Microbiota and Mycobiota of Soy Sauce-Supplied Lactic Acid Bacteria Treated with High Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Koji Fermentation
2.2. Bacteria Culture and HPP Treatment
2.3. Assays for Levels of Total Aerobic Plate Counts, Yeast, and Lactic Acid Bacteria
2.4. Measured for EC and L-Citrulline
2.5. Statistical Analysis
3. Results and Discussion
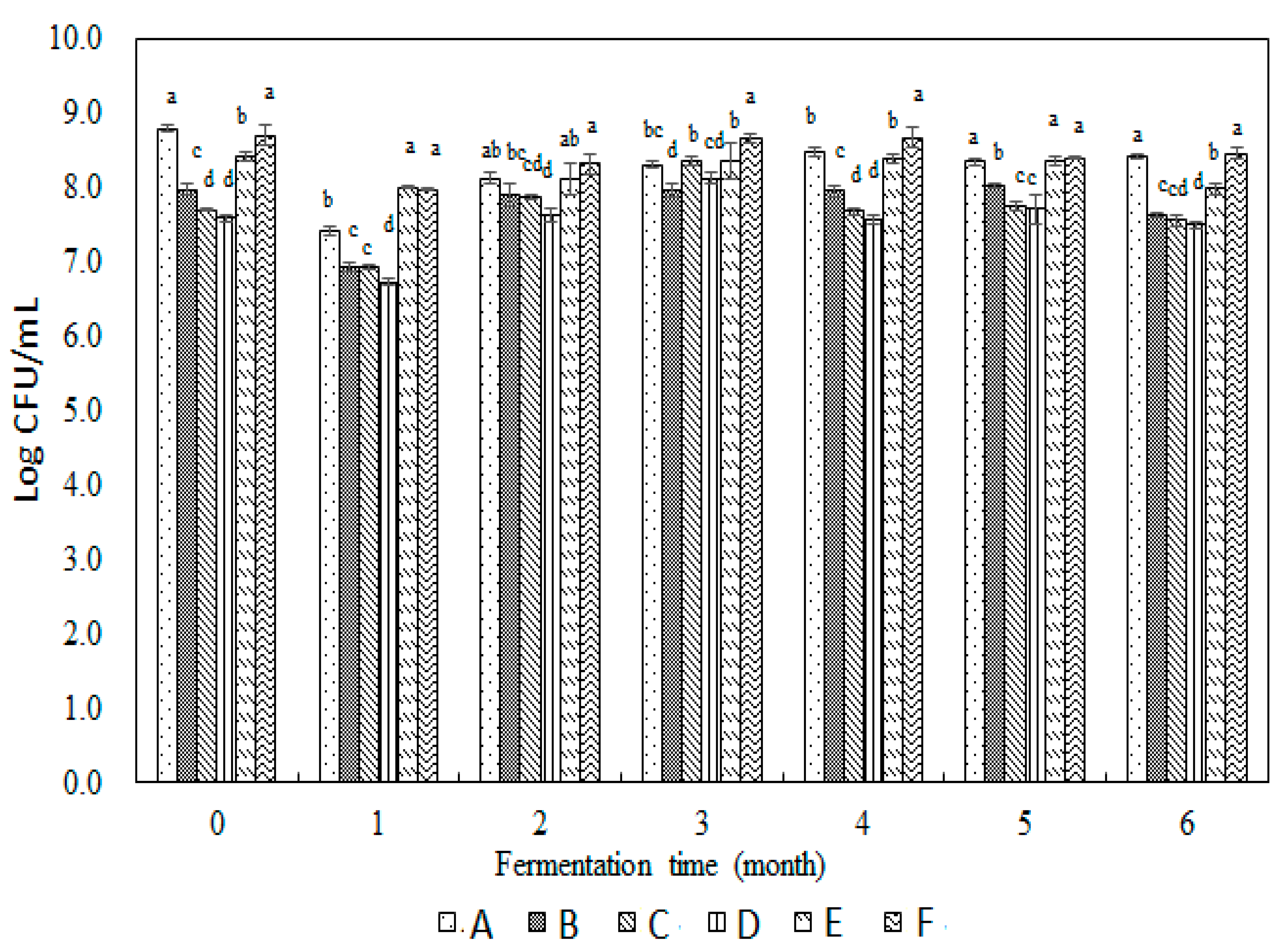
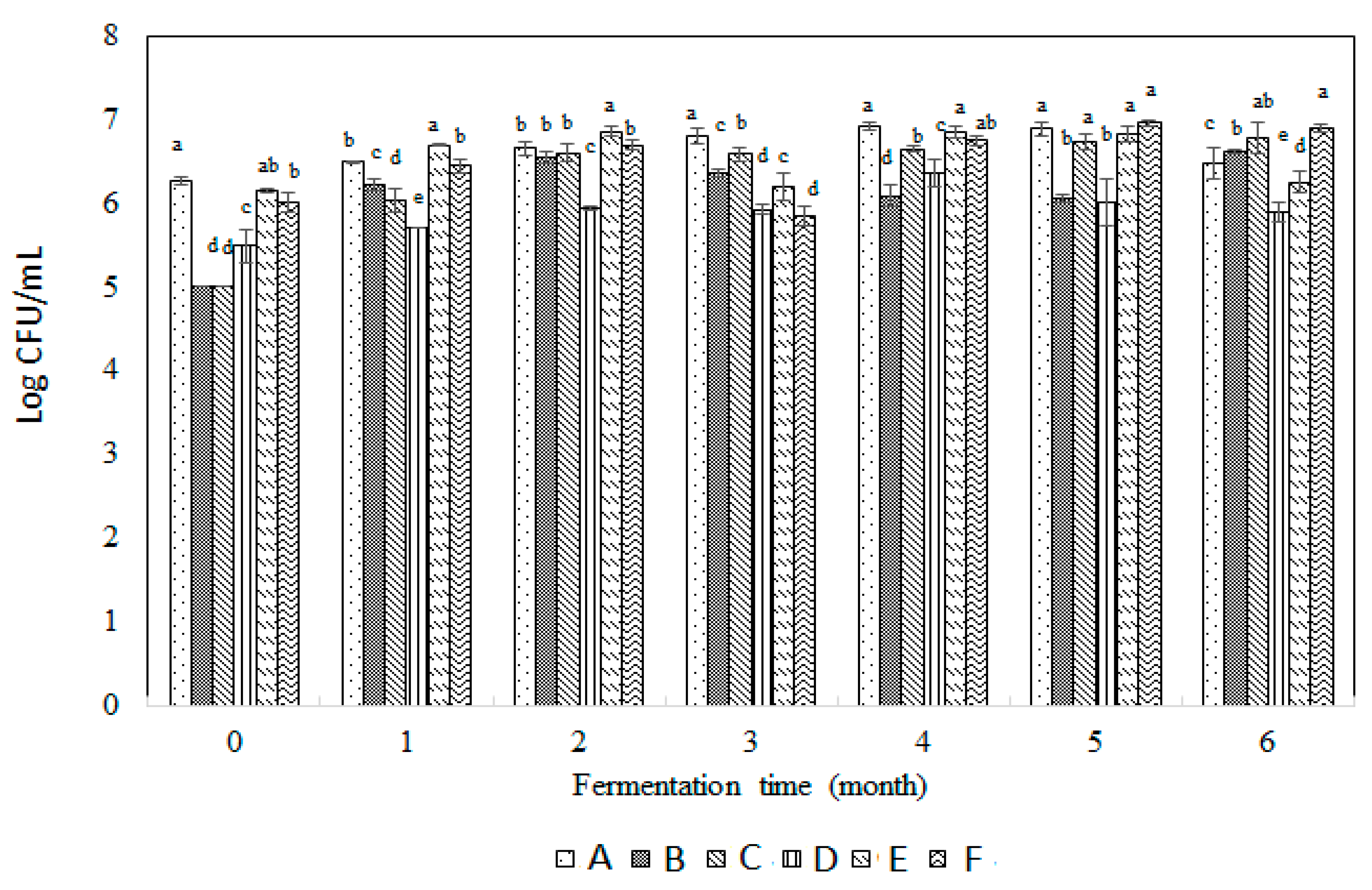
3.1. Assays for Total Aerobic Plate Counts, Yeast, and Lactic Acid Bacteria
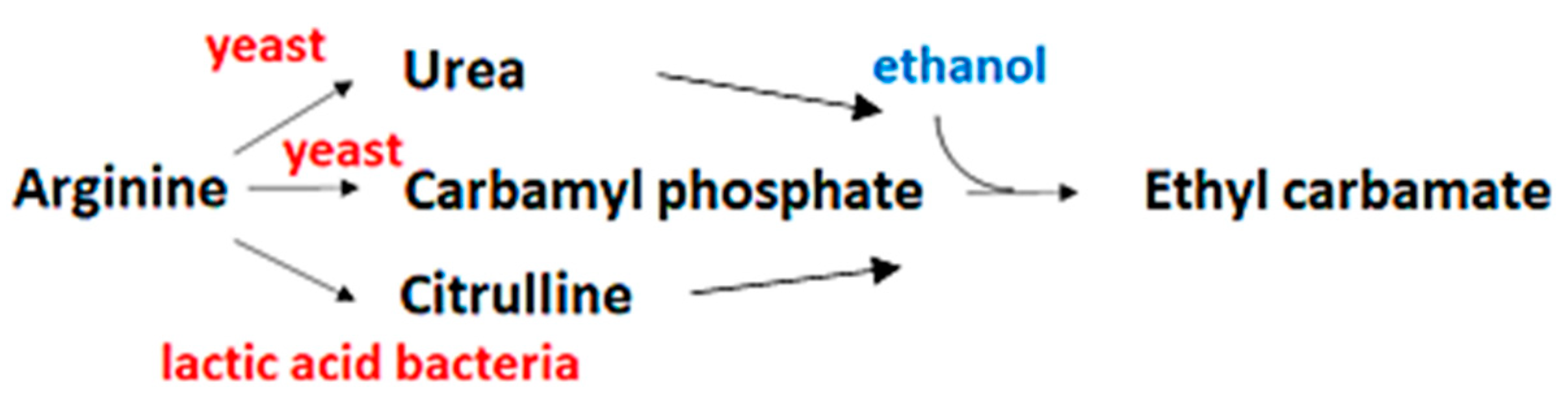
3.2. L-Citrulline and EC Contents in Soy Sauce
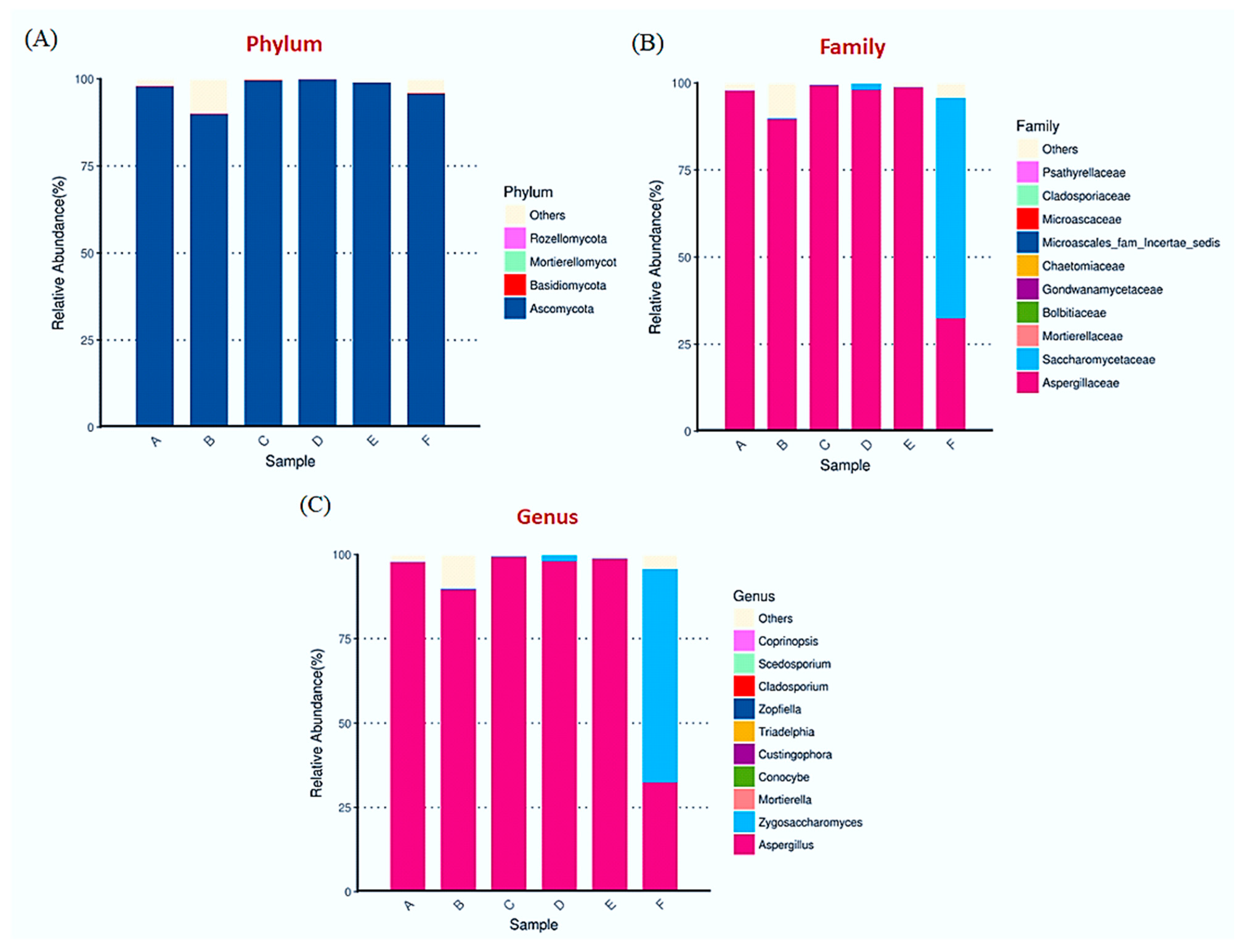
3.3. Diversification of Fungal Diversity by ITS Assay in Soy Sauce
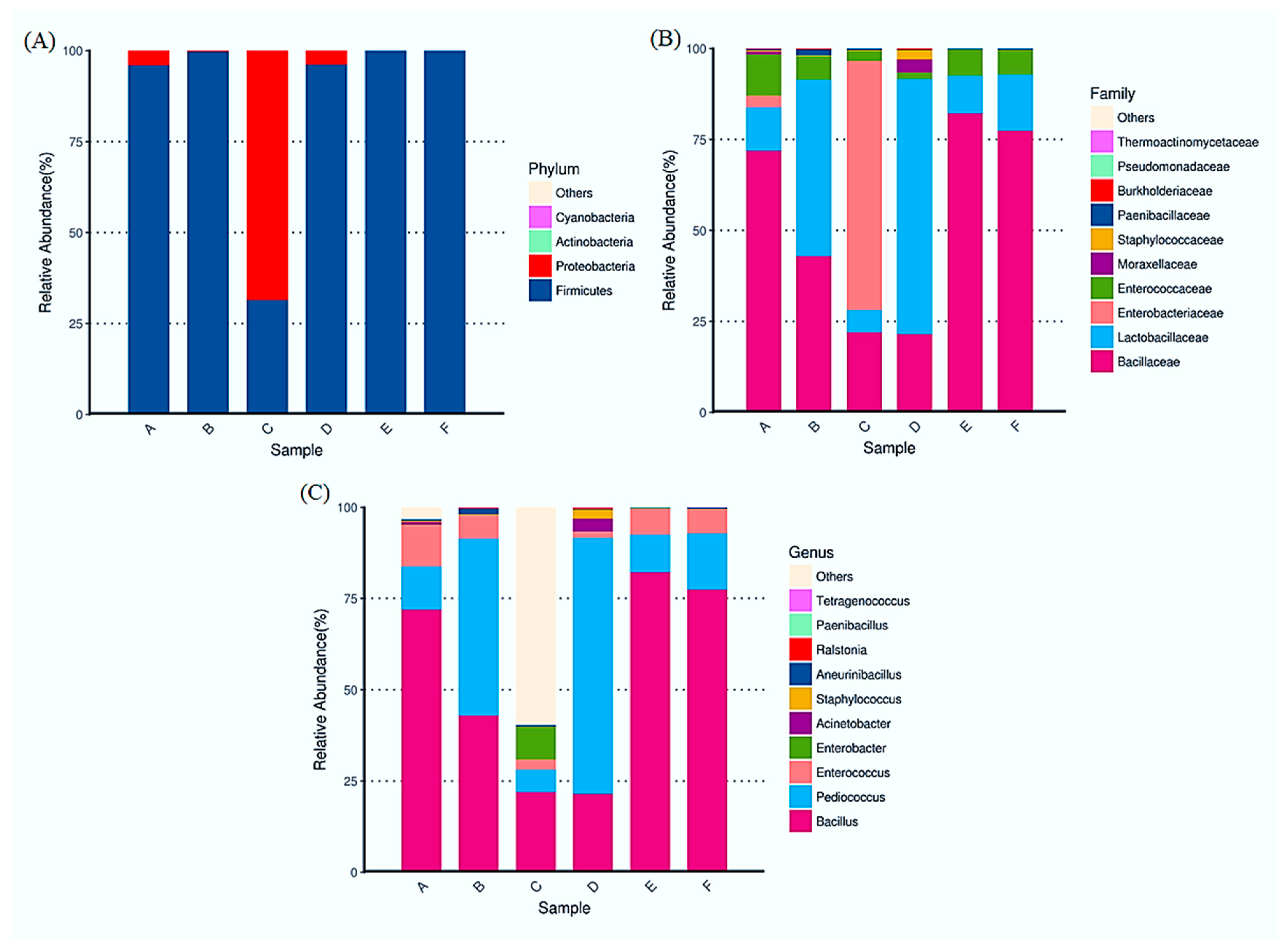
3.4. Diversification of Bacterial Diversity by 16S Assay in Soy Sauce
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yang, L.; Yang, H.L.; Tu, Z.C.; Wang, X.L. High-throughput sequencing of microbial community diversity and dynamics during douchi fermentation. PLoS ONE 2016, 11, e0168166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beland, F.A.; Benson, R.W.; Mellick, P.W.; Kovatch, R.M.; Roberts, D.W.; Fang, J.L.; Doerge, D.R. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. 2005, 43, 1–19. [Google Scholar] [CrossRef]
- Riachi, L.G.; Santos, A.; Moreira, R.F.; De Maria, C.A. A review of ethyl carbamate and polycyclic aromatic hydrocarbon contamination risk in cachaça and other Brazilian sugarcane spirits. Food Chem. 2014, 149, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Ren, Y.P.; Zhong, Q.D.; Shao, Y.; Zhao, Y.F.; Wu, Y.N. Ethyl carbamate in alcoholic beverages from China: Levels, dietary intake, and risk assessment. Food Control 2017, 72, 283–288. [Google Scholar] [CrossRef]
- Hasnip, S.; Crews, C.; Potter, N.; Christy, J.; Chan, D.; Bondu, T.; Matthews, W.; Walters, B.; Patel, K. Survey of ethyl carbamate in fermented foods sold in the United Kingdom in 2004. J. Agric. Food Chem. 2007, 55, 2755–2759. [Google Scholar] [CrossRef]
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl carbamate in fermented beverages: Presence, analytical chemistry, formation mechanism, and mitigation proposals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 611–626. [Google Scholar] [CrossRef]
- Ryu, D.; Choi, B.; Kim, E.; Park, S.; Paeng, H.; Kim, C.I.; Lee, J.Y.; Yoon, H.J.; Koh, E. Determination of ethyl carbamate in alcoholic beverages and fermented foods sold in Korea. Toxicol. Res. 2015, 31, 289–297. [Google Scholar] [CrossRef]
- Weber, J.V.; Sharypov, V.I. Ethyl carbamate in foods and beverages—A review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 429–452. [Google Scholar]
- Wu, P.G.; Pan, X.D.; Wang, L.Y.; Shen, X.H.; Yang, D.J. A survey of ethyl carbamate in fermented foods and beverages from Zhejiang, China. Food Control 2012, 23, 286–288. [Google Scholar] [CrossRef]
- Zhao, X.R.; Du, G.C.; Zou, H.J.; Fu, J.W.; Zhou, J.W.; Chen, J. Progress in preventing the accumulation of ethyl carbamate in alcoholic beverages. Trends Food Sci. Technol. 2013, 32, 97–107. [Google Scholar] [CrossRef]
- Matsudo, T.; Aoki, T.; Abe, K.; Fukuta, N.; Higuchi, T.; Sasaki, M.; Uchida, K. Determination of ethyl carbamate in soy-sauce and its possible precursor. J. Agric. Food Chem. 1993, 41, 352–356. [Google Scholar] [CrossRef]
- Zhang, J.R.; Fang, F.; Chen, J.; Du, G.C. The arginine deiminase pathway of koji bacteria is involved in ethyl carbamate precursor production in soy sauce. FEMS Microbiol. Lett. 2014, 358, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.; Zhang, J.; Zhou, J.; Zhou, Z.; Li, T.; Lu, L.; Zeng, W.; Du, G.; Chen, J. Accumulation of citrulline by microbial arginine metabolism during alcoholic fermentation of soy sauce. J. Agric. Food Chem. 2018, 66, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Arbol, J.T.; Pulido, R.P.; La Storia, A.; Burgos, M.J.G.; Lucas, R.; Ercolini, D.; Galvez, A. Changes in microbial diversity of brined green asparagus upon treatment with high hydrostatic pressure. Int. J. Food Microbiol. 2016, 216, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.P.; Burgos, M.J.G.; Galvez, A.; Lucas, R. Changes in bacterial diversity of refrigerated mango pulp before and after treatment by high hydrostatic pressure. LWT Food Sci. Technol. 2017, 78, 289–295. [Google Scholar] [CrossRef]
- Shi, Y.C.; Lai, C.Y.; Lee, B.H.; Wu, S.C. The bacterial and fungi microbiota of soy sauce-supplied lactic acid bacteria treated with high-pressure process. Fermentation 2022, 8, 97. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Pan, G.; Tu, C.; Zhou, X.; Mo, L. Study on the changing concentration of ethyl carbamate in yellow rice wine during production and storage by gas chromatography/mass spectrometry. Eur. Food Res. Technol. 2012, 235, 779–782. [Google Scholar] [CrossRef]
- Ballini, A.; Tete, S.; Scattarella, A.; Cantore, S.; Mastrangelo, F.; Papa, F.; Nardi, G.M.; Perillo, L.; Crincoli, V.; Gherlone, E.; et al. The role of anti-cyclic citrullinated peptide antibody in periodontal disease. Int. J. Immunopathol. Pharmacol. 2010, 23, 677–681. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, X.; Li, B.; Shen, C.; Sun, Y.M.; Yang, J.; Xu, Z.L. Citrulline accumulation mechanism of Pediococcus acidilactici and Weissella confusa in soy sauce and the effects of phenolic compound on citrulline accumulation. Front. Microbiol. 2021, 12, 757542. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Linforth, R.; Onyeaka, H.; Gkatzionis, K. Effects of co-inoculation and sequential inoculation of Tetragenococcus halophilus and Zygosaccharomyces rouxii on soy sauce fermentation. Food Chem. 2018, 240, 1–8. [Google Scholar] [CrossRef]
- O’toole, D.K. The role of microorganisms in soy sauce production. In Advances in Applied Microbiology; Meidleman, S.L., Laskin, A.I., Eds.; Academic Press: New York, NY, USA, 1997; Volume 45, pp. 87–152. [Google Scholar]
- Singracha, P.; Niamsiri, N.; Visessanguan, W.; Lertsiri, S.; Assavaning, A. Application of lactic bacteria and yeasts as starter cultures for reduced-salt soy sauce (moronic) fermentation. LWT Food Sci. Technol. 2017, 78, 181–188. [Google Scholar] [CrossRef]
- Ough, C.S.; Crowell, E.A.; Gutlove, B.R. Carbamyl compound reactions with ethanol. Am. J. Enol. Vitic. 1988, 39, 239–242. [Google Scholar]
- Kitamoto, K.; Oda, K.; Gomi, K.; Takahashi, K. Genetic engineering of a sake yeast producing no urea by successive disruption of arginase gene. Appl. Environ. Microbiol. 1991, 57, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mira de Orduna, R.; Liu, S.Q.; Patchett, M.L.; Pilone, G.J. Ethyl carbamate precursor citrulline formation from arginine degradation by malolactic wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 183, 31–35. [Google Scholar] [CrossRef]
- Vrancken, G.; Rimaux, T.; Wouters, D.; Leroy, F.; De Vuyst, L. The arginine deiminase pathway of Lactobacillus fermentum IMDO 130101 responds to growth under stress conditions of both temperature and salt. Food Microbiol. 2009, 26, 720–727. [Google Scholar] [CrossRef]
- Sulaiman, J.; Gan, H.M.; Yin, W.F.; Chan, K.G. Microbial succession and the functional potential during the fermentation of Chinese soy sauce brine. Front. Microbiol. 2014, 5, 556. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.; Manca de Nadra, M. Influence of ethanol and low pH on arginine and citrulline metabolism in lactic acid bacteria from wine. Res. Microbiol. 2005, 156, 858–864. [Google Scholar] [CrossRef]
- Cottenceau, G.; Dherbomez, M.; Lubochinsky, B.; Lettellier, F. Immobilization and treatment of Streptococcus faecalis for the continuous conversion of arginine into 420 citrulline. Enzyme Microb. Technol. 1990, 12, 355–360. [Google Scholar] [CrossRef]
- Zhang, J.; Du, G.; Chen, J.; Fang, F. Characterization of a Bacillus amyloliquefaciens strain for reduction of citrulline accumulation during soy sauce fermentation. Biotechnol. Lett. 2016, 38, 1723–1731. [Google Scholar] [CrossRef]
- Liu, X.; Bai, W.; Zhao, W.; Qian, M.; Dong, H. Correlation analysis of microbial communities and precursor substances of ethyl carbamate (EC) during soy sauce fermentation. LWT-Food Sci. Technol. 2021, 152, 112288. [Google Scholar] [CrossRef]
- Liu, X.; Qian, M.; Dong, H.; Bai, W.; Zhao, W.; Li, X.; Liu, G. Effect of ageing process on carcinogen ethyl carbamate (EC), its main precursors and aroma compound variation in Hakka Huangjiu produced in southern China. Int. J. Food Sci. Technol. 2020, 55, 1773–1780. [Google Scholar] [CrossRef]

| Time | Groups/L-Citrulline (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Months | A | B | C | D | E | F |
| 0 | 91 ± 24 a | 76 ± 19 a | 78 ± 40 a | 85 ± 30 a | 160 ± 99 a | 120 ± 43 a |
| 1 | 94 ± 6 b | 100 ± 13 b | 88 ± 23 b | 153 ± 40 a | 104 ± 20 b | 124 ± 9 a,b |
| 2 | 98 ± 17 a | 89 ± 33 a | 104 ± 19 a | 96 ± 19 a | 103 ± 16 a | 104 ± 27 a |
| 3 | 96 ± 37 a | 98 ± 34 a | 111 ± 14 a | 112 ± 27 a | 107 ± 32 a | 83 ± 25 a |
| 4 | 89 ± 26 a b | 86 ± 18 a,b | 94 ± 6 a,b | 86 ± 8 a,b | 72 ± 9 b | 104 ± 15 a |
| 5 | 81 ± 11 a | 98 ± 7 a | 84 ± 39 a | 80 ± 14 a | 104 ± 28 a | 101 ± 14 a |
| 6 | 92 ± 8 c | 99 ± 17 a,b | 123 ± 19 a | 99 ± 18 a,b | 104 ± 9 a,b | 102 ± 10 a,b |
| Time | Groups/EC Content (mg/mL) | |||||
|---|---|---|---|---|---|---|
| Months | A | B | C | D | E | F |
| 0 | 33.75 ± 1.56 a | 3.35 ± 1.01 c | 11.50 ± 0.56 b | 10.93 ± 1.64 b | 12.61 ± 5.26 b | 6.01 ± 0.41 c |
| 1 | 44.01 ± 4.54 a | 10.39 ± 4.50 b | 1.05 ± 0.08 c | 0.16 ± 0.05 c | 0.65 ± 0.02 c | 3.03 ± 2.95 c |
| 2 | 0.21 ± 0.16 c | 0.29 ± 0.01 c | 5.27 ± 0.25 b | 1.47 ± 0.20 c | 16.37 ± 3.86 a | 2.22 ± 1.33 c |
| 3 | 5.35 ± 0.77 b,c | 12.25 ± 1.34 a,b | 10.31 ± 0.60 b | 1.88 ± 0.12 c | 18.11 ± 8.83 a | 1.86 ± 1.17 c |
| 4 | 24.48 ± 7.79 a | 4.24 ± 1.50 b,c | 9.28 ± 0.96 b | 9.75 ± 1.60 b | 4.46 ± 2.20 b,c | 0.82 ± 0.12 c |
| 5 | 30.19 ± 24.52 a | 1.27 ± 0.60 b | 0.37 ± 0.31 b | 1.16 ± 0.82 b | 1.52 ± 0.22 b | 0.38 ± 0.31 b |
| 6 | 7.85 ± 0.64 b | 13.16 ± 1.77 a | 2.35 ± 0.92 c | 0.40 ± 0.05 d | 1.08 ± 035 d | 0.69 ± 0.33 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-Y.; Hou, C.-Y.; Chuang, P.-T.; Hsu, W.-H.; Wu, S.-C. Microbiota and Mycobiota of Soy Sauce-Supplied Lactic Acid Bacteria Treated with High Pressure. Fermentation 2022, 8, 338. https://doi.org/10.3390/fermentation8070338
Lai C-Y, Hou C-Y, Chuang P-T, Hsu W-H, Wu S-C. Microbiota and Mycobiota of Soy Sauce-Supplied Lactic Acid Bacteria Treated with High Pressure. Fermentation. 2022; 8(7):338. https://doi.org/10.3390/fermentation8070338
Chicago/Turabian StyleLai, Chiung-Yu, Chih-Yao Hou, Pei-Ting Chuang, Wei-Hsuan Hsu, and She-Ching Wu. 2022. "Microbiota and Mycobiota of Soy Sauce-Supplied Lactic Acid Bacteria Treated with High Pressure" Fermentation 8, no. 7: 338. https://doi.org/10.3390/fermentation8070338
APA StyleLai, C.-Y., Hou, C.-Y., Chuang, P.-T., Hsu, W.-H., & Wu, S.-C. (2022). Microbiota and Mycobiota of Soy Sauce-Supplied Lactic Acid Bacteria Treated with High Pressure. Fermentation, 8(7), 338. https://doi.org/10.3390/fermentation8070338








