Abstract
Fusicocca-2,10(14)-diene (FCdiene) is a tricyclic diterpene which has many pharmaceutical applications, for example, it is a precursor for different anticancer drugs, including fusicoccin A. Chemical synthesis of this diterpene is not economical as it requires 14 steps with several stereospecific reactions. FCdiene is naturally produced at low titers in phytopathogenic filamentous fungi. However, production of FCdiene can be achieved via expression of fusicoccadiene synthase in yeast. The objective of this study is to increase FCdiene production by optimizing the yeast fermentation process. Our preliminary fermentations showed influences of carbon sources, buffer agents, and oxygen supply on FCdiene production. Buffer agents as well as oxygen supply were investigated in detail at 0.2 and 1.8 L cultivation volumes. Using glucose as the carbon source, FCdiene concentrations were increased to 240 mgFCdiene/L by optimizing pH and oxygen conditions. In situ extraction and adsorption techniques were examined at the 0.2 L scale to determine if these techniques could improve FCdiene yields. Different adsorbents and solvents were tested with in situ product recovery and 4-fold increases in FCdiene productivity could be shown. The results generated in this work provide a proof-of-concept for the fermentative production of FCdiene from S. cerevisiae as a practical alternative to chemical synthesis.
1. Introduction
Terpenes are a large group of structurally and functionally diverse hydrocarbons derived from the 5-carbon containing building blocks isopentenyl pyrophosphate and its isomer dimethylallyl pyrophosphate [1,2]. Fusicocca-2,10(14)-diene (FCdiene) belongs to the family of diterpenes and has a tricyclic chemical character. Its chemical structure is 5-8-5 and, therefore, belongs to the group of fusicoccanes, and it is the precursor of several fusicoccane diterpenoids [3].
Fusicoccane based diterpenoids have come into research focus as interesting compounds because of their apoptotic and anticancer effects on mammalian cells. Three examples of FCdiene derived fusicoccanes are the diterpenoid glucosides fusicoccin A, cotylenin A, and brassicicene C [4,5]. Fusicoccin A was isolated from Fusicoccum amygdali, brassicicene C was found in Alternaria brassicicola. Both fungi express an identical bifunctional fusicocca-2,10(14)-diene synthase which is capable of the production of FCdiene [6,7]. The titer of the naturally produced terpenes from fungal hosts is too low for industrial biosynthesis. Chemical synthesis is possible at lab scale, but is too complex to be economical at larger scales [5,8]. Synthesis of FCdiene via heterologous expression of FCdiene synthase in S. cerevisiae may be a promising alternative [4]. Heterologous terpenoid production in S. cerevisiae is possible by converting the native C20 carbon backbone geranylgeranyl pyrophosphate produced by the mevalonate pathway from acetyl-CoA into heterologous diterpenoids by expression of diterpene synthases like the FCdiene synthase. Fermentative production of heterologous terpenes using yeast cells has been thoroughly investigated in recent years, sparked by production of the sesquiterpenoid artemisinic acid [9,10,11,12]. Although numerous examples of diterpenoids have been produced via heterologous expression from yeasts, only one other diterpenoid has been reported to be produced in literature; it was produced at a larger scale and named taxadiene, the precursor of taxol. Taxadiene was previously produced at a 5 L scale in S. cerevisiae where 72.8 mgTaxadiene/L were detected [13]
The S. cerevisiae strain employed for FCdiene production here is based on a strain that has been shown to produce higher levels of triterpenes [14] and was used to determine its capacity for the production of diterpenes as well. It has been previously shown that combining metabolic manipulations in an optimized strain with fermentation process optimizations can increase terpene product titers [11].
A previous report suggested that FCdiene could be readily produced from S. cerevisiae, however, fermentation parameters were not optimized [5,15]. Fermentation parameters such as pH, dissolved oxygen tension (DOT), ethanol, as well as in situ product removal were also shown to strongly influence the production of a triterpenoid, betulinic acid, from S. cerevisiae. Therefore, the influence of DOT and pH were investigated here at shake flaks and stirred tank reactor scales. In situ product removal was also investigated to determine if this could influence FCdiene production rates. Extraction and adsorption processes were investigated at the shake flask scale and several solvents as well as adsorbents were tested for FCdiene capture and release.
2. Materials and Methods
2.1. Fermentation Conditions
S. cerevisiae CEN.PK2-1c [pRS313-upc2.1, pRS315-thmgr, pVV214-abfs], kindly provided by Prof. Dr. Frank Schulz from Ruhr University of Bochum, Germany (further information in Appendix A.1), was used for the fermentations [4]. Cultivations were conducted at 30 °C. For all experiments, synthetic dropout (SD) medium was used (Appendix A.2). The medium had a pH of ~4.0.
2.2. Investigation of Effects of DOT and pH
For investigation of the effect of oxygen supply, the experiments have been divided into two groups. First, different flasks with different fill volumes were used to produce three different aeration rates. Erlenmeyer flasks (50 mL) with a total working volume of 50 mL were used for decreased aeration. For the standard conditions, 1 L Erlenmeyer flasks with 200 mL working volume were used. To increase the oxygen supply to see the effect on FCdiene production, 1 L baffled flasks were used with 200 mL working volume.
The 1 L flask cultivations were incubated in a GLF 3033 shaking incubator (GLF mbH, Burgwedel, Germany) at 120 rpm with a shaking amplitude of 2.5 cm. The 50 mL flasks were incubated as above in a separate JRC-1-T shaking incubator (Adolf Kühner AG, Basel, Switzerland). All shake flask experiments were performed with either glucose (D(+)-glucose monohydrate, Carl Roth GmbH + Co. KG, Karlsruhe, Germany) or ethanol (denatured, Carl Roth GmbH + Co. KG, Karlsruhe, Germany) as a carbon source. The selected concentrations were 0.14 M (25 g/L) glucose, and the equivalent carbon amount of 0.42 M (19.18 g/L) ethanol. Additionally, 0.86 M (39.47 g/L) and 1.71 M (78.93 g/L) ethanol were selected to determine if an influence on FCdiene production could be observed. These two concentrations were equal 0.29 M (51.42 g/L) and 0.57 M (102.85 g/L) glucose, respectively. The amino acid concentrations were doubled to avoid potential limitation during the fermentations.
In a second series of experiments, depending on the results of the first group of experiments, oxygen and carbon dioxide concentrations in the gas phase were measured for more precise results. Therefore, 1 L baffled and unbaffled Erlenmeyer flasks with on-flask gas sensors (BCP-CO2 and BCP-O2 BlueSens gas sensor GmbH, Herten, Germany) were used. Here, the shaking frequency was varied between 60 and 180 rpm in the shaking incubator JRC-1-T with shaking amplitude of 2.5 cm.
For investigation of the influence of pH, 1 L Erlenmeyer flasks with 200 mL working volume were employed. SD medium with doubled amino acid concentrations was used again to avoid potential growth limitation. The fermentation duration was 72 h with glucose at 0.14 M (25 g/L) or ethanol at 0.42 M (19.18 g/L). The pH was modulated by addition of succinate (40 mM) because it is considered a metabolic buffer with potential influence on FCdiene synthesis [5]. The addition of 2 M NaOH was used to adjust pH to 6.9 before fermentation. Additionally, a phosphate buffer (0.2 M, Appendix A.3) or CaCO3 (0.08 g, Fluka by Sigma Aldrich Chemie GmbH, Steinheim, Germany) was added after observed pH shifts with 2 M NaOH. Alternatively, the pH was controlled using CaCO3 for pH shift and buffering action. All buffering agents were added to the SD medium directly before inoculation
2.3. In situ Product Removal
The in situ product removal can be performed via extraction or adsorption processes [16]. In both cases, 300 mL Erlenmeyer flasks with a working volume of 50 mL were used. The initial glucose concentration was 0.14 M (25 g/L), the amino acid concentrations were doubled (Appendix A.4) as described above, and the pH was unregulated as this has been previously shown to positively impact FCdiene production.
For adsorption experiments, the flasks were incubated in a JRC-1-T shaking incubator at 120 rpm with shaking amplitude of 2.5 cm for 72 h. The applied adsorbents were Europrep 60–60 C18 ECO (Europrep, fractional silica gel), Lupolen 4261 A IM (Lupolen, fractional high density polyethylene), and Lewatit® VP OC 1064 MD PH (Lewatit®, spherical polystyrene resin) to allow for comparison with Arens et al. [5]. The adsorbent was added at the start of fermentation using three different masses (2 g, 4 g, and 6 g), packed into cellulose bags, for separation of the adsorbents from the culture. After fermentation, the cellulose bags were placed into 20 mL ethyl acetate and FCdiene was eluted for 12 h under stirring with a magnetic stir bar.
For extraction experiments, the medium was stirred with a magnetic stir bar at 700 rpm. The experiments lasted 96 h, and 50 mL of organic solvent was added after 24 h for in situ product removal. For this reason, both separation processes used the same amount of time. Nine organic solvents with octanol-water partition coefficients between 0.73 and 7.88 and boiling points between 69 and 385 °C were chosen for in situ extraction: ethyl acetate, methyl propionate, diisopropyl ether, butyl acetate, isoamyl acetate, toluene, dibutyl ether, n-heptane, and bis(2-ethylhexyl)phthalate.
2.4. Batch Fermentations
The 3.1 L fermenters (KLF 2000, Bioengineering AG, Wald, Switzerland) were used in order to continuously measure and regulate the pH as well as DOT. The fermenter was equipped with two six-bladed disk turbine impellers (pH measurements: outer diameter: 48 mm, agitation blade: 15 mm × 12 mm; DOT measurements: outer diameter 40 mm, agitation blade: 11 mm × 8 mm) and four baffles. The temperature was held at 30 °C. Airflow was controlled at 0.3 L/min using a mass-flow controller (MASS-VIEW®, Bronkhorst High-Tech B.V., Ruurlo, The Netherlands). The pH was measured in-line with a SteamLine pH electrode (SI Analytics GmbH, Weilheim, Germany) and was equipped with a peristaltic pump for automatic titrations. Concentrations of oxygen, carbon dioxide, and ethanol in the exhaust gas were measured online using three BlueSens gas sensors as above. Sterile filtered SD medium (1.8 L) was used [5].
For further investigation of the influence of DOT on FCdiene production, three different constant stirrer rates were used: 350 rpm, 600 rpm, and 850 rpm. The initial glucose concentration was 0.0278 M (5 g/L), and the amino acid concentrations were doubled for fermentation duration of 24 h. The pH was not regulated.
To examine the influence of pH on FCdiene production, it was adjusted to 6.7 by 2 M NaOH or left unregulated. The pH of 6.7 was chosen to avoid precipitation of medium components. The stirring rate was controlled between 150 and 550 rpm depending on the DOT in the medium, which was set to 10% and monitored with a dissolved oxygen (DO)-sensor (Bioengineering AG, Wald, Switzerland).
All fermentations described were inoculated using an initial cell dry weight (CDW) of 0.025 g/L. Preparations of pre-cultures were performed in 1 L Erlenmeyer flasks with an operating volume of 200 mL and an initial glucose concentration of 0.14 M (25 g/L) for 24 h.
2.5. FCdiene Quantification
For analyses of FCdiene, a gas-chromatographic system GC-2025 with a flame ionization detector (Shimadzu Corp., Kyoto, Japan) and a Zebron ZB-SemiVolatiles column (length 30 m, inner diameter 0.25 mm, film thickness 0.25 µm) (Phenomenex, Inc., Torrance, CA, USA) was used. The temperature program used was: 75 °C for 1 min, increasing to 270 °C at a heating rate of 40 °C/min, and holding at 270 °C for 2 min, followed by an increase to 300 °C at 40 °C/min with a final hold of 3 min. Nitrogen was used as a carrier gas. FCdiene standard was kindly provided by the group of Prof. F. Schulz (Ruhr University Bochum, Germany), and cycloundecane (Sigma Aldrich Chemie GmbH, Steinheim, Germany) was used as an internal standard as previously described [5]. The calibration curve reached an accuracy of 99.94 % and is given in Equation (1). The calibration was carried out in triplicate.
FCdiene samples were collected from culture broth using Europrep cartridges for adsorption (Europrep, Dr.-Ing. Herbert Knauer GmbH, Berlin, Germany; empty cartridges, Macherey-Nagel, Düren, Germany). C18 SPE cartridges showed higher FCdiene recoveries up to 160% compared to extractions using n-pentane or n-heptane as solvents. In addition, after the adsorption process, the aqueous phase was extracted to determine the amount of remaining FCdiene. However, no further FCdiene could be detected, indicating a complete separation of FCdiene. We employed adsorption instead of an extraction protocol due to the scalability of this technology in comparison to solvent extraction.
Prior to sample collection, cartridges were equilibrated using 5 mL aqua bidest., followed by 1 mL ethyl acetate and again 5 mL aqua bidest. with a flow rate of 1 mL/min. Cartridges were subsequently dried for 10 min under vacuum of approximately 0.98 bar. Then, 2 mL of the cell-free sample were adsorbed six times each through a cartridge at a flow rate of 1 mL/min. Cell free samples were used to prevent clogging of the adsorbent particles.
The cartridges were then washed with 2 mL aqua bidest. to wash the samples of bound hydrophilic compounds. FCdiene was eluted using 1 mL ethyl acetate at a flow rate of 1 mL/min. Then, 90 µL of this eluate was mixed with 10 µL of the internal standard cycloundecane (1 g/L) for gas-chromatographic system GC-2025 with a flame ionization detector (GC-FID) analysis. Specific productivity (P) was calculated based on the data obtained in the GC-FID analyses using the following equation:
is the produced FCdiene mass in the observed time interval t, and is the grown cell mass which catalyzes the FCdiene synthesis.
3. Results
3.1. The Influence of Culture pH on FCdiene Productivities
An important aspect in microbial fermentations is the pH, in particular because the acidic conditions can lead to isomerization of terpene products. It was previously determined that no isomerization occurred in FCdiene production when SD medium was used [5]. We found the FCdiene production was increased by using doubled amino acid concentrations and sterile filtered yeast nitrogen base in contrast to autoclaved base (Figure 1).
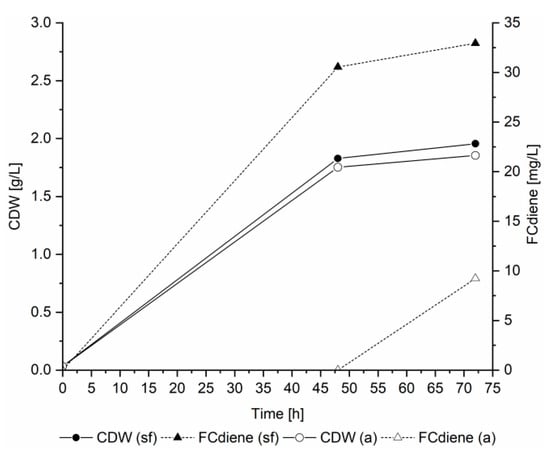
Figure 1.
Influence of sterile filtered (sf) and autoclaved (a) yeast nitrogen base on yeast growth and FCdiene production: Fermentations in 1 L unbaffled shake flasks (120 rpm, shaking amplitude: 2.5 cm) using glucose as a carbon source at 30 °C (single determination under these conditions).
For the investigation of the influence of pH on FCdiene production, in 1 L shake flasks with 200 mL working volume, cells were supplied with different buffer agents. First, the pH was shifted to 6.8 using 2 M NaOH before adding 0.2 M phosphate buffer or CaCO3 as buffering agents. pH shift was stopped at pH 6.8 because at higher pH, first media components were observed to precipitate. These fermentations resulted in FCdiene concentrations up to 46.41 ± 4.98 mgFCdiene/L (phosphate buffer) and 42.83 ± 8.53 mgFCdiene/L (CaCO3) with productivities of 0.21 ± 0.02 mgFCdiene/(gCDW h) and 0.16 ± 0.02 mgFCdiene/(gCDW h), respectively. These concentrations were 2X greater than the highest previously reported concentrations [5]. All results are summarized in Table 1. Using CaCO3 for pH shift and CaCO3 as a buffer increased the FCdiene concentration further up to 54.42 ± 6.73 mgFCdiene/L, however, the productivity remained at 0.16 ± 0.02 mgFCdiene/(gCDW h). An increase in productivity was reached using only a pH shift to 6.8 with 2 M NaOH. This resulted in a doubled productivity of 0.40 ± 0.10 mgFCdiene/(gCDW h). The FCdiene concentration increased more than two times to 97.40 ± 24.81 mgFCdiene/L. As a last buffer agent, 40 mM sodium succinate was used, but it must be noted that succinate can be used as a carbon source by several organisms [5,17,18]. Therefore, an experiment using succinate as a carbon source was conducted and resulted in 0.76 gCDW/L during 48 h with no FCdiene production, indicating sodium succinate did not act as an additional carbon source in S. cerevisiae.

Table 1.
Influence of the pH on the production of FCdiene in 1 L shaking flasks with 200 mL synthetic dropout (SD) medium. Values after 72 h of fermentation time and all experiments have been performed in triplicate. P stands for productivity.
Buffering with sodium succinate increased the productivity and FCdiene concentration further up to 0.83 ± 0.08 mgFCdiene/(gCDW h) and 212.45 ± 33.28 mgFCdiene/L. This was unexpected because all other buffer agents led to lower FCdiene concentrations.
In order to understand the influence of the pH on FCdiene production, another experiment without pH control was additionally conducted. After 72 h, 153.47 ± 7.20 mgFCdiene/L (0.92 ± 0.06 mgFCdiene/(gCDW h)) was reached. FCdiene concentrations up to 200 mgFCdiene/L could be reached after 96 h, an increase of 8.5X in comparison to previously reported yields [5].
In cultivations using ethanol, phosphate buffer had a negative influence on the production of FCdiene. Only 7.55 ± 0.95 mgFCdiene/L could be detected. Cultivations with CaCO3 as a buffer agent reached FCdiene concentrations of 30.93 ± 9.24 mgFCdiene/L for pH shift with CaCO3 and increased to 68.90 ± 2.10 mgFCdiene/L for pH shift using 2 M NaOH. In cultivations with ethanol, NaOH mediated pH adjustment from 3.7 to approximately 6.8, resulting in increased FCdiene concentration to its maximum with ethanol of 112.84 ± 7.98 mgFCdiene/L and a productivity of 0.57 ± 0.02 mgFCdiene/(gCDW h). Cultivations with ethanol and without pH regulation only reached a productivity of 0.32 ± 0.02 mgFCdiene/(gCDW h) and a FCdiene concentration of 60.31 ± 2.53 mgFCdiene/L.
In order to determine whether the specific buffer agents or the general state of higher pH was responsible for lower FCdiene production using glucose as the sole carbon source, two conditions were compared. For this comparison, fermentations were conducted in a KLF 2000 fermenter with 1.8 L working volume. Either pH was unregulated or a constant pH of 6.6 was maintained using regulated addition of 2 M NaOH (Figure 2). As shown in Figure 2, pH-unregulated fermentations, independent of buffer choice or use of NaOH, consistently led to higher FCdiene production than did regulated fermentations. In contrast to the findings of Arens et al. [5], FCdiene concentrations increased up to 90 mgFCdiene/L with a pH drop to pH 2.7. The FCdiene concentration was more than 4.5X higher in comparison to the fermentation regulated to pH 6.6. The results indicate that unregulated pH shifts promote higher FCdiene titers accumulating in the culture medium throughout fermentation.
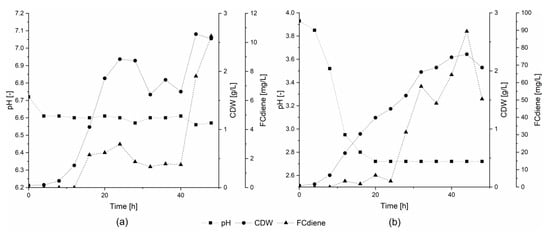
Figure 2.
Influence of pH on FCdiene production. Fermentations in a KLF2000 fermenter (working volume 1.8 L) using glucose as a carbon source at 30 °C and 10% dissolved oxygen tension (DOT). Aeration using sterile air. (a) with regulated pH at 6.6; (b) without pH regulation (single determination).
3.2. Investigation of Oxygen Supply Level
Optimization of DOT in the cultivation may have an effect on terpene productivity. In initial shake flask experiments under batch conditions, lower and higher oxygen supply was investigated by adjusting different culture volumes or flask types, but no significant differences were observed in FCdiene productivity in these cultivations. In cultivations with 0.57 M (102.85 g/L) glucose, low DOT cultivations accumulated 229.09 ± 16.67 mgFCdiene/L while high DOT cultivations reached 185.38 ± 22.47 mgFCdiene/L. However, when 0.29 M (51.42 g/L) glucose was used, up to 188.21 ± 16.00 mgFCdiene/L could be produced regardless of DOT, and up to 170.11 ± 12.22 mgFCdiene/L could be measured when cells were grown with 0.14 M (25 g/L) glucose.
Using ethanol as a carbon source, concentrations of FCdiene up to 144.38 ± 95.99 mgFCdiene/L could be reached with an ethanol concentration of 0.42 M (19.18 g/L). Using higher ethanol concentrations, lower yields of FCdiene were observed for all DOT conditions, potentially indicating a toxicity effect (data not shown).
For further investigation of DOT influence using glucose as a carbon source, a second series of experiments with off-gas analysis was conducted using 1 L unbaffled and baffled Erlenmeyer flasks. The flasks were incubated with a shaking amplitude of 2.5 cm at three different shaking frequencies: 60, 120, and 180 rpm. The highest concentrations of FCdiene were reached in both flask types at a shaking frequency of 180 rpm (Figure 3a). Without baffles, great variability was observed in culture productivities, however, for the baffled flasks at 180 rpm, more than two-times higher FCdiene concentrations (239.59 mgFCdiene/L) were observed compared to shaking frequencies of 60 rpm and 120 rpm (100.80 mgFCdiene/L). Figure 3b depicts the carbon dioxide concentrations in the head space of each flask. This is in contrast to previously reported results [5], where no significant influence on FCdiene production was observed for different oxygen input in shake flasks. The findings shown in this work indicate lower oxygen input decreases the FCdiene productivity.
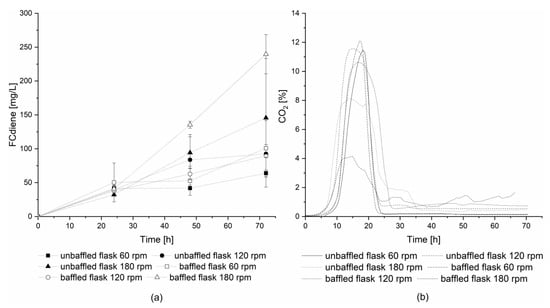
Figure 3.
Influence of different oxygen conditions on FCdiene production: Fermentations in 1 L shake flasks using 200 mL SD medium and glucose as a carbon source at 30 °C, (a) FCdiene concentration; (b) CO2 in gas phase.
Therefore, the effects of culture mixing and DOT were determined in three scale-up experiments in a 3.1 L KLF 2000 fermenter. The stirring speed was set to 350, 600, or 850 rpm and an airflow rate of 0.3 L/min was used for all three fermentations. In contrast to the findings of Arens et al. [5], the highest FCdiene concentration was reached in the fermentation with 600 rpm stirring speed, as depicted in Figure 4a. Figure 4b depicts the DOT over the fermentation time and the cell dry weight. In the reactor set to 600 rpm, micro-aerobic conditions were maintained throughout fermentation. For 350 rpm, the DOT reached 0% after 8 h, and at 850 rpm, the DOT did not fall below a level of 50%. Hence, micro-aerobic conditions seemed to favor FCdiene production. The cell dry weight increased as expected with increasing stirring speed. Thus, FCdiene production did not have a direct correlation with biomass formation.
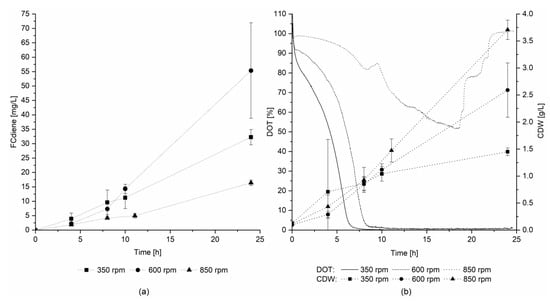
Figure 4.
Influence of different oxygen conditions on FCdiene production in a stirred tank reactor: Fermentations in the KLF2000 fermenter (working volume 1.8 L) using glucose as a carbon source at 30 °C and 0.116 vvm aeration with sterile air, (a) FCdiene concentration; (b) DOT in medium and cell dry weight.
3.3. In situ Product Removal
Europrep, Lewatit®, and Lupolen in cellulose bags were investigated for in situ FCdiene capture. Cellulose bags alone were tested as a control. Interestingly, cellulose bags inhibited the growth and showed nearly half the FCdiene productivity (0.50 mgFCdiene/(gCDW h)) in comparison to that of the shake flasks without adsorbent (0.90 mgFCdiene/(gCDW h)). Culture growth was slightly increased when Europrep or Lewatit® were used, however, Lupolen resulted in decreased growth using 4 g or more. FCdiene was eluted with 20 mL ethyl acetate and measured in the eluent as well as in the remaining fermentation medium. Detectible FCdiene production was lower with all three adsorbents than an adsorbent free control. The highest FCdiene concentrations using 2 to 6 g of Lupolen reached only 48.6 to 62.7% of the FCdiene concentration of the glucose reference. Lewatit® reached a range of 20.1 to 24.4% and Europrep a range of 19.7 to 29.6% of the FCdiene concentration. Europrep was found to be the best adsorbent, with up to 80.5 to 92.8% of the synthesized FCdiene. However, the synthesized FCdiene titer was significantly lower than that without adsorbens (up to 43 mgFCdiene/L compared to 153 mgFCdiene/L). Lupolen and Lewatit® adsorbed 49.4 and 75.5% of the produced FCdiene, respectively, which is in the range of Europrep. Lupolen and Europrep indicated higher deviations within three identical experiments than did Lewatit®, likely due to effects on the fermentation process.
Stirred fermentations in 300 mL shake flasks show FCdiene concentrations up to 80 mgFCdiene/L lower than those incubated in a shaking incubator. This has to be taken into account in the examination of the results of the in situ extractions.
Two solvents comparing the productivities of the in situ extraction experiments showed higher values than those of the stirred flask fermentation without any solvent. Ethyl acetate showed an up to 4X higher yield compared to that of the stirred flask fermentation without any solvent. The highest value of 0.96 ± 0.21 mgFCdiene/(gCDW h) is followed by bis(2-ethylhexyl)phthalate (0.44 ± 0.09 mgFCdiene/(gCDW h)).
Culture growth under influence of organic solvents was also investigated. Yeast treated with bis(2-ethylhexyl)phthalate (3.68 gCDW/L) exhibited higher growth than that shown in fermentations without solvents (2.76 gCDW/L). For the other solvents, the yeast stopped growing when the other solvents were added. The CDW concentration reached a value of 1.33 gCDW/L for ethyl acetate.
4. Discussion
In this study, two optimization parameters of the fermentative production of FCdiene from S. cerevisiae and its in situ capture were investigated.
The use of sterile filtered instead of autoclaved yeast nitrogen base increased the FCdiene titers. In addition, double of the amino acid concentration was used to prevent growth limitations in extended fermentation durations (48 to 96 h) and resulted in increased FCdiene titers. The FCdiene quantification technique used here showed higher FCdiene recovery than previously reported using n-pentane extraction [5].
The results of this study indicate that unregulated pH in shake flasks while using glucose as the sole carbon source increased FCdiene concentrations up to 153.47 ± 7.20 mgFCdiene/L, equal to 358% in comparison to that of the buffered fermentations, and increased 767% in contrast to the highest previously reported findings [5]. By extending fermentation time, FCdiene concentrations up to 200 mgFCdiene/L were reached: 10X higher concentrations than previously reported [5]. Succinate addition increased the FCdiene production by ~38% compared to the pH unregulated fermentation. It has been previously reported that succinate increases the respiration rate of S. cerevisiae [19], which may explain the results observed here as it is unlikely that pH buffering increased productivity. In support of this, pH unregulated cultivations using 1.8 L working volume showed up to 90 mg FCdiene/L, 9X higher than that of pH regulated fermentations, and the highest FCdiene concentrations yet reported in a stirred tank reactor. This indicates that higher FCdiene concentrations are achieved when pH is unregulated regardless of any buffering system.
Additionally, ethanol was tested as a carbon source under similar conditions because it potentially supports the FCdiene production as reported for the production of betulinic acid [15]. Due to the Crabtree effect, ethanol is produced from glucose by S. cerevisiae and consequently consumed after glucose depletion. FCdiene is also formed during this phase of fermentation, and the influence of ethanol metabolism on FCdiene production was investigated. In comparison to glucose as a carbon source in shake flask cultivations, slightly different results were obtained when cells were fed with ethanol. Adjusting the medium pH from 3.8 to 6.9 before inoculation increased the FCdiene production up to 113 mgFCdiene/L and the productivity to 0.57 mgFCdiene/(gCDW h). However, pH regulation did not result in higher FCdiene concentrations (max. 69 mgFCdiene/L). Therefore, independent of the carbon source, an unregulated pH drop during cultivation seems to enhance the extracellular FCdiene from S. cerevisiae. It is unclear whether this is due to increased activity of the terpene precursor pathways, active secretion of FCdiene, or something else.
Dissolved oxygen supply was examined in shake flasks (50 mL to 200 mL working volume) and 1.8 L fermenter scales while glucose and ethanol as carbon sources were tested. It was observed that ethanol concentrations of 0.86 M and 1.71 M inhibit the production of FCdiene, likely due to toxicity effects for S. cerevisiae. However, using an ethanol concentration of 0.42 M, FCdiene concentrations up to 129.95 mgFCdiene/L were reached. A direct influence of the DOT on the FCdiene production was not observed when ethanol was used as a carbon source.
The higher shaking frequencies with glucose as a carbon source lead to higher FCdiene concentrations, likely the result of increased culture mixing and nutrient availability. This is the opposite of previously reported findings [5]. Therefore, the influence of DOT on glucose cultivations was also determined in stirred tank reactors at different stirring rates with continuous recording of DOT. The highest FCdiene concentration was not found at the highest stirring rate but at 600 rpm with micro-aerobic conditions slightly above 0% DOT. Micro-aerobic conditions with restricted oxygen supply seemed to be the preferred condition for FCdiene production from S. cerevisiae, similar to results previously obtained for squalene synthesis as well [20]. High productivity levels were observed for shake flasks cultivations, which may have maintained micro-aerobic conditions (Figure 3). It was observed that too high oxygen supply has a negative effect on FCdiene production, in line with previous reports [5]. However, the results of this work show new findings that indicate that regulating DOT to micro-aerobic levels in fermentations may be advantageous for higher FCdiene productivities. The results could be improved up to nine fold in the stirred tank reactor.
Three adsorbents were tested for in situ adsorption. It was found that Europrep adsorbed most FCdiene out of the fermentation broth, however, the spherical adsorbent Lewatit® exhibited higher reproducibility. The cell growth was rarely affected at higher concentrations of Lupolen. For further experiments, a spherical adsorbent with similar properties to Europrep would be of interest. Performing an in-line separation of FCdiene by leading a cell free fermentation medium through a fixed bed adsorber may be a possible process alternative concerning the problem of free particles in a stirred system.
Terpene product removal using in situ extraction is commonly used in metabolic engineering studies of numerous microorganisms [21,22,23]. Here, we determined that higher FCdiene concentrations can be achieved using a solvent phase. Each solvent had different effects on the culture productivity; however, most caused growth cessation when the solvent was applied. The only exception was bis(2-ethylhexyl)phthalate, which also exhibited 2X higher FCdiene productivity compared to that of fermentations without a solvent phase. Ethyl acetate showed a 4X higher FCdiene productivity without further cell growth. These results are promising as they indicate that even higher yields of FCdiene may be possible from yeast cultures when solvent overlays are optimized, however, downstream purification of FCdiene from the solvent has to be taken into account. With ex situ n-pentane extractions, purities up to 80% could be reached, as determined by H-NMR.
5. Conclusions
Concentrations presented in this work represent significant improvements over previous studies. It was shown that the FCdiene titers could be increased up to 10-fold in shaking flask and up to 3-fold in batch fermentations with 1.8 L working volume compared to previous reports [5]. Indeed, possibilities for further process optimization, for example in FCdiene purification, exist as well. The unregulated pH drop characteristic of SD medium would appear to present the mutualistic benefit of favorable terpene product yields from S. cerevisiae and a reducing potential for contaminations in larger scale cultivations. The insights elucidated in this work may assist the development of scalable and more reliable production of FCdiene.
Author Contributions
Conceptualization, L.H.; Validation, L.H.; Formal Analysis, L.H.; Data Curation, L.H.; Writing-Original Draft Preparation, L.H.; Writing-Review and Editing, R.W.; Visualization, L.H.; Supervision, R.W.; Project Administration, R.W.
Funding
This research received no external funding.
Acknowledgments
The authors thank the collaboration partners Frank Schulz and Julia Arens from Ruhr University of Bochum, Germany for providing the S. cerevisiae strain, Kyle J. Lauersen and Eva Maria del Amor Villa for critical manuscript reading and English editing, and BlueSens gas sensor GmbH, Germany for providing their gas sensors. The authors would also like to thank Lara Feliczak, Lukas Greiwe, Alica Kleinschmidt, Sven Kockelke, Klaas Nolte, Nicole Piechulik, Jascha Rolf, Hanna Schnittger, and Hannah Weber for their assistance with several experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. FCdiene Producing Saccharomyces Cerevisiae
S. cerevisiae CEN.PK2-1c [pRS313-upc2.1, pRS315-thmgr, pVV214-abfs] is engineered with a truncated HMG-CoA reductase (tHMGR) for increased flux to terpenes by regulating the limiting step of the mevalonate pathway. It carries the UPC2.1 mutation to provide 100-fold increased geranylgeraniol concentrations and the abfs gene for the FCdiene synthase.
Appendix A.2. Synthetic Dropout Medium
The medium consisted of 6.7 g/L yeast nitrogen base without amino acids; 50 mg/L L-arginine HCl; 80 mg/L L-aspartic acid; 50 mg/L L-isoleucine; 50 mg/L L-lysine HCl; 20 mg/L L-methionine; 50 mg/L L-phenylalanine; 100 mg/L L-threonine (all obtained from Carl Roth GmbH + Co. KG, Karlsruhe, Germany); 50 mg/L L-tyrosine (Merck KGaA, Darmstadt, Germany); 140 mg/L L-valine; 20 mg/L L-tryptophan (Carl Roth GmbH + Co. KG, Karlsruhe, Germany); 95 mg/L MgCl2 (Merck KGaA, Darmstadt, Germany), and 10 mg/L adenine (Fluka by Sigma Aldrich Chemie GmbH, Steinheim, Germany). All components were sterile filtered.
Appendix A.3. Phosphate Buffer
Concentration: 0.2 M; Containing 0.09 M (1.08 g) NaH2PO4 (Sigma Aldrich, Steinheim, Germany) and 0.11 M (1.56 g) Na2HPO4 (Na2HPO4 12 H2O, Merck KGaA, Darmstadt, Germany).
Appendix A.4. Synthetic Dropout Medium with Doubled Amino Acid Concentrations
The meduium consisted of 6.7 g/L yeast nitrogen base without amino acids; 100 mg/L L-arginine HCl; 160 mg/L L-aspartic acid; 100 mg/L L-isoleucine; 100 mg/L L-lysine HCl; 40 mg/L L-methionine; 100 mg/L L-phenylalanine; 200 mg/L L-threonine (all obtained from Carl Roth GmbH + Co. KG, Karlsruhe, Germany); 100 mg/L L-tyrosine (Merck KGaA, Darmstadt, Germany); 280 mg/L L-valine; 40 mg/L L-tryptophan (Carl Roth GmbH + Co. KG, Karlsruhe, Germany); 95 mg/L MgCl2 (Merck KGaA, Darmstadt, Germany), and 10 mg/L adenine (Fluka by Sigma Aldrich Chemie GmbH, Steinheim, Germany). All components were sterile filtered.
References
- Breitmaier, E. Terpene: Aromen, Düfte, Pharmaka, Pheromone, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2005; pp. 2–3. ISBN 978-3-527-31498-0. [Google Scholar]
- Ruzicka, L. The Isoprene Rule and the Biogenesis of Terpenic Compounds. Experientia 1953, 9, 357–396. [Google Scholar] [CrossRef] [PubMed]
- Muromtsev, G.; Voblikova, V.; Kobrina, N.; Koreneva, V.; Krasnopolskaya, L.; Sadovskaya, V. Occurrence of Fusicoccanes in Plants and Fungi. J. Plant Growth Regul. 1994, 13, 39–49. [Google Scholar] [CrossRef]
- Arens, J.; Engels, B.; Klopries, S.; Jennewein, S.; Ottmann, C.; Schulz, F. Exploration of Biosynthetic Access to the Shared Precursor of the Fusicoccane Diterpenoid Family. Chem. Commun. 2013, 49, 4337–4339. [Google Scholar] [CrossRef] [PubMed]
- Arens, J.; Bergs, D.; Mewes, M.; Merz, J.; Schembecker, G.; Schulz, F. Heterologous Fermentation of a Diterpene from Alternaria brassisicola. Mycology 2014, 5, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, T.; Tsukahara, M.; Kaneko, A.; Niida, R.; Mitsuhashi, W.; Dairi, T.; Kato, N.; Sassa, T. Fusicoccins are Biosynthesized by an Unusual Chimera Diterpen Synthase in Fungi. Proc. Natl. Acad. Sci. USA 2007, 104, 3084–3088. [Google Scholar] [CrossRef] [PubMed]
- Minami, A.; Tajima, N.; Higuchi, Y.; Toyomasu, T.; Sassa, T.; Kato, N.; Dairi, T. Identification and Functional Analysis of Brassicicene C Biosynthetic Gene Cluster in Alternaria brassicicola. Bioorg. Med. Chem. Lett. 2009, 19, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Zhang, C.; Matsui, T.; Iwabuchi, H.; Mori, A.; Ballio, A.; Sassa, T. Isolation of (+)-fusicocca-2,10(14)-diene, a 5-8-5 Tricyclic Diterpene Hydrocarbon Biosynthetically Related to the Fusicoccin Aglycon from Fusicoccum amygdali and Confirmation of its Structure by Total Synthesis. J. Chem. Soc. Perkin. Trans. 1 1998, 2473–2474. [Google Scholar] [CrossRef]
- Paddon, C.; Westfall, P.; Pitera, D.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.; Tai, A.; Main, A.; Eng, D.; et al. High-level Semi-synthetic Production of the Potent Antimalarial Artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Ro, D.; Paradise, E.; Ouellet, M.; Fisher, K.; Newman, K.; Ndungu, J.; Ho, K.; Eachus, R.; Ham, T.; Kirby, J.; et al. Production of the Antimalarial Drug Precursor Artemisinic acid in Engineered Yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Westfall, P.; Pitera, D.; Lenihan, J.; Eng, D.; Woolard, F.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.; Owens, A.; et al. Production of Amorphadiene in Yeast, and its Conversion to Dihydroartemisinic acid, Precursor to the Antimalarial Agent Artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, N.; Kroukamp, H.; Pretorius, I.S. The Smell of Synthetic Biology: Engineering Strategies for Aroma Compound Production in Yeast. Fermentation 2018, 4, 54. [Google Scholar] [CrossRef]
- Ding, M.; Yan, H.; Li, l.; Zhai, F.; Shang, L.; Yin, Z.; Yuan, Y. Biosynthesis of Taxadiene in Saccharomyces cerevisiae: Selection of Geranylgeranyl Diphosphate Synthase Directed by a Computer-Aided Docking Strategy. PLoS ONE 2014, 9, e109348. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.A.; Hampton, R.Y.; Fritz, I.B. Effects of Overproduction of the Catalytic Domain of 3-Hydroxy-3-methylglutaryl Coenzym A Reductase on Squalene Synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1997, 63, 3341–3344. [Google Scholar] [PubMed]
- Czarnotta, E.; Dianat, M.; Korf, M.; Granica, F.; Merz, J.; Maury, J.; Jacobsen, S.A.B.; Frster, J.; Ebert, B.E.; Blank, L.M. Fermentation and Purification Strategies for the Productio of Betulinic Acid and its Lupane-type Precursor in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Woodley, J.M.; Lilly, M.D. In Situ Product Removal as a Tool for Bioprocessing. Nature 1993, 11, 1007–1012. [Google Scholar] [CrossRef]
- Han, S.; Inui, M.; Yukawa, H. Effect of Carbon Source Availability and Growth Phase on Expression of Corynebacterium glutamicum Genes Involved in the Tricarboxylic Acid Cycle and Glyoxylate Bypass. Microbiology 2008, 154, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kobayashi, K.; Ogasawara, N. The Bacillus subtilis YufLM Two-component System Regulates the Expression of the Malate Transporters MaeN (YufR) and YflS, and is Essential for Utilization of Malate in Minimal Medium. Microbiology 2003, 149, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Aliverdieva, D.A.; Mamaev, D.M.; Lagutina, L.S.; Bondarenko, D.I. Transport of Dicarboxylates in Saccharomyces cerevisiae. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Formatex, Spain, 2010; pp. 1611–1620. [Google Scholar]
- Mantzouridou, F.; Naziri, E.; Tsimidou, M.Z. Squalene versus Ergosterol Formation Using Saccharomyces cerevisiae: Combined Effect of Oxygen Supply, Inoculum Size, and Fermentation Time on Yield and Selectivity of the Bioprocess. J. Agric. Food Chem. 2009, 57, 6189–6198. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J.; Baier, T.; Wichmann, J.; Wördenweber, R.; Hübner, W.; Huser, T.; Kruse, O. Efficient Phototrophic Production of a High-value Sesquiterpenoid from the Eukaryotic Microalga Chlamydomonas reinhardtii. Metab. Eng. 2016, 38, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Marshall, J.; Chang, M.; Nowroozi, F.; Paradise, E.; Pitera, D.; Newman, K.L.; Keasling, J.D. High-Level Production of Amorpha-4,11-Diene in a Two-Phase Partitioning Bioreactor of Metabolically Engineered Escherichia coli. Biotechnol. Bioeng. 2006, 95, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Baier, T.; Wentnagel, E.; Lauersen, K.J.; Kruse, O. Tailored Carbon Partitioning for Phototrophic Production of (E)-α-bisabolene from the Green Microalga Chlamydomonas reinhardtii. Metab. Eng. 2018, 45, 211–222. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).