Abstract
The fermentation of fruit processing residuals (FPRs) with filamentous fungi can provide protein-rich food products. However, FPRs that contain bioactive compounds with antimicrobial properties present a major challenge. In this work, the resistance of two edible filamentous fungi, Rhizopus oligosporus and Neurospora intermedia, to 10 typically inhibiting bioactive compounds available in FPRs (epicatechin, quercetin, ellagic acid, betanin, octanol, hexanal, D-limonene, myrcene, car-3-ene, and ascorbic acid) was examined. These compounds’ inhibitory and stimulatory effects on fungal growth were examined individually. Three different concentrations (2.4, 24, and 240 mg/L) within the natural concentration range of these compounds in FPRs were tested. These bioactive compounds stimulated the growth yield and glucose consumption rate of R. oligosporus, while there was no increase in the biomass yield of N. intermedia. Ellagic acid caused an up to four-fold increase in the biomass yield of R. oligosporus. In addition, octanol and D-limonene showed antifungal effects against N. intermedia. These results may be helpful in the development of fungus-based novel fermented foods.
1. Introduction
The increasing global population has created demand for an increased food supply. By 2050, food scarcity is expected, as demand will be 56% higher than it was in 2010 [1]. Current animal-based nutrient sources are not sufficient to meet this increasing demand [2] and have negative impacts on the environment, such as greenhouse gas emissions and high land and water utilization of the meat industry [3]. Therefore, new food sources—developed sustainably and cost-efficiently—are needed to meet the projected nutrition demand [4]. Plant-based and fermented protein-rich foods are alternative food sources that can help to meet these demands, while fermentation with filamentous fungi can provide products that are superior to plant-based foods in terms of their nutrient content, essential amino acids, reduced antinutrients, and enriched antioxidants, vitamins, and lipids [4,5].
Filamentous fungi can convert various carbon sources (e.g., glucose, xylose, fructose, etc.) into proteins, enzymes, organic acids, antioxidants, etc. [6]. They can provide protein-rich food/feed sources with adequate nutrients while having less of a negative impact on the environment [4]. Moreover, filamentous fungi are known to improve the nutrient profiles of raw materials and produce several bioactive compounds, such as antioxidants, vitamins, polyunsaturated fatty acids, etc. Due to the fact that fungi-fermented products have health-improving effects, they are called functional foods [4]. Filamentous fungi have a long history in Southeast and East Asian cuisine. Some examples of traditional fungus-based food products include red fermented rice (angkak) produced by Monascus purpureus, koji and miso by Aspergilus oryzae, tempeh by R. oligosporus, and oncom by N. intermedia [7,8]. Another fungus-based food product is Quorn, which is produced by Fusarium venenatum using a synthetic medium and is commercially available in several countries [9,10]. Alternatively, recent studies have investigated fungus-based feed/food products using less costly substrates from food industry side-streams, e.g., bread waste, brewery spent grains, and olive oil mill wastewater [11,12,13]. Another substrate for feed/food production by filamentous fungi could be fruit and vegetable processing residuals (FPRs), e.g., cashew byproduct or apple pomace [6,14,15].
Fruit/FPRs contain various bioactive compounds, including some flavor compounds. Flavor compounds are known for their contribution to the specific tastes of fruits and vegetables [16,17]. These compounds belong to various different chemical groups, including “alcohols, aldehydes, ketones, lactones, terpenoids, and esters” [17,18]. Bioactive compounds such as limonene and ellagic acid can be obtained from different parts of fruits, including the peels and seeds [19,20]. They form when the fruit is ripening to provide protection against environmental factors, i.e., via antimicrobial action [21]. Furthermore, their antioxidant and anti-inflammatory characteristics have positive effects on human health [21]. Bioactive compounds that are extracted from fruit/FPRs can be utilized in the pharmaceutical, dietary supplement, food, and cosmetics industries due to their aforementioned properties [22,23]. On the other hand, some studies have investigated the fermentation of FPRs without the extraction of bioactive compounds [14,24]. As an alternative to extraction of bioactive compounds, FPRs could be a potential material for the production of fermented foods with bioactive properties.
Some bioactive compounds in fruit and vegetables have been reported to possess an antimicrobial effect [17,25]. This antimicrobial effect hinders the valorization of fruit processing residuals via microbial conversion. There are a limited number of studies about the valorization of fruit/FPRs, e.g., mango waste, apple pomace, and grape residuals from wineries, by filamentous fungi [26,27,28]. Furthermore, studies that focus on inhibiting or enhancing effect of bioactive compounds from fruit/FPRs are scarce in the literature; however, one study reported that octanol showed an antifungal effect [29]. To be able to implement efficient fermentation processes, knowledge about the effects of potentially inhibitory compounds in nutrient media on filamentous fungi is necessary. Hence, an extended screening of filamentous fungus strains that are resistant to inhibition is required for the development of processes that can utilize nutrient media with bioactive compounds.
Therefore, in this study, 10 common bioactive compounds found in fruit/FPRs (octanol, ellagic acid, (-)-epicatechin, quercetin, betanin, ascorbic acid, limonene, hexanal, car-3-ene, and myrcene) were investigated to observe their effects on N. intermedia and R. oligosporus. To determine their activating or inhibiting effects, bioactive compounds were added to a synthetic medium at different concentrations.
2. Materials and Methods
2.1. Microorganisms
Two strains of edible filamentous fungi, Neurospora intermedia CBS 131.92 and R. oligosporus CBS 112586 (Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands), were used. Potato dextrose agar (PDA) plates (20 g/L glucose, 15 g/L agar, and 4 g/L potato extract) were used to cultivate the fungi [30]. The fungal plates were incubated at 30 °C for 3 days; they were then preserved at 4 °C and plates were renewed after 1 month. A fungal spore solution was prepared by flooding 20 mL of sterile milli-Q® water into the fungal plates and mixing the spores with water using an L-shaped spreader. The spore solution was transferred to a sterile centrifuge tube and vortexed prior to being added to the cultivation media in shaking flasks.
2.2. Cultivation Medium and Chemicals
A wide-neck, cotton-plugged 250 mL Erlenmeyer flask was used for submerged cultivation. The cultivation medium used was a synthetic medium with a composition of 30 g/L glucose, 7.5 g/L (NH4)2SO4, 3.5 g/L KH2PO4, 2.2 g/L CaCl2·2H2O, 1 mL/L vitamin solution, and 10 mL/L trace metal solution, according to [31]. The volume of the synthetic medium added to each flask was 100 mL, set to pH 5.5. Fungal cultivation was conducted as described in [32,33]. Cultivation was conducted for 72 h at 35 °C in a shaking water bath (125 rpm). Two milliliters of spore solution was added to 100 mL of medium with a concentration of 4.1–7.4 × 106 spores/mL and 3.7–5.2 × 106 spores/mL for N. intermedia and R. oligosporus, respectively. All chemical compounds including bioactive compounds were purchased from Sigma Aldrich (St. Louis, MO, USA), except ascorbic acid (Acros Organic, NJ, USA), and ellagic acid (Acros Organic, NJ, USA). Ten bioactive compounds (D-limonene, myrcene, car-3-ene, quercetin, epicatechin, ellagic acid, octanol, betanin, hexanal, and L-ascorbic acid) were examined individually at three different concentrations (2.4, 24, and 240 mg/L). For each compound, a stock solution was prepared according to the procedure described by Bulkan et al. [29]. For those compounds with low solubility in milli-Q® water (D-limonene, quercetin, and epicatechin), ethanol was used as the solvent. Initially, 10% (w/v) limonene and 10% (w/v) quercetin solutions were prepared in absolute ethanol, besides 1% (w/v) epicatechin was dissolved in 20% aqueous ethanol. Following this step, the stock solutions were prepared by diluting the aforementioned concentrated solutions into 0.5% (w/v) of each bioactive compound.
In order to reach the 240 mg/L final bioactive compound concentration, 5 mL of the stock solution was added to the cultivation medium. For the remaining concentration levels (2.4 and 24 mg/L), 0.5 and 0.05 mL solutions were taken from the stock solution and completed to 5 mL with milli-Q® water and ethanol to reach to the same ethanol concentration as the highest concentration level [32,33]. Myrcene, car-3-ene, hexanal, and octanol have low solubility in water due to their hydrophobic nature. Stock solutions for each of these compounds (0.5% (w/v) bioactive compound) were prepared using milli-Q® water containing 0.5% (v/v) Tween 80 (Sigma Aldrich, St. Louis, MO, USA). Tween 80 is a surfactant that has been reported to enhance the solubility of an hydrophobic compound [34]. The preparation of the cultivation media containing 2.4, 24, and 240 mg/L bioactive compounds was carried out in a similar fashion to the compounds with ethanol solvent. The stock solutions of the remaining compounds were prepared using milli-Q® water, except for ellagic acid, which was dissolved in 1 M NaOH solution [29].
2.3. Analytical Methods
After cultivation, the biomass was harvested using a sieve and the collected biomass was washed with milli-Q® water and dried in a 70 ℃ oven [35]. Biomass production was reported as g/L and the yield was reported as g biomass/g initial glucose. The liquid samples were analyzed using high-performance liquid chromatography (HPLC) to determine the glucose, glycerol, lactic acid, acetic acid, and ethanol concentrations. For HPLC, a hydrogen-ion-based ion-exchange column (Aminex HPX-87H, Bio-Rad, Hercules, CA, USA) was used at 60 °C with 0.6 mL/min of 5 mM H2SO4 as an eluent [36].
2.4. Statistical Analysis
All experiments were carried out in duplicate. The standard deviation is shown in the figures with a 95% confidence interval. The data were analyzed with one-way ANOVA using the MINITAB software (Minitab Ltd., Coventry, UK) (p < 0.05).
3. Results and Discussions
In this work, the effects on N. intermedia and R. oligosporus of 10 different bioactive compounds found in fruit and vegetables were examined. The compounds were tested at different concentrations (2.4, 24, and 240 mg/L), which were within the concentration ranges naturally found in fruits/FPRs. The changes in glucose consumption and biomass and metabolite yields (i.e., ethanol, lactic acid, etc.) were analyzed. Furthermore, the morphological changes observed during cultivation were reported. In general, the effects of the bioactive compounds on N. intermedia and R. oligosporus differed significantly.
3.1. The Effect of Bioactive Compounds on R. oligosporus
In this study, the biomass yield of R. oligosporus in synthetic medium was lower in comparison to that reported in a previous study, with 0.17 g biomass/initial glucose [37]. The difference in biomass yields might be related to the existence of yeast extract in the cultivation medium used by the authors of [37]. The same biomass yield (0.17 g biomass/initial glucose) was reported by Ferreira et al. [36] for Rhizopus sp. in a semisynthetic medium containing yeast extract over 144 h.
Among the compounds examined, ellagic acid showed the highest effect on R. oligosporus. The biomass yield was enhanced with all concentrations of ellagic acid (2.4, 24, and 240 mg/L) and increased with increasing ellagic acid concentration. The highest biomass yield was four-fold higher than that of the control. Betanin enhanced the biomass yield in the 24–240 mg/L concentration range by 2.5- and 2.7-fold, respectively. Following ellagic acid and betanin, quercetin and octanol increased the biomass yield by 2.1- and 2.2-fold in comparison to their respective control. Limonene, myrcene, hexanal, and ascorbic acid increased the biomass yield in the range of 38–92% at the 240 mg/L concentration in comparison to their respective controls. Epicatechin and car-3-ene were the only compounds that did not cause any change in the biomass yield of R. oligosporus. The changes in biomass yield for R. oligosporus are shown in Figure 1.
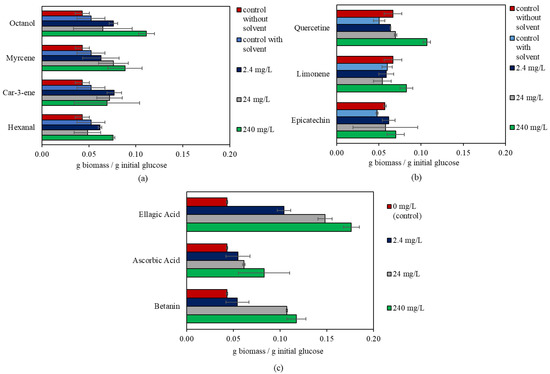
Figure 1.
Change in the biomass yield of R. oligosporus in the presence of bioactive compounds. (a) Compounds with Tween 80 in their stock solutions. (b) Compounds with ethanol in their stock solutions (different concentration for each compound). (c) Compounds dissolved in milli-Q® water.
Ellagic acid is a phenolic compound that is associated with various health effects due to its anticarcinogenic, antimutagenic, and antioxidant properties, in addition to its potential beneficial effects on chronic diseases such as diabetes [38,39]. The compound naturally exists in various fruits, e.g., pomegranates, raspberries, cranberries, etc., in free form, and it can also be obtained by the hydrolysis of ellagitannin [24,38]. The antimicrobial activity of ellagic acid against some microorganisms, including bacteria and filamentous fungi, is well known [40]. Its inhibitory effect against fungi may be related to decreased CYP51 (from the cytochrome P450 enzyme family) activity and ergosterol in the cell membranes of fungi. Disintegration of the cell membrane may occur due to low ergosterol levels [40]. On the other hand, the results of this study suggest that R. oligosporus is resistant to the existence of ellagic acid. The increasing ellagic acid content during cranberry fermentation by R. oligosporus aligns with this result [24]. Similarly, in previous studies, Aspergillus spp. and Candida glabrata were shown to be resistant to ellagic acid [29,40]. One reason for our results contradicting previous work reporting an antifungal effect may be related to the differences in the cell wall composition of different fungal species [41]. The cell wall plays a significant role in protection against the effects of external factors [41,42]. Fungal cell walls are commonly composed of glucans, chitin, and glycoproteins [42]. The unique structure of the fungal cell wall is a common target for antifungal agents, which affect fungi but not other living beings, e.g., humans or bacteria [42]. The cell wall composition and configuration can differ between different fungal species [41,42,43].
Betanin is a natural colorant commonly used in the food industry. It belongs to the betacyanin group of betalains and possesses antioxidant properties. It is commonly found in fruits and vegetables, such as beetroot, pitahaya, tubers, etc. [29]. Unlike R. oligosporus, some microorganisms are susceptible to betanin due to its antimicrobial properties. The antimicrobial activity of betanin is not well described in the literature; however, it may be related to the activity of betalains, which bond with metal ions such as Fe2+, Ca2+, and Mg2+. Insufficient access to these necessary compounds—due to their bonding with betalains—can cause bacteria to die [44].
Quercetin is a bioactive compound found in various fruits, such as peaches, apples, pears, plums, grapes, cashew apples, etc. In quercetin-containing medium, R. oligosporus showed an enhanced biomass yield, which has not been reported in the literature before. Quercetin is known to have antimicrobial effects on some microorganisms [45], while others have the ability to produce or transform quercetin [46,47]. One study reported that its antimicrobial effect targeted the function of mitochondria and nucleic acid synthesis [48]. It is unknown why R. oligosporus is resistant to this compound, even showing improved growth potential. It could be that the fungus has the ability to transform quercetin. R. oryzae was reported to convert phenolic compounds in cauliflower outer leaves and onion into quercetin [49]. The results of our study indicate a potential opportunity to use quercetin-containing materials, such as peach juice industrial waste, for producing food products with R. oligosporus.
Octanol is a flavor compound naturally found in grapes, plums, strawberries, etc. [17]. Bulkan et al. [29] stated that the presence of 240 mg/L octanol inhibited the growth of Aspergillus oryzae and A. niger. It has been suggested that the antifungal effect of octanol is associated with increased plasma membrane fluidity [50]. In this study, the growth of R. oligosporus was enhanced, in contrast to the potential inhibitory effect reported in earlier studies [29,50].
D-limonene is a flavor compound found in citrus peels. It has various bioactive properties, including antioxidant, anticarcinogenic, and antimicrobial effects. Miller et al. [51] mentioned early-stage investigations on the chemotherapeutic effects of D-limonene when taken orally. Here, edible fungus (R. oligosporus) cultivation in the presence of 240 mg/L limonene resulted in enhanced biomass growth. Lennartsson et al. [52] reported the tolerance of Rhizopus sp. to up to 2% D-limonene in a semisynthetic medium. Some fungi are capable of converting limonene into other aroma compounds. Phomopsis sp. was reported to transform limonene into carvone and α-terpineol [53]. Considering the market demand for natural flavors [53], R. oligosporus is worthy of further investigation. While the reason behind the enhanced growth of R. oligosporus in the presence of limonene is still unknown, it might be possible that the fungus is capable of converting D-limonene or using it as a substrate.
Two common sources of fruit processing industrial waste, orange and mango, contain myrcene. This compound has been found to be inhibitory to other microbial processes, particularly in biogas production [17]. Therefore, materials that contain myrcene require an additional processing step to remove the compound, or the use of a microorganism resistant to myrcene. R. oligosporus could be a potential candidate for this purpose, since it was found to be resistant to myrcene up to 240 mg/L; in addition, its biomass yield was even enhanced in these conditions.
Various industrially important fruits contain hexanal, i.e., apples, grapes, oranges, strawberries, etc. This compound has been reported to exert antimicrobial effects on bacteria and fungi [17,54]. One potential reason for this inhibition could be the negative effect of hexanal on cell membrane formation [55]. However, in this study, the growth of R. oligosporus was enhanced, instead of being inhibited. Although the reason behind this resistance is still unknown, the enhanced biomass yield of R. oligosporus in the presence of hexanal is promising for possible food production utilizing a hexanal-containing medium.
The results of the statistical analysis showing the significant changes caused by the different concentrations of the bioactive compounds can be found in Table 1 and Table 2 for biomass and ethanol yield, respectively. The presence of bioactive compounds in cultivation media had some effects on the glucose consumption trends of R. oligosporus. The changes observed are shown in Figure 2. The presence of betanin, octanol, ellagic acid, ascorbic acid, and hexanal increased the glucose consumption speed, while the remaining compounds did not show a considerable effect. All glucose was consumed within 48 h in the presence of 240 mg/L ellagic acid, octanol, and hexanal in comparison to the control, which needed more than 72 h. Faster consumption of glucose can provide shorter fermentation times, and thus more cost-efficient processes.

Table 1.
Statistical analysis results for the varying concentrations of bioactive compounds that showed significant effects on R. oligosporus biomass.

Table 2.
Statistical analysis results for the varying concentrations of bioactive compounds that showed significant effects on Rhizopus oligosporus ethanol and lactic acid yield.
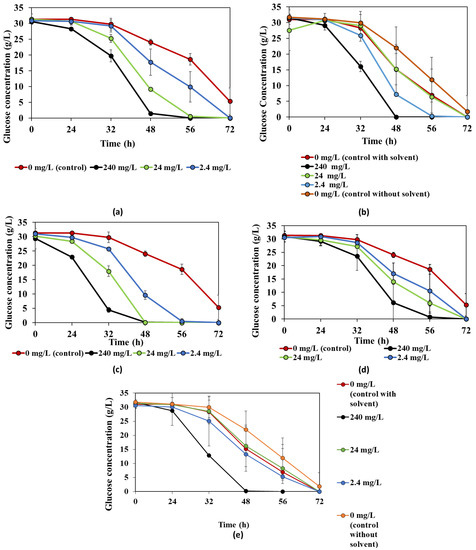
Figure 2.
Changes in the glucose consumption trend of R. oligosporus during cultivation in the presence of bioactive compounds: (a) betanin, (b) octanol, (c) ellagic acid, (d) ascorbic acid, and (e) hexanal.
Rhizopus spp. are well known for their capability to produce ethanol [56,57]. Various studies investigating Rhizopus spp. have found that they can produce ethanol from lignocellulose, thin stillage, spent sulfite liquor, etc. [36,56,57]. In this study, the ethanol yield in synthetic medium was 0.23 ± 0.09 g ethanol/g initial glucose, which is lower than the value of 0.37–0.43 g ethanol/g glucose reported for Rhizopus spp. in a previous study [56]. On the other hand, it is higher than the value of 0.14 g ethanol/g initial glucose reported in [36]. The difference between the results of this study and those of previous studies could be related to the species of fungus, the cultivation medium (with or without yeast extract), and oxygen availability. In a previous study, it was reported that in the presence of yeast extract, a higher ethanol yield was obtained by R. oryzae [58]. The ethanol yields for the bioactive-compound-containing media varied between 0.12 and 0.31 g ethanol/initial glucose.
Furthermore, in the presence of ellagic acid and betanin, the maximum ethanol yield increased. At up to 24 mg/L betanin, the ethanol yield increased by 32% in comparison to the control (0.21 ± 0.04 g ethanol/g initial glucose). The ethanol yield was not further improved by betanin at a concentration of 240 mg/L, and it was only 29% higher than in the control. Similarly, 480 mg/L betanin was the limiting concentration for biomass growth, and the biomass yield did not increase further after a betanin concentration of 240 mg/L. In the presence of 240 mg/L hexanal, the maximum ethanol production was achieved earlier; instead of 72 h, it was at 48 h. Thus, it can be concluded that the cultivation conditions can be adjusted according to the final target, i.e., biomass or ethanol.
R. oligosporus is capable of producing lactic acid, similarly to other Rhizopus spp. used for lactic acid production in industry [59]. In this study, the lactic acid yield varied between different conditions in terms of the bioactive compounds and their concentrations. In the presence of 240 mg/L betanin, the lactic acid yield increased by 30% in comparison to a betanin concentration of 2.4 mg/L, which resulted in 0.18 ± 0.01 g lactic acid/g initial glucose. In the presence of 24 mg/L and 240 mg/L ellagic acid, the lactic acid yield decreased by 32% and 63%, respectively, in comparison to the control with 0.18 ± 0.03 g lactic acid/g initial glucose, in contrast to the increase in ethanol yield at an ellagic acid concentration of 24 mg/L. Decreased lactic acid production in the presence of 240 mg/L ellagic acid may have been related to the morphology of the fungi, which was in form of clumps. Zhang et al. [59] stated that the small and loose pellets of Rhizopus spp. are more preferable for lactic acid production. Table 2 shows the statistical analysis results for the ethanol and lactic acid yields of R. oligosporus. The mechanism by which fungal growth was enhanced is still unknown. It is possible that the fungi converted bioactive compounds as carbon source; however, this requires further investigation, since the glucose consumption rate increased considerably in the presence of some compounds.
3.2. The Effect of Bioactive Compounds on N. intermedia
The biomass yield of N. intermedia in synthetic medium was similar to that reported in another study, around 0.17 g biomass/g glucose [37]. In the presence of the bioactive compounds, N. intermedia showed no significant increase in biomass yield. The changes in biomass yield for N. intermedia are shown in Figure 3. Unlike R. oligosporus, N. intermedia consumed all the glucose within 48 h. Glucose consumption was slightly delayed in the presence of 240 mg/L hexanal. The statistical analysis results for the effect of the bioactive compounds in a concentration range of 0–240 mg/L on N. intermedia biomass yield are shown in Table 3. Regarding ethanol production by N. intermedia, the only significant change was observed in the presence of ascorbic acid at 24 mg/L. The ethanol yield decreased by 17% in comparison to the control, with 0.27 ± 0.02 g ethanol/g initial glucose. Moreover, since biomass growth was inhibited in the presence of 240 mg/L octanol and limonene, there was no ethanol production.
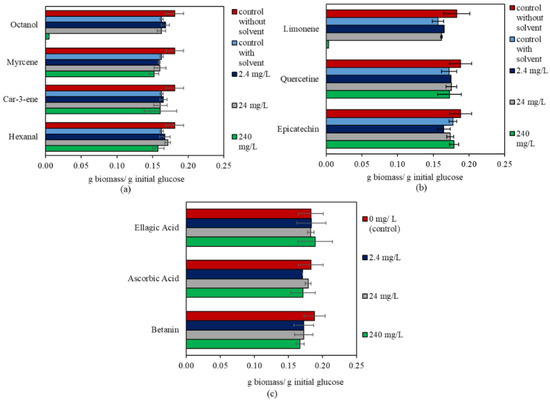
Figure 3.
The effect of bioactive compounds in the 0–240 mg/L concentration range on N. intermedia. (a) Compounds with Tween 80 in their stock solutions. (b) Compounds with ethanol in their stock solutions (different concentration for each compound). (c) Compounds dissolved in milli-Q® water.

Table 3.
Statistical analysis results for the varying concentrations of bioactive compounds that showed significant effects on Neurospora intermedia biomass yield.
Octanol and limonene inhibited the growth of N. intermedia by 97–98% at a concentration of 240 mg/L. Weak biomass formation was also observed (less than 0.005 g biomass/g initial glucose (Figure 3)). This inhibitory effect is in agreement with the results of previous studies. Similarly, two other Ascomycete fungi, Aspergillus oryzae and A. niger, were reported to be inhibited by 240 mg/L octanol [29]. The antifungal mechanism of octanol was mentioned in a study with S. cerevisiae, where the plasma membrane fluidity of the spheroplast form was shown to be elevated as a mechanism of inhibition [50]. D-limonene has been reported to have antimicrobial effects as well. This inhibition occurs due to the effects on fatty acid content, enzymes, and permeability of the cell membrane [60].
The inhibitory effect of octanol on N. intermedia growth provides an opportunity to prevent N. intermedia contamination with natural antifungal sources. Yassin et al. [61] reported N. intermedia as one of the most common contaminating fungi together with N. sitophila, following N. crassa. Octanol can be obtained from fruits or vegetables/residuals. Mixing them with the raw material could create a solution while also providing new food products. The co-cultivation of bakery microorganisms and Pleurotus ostreatus might be another approach, since the mushroom was reported to produce octanol at inhibitory concentrations in this study [62]. On the other hand, N. intermedia is currently used in fermented food production, and several studies have investigated the production of food and feed using various industrial side-streams, e.g., thin stillage, bread waste, brewery spent grains (BSGs), etc. [5,12,35]. When substrates containing octanol or D-limonene, e.g., citrus, grape, plum, etc., are used for N. intermedia cultivation, the aforementioned compounds should be removed prior to cultivation. Another solution could be to dilute the material by mixing it with other substrates, as fungi can grow at up to a 24 mg/L concentration of octanol and limonene.
The bioactive compounds—except limonene and octanol—did not affect the biomass yield significantly, possibly indicating that the fungi did not consume them. However, the adsorption of some phenolic compounds onto the fungal biomass was observed visually. Figure 4 shows the fungal biomass obtained from the cultivation media containing epicatechin. The pictures were taken after the biomass was washed with milli-Q® water. The fungus shows potential for color removal from fruit industry waste; however, it should be further investigated for efficiency. Nweke et al. [63] stated that dead fungi showed better potential for biosorption in comparison to live fungi. Hence, it might be interesting to investigate the adsorption potential of the dead biomass of N. intermedia further for the enrichment of edible biomass with phenolic content from fruit processing side-streams.
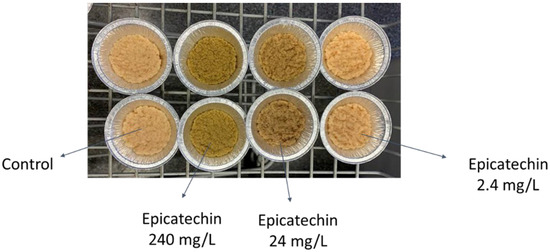
Figure 4.
Fungal biomass of N. intermedia obtained after 72 h of cultivation in epicatechin-containing media.
3.3. Comparison of R. oligosporus and N. intermedia
The two fungi responded to the presence of the flavor compounds differently. The compounds which inhibited N. intermedia did not show any inhibition of R. oligosporus. Differences in cell wall composition may be one reason behind the varying tolerances of these two fungi toward different compounds. In the fungal kingdom, Zygomycetes, a class of Zygomycota (which R. oligosporus belongs to), are the only fungi that have chitin–chitosan as a cell wall polysaccharide. In Zygomycetes, glucan is replaced by chitosan at a rate up to three times that of chitin [41]. On the other hand, N. intermedia belongs to Ascomycota. As a model fungus, Neurospora crassa was reported to have β-1,3-glucan and lichenin (mixed β-1,3-/β-1,4- glucan) in up to 65% of its dry cell wall mass [43], and chitin in up to 4% in vegetative state of the fungus [43,64].
R. oligosporus was not only resistant to limonene and octanol, but also provided a higher biomass yield at 240 mg/L. A comparison of biomass yields is shown in Figure 5a. Among the other compounds, the biggest difference was observed in the presence of ellagic acid. While there was no significant change in the biomass and ethanol yield of N. intermedia, R. oligosporus showed a four-fold and 38% increase, respectively. These results suggest that when the R. oligosporus flavor and properties are preferred, R. oligosporus can provide the same biomass yield as N. intermedia at 240 mg/L ellagic acid concentration.
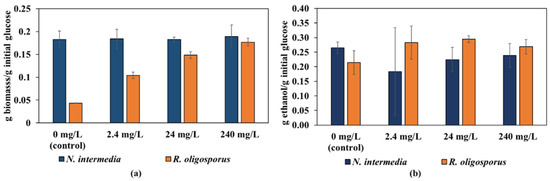
Figure 5.
Comparison of R. oligosporus and N. intermedia in the presence of ellagic acid: (a) biomass yields and (b) ethanol yields.
The different response of R. oligosporus in comparison to N. intermedia and previous studies [29] could be interpreted as R. oligosporus having an ability to secrete more diverse enzymes, and thus pathways to degrade more substrate types. The degradation might include enzymes such as phenol oxidase, alcohol dehydrogenase, aldehyde dehydrogenases, etc. [65,66,67,68]. R. oligosporus is already known for its ability to assimilate carboxylic acids, e.g., acetic acid, butyric acid, and caproic acids (hexanoic acid), as a carbon source [69]. However, knowledge is scarce about its ability to use hexanal and octanol as carbon sources.
3.4. Morphology
Although the morphology of the fungi was not the focus of this work, some alterations were observed at varying concentrations of the bioactive compounds. The morphology of fungi differs in submerged cultivation based on the cultivation conditions and species of fungus [70]. The growth form can be filamentous mycelia, mycelial clumps, or pellets, as well as intermediate forms, e.g., granular and flocculant growth [59,71]. There are three categories of pellets: The first group of pellets have a compact central core, and their outer surface has loose and fluffy (hairy) filaments. The second group of pellets also have a compact core, but with a smooth outer surface. The last group of pellets arises from the previous two groups; they have a hollow core, where the inner cells have been autolyzed [71,72]. One reason for this autolysis is the insufficient transfer of nutrients and oxygen to the inner cells. Therefore, controlling pellet size is important in bioprocesses [72]. In bioprocesses, pellet form is more preferable since the pellets are easy to harvest and the viscosity of the medium is lower, leading to higher oxygen transfer and increased product yields [71]. The factors affecting pellet formation include temperature, pH, oxygen level, trace metals, carbon source, etc. [71]. Pellet formation in Rhizopus spp. has been mentioned in many studies [59,73]. Souza Filho et al. [73] reported the effect of potato protein liquor (PPL) concentration on the morphology of Rhizopus oryzae; filaments, mixture of pellets and filaments, and pellets were reported for increasing three different concentrations of PPL, respectively. However, in this study, an increasing concentration of ellagic acid resulted in changes in the shape of R. oligosporus pellets; at the highest concentration (240 mg/L), growth was in the form of clumps, while spherical and ellipsoidal pellets were formed at other concentrations. Table 4 shows the morphological changes and their respective cultivation conditions. Figure 6a shows the pictures taken at 72 h of cultivation.

Table 4.
Morphological changes in fungi.
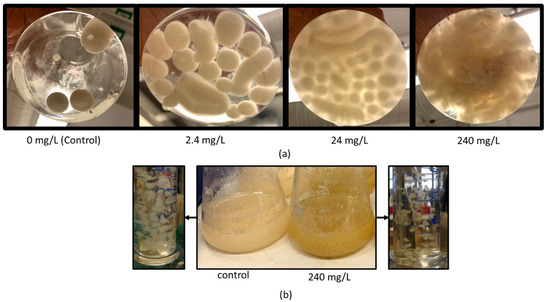
Figure 6.
Morphological changes in fungi: (a) R. oligosporus in the presence of ellagic acid and (b) N. intermedia in the presence of quercetin.
N. intermedia has been reported to grow in both filament and pellet forms in previous studies [71,74]. pH was reported to be a significant factor in the pellet formation of N. intermedia, where a pH of 3.5–4 resulted in the formation of pellets with a smooth outer surface [71]. In this study, N. intermedia grew in small, loose, spherical mycelium pellets with fluffy outer surface and a mixture of these pellets with nonuniform mycelium clumps as well. The sizes of the pellets varied. Although there was no change in the biomass yield of N. intermedia, the morphology changed slightly in the presence of quercetin. The highest concentration of quercetin resulted in smaller pellets (Figure 6b).
The size and shape of pellets can be helpful in choosing a preferred fungal strain and selecting optimal process conditions. In the presence of quercetin, for instance, the pellet form of R. oligosporus can be more advantageous in terms of harvesting fungi, although the biomass yield was higher with N. intermedia. The pellet form provides ease of biomass collection, as the mycelium form or very small pellets are likely to be lost during this process. At the highest concentration of ellagic acid, R. oligosporus had its highest biomass yield; however, it was in clump form. Therefore, 24 mg/L may be a more preferable concentration; at this concentration, the biomass yield was 0.15 ± 0.01 g biomass/g glucose and the fungus was still in pellet form. On the other hand, higher ellagic acid concentrations should be investigated in solid-state fermentation to observe the enhanced biomass growth.
3.5. Potential Applications of Fruit Bioactive Compounds in the Food Industry
The fermentation of materials for food production requires food-grade microorganisms which are resistant to the inhibitory components in these materials. In this study, R. oligosporus and N. intermedia were shown to be resistant to the fruit bioactive compounds in the range of the concentrations studied. Hence, they can be used in the food industry to utilize materials containing fruit bioactive compounds, e.g., fruit processing residuals. Octanol and D-limonene were inhibitory for N. intermedia at 240 mg/L. Considering the sensitivity of N. intermedia and other edible filamentous fungi (e.g., A. oryzae and A. niger) to octanol [29], R. oligosporus seems to be more promising in food fermentation with its enhanced biomass yield. Furthermore, food products containing octanol may have potential applications in medicine. Bushara et al. [75] reported that the oral intake of octanol was helpful in controlling essential tremor, a neurological disorder. However, its application for health-improving effects and biosorption potential should be investigated further.
The adsorption of phenolic compounds with bioactive properties into edible fungal biomass could lead to the production of novel foods with functional properties. As a low-value and environmentally friendly raw material, fruit processing industry residuals can be utilized. In this regard, N. intermedia shows potential in adsorbing phenolic compounds, e.g., epicatechin.
The bioactive compounds that increased the biomass yield of R. oligosporus, particularly ellagic acid, could be used as a growth booster for fungi in the food industry, e.g., in tempeh and novel fermented food production. Moreover, the fermentation of fruit/FPRs by R. oligosporus could be advantageous due to the ability of fungi to release phenolic compounds from fruit residuals [24]. Vattem et al. [24] reported that extractable phenolics, including ellagic acid, were increased during the solid-state fermentation of cranberry pomace by R. oligosporus. The increased total phenolic and antioxidant content could be due to higher ß-glucosidase activity, since ß-glucosidase can hydrolyze phenolic glycosides and release phenolic compounds.
R. oligosporus and N. intermedia produced ethanol in concentrations of up to 10 g/L. Following biomass separation after cultivation, the remaining liquid fraction might be interesting to evaluate as a nonalcoholic or low-alcohol fermented drink [12]. The possibility of having a fruity aroma in this fermented drink would also be intriguing to be investigated.
4. Conclusions
Two food-grade fungi were examined for their reactions to fruit bioactive compounds known for their antimicrobial properties. Both fungi showed the potential to be used to cultivate materials with bioactive compound contents. However, some limitations to the application of N. intermedia were observed. For limonene- and octanol-containing media at 240 mg/L or above, the compounds should be removed prior to N. intermedia cultivation. On the other hand, R. oligosporus may be preferred, since its fungal biomass yield was increased in the presence of octanol and limonene. Furthermore, the increasing biomass yield of R. oligosporus in the presence of bioactive compounds shows the potential of the fungus for fermenting food from materials with bioactive compound contents, e.g., fruit processing residuals. Ellagic acid can be utilized to enhance growth in the fermented food industry, as evidenced by the four-fold increase in biomass yield observed in the presence of this compound. Moreover, the faster glucose consumption trend of R. oligosporus in the presence of bioactive compounds could lead to a shorter fermentation time, and therefore less costly industrial-scale processes.
Author Contributions
Conceptualization, G.B. and M.J.T.; formal analysis, G.B., S.S. and G.T.Y.; funding acquisition, M.J.T.; investigation, G.B., S.S. and G.T.Y.; methodology, G.B., S.S. and G.T.Y.; project administration, M.J.T.; supervision, G.B., R.M., R.W. and M.J.T.; visualization, G.B., S.S. and G.T.Y.; writing—original draft, G.B.; writing—review and editing, R.M., R.W. and M.J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Swedish Agency for Economic and Regional Growth (Tillväxtverket) (Grant number: 20201656) through a European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Javourez, U.; O’Donohue, M.; Hamelin, L. Waste-to-nutrition: A review of current and emerging conversion pathways. Biotechnol. Adv. 2021, 53, 107857. [Google Scholar] [CrossRef] [PubMed]
- Hashempour-Baltork, F.; Khosravi-Darani, K.; Hosseini, H.; Farshi, P.; Reihani, S.F.S. Mycoproteins as safe meat substitutes. J. Clean. Prod. 2020, 253, 119958. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; De Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- Rousta, N.; Hellwig, C.; Wainaina, S.; Lukitawesa, L.; Agnihotri, S.; Rousta, K.; Taherzadeh, M.J. Filamentous fungus aspergillus oryzae for food: From submerged cultivation to fungal burgers and their sensory evaluation—A pilot study. Foods 2021, 10, 2774. [Google Scholar] [CrossRef] [PubMed]
- Gmoser, R.; Sintca, C.; Taherzadeh, M.J.; Lennartsson, P.R. Combining submerged and solid state fermentation to convert waste bread into protein and pigment using the edible filamentous fungus N. intermedia. Waste Manag. 2019, 97, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for future foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Ferreira, J. Integration of Filamentous Fungi in Ethanol Dry-Mill Biorefinery. Ph.D. Thesis, University of Borås, Borås, Sweden, 2015. [Google Scholar]
- Wikandari, R.; Hasniah, N.; Taherzadeh, M.J. The role of filamentous fungi in advancing the development of a sustainable circular bioeconomy. Bioresour. Technol. 2021, 345, 126531. [Google Scholar] [CrossRef]
- Wiebe, M. Myco-protein from Fusarium venenatum: A well-established product for human consumption. Appl. Microbiol. Biotechnol. 2002, 58, 421–427. [Google Scholar] [CrossRef]
- Wiebe, M.G. Quorn TM myco-protein-overview of a successful fungal product. Mycologist 2004, 18, 17–20. [Google Scholar] [CrossRef]
- Gmoser, R.; Fristedt, R.; Larsson, K.; Undeland, I.; Taherzadeh, M.J.; Lennartsson, P.R. From stale bread and brewers spent grain to a new food source using edible filamentous fungi. Bioengineered 2020, 11, 582–598. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.; Taherzadeh, M. Brewing process development by integration of edible filamentous fungi to upgrade the quality of Brewer’s spent grain (BSG). BioResources 2021, 16, 1686–1701. [Google Scholar] [CrossRef]
- Sar, T.; Ozturk, M.; Taherzadeh, M.J.; Ferreira, J.A. New insights on protein recovery from olive oil mill wastewater through bioconversion with edible filamentous fungi. Processes 2020, 8, 1210. [Google Scholar] [CrossRef]
- Borujeni, N.E.; Karimi, K.; Denayer, J.; Kumar, R. Apple pomace biorefinery for ethanol, mycoprotein, and value-added biochemicals production by Mucor indicus. Energy 2021, 240, 122469. [Google Scholar] [CrossRef]
- Lima, T.M.D.; Almeida, A.B.D.; Peres, D.S.; Oliveira, R.M.D.S.F.D.; Sousa, T.L.D.; Freitas, B.S.M.D.; Silva, F.G.; Egea, M.B. Rhizopus oligosporus as a biotransforming microorganism of Anacardium othonianum Rizz. byproduct for production of high -protein, -antioxidant, and -fiber ingredient. LWT 2021, 135, 110030. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, J. Fruits and fruit flavor: Classification and biological characterization. In Handbook of Fruit and Vegetable Flavors; John Wiley and Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Wikandari, R. Effect of Fruit Flavors on Anaerobic Digestion: Inhibitions and Solutions. Ph.D. Thesis, University of Borås, School of Engineering, Borås, Sweden, 2014. [Google Scholar]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Balaban, M.; Koc, C.; Sar, T.; Yesilcimen Akbas, M. Screening for bioactive compound rich pomegranate peel extracts and their antimicrobial activities. Johns. Matthey Technol. Rev. 2021, 66, 81–89. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Balaban, M.; Koc, C.; Sar, T.; Yesilcimen Akbas, M. Antibiofilm effects of pomegranate peel extracts against B. cereus, B. subtilis and E. faecalis. Int. J. Food Sci. Technol. 2021, 56, 4915–4924. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Vattem, D.A.; Shetty, K. Solid-state production of phenolic antioxidants from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 2002, 16, 189–210. [Google Scholar] [CrossRef]
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of biogas production from orange peel waste by leaching of limonene. BioMed Res. Int. 2015, 2015, 494182. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.M.; Koch, F.; Trossini, T.G.; Esposito, E.; Ninow, J.L. Production of Rhizopus oligosporus protein by solid state fermentation of apple pomace. Braz. Arch. Biol. Technol. 2006, 49, 91–100. [Google Scholar]
- Jin, B.; Zepf, F.; Bai, Z.; Gao, B.; Zhu, N. A biotech-systematic approach to select fungi for bioconversion of winery biomass wastes to nutrient-rich feed. Process Saf. Environ. Prot. 2016, 103, 60–68. [Google Scholar] [CrossRef]
- Kayode, R.M.O.; Sani, A. Physicochemical and proximate composition of mango (Mangifera indica) kernel cake fermented with mono-culture of fungal isolates obtained from naturally decomposed mango kernel. Life Sci. J. 2008, 5, 55–63. [Google Scholar]
- Bulkan, G.; Sitaresmi, S.; Yudhanti, G.T.; Millati, R.; Wikandari, R.; Taherzadeh, M.J. Enhancing or inhibitory effect of fruit or vegetable bioactive compound on aspergillus niger and A. Oryzae. J. Fungi 2022, 8, 12. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Production of ethanol and biomass from thin stillage using food-grade Zygomycetes and Ascomycetes filamentous fungi. Energies 2014, 7, 3872–3885. [Google Scholar] [CrossRef] [Green Version]
- Sues, A.; Millati, R.; Edebo, L.; Taherzadeh, M.J. Ethanol production from hexoses, pentoses, and dilute-acid hydrolyzate by Mucor indicus. FEMS Yeast Res. 2005, 5, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Yudhanti, G.T. Effect of Terpenoids, Aldehyde, and Organic Acid on Filamentous Fungal Growth and Ethanol Production. Bachelor’s Thesis, Universitas Gadjah Mada, Sleman, Indonesia, 2021. [Google Scholar]
- Sitaresmi, S. Effect of Alcohol, Phenolic Compounds, and Glucoside on Filamentous Fungal Growth and Ethanol Production: Beneficial or Detrimental? Bachelor’s Thesis, Universitas Gadjah Mada, Sleman, Indonesia, 2021. [Google Scholar]
- Ünal, M.Ü.; Ucan, F.; Şener, A.; Dincer, S.J. Research on antifungal and inhibitory effects of DL-limonene on some yeasts. Turk. J. Agric. For. 2012, 36, 576–582. [Google Scholar]
- Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Production of ethanol and biomass from thin stillage by Neurospora intermedia: A pilot study for process diversification. Eng. Life Sci. 2015, 15, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.A.; Lennartsson, P.R.; Niklasson, C.; Lundin, M.; Edebo, L.; Taherzadeh, M.J. Spent sulphite liquor for cultivation of an edible Rhizopus sp. BioResources 2012, 7, 173–188. [Google Scholar]
- Rousta, N.; Ferreira, J.A.; Taherzadeh, M.J. Production of L-carnitine-enriched edible filamentous fungal biomass through submerged cultivation. Bioengineered 2021, 12, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Ellagic Acid and Its Role in Chronic Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 473–479. [Google Scholar]
- Ramadan, D.T.; Ali, M.A.M.; Yahya, S.M.; El-Sayed, W.M. Correlation between Antioxidant/Antimutagenic and Antiproliferative Activity of Some Phytochemicals. Anti-Cancer Agents Med. Chem. 2019, 19, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Guo, X.; Dawuti, G.; Aibai, S. Antifungal activity of ellagic acid in vitro and in vivo. Phytother. Res. 2015, 29, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A. Superabsorbent polymers from the cell wall of zygomycetes fungi. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2010. [Google Scholar]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, cryptococcus, and aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Patel, P.K.; Free, S.J. The Genetics and biochemistry of cell wall structure and synthesis in Neurospora crassa, a model filamentous fungus. Front. Microbiol. 2019, 10, 2294. [Google Scholar] [CrossRef]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic application of betalains: A review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of anti-staphylococcus aureus activity of quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Kapešová, J.; Petrásková, L.; Markošová, K.; Rebroš, M.; Kotik, M.; Bojarová, P.; Křen, V. Bioproduction of quercetin and rutinose catalyzed by rutinosidase: Novel concept of “solid state biocatalysis”. Int. J. Mol. Sci. 2019, 20, 1112. [Google Scholar] [CrossRef] [Green Version]
- Purewal, S.S.; Salar, R.K.; Bhatti, M.S.; Sandhu, K.S.; Singh, S.K.; Kaur, P. Solid-state fermentation of pearl millet with Aspergillus oryzae and Rhizopus azygosporus: Effects on bioactive profile and DNA damage protection activity. J. Food Meas. Charact. 2020, 14, 150–162. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.d.A.; de Aguiar Cordeiro, R.; Castelo-Branco, D.d.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Bioconversion of kaempferol and quercetin glucosides from plant sources using rhizopus spp. Fermentation 2018, 4, 102. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.I.; Fujita, T.; Kubo, I. Antifungal activity of alkanols against Zygosaccharomyces bailii and their effects on fungal plasma membrane. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Thompson, P.A.; Hakim, I.A.; Chow, H.H.S.; Thomson, C.A. d-Limonene: A bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol. Rev. 2011, 5, 31–42. [Google Scholar] [CrossRef]
- Lennartsson, P.R.; Ylitervo, P.; Larsson, C.; Edebo, L.; Taherzadeh, M.J. Growth tolerance of zygomycetes mucor indicus in orange peel hydrolysate without detoxification. Process Biochem. 2012, 47, 836–842. [Google Scholar] [CrossRef]
- Bier, M.C.J.; Medeiros, A.B.P.; Soccol, C.R. Biotransformation of limonene by an endophytic fungus using synthetic and orange residue-based media. Fungal Biol. 2017, 121, 137–144. [Google Scholar] [CrossRef]
- Setzer, W.N.; Vogler, B.; Schmidt, J.M.; Leahy, J.G.; Rives, R. Antimicrobial activity of Artemisia douglasiana leaf essential oil. Fitoterapia 2004, 75, 192–200. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhang, S.-B.; Zhai, H.-C.; Lv, Y.-Y.; Hu, Y.-S.; Cai, J.-P. Hexanal induces early apoptosis of Aspergillus flavus conidia by disrupting mitochondrial function and expression of key genes. Appl. Microbiol. Biotechnol. 2021, 105, 6871–6886. [Google Scholar] [CrossRef]
- Millati, R.; Edebo, L.; Taherzadeh, M.J. Performance of rhizopus, rhizomucor, and mucor in ethanol production from glucose, xylose, and wood hydrolyzates. Enzym. Microb. Technol. 2005, 36, 294–300. [Google Scholar] [CrossRef]
- Pietrzak, W.; Kawa-Rygielska, J. Backset valorization in dry-grind ethanol process by co-culture of edible filamentous fungi and fodder yeast. J. Clean. Prod. 2019, 220, 376–385. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Fox, M.; Hjorth, H.; Edebo, L. Production of mycelium biomass and ethanol from paper pulp sulfite liquor by Rhizopus oryzae. Bioresour. Technol. 2003, 88, 167–177. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jin, B.; Kelly, J.M. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 2007, 35, 251–263. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Rasoul, M.A.A.; Abdelgaleil, S.A. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Yassin, S.; Wheals, A. Neurospora species in bakeries. J. Appl. Bacteriol. 1992, 72, 377–380. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; Estarron-Espinosa, M.; Ogura, T. Volatile compounds secreted by the oyster mushroom (Pleurotus ostreatus) and their antibacterial activities. J. Agric. Food Chem. 1997, 45, 4049–4052. [Google Scholar] [CrossRef]
- Nweke, C.O.; Okpokwasili, G.C. Removal of phenol from aqueous solution by adsorption onto activated carbon and fungal biomass. Int. J. Biosci. 2013, 3, 11–21. [Google Scholar]
- Aranda-Martinez, A.; Lopez-Moya, F.; Lopez-Llorca, L.V. Cell wall composition plays a key role on sensitivity of filamentous fungi to chitosan. J. Basic Microbiol. 2016, 56, 1059–1070. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Hashem, A.H.; Abd El-Sayed, E.S.; El-Saied, H. Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus microsporus AH3: Efficiency and characteristics. Cellulose 2020, 27, 4443–4453. [Google Scholar] [CrossRef]
- Jiménez, M.; García-Carmona, F. Oxidation of the flavonol quercetin by polyphenol oxidase. J. Agric. Food Chem. 1999, 47, 56–60. [Google Scholar] [CrossRef]
- Nedele, A.-K.; Schiebelbein, R.; Bär, A.; Kaup, A.; Zhang, Y. Reduction of aldehydes with green odor in soy products during fermentation with Lycoperdon pyriforme and analysis of their degradation products. Food Res. Int. 2022, 152, 110909. [Google Scholar] [CrossRef]
- Thitiprasert, S.; Sooksai, S.; Thongchul, N. In vivo regulation of alcohol dehydrogenase and lactate dehydrogenase in rhizopus oryzae to improve L-lactic acid fermentation. Appl. Biochem. Biotechnol. 2011, 164, 1305–1322. [Google Scholar] [CrossRef]
- Wainaina, S.; Kisworini, A.D.; Fanani, M.; Wikandari, R.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Utilization of food waste-derived volatile fatty acids for production of edible Rhizopus oligosporus fungal biomass. Bioresour. Technol. 2020, 310, 123444. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.B.; Lennartsson, P.R.; Taherzadeh, M.J. Mycelial pellet formation by edible ascomycete filamentous fungi, Neurospora intermedia. AMB Express 2016, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, P.W.; Thomas, C.R. Classification and measurement of fungal pellets by automated image analysis. Biotechnol. Bioeng. 1992, 39, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Souza Filho, P.F.; Zamani, A.; Taherzadeh, M.J. Production of edible fungi from potato protein liquor (PPL) in airlift bioreactor. Fermentation 2017, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.B.; Osadolor, O.A.; Ravula, V.K.; Lennartsson, P.R.; Taherzadeh, M.J. Lignocellulose integration to 1G-ethanol process using filamentous fungi: Fermentation prospects of edible strain of Neurospora intermedia. BMC Biotechnol. 2018, 18, 49. [Google Scholar] [CrossRef] [Green Version]
- Bushara, K.O.; Goldstein, S.R.; Grimes, G.J.; Burstein, A.H.; Hallett, M. Pilot trial of 1-octanol in essential tremor. Neurology 2004, 62, 122–124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).