Abstract
In this study, an innovative green biorefinery system was successfully developed to process the green biomass into multiple biofuels and bioproducts. In particular, fresh giant miscanthus was separated into a solid stream (press cake) and a liquid stream (press juice) using a screw press. The juice was used to cultivate microalga Chlorella vulgaris, which was further thermochemically converted via thermogravimetry analysis (TGA) and pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) analysis, resulting in an approximately 80% conversion. In addition, the solid cake of miscanthus was pretreated with dilute sulfuric acid and used as the feedstock for bioethanol production. The results showed that the miscanthus juice could be a highly nutritious source for microalgae that are a promising feedstock for biofuels. The highest cell density was observed in the 15% juice medium. Sugars released from the miscanthus cake were efficiently fermented to ethanol using Saccharomyces cerevisiae through a simultaneous saccharification and fermentation (SSF) process, with 88.4% of the theoretical yield.
1. Introduction
Fossil fuels are the major source for our energy need at present, which currently contribute about 80% of the global energy demand [1]. According to the International Energy Agency (IEA), this demand will be increased by 40% by the year 2035, with fossil fuels contributing 75% [2]. This over dependence and increasing use of fossil fuels in the US and globally serves as a source of worry, as it is predicted to reach a crisis point in the near future [3].
Fossil fuels are also the major source of environmental pollutants and greenhouse gases. Sustainable developments requires the use of renewable biomass-based resources for fuels, chemicals and material production [4]. Renewable biomass resources can be converted to fuels and are a logical choice to replace oil. Considerable attention has been given to lignocellulosic biomass such as agricultural residues and energy crops for biofuel production.
In order to derive full benefits from the use of biomass in the biofuel industry, it is imperative that different byproducts are produced from the feedstock in a way analogous to the petroleum refinery platform. This concept of producing various bioproducts from a single biomass feedstock is known as the biorefinery concept. A biorefinery is a facility that can convert biomass into multiple biofuels, bioproducts, power, and chemicals by integrating various biomass conversion processes [1].
Currently, the biorefinery platform is aimed at replacing the petroleum refineries and reducing the fossil fuel intensity in different production areas [2]. As such, it is vital to investigate and develop biorefinery platforms based on lignocellulosic biomass and improve on their economic potentials to make them competitive with the petroleum refining industry.
One major type of the biorefinery concepts being explored is the green biorefinery. A green biorefinery uses “nature-wet” biomasses such as green grasses as feedstock, and employs a wet-fractionation technology as a first step to isolate the content-substances in their natural form into fiber-rich cake and a nutrient-rich green juice which are then converted by different processes into various products [5,6]. Using the nutrient-rich green juice for products generation eliminates the need for energy intensive drying processes, which could greatly improve the economic viability of this biorefinery platform type.
Using perennial energy grasses as feedstock for biorefinery processes has attracted tremendous attention due to their superior advantages such as broad adaptability, high water and fertilizer use efficiency, and tremendous biomass production. Miscanthus x. giganteus (MxG) is a perennial warm season grass in the sugarcane family that has been identified as a primary biomass crop for development in the US [7].
In order to enhance the versatility of the green biorefinery and increase the economics of the green biorefinery, it is essential to process both the press cake and the press juice into a variety of final products or other intermediary products that could be used as feedstock for further downstream processes [8]. The press cake has been primarily used for production of fodder pellet and biogas [9]. Little or no attention has yet been focused on the utilization of the press cake for biofuel purpose, especially as raw materials for bioethanol production. Bioethanol is by far the most widely used biofuel for transportation worldwide. Bioethanol and bioethanol/gasoline blends have a long history as alternative transportation fuels [10]. Producing bioethanol as a transportation fuel can help reduce CO2 buildup in two important ways: by displacing the use of fossil fuels, and by recycling CO2 that is released when it is combusted as fuel. The efficiency of conversion of biomass to ethanol depends upon feedstock characteristics and composition, pretreatment processes, and the fermentation technologies [11]. Research focus on the use of the press juice is on the increase now. Various uses of juice extracted from biomass have been investigated in the biorefinery platform. For example, the extracted banagrass juice has been used for the cultivation of an edible fungus [12]. Our previous study observed that the cattail juice held great potential as alternative growth media for microalgae and bacteria [13]. The juice from Italian rye grass, clover grass and alfalfa has been used for the production of lactic acids and other value added products [14].
In green biorefinery, wet fractionation technology is used as the first step to separate the juice from the green biomass. Screw press was the most common method used to press the green juice out of the green biomass [14]. Besides the screw press, filter presses, belt presses, centrifuges, Hammer mill, the thermal mechanical dewatering method, simultaneous application of a pulsed electric field, and superimposition of ultrasounds were also used to separate the juice from green biomass [15,16]. However, most of the publications did not provide a detailed description of the fractionation process, such as equipment operation, product recovery rates and analysis. Furthermore, to the best of our knowledge, there has not been any work done so far on the wet fractionation of MxG.
With the aim of overcoming the problems outlined above, the intent of this research is to develop a green biorefinery platform in an economically viable and environmentally sustainable manner, using MxG as a feedstock. Particularly, we separated MxG into pressed cake and juice, and accessed the viability of using the juice to grow microalgae for hydrocarbons production, and using the press cake to produce bioethanol using some biochemical processes. To the best of our knowledge, this use of juice from MxG has not been investigated yet.
2. Materials and Methods
2.1. Miscanthus Harvest and Processing
MxG was harvested from North Carolina A&T State University farm during the early summer in 2015 using a Tanaka TPH 270s-pole hedge trimmer to achieve consistent cuts. The MxG was then shred into smaller size using a DR Wood chipper/shredder (14.50 Pro Manual Start, DR Power Equipment, Vergennes, VT, USA). A Carver press (#2094 Cage Equipment, Carver Inc., Wabash, IN, USA) was used to press and separate the shredded biomass into a green juice liquid fraction and a solid cake fraction, at an optimized force of 30,000 lbs for a residence time of 15 min to allow for effective separation. The green juice was stored in a freezer at −20 °C until use. Then, 100 g of deionized water was added to 50 g of the pressed miscanthus solid cake. The 2:1 mixtures were thoroughly mixed and chopped in a rotary knife mill Grindomix GM 200 (Retsch®, Verder Scientific Inc. Newtown, PA, USA) at a speed of 9000 RPM for 2 min. Subsequent juice separation was conducted using a centrifuge (Centra-GP8R Centrifuge, ThermoIEC, Champaign, IL, USA). The centrifugation was carried out at a rotational speed of 2600 RCF for 10 min at 25 °C. The solid cake was stored in sealable containers at −4 °C for further analysis and downstream processing. Figure 1 illustrates the procedure taken in this work, including mechanical separation, microalgae cultivation, thermochemical processing and bioethanol fermentations.
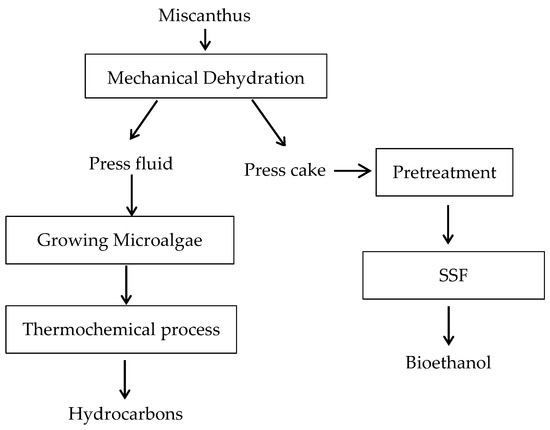
Figure 1.
Flow chart of the integrated generation of bioethanol and hydrocarbons from uses of MxG’ solid and liquid fractions. (SSF: Simultaneous saccharification and fermentation).
2.2. Biomass Analytical Procedures
The solid fraction and juice fraction of miscanthus after separation were analyzed for minerals (e.g., K, Mg, Ca, Cl, S, P), elemental composition (e.g., C, H, O, N), solids content, ash content, volatile content, and carbohydrates (cellulose, hemicellulose, lignin). Two stages of acid hydrolysis were performed for determining the structural carbohydrates and lignin content on the alfalfa samples according to NREL Ethanol Project Laboratory Analytical Procedure. In addition to chemical analyses, the mass flow of dry matter from the MxG into the juice and solid cake were calculated.
The elemental composition (C, H, O, N) of the solid cake samples was determined using a PE 2400 II CHNS/O analyzer (Perkin Elmer Japan Co., Ltd., Yokohama, Japan). The miscanthus samples were digested with HNO3/HCl in a microwave oven (200 °C, 2 MPa) and analyzed by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (ARL 3560, Waltham, MA, USA) for minerals.
Compounds in MxG juice was determined using LC/MS. The concentration of monomeric sugars, including cellobiose, glucose, arabinose and xylose in all liquid fractions as well as the concentration of ethanol were all determined using a Dionex Ultimate 3000 (UHPLC) (Thermo Fisher Scientific, Bannockburn, IL, USA) equipped with a Shodex Sugar SH 1218 ion exclusion column and a Shodex RI-101 refractive index detector [17]. The samples were analyzed using a UPLC-QTOF-MS system (ACQUITY UPLC-SYNAPT MS, Waters Corp., Milford, MA, USA). An ACQUITY UPLC BEH C18 1.7 μM VanGuard pre-column and an ACQUITY UPLC BEH C18 1.7 μM analytical column were used for the analyses at column temperature of 40 °C and a flowrate of 0.4 (mL/min). The system was operated in both electrospray ionization (ESI) positive (mobile phases: 0.1% formic acid in water and 0.1% formic acid in acetonitrile) and negative modes (mobile phases 1 mM ammonium fluoride in water and acetonitrile) to provide comprehensive coverage. Metabolite annotation for UPLC-QTOF-MS analysis was performed by comparing mass spectra and retention time of each detected signal from the MxG samples to those of reference standards in an in-house library, which contained 500+ endogenous metabolites and plant metabolites.
The mobile phase was 0.01N H2SO4 at a flowrate of 1 mL/min. The temperature of the detector and column were maintained at 50 °C and 75 °C, respectively. All experiments and analyses were performed in duplicate.
2.3. Microalgae Cultivation
Chlorella vulgaris (UTEX 2714), which was obtained from the Culture Collection of Algae at the University of Texas at Austin, was used in this study. The MxG juice was diluted to DI water to prepare 1, 2, 5 and 10% (v/v) juice media. A 10 vol % algal inoculum was used for scaling-up the culture to a larger volume. Microalgae cultivation was carried out in 150 mL Wheaton glass bottles containing 100 mL algal culture (i.e., 90 mL of medium plus 10 mL of algal inoculum) at room temperature and 200 μmol m−2 s−1 continuous cool-white fluorescent light illumination. Agitation of the bottles was carried out manually once daily and the optical density (OD) of the microalgal culture was measured everyday using a Thermo Scientific GENESYS 20 Spectrophotometer (Waltham, MA, USA). A previously established correlation (Equation (1)) between the optical density of this particular C. vulgaris at 680 nm and the cell number was used for analysis [18].
Cell number (cell/mL) = 8 × 106 OD680 + 425,897
At the end of the microalgal cultivation, the microalgal broth was centrifuged at 2300× g and 20 °C for 15 min. Supernatants were separated, and the collected microalgal cells were dried at 60 °C for 48 h until the sample reached equilibrium moisture content.
2.4. Thermalchemical Conversion Analysis of Microalgae via Py-GC/MS and TGA
The pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) analysis of microalgal biomass was carried out in a Frontier EGA/PY-3030D multi-shot pyrolyzer (Fukushima, Japan), which was coupled with an Agilent 7890A gas chromatography/5975c mass spectrometer (GC/MS) with a DB-5MS capillary column (Santa Clara, CA, USA). For each experiment, the pyrolyzer was pre-heated to desired temperatures of 500 °C, following by dropping approximately 0.3 mg of sample into the pyrolysis part of the reactor. The sample was volatized immediately, giving off pyrolysis products as the vapor, which was injected directly into GC/MS. The compounds were identified by comparison with the mass spectral database of the National Institute of Standards and Technology (Gaithersburg, MD, USA).
Thermogravimetry analysis (TGA) was performed by using a SDT Q600 thermalgravimetric analyzer (TA Instruments, New Castle, DE, USA), in which the sample was heated to 600 °C at a heating rate of 5 °C/min in nitrogen.
2.5. Dilute Sulfuric Acid Pretreatment
A Dionex ASE 350 Accelerated Solvent Extractor (Dionex Corporation, Sunnyvale, CA, USA) was used to do the dilute sulfuric acid pretreatment. A 1% (v/v) sulfuric acid solution was prepared to pretreat the biomass, at a pretreatment temperature of 160 °C for 10 min. Approximately 30 g of blended wet miscanthus was placed into a tared 66 mL Dionex extraction cell containing a glass fiber filter. Then the appropriate number of 150 mL collection vials were weighed and placed onto the ASE system. The extractor passed 60 mL of dilute sulfuric acid solution into each cell containing the biomass. Then the cells were heated to the desired temperature (160 °C) at a heating rate of 25 °C/min, with the temperature maintained for 15 min. After this treatment, 40 mL of the dilute sulfuric acid solution was passed into the cells to rinse the biomass. The resulting extractive and the rinsing solution (total about 100 mL) were collected in the collection vials. The extraction cells were cooled down to 25 °C. The recovered biomass samples were stored in sealable containers and kept at −4 °C for further downstream processing and analyses such as compositional analysis and fermentation.
2.6. Fermentation of MxG Solid Fraction to Produce Ethanol
MxG solid cake was taken through simultaneous saccharification and fermentation (SSF) to produce ethanol using S. cerevisiae microorganisms. For SSF, a pretreated biomass loading of 5 g (wet basis) with a total working volume of 50 mL and a pH adjusted to 4.5 by the addition of 0.05 M citric buffer was used. Wheaton septum glass bottles (125 mL) were used as fermentation reaction vessels. A cocktail of enzymes including cellulose (Novozyme NS 50013) at a loading of 60 FPU/g glucan, hemicellulase (Novozyme NS 22002) at a loading of 2.5 FBG/g glucan and β-glucosidase (Novozyme NS 50010) at 4.5 CBU/g glucan was used for enzymatic hydrolysis. Saccharomyces cerevisiae (ATCC 24858) was then added to the reaction vessel to begin the SSF process.
The fermentation cultures were placed in a rotary shaker and incubated at 35 °C and 200 rpm and grown aerobically. Samples were taken at predetermined intervals (0, 3, 6, 12, 24, 48, 72, and 96 h) and collected by filtering through 0.45-μm nylon membranes for ethanol and sugars analysis by HPLC. Saccharomyces cerevisiae cultured with YM broth was also used for SSF to serve as control and allow for comparison. The ethanol yield was expressed as the percentage of the theoretical yield using the following formula:
where Cethanol,f is the ethanol concentration at the end of the fermentation (g/L), Cethanol,i is the ethanol concentration at the beginning of the fermentation (g/L), Cbiomass is the dry biomass concentration at the beginning of the fermentation (g/L), f is the cellulose fraction of the dry biomass (g/g), and 0.568 is the conversion factor from cellulose to ethanol. All experiments were carried out in duplicates.
3. Results and Discussion
3.1. Characteristics of Raw Miscanthus, Miscanthus Cake and Juice
The mechanical press was largely effective in separating freshly harvested MxG into juice and cake with a percentage mass distribution of between 0.5 g/g of liquid and 0.5 g/g of solid [17]. The compositions of the separated MxG solid cake and juice are listed in Table 1. One of the most notable differences between the cake and juice was the significantly lower total solids content of the MxG juice (~0.1%). Differences also existed in the carbohydrates group (cellulose, hemicellulose, lignin) among the cake and juice samples. As expected, the carbohydrates of the cake are higher than those of the juice. This is mainly because the cellulosic fibers are less extractable due to the polysaccharide matrix structure in biomass [13]. The elemental compositions of the two fractions are comparable, with a higher carbon content of the solid cake. MxG has been noted to be a good candidate for bioethanol production due to its high carbon content. The juice fraction showed a higher amount of crude protein content than the solid cake, indicating proteins in the raw miscanthus were likely extracted in to the juice fraction.

Table 1.
Characteristics of MxG cake and juice.
According to the ICP analysis, the Calcium (Ca), Potassium (K), and Magnesium (Mg) concentrations of the MxG juice were much higher than the solid cake, while the Sodium (Na) concentrations of The MxG juice was significantly lower than the solid cake. Carbon, Nitrogen and Phosphorus are the three primary nutrients for algae cultivation. Other micronutrients also required include silica, calcium, magnesium, potassium, iron, manganese, sulfur, zinc, copper and cobalt [19].
The LC/MS further confirmed that the MxG juice contains trace amount of amino acids, organic acids, and metabolites, which are good feed supplements for microorganism growth (Table 2).

Table 2.
LC/MS analysis of MxG juice.
3.2. Miscanthus Juice as a Nutrition Supplement for Microalgal Growth
Figure 2 shows the growth curves of C. vulgaris in the MxG juice media. As the control, DI-water alone cannot support the algal growth. The solid content of the MxG juice is 0.1 wt %, meaning that the actual solid contents in 1%, 2%, 5%, 10% and 15% juice media were 0.001%, 0.002%, 0.005%, 0.01% and 0.015%, respectively. It was observed that the higher concentration on MxG juice, the higher the microalgal growth rate. The highest cell density was observed in the 15% juice medium. A similar growth pattern was observed in the 1% and 5% juice medium. These results indicate that MxG juice was able to supple the microalgae with the necessary nutrients for its growth. Work done by Rahman et al. (2015), using cattail juice, showed that the cattail juice was similar to proteose medium, which was rich enough to support high-cell-density growth of numerous microalgae strains [18]. Juice extracts from plants therefore hold great potential as alternative growth media for microalgae and bacteria.
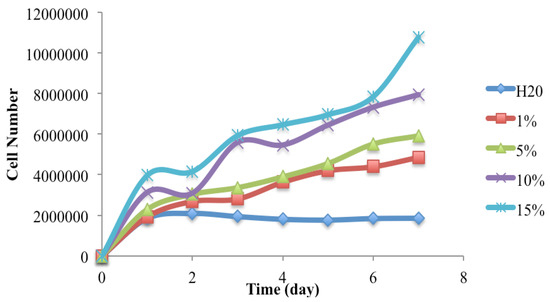
Figure 2.
Growth curves of C. vulgaris in DI water with MxG juice at room temperature and 600 μmol m−2 s−1 light intensity.
3.3. Thermochemical Conversion of Microalgae Grown on MxG Juice
C. vulgaris grown on MxG juice contained approximately 64.6% protein, 8.9% carbohydrates and 12.3% lipids (Table 3). Its biochemical and elemental compositions were similar to the same species grown on other media [15].

Table 3.
Elemental and composition analysis of microalgae (moisture free basis, % by weight).
TGA study of C. vulgaris used a condition that was similar to slow pyrolysis. Figure 3 shows the Thermogravimetry (TG) and Differential thermogravimetry (DTG) curves of microalgae that exhibited its weight loss characteristics. The decomposition of this microalgal biomass can be divided into two phases. The first phase (T < 180 °C) was moisture removal. The decomposition phase occurred between 180 and 350 °C with a weight loss of 45 wt %. The results indicated that applying the slow pyrolysis process to C. vulgaris might result in a residue yield of 20.1 wt % of the starting material (i.e., the conversion ratio was 79.9 wt %). The possible reasons for the high residue yield are the ash content and the high protein content in feedstock.
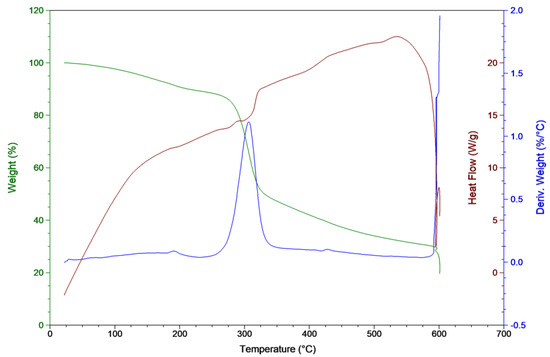
Figure 3.
Thermogravimetry (TG) and DTG curves of microalgae.
Py-GC/MS analysis of microalgae C. vulgaris was performed at 500 °C, resulting in over 100 different chemicals (Figure 4). The top 20 pyrolytic products representing 48% (area) of all products are summarized in Table 4. Pyrolysis of microalgae could form a complex organic mixture. The chemicals in the mixture can be generally categorized as fatty acids (such as C16 hexadecenoic acid and C14 tetradecanoic acid), hydrocarbons (like toluene, butane, cresol and octadecane), nitrogenated compounds (such as pyridines, pyrazines, pyrroles, indoles and their derivatives), and oxygenated compounds (organic acids, ketones, furfurals, aldehydes and phenols). Fatty acids are degradation products of microalgal lipids, and C16 and C14 fatty acids are major components. Nitrogenated compounds are derived from microalgal proteins. Although a fair amount of hydrocarbons was produced via pyrolysis of microalgae, the products are still combined with hundreds of other chemicals. To use microalgal pyrolysis bio-oil as the transportation fuel, an upgrading process is still required [20].
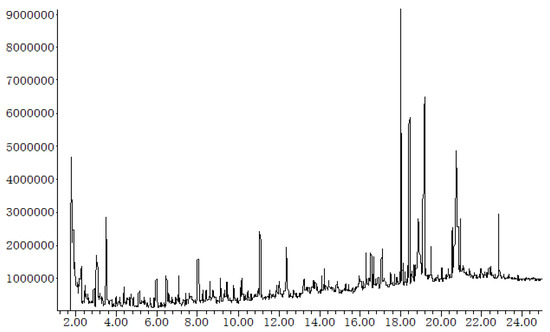
Figure 4.
Gas chromatography (GC) profiles of pyrolytic products of microalgae.

Table 4.
Pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) analysis of microalgae C. vulgaris.
3.4. Effect of Pretreatment on the Cake of Miscanthus X Giganteus
The purpose of the pretreatment was to remove lignin and/or hemicellulose, to disrupt the crystalline structure of cellulose, and to increase the porosity of the material, making it more accessible to enzyme attack [21]. The compositions of miscanthus before and after dilute sulfuric acid pretreatment are given in Table 5.

Table 5.
Composition of MxG before and after dilute sulfuric acid pretreatment.
Untreated MxG contained approximately 49% cellulose, 32.75% hemicellulose, and 15.25% lignin (Table 5). Miscanthus, with its cellulose content of approximately 40% and above, is considered useful for biofuel production [22]. The compositions of the raw miscanthus in the present study is consistent with previous findings reported so far [23].
After dilute sulfuric acid pretreatment, the composition of the biomass changed, with an increase in the cellulose and lignin contents and a decrease in the hemicellulose content the biomass samples. The hemicellulose of biomass decreased from about 32.75% in the untreated samples to approximately 1% in the pretreated samples, indicating almost complete hydrolysis of hemicellulose fraction of the biomass This removal of hemicellulose increases porosity and improves enzymatic digestibility. The complete removal of hemicellulose usually results in a maximum enzymatic digestibility [24], while the increase of the porosity of the sample can cause an increase in the lignin content of the sample. Studies of dilute acid pretreatment show the increase in the lignin content would not affect the ethanol yield and concentration [25,26,27].
3.5. Fermentation of MxG Cake for Ethanol Production
The MxG cake was treated with dilute acid first, and then used to produce bioethanol via a simultaneous saccharification and fermentation (SSF) process. Bioethanol was rapidly produced within the first 24 h, while the concentration of glucose reduced rapidly as well, with the measured xylose concentration remaining stable throughout the period (Figure 5). The production of bioethanol began to level off during the following 72 h, with a continued reduction in glucose concentration and a steadiness of the xylose concentration (Figure 5). The final ethanol yield was 88.4% of the theoretical value. This result suggests that sugars produced from the MxG cake can be efficiently fermented to ethanol.
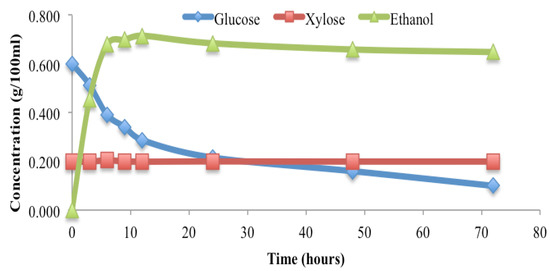
Figure 5.
Ethanol, glucose and xylose profiles during the saccharification and fermentation (SSF) of the MxG cake.
4. Conclusions
Freshly harvested MxG was fractionated using mechanical press, whereby MxG was separated into a fiber-rich cake and a nutrient-rich juice. The mechanical pressing proved to be very efficient at reducing the solids mass transfer to the juice, resulting in a very low solid content of the juice.
The MxG juice was used to cultivate C. vulgaris in different media. Results showed the juice was highly nutritious and supported the growth of microalgal culture. Thus the juice could be a highly nutritious source for the microorganisms and bacteria. The uses of the MxG juice eliminate the extracted juice as a waste stream within a green biorefinery platform, making the concept more economical and environmentally friendly. Further, microalgae grown with the juice were studied for its thermochemical conversion behaviors via Py-GC/MS and TGA. Pyrolysis of this microalgae might result in approximately 79.9% conversion and a bio-oil containing over 100 chemicals.
The MxG cake was pretreated with dilute acid, and used as the feedstock for ethanol production through SSF using yeasts. Glucose from MxG solid cake can be efficiently fermented to ethanol with 88.4% of the theoretical yield. The dilute acid pretreatment was sufficient for pretreating MxG cake, resulting in very low hemicellulose content and high cellulose content of the pretreated cake.
Acknowledgments
This work is supported by the USDA National Institute of Food and Agriculture, (Evans-Allen) project (Grant No. NCX-272-5-13-130-1 and NC.X-303-5-17-130-1).
Author Contributions
Shuangning Xiu devised and drafted the manuscript; Bo Zhang performed the microalgae analysis and thermochemical conversion process. Nana Abayie Boakye-Boaten performed the experiments of pretreatment and bioethanol production and analyzed the data; Bo Zhang and Abolghasem Shahbazi revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Parajuli, R.; Dalgaard, T.; Jørgensen, U.; Adamsen, A.P.S.; Knudsen, M.T.; Birkved, M.; Gylling, M.; Schjørring, J.K. Biorefining in the prevailing energy and materials crisis: A review of sustainable pathways for biorefinery value chains and sustainability assessment methodologies. Renew. Sustain. Energy Rev. 2015, 43, 244–263. [Google Scholar]
- McLaren, J.S. Crop biotechnology provides an opportunity to develop a sustainable future. Trends Biotechnol. 2005, 23, 339–342. [Google Scholar] [PubMed]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Kamm, B.; Kamm, M. Biorefineries—Multi product processes. In White Biotechnology; Ulber, R., Sell, D., Eds.; Springer: Berlin, Germany, 2007; pp. 175–204. [Google Scholar]
- Xiu, S.; Shahbazi, A. Development of Green Biorefinery for Biomass Utilization: A Review. Trends Renew. Energy 2015, 1, 4–15. [Google Scholar] [CrossRef]
- Khanna, M.; Dhungana, B.; Clifton-Brown, J. Costs of producing miscanthus and switchgrass for bioenergy in Illinois. Biomass Bioenergy 2008, 32, 482–493. [Google Scholar] [CrossRef]
- Boakye-Boaten, N.A.; Xiu, S.; Shahbazi, A.; Wang, L.; Li, R.; Schimmel, K. Uses of miscanthus press juice within a green biorefinery platform. Bioresour. Technol. 2016, 207, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wachendorf, M.; Richter, F.; Fricke, T.; Graß, R.; Neff, R. Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. I. Effects of hydrothermal conditioning and mechanical dehydration on mass flows of organic and mineral plant compounds, and nutrient balances. Grass Forage Sci. 2009, 64, 132–143. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Xiu, S.; Boakye-Boaten, N.A.; Shahbazi, A. Separate hydrolysis and fermentation of untreated and pretreated alfalfa cake to produce ethanol. In Proceedings of the 2013 National Conference on Advances in Environmental Science and Technology; Uzochukwu, G.A., Schimmel, K., Kabadi, V., Chang, S.-Y., Pinder, T., Ibrahim, S.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 233–240. [Google Scholar]
- Takara, D.; Khanal, S.K. Green processing of tropical banagrass into biofuel and biobased products: An innovative biorefinery approach. Bioresour. Technol. 2011, 102, 1587–1592. [Google Scholar] [PubMed]
- Rahman, Q.M.; Wang, L.J.; Zhang, B.; Xiu, S.N.; Shahbazi, A. Green biorefinery of fresh cattail for microalgal culture and ethanol production. Bioresour. Technol. 2015, 185, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.; Kiel, P. Integrated utilisation of green biomass in the green biorefinery. Ind. Crops Prod. 2000, 11, 129–137. [Google Scholar] [CrossRef]
- Arlabosse, P.; Blanc, M.; Kerfaï, S.; Fernandez, A. Production of green juice with an intensive thermo-mechanical fractionation process. Part I: Effects of processing conditions on the dewatering kinetics. Chem. Eng. J. 2011, 168, 586–592. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Boakye-Boaten, N.A. Effects of Fractionation Methods on the Isolation of Fiber-Rich Cake from Alfalfa and Ethanol Production from the Cake. BioResources 2014, 9, 3407–3416. [Google Scholar] [CrossRef]
- Boakye-Boaten, N.A.; Xiu, S.; Shahbazi, A.; Wang, L.; Li, R.; Mims, M.; Schimmel, K. Effects of fertilizer application and dry/wet processing of miscanthus x giganteus on bioethanol production. Bioresour. Technol. 2016, 204, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Zhang, B.; Wang, L.; Shahbazi, A. Bioremediation of swine wastewater and biofuel potential by using chlorella vulgaris, chlamydomonas reinhardtii, and chlamydomonas debaryana (corrected version). J. Pet. Environ. Biotechnol. 2014, 5, 175–180. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Hasan, R.; Shahbazi, A. Characterization of a native algae species chlamydomonas debaryana: Strain selection, bioremediation ability, and lipid characterization. BioResources 2014, 9, 6130–6140. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Li, R.; Rahman, Q.M.; Shahbazi, A. Catalytic conversion of chlamydomonas to hydrocarbons via the ethanol-assisted liquefaction and hydrotreating processes. Energy Fuels 2017, 31, 12223–12231. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Saida, L.; Bansal, S.; Hughes, J.D. Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Manag. 2011, 31, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.; Teller, P.J.; Hilstrøm, T.; Ahring, B.K. Hydrolysis of miscanthus for bioethanol production using dilute acid presoaking combined with wet explosion pre-treatment and enzymatic treatment. Bioresour. Technol. 2008, 99, 6602–6607. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Effectiveness of dilute oxalic acid pretreatment of miscanthus × giganteus biomass for ethanol production. Biomass Bioenergy 2013, 59, 540–548. [Google Scholar] [CrossRef]
- Ballesteros, I.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Oliva, J.M.; Sáez, F. Dilute sulfuric acid pretreatment of cardoon for ethanol production. Biochem. Eng. J. 2008, 42, 84–91. [Google Scholar] [CrossRef]
- Zhu, L.; O’Dwyer, J.P.; Chang, V.S.; Granda, C.B.; Holtzapple, M.T. Structural features affecting biomass enzymatic digestibility. Bioresour. Technol. 2008, 99, 3817–3828. [Google Scholar] [CrossRef] [PubMed]
- Boonsawang, P.; Subkaree, Y.; Srinorakutara, T. Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF). Biomass Bioenergy 2012, 40, 127–132. [Google Scholar] [CrossRef]
- Torr, K.M.; Love, K.T.; Simmons, B.A.; Hill, S.J. Structural features affecting the enzymatic digestibility of pine wood pretreated with ionic liquids. Biotechnol. Bioeng. 2016, 113, 540–549. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).