Abstract
Labneh is positioned in the top ranking of the bestselling dairy products all over the world due to its health benefits and delicious taste. Labneh production depends mainly on probiotic bacteria in the fermentation of milk. Probiotic bacteria have many health benefits, which are driven by their selective bioactive metabolites that quantitively affect the fermentation products. The current investigation aimed to study the implementation of photobiomodulation through the irradiation of Lacticaseibacillus casei NRRL-B-1922 by a He–Ne laser (630 nm) with different laser doses (3, 6 & 12 J/cm2) prior to milk fermentation. This procedure sought to improve the probiotic bacteria’s activities while enhancing the labneh’s characteristics and consequently produce a more favorable labneh product with better marketing qualities. The photobiostimulated bacterial starter was found to induce increased titratable acidity with the moisture reduction of the labneh product under cold storage conditions for 20 days. The effect was most prominent when using a 12 J/cm2 laser dose. The flavor-aiding components, mainly diacetyl and acetaldehyde compounds, and sensory scores were increased in the labneh produced by irradiated L. casei when compared to the non-radiated probiotic culture after storing the products under cold conditions for 20 consecutive days. Moreover, the antioxidant and proteolytic activities of labneh produced by treated L. casei (12 J/cm2 laser dose) after cold storage were significantly elevated by 41 and 14%, respectively. In conclusion, we can report significantly improved selected characteristics in the final products after the employment of photobiomodulation process, the potential application of this concept on the industrial scale, and its implications on lengthening the product shelf life with improved qualities.
1. Introduction
Labneh is a strained and/or concentrated yogurt produced from fermented milk. It is a widely used product in the Mediterranean region, especially in Egypt. Labneh is produced by draining the water-soluble components from yogurt to obtain a semi-solid structure. The removal of the whey from the yogurt continues until the fat content reaches a range of 9–11% and the total solid content becomes 23–25% [1]. Variable milk sources are used in the industrial production of labneh, such as goat, cow, and sheep milk [2]. This product has many nutritional benefits as it has double the protein content of common yogurt and more viable beneficial microorganisms with a lower lactose percentage [3]. The final product is a smooth, white textured paste. It has a sharp taste that ranges between that of cottage cheese and sour cream. Industrially, the product’s taste is controlled by the fermentation diacetyl metabolite [4].
Recently, the European market has seen more interest in labneh, and such interest has been reflected in the market share of the yogurt sector [5]. There are several labneh products in the market based on their textures and fat contents. Because of the increased European market demand and health-related concerns toward fat products, there is a growing trend to produce labneh with a low fat content. Fat itself, as a flavor precursor, plays a crucial role in the physical characteristics, such as the consistency, opacity, and texture, besides the taste to the consumers. However, it is not easy to produce low-fat labneh products with the same taste as full-fat labneh [6].
Starter bacteria with yeast and mold impurities when combined with storage and the packaging factors may reduce the shelf-life of the product [7]. Choosing the starter culture is a crucial step in obtaining a labneh product with high quality due to the usage of pasteurized milk. Several strains of thermophilic lactic acid bacteria are used to ferment lactose to lactic acid. Mixtures of Lactobacillus delbrueckii spp. bulgaricus and Streptococcus thermophilus are widely used with fermented milk in the labneh industry [8]. Mixed microorganisms in the starter reduce the time of milk coagulation significantly when compared to the usage of a single culture [9]. Several researchers have studied the use of different microorganism mixtures in the starter culture [3]. Typically, labneh would contain more viable lactic acid bacteria than normal yogurt products and, likewise, it may hold 2000 million bacteria/gram of each of L. delbrueckii spp. bulgaricus and S. thermophilus, since these bacteria stay in the curd instead of being removed in the concentration step [10]. Previous studies showed that those bacteria are lost during the processing procedure of the product and, hence, the bacterial count could be similar when compared to that of normal yogurt [11]. The minor reduction in the viable bacterial count of labneh by the expiration date demonstrates the resistance of those bacteria to acidity and low temperatures.
Probiotic bacteria are living bacteria that have beneficial health effects in the administered hosts when taken in suitable amounts. Probiotic bacteria, after surviving the host’s gastrointestinal tract, modulate intestinal microorganisms, decrease the frequency of diarrhea, and improve some metabolic diseases, such as obesity and diabetes [12]. Therefore, they are used extensively in the food industry and dairy products. Limited bacterial species can meet the above definition; among which are Bifidobacterium and Lactobacillus genera, which are abundantly present in the human intestine [13]. Other bacterial strains, such as Lactococcus, Streptococcus, Enterococcus, and some non-pathogenic Escherichia coli strains, and some yeast and bacilli strains may have probiotic behavior; however, they are not found in the host’s gastrointestinal tract [14]. One of the most principal and prevalent applications of probiotic bacteria is dairy products, specifically fermented ones [15]. The wide usage of these bacteria is due to their positive impact on human health via many routes. They include vitamin K biosynthesis, the induction and modulation of the immune reaction, xenobiotic detoxification, pathogenic bacteria antagonism, and intestinal peristalsis effects [16].
Photobiomodulation (PBM) is comprised of light application, mainly monochromatic laser or LED light of low power. Light of different wavelengths has been demonstrated to exert many biological impacts. For instance, ultraviolet-B can induce the synthesis of vitamin D. Red and near-IR light wavelengths have various physiological impacts, especially in that this wavelength range can penetrate human skin and tissues. Then, light can affect and modulate the body’s inflammatory response, and cellular metabolism and signaling [17]. The exact mechanism of PBM is still not certain, but there are many postulations and evidence that favor certain mechanistic pathways. One of the commonly employed pathways for red and IR light is the enhancement of electron transport after increasing the membrane potential of the mitochondria and increased levels of ATP and oxygen utilization [18]. On the other hand, other light wavelengths can hinder mitochondria electron transport and can thus be used in tissue damage-related diseases [19]. To date, there are no experimental data exploring the effect of red laser beams on the characteristics of different starter cultures, which consequently could enhance selected properties of fermented dairy products. The current research aimed to investigate the effect of a He–Ne laser with different doses on the biomodulation of L. casei that is used in milk fermentation for labneh production and enriching its properties.
2. Materials and Methods
2.1. Milk
Fresh cow milk (3.2% fat and 12.1% total solids) was obtained from the herd at the Faculty of Agriculture, Cairo University, Giza, Egypt.
2.2. Chemicals and Bacteriological Media
1,1-diphenyl-2- picrylhydrazyl (DPPH) and o-phthaldialdehyde (OPA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The bacteriological media, de Man, Rogosa, and Sharpe (MRS), skim milk broth medium, and MacConkey agar, as well as Vancomycin, were purchased from Merck Co. (Darmstadt, Germany)
2.3. Bacterial Strains
Lactocaseibacillus (L.) casei NRRL-B-1922 was obtained from the Northern Regional Research Laboratory (NRRL), Peoria, IL, USA. Yogurt culture YC-380 was purchased from Chr. Hansen Co., Copenhagen, Denmark. Bacillus subtilis EMCCN 1152 and Escherichia coli ATCC 25922 were obtained from the Egyptian Microbial Culture Collection (EMCC), Microbiological Resources Center (MIRCEN), Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
2.4. Laser Exposure
We used the same experimental setup that was described in our earlier study [14]. Briefly, we used a He–Ne laser that emits at 630 nm wavelength with 40 mW. The laser doses used throughout this study were 3, 6, and 12 J/cm2. These doses were achieved by calculating the irradiation time via the following equations:
Laser power/Irradiated area = Y mW/cm2
Y mW/cm2/1000 = Y W/cm2
Work (W/cm2) = Joules (J)/Time (s)
Time (s) = Joules (J)/Work (W/cm2)
Ten milliliters of bacterial culture with an optical density of 0.6 placed in a small cultural flask were irradiated at 3, 6, and 12 J/cm2 after calculating the required irradiation times. The cultural flasks were placed on a magnetic stirrer to ensure uniform and coherent laser irradiation, as shown in Figure 1. The laser beam was diverged by a suitable diverging lens to ensure that the bacterial culture that lay beneath was fully covered by the laser beam.
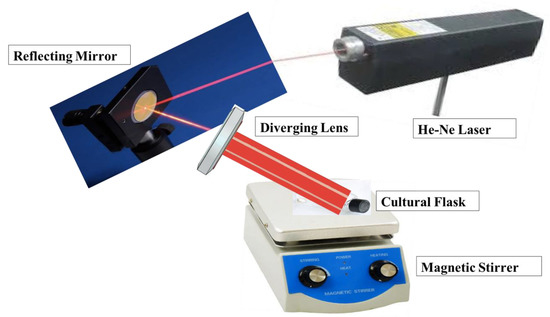
Figure 1.
Laser irradiation experimental setup.
2.5. Propagation of Bacterial Cultures
L. casei and yogurt culture YC-380 were cultivated using skim milk broth medium and incubated at 37 °C for 24 h. E. coli and B. subtilis, were cultivated in nutrient broth (Oxoid, Hampshire, UK) at 37 °C for 24 h.
2.6. Manufacture of Labneh
Fresh cow’s milk was heated to 90 °C for 20 min, cooled to 40 °C, and then divided into four equal portions. The first portion served as the control, where the milk was inoculated with 0.5% YC-380 and untreated 0.5% L. casei. The second, third, and fourth portions were inoculated with 0.5% YC-380 and laser-treated 0.5% L. casei with 3 J/cm2, 6 J/cm2, and 12 J/cm2 respectively. The milk was incubated at 37 °C for 4 h until complete coagulation. The resultant coagulant was mixed thoroughly with 0.5% NaCl (Figure 2). Samples were collected for analysis either fresh (day 1) or during the twenty days of cold storage [20].
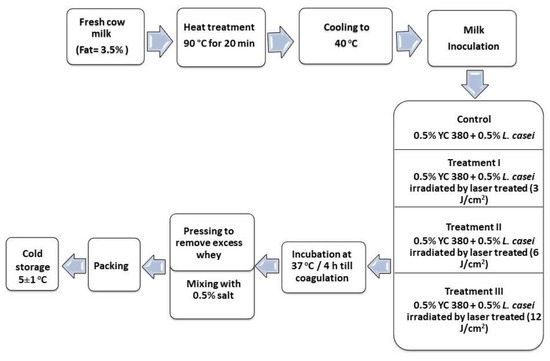
Figure 2.
Graphical diagram for the manufacture of labneh.
2.7. Measurement of Titratable Acidity and Moisture Levels of Different Labneh Treatments
The titratable acidity (% lactic acid) and moisture of the labneh samples was measured according to A.O.A.C. [21].
2.8. Acetaldehyde and Diacetyle Content of Different Labneh Treatments
In order to evaluate the development of flavor components, the acetaldehyde content (µmol 100 g−1) of the labneh was measured in accordance with the procedure described by Lee and Jago [22], and the diacetyl content (mmol 100 g−1) of the labneh was determined as described by Pack et al. [23] before and after storage in each laser treatment.
2.9. Viable Counts (Log CFU/g) of Starter Culture of Different Labneh Treatments
Viable counts of L. delbrueckii ssp. bulgaricus were enumerated using MRS agar at pH 5.2 (aerobic, 45 °C for 72 h). MRS agar supplemented with vancomycin was used for the enumeration of L. casei (anaerobic, 37 °C for 72 h). Streptococcus thermophilus (ST) agar was used for the enumeration of S. thermophilus (aerobic, 37 °C for 24 h). All microbiological media were used as described by Tharmaraj and Shah [24].
2.10. Antioxidant Activity as DPPH Scavenging Activity of Different Labneh Treatments
The crude extract from each labneh sample (50 gm) was prepared by centrifugation at 6000× g for 20 min at 5 °C, and then filtered through a 0.45 µm pore-diameter syringe filter. The DPPH scavenging activity of the different labneh treatments was assessed as described by Lee et al. [25]. The absorbance of the samples was measured at 517 nm (Jenway spectrophotometer 6300, Staffordshire, UK). Ascorbic acid was used as a positive control, and bacteria-free fresh milk was used as a negative control.
2.11. Proteolytic Activity of Crude Extract Collected from Different Labneh Treatments
The degree of protein hydrolysis (DH) of different labneh treatments was assessed using the o-phthaldialdehyde OPA assay as described by Donkor et al. [26]. The crude extract of each sample was incubated at room temperature with OPA for 4 min, and the absorbance of the mixture was measured at 340 nm. The degree of protein hydrolysis (%DH) was calculated by the following equation:
DH% = Asample − Ablank/Atotal − Ablank × 100
2.12. Sensory Evaluation
Fresh samples of different Labneh treatments were sensory-evaluated before cold storage using a hedonic scale (1–9) according to Dagher and Ali [27]. Briefly, a total of fifteen trained panelists participated in this study in order to evaluate the overall acceptability of the labneh samples using the 9-point hedonic scales, where 1 means extremely dislike and 9 means extremely like [27].
2.13. Induced Labneh Spoilage by B. subtilis and E. coli during Cold Storage
In order to evaluate the probability of labneh contamination after cold storage, separate patches of labneh (treated and non-treated) were subjected to induced bacterial contamination using two different bacterial strains. The active cultures of B. subtilis and E. coli (viable counts were 6.30 Log CFU/mL for B. subtilis and 6.50 Log CFU/mL for E. coli) were separately inoculated by 2% to each labneh treatment. The enumeration of E. coli was measured using MacConkey agar; however, plate-count milk agar supplemented with 0.2% starch was used for the enumeration of B. subtilis [28] after the cold storage period.
2.14. Data Analyses
All the experimental assays were performed in at least triplicate independent experiments (n = 3) and analyzed using the mean variance of ANOVA, where a p-value of <0.05 was set as the significant level. Tukey’s test was performed using the Statistical Analysis System.
3. Results
3.1. Titratable Acidity and Moisture Content
After 20 days of cold storage (5 ± 1 °C), the levels of titratable acidity were increased while the levels of moisture were decreased, as shown in Table 1. The labneh samples manufactured using laser-treated L. casei had a significantly (p < 0.05) higher acidity and lower moisture content than the control at the end of cold storage.

Table 1.
Changes in the titratable acidity (% lactic acid) and moisture levels of different labneh treatments.
3.2. Acetaldehyde and Diacetyl
The levels of the flavor components (acetaldehyde and diacetyl) were significantly increased during the cold storage period. Laser treatment of the L. casei strain had an enhancement effect to increase the produced levels of acetaldehyde and diacetyl, as shown in Table 2.

Table 2.
Changes in the acetaldehyde and diacetyl profiles (µmol.100 g−1) of different labneh treatments.
3.3. Viability of Starter Culture after Cold Storage
After 20 days of cold storage, the viable counts (Log CFU/g) of all bacterial strains were decreased, but not significantly, when compared to the counts before cold storage, as shown in Figure 3. Furthermore, at the end of the cold storage period, there were no significant differences in the viable counts of L. delbrueckii ssp. bulgaricus, S. thermophilus, and L. casei between the treatments in comparison with the control.
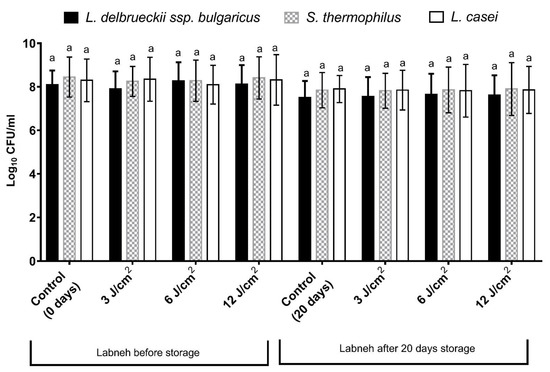
Figure 3.
Changes in the viable counts (Log10 CFU/g) of the starter cultures of different labneh treatments before and after 20 days of cold storage. The bars on the graph represent the mean ± SD as a Log10 CFU/g of triplicate independent experiments (n = 3). The common letters above the bars are not significantly different (p < 0.05) within the same bacterial species.
3.4. Antioxidant Activity
The levels of antioxidant activity (measured as % DPPH scavenging activity) at the beginning of cold storage were significantly increased when L. casei was treated with a laser beam in comparison with the control. Furthermore, a significant increase in the values of antioxidant activity was observed at the end of the cold storage period (Table 3).

Table 3.
Antioxidant activity as the DPPH-scavenging activity of different labneh treatments.
3.5. Proteolytic Activity
The data in Table 4 show that the levels of proteolytic activity (measured as the percentage degree of protein hydrolysis) were significantly increased when L. casei was treated with a laser beam only after 20 days of cold storage compared with control in the same storage period.

Table 4.
Proteolytic activity of the crude extract collected from different labneh treatments.
3.6. Sensory Evaluation of Labneh Produced by L. casei Irradiated with Different Laser Doses
In order to evaluate the effect of laser exposure of L. casei on the produced labneh, the general sensory properties were assessed compared to non-irradiated L. casei produced labneh as a control. The results revealed that no negative effect was observed between the treated and non-treated labneh. In contrast, by increasing the irradiation levels to 12 J/cm2, the overall acceptability was significantly increased (Table 5).

Table 5.
Overall acceptability score of different labneh treatments.
3.7. Induced Labneh Spoilage by B. subtilis and E. coli during Cold Storage
After 20 days of cold storage, no colonies were detected for E. coli ATCC 25922 in the different Labneh treatments. However, the viable counts of spore-forming B. subtilis EMCCN 1152 were significantly increased in the control sample by ca. two log cycles (Table 6). However, B. subtilis had relative stability in the labneh samples manufactured with laser-treated L. casei.

Table 6.
Growth of B. subtilis and E. coli (Log CFU/g) in different Labneh treatments during the shelf-life period.
4. Discussion
The growing interest in healthy food, especially that containing probiotics, has forced many researchers to improve the viability of probiotic strains and prolong the shelf life of dairy products. Labneh, concentrated yogurt, is a popular Mitterrandian fermented milk product that has a short shelf life, even at low temperatures. Many attempts have been made to improve its probiotic viability, microbiological properties, and flavor during cold storage by the addition of different natural products as bio-preservatives in the manufacturing process, such as essential oils [29] and plant extracts [30]. However, few studies have been performed regarding the implementation of the photobiomodulation of the bacterial starter culture in the fermentation process [14]. Thus, the current study shows a positive correlation between the treatment of L. casei by low laser doses and the beneficial impact on the manufactured labneh product, at the beginning, as well as after 20 days of cold storage.
The change in the TA and moisture content of Labneh is an important factor of its quality, since it affects the acceptability and the shelf life of Labneh products [31]. It was observed that no significant change in both factors was recorded after the manufacturing process. However, increasing the storage period significantly increased the acidity and decreased the moisture content in all labneh samples manufactured using laser-treated L. casei compared to the control, suggesting that laser treatment had a stimulatory effect on the bacterial culture’s viability, activities, and acid-production dynamics. These findings were in accordance with the results found before, that TA gradually increased with the storage time due to the activity of both the L. casei and starter cultures [29,31,32] or after the addition of bacterial stimulating supplements [29]. Previous observations that laser-treated L. casei could produce high levels of acidity after skim milk fermentation attributed this effect to the increase of β-galactosidase activity, which is a key factor in lactose fermentation [14]. It was observed that the increased levels of pH in the labneh samples manufactured with laser-treated L. casei significantly affected the moisture content compared to the control (Table 1). Similar observations attributed the increase in acidity to the low moisture content of cheese [31,32,33,34]. The change in the levels of pH and moisture was not significant between different laser dosage treatments, indicating a maximum threshold level that can be reached after prolonged storage. Indeed, the maximum recorded value of TA (1.50 ± 0.03%) after L. casei laser irradiation of 12 J/cm2 was lower than those reported in the literature after 20 days storage (1.69%).
The flavor composition of fermented dairy products is a complicated characteristic that contains acids, aldehydes, ketones, alcohols, esters, and hydrocarbons [32,35]. The fermentation of lactose by L. casei results in the formation of acetaldehyde and diacetyl compounds [36], which are the main flavor components in labneh. Acetaldehyde is produced in labneh as a result of the activities of both starter cultures and L. casei; these strains lack the alcohol dehydrogenase enzyme, which is necessary for acetaldehyde conversion into ethanol [31,32]. Acetaldehyde provides fermented milk an ethereal, fresh, and pungent flavor and diacetyl provides the buttery flavor, which are considered the typical flavor and aroma of labneh [32,35]. Labneh samples manufactured with-laser treated L. casei strains with a dose of 12 J/cm2 contained the highest levels of acetaldehyde (33.41 ± 0.91) and diacetyl (17.30 ± 1.41), which may be due to the increased activity of the threonine aldolase enzyme [29]. Interestingly, the levels of flavor compounds were increased in a dose–response relationship, indicating the positive effect of the red laser on bacterial enzymatic activities.
The cold-storage of different dairy products has a negative effect on the viability of different probiotic strains, as previously observed in fermented milk [26,37], bio-yogurt [38], labneh [29,31], and a wide range of cheeses [39]. Figure 3 shows a decrease (not significant) in the viability of L. delbrueckii ssp. Bulgaricus, S. thermophilus, and L. casei strains in both the control and treated samples. The viable counts of L. casei were above 106 CFU/g, as the recommended level for probiotics [40]. At the end of cold storage, there was no significant difference between the counts of L. casei in the control samples and laser-treated samples. Laser treatment did not have any negative effect on the viability of L. casei, which is in line with the results demonstrated in a previous study on L. acidophilus and L. reuteri irradiated at a 660 nm wavelength with different dosages of 1, 5, and 10 J/cm2 [41].
After the cold storage period, a significant increase in the values of antioxidant capacity was detected in the labneh samples manufactured with laser-treated L. casei. The increase in the antioxidant activities reached 41% in the Labneh samples manufactured with laser-treated L. casei strains with a dose of 12 J/cm2. It was reported that the levels of antioxidant activity of crude extracts of different fermented milks and labneh were significantly increased during the cold storage period as a result of the accumulation of different metabolites with antioxidant capacity [42,43]. Laser-treated L. casei NRRL-B-1922 could enhance the levels of antioxidant capacity in fermented skim milk, as previously observed by Mohamed et al. [14]. The increase in the antioxidant capacity of the produced Labneh may be attributed to the significant observed elevation of the proteolytic bacterial activity (Table 4) that releases several antioxidant bioactive peptides from milk proteins [37,44].
The proteolytic activity of different probiotic Lactocaseibacillus strains plays an essential role in the development of flavor and health benefits through the release of bioactive peptides in a wide range of fermented dairy products [44,45]. The enhancement of proteolytic activity in labneh samples manufactured with the laser-treated L. casei strain was pronounced after 20 days of cold storage. The proteolytic activity of labneh produced by L. casei treated with 12 J/cm2 was increased by 14% at the end of cold storage compared to the untreated control (Table 4). These results agree with the literature, where the proteolytic activity of L. casei in fermented milk was improved by increasing the photon energy of the laser source [14]. On the other hand, other microorganisms, such as Saccharomyces cerevisiae, increased their fermentation products (ethanol) after exposure to a low-dosage red laser prior to the fermentation of agro-industrial wastes, and it was reported that laser irradiation can increase bioethanol production by 13.13% compared to the non-treated control [46]. Taken together, the exposure of microorganisms to low dosages of a red laser can enhance the fermentation process by triggering the enzymatic activities of microorganisms and the overall metabolic process.
The sensory evaluation of labneh produced by the prior laser irradiation of L. casei indicated that no undesirable overall taste was observed compared to the control without treatment. Indeed, the overall acceptability was significantly increased at laser dosages of 6 and 12 J/cm2. This increase in the overall acceptability was in line with the higher levels of flavor components (acetaldehyde and diacetyl) detected after increasing the laser dosages compared to the non-treated control (Table 2). In this regard, the results obtained by El-Shafei et al. [31] reported that the addition of free or microencapsulated L. casei did not have any negative effect on the organoleptic properties of labneh.
E. coli is a main member of the coliform group which causes spoilage in a wide range of dairy products. Additionally, the detection and enumeration of the coliform group are required by International Dairy Federation. The E. coli strain lost its survivability in different labneh treatments (Table 5) after the induction of spoilage, which may be due to the high acidity detected after cold storage. Non-pathogenic E. coli is less acid-tolerant than pathogenic E. coli in different food matrices [47]. B. subtilis, as a spore-forming bacteria, can contaminate milk during transport from the farm to dairy-processing units or equipment by the bacterial biofilm matrix [48]. The spore coat is a complex layer found on all endospores of B. subtilis and contributes to the microbe’s resistance to chemicals and enzymes [49]. The results of our previous study showed that laser-treated L. casei had higher antibacterial activity against B. subtilis and E. coli than the laser-untreated strain [14].
5. Conclusions
In conclusion, the labneh product produced by L. casei NRRL-B-1922 was significantly improved after exposure to laser doses of 12 J/cm2 at the beginning, as well as after 20 days of cold storage. This photobiomodulation improvement was found to induce increased titratable acidity with the moisture reduction of the labneh product after cold storage for 20 days. Furthermore, the flavor, antioxidant capacity, and proteolytic activities of fermented labneh were increased when compared to non-radiated probiotic cultures. The induction of labneh spoilage by B. subtilis and E. coli demonstrated no significant change in the B. subtilis count, and E. coli was not detected after cold storage. The photobiomodulation potential application on the industrial scale and its implications on lengthening the product shelf life with improved quality is a promising area of research. However, in large-scale labneh production, a cost-controlled and feasible method for fermentation plants will be a future challenge, and proteomics analysis of the laser-treated L. casei strain will be our future study.
Author Contributions
All authors were involved in the conception of the research idea and methodology design. F.M.F.E., A.E.-H. and M.S.M.M. carried out the laboratory work. F.M.F.E., A.E.-H. and M.S.M.M. interpreted the results and prepared the manuscript for publication. A.E.-H., M.S.M.M. and F.M.F.E. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the conclusions of this article are included in the manuscript.
Conflicts of Interest
The authors declare there is no conflict of interest.
References
- Al-Kadamany, E.; Toufeili, I.; Khattar, M.; Abou-Jawdeh, Y.; Harakeh, S.; Haddad, T. Determination of Shelf Life of Concentrated Yogurt (Labneh) Produced by In-Bag Straining of Set Yogurt using Hazard Analysis. J. Dairy Sci. 2002, 85, 1023–1030. [Google Scholar] [CrossRef]
- Kaaki, D.; Baghdadi, O.K.; Najm, N.; Olabi, A. Preference mapping of commercial Labneh (strained yogurt) products in the Lebanese market. J. Dairy Sci. 2012, 95, 521–532. [Google Scholar] [CrossRef]
- Nsabimana, C.; Jiang, B.; Kossah, R. Manufacturing, properties and shelf life of labneh: A review. Int. J. Dairy Technol. 2005, 58, 129–137. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yogurt Science and Technology, 3rd ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2007; ISBN 978-184-569-213-1. [Google Scholar]
- Mohameed, H.A.; Abu-Jdayil, B.; Al-Shawabkeh, A. Effect of solids concentration on the rheology of labneh (concentrated yogurt) produced from sheep milk. J. Food Eng. 2004, 61, 347–352. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, R.; Ren, F.; Mao, X. Addition of buttermilk improves the flavor and volatile compound profiles of low-fat yogurt. LWT 2018, 98, 9–17. [Google Scholar] [CrossRef]
- Ozer, B.H.; Bell, A.E.; Grandison, A.S.; Robinson, R.K. Rheological properties of concentrated yoghurt (labneh). J. Texture Stud. 1998, 29, 67–79. [Google Scholar] [CrossRef]
- Radke-Mitchell, L.; Sandine, W.E. Associative Growth and Differential Enumeration of Streptococcus thermophilus and Lactobacillus bulgaricus: A Review. J. Food Prot. 1984, 47, 245–248. [Google Scholar] [CrossRef]
- Lange, I.S.; Mleko, M.; Tomczyńska-Mleko, G.; Janas, P.P.; Ozimek, L. Technology and factors influencing Greek-style yogurt—A Review. Ukr. Food J. 2020, 9, 7–35. [Google Scholar] [CrossRef]
- Yamani, M.I.; Abu-Jaber, M.M. Yeast Flora of Labaneh Produced by In-Bag Straining of Cow Milk Set Yogurt. J. Dairy Sci. 1994, 77, 3558–3564. [Google Scholar] [CrossRef]
- Yerlikaya, O. Starter cultures used in probiotic dairy product preparation and popular probiotic dairy drinks. Food Sci. Technol. 2014, 34, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Howarth, G.S.; Wang, H. Role of Endogenous Microbiota, Probiotics and Their Biological Products in Human Health. Nutrients 2013, 5, 58–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, K.T.; Pereira, G.V.D.M.; Campos, C.R.; Dragone, G.; Schwan, R.F. Brazilian kefir: Structure, microbial communities and chemical composition. Braz. J. Microbiol. 2011, 42, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.S.; Elshaghabee, F.M.; Alharbi, S.A.; El-Hussein, A. The Prospective Beneficial Effects of Red Laser Exposure on Lactocaseibacillus casei Fermentation of Skim Milk. Biology 2020, 9, 256. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Cunningham-Rundles, S.; Ahrné, S.; Johann-Liang, R.; Abuav, R.; Dunn-Navarra, A.-M.; Grassey, C.; Bengmark, S.; Cervia, J.S. Effect of Probiotic Bacteria on Microbial Host Defense, Growth, and Immune Function in Human Immunodeficiency Virus Type-1 Infection. Nutrients 2011, 3, 1042–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, T.H.; Wider, J.M.; Lee, I.; Reynolds, C.A.; Liu, J.; Lepore, B.; Tousignant, R.; Bukowski, M.J.; Johnston, H.; Fite, A.; et al. Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury. Sci. Rep. 2018, 8, 3481. [Google Scholar] [CrossRef]
- Ojha, N.K.; Nematian-Ardestani, E.; Neugebauer, S.; Borowski, B.; El-Hussein, A.; Hoshi, T.; Leipold, E.; Heinemann, S.H. Sodium channels as gateable non-photonic sensors for membrane-delimited reactive species. Biochim. Biophys. Acta 2014, 1838, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoto, S.L.; El-Hussein, A.; Malabi, R.; Thobakgale, L.; Ombinda-Lemboumba, S.; Attia, Y.A.; Kasem, M.A.; Mthunzi-Kufa, P. Exploring optical spectroscopic techniques and nanomaterials for virus detection. Saudi J. Biol. Sci. 2021, 28, 78–89. [Google Scholar] [CrossRef]
- Tamime, A.Y. Fermented milks: A historical food with modern applications—A review. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 4), S2–S15. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Lee, G.I.; Jago, G.R. Methods for the estimation of acetaldehyde in cultured dairy products. Aust. J. Dairy Technol. 1969, 24, 181–190. [Google Scholar]
- Pack, M.Y.; Sandine, W.E.; Elliker, P.R.; Day, E.A.; Lindsay, R.C.O. Method for diacetyl determination in mixed strain starters. J. Dairy Sci. 1964, 44, 15–26. [Google Scholar]
- Tharmaraj, N.; Shah, N. Selective Enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and Propionibacteria. J. Dairy Sci. 2003, 86, 2288–2296. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Hwang, W.-I.; Lim, S.-T. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J. Ethnopharmacol. 2004, 93, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Le Lait 2007, 87, 21–38. [Google Scholar] [CrossRef]
- Dagher, S.; Ali, A. Effect of Pasteurization, Centrifugation and Additives on Quality of Concentrated Yogurt (Labneh). J. Food Prot. 1985, 48, 300–302. [Google Scholar] [CrossRef]
- Eijlander, R.T.; Van Hekezen, R.; Bienvenue, A.; Girard, V.; Hoornstra, E.; Johnson, N.B.; Meyer, R.; Wagendorp, A.; Walker, D.C.; Wells-Bennik, M.H.J. Spores in dairy—New insights in detection, enumeration and risk assessment. Int. J. Dairy Technol. 2019, 72, 303–315. [Google Scholar] [CrossRef]
- Al Otaibi, M.; El Demerdash, H. Improvement of the quality and shelf life of concentrated yoghurt (Labneh) by the addition of some essential oils. Afr. J. Microbiol. Res. 2008, 2, 156–161. [Google Scholar]
- El-Montaleb, H.S.A.; Abbas, K.A.-E.; Mwaheb, M.A.; Hamdy, S.M. Production and characteristic quality of probiotic Labneh cheese supplemented with broccoli florets. Br. Food J. 2021, in press. [Google Scholar] [CrossRef]
- El-Shafei, K.; Elshaghabee, F.M.F.; El-Sayed, H.S.; Kassem, J.M. Assessment the viability properties of Lactobacillus casei strain using labneh as a carrier. Acta Sci. Pol. Technol. Aliment. 2018, 17, 267–276. [Google Scholar] [CrossRef]
- Dan, T.; Ren, W.; Liu, Y.; Tian, J.; Chen, H.; Li, T.; Liu, W. Volatile Flavor Compounds Profile and Fermentation Characteristics of Milk Fermented by Lactobacillus delbrueckii subsp. bulgaricus. Front. Microbiol. 2019, 10, 2183. [Google Scholar] [CrossRef]
- Ramkumar, C.; Creamer, L.K.; Johnston, K.A.; Bennett, R.J. Effect of pH and time on the quantity of readily available water within fresh cheese curd. J. Dairy Res. 1997, 64, 123–134. [Google Scholar] [CrossRef]
- Pastorino, A.; Hansen, C.; McMahon, D. Effect of pH on the Chemical Composition and Structure-Function Relationships of Cheddar Cheese. J. Dairy Sci. 2003, 86, 2751–2760. [Google Scholar] [CrossRef] [Green Version]
- Valero, E.; Villamiel, M.; Miralles, B.; Sanz, J.; Martínez-Castro, I. Changes in flavour and volatile components during storage of whole and skimmed UHT milk. Food Chem. 2001, 72, 51–58. [Google Scholar] [CrossRef]
- Amrita, V.; Santosh, K.; Jain, P.K. Key pretreatment technologies on cellulosic ethanol production. J. Sci. Res. 2011, 55, 57–63. [Google Scholar]
- Elshaghabee, F.; El-Maksoud, A.A.; Alharbi, S.; Alfarraj, S.; Mohamed, M. Fortification of Acidophilus-bifidus-thermophilus (ABT) Fermented Milk with Heat-Treated Industrial Yeast Enhances Its Selected Properties. Molecules 2021, 26, 3876. [Google Scholar] [CrossRef] [PubMed]
- El-Deib, S.M.; Abd Rabo, F.H.R.; Badran, S.M.; Abd El-Fattah, A.M.; Elshaghabee, F.M.F. The growth behavior and enhancement of probiotic viability in bio-yoghurt. Int. Dairy J. 2012, 22, 44–47. [Google Scholar] [CrossRef]
- Homayouni, A.; Ansari, F.; Azizi, A.; Pourjafar, H.; Madadi, M. Cheese as a Potential Food Carrier to Deliver Probiotic Microorganisms into the Human Gut: A Review. Curr. Nutr. Food Sci. 2020, 16, 15–28. [Google Scholar] [CrossRef]
- Boylston, T.D.; Vinderola, C.G.; Ghoddusi, H.; Reinheimer, J.A. Incorporation of bifidobacteria into cheeses: Challenges and rewards. Int. Dairy J. 2004, 14, 375–387. [Google Scholar] [CrossRef]
- Supino, C.; Pacheco-Soares, C.; da Silva, N.S. Effects of photobiomodulation on the growth of intestinal bacteria. Res. Soc. Dev. 2021, 10, e56810817103. [Google Scholar] [CrossRef]
- Hassan, H.M.M.; Elshaghabee, F.M.F. Influence of cold storage on antimicrobial, antioxidant and proteolytic activities of three different probiotic fermented milks. Adv. Food Sci. 2016, 38, 82–89. [Google Scholar]
- El-Sayed, E.; Ali, A.; Elshaghabee, F. Influence of Addition of Egyptian Grape Seed Extract on the Antioxidant, Antibacterial Activities and Shelf Life of Traditional Labneh. Asian J. Dairy Food Res. 2019, 38, 295–300. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Isono, H.; Miyata, T. Potential antioxidant bioactive peptides from camel milk proteins. Anim. Nutr. 2018, 4, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T. Profiles of Volatile Flavor Compounds in Milk Fermented with Different Proportional Combinations of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Molecules 2017, 22, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, S.; Samer, M.; Mohamed, M.S.M.; Abdelsalam, E.; Mohamed, Y.M.A.; Abdel-Hafez, S.H.; Attia, Y.A. Implementation of graphitic carbon nitride nanomaterials and laser irradiation for increasing bioethanol production from potato processing wastes. Environ. Sci. Pollut. Res. 2022, in press. [Google Scholar] [CrossRef]
- Pantoja, J.; Reinemann, D.; Ruegg, P. Factors associated with coliform count in unpasteurized bulk milk. J. Dairy Sci. 2011, 94, 2680–2691. [Google Scholar] [CrossRef]
- Faille, C.; Bénézech, T.; Midelet-Bourdin, G.; Lequette, Y.; Clarisse, M.; Ronse, G.; Ronse, A.; Slomianny, C. Sporulation of Bacillus spp. within biofilms: A potential source of contamination in food processing environments. Food Microbiol. 2014, 40, 64–74. [Google Scholar] [CrossRef]
- Setlow, P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007, 15, 172–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).