Abstract
Diabetes is one of the most common chronic metabolic diseases, and its occurrence rate has increased in recent decades. Sidr (Ziziphus spina-christi L.) is a traditional herbaceous medicinal plant. In addition to its good flavor, sidr has antidiabetic, anti-inflammatory, sedative, analgesic, and hypoglycemic activities. Camel milk has a high nutritional and health value, but its salty taste remains the main drawback in relation to its organoleptic properties. The production of flavored or fortified camel milk products to mask the salty taste can be very beneficial. This study aimed to investigate the effects of sidr fruit pulp (SFP) on the functional and nutritional properties of fermented camel milk. SFP was added to camel milk at rates of 5%, 10%, and 15%, followed by the selection of the best-fermented product in terms of functional and nutritional properties (camel milk supplemented with 15% SFP), and an evaluation of its hypoglycemic activity in streptozotocin (STZ)-induced diabetic rats. Thirty-two male adult albino rats (weighing 150–185 g) were divided into four groups: Group 1, nontreated nondiabetic rats (negative control); Group 2, diabetic rats given STZ (60 mg/kg body weight; positive control); Group 3, diabetic rats fed a basal diet with fermented camel milk (10 g/day); and Group 4, diabetic rats fed a basal diet with fermented camel milk supplemented with 15% SFP (10 g/day). The results revealed that supplementation of camel milk with SFP increased its total solids, protein, ash, fiber, viscosity, phenolic content, and antioxidant activity, which was proportional to the supplementation ratio. Fermented camel milk supplemented with 15% SFP had the highest scores for sensory properties compared to other treatments. Fermented camel milk supplemented with 15% SFP showed significantly decreased (p < 0.05) blood glucose, malondialdehyde, low-density lipoprotein-cholesterol, cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, creatinine, and urea, and a significantly increased (p < 0.05) high-density lipoprotein-cholesterol, total protein content, and albumin compared to diabetic rats. The administration of fermented camel milk supplemented with 15% SFP in diabetic rats restored a series of histopathological changes alonsgside an improvement in various enzyme and liver function tests compared to the untreated group, indicating that fermented camel milk supplemented with 15% SFP might play a preventive role in such patients.
1. Introduction
Diabetes is one of the most common chronic metabolic diseases, with an increasing occurrence rate in recent decades [1]. This metabolic disorder has three main types (types 1–3) and is characterized by an abnormal long-term increase in plasma glucose levels [2]. Many factors, such as fat and protein metabolism, altered carbohydrate metabolism, and insulin deficiency or resistance, are the common reasons for the high blood glucose levels leading to diabetes. Chronic hyperglycemia related to diabetes is often associated with many other complications, such as neurological, renal, retinal, cardiovascular, dermatological, and nerve diseases [3]. In type 2 diabetes, a progressive decline in insulin action referred to as insulin resistance and pancreatic β-cell dysfunction leads to increased blood glucose levels [4]. The current treatment for type 2 diabetes includes insulin and oral hypoglycemic drugs, such as thiazolidinediones, biguanides, sulfonylurea derivatives, and α-glucosidase inhibitors. However, these medications have several side effects, including sodium retention, osteoporosis, risk of developing lactic acidosis, an induction of obesity, and higher incidences of severe hypoglycemia [5]. There is an increasing need to search for effective antidiabetic agents exhibiting fewer side effects. As an alternative, plant-based remedies for this metabolic disorder have been adopted [6,7,8,9]. Antioxidants can reduce oxidative stress markers in experimental and clinical models of diabetes [10]. Bioactive components from natural sources have also captivated scientific attention because of their functional, pharmacological, and biological properties [11]. Among others, sidr (Ziziphus spina-christi) is known as a multifunctional tree. Its leaves, fruits, and fruit juices are considered good sources of bioactive components, such as phenolics, flavonoids, alkaloids, cyclopeptides, steroid tannins, betulinic acid, triterpenoid saponin, glycosides, vitamin C, and bioactive polysaccharides [12,13,14]. These bioactive components have shown potential activities against liver complaints, urinary issues, digestive syndromes, weakness, obesity, diabetes, skin infection, appetite loss, fever, bronchitis, pharyngitis, anemia, insomnia, and diarrhea as well as immunomodulation, antioxidation, antitumor, and hepatoprotection [15,16]. In many countries, bioactive polysaccharides or polyphenol-enriched foods are traditionally mixed and consumed with milk [17,18,19,20,21,22,23,24].
Camel milk is characterized by its higher nutritional and health value compared to cow’s milk because it contains immune proteins with antioxidant and anti-inflammatory activities. Immunoglobulins, without β-lactoglobulin (β-LG), may cause allergic reactions in some people and contain large amounts of iron, potassium, and vitamins C, E, and A [25,26,27]. Camel milk also contains good amounts of lactoferrin, lactoperoxidase, lysozyme, and immunoglobulins G and A, and has more free amino acids and peptides compared to bovine milk [28,29,30]. Moreover, non-protein-bound amino acids in camel milk are easily digested by microorganisms, alongside the higher number of peptides and increased metabolic activity when used in a starter culture preparation. Therefore, camel milk can be used in fermented milk production [31,32]. Camel milk is traditionally drunk either raw or naturally fermented [33], and some products have been manufactured from camel milk such as pasteurized milk [34], fermented milk [35,36], cheese [37], yogurt [29], and powdered milk [27,38]. However, camel milk is characterized by a salty taste compared to cow’s milk due to its higher Na and K salt contents. Therefore, it is important to produce flavored or fortified camel milk products to mask the salty taste [17,18,19,20,21]. Furthermore, fermented camel milk has been proven to have health benefits, including angiotensin I-converting enzyme inhibitory activity, hypocholesterolemic effects, and antimicrobial, antioxidant, antidiarrheal, and anticancer activities [39,40]. However, there is no available information about the potential influence of the addition of sidr fruit pulp to fermented camel milk manufacture. Given the above information, this study aimed to enhance the functional properties of fermented camel milk via supplementation with bioactive sidr fruit pulp (SFP) and to evaluate the improvement of its bioavailability and protective effects against diabetes and liver conditions.
2. Materials and Methods
2.1. Materials and Reagents
Fresh camel milk was obtained from a healthy herd of camels from a private farm in El-Arish, North Sinai Governorate, Egypt. Ripening sidr fruit (5 kg) was obtained from a local market in Sharkia, Egypt. The starter culture “Yo-Flex culture (YC-X11)”, which contains a mixed strain of Streptococcus thermophilus and Lactobacillus delbruekii subsp bulgaricus at a ratio of 50:50, was obtained freeze-dried from Hansen’s Laboratories (Copenhagen, Denmark) and was used as a yogurt starter. Other chemicals and reagents were purchased from Sigma-Aldrich.
2.2. Preparation of High-Viscosity SFP
Sidr fruits were first washed. Fresh peel and pulp were separated from the seeds, mixed with distilled water at a 1:1 (w/v) ratio to produce high-viscosity pulp, and stored overnight under controlled cooling conditions (4–5 °C). Both peel and pulp were blended using a laboratory blender (Toshiba Mixie©, Tokyo, Japan) and kept at 4–5 °C until further use in fermented camel milk manufacture.
2.3. Fermented Camel Milk Manufacture
Fresh camel milk was divided into four portions as follows: the first portion was left without an additive as a control (C), and SFP was added to the other three portions at rates of 5%, 10%, and 15% (T1–T3). The fortified camel milk bases were homogenized at 55–60 °C for 2 min using a high-speed mixer (22,000 rpm); heat-treated in a thermostatically controlled water bath at 85 °C for 30 min; cooled to 42 °C in an ice bath; inoculated with 2.5% (w/v) starter culture containing S. thermophilus and L. delbruekii subsp bulgaricus, which dissolved in 50 mL of sterilized skimmed milk (121 °C for 15 min) (rated 50 U from starter to 250 L/66 gal); activated at 42 °C for 15 min before use; and then incubated at 42 °C for 6–8 h until a firm curd was obtained. The curd was refrigerated at 4 °C overnight, stirred using a mixer, poured into 100 mL plastic bottles with covers, stored at 4 ± 1 °C, and analyzed 1 day after their manufacture [41]. The experiments were repeated in triplicates and each analysis duplicates.
In accordance with the preparation schedule of fermented camel milk, camel milk was mixed with 5,10, and 15% sidr fruit pulp, homogenized at 55–60 °C, heat-treated at 85 °C for 30 min, cooled to 42 °C and inoculated with 2.5% yoghurt culture containing S. thermophilus and Lb. delbrueckii subsp. bulgaricus
 |
| Incubation at 42 °C for (6–8 h) |
 |
| The curd was refrigerated (4 ± 1 °C) overnight |
 |
| Mixing resultant curd with stirred with electrical mixer |
 |
| Filling in 100 mL. plastic bottles with cover and 4 ± 1 °C |
 |
| The resultant product of all treatments was analyzed after 1 day from manufacture for physicochemical, phytochemical, and sensory properties. |
2.4. Chemical Composition, Physicochemical and Phytochemical Analysis, and Sensory Evaluation of Fermented Camel Milk Treatments
This step involved the determination of total protein, total solids (TS), fat, ash, fiber, carbohydrate, and titratable acidity (TA) for fermented camel milk samples, following the protocol described elsewhere [42]. The total solids of sidr fruit pulp, camel milk, and fermented camel milk samples were determined using a drying oven at 100 °C for 24 h. The percentage of moisture content was calculated by the following formula.
where, W1 = initial weight of sample; W2 = weight of the dried sample
% moisture = W1 − (W2 × 100)/W1
Total solids % = 100 − moisture content.
The total nitrogen content (TN) was determined by using the micro-Kjeldahl method, and the protein content was calculated by multiplying the percentage of TN by 6.38 for milk components and 6.25 for sidr fruit pulp. The Gerber method was used to determine the percentage of fat in camel milk and fermented camel milk treatments, while the Soxhlet method was used to determine the percentage of fat in sidr pulp. To determine ash content, about 5 g of the sample was heated in a muffle furnace at 550 °C overnight. The difference between the product of subtracting the weight of ash from the weight of the insoluble material was expressed as the proportion of crude fiber from the original weight content. Carbohydrate content was calculated according to [43] by the following formula:
Total carbohydrates% = 100 − (%fat + %protein + %ash + %fiber + %moisture)
The titratable acidity was determined by mixing 10 g of fermented camel milk with 10 mL of distilled water and titrating it with 0.1 N NaOH using phenolphthalein as an indicator to an end-point of faint pink color, and expressed as a percentage of lactic acid. Their pH values were monitored using a pH meter equipped with a glass electrode (HANNA Instrument, Portugal). The viscosity of the fermented camel milk was determined according to Aryana [44] using Rotational Viscometer Type Lab, Line Model 5437. Measurements were determined at a temperature of 30 °C after 15 s; the results were expressed as centipoise (cP). To determine phytochemical properties of fermented camel milk samples, samples were centrifuged at 20,000× g for 60 min at 4 °C. The supernatants were then filtered using a 0.45 µm syringes filter and kept at −20 °C until further testing. The total phenolic content (TPC), expressed as mg gallic acid equivalents/100 g, was assessed according to Maksimović et al. [45], with minor modifications. Briefly, 100 μL of different concentrations of the test sample was mixed with 1 mL of diluted FC reagent (1:10). After 10 min, 1 mL of 7.5% (w/v) sodium carbonate solution was added to the mixture and incubated in the dark for 90 min. Absorbance was recorded at 725 nm. TPC was calculated from the calibration curve of gallic acid:
Y = 0.2808X + 0.0301; R2 = 0.9983
In addition, the antioxidant (AO) activity (%) of the prepared yogurts was assessed according to Apostolidis et al. [46], and absorbance was noted at 517 nm using a spectrophotometer (Thermo Scientific, Wilmington, NC, USA). Scavenging activity was calculated using the following formula:
DPPH radical scavenging % = 1 − (Absorbance of sample − Absorbance of blank)/Absorbance of control × 100
Total flavonoid content was determined by using the aluminum chloride calorimetric method, as described by [47]. The yogurt sample’s sensory evaluation was studied in terms of flavor (50), texture (30), and appearance (20), following the method of Tamime and Robinson [41]. Sensory evaluation was carried out by a team of 20 professional and trained panelists. The samples were packed in plastic cups and coded with a three-digit code. Each cup contained 100 mL of yoghurt samples freshly removed from the refrigerator. The sensory evaluation of the different descriptors relied on the pre-selected descriptors: flavor (sweetness, acidity, and bitterness), texture (absence of curd homogeneity, lumps, and bubbles), appearance (wheying-off, white color, and reddish color) and overall acceptability (the sum of all the character results). The coded samples were presented in a tray to the panelists. After each sample testing, the panelists were offered plain water to cleanse their palate before moving on to the next sample. The data were collected in specially designed ballots.
2.5. Experimental Design of the Biological Study
Thirty-two male adult albino rats weighing 150–185 g were provided by the Agricultural Research Center (Giza, Egypt). All animals were kept under controlled light conditions (12 h light and 12 h darkness) with an ambient temperature of 22 ± 2 °C, relative humidity of 40–60%, and free access to water and food in the animals’ room. All animals were allowed free access to a standard diet according to AIN-93 guidelines [48]. After acclimation to a basal diet for 7 days, albino rats were classified into two main groups. The first group (n = 8) was given only the standard diet and served as the normal control group, and the second group (hyperglycemic rats, n = 24) was subjected to an intraperitoneal injection of streptozotocin (STZ) at a dose of 60 mg/kg body weight (BW). After 24–48 h, rats with a fasting blood glucose of >200 mg/dL were considered diabetic rats. This main group of diabetic rats was divided into three subgroups (n = 8 each). The first subgroup did not receive treatment and served as the hyperglycemic positive control. The second and third subgroups were given fermented camel milk (10 g/day) and fermented camel milk (10 g/day) supplemented with 15% SFP, respectively. The BW of rats was measured at the beginning of the experiment and at 7-day intervals. At the end of the experiment and after overnight fasting (10 h), rats were sacrificed. Blood samples were collected and centrifuged at 3000 rpm to obtain sera, which were stored at −20 °C for further biochemical analysis.
2.6. Biochemical Analysis
Blood glucose level was determined as described elsewhere [49]. Insulin was determined in blood samples according to Thomas et al. [50]. Malondialdehyde (MDA) was determined in serum according to Satoh [51]. Furthermore, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes were measured according to Bergmeyer and Harder [52]. Serum creatinine level was measured according to Baranowski and Westenfelder [53]. Serum urea was determined according to Marsch et al. [54]. Total cholesterol was also measured according to the enzymatic colorimetric method [55]. Total lipids and triglycerides (TG) were determined according to Devi and Sharma [56]. Low-density lipoprotein-cholesterol (LDL-C) was also calculated using the Friedewald formula [57] as follows:
LDL-C = Total cholesterol − [High-density lipoprotein-cholesterol (HDL-C)] − (TG/5).
2.7. Histological Evaluation of the Pancreas
Pancreatic specimens from rats were fixed in 10% neutral buffered formalin solution for 24 h to ensure perfect fixation, after which they were dehydrated in a graded alcohol series, cleared in xylene, and finally embedded in paraffin. Tissue was cut into 3-μm-thick sections and stained with hematoxylin and eosin [58]. Tissue sections were examined for histopathological changes using an Olympus BX51 microscope and photographed with an Olympus DP72 camera adapted to the microscope, as described elsewhere [59].
2.8. Statistical Analysis
Data were expressed as the mean ± standard error of the mean via analysis of variance using SPSS version 20 (2012) [60]. Significant treatment, followed by Duncan’s new multiple range tests, was used to evaluate the statistical significance of the results. Differences were considered significant at p ≤ 0.05.
3. Results and Discussion
3.1. Chemical Composition and Phytochemical Properties of Camel Milk and SFP
Table 1 shows the chemical composition and phytochemical properties of camel milk and SFP. The TS, protein, fat, ash, and carbohydrate contents of camel milk were 12.36, 3.20, 4.16, 0.88, and 4.14 g/100 g, respectively. These results agreed with those obtained by Zang et al. [61], who found that the protein, lactose, fat, ash, and TS contents of camel milk were 3.55, 4.24, 5.65, 0.87, and 14.31 g/100 g, respectively. In addition, Karaman et al. [62] found that the TS, protein, fat, and ash contents of camel milk were 11.83, 3.10, 3.28, and 0.83 g/100 g, respectively. Various factors, such as genetic factors, the lactation stage, the animal’s health status, and environmental factors, could influence this wide variation in milk composition [63]. As for the chemical composition of SFP, the TS, protein, fat, ash, fiber, and carbohydrate contents were 74.2, 2.35, 0.40, 2.11, 3.2, and 39.08 g/100 g, respectively. Several previous studies [12,64] reported that the TS, protein, fat, ash, and fiber contents of SFP ranged from 73.00 to 94.0, 4.1 to 5.40, 0.66 to 0.94, 3.79 to 7.92, and 4.1 to 6.90 g/100 g, respectively. These results were lower than those in previous studies by approximately half because the fruit pulp was mixed with distilled water at a ratio of 1:1 (w/v) to produce high-viscosity pulp to improve the properties of the resulting fermented milk. In this study, TPC, TF, and DPPH% of camel milk were 0.85, 0.08 mg/g FW, and 6.34%, respectively. These results were in line with those obtained by El-Fattah et al. [65], who found that the DPPH% inhibition of camel milk was 18.57% whereas the TPC, TF, and DPPH% of SFP were 10.40, 1.24 mg/g FW, and 62.02%, respectively. This high phenolic and flavonoid component content of SFP agreed with that of many researchers [13,14,16], who explained that sidr fruit has a high content of bioactive components, such as phenolics and flavonoids.

Table 1.
Chemical composition and phytochemical properties of camel milk and sidr fruit pulp (SFP).
3.2. Chemical Composition and Physicochemical and Phytochemical Properties of Fermented Camel Milk Supplemented with SFP
Table 2 shows the effects of fortifying camel milk with SFP on the chemical composition and the physicochemical and phytochemical properties of fermented camel milk. In Table 2, supplementation of camel milk with SFP significantly (p ≤ 0.05) increased the TS, ash, fiber, and carbohydrate contents from 12.44, 0.94, 0.00, and 4.10 in the control samples to 23.40, 1.33, 0.48, and 8.76 in the samples fortified with 15% SFP (T3). The possible explanation is the high TS, ash, fiber, and carbohydrate contents of fermented camel milk samples supplemented with SFP compared to those of unfermented camel milk [66]. By contrast, the addition of SFP at different proportions did not affect the protein and fat contents of fermented camel milk because of the low protein and fat contents of SFP. These results agreed with Soliman and Shehata [67] and Aljutaily et al. [68], who found that fermented camel milk fortified with kiwi and avocado fruits or Sukkari date increased the TS, ash, dietary fiber, and carbohydrate contents compared to plain fermented camel milk.

Table 2.
Chemical composition, physicochemical, phytochemical, and sensory properties of fermented camel milk supplemented with sidr fruit pulp.
Concerning TA, Table 2 indicates that the acidity of fermented camel milk fortified with SFP significantly decreased (p ≤ 0.05) by increasing the fortification ratio. This could be attributed to the SFP content of the antimicrobial substance, which might reduce the viability of the starter culture [69,70]. The TA of plain fermented camel milk had a higher value than that of fortified treatments. By contrast, the pH values increased in the treatments fortified with SFP, as a function of the fortification percentage, compared to plain fermented camel milk. Similar results were obtained in a previous work [71], which found that yogurt supplementation with dried sidr fruit increased the pH and decreased the TA values of yogurt. Another previous work [29] reported that the fortification of camel milk yogurt with monk fruit sweetener increased the pH and decreased the TA values of camel milk yogurt. The viscosity values of the treatments fortified with SFP significantly increased (p ≤ 0.05), and this increase was proportional to the fortification ratios as a result of the high-viscosity conditions resulting from mixing SFP with water [72], which consequently increased the viscosity values of the fortified fermented camel milk treatments. Similarly, other previous studies [29,71] observed that the addition of dried sidr fruit or monk fruit sweetener to cow or camel milk yogurt significantly increased (p ≤ 0.05) the viscosity of the final product.
In Table 2, the TFC and DPPH% of fermented camel milk supplemented with SFP significantly increased (p ≤ 0.05) when increasing the supplementation ratio compared to plain fermented camel milk. The possible explanation could be the high TPC of SFP [73] compared to that of camel milk [67]. These results agreed with a previous work [71], which found that adding dried sidr fruit to yogurt milk increased the TFC and DPPH% of yogurt. In addition, another previous study [67] found that the addition of kiwi and avocado to camel milk increased the TFC and DPPH% of fermented camel milk.
In Table 2, regarding the sensory characteristics, the supplementation of camel milk with SFP significantly increased (p ≤ 0.05) the sensory attributes of the resultant fermented camel milk, particularly consistency and flavor, compared to plain fermented camel milk. Furthermore, this improvement was proportional to the supplementation ratio, and plain fermented camel milk had lower sensory property scores. This could result from the weak body and texture and inferior flavor of the curd produced from camel milk. In addition, plain fermented camel milk produced a watery and fragile consistency, resulting in a poor structure, most likely due to the small size of the camel fat globules, relative distribution of casein fractions, large casein micelles, and the absence of β-LG in camel milk [35,68]. These results were consistent with several previous reports [67,68], which revealed that the fortification of camel milk with kiwi and avocado fruits or Sukkari date increased the sensory attributes of fermented camel milk.
3.3. Effects of Fermented Camel Milk Supplemented with SFP on the Final Weight and BW Gain (BWG) of Diabetic Rats
Table 3 shows the effects of fermented camel milk supplemented with SFP on the final weight and BWG of diabetic rats. The results showed that the initial weights of nontreated nondiabetic rats (negative control), diabetic rats (positive control), diabetic rats given fermented camel milk, and diabetic rats given fermented camel milk supplemented with 15% SFP were 158.5 ± 3.8, 159.3 ± 2.9, 158.8 ± 4.3, and 159.6 ± 4.6 g, respectively. The FW and BWG of rats were significantly (p < 0.05) affected by the treatments. The use of 10 g/day fermented camel milk supplemented with 15% SFP in diabetic rats induced the best values of FW (214.4 ± 4.4 g) and BWG (25.55%), followed by rats given 10 g/day fermented camel milk with 209.5 ± 4.8 g and 24.20%, respectively. By contrast, the positive control group had lower FW (204.6 ± 3.5 g) and BWG (by 22.14%) compared to animals given fermented camel milk supplemented with 15% SFP. This improvement in the final weight and BWG of diabetic rats given fermented camel milk supplemented with 15% SFP might be attributed to the high SFP content from bioactive compounds, such as phenolic acids, flavonoids, vitamins, and minerals, which may prevent or avoid the body’s cell damage by free radicals [73]. In addition, the high vitamin C and mineral contents of camel milk might act as an antioxidant, which targets the removal of free radicals [74], increasing BWG in diabetic rats compared to the positive control group. Accordingly, Guizani et al. [75] found that Z. spina-christi extract enhanced the nutritional status and increased the BWG of diabetic rats. Another study [76] found that camel milk curd enhanced the nutritional status and increased the BWG of diabetic rats. Collectively, diabetic rats given fermented camel milk and supplemented with 15% SFP induced the best results in terms of FW and BWG compared to other diabetic rats.

Table 3.
Final weight and body weight gain of diabetic rats treated with fermented camel milk containing SFP.
3.4. Effects of Fermented Camel Milk Supplemented with SFP on Blood Glucose and Insulin Levels in Diabetic Rats
It is noteworthy to mention that many people have a common belief that high-calorie foods increase blood sugar levels, but in general, foods that increase blood sugar are those that contain a high percentage of carbohydrates that quickly turn into energy [77]. Revising the availble literature, previous studies have shown that some types of fruits can lower blood sugar, such as sidr fruit [16,78]. Moreover, other previous studies have also demonstrated the potential benefits and role of camel milk in the reduction of blood sugar levels in patients with type 2 diabetes [74,79,80]. Table 4 shows that diabetic rats (positive control) had higher (p ≤ 0.05) blood glucose levels and lower insulin levels of 236.2 ± 7.55 mg/dL and 10.26 ± 0.64d μu/mL compared to nondiabetic rats (negative control) and other diabetic rats. The administration of fermented camel milk and fermented camel milk supplemented with SFP in diabetic groups led to a significant decrease (p ≤ 0.05) in serum glucose levels and increased insulin levels compared to the positive control group. The mechanisms underlying the obvious effects of sidr fruit on lowering blood glucose might be attributed to its ability to reduce glycosylated hemoglobin [16] and inhibit α-glucosidase and α-amylase [81], in addition to the fact that fruits are rich in several secondary metabolites, including flavonoids and polyphenols, which have significant antihyperglycemic activity [82]. In addition, camel milk does not form coagulum in the stomach or acidic medium because camel milk contains a high insulin (52 units/L) concentration and the amino acid sequences of some camel milk proteins are rich in half-cystine, which has a superficial similarity to the insulin family of peptides and thereby prevents insulin degradation in the stomach [28,83]. In line with this study, Ashraf et al. [74] and Bencheikh et al. [16] found that diabetic rats given fermented camel milk or sidr fruits showed a significant decrease in blood sugar levels compared to untreated diabetic rats. Diabetic rats given fermented camel milk supplemented with 15% SFP achieved the best results in terms of blood sugar and insulin levels compared to the other diabetic rats.

Table 4.
Effect of fermented camel milk supplemented with SFP on blood glucose and insulin of rate groups.
3.5. Effects of Fermented Camel Milk Supplemented with SFP on the Serum Lipid Profile in Diabetic Rats
In Table 5, diabetic rats given fermented camel milk supplemented with SFP had a lower total cholesterol level (72.2 ± 2.6 mg/dL) compared to the positive control (94.6 ± 3.2 mg/dL). In addition, the positive control group had higher triacylglyceride and LDL contents (103.5 ± 3.6 and 43.30 ± 1.2 mg/dL, respectively) compared to diabetic rats given fermented camel milk and fermented camel milk supplemented with SFP, with a significant decrease in triacylglyceride and LDL contents (90.8 ± 3.2 and 84.4 ± 3.3, and 29.14 ± 1.04 and 20.52 ± 0.86 mg/dL, respectively). The positive control group had a lower HDL-C content (30.6 ± 1.5 mg/dL) compared to the normal and diabetic groups. In this respect, rats given fermented camel milk supplemented with SFP had a significant (p ≤ 0.05) increase in HDL levels (37.4 ± 1.3 mg/dL). There was a significant increase (p ≤ 0.05) in serum triacylglyceride, cholesterol, and LDL-C levels and a decrease in HDL-C levels in the positive control group compared to the normal group and diabetic groups given fermented camel milk and fermented camel milk supplemented with SFP. The antihyperlipidemic effects observed in SFP might be attributed to the richness of SFP in many components, such as minerals, vitamins, polyphenols, and flavonoids, and a large amount of catechin, which can form complexes with lipids and their degrading enzymes, causing fat emulsification, hydrolysis, and micelle dissolution, followed by absorption [84]. In addition, the antihyperlipidemic action might be driven by stopping the chain reaction of lipid oxidation by capturing free radicals and thus maintaining the activity of HDL-binding paroxonase by chelating oxidized metal ions, preventing LDL oxidation [85]. Furthermore, the high insulin concentration of camel milk can cause the activation of the lipoprotein lipase enzyme [28,86]. Moreover, the high mineral content of camel milk (sodium, potassium, zinc, copper, and magnesium) and vitamin C intake might act as an antioxidant, thereby targeting the removal of free radicals [74]. Similar results were obtained by Bencheikh et al. [16] and Dikhanbayeva et al. [76], who found that sidr fruits or fermented camel milk had a hypocholesterolemic effect.

Table 5.
Effect of fermented camel milk supplemented with SFP on the serum lipid profile in diabetic rats.
3.6. Effects of Fermented Camel Milk Supplemented with SFP on Liver Function Parameters in Diabetic Rats
The effects of fermented camel milk supplemented with SFP on the liver function parameters in diabetic rats are shown in Table 6. STZ injection in rats provoked a significant increase (p ≤ 0.05) in plasma AST and ALT levels and a significant decrease in total protein and albumin compared to the control group. However, the administration of fermented camel milk supplemented with SFP showed a significant reduction in plasma AST and ALT levels as liver markers and a significant increase in plasma total protein and albumin levels compared to the positive control group. The hepatoprotective effects of SFP can be attributed to the high phenolic acid and flavonoid contents of SFP, which possess antioxidant properties by trapping free radicals [15]. In addition, the high vitamin C content of camel milk might act as an antioxidant and might therefore decrease aminotransferase enzymes [83]. These results agreed with Bencheikh et al. [87] and Dikhanbayeva et al. [76], who found that Sider fruit extract or fermented camel milk had hepatoprotective effects. Collectively, diabetic rats given fermented camel milk supplemented with 15% SFP showed improved liver function test results compared to other diabetic rats.

Table 6.
Effect of fermented camel milk supplemented with SFP on liver function parameters in diabetic rats.
3.7. Effects of Fermented Camel Milk Supplemented with SFP on Kidney Function Parameters in Diabetic Rats
The nephroprotective effects of fermented camel milk supplemented with SFP were evaluated by measuring the plasma levels of renal function biomarkers, such as creatinine and urea (Table 7). The positive control group showed a significant increase in plasma creatinine (p ≤ 0.05) and urea concentrations. In addition, the diabetic group given fermented camel milk (Group 3) experienced a significant decrease in these measured parameters. However, the diabetic group given fermented camel milk supplemented with SFP (Group 4) exhibited a highly significant decrease (p ≤ 0.05) in creatinine and urea. Furthermore, a significant (p ≤ 0.05) increase in lipid peroxidation in the positive control group was observed, as indicated by its high MDA levels compared to the diabetic groups. Meanwhile, the diabetic group given fermented camel milk supplemented with SFP showed a significant (p ≤ 0.05) reduction in MDA levels. The formation and increase of MDA levels can lead to oxidative mechanisms, high cytotoxicity, and inhibitory actions, which are inhibited by natural antioxidants [88]. This conspicuous change might be attributed to the richness of SFP in bioactive components, such as flavonoids, phenolics, minerals, and vitamins [73], which act as superoxide scavengers, resulting in the suppression of reactive oxygen species and uric acid formation [89]. Moreover, camel milk had nephroprotective effects due to its antioxidant activity, which was attributed to its high vitamin C and mineral contents [83]. These results were consistent with Bencheikh et al. [87] and El-Zahar et al. [90], who found that sidr fruit extract or fermented camel milk had nephroprotective effects. Collectively, diabetic rats given fermented camel milk supplemented with 15% SFP induced the best results in terms of serum kidney function compared to other diabetic rats.

Table 7.
Effect of fermented camel milk supplemented with SFP on kidney function parameters in diabetic rats.
3.8. Histological Evaluation of the Pancreas under the Influence of Fermented Camel Milk Supplemented with SFP for Diabetic Rats
Histological examination of pancreatic tissue sections from control rats revealed the typical histological architecture of the pancreatic exocrine parenchyma with the numerous pancreatic acini of the pyramidal acinar cells, whereas the endocrine islets of Langerhans appeared as large pale oval areas with a well-defined contour between the exocrine acini. They consisted of multiple, small, and pale β cells and a few large, acidophilic, and rounded α cells around small blood capillaries (Figure 1). The sections from the Group 2 diabetic rats without any treatment showed several pathological findings, including distorted pancreatic lobules and acini separated by edema and contained dilated ducts. The acinar cells showed vacuolar degeneration and desquamated cells. The islets of Langerhans were atrophied and revealed depleted β cells with a reduced number of other cells (Figure 2). The concomitant administration of fermented camel milk to Group 3 rats ameliorated the previously mentioned pathological findings recorded in Group 2, in which multiple normal pancreatic lobules with thin separated interlobular septa were observed. The exocrine parenchyma had numerous pancreatic acini of the pyramidal acinar cells, whereas the islets of Langerhans appeared as a well-defined large pale area between the exocrine acini with an observed improvement in its cellular population (Figure 3). By contrast, microscopic sections from diabetic rats given fermented camel milk coadministered with SFP showed greater improvement in the histologic structure in the form of multiple normal pancreatic lobules exhibiting numerous exocrine pancreatic acini. By contrast, the islets of Langerhans appeared as well-defined large pale oval areas around small blood capillaries and between the exocrine acini with normal cellular structure and density (Figure 4). These results made it clear to the authors’ knowledge that diabetes induced changes in the size and structure of exocrine and endocrine pancreatic cells. However, concomitant treatment with fermented camel milk and/or camel milk fortified with SFP may improve the size of the islets of Langerhans with its cellular density, as in previous studies [91].
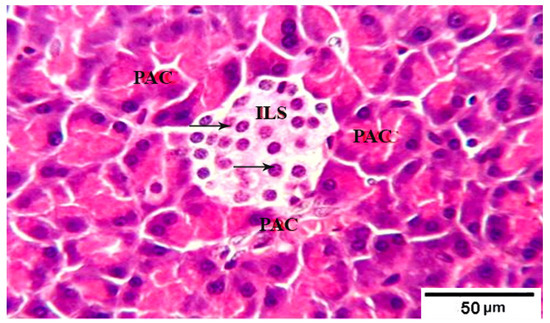
Figure 1.
Photomicrograph of pancreatic tissue sections from control group 1 rats showing: well defined normal large pale islets of Langerhans (ILs) with normal cellular shape and density (arrows) in between the acidophilic pancreatic acini (PAC). H&E stain. The bar size is indicated under picture.
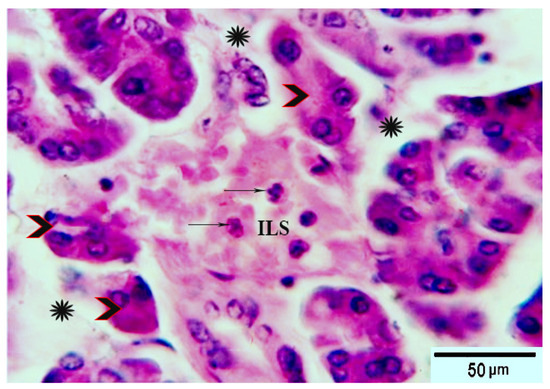
Figure 2.
Photomicrograph of pancreatic tissue sections from group 2 rats showing: pale distorted islets of Langerhans (ILs) in between the acidophilic pancreatic acini (arrowheads), separated by edema (stars). The acinar cells had vacuolar degeneration and dissociation (arrowheads) and the islets showing depletion of its constituent cells (arrows). H&E stain. The bar size is indicated under picture.
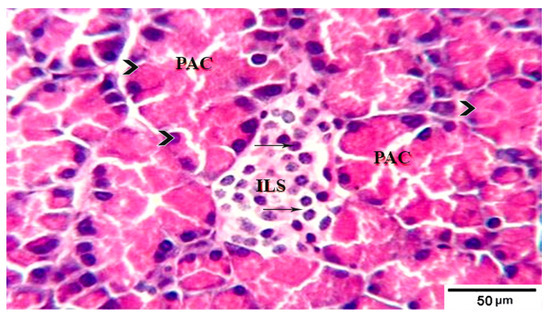
Figure 3.
Photomicrograph of pancreatic tissue sections from treated group 3 rats showing well defined pale islets of Langerhans (ILs) with proliferated cell population (arrows) in between normal pyramidal acidophilic pancreatic acini (PAC) (arrowheads)H&E stain. The bar size is indicated under picture.
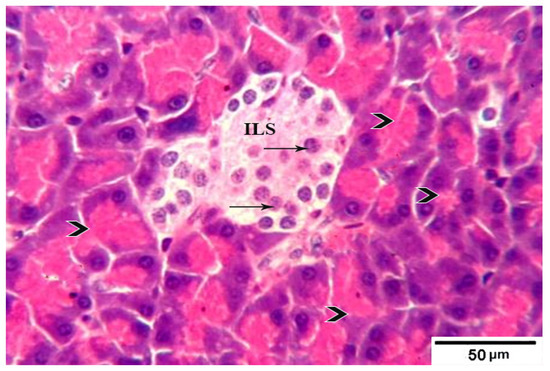
Figure 4.
Photomicrograph of pancreatic tissue sections from treated group 4 rats showing islets of Langerhans (ILs) restored its normal size and with normal cellular structure and density (arrows) in between intact normal acidophilic pancreatic acini (arrowheads) H&E stain. The bar size is indicated under picture.
4. Conclusions
Given the above findings, the present study revealed that fermented camel milk has hypoglycemic properties, and that sidr fruit also has the ability to reduce blood sugar. The fortififcation of fermented camel milk with sidr fruit pulp resulted in a fermented product with good physicochemical and sensory properties and a high nutritional value, which has an improved ability to lower blood sugar levels. Furthermore, this study concluded that SFP has a high content of bioactive compounds, such as phenolic compounds, flavonoids, dietary fiber, and polysaccharides. More importantly, the fortification of camel milk with SFP at a rate of 15% to manufacture fermented camel milk improved the chemical, nutritional, antioxidant, rheological, and sensory properties, and added the nutritive and healthy benefits of fermented camel milk. The consumption of fermented camel milk supplemented with 15% SFP (10 g/day) in diabetic rats induced by STZ significantly decreased blood glucose, MDA, cholesterol, TG, LDL-C, AST, ALT, creatinine, and urea levels, and increased HDL-C, total protein, and albumin compared to diabetic rats. Further research is recommended to explore the effects of the incorporation of SFP in other dairy products.
Author Contributions
E.S.H.A., M.R.S. and M.A.A.H. were involved in the conception of the research idea and methodology design, supervision, and the performance of the data analysis and interpretation and prepared the manuscript for publication and revision. B.M.A., M.A.A., N.D., F.A.Z.A. and E.K.E. were involved in the methodology and drafted and prepared the manuscript for publication and revision. All authors have read and agreed to the published version of the manuscript. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
Thanks and appreciation to the Saudi Ministry of Environment, Water, and Agriculture, facilitated by General Administration of Animal Productions for enabling research and using its facilities.
Institutional Review Board Statement
This study was conducted with the approval of the approval of the Faculty of Veterinary Medicine, Suez Canal University, and the institutional Review Board Number 2022005.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are contained within the article.
Acknowledgments
The authors thank the Taif University Researchers supporting project number (TURSP-2020/93), Taif University, Taif, Saudi Arabia, for support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bullard, K.M.; Cowie, C.C.; Lessem, S.E.; Saydah, S.H.; Menke, A.; Geiss, L.S.; Orchard, T.J.; Rolka, D.B.; Imperatore, G. Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 359. [Google Scholar] [CrossRef]
- Chamberlain, J.J.; Doyle-Delgado, K.; Peterson, L.; Skolnik, N. Diabetes technology: Review of the 2019 American Diabetes Association standards of medical care in diabetes. Ann. Intern. Med. 2019, 171, 415–420. [Google Scholar] [CrossRef]
- Mauricio, D.; Alonso, N.; Gratacòs, M. Chronic diabetes complications: The need to move beyond classical concepts. Trends Endocrinol. Metab. 2020, 31, 287–295. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Bandawane, D.; Mooliya, S.; Jadhav, S. Protective role of berberine in ameliorating diabetic complications in streptozotocin-high fat diet model in experimental animals. Int. J. Pharm. Pharm. Sci. 2020, 12, 41–48. [Google Scholar] [CrossRef]
- Agil, A.; Elmahallawy, E.K.; Rodriguez-Ferrer, J.M.; Adem, A.; Bastaki, S.M.; Al-Abbadi, I.; Fino Solano, Y.A.; Navarro-Alarcon, M. Melatonin increases intracellular calcium in the liver, muscle, white adipose tissues and pancreas of diabetic obese rats. Food Funct. 2015, 6, 2671–2678. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Albarakati, A.J.A.; Daabo, H.M.A.; Baty, R.S.; Salem, F.E.H.; Habotta, O.A.; Elmahallawy, E.K.; Abdel-Mohsen, D.M.; Taha, H.; Akabawy, A.M.A.; et al. Neuromodulatory effects of green coffee bean extract against brain damage in male albino rats with experimentally induced diabetes. Metab. Brain Dis. 2020, 35, 1175–1187. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcon, M.; Ali, F.A.Z.; Albrakati, A.; Salagre, D.; Campoy, C.; Elmahallawy, E.K. Melatonin Enhances the Mitochondrial Functionality of Brown Adipose Tissue in Obese-Diabetic Rats. Antioxidants 2021, 10, 1482. [Google Scholar] [CrossRef]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef]
- Xie, J.-H.; Tang, W.; Jin, M.-L.; Li, J.-E.; Xie, M.-Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Singh, V.; Guizani, N.; Essa, M.M.; Rahman, M.S.; Selvaraju, S. In vitro antioxidant activities of Ziziphus spina-christi fruits (red date) grown in Oman. Biotechnology 2012, 11, 209–216. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Moustaid, K.; Essamadi, A.K.; Nasser, B. Comparative study of phytochemical profile between Ziziphus spina christi and Ziziphus lotus from Morocco. J. Food Meas. Charact. 2019, 13, 121–130. [Google Scholar] [CrossRef]
- Cadi, H.E.; Bouzidi, H.E.; Selama, G.; Cadi, A.E.; Ramdan, B.; Oulad El Majdoub, Y.; Alibrando, F.; Dugo, P.; Mondello, L.; Fakih Lanjri, A. Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules 2020, 25, 5237. [Google Scholar] [CrossRef]
- Khouchlaa, A.; Talbaoui, A.; El Yahyaoui El Idrissi, A.; Bouyahya, A.; Ait Lahsen, S.; Kahouadji, A.; Tijane, M. Détermination des composés phénoliques et évaluation de l’activité litholytique in vitro sur la lithiase urinaire d’extrait de Zizyphus lotus L. d’origine marocaine. Phytothérapie 2017, 16, 1–6. [Google Scholar]
- Bencheikh, N.; Bouhrim, M.; Merrouni, I.A.; Boutahiri, S.; Kharchoufa, L.; Addi, M.; Tungmunnithum, D.; Hano, C.; Eto, B.; Legssyer, A. Antihyperlipidemic and antioxidant activities of flavonoid-rich extract of Ziziphus lotus (L.) lam. fruits. Appl. Sci. 2021, 11, 7788. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, Physicochemical and Sensory Properties of Probiotic Yoghurt Made from Whole Milk Powder Reconstituted in Aqueous Fennel Extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; El-Zahar, K.M.; Elmaadawy, A.A.; Hijazy, H.H.A.; Sitohy, M.Z.; Albrakati, A.; Elmahallawy, E.K. Remedial Action of Yoghurt Enriched with Watermelon Seed Milk on Renal Injured Hyperuricemic Rats. Fermentation 2022, 8, 41. [Google Scholar] [CrossRef]
- Swelam, S.; Zommara, M.A.; Abd El-Aziz, A.E.-A.M.; Elgammal, N.A.; Baty, R.S.; Elmahallawy, E.K. Insights into Chufa Milk Frozen Yoghurt as Cheap Functional Frozen Yoghurt with High Nutritional Value. Fermentation 2021, 7, 255. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.; Hernández-Mendoza, A.; Torres-Llanez, M.; González-Córdova, A.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Radwan, H.A.; Elmeligy, A.A.; Hafiz, A.A.; Albrakati, A.; Elmahallawy, E.K. Production of a Yogurt Drink Enriched with Golden Berry (Physalispubescens L.) Juice and Its Therapeutic Effect on Hepatitis in Rats. Fermentation 2022, 8, 112. [Google Scholar] [CrossRef]
- Elkot, W.F.; Ateteallah, A.H.; Al-Moalem, M.H.; Shahein, M.R.; Alblihed, M.A.; Abdo, W.; Elmahallawy, E.K. Functional, Physicochemical, Rheological, Microbiological, and Organoleptic Properties of Synbiotic Ice Cream Produced from Camel Milk Using Black Rice Powder and Lactobacillus acidophilus LA-5. Fermentation 2022, 8, 187. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Elkot, W.F.; Hijazy, H.H.A.; Kassab, R.B.; Alblihed, M.A.; Elmahallawy, E.K. The Impact of Date Syrup on the Physicochemical, Microbiological, and Sensory Properties, and Antioxidant Activity of Bio-Fermented Camel Milk. Fermentation 2022, 8, 192. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.-S.H.; Babalghith, A.O.; ALRashdi, B.M.; Radwan, H.A.; Umair, M.; Abdalmegeed, D.; Mahfouz, H.; Dahran, N.; Cacciotti, I.; et al. Impact of incorporation of Hawthorn (C. oxyanatha) leaves aqueous extract on yogurt properties and its therapeutic effects against oxidative stress in Rats induced by carbon tetrachloride. Fermentation 2022, 8, 200. [Google Scholar] [CrossRef]
- Salem, S.; Meead, G.; El-Rashody, F.M. Physicochemical and sensory properties of ice cream made from camel milk and fortified with dates products. Int. J. Humanit. Arts Med. Sci. 2017, 5, 29–40. [Google Scholar]
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Zouari, A.; Mtibaa, I.; Triki, M.; Jridi, M.; Zidi, D.; Attia, H.; Ayadi, M.A. Effect of spray-drying parameters on the solubility and the bulk density of camel milk powder: A response surface methodology approach. Int. J. Dairy Technol. 2020, 73, 616–624. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Saran, S.; Sharma, P.; Gupta, R.P.; Kochar, D.K.; Sahani, M.S. Effect of camel milk on residual β-cell function in recent onset type 1 diabetes. Diabetes Res. Clin. Pract. 2007, 3, 494–495. [Google Scholar] [CrossRef]
- Buchilina, A.; Aryana, K. Physicochemical and microbiological characteristics of camel milk yogurt as influenced by monk fruit sweetener. J. Dairy Sci. 2021, 104, 1484–1493. [Google Scholar] [CrossRef]
- Meena, S.; Rajput, Y.; Sharma, R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int. Dairy J. 2014, 35, 153–156. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Verma, A.K.; Chatli, M.K.; Singh, R.; Kumar, P.; Mehta, N.; Malav, O.P. Camel milk: Alternative milk for human consumption and its health benefits. Nutr. Food Sci. 2016, 46, 76268245. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B. Recent advances in camel milk processing. Animals 2021, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-R.; Yue, H.-T.; Shi, Z.-Y.; Shen, T.; Yao, H.-B.; Zhang, J.-W.; Gao, Y.; Yang, J. Protein profile of whole camel milk resulting from commercial thermal treatment. LWT 2020, 134, 110256. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Alrashdi, B.M.; Ramadan, M.F.; Abd El-Sattar, E.S.; Siam, A.A.H.; Alblihed, M.A.; Elmahallawy, E.K. Effect of Fermented Camel Milk Containing Pumpkin Seed Milk on the Oxidative Stress Induced by Carbon Tetrachloride in Experimental Rats. Fermentation 2022, 8, 223. [Google Scholar] [CrossRef]
- Mbye, M.; Sobti, B.; Al Nuami, M.K.; Al Shamsi, Y.; Al Khateri, L.; Al Saedi, R.; Saeed, M.; Ramachandran, T.; Hamed, F.; Kamal-Eldin, A. Physicochemical properties, sensory quality, and coagulation behavior of camel versus bovine milk soft unripened cheeses. NFS J. 2020, 20, 28–36. [Google Scholar] [CrossRef]
- Perusko, M.; Ghnimi, S.; Simovic, A.; Stevanovic, N.; Radomirovic, M.; Gharsallaoui, A.; Smiljanic, K.; Van Haute, S.; Stanic-Vucinic, D.; Velickovic, T.C. Maillard reaction products formation and antioxidative power of spray dried camel milk powders increases with the inlet temperature of drying. LWT 2021, 143, 111091. [Google Scholar] [CrossRef]
- Solanki, D.; Hati, S. Fermented camel milk: A Review on its bio-functional properties. Emir. J. Food Agric. 2018, 30, 268–274. [Google Scholar]
- Mohamed, H.; Ranasinghe, M.; Amir, N.; Nagy, P.; Gariballa, S.; Adem, A.; Kamal-Eldin, A. A study on variability of bioactive proteins in camel (Camelus dromedarius) milk: Insulin, insulin-like growth factors, lactoferrin, immunoglobulin G, peptidoglycan recognition protein-1, lysozyme and lactoperoxidase. Int. J. Dairy Technol. 2022, 75, 289–297. [Google Scholar] [CrossRef]
- Tamime, A.; Robinson, R. Yoghurt. Science and Technology; Woodhead Publishing Limited England: Cambridge, UK, 1999. [Google Scholar]
- Chemists, A.; Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Ceirwyn, S. Analytical Chemistry of Foods, Part I in Book; Springer: Berlin/Heidelberg, Germany, 1995. (In English) [Google Scholar]
- Aryana, K.J. Folic acid fortified fat-free plain set yoghurt. Int. J. Dairy Technol. 2003, 56, 219–222. [Google Scholar] [CrossRef]
- Maksimović, Z.; Malenčić, Đ.; Kovačević, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.-I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Reeves, P.G. Purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Thomas, A.; Schänzer, W.; Thevis, M. Determination of human insulin and its analogues in human blood using liquid chromatography coupled to ion mobility mass spectrometry (LC-IM-MS). Drug Test. Anal. 2014, 6, 1125–1132. [Google Scholar] [CrossRef]
- Sotoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar]
- Bergmeyer, H.; Harder, M. A colorimetric method of the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Clin. Biochem. 1986, 24, 1–488. [Google Scholar]
- Baranowski, R.L.; Westenfelder, C. A micro method to measure para-amino hippurate and creatinine in plasma and urine. Kidney Int. 1986, 30, 113–115. [Google Scholar] [CrossRef]
- Marsh, W.H.; Fingerhut, B.; Miller, H. Automated and manual direct methods for the determination of blood urea. Clin. Chem. 1965, 11, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, T.V.; Bankolé, H.S.; Klotoé, J.R.; Sènou, M.; Fah, L.; Koudokpon, H.; Akpovi, C.; Dougnon, T.J.; Addo, P.; Loko, F. Treatment of hypercholesterolemia: Screening of Solanum macrocarpon Linn (Solanaceae) as a medicinal plant in Benin. Avicenna J. Phytomedicine 2014, 4, 160. [Google Scholar] [CrossRef]
- Devi, R.; Sharma, D. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J. Ethnopharmacol. 2004, 90, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.; Layton, C. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone Elsevier: London, UK, 2013. [Google Scholar]
- Drury, R.; Wallington, E.; Cameron, R. Carleto’s Histological Technique, 4th ed.; Oxford University Press: Oxford, UK, 1967. [Google Scholar]
- Package, S. Software Package for Social Science for Windows; SPSS Amos: Singapore, 2012. [Google Scholar]
- Zhang, H.; Yao, J.; Zhao, D.; Liu, H.; Li, J.; Guo, M. Changes in chemical composition of Alxa Bactrian camel milk during lactation. J. Dairy Sci. 2005, 88, 3402–3410. [Google Scholar] [CrossRef]
- Karaman, A.D.; Yildiz Akgül, F.; Öğüt, S.; Seçilmiş Canbay, H.; Alvarez, V. Gross composition of raw camel’s milk produced in Turkey. Food Sci. Technol. 2021, 42, e59820. [Google Scholar] [CrossRef]
- Mehta, B.M. Chemical composition of milk and milk products. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 511–553. [Google Scholar]
- Hussein, A.S. Ziziphus spina-christi: Analysis of bioactivities and chemical composition. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Berlin/Heidelberg, Germany, 2019; pp. 175–197. [Google Scholar]
- El-Fattah, A.A.; Azzam, M.; Elkashef, H.; Elhadydy, A. Antioxidant properties of milk: Effect of milk species, milk fractions and heat treatments. Int. J. Dairy Sci. 2020, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Osman, M.A.; Ahmed, M.A. Chemical and proximate composition of (Zizyphus spina-christi) nabag fruit. Nutr. Food Sci. 2009, 39, 70–75. [Google Scholar] [CrossRef]
- Soliman, T.N.; Shehata, S.H. Characteristics of fermented camel’s milk fortified with kiwi or avocado fruits. Acta Sci. Pol. Technol. Aliment. 2019, 18, 53–63. [Google Scholar]
- Aljutaily, T.; Barakat, H.; Moustafa, M.M.; Rehan, M. Incorporation of Sukkari Date in Probiotic-Enriched Fermented Camel Milk Improves the Nutritional, Physicochemical, and Organoleptical Characteristics. Fermentation 2021, 8, 5. [Google Scholar] [CrossRef]
- Nazif, N.M. Phytoconstituents of Zizyphus spina-christi L. fruits and their antimicrobial activity. Food Chem. 2002, 76, 77–81. [Google Scholar] [CrossRef]
- Ali, A.B.; Almagboul, A.Z.; Mohammed, O.M. Antimicrobial activity of fruits, leaves, seeds and stems extracts of Ziziphus spina-christi. Arab. J. Med. Aromat. Plants 2015, 1, 94–107. [Google Scholar]
- Hussein, H.; El-Sayed, E.; Said, A. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Zizyphus spina christi and Zizyphus jujuba in alloxan diabetic rats. Int. J. Pharmacol. 2006, 2, 563–570. [Google Scholar]
- El-Maksoud, A.A.A.; Korany, R.M.; El-Ghany, I.H.A.; El-Beltagi, H.S.; Ambrósio, F. de Gouveia, G.M. Dietary solutions to dyslipidemia: Milk protein–polysaccharide conjugates as liver biochemical enhancers. J. Food Biochem. 2020, 44, e13142. [Google Scholar] [CrossRef]
- Zandiehvakili, G.; Khadivi, A. Identification of the promising Ziziphus spina-christi (L.) Willd. genotypes using pomological and chemical proprieties. Food Sci. Nutr. 2021, 9, 5698–5711. [Google Scholar] [CrossRef]
- Ashraf, A.; Mudgil, P.; Palakkott, A.; Iratni, R.; Gan, C.-Y.; Maqsood, S.; Ayoub, M.A. Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. J. Dairy Sci. 2021, 104, 61–77. [Google Scholar] [CrossRef]
- Guizani, N.; Waly, M.I.; Singh, V.; Rahman, M.S. Nabag (Zizyphus spina-christi) extract prevents aberrant crypt foci development in colons of azoxymethane-treated rats by abrogating oxidative stress and inducing apoptosis. Asian Pac. J. Cancer Prev. 2013, 14, 5031–5035. [Google Scholar] [CrossRef]
- Dikhanbayeva, F.; Zhaxybayeva, E.; Smailova, Z.; Issimov, A.; Dimitrov, Z.; Kapysheva, U.; Bansal, N. The effect of camel milk curd masses on rats blood serum biochemical parameters: Preliminary study. PLoS ONE 2021, 16, e0256661. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Z.; Sang, D.; Gao, Q.; Li, Q. The role of nutrition in the prevention and intervention of type 2 diabetes. Front. Bioeng. Biotechnol. 2020, 8, 1054. [Google Scholar] [CrossRef]
- Visvanathan, R.; Williamson, G. Effect of citrus fruit and juice consumption on risk of developing type 2 diabetes: Evidence on polyphenols from epidemiological and intervention studies. Trends Food Sci. Technol. 2021, 115, 133–146. [Google Scholar] [CrossRef]
- Mirmiran, P.; Ejtahed, H.-S.; Angoorani, P.; Eslami, F.; Azizi, F. Camel Milk Has Beneficial Effects on Diabetes Mellitus: A Systematic Review. Int. J. Endocrinol. Metab. 2017, 15, e42150. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Naslaji, A.N.; Mirmiran, P.; Yeganeh, M.Z.; Hedayati, M.; Azizi, F.; Movahedi, A.M. Effect of camel milk on blood sugar and lipid profile of patients with type 2 diabetes: A pilot clinical trial. Int. J. Endocrinol. Metab. 2015, 13, e21160. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; Kharbach, M.; El Jemli, M.; Bouyahya, A.; Cherrah, Y.; Bouklouze, A.; Vander Heyden, Y.; Faouzi, M.E.A. Antidiabetic, dermatoprotective, antioxidant and chemical functionalities in Zizyphus lotus leaves and fruits. Ind. Crops Prod. 2019, 132, 134–139. [Google Scholar] [CrossRef]
- Dahlia, F.; Barouagui, S.; Hemida, H.; Bousaadia, D.; Rahmoune, B. Influence of environment variations on anti-glycaemic, anti-cholesterolemic, antioxidant and antimicrobial activities of natural wild fruits of Ziziphus lotus (L.). S. Afr. J. Bot. 2020, 132, 215–225. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Bouhaddaoui, S.; Chabir, R.; Errachidi, F.; El Ghadraoui, L.; El Khalfi, B.; Benjelloun, M.; Soukri, A. Study of the biochemical biodiversity of camel milk. Sci. World J. 2019, 2019, 2517293. [Google Scholar] [CrossRef]
- Bencheikh, N.; Bouhrim, M.; Kharchoufa, L.; Choukri, M.; Bnouham, M.; Elachouri, M. Protective effect of Zizyphus lotus L.(Desf.) fruit against CCl4-induced acute liver injury in rat. Evid. Based Complement. Altern. Med. 2019, 2019, 6161593. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, D.; Yang, H.; Wang, X.; Zhu, M.; Liu, X. The dose-dependent effects of polyphenols and malondialdehyde on the emulsifying and gel properties of myofibrillar protein-mulberry polyphenol complex. Food Chem. 2021, 360, 130005. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary flavonoids as xanthine oxidase inhibitors: Structure–affinity and structure–activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef] [PubMed]
- El-Zahar, K.M.; Hassan, M.F.; Al-Qaba, S.F. Protective Effect of Fermented Camel Milk Containing Bifidobacterium longum BB536 on Blood Lipid Profile in Hypercholesterolemic Rats. J. Nutr. Metab. 2021, 2021, 1557945. [Google Scholar] [CrossRef] [PubMed]
- Goli-malekabadi, N.; Asgary, S.; Rashidi, B.; Rafieian-Kopaei, M.; Ghannadian, M.; Hajian, S.; Sahebkar, A. The protective effects of Ziziphus vulgaris L. fruits on biochemical and histological abnormalities induced by diabetes in rats. J. Complement. Integr. Med. 2014, 11, 171–177. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).