, in which legislation and consumer demand call for the reduction and even the elimination of sulfur in wines, different alternatives are being investigated to achieve these objectives. The initial stage of the vinification process, prior to the domination of the medium by yeasts of the Saccharomyces (S.) cerevisiae species, represents a high risk for the development of spoilage microorganisms and it is the key point where the use of sulfur inhibits their development. To replace the antimicrobial activity of SO2, one of the options that is being considered is the use of bioprotection agents. These microorganisms prevent food spoilage through different mechanisms that can be divided into active, such as the production of antimicrobial molecules, or passive, such as competition for space, nutrients and oxygen [5]. Some microorganisms are able to restrict competitors’ access to nutrients through the secretion of digestive enzymes or siderophores [6]. As an example, the antimicrobial activity of Metschnikowia pulcherrima is attributed mainly to the production of a brown–red pigment pulcherrimin, which causes iron depletion [7]. Passive competition strategies are exerted by all microorganisms, while active competition strategies are performed only by some microorganisms and include the production of antimicrobial compounds or the disruption of signaling molecules through quorum quenching [5]. Non-Saccharomyces (NS) yeasts naturally dominate the early stages of spontaneous fermentation, making them ideal candidates as bioprotective inoculums for wine. Studies such as those carried out by Simonin et al. [8,9] have evaluated the bioprotective capacity of NS yeasts in wine with promising results. The inoculation of NS yeast species such as Torulaspora delbrueckii or Metschnikowia pulcherrima at the beginning of the vinification limited the proliferation of spoilage microorganisms with an effect similar to that of the addition of SO2.
The current work is aimed at evaluating the bioprotective effect of an NS-mixed inoculum formed by Torulaspora (T.) delbrueckii and Lachancea (L.) thermotolerans previously selected by our research team because of its enological properties [10]. For this purpose, the population and species composition of yeasts, lactic acid bacteria (LAB) and acetic acid bacteria (AAB) were studied in vinifications made following different inoculation strategies.
2. Materials and Methods
2.1. Microorganisms
All the microorganisms used in this study had been previously selected by our research group and all of them were autochthonous strains from the Denominación de Origen Calificada Rioja (D.O.Ca. Rioja). The non-Saccharomyces yeasts inoculum was composed of T. delbrueckii and L. thermotolerans in a 70/30 ratio [10], the commercial strain of S. cerevisiae used was Uvaferm VRB [11] and to induce malolactic fermentation (MLF), the wines were inoculated with the commercial bacteria Oenococcus (O.) oeni SILKA [12].
2.2. Vinifications
The assay was designed with the aim of studying the bioprotective effect of the mixed non-Saccharomyces inoculum in vinifications without SO2 and comparing it with the effect of SO2 in sulfite fermentations. To do so, vinifications with red Tempranillo grapes were carried out in triplicate in 50 L tanks following threedifferent inoculation strategies:
- -
- Initial spontaneous fermentation without addition of SO2 and inoculation after 72 h with the commercial yeast S. cerevisiae Uvaferm VRB at a dose of 4 × 106 cells/mL (S).
- -
- Initial spontaneous fermentation with addition of SO2 at a dose of 50 mg/L and inoculation after 72 h with the commercial yeast S. cerevisiae Uvaferm VRB (4 × 106 cells/mL) (SS).
- -
- Inoculation of the NS-mixed starter at vatting at 2 × 106 cells/mL without addition of SO2 and inoculation of S. cerevisiae Uvaferm VRB (4 × 106 cells/mL) at 72 h (TL).
The non-Saccharomyces inoculum was prepared by growing the yeasts in a liquid YPD medium, incubating the culture at 25 °C for 48 h before inoculating the deposits. To determine the concentration, the cells were counted in a Neubauer chamber. After 72 h vatting, the commercial S. cerevisiae yeast was prepared following the manufacturer’s instructions and inoculated in the corresponding deposits at a concentration of 4 × 106 cells/mL.
Alcoholic fermentation (AF) was monitored daily by measuring density and temperature. Once AF was completed, the tanks were devatted, pressed and inoculated with commercial LAB of the species O. oeni to induce MLF. The evolution of MLF was monitored by enzymatic analysis of malic and lactic acids. After completion of MLF (malic acid < 0.2 g/L), the wines were stabilized for a month at 8–10 °C before bottling.
2.3. Microorganism Count
Sampling for the count of microorganisms was carried out four times: initial must, at 24 and 72 h after vatting, and at the end of AF. The samples were collected in sterile tubes, serial dilutions were made and they were seeded in Petri dishes with different culture media: Chloramphenicol Glucose Agar (CGA) (D-glucose 20 g/L, yeast extract 5 g/L, agar 15 g/L, chloramphenicol 0.2 g/L) for the yeast count, Mannitol (D-mannitol 25 g/L, peptone 3 g/L, yeast extract 5 g/L, penicillin 3 U/mL) for the acetic acid bacteria count and MRS agar (MRS broth 52 g/L, agar 20 g/L, pymaricine 50 mg/L) for the lactic acid bacteria count. The CGA and Mannitol plates were incubated for 48 h at a temperature of 25 °C. In the case of MRS plates, incubation was carried out under anaerobic conditions (Gas Pak System, Oxoid Ltd., Basingstoke, UK) for 10 days at 30 °C. After the corresponding incubation period in each case, the counts were carried out on the plates whose growth was between 30 and 300 colonies, expressing the results in colony-forming units per milliliter (CFU/mL).
2.4. Microorganism Identification
In the plates where the counts were made, 15 colonies per replicate were randomly isolated in each culture medium in the sampling carried out 24 h after vatting. This point was selected for the identification of the microorganisms present in the deposits because it is a key point when the development of spoilage microorganisms can occur. Therefore, it is an appropriate moment to detect the bioprotective effect of the inoculum studied.
For the identification of microorganisms, DNA amplification was first carried out by PCR. In the case of yeasts, NL1 and NL4 [13] primers were used, and for lactic acid and acetic acid bacteria, WLAB1–WLAB2 and WBAC1–WBAC2 primer pairs were used as described by López et al. [14]. The sequencing of the amplicons obtained was carried out by Macrogen Spain. The results obtained were compared with the available databases using the BLAST program [15]. The identifications were considered correct when they exceeded 98% identity.
2.5. Physicochemical Analysis
In the final wines, the general parameters and those related to the color were determined. Alcoholic strength, pH, total acidity, volatile acidity, color intensity (CI), hue and total polyphenol index (TPI) were analyzed according to the official methods of the EEC [16]. Tartaric acid was determined using the Rebelein method [17] and malic and lactic acid, glycerol and acetaldehyde were determined enzymatically using an autoanalyzer (Miura One, Spain). Free SO2 was determined by automated iodometric titration in acid medium using an Iodo M 920 potentiometer (Oeno-Bio), and the concentration of total SO2 was determined using the same technique, performing previous alkaline hydrolysis of the sample with NaOH 2 N. Total anthocyanins were measured by decoloring using SO2 [18], the ionization index was determined according to the method described by Glories [19] and the polymerization index following the method proposed by Ruiz [20].
2.6. Statistical Analysis
The statistical analysis of the data obtained was carried out with the IBM SPSS Statistics program (version 23). The analysis of variance (ANOVA) was carried out in the parameters studied. Significant differences were established by using the Tukey post-hoc test (p ≤ 0.05)
3. Results and Discussion
3.1. AF Kinetics
The evolution of density throughout AF is shown in Figure 1. As can be observed, during the first 24 h the density remained at the initial values in all treatments. At 72 h, differences began to be observed between the samples, and the wines made by spontaneous fermentation had slower kinetics, especially in the case of sulfite samples (SS), which had a more gradual decrease in density in the early stages. After the sampling at 72 h, when the commercial S. cerevisiae yeast was inoculated in all the wines, a more rapid decrease in density was observed. Despite these differences in the process of AF, all wines finished after 9 days. The slower initial kinetics in spontaneous sulfite fermentations can be explained by the inhibitory effect of sulfur on yeast multiplication. On the other hand, the low-density variation during the first days in the wines inoculated with the NS yeasts and in the non-sulfite spontaneous fermentation could be due to the reduced fermentative power of both the NS inoculum yeasts and the indigenous yeasts present in the first stages of AF.
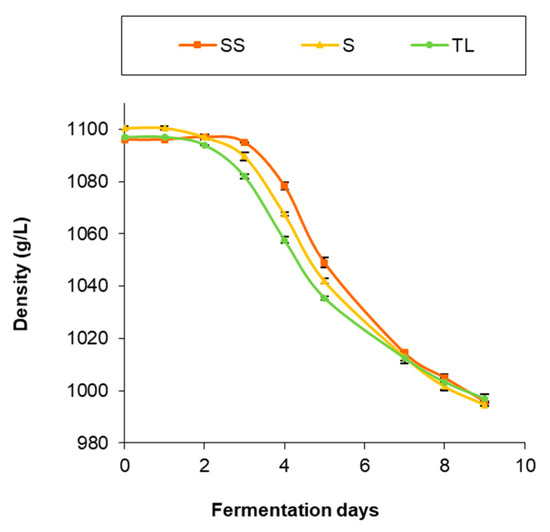
Figure 1.
Evolution of density during AF in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.2. Yeasts
3.2.1. Evolution of the Yeast Population during Fermentation
Figure 2 shows the yeast counts (log CFU/mL) performed at different moments of AF. In the early stages of fermentation, the yeast population had a similar evolution in the vinifications studied, increasing from the moment of vatting to 72 h. The sampling carried out 72 h after vatting showed the greatest differences in the yeast population between the different vinifications. In the sulfite wines (SS) the yeast population did not exceed seven logarithmic units, while in wines with spontaneous fermentation without sulfite (S) and wines made with the NS inoculum (TL), the yeast counts showed values above that level. In the sampling carried out at the end of AF (day 9), the yeast population was the same in all treatments.
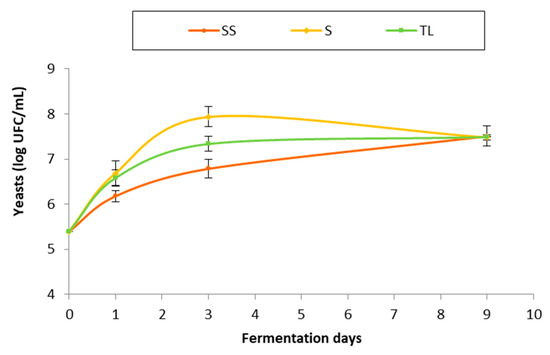
Figure 2.
Yeast population (log CFU/mL) throughout AF in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.2.2. Yeast Species Present in the Tanks 24 h after Vatting
The results of the identification of the yeast colonies isolated in CGA at 24 h after vatting showed differences in the species and the proportions present in the different vinifications studied (Figure 3). Hanseniaspora (H.) uvarum was found in all samples in different percentages, being the majority species in the two spontaneous fermentations (sulfite and non-sulfite). The other species present in the spontaneous fermentations was Candida (C.) zemplinina. In the sulfite spontaneous fermentations, a reduction of the population of H. uvarum was observed, with a higher presence of C. zemplinina in the samples. This result can be explained by the greater tolerance to SO2 of the C. zemplinina strain present in the must. In the work carried out by Grangeteau et al. [21], a greater tolerance to SO2 was observed in the strains of C. zemplinina included in the study compared to the strains of the Hanseniaspora genus, which is consistent with the results obtained in the present study.
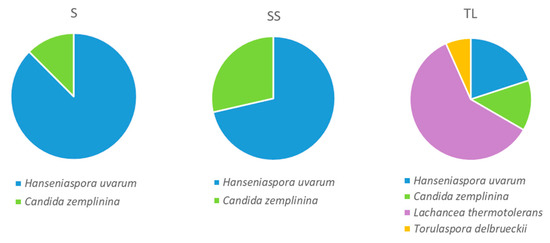
Figure 3.
Proportion of yeast species at 24 h after vatting in the in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
The main species found in the TL samples was L. thermotolerans, one of the yeasts present in the inoculum, followed by those present in the spontaneous fermentations, H. uvarum and Candida zemplinina, and finally, T. delbrueckii, the other species that was part of the mixed inoculum. In wines made with the non-Saccharomyces-mixed inoculum, a significant reduction in the proportion of H. uvarum was observed. Some studies have shown the production of high levels of ethyl acetate and acetic acid by indigenous apiculate yeasts during the early stages of fermentation [22,23], therefore, the reduction of the population achieved with the inoculation would be positive in terms of its bioprotective effect.
3.3. Lactic Acid Bacteria
3.3.1. Evolution of the Lactic Acid Bacteria Population during Fermentation
Figure 4 shows the population of LAB (log CFU/mL) detected at different times of fermentation. At the time of vatting, the population of LAB in the initial must was between four and five logarithmic units. This population is slightly higher than that normally found in the must, which usually ranges between three and four logarithmic units [24,25]. As can be observed in the data obtained in the sampling carried out at 72 h, the effect of the addition of the NS inoculum (TL) on the population of LAB was comparable to that exerted by SO2 (SS). In the samples corresponding to the spontaneous fermentation without sulfite, higher populations of LAB were observed than in the rest of the treatments. In the sampling at the end of AF, the populations of LAB were reduced to equalize, with values around three logarithmic units in all the treatments. This decrease in the population of LAB is due to the colonization of the medium by the inoculated commercial yeasts, which during AF raise the concentration of ethanol and release toxic compounds for bacteria [26].
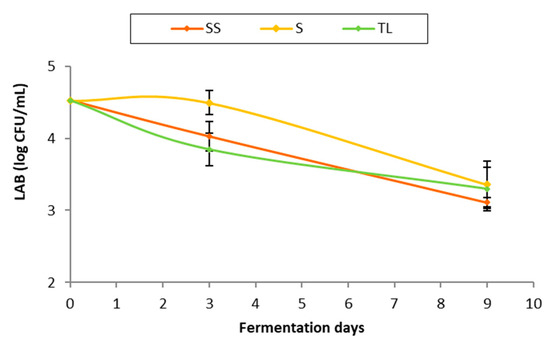
Figure 4.
LAB population (log CFU/mL) throughout AF in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.3.2. Lactic Acid Bacteria Present in the Tanks 24 h after Vatting
The results of the identification of the isolates from the samples taken 24 h after vatting indicated that the main species in all the treatments was Pediococcus (P.) pentosaceus (Figure 5). In general, the species of the genus Pediococcus such as P. parvulus, P. inopinatus, P. damnosus or P. pentosaceus are considered spoilage microorganisms, since they can produce excessive amounts of diacetyl, biogenic amines or exopolysaccharides, giving rise to a negative impact on wine quality [27]. However, it is important to note that this negative impact depends on the strain of Pediococcus spp. that is present in the wine since there are studies in which different strains have carried out malolactic fermentation without harming the wines [28,29]. The most abundant genus after Pediococcus in the samples inoculated with NS and in the sulfite samples was Lactococcus (L.), while in the spontaneous fermentations without sulfite it was Lactobacillus (Lb.). Some bacteria of the genus Lactobacillus are considered responsible for the production of biogenic amines, volatile acidity or the appearance of organoleptic defects in wine such as the “mousy” off flavor [30]. The species that have shown the highest levels of these compounds are Lb. hilgardii and Lb. brevis [31], the latter detected in wines made from spontaneous fermentation. In the sulfite vinifications and in those inoculated with NS, the Lactobacillus species that was present in the samples was Lb. plantarum. Different strains of this species have been studied in recent years for their application as microbial starters in malolactic fermentation [32,33,34]. In the case of the vinifications with the NS inoculum, the percentage of P. pentosaceus decreased, and a high percentage of Lactococcus was found, represented by the species L. lactis. On the other hand, in TL samples it was observed a higher presence of genera belonging to other groups such as environmental bacteria.
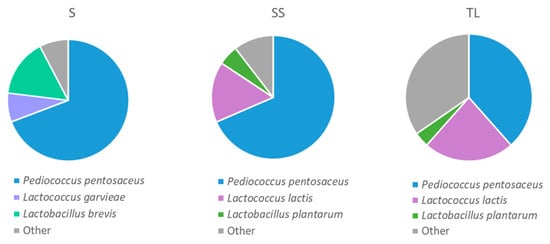
Figure 5.
Proportion of LAB species isolated in MRS at 24 h after vatting in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.4. Acetic Acid Bacteria
3.4.1. Evolution of the Acetic Acid Bacteria Population during Fermentation
The counts of the bacteria grown in the Mannitol medium are shown in Figure 6. The population of bacteria in the initial must was close to five logarithmic units, which can be considered high, taking into account that in healthy vintages it is usually between two and three logarithmic units [35]. The results obtained in the 24 h sampling show a notable decrease in the population of bacteria in the samples of the spontaneous sulfite fermentation, probably due to the high sensitivity of AAB to SO2. In TL samples, the population remained below five logarithmic units, while the spontaneous fermentation without sulfur (S) had the highest population, with over five logarithmic units. The population of bacteria reached the highest values in the sampling carried out at 72 h. The highest values were those obtained in the spontaneous fermentation without sulfite (S), close to six logarithmic units, followed by the spontaneous sulfite fermentation (SS). The effect of the sulfite could be observed in SS samples, with lower bacterial populations than those detected in the spontaneous fermentation without sulfite. The vinification with the mixed inoculum of NS gave rise to lower bacterial populations than those detected in samples S and SS, which indicates a protective effect comparable to and even greater than that exerted by the addition of SO2. After the sampling was carried out at 72 h, the inoculation with the commercial S. cerevisiae strain and the anaerobic conditions generated during the fermentation caused a decrease in the population of AAB, which remained at values close to two logarithmic units in all treatments at the end of AF.
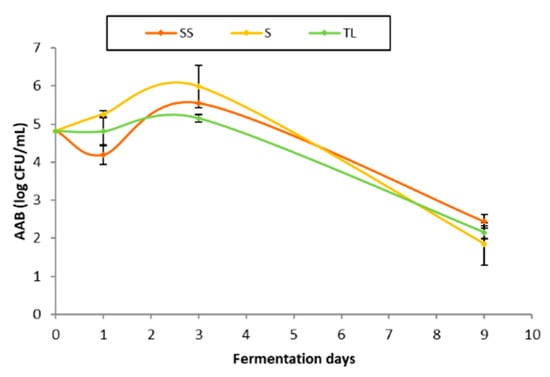
Figure 6.
AAB population (log CFU/mL) throughout AF in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.4.2. Acetic Acid Bacteria Present in the Tanks 24 h after Vatting
The identification of the bacteria isolated in the Mannitol medium showed a greater proportion of species belonging to other groups of bacteria than that found in the media for the isolation of LAB. Mainly wine acetic acid bacteria grow in this medium, but some environmental bacteria are also capable of growing in it [24]. In the samples of the spontaneous fermentation with SO2 (SS), the genera belonging to groups different from AAB accounted for a proportion that reached 75% of the identified isolates (Figure 7). The main species found was Tatumella (T.) terrea, with a proportion of 50% of the total isolates. T. terrea is an environmental bacterium that has previously been detected in enological environments such as stored grape pomace [36] or wine [37]. The main AAB genus found in the rest of the samples was Gluconobacter (G.), represented by the species G. oxydans and G. cerinus. This difference between the predominant species in SS samples and the rest of the samples may be due to the effect of SO2. In a study carried out by Takashi et al. [37], in which the microbial diversity in sulfite and non-sulfite wines was evaluated, T. terrea was found in high proportions in both wines, which points to high tolerance to SO2. On the other hand, the inoculation with the NS inoculum (TL) gave rise to a reduction in the proportion of AAB of the Gluconobacter genus with respect to spontaneous fermentation (S), which represented more than 69% of the isolates identified. In TL samples bacteria of the Acetobacter genus were also detected.
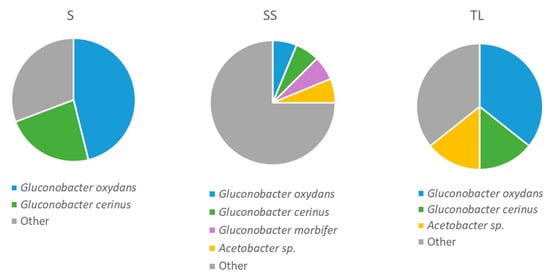
Figure 7.
Proportion of AAB species at 24 h after vatting in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.5. Malolactic Fermentation
MLF monitoring was performed after the inoculation of commercial LAB by analyzing the concentration of malic acid in the wines. The MLF duration was 16 days, with similar kinetics in all the inoculation strategies (Figure 8). In the case of the vinifications with the NS inoculum, the MLF slowed down slightly compared to the two spontaneous vinifications from day 10, possibly due to a higher concentration of lactic acid in the medium produced by L. thermotolerans. This compound, either due to its direct inhibitory effect on the metabolic activity of lactic acid bacteria or due to the consequent drop in pH [25,38,39], could have caused this slowdown in malic acid degradation. However, it did not have a significant influence, since the MLF ended at the same time as in the spontaneous fermentations.
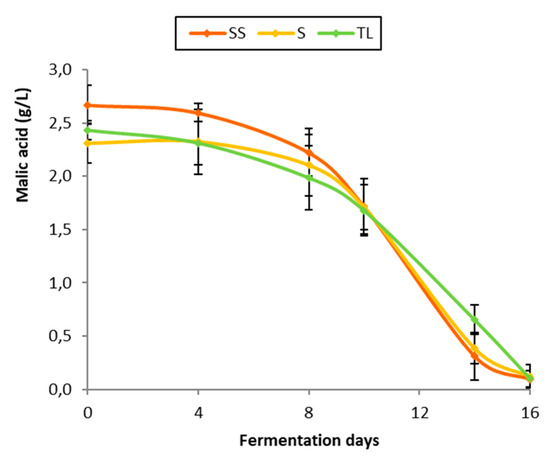
Figure 8.
Evolution of malic acid concentration during MLF in the different inoculation strategies (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.6. Characteristics of the Wines
3.6.1. Physical Chemical Parameters
Once the MLF was finished and after a month of stabilization, the wines produced were analyzed. The results obtained showed significant differences between the samples of the different inoculation strategies in parameters such as pH, total acidity, lactic acid, glycerol and acetaldehyde (Table 1). The pH was lower in TL wines, with statistically significant differences compared to the wines from spontaneous fermentations. Total acidity and lactic acid concentration were higher in the case of TL wines, with significant differences with respect to the rest of the wines. These differences can be explained by the production of lactic acid by the yeast of the mixed inoculum L. thermotolerans. In the case of glycerol, the highest concentrations were found in TL and S wines. The NS inoculum yeast T. delbrueckii is capable of producing glycerol [40,41], therefore, its presence in TL wines would explain the highest concentration of this compound. The highest concentrations of acetaldehyde were also found in TL and S wines, with significant differences with respect to wines with spontaneous sulfite fermentation (SS). Nonetheless, the concentration of this compound remained below the perception threshold in all the wines, so it did not compromise the quality of the wine. The lower concentration of this compound in SS wines could be explained by the combination of the added SO2 with acetaldehyde [42], forming a stable compound and giving rise to a lower presence of acetaldehyde in the wine.

Table 1.
Values of the physical–chemical parameters analyzed in the wines (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
3.6.2. Color Parameters
The results of the analysis of the parameters related to color showed differences between the inoculation strategies in most of them, except for the polymerization index (Table 2). The levels of anthocyanins and total polyphenol index (TPI) were higher in SS wines with significant differences compared to TL wines, which could be due to a more intense extraction caused by SO2. The color intensity values were higher in TL wines, with significant differences with respect to SS wines. Color intensity (CI) of anthocyanins is pH dependent [39], which is why the ability of the NS inoculum yeast L. thermotolerans to lower the pH of the wine affects wine color in an important way. TL wines presented a higher anthocyanin ionization index, with significant differences compared to the wines from spontaneous fermentations (S and SS). The ionization index indicates the proportion of anthocyanins that are ionized in the flavylium cation state, contributing to the red color of the wine, which indicates a positive influence of the NS inoculum on this color parameter. The hue values were lower in TL wines, with significant differences compared to the other inoculation strategies, which presented values above 0.7, typical of more evolved wines. This result could also be related to the differences in the pH values between the wines, since, as described by Glories [43], the increase in pH produces an increase in hue.

Table 2.
Values of the parameters related to the color analyzed in the wines (SS: spontaneous sulfite, S: spontaneous without SO2, TL: inoculation with T. delbrueckii/L. thermotolerans at vatting without SO2).
4. Conclusions
This work was aimed at studying the potential of an NS-mixed inoculum to control the proliferation of spoilage microorganisms in wine. The results showed a bioprotective effect of the NS-mixed inoculum comparable to that of SO2 in the control of populations of LAB and AAB in the early stages of fermentation. In the case of yeasts, the addition of the NS inoculum allowed the control of the proliferation of indigenous apiculate yeasts such as H. uvarum, which can lead to defects in wine.
In previous studies, this inoculum had shown the ability to improve certain parameters related to the quality of red wine [10]. With this study, it was shown that it can also exert an antimicrobial activity similar to that of SO2 in non-sulfite vinifications.
Author Contributions
R.E.-V. was in charge of the methodology and the original draft preparation. L.G.-A., P.G. and L.F. were also responsible for the methodology. R.L. and P.S. researched and were in charge of resources. A.R.G. was responsible for the project administration, and developed the formal analysis, resources and reviewed and edited the draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Nacional de Investigaciones Agrarias (INIA) (Project RTA2013-0053-C03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hidalgo, J. Tratado de Enología, 2nd ed.; Mundi Prensa: Madrid, España, 2011. [Google Scholar]
- Carrascón, V.; Vallverdú-queralt, A.; Meudec, E.; Sommerer, N. The kinetics of oxygen and SO2 consumption by red wines. What do they tell about oxidation mechanisms and about changes in wine composition? Food Chem. 2018, 241, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Gama, J.; Pinto, N.; Pivi, G.; Brancal, H.; Carvalho, L.; Loureiro, V.; Vaz Patto, M. Sulfite concentration and the occurrence of headache in young adults: A prospective study. Eur. J. Clin. Nutr. 2019, 73, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.A.; Kainz, K.; Carmona-Gutiérrez, D.; Madeo, F. Microbial wars: Competition in ecological niches and within the microbiome. Microbial Cell. 2018, 5, 215–219. [Google Scholar] [CrossRef]
- Ghoul, M.; Mitri, S. The Ecology and Evolution of Microbial Competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef]
- Di Gianvito, P.; Englezos, V.; Rantsiou, K.; Cocolin, L. Bioprotection strategies in winemaking. Int. J. Food Microbiol. 2022, 364, 109532. [Google Scholar] [CrossRef]
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora delbrueckii as a bio-protection agent in winemaking. Food Res. Int. 2018, 107, 451–461. [Google Scholar] [CrossRef]
- Simonin, S.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Quintanilla-Casas, B.; Vichi, S.; Peyron, D.; Alexandre, H.; Tourdot-Maréchal, R. Bio-Protection as an Alternative to Sulphites: Impact on Chemical and Microbial Characteristics of Red Wines. Front. Microbiol. 2020, 11, 1308. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; González-Arenzana, L.; Garijo, P.; López, R.; Santamaría, P.; Gutiérrez, A.R. Selection process of a mixed inoculum of non-Saccharomyces yeasts isolated in the D.O.Ca. Rioja. Fermentation 2021, 7, 148. [Google Scholar] [CrossRef]
- Gutiérrez, A.R. Selección de Levaduras Vínicas en la Denominación de Origen Rioja; Universidad del País Vasco: Bilbao, Spain, 1994. [Google Scholar]
- González-Arenzana, L.; Santamaría, P.; López, R.; López-Alfaro, I. Indigenous lactic acid bacteria communities in alcoholic and malolactic fermentations of Tempranillo wines elaborated in ten wineries of La Rioja (Spain). Food Res. Int. 2013, 50, 438–445. [Google Scholar] [CrossRef]
- Cocolin, L.; Bisson, L.F.; Mills, D.A. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol. Lett. 2000, 189, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Ruiz-Larrea, F.; Cocolin, L.; Orr, E.; Phister, T.; Marshall, M.; VanderGheynst, J.; Mills, D.A. Design and Evaluation of PCR Primers for Analysis of Bacterial Populations in Wine by Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2003, 69, 6801–6807. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No. 2676/90 determining Community methods for the analysis of wines. Off. J. Eur. Union 1990, 272, 1–192.
- Lipka, Z.; Tanner, H. Une nouvelle méthode de dosage rapide de l’acide tartrique dans les moûts, les vins et autres boissons (selon Rebelein). Rev. Suisse Vitic Arboric Hortic. 1974, 6, 5–10. [Google Scholar]
- Somers, T.C.; Evans, M.E. Wine quality: Correlations with colour density and anthocyanin equilibria in a group of young red wines. J. Sci. Food Agric. 1974, 25, 1369–1379. [Google Scholar] [CrossRef]
- Glories, Y. Recherches sur la Matière Colorantes des Vins Rouges; Université de Bourdeaux II: Bordeaux, France, 1978. [Google Scholar]
- Ruiz, M. La Crianza del Vino Tinto Desde la Perspectiva Vitícola; AMV Ediciones: Madrid, Spain, 1999. [Google Scholar]
- Grangeteau, C.; Gerhards, D.; Von Wallbrunn, C.; Alexandre, H.; Rousseaux, S.; Guilloux-Benatier, M. Persistence of two non-Saccharomyces yeasts (Hanseniaspora and Starmerella) in the cellar. Front. Microbiol. 2016, 7, 268. [Google Scholar] [CrossRef]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R. Higher alcohol and acetic acid production by apiculate wine yeasts. J. Appl. Bacteriol. 1992, 73, 126–130. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R.; González-Arenzana, L. Impact of Chemical and Biological Fungicides Applied to Grapevine on Grape Biofilm, Must, and Wine Microbial Diversity. Front. Microbiol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Muñoz, R.; Moreno-Arribas, M.V.; De Las Rivas, B. Bacterias lácticas. In Microbiología del Vino; Carrascosa, A.V., Muñoz, R., González, R., Eds.; AMV Ediciones: Madrid, Spain, 2005. [Google Scholar]
- Costello, P.J.; Henschke, P.A.; Markides, A.J. Standardised methodology for testing malolactic bacteria and wine yeast compatibility. Aust. J. Grape Wine Res. 2003, 9, 127–137. [Google Scholar] [CrossRef]
- Wade, M.E.; Strickland, M.T.; Osborne, J.P.; Edwards, C.G. Role of Pediococcus in winemaking. Aust. J. Grape Wine Res. 2018, 25, 7–24. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.G.; Peterson, J.C.; Boylston, T.D.; Vasile, T.D. Interactions Between Leuconostoc oenos and Pediococcus spp. During Vinification of Red Wines. Am. J. Enol. Vitic. 1994, 45, 49 LP–55. [Google Scholar]
- Juega, M.; Costantini, A.; Bonello, F.; Cravero, M.-C.; Martinez-Rodriguez, A.; Carrascosa, A.; Garcia-Moruno, E. Effect of malolactic fermentation by Pediococcus damnosus on the composition and sensory profile of Albariño and Caiño white wines. J. Appl. Microbiol. 2014, 116, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Costello, P.J.; Henschke, P.A. Mousy off-flavor of wine: Precursors and biosynthesis of the causative N-Heterocycles 2-ethyltetrahydropyridine, 2-acetyltetrahydropyridine, and 2-acetyl-1-pyrroline by Lactobacillus hilgardii DSM 20176. J. Agric. Food Chem. 2002, 50, 7079–7087. [Google Scholar] [CrossRef] [PubMed]
- Costello, P.J.; Lee, T.H.; Henschke, P. Ability of lactic acid bacteria to produce N-heterocycles causing mousy off-flavour in wine. Aust. J. Grape Wine Res. 2001, 7, 160–167. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef] [Green Version]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus plantarum, a new biological tool to control malolactic fermentation: A review and an outlook. Beverages 2020, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Guillamón, J.M.; Mas, A. Bacterias acéticas. In Microbiología del Vino; Carrascosa, A.V., Muñoz, R., González, R., Eds.; AMV Ediciones: Madrid, Spain, 2005. [Google Scholar]
- Maragkoudakis, P.A.; Nardi, T.; Bovo, B.; D’Andrea, M.; Howell, K.S.; Giacomini, A.; Corich, V. Biodiversity, dynamics and ecology of bacterial community during grape marc storage for the production of grappa. Int. J. Food Microbiol. 2013, 162, 143–151. [Google Scholar] [CrossRef]
- Takahashi, M.; Ohta, T.; Masaki, K.; Mizuno, A.; Goto-Yamamoto, N. Evaluation of microbial diversity in sulfite-added and sulfite-free wine by culture-dependent and -independent methods. J. Biosci. Bioeng. 2014, 117, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Shelef, L.A. Antimicrobial Effects of Lactates: A Review. J. Food Prot. 1994, 57, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non-Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef]
- Ochando, T.; Mouret, J.R.; Humbert-Goffard, A.; Aguera, E.; Sablayrolles, J.M.; Farines, V. Comprehensive study of the dynamic interaction between SO2 and acetaldehyde during alcoholic fermentation. Food Res. Int. 2020, 136, 109607. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. 2e partie: Mesure, origine et interprétation. OENO One 1984, 18, 253. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).