1. Introduction
In many countries nowadays, alcohol-free beer (AFB) is no longer just a niche product in the beer market. For brewers, this product category offers economic benefits in the form of a steadily growing market and often a lower tax burden. At the same time, consumer preference for low-alcohol and alcohol-free beer is increasing due to greater interest in health, concern about weight, and considering the encouragement of responsible drinking, especially when driving. Furthermore, consumers benefit from the health effects of alcohol-free beers, which lie in the healthy beer components (antioxidants, soluble fiber, vitamins, and minerals), lower energy intake and absence of negative aspects of alcohol consumption [
1].
The terminology of alcohol-free beer and the corresponding alcohol limits are not uniform. The classifications of alcohol-free beers are defined in the statutory regulations of the individual countries. In many European countries such as Germany, Switzerland, Austria, Finland, and Portugal, the term “alcohol-free” describes a maximum alcohol limit of 0.5% (
v/
v) ethanol. In Denmark and in the Netherlands the term “alcohol-free” may be applied to beers with <0.1% (
v/
v) [
2]. In the UK, the term “alcohol-free” can be applied to beer with <0.05% (
v/
v) alcohol and the term “de-alcoholised” when the alcohol content is <0.5% (
v/
v) [
3]. In the USA and China, the limit of <0.5% (
v/
v) is described by the term “non-alcoholic”. Other countries like Spain or France are more tolerant towards the term “alcohol-free” with limits of 1.0% and 1.2% (
v/
v), respectively [
2].
The strategies to produce alcohol-free beers can be divided into two main groups: physical and biological processes. The physical processes, divided into thermal and membrane-based methods, are based on the removal of alcohol from regular beer and require considerable investments into special equipment [
4]. In the case of thermal processes, the beer is heated to evaporate the ethanol, whereby also volatile aroma components are partly or completely evaporated. During membrane-based processes, ethanol (as well as aroma components) is removed mainly by its molecular size. Both cases can lead to less aromatic beers with reduced body and a significant acidity [
2]. The most widespread biological approaches are based on limited ethanol formation by the yeast during the beer fermentation. Limited fermentation is usually performed in traditional brewery equipment and hence does not require additional investment. However, the beers are often perceived as sweet because of the interruption of the fermentation; fermentable sugars are not or only partly metabolized by the yeast, and the aromatic secondary metabolites are formed only in small quantities or have not yet been generated due to the short fermentation time. In the field of limited fermentation different approaches are being pursued to improve the taste impression, which include the reduction of worty taste caused by strecker aldehydes [
5,
6], the use of immobilized yeasts [
7], and the use of alternative yeast strains or yeast mutants [
8]. The use of non-
Saccharomyces yeasts (other than
Saccharomycodes ludwigii) for the production of AFB has not been studied to a great extent, though changing the yeast is an easy adjustment for breweries to make. By using yeast strains which are unable to ferment the most abundant wort sugars maltose and maltotriose, a natural fermentation limit is set. It is unnecessary to stop the fermentation by cooling or yeast separation, since the fermentation will naturally come to a halt by the depletion of the fermentable sugars. However, the challenge is to discover non-
Saccharomyces yeasts, that are able to produce flavors that can mask the wort-like off-flavors created by residual wort sugars and aldehydes [
5,
6].
There are few published studies on the application of non-
Saccharomyces yeasts in the production of alcohol-free beer [
9]. Mostly known as spoilage yeasts for beer or other beverages, they can form a range of flavors which could potentially benefit the alcohol-free beer [
10,
11,
12]. In a recent patent application, Saerens and Swiegers [
13] used
Pichia kluyveri to produce a low-alcohol or alcohol-free beer with a flavor profile very close to a beer of at least 4% (
v/
v) alcohol. Another patent by Li et al. [
14] suggests the use of
Candida shehatae to produce an alcohol-free beer. Sohrabvandi et al. [
15] investigated the use of
Zygosaccharomyces rouxii in a successive application after
Saccharomyces cerevisiae in order to produce an alcohol-free beer. A significant alcohol reduction could be shown, however, the taste was compromised. De Francesco et al. [
3] investigated strains of
Z. rouxii and
Saccharomycodes ludwigii for the production of low-alcohol beers. In contrast to the results from Sohrabvandi et al. [
15],
Z. rouxii strains were found unsuitable to produce low alcohol beer due to the production of a high concentration of ethanol, however,
S. ludwigii was identified as a yeast species with great potential for the production of low-alcohol and alcohol-free beer.
In this study, five non-
Saccharomyces yeast strains isolated from kombucha, namely
Hanseniaspora valbyensis KBI 22.1,
Hanseniaspora vineae KBI 7.1,
Torulaspora delbrueckii KBI 22.2,
Zygosaccharomyces bailii KBI 25.2 and
Zygosaccharomyces kombuchaensis KBI 5.4, were investigated for their application in the production of an alcohol-free beer. Kombucha is effervescent, slightly sweet, and slightly acidic fermented tea, comprising a SCOBY, a Symbiotic Culture of Bacteria and Yeast, consisting of ethanol fermenting yeast and bacteria originating from the acetic acid bacteria family, which are enclosed in a thick cellulose containing pellicle [
16]. The yeasts were characterized, and fermentation performance and final beer quality were compared to two commercially applied yeast strains.
Saccharomyces cerevisiae WLP001 (California Ale Yeast
®) is a broadly applied, maltose-positive brewer’s yeast and
Saccharomycodes ludwigii TUM SL 17 is a maltose-negative yeast, which is commercially applied in AFB brewing [
17].
The objective of this study was to compare the selected non-Saccharomyces yeasts to the commercially applied AFB strain TUM SL 17 and to evaluate their general applicability in AFB brewing.
2. Materials and Methods
2.1. Materials
All reagents used in this study were at least analytical grade from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. Malt extract used for the flocculation test, hop resistance test, and propagation was supplied by Muntons (Spraymalt Light, Muntons plc, Suffolk, UK). Pilsner malt for wort production was sourced from Weyermann® (Malzfabrik Weyermann, Bamberg, Germany).
2.2. Yeast Strains
The yeast strains investigated in this study were isolated from kombucha. DNA of the isolates was extracted using an extraction kit (Yeast DNA Extraction Kit, Thermo Fisher Scientific, Waltham, MA, USA). To amplify the D1/D2 domain of the 26S rRNA gene the primers NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) were used. PCR was performed using the temperature protocol: 95 °C/2 min; 30 cycles of 95 °C/30 s, 56 °C/15 s; 72 °C/60 s; 72 °C/5 min.
Stocks were kept in glycerol at −80 °C.
Table 1 lists the yeast strains that were used in this study. Strains were grown on PDA agar plates for 72 h at 25 °C and stored in a sterile environment at 2–4 °C. During this study, strains were subcultured at intervals of two weeks. The strains were chosen from a collection of 64 isolated strains by their performance in a pre-screening in wort (data not shown).
2.3. Flocculation Test
Flocculation of the yeast strains was evaluated using a slightly modified Helm’s assay [
18,
19]. Essentially, all cells were washed in EDTA and the sedimentation period was extended to 10 min to allow slowly flocculating strains to show their potential. Fermentation wort was 75 g spray-dried malt extract (Spraymalt Light, Muntons plc, Suffolk, UK) in 1000 mL brewing water with 30 IBU (30 mg/mL iso-α-acids; from 30% stock solution; Barth-Haas Group, Nürnberg, Germany). Cultures recovered from fermentation were washed with 5 mM EDTA (pH 7) to break the cell aggregates. Flocculation was assayed by first washing the yeast pellets with 3.7 mM CaSO
4 solution and resuspending them in flocculation solution containing 3.7 mM CaSO
4, 6.8 g/L sodium acetate and 4.05 g/L acetic acid (pH 4.5). Yeast cells in control tubes were resuspended in 5 mM EDTA (pH 7) without undergoing the flocculation step with CaSO
4. After a sedimentation period of 10 min, samples were taken from just below the meniscus and dispersed in 5 mM EDTA. The absorbance at 600 nm was measured (Helios Gamma Spectrophotometer, Thermo Fisher Scientific, Waltham MA, USA), and percentage of flocculation was determined from the difference in absorbance between the control and flocculation tubes.
2.4. Sugar Utilization
Substrate utilization tests YT MicroPlate™ (Biolog Inc., Hayward, CA, USA) were used to analyze the biochemical spectrum of the yeast isolates. The yeast strains were cultured on Sabouraud agar for 72 h at 25 °C. Individual colonies were taken from the surface using sterile inoculation loops and suspended in 20 mL of sterile water. Colonies were gradually added to increase the turbidity until 46 ± 1%. From this yeast solution, 100 µL were added to each of the 96 wells of the YT MicroPlate™. After incubation at 25 °C for 72 h, the YT MicroPlate™ was read with the Microplate reader (Multiskan FC, Thermo Fischer Scientific) at a wavelength of 590 nm. Results are shown as “+” for a significant increase in optical density (OD) compared to the OD of the water control and a “−” for showing no difference. The substrate utilization test was carried out in duplicate.
2.5. Hop Resistance
Three 100 mL flasks containing sterile filtered wort (75 g Muntons Spraymalt Light in 1000 mL brewing water) were adjusted to 0, 50, and 100 mg/L iso-α-acids respectively by using an aliquot of a stock solution of 3% iso-α-acids in 96% (v/v) ethanol (Barth-Haas Group, Nürnberg, Germany). The pure grown yeast cells were added to a total cell count of 105 cells/mL. Optical density (OD600) was measured every 40 min at 25 °C without shaking over a time period of 96 h (Multiskan FC, Thermo Scientific, Waltham, MA, USA).
2.6. Phenolic Off-Flavor Test
The phenolic off-flavor (POF) test was conducted according to Meier-Dörnberg et al. [
20]. Yeast strains were spread on yeasts and mold agar plate (YM-agar) containing one of the following precursors: ferulic acid, cinnamic acid or coumaric acid. After three days of incubation at 25 °C, plates were evaluated by sniffing to detect any of the following aromas: ferulic acid becomes 4-vinylguaiacol (clove-like), cinnamic acid becomes 4-vinylstyrene (Styrofoam-like), and coumaric acid becomes 4-vinylphenol (medicinal-like). TUM 68 (Research Center Weihenstephan for Brewing and Food Quality, Freising-Weihenstephan, Germany) was used as a positive control.
2.7. Propagation
Propagation wort was prepared by dissolving 75 g spray-dried malt (Muntons Spraymalt light, Muntons plc, Suffolk, UK) and 30 g glucose (Gem Pack Foods Ltd., Dublin, Ireland) in 1000 mL brewing water, followed by sterilization (15 min, 121 °C). Investigated pure yeast strains were inoculated into a 140 mL of sterile propagation wort. The flask was covered with sterile cotton and placed in an incubator with orbital shaker (ES-80 shaker-incubator, Grant Instruments (Cambridge) Ltd., Shepreth, UK) and incubated for 48 h at an orbital agitation of 170 rpm and 25 °C. Viability was measured by staining with Löffler’s methylene blue solution (MEBAK 10.11.3.3) and cells were counted with a Hemocytometer (Blaubrand, Thoma pattern, Sigma-Aldrich, St. Louis, MO, USA).
2.8. Wort Production
Wort for fermentation trials was produced on a 60 L pilot-scale brewing plant comprising of a combined mash-boiling vessel, a lauter tun and a whirlpool tank. Weyermann® Pilsner Malt was milled with a two-roller mill fitted with a 0.8 mm gap size between the rollers. Seven kg of malt were mashed in with 40 L of brewing water. The following mashing regime was employed: 40 min at 50 °C, 20 min at 62 °C, 20 min at 72 °C and 5 min at 78 °C for mashing off. The heating rate was 1 °C/min between the temperature rests. The mash was pumped in the lauter tun and lautering was performed using three sparging steps of 5 L each. Collected wort was boiled for 45 min. 25 g Magnum hop pellets (10.5% iso-α-acids) were added at the start of the boil for a calculated IBU content of 10.4. Hot trub precipitates and hop residue were removed by means of the whirlpool with a rest of 20 min. Wort was pumped back to the boiling vessel, corrected to a specific gravity of 6.6 °P extract by the addition of brewing water, and heated to 100 °C before filling into sterile 5 L containers, which were kept for short-term storage at 2 °C.
2.9. Fermentation
Fermentation trials were carried out in 2-L sterile Duran glass bottles (Lennox Laboratory Supplies Ltd., Dublin, Ireland), equipped with an air lock to control CO
2 under sterile conditions. Bottles were filled with 1600 mL wort. Respective fermentation temperature was 25 °C, a temperature that suits most non-
Saccharomyces species [
21]. Fermentation was performed until no change in extract could be measured for 24 h. Yeast cells for pitching were washed by centrifugation at 5000
g for 5 min and resuspension in sterile water. Supernatant was discarded to ensure no carryover of sugars from the propagation wort into the fermentation wort and yeast cells were resuspended in sterile water. The pitching volume was 30 mL with a pitching rate of 8 × 10
6 CFU/mL at a viability of at least 96% for all fermentations.
2.10. Analyses of the Produced Beers
50 mL samples of each fermentation were withdrawn every day. Cell count was performed using the Hemocytometer (Blaubrand, Thoma pattern). Yeast was separated by centrifugation at 5000 g for 5 min and specific gravity and ethanol content of the supernatant were measured using a density meter DMA 4500 M with Alcolyzer Beer ME (Anton-Paar GmbH, Graz, Austria). The pH value was determined using a digital pH meter (Mettler Toledo LLC, Columbus, OH, USA).
Analyses of the final beers were performed by the following methods. Sugars and ethanol were determined by high performance liquid chromatography HPLC Agilent 1260 Infinity (Agilent Technologies, Santa Clara, CA, USA) equipped a refractive index detector (RID) and a Sugar-Pak I 10 µm, 6.5 mm × 300 mm column (Waters, Milford, MA, USA) with 0.1 mM Ca-EDTA as mobile phase and a flow rate of 0.2 mL/min. Differentiation of maltose and sucrose was achieved with a Nova-Pak 4 µm, 4.6 mm × 250 mm column (Waters) with acetonitrile/water 75:25 (v/v) as mobile phase and a flow rate of 1.2 mL/min.
Free vicinal diketones were quantified by a Clarus 500 gas chromatograph (Perkin-Elmer, Waltham, MA, USA) with a headspace unit and Elite-5 60 m × 0.25 mm, 0.5 µm column using a 2,3-hexanedione internal standard. The final concentrations of fermentation by-products (e.g., acetaldehyde, ethyl acetate, n-propanol, i-butanol, isoamyl acetate, amyl alcohols) were quantified using a gas chromatograph with a headspace unit and INNOWAX cross-linked polyethylene-glycol 60 m × 0.32 mm 0.5 μm column (Perkin-Elmer). The amino acid content was quantified using the HPLC MEBAK 2.6.4.1 method. Free amino nitrogen (FAN) was measured using a ninhydrin-based dying method where absorbance is measured at 570 nm against glycine (MEBAK 2.6.4.1). Free vicinal diketones, fermentation by-products and amino acids were quantified in duplicate.
2.11. Sensory Evaluation
All beer samples were tasted and judged by a sensory panel of 11 panelists with long-standing experience in the sensory analysis of beer. “Fruity”, “floral”, and “wort-like” were chosen as attributes for the smell. “Acidic/sour” and “sweet” were chosen as attributes for the taste and the panelists were additionally asked to evaluate the “body”. Panelists were asked to evaluate the attributes in its intensity on a scale from 0, nothing, to 10, extremely. Before the evaluation of the intensity, a descriptive sensory was performed where the panelists were asked to record the flavors they perceived from the samples. Samples were given in dark glasses with a three-digit code.
2.12. Statistical Analyses
Fermentations and analyses were carried out in triplicate, unless stated otherwise. The data was statistically analyzed using RStudio, Version 1.1.423 with R version 3.4.4 (RStudio Inc., Boston, MA, USA; R Core Team, r-project). For the analysis of sensory data and constructing the multidimensional sensory profile, the R package “SensoMineR” was used [
22]. One-way ANOVA was used to compare means and Tukey’s test with 95% confidence intervals was applied for the pairwise comparison of means. The statistical significance value for both ANOVA and multiple comparison analysis was set at
p = 0.05. Values are given as means ± standard deviation.
3. Results and Discussion
3.1. Yeast Characterization
When characterizing non-
Saccharomyces yeasts for their suitability in alcohol-free beer production, several key attributes should be investigated. The first attribute is the ability to utilize the sugars in the wort, as for all-malt beers the average composition of fermentable wort sugars is 12% glucose and fructose (0.8–2.8%), 5% sucrose, 65% maltose, and 17.5% maltotriose [
23]. For its suitability to produce alcohol-free beers it should not be able to ferment maltose. Considering the sugars that are important for brewing (glucose, fructose, sucrose, maltose, maltotriose), all strains were capable of fermenting glucose and fructose (
Table 2).
All investigated strains except KBI 22.1 and KBI 7.1 (
Hanseniaspora spp.) were able to ferment sucrose. The inability to ferment sucrose by KBI 22.1 and KBI 7.1 can be traced to the absence of the enzyme invertase, which converts sucrose into glucose and fructose [
24]. In kombucha (source of investigated yeasts), where sucrose is the main or only sugar source, the conversion of sucrose by yeast invertase is required for
Acetobacter spp. to subsequently produce acetic acid [
25]. Looking at the main sugars of wort, only the control strain WLP001 was able to ferment maltose and maltotriose. The disability to ferment maltose and maltotriose indicates the absence of a maltose transporter and the enzyme maltase [
26,
27]. The sugar fermentation patterns were confirmed by the sugar analysis of the final beers.
The second criterion for a yeast to be applied in brewing is its capability of growing in the presence of hop-derived iso-α-acids. The resistance against iso-a-acids and their induced weak organic acid stress were studied for the
Saccharomyces species but it has barely been investigated for non-
Saccharomyces species [
28,
29,
30]. All investigated strains were able to grow in wort with 0, 50 and 100 IBU (international bitterness units).
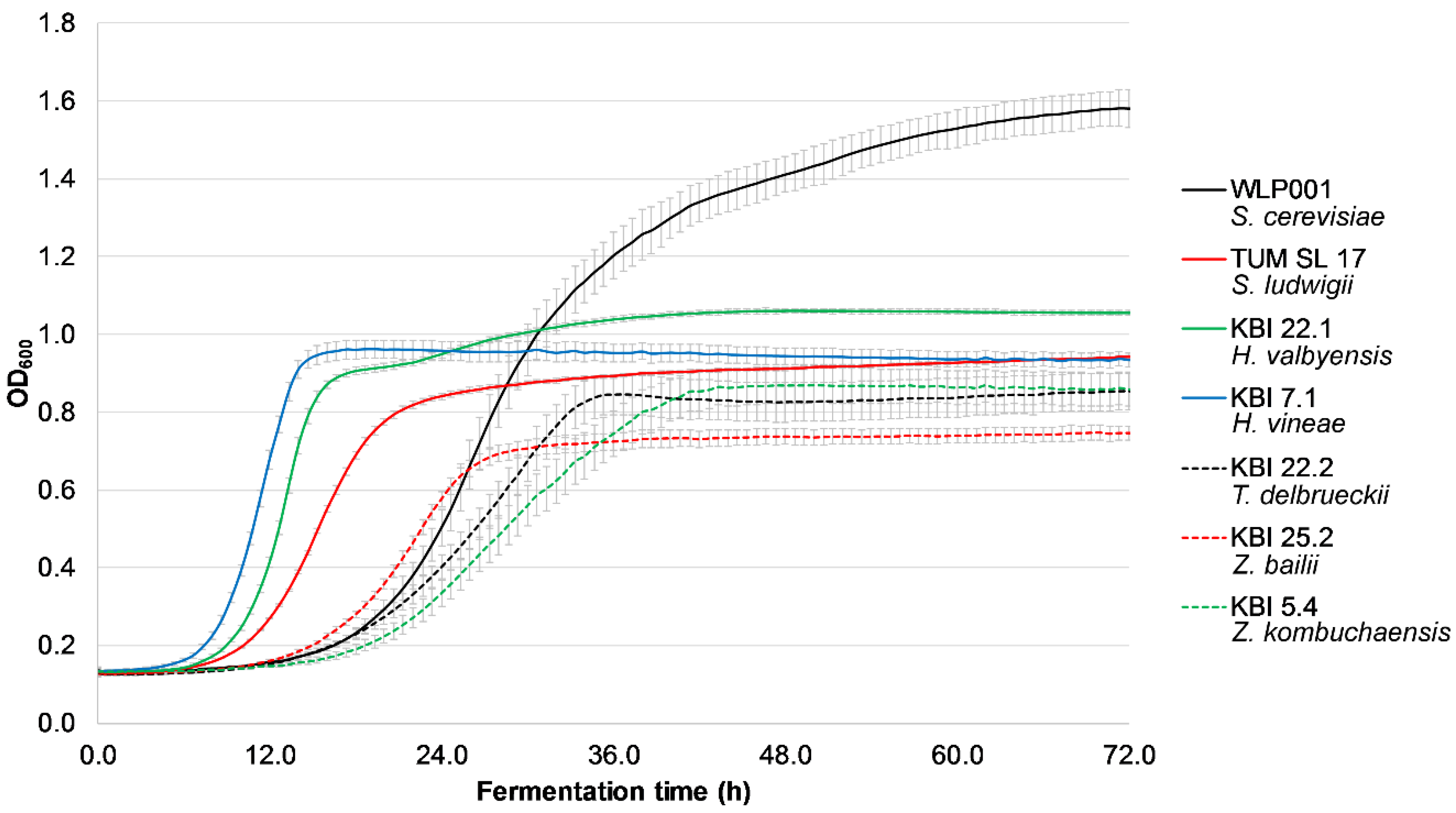
Figure 1 shows the exemplary growth of the investigated strains at 50 IBU (due to all strains exhibiting the same behavior at different IBU values, the rest of the data is not shown).
KBI 7.1, KBI 22.1 and TUM SL 17 had the shortest lag time with log phases starting between 8 and 13 h after inoculation, followed by the rest of the investigated strains with log phases between 19 and 23 h. However, all the investigated yeast strains were able to grow in high iso-α-acid concentrations and are therefore able to ferment even highly hopped worts. The presence of iso-α-acids did not have any influence on the growth of the investigated yeast strains. This is in contrast to a study by Michel et al. [
29], where the presence of 90 IBU resulted in a longer log phase as well as a lower slope during log phase compared with 50 and 0 IBU with several
Torulaspora delbrueckii strains.
None of the investigated yeast strains, except the positive control TUM 68, showed any positive POF behavior on plate when exposed to precursors, suggesting the absence of a functional
POF1 gene [
31] (
Table 2). Those results were consistent with the sensory of the final beers where no panelist detected any phenolic off-flavors. POF are produced by decarboxylation of ferulic acid, coumaric acid and cinnamic acid, which are present in beer wort. Ferulic acid becomes 4-vinylguaiacol, which is described as having a clove-like flavor [
32]. Apart from the wheat beer style this flavor is usually unwanted [
33]. Coumaric acid is decarboxylated to 4-vinylphenol, having a solvent-like flavor, and cinnamic acid becomes 4-vinylstyrene, which has a Styrofoam-like flavor [
34].
In terms of flocculation, a prerequisite for bulk sedimentation of yeast during brewery fermentation, the control yeast WLP001 performed as most flocculent of all the investigated strains. The method defines flocculation values of 85–100% as “very flocculent”, 20–80% as “moderately flocculent” and less than 20% as “non-flocculent” yeasts [
18]. By that definition WLP001 was with 83.3% at the very upper scale of moderately flocculent yeasts. KBI 22.1 and KBI 22.2 fell with 11.0% and 17.0%, respectively into the category of non-flocculent yeasts, while the rest qualified as moderately flocculent (
Table 2). The most common mechanism of yeast flocculation is generally accepted to be the lectin-mediated adhesion of adjacent yeast cells to form large cell aggregations [
35]. The flocculation characteristics of yeast are strongly strain-dependent and largely defined by which members of the
FLO genes, which encode for lectin proteins, are functional in each strain. Rossouw et al. [
36] showed that for 17 out of 18 investigated, non-
Saccharomyces strains the flocculation phenotypes were calcium-dependent, thus indicating a
FLO-dependency much like in
Saccharomyces cerevisiae.
3.2. Fermentation Performance
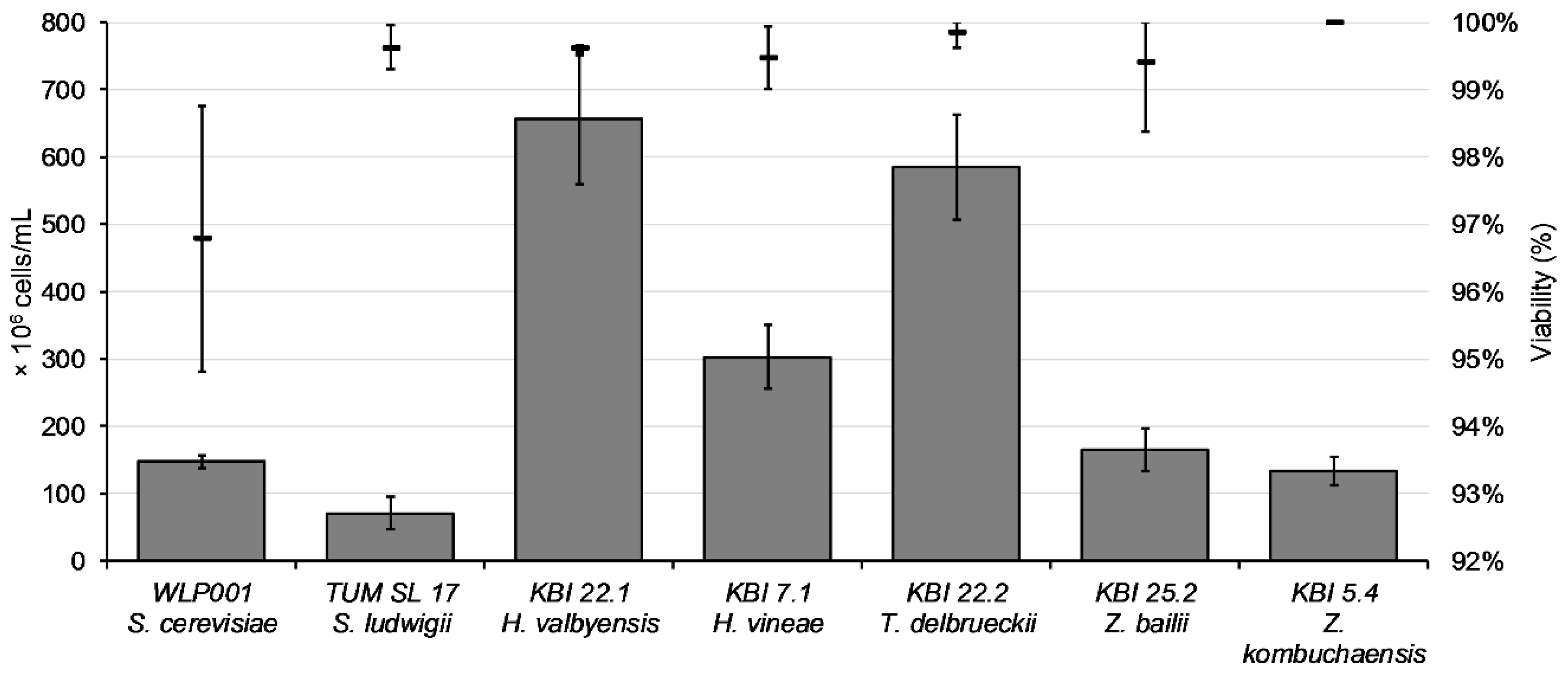
The aim of propagation is to get a high quantity of yeast cells with high viability and vitality. After propagation for 48 h, cell counts ranged from 7.1 × 10
7 cells/mL for TUM SL 17 to 6.5 × 10
8 cells/mL for KBI 22.1, as illustrated in
Figure 2.
Except for WLP001 with a viability value around 97%, viability values after propagation were over 99%. The composition of the wort used for the fermentation trials is shown in
Table 3.
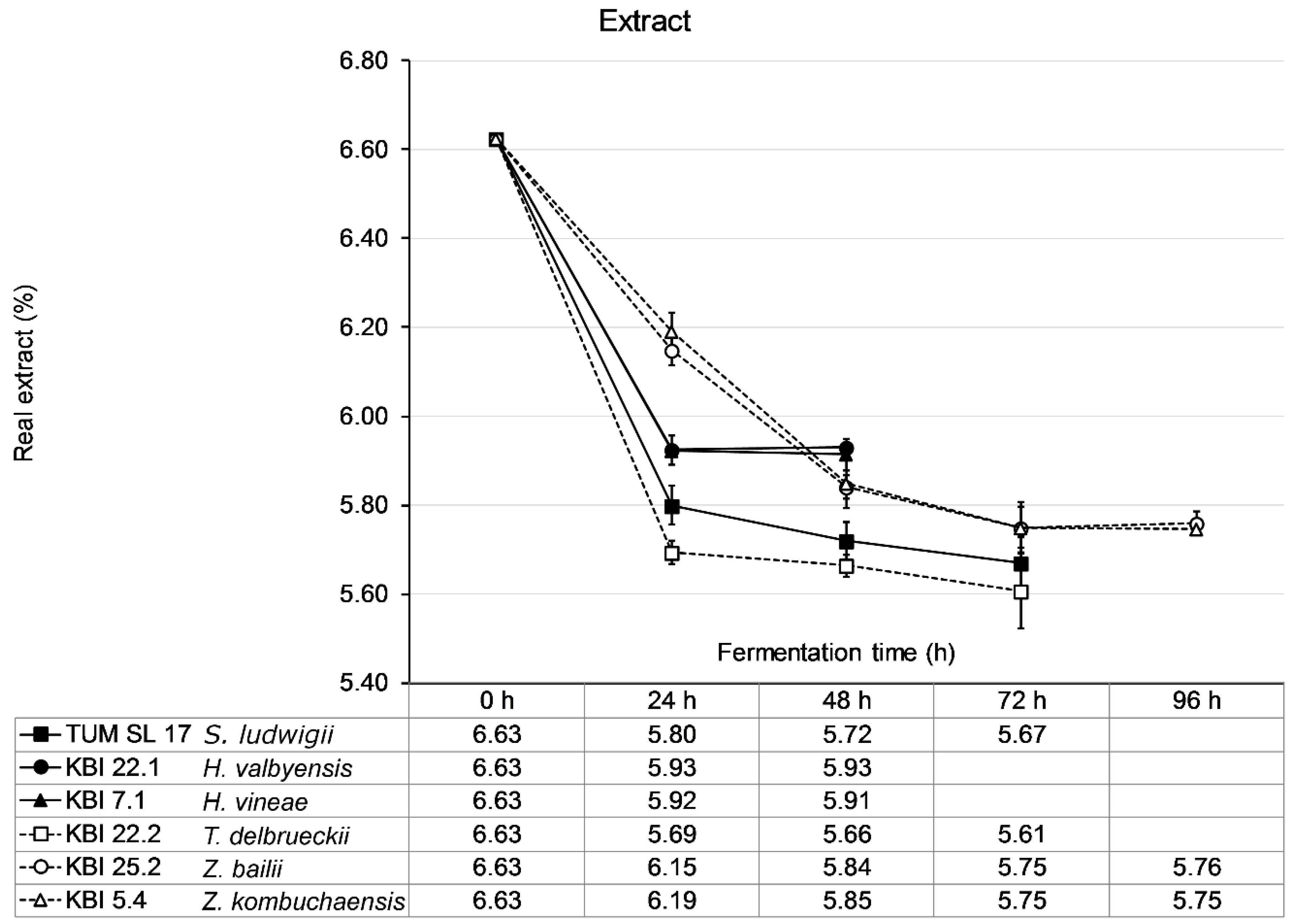
The wort was fermented until no change in extract was measurable for 24 h. KBI 22.2 showed the steepest decrease in extract with a drop in extract of nearly 1 °P extract in the first 24 h followed by TUM SL 17 (0.8 °P) and the
Hanseniaspora spp. KBI 22.1 and KBI 7.1 (0.7 °P) (
Figure 3). The
Zygosaccharomyces spp., KBI 25.2 and KBI 5.4 followed a lesser decrease in extract with a linear decrease of about 0.45 °P per 24 h for the first 48 h. Consequently, KBI 22.2 reached an ethanol concentration of 0.42% (
v/
v) after 24 h, while KBI 25.2 and KBI 5.4 produced only 0.21% (
v/
v) and 0.20% (
v/
v), respectively. Fermentation ceased fastest for KBI 22.1 and KBI 7.1 after 24 h when fructose and glucose were depleted while sucrose remained untouched. TUM SL 17 and KBI 22.2 reached their final extract after 48 h of fermentation. KBI 25.2 and KBI 5.4, demonstrating the slowest metabolism, ceased fermentation after 72 h. WLP001 fermented the wort to a final extract (real) of 2.13 °P after 96 h (data not shown).
The pH value dropped during the first 24 h of fermentation by values ranging from 0.7 for KBI 5.4 to 1.0 for KBI 22.2 with only marginal changes thereafter (data not shown). Due to the high starting pH of the wort of 5.7, the beers, except WLP001, did not reach pH values below 4.5, which are desired in order to serve as one of the microbial hurdles for beer spoiling bacteria to overcome [
37]. However, lower pH values can be reached with a lower starting pH of the wort, which can be adjusted; i.e., by lactic acid, the use of sour malt or biological acidification. Visual evaluation of the finished beers matched the analyzed flocculation behavior from
Table 2. KBI 22.1 and KBI 22.2 showed the highest turbidity and cells in suspension while TUM SL 17 and WLP001 were the clearest beers with a layer of flocculated yeast at the bottom.
Sugar analysis of the final beers revealed a complete depletion of all fermentable sugars by WLP001. Consistent with the sugar utilization patterns from
Table 2, the other investigated strains showed a complete depletion of monosaccharides. Sucrose was not fermented by the
Hanseniaspora spp. KBI 22.1 and KBI 7.1 but was depleted by the other strains as predicted before.
Analyses of the ethanol content of the final beers showed all investigated maltose-negative strains at or below 0.5% (
v/
v). WLP001, the maltose-positive control reached an ethanol content of 2.61% (
v/
v). The maltose-negative control, TUM SL 17, together with KBI 22.2 showed an ethanol content of 0.50% (
v/
v), followed by KBI 5.4 and KBI 25.2 with 0.48% (
v/
v) and 0.42% (
v/
v), respectively. The least ethanol content showed KBI 7.1 and KBI 22.1 with 0.34% (
v/
v) and 0.35% (
v/
v), respectively. The lower ethanol production by KBI 7.1 and KBI 22.1 was due to the inability to ferment sucrose, which reflected in a higher final gravity of the beers (
Table 4). Corresponding to a lower degree of fermentation, pH values for the alcohol-free beers are higher, ranging between 4.61 (KBI 5.4), and 4.84 (KBI 22.1).
3.3. Amino Acid Metabolism
The amino acid (AA) catabolism is very important for the formation of higher alcohols in the final beer. AA are important for the formation of higher alcohols such as propanol, isobutanol, and isoamyl alcohol via the Ehrlich pathway [
38]. The AA are transaminated to α-keto acids and decarboxylated to form the respective aldehyde, which are further reduced to higher alcohols [
10]. AA analysis revealed a substantial AA consumption only by WLP001 with a consumption of 76.4% of AA and depleting six AA namely aspartic and glutamic acid, asparagine, methionine, leucine, and isoleucine (
Table 5), owing to its longer fermentation time and higher sugar uptake. WLP001 also formed higher concentrations of higher alcohols (4 times higher) than the other strains, as seen in
Table 6. Adequate levels of amino acids and free amino nitrogen (FAN) in wort are necessary for a “healthy” fermentation [
39,
40,
41]. Only one depletion of methionine for KBI 22.1 revealed that, for the low-alcohol strains, every AA was available in the wort in sufficient amounts. The high amount of residual amino acids and FAN after fermentation indicated that the diluted wort (6.64 °P) used in this study held a sufficient amount of amino acids and free amino nitrogen for a healthy fermentation. Generally, AA consumption was strain dependent with TUM SL 17 and KBI 22.2 being on the higher end with 26.6% and 25.5% of consumption, respectively. KBI 5.4 consumed with 11.2% the lowest amount of AA (
Table 5). KBI 7.1 formed serine, which is shown at a significantly higher value after fermentation in
Table 5.
3.4. Volatile Compounds
Analysis of the volatile fraction of the beers fermented with the different yeasts showed mostly only small differences in higher alcohols, esters, and diacetyl (
Table 6). Regarding higher alcohols, n-propanol, isobutanol, and isoamyl alcohol contents were significantly higher for the maltose-positive control WLP001, owing to the extensive fermentation compared to the low-alcohol strains, which showed no significant differences amongst each other for n-propanol and isobutanol. Small, yet significant differences could be found for isoamyl alcohol values with KBI 22.1 exhibiting highest (16.5 mg/L) and KBI 22.2 exhibiting lowest (10.4 mg/L) values amongst the strains. The odor threshold for isoamyl alcohol, which is considered to have a fruity, brandy-like aroma, is reported to lay between 50–70 mg/L [
10]. All the investigated low-alcohol yeasts produced a fifth to a third of the odor threshold of isoamyl alcohols. In sum, the low-alcohol strains produced an average of 21 mg/L of higher alcohols compared to 82 mg/L by WLP001. Other major contributors to the aroma of beer are acetate esters [
11]. Volatile esters are the product of an enzyme-catalyzed condensation reaction between acyl-CoA—a product of the sugar and lipid metabolism—and a higher alcohol, originating from the nitrogen metabolism [
42,
43]. Ethyl acetate represents approximately one third of all esters in beers [
44]. Sum of acetate ester concentration was low in all the beers ranging from 0.77 mg/L for KBI 22.2 to 6.00 mg/L for KBI 7.1 (
Table 6).
Ethyl acetate production by KBI 7.1 was with 6.00 mg/L the highest of all investigated strains and outperformed even the maltose-positive control yeast WLP001 with 4.05 mg/L, which is described by the supplier to have a clean taste and has been reported to produce low concentrations of esters in previous studies [
45]. Threshold values for ethyl acetate in beer range from 21–30 mg/L which is usually higher than the amount found in alcohol-free beers [
11]. However, synergistic effects of different volatile aroma compounds could contribute to the overall flavor, as suggested by Sterckx et al. [
46]. The concentration of isoamyl acetate was below the detection level of 0.1 mg/L in all alcohol-free beers. The concentrations of ethyl formate (light estery, fruity, solvent) were with 1 mg/L and lower far below their individual threshold of 150 mg/L [
47]. The concentration of ethyl propionate, ethyl butyrate and ethyl caproate, did not reach higher than the LOD of 0.01 mg/L in either of the beers (data not shown). Diacetyl levels were strain dependent with KBI 22.1 and KBI 5.4 producing values above the flavor threshold in light beers of 0.1 mg/L with 0.21 mg/L and 0.15 mg/L, respectively, while diacetyl production of the other strains stayed below the threshold [
48]. Diacetyl is known for its undesired buttery flavor, which usually undergoes reduction during maturation of the beer [
49]. Acetaldehyde is the most important aldehyde of beer and is formed in the metabolic pathway leading from carbohydrate to ethanol. Its level varies during fermentation and aging and in beers, it usually lies in the range 2–20 mg/L, while its threshold lies between 10–25 mg/L [
44,
47]. Acetaldehyde concentrations were below the threshold for all beers produced (
Table 6). The overall flavor of beer depends on the relative contents of all the flavor-active compounds [
44]. The presence of different esters can have a synergistic effect on the individual flavors, which means that esters can also have a positive effect on beer flavor, even at amounts below their individual threshold concentrations [
50].
3.5. Sensory
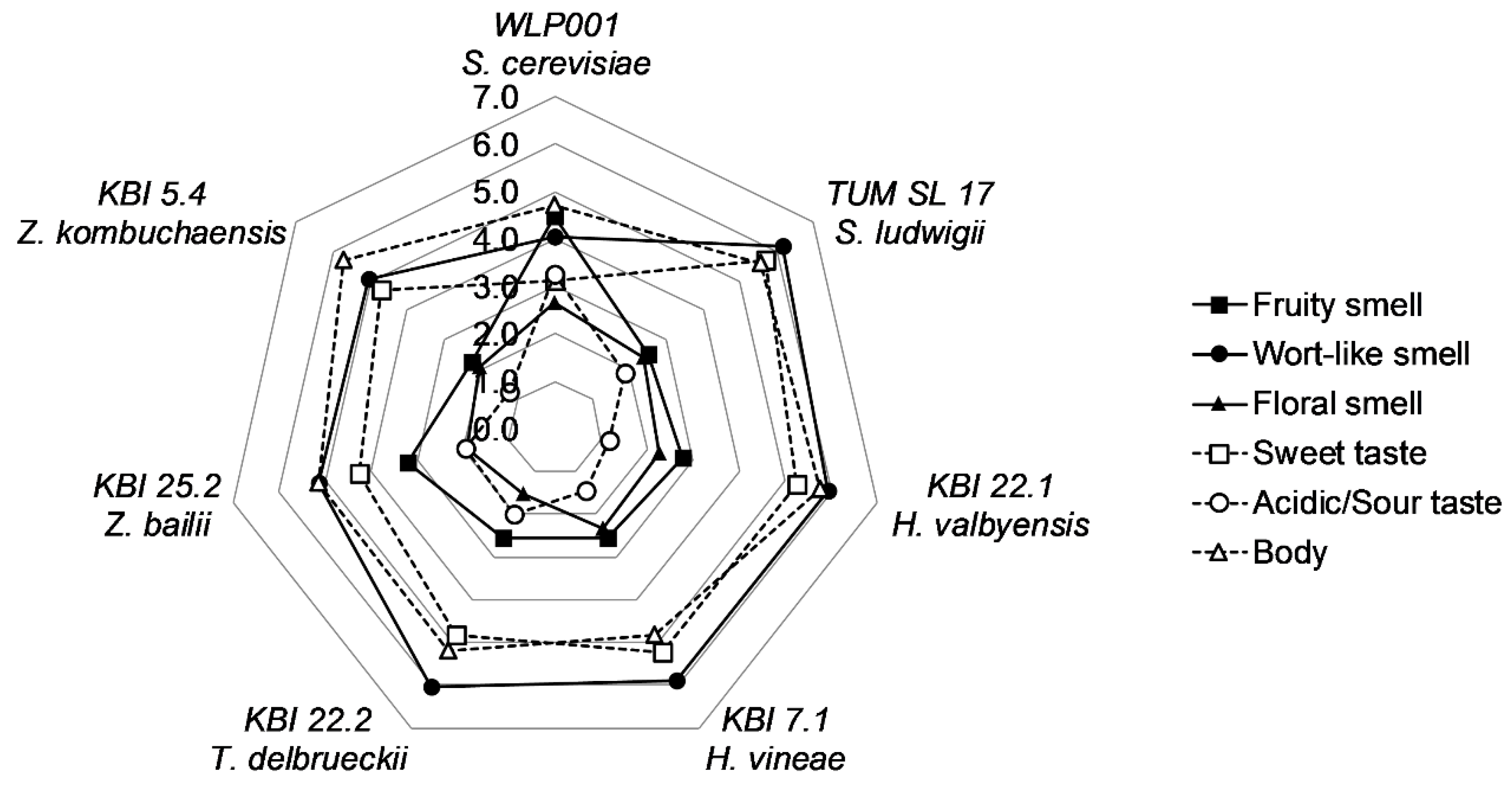
To evaluate and compare the flavor of the beers, a panel of 11 trained and experienced beer tasters judged the beers by individual description of the aroma, followed by the evaluation of the intensity descriptors “fruity”, “wort-like” and “floral” smell, “sweet” and “acidic/sour” taste, and the body of the beer. Each descriptor was given a value on a scale from 0 (nothing) to 10 (extremely). A spider web graph of the means for the descriptors is shown in
Figure 4.
WLP001 showed to have a less wort-like and fruitier smell and a less sweet, but more acidic/sour taste, owing to the longer fermentation time and higher extract consumption. The body of the beers was evaluated as being a little lower compared to the alcohol-free beers. Floral smell and acidic/sour taste were generally described to be low in intensity. Overall, the differences between the alcohol-free beers were small. KBI 25.2 was described to have a slightly fruitier smell and lower wort-like smell and sweet taste amongst the alcohol-free beers. However, analysis of variance (ANOVA) revealed no significant difference (p ≤ 0.05) between the AFB. ANOVA analysis between all beer samples revealed significant differences in acidic/sour taste (p < 0.001) and differences in sweet taste (p < 0.1) and fruity smell (p < 0.1).
To create a multidimensional sensory profile of all beers, a principal component analysis (PCA) was conducted. PCA is a tool used to transform and combine a large amount of data into new components, based on variation and correlation within a data set. As descriptors, wort-like and fruity smell were selected as well as sweet and acidic/sour taste and body. If descriptors do not discriminate the products, they cause distortion in the PCA. Hence the descriptor “flora smell”, having a P value for the F-test of the product effect greater than the default value of 0.5, was excluded from the PCA [
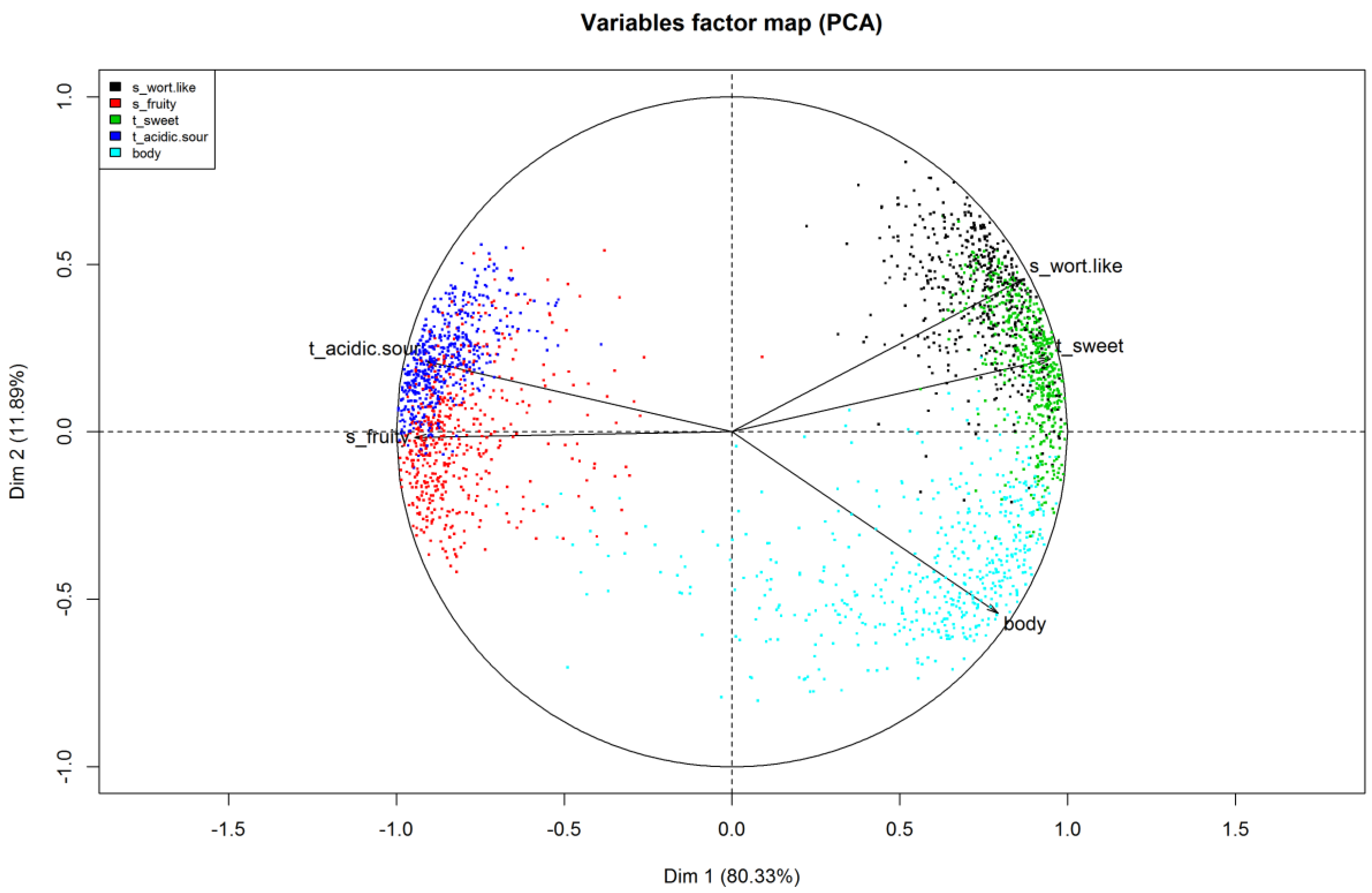
22]. The Variables factor map (
Figure 5) presents the observed variables projected into the plane, spanned by the first two principal components. It shows the structural relationship between the variables and helps to name the components. The projection of a variable vector onto the component axis allows to directly read the correlation between the variable and the component.
The variables factor map should be interpreted in terms of angles, either between each variable or between a variable and the component axes. Narrow angles reflect positively linked variables (i.e., sweet taste and wort-like smell). Right angles depict variables that are unrelated to each other (i.e., body and wort-like smell) and obtuse angles represent negative relationships (i.e., wort-like and fruity smell). The first principal component described about 80% of the total variation and showed an almost perfect correlation to the variable fruity smell and a very strong correlation to sweet and acidic/sour taste and wort-like smell. The second principal component explained an additional 12% of the total variation with a correlation to the body of the beers. Combined, the first two principal components explained about 92% of the total variance of the data.
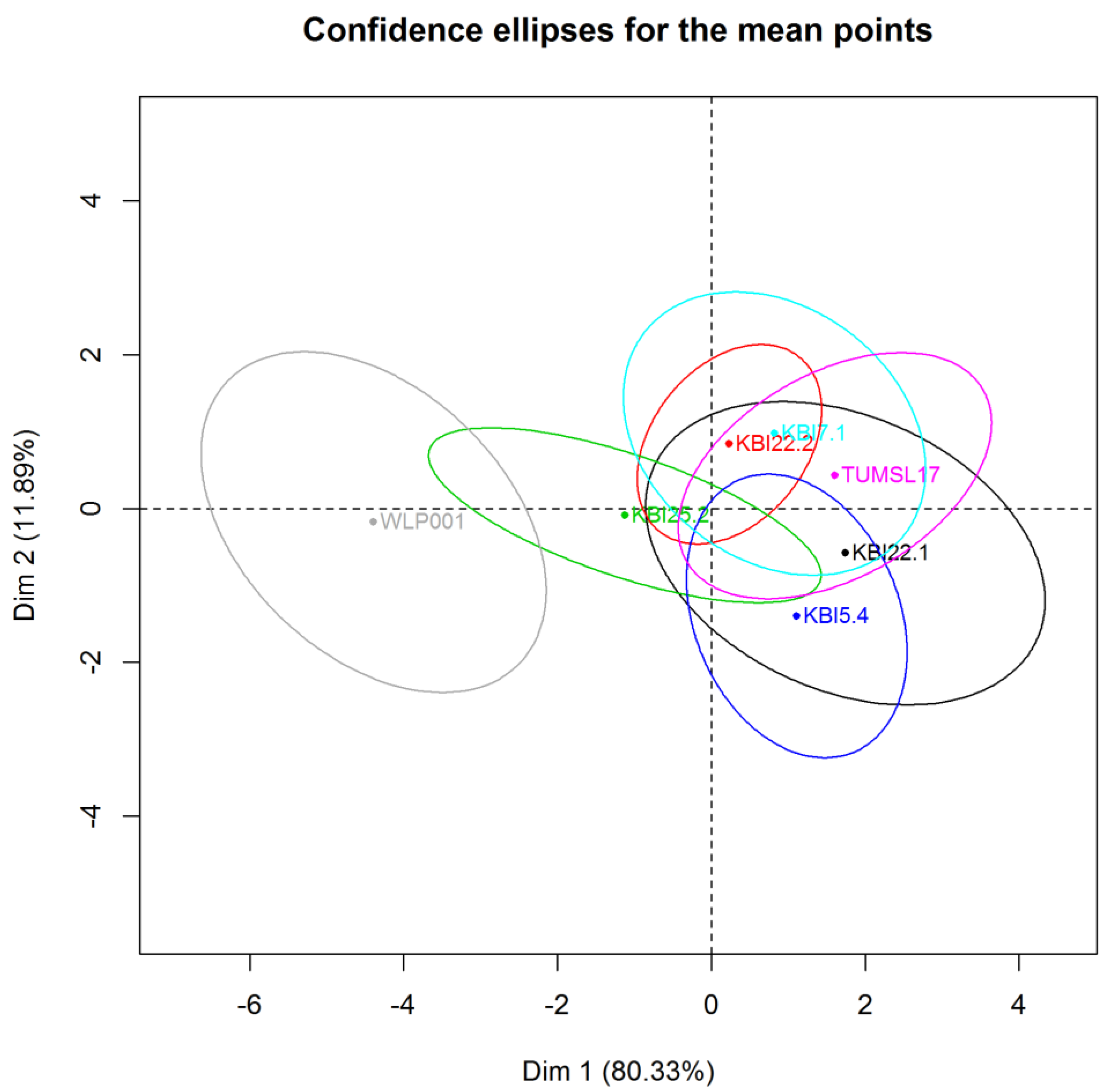
In the PCA graph (
Figure 6), confidence ellipses (α = 0.05) around each beer were added to visualize the uncertainty as for the position of the beer given by the panel. Well separated confidence ellipses indicate a great discriminant power of the panel.
As expected, WLP001 could be well discriminated from the other beers in the direction of a fruitier smell, more acidic/sour taste and away from a sweet taste and wort-like smell. The alcohol-free beers were not well discriminated, but were all located in the direction of a sweeter taste and wort-like smell. However, a tendency of KBI 25.2 separating from the group of alcohol-free beers in the direction of WLP001 could be observed. The highest means for body by KBI 5.4 and KBI 22.1 also reflected in the PCA. The results of the sensory reflect the marginal differences of the alcohol-free beers between each other from the analyses of secondary metabolites. However, the significantly higher ester content of the beer fermented with KBI 7.1 did not show in the sensory analyses of the different beers.
In the descriptive part of the sensory, the panelists gave all the alcohol-free beers attributes like: “wort-like”, “bread-like”, and “honey-like”. TUM SL 17 was described using at least one of those attributes, by 90% of the panelists. KBI 22.1 was additionally given a “cereal-like” character and half of the panel detected the diacetyl flavor as expected from the metabolites analysis (
Table 6). KBI 7.1 was also described with attributes like “black tea” and “caramel”. KBI 25.2 was given additional attributes like “slightly grassy”, “fruity” and “white wine”. The elevated diacetyl values for KBI 5.4 were again detected by 50% of the panelists. The problem of wort-like off-flavor in alcohol-free beers is very common. Aldehydes are reported to be the cause, with 3-methylthiopropionaldehyde seemingly being the key compound responsible for the worty off-flavor [
51,
52]. Wort aldehydes form mainly during mashing and boiling, but are also partially formed during fermentation by the yeast. They can originate from oxo-acids via the anabolic process, and from exogenous amino acids via the catabolic pathway [
53]. Ethanol plays a significant role in the reduction of the worty character of the beer. As a flavor component, it contributes directly to the flavor of beer, giving rise to a warming character and influencing the partitioning of flavor components between the liquid beer, foam, and the headspace above the beer [
54]. Additionally, Perpète and Collin [
6] reported, that aldehyde retention caused by its solubilization in ethanol leads to a lower perception of the worty taste. In regular beers the retention of aldehydes is 32–39% as opposed to 8–12% retention in alcohol-free beers [
4]. It is also known that yeast metabolism reduces wort aldehydes to less flavor active ones [
55]. The absence of ethanol, the lack of aldehyde reduction due to shortened fermentation times and the higher level of mono and disaccharides such as maltose intensify undesirable worty flavors [
6]. The results of the sensory indicate, that none of the investigated maltose-negative strains were able to mask the worty off-flavors. However, they neither stood out negatively compared to TUM SL 17, which is already commercially applied in the production of alcohol-free beers. KBI 25.2 showed the highest potential of non-
Saccharomyces yeasts to become a serious alternative in the brewing of alcohol-free beer with an improved sensorial profile.
3.6. Concluding Remarks
This study on the application of five non-Saccharomyces yeasts in the production of AFB, gave a comprehensive overview of their suitability and characteristics. After ruling out undesirable traits during characterization, such as POF production and hop sensitivity, the non-Saccharomyces yeasts showed excellent performance during propagation, outperforming TUM SL 17 in cell numbers and showing very high viability rates. In fermentation trials the non-Saccharomyces yeasts exhibited a comparable performance, and analysis of volatile compounds revealed only marginal differences. The AFB fermented with the commercial AFB yeast (TUM SL 17) could not be discriminated from the alcohol-free beers fermented with the investigated non-Saccharomyces yeasts, which indicates the potential of their application in alcohol-free beer brewing. All fermentations were performed at 25 °C to be able to compare the strains. Twenty-five degrees Celsius most likely was not the optimum for each of the yeast strains in terms of fermentation performance or production of secondary metabolites, but it allows an indication of the suitability of the investigated strains in alcohol-free beer production.