Abstract
The industrial transition to advanced biofuels is currently limited by the metabolic constraints and low inhibitor tolerance of wild-type microbial hosts. This review justifies the necessity of Precision Fermentation (PF) as the pivotal technological framework to overcome these barriers, providing a systematic synthesis of high-resolution genetic tools and intelligent bioprocess architectures. We analyze how the integration of CRISPR-Cas9, retron-mediated recombineering, and synthetic regulatory circuits enables the development of specialized microbial “chassis” capable of achieving 10- to 100-fold higher yields compared to native organisms, with industrial titers reaching 50 g/L for isobutanol and 25 g/L for farnesene. A major novelty of this work is the critical evaluation of Artificial Intelligence (AI), Soft Sensing, and Digital Twins in orchestrating real-time metabolic control and mitigating the toxic effects of advanced alcohols and drop-in hydrocarbons (C15–C20). Furthermore, the study concludes that the “scale-out” modular strategy, when integrated into hybrid thermochemical-biochemical biorefineries, allows for the full valorization of C5/C6 sugars and lignin, achieving a Minimum Selling Price (MSP) competitive with fossil fuels. By mapping the synergy between advanced metabolic engineering and data-driven process optimization, this review establishes PF as an indispensable driver for achieving carbon-neutral and carbon-negative energy systems in the circular bioeconomy.
1. Introduction
The environmental and health consequences of reliance on nonrenewable energy sources are evident in the heightened frequency and severity of extreme climatic events. The primary cause of this phenomenon is the combustion of fossil fuels, intensified by climate change and the release of harmful atmospheric pollutants in recent decades [1]. The World Meteorological Organization (WMO) states that the climate crisis has exacerbated droughts, floods, and heatwaves, emphasizing the imminent threat these events pose to lives and livelihoods and highlighting the need for urgent measures to mitigate the damage [2].
In response to the unsustainability of fossil-based energy sources and the need to mitigate greenhouse gas (GHG) emissions, with targets of a 45% reduction by 2030 and the achievement of global net-zero emissions by 2050, the search for renewable alternatives, such as biofuels, has gained prominence [3,4]. This option is considered promising for the transition toward a cleaner and more sustainable energy matrix, particularly considering the growing contribution of renewables, which accounted for approximately 11.2% of global final energy consumption in 2019 and about 29% of worldwide electricity generation in 2020 [3,5]. The large-scale utilization of biofuels is not merely an ambition but a consolidated industrial reality, as evidenced by current production volumes. In 2024, the United States led the global market with a production output of 1.917 petajoules, followed by Brazil and Indonesia, which produced 1.143 and 459 petajoules, respectively. Within the European context, Germany emerged as the leading producer, ranking among the top five global producers with an output of 149 petajoules [6].
Within this context, biofuels emerge as a strategic component of the renewable energy portfolio, as they are derived from renewable resources and generally exhibit a lower environmental impact, contributing to the reduction in greenhouse gas (GHG) emissions, by up to 86% [7,8,9]. Among biofuels, bioethanol remains the dominant liquid biofuel worldwide, accounting for approximately 65% of global production; however, first-generation (1G) routes based on food crops face sustainability constraints related to land use, food security, and environmental degradation [10]. Consequently, increasing attention has been directed toward next-generation biofuels produced from lignocellulosic biomass, an abundant and low-cost resource that does not compete with food supply, although its complex structure still imposes significant techno-economic challenges due to the energy-intensive steps required for pretreatment, hydrolysis, and fermentation [11,12].
Despite biofuels being a more environmentally friendly alternative to fossil fuels, they still face significant challenges related to sustainability and large-scale economic viability. Microbial bioconversion processes are crucial for producing low-cost biofuels but require significant capital and operational investments, which significantly increase the overall process cost. Numerous microbial species can transform lignocellulosic substrates into various biofuels; however, the efficiency of this conversion is directly contingent upon the inherent metabolic traits of each microorganism [13].
Precision fermentation (PF) and metabolic engineering emerge as promising strategies to accelerate the development of bioenergy, expanding microbial bioconversion beyond advanced alcohols to include syngas-derived fuels and biohydrogen as strategic energy carriers [14,15,16,17]. In this scenario, wild microorganisms, naturally limited by low productivity and inefficient regulation, can be enhanced through the insertion of specific genes, enabling the simultaneous utilization of C5 and C6 sugars, increasing tolerance to inhibitory compounds, and improved titer, rate, and yield (TRY) [18,19,20,21,22,23,24,25].
Simultaneously, synthetic biology expands the potential of these strategies by enabling the design and construction of artificial genetic circuits using modern tools such as CRISPR–Cas9, MAGE (Multiplex Automated Genome Engineering), and comprehensive “omics” platforms (genomics, transcriptomics, proteomics, and metabolomics). This integration has facilitated the development of highly efficient microorganisms capable of converting renewable feedstocks into biofuels in a more economical, scalable, and environmentally sustainable manner [18,26].
Although several reviews have addressed individual aspects of microbial biofuel production, most focus on specific fuel classes, organisms, or genetic tools in isolation. Given this panorama, the present review article provides a comprehensive and integrative analysis of recent advances, challenges, and future perspectives in biofuel production, positioning precision fermentation as a technological frontier in the transition toward a low-carbon and potentially carbon-neutral energy matrix. Specifically, this review addresses the fundamental principles of precision fermentation, the main strategies of microbial engineering, and the application of modern molecular and digital tools, while systematically integrating these advances across multiple biofuel production pathways and their incorporation into biorefinery systems.
2. Fundamentals of Precision Fermentation
Precision Fermentation (PF) comprises strategies designed to optimize the production of high-value compounds such as food ingredients, enzymes, bioactive metabolites, pigments, and biofuels, while ensuring high yields, purity, and traceability. This approach is intricately connected to metabolic engineering and incorporates various disciplines and tools (Figure 1), including bioinformatics, molecular analyses, omics studies, kinetic modeling, bioprocess development, genetic engineering, and, more recently, the use of artificial intelligence (AI) [27].
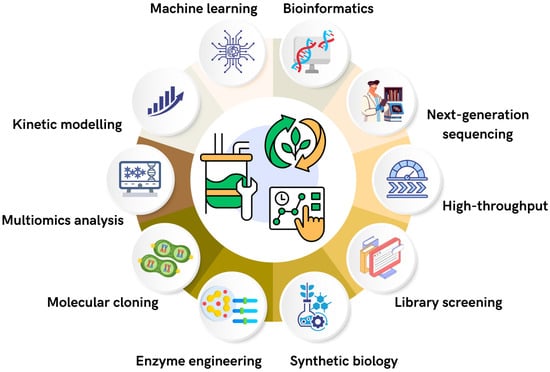
Figure 1.
Key technological pillars supporting precision fermentation: integration of computational, molecular, and bioprocess tools for high-value bioproduct synthesis.
In the realm of biofuels, PF has focused on increasing production efficiency and yield by refining industrial processes through enhanced cellular stress tolerance, improved conversion of fermentable sugars, and metabolic optimization of biosynthetic pathways [28]. Another important aspect involves the development of bioengineered enzymes (e.g., xylanases, cellulases, pectinases, and amylases) to enhance stability, specificity, and catalytic efficiency in the transformation of lignocellulosic biomass into fermentable sugars [29].
This approach supports productive value chains by enabling the use of agricultural byproducts and other organic residues, in which microorganisms are genetically modified (GM) to grow and produce target metabolites under critical conditions, thereby reducing bottlenecks and enhancing the utilization of natural resources [30]. Thus, PF aligns with the global demand for eco-efficient and sustainable processes capable of delivering clean, traceable, and environmentally responsible production [31].
2.1. Microbial Engineering: Common Platforms (Yeasts, Bacteria, Algae, Synthetic Consortia)
PF integrates multiple engineering steps (Figure 2), which typically begin with the selection of a microbial platform capable of hosting and expressing the genes responsible for producing the target compound, commonly referred to as the “chassis”. Microorganisms classified as GRAS (Generally Recognized as Safe) are preferentially employed due to their well-established history of industrial use, including bacteria, yeasts, and filamentous fungi [32]. The continuous advancement of the field has driven the exploration of new chassis and the development of more efficient biotechnological processes [33]. The choice of a chassis depends on factors such as ease of genetic manipulation, the presence of native biosynthetic pathways, safety, and adaptability to fermentation conditions [34]. Table 1 lists examples of metabolic engineering strategies in different species.
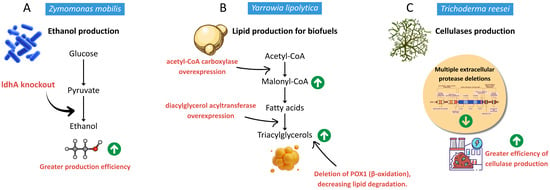
Figure 2.
Representative metabolic engineering strategies in precision fermentation. (A) Ethanol pathway optimization in Zymomonas mobilis through deletion of ldh, reducing lactate formation and redirecting carbon flux to ethanol [35]. (B) Lipid overproduction in Yarrowia lipolytica via ACC1 and DGA1 overexpression combined with POX1 deletion, enhancing triacylglycerol accumulation [36]. (C) Improved cellulase secretion in Trichoderma reesei through deletion of protease-encoding genes and promoter strengthening of cbh1 [37].
2.1.1. Yeast Chassis: Saccharomyces cerevisiae and Emerging Species
Saccharomyces cerevisiae is recognized as a model yeast due to its ease of cultivation, robust stress tolerance, well-characterized metabolism, and rapid growth. The Brazilian strain UFMG-CM-Y257 has been reported to exhibit a high specific growth rate on sucrose (0.57 ± 0.02 h−1) together with a high ethanol yield (1.65 ± 0.02 mol ethanol per mol of hexose equivalent) [38,39]. A study comparing ethanol production across Candida stellata, Hanseniaspora uvarum, Kloeckera apiculata, Saccharomycodes ludwigii, Torulaspora delbrueckii and S. cerevisiae reported values of 5.50, 4.60, 4.82, 6.50, 6.38 and 6.42 percent v/v, respectively. S. cerevisiae also showed the highest specific growth rate, reinforcing its broader advantage in fermentation processes [40].
Besides S. cerevisiae, several emerging yeast species have demonstrated strong potential as microbial chassis for PF. Yarrowia lipolytica can accumulate lipids and metabolize lignocellulosic waste streams, such as fatty acids, glycerol, and acetate, which are key substrates for the production of biofuels and oleochemicals. Kluyveromyces marxianus is thermotolerant and grows rapidly on different substrates, including agricultural residues and dairy byproducts, showing potential for bioethanol production [41]. Yeasts of the genus Rhodotorula stand out for performing high-cell-density fermentations, converting substrates such as lignocellulose and glycerol, and enhancing the synthesis of fatty acids and alcohols when GM [42].
2.1.2. Bacterial Chassis: Escherichia coli and Solventogenic Strains
Bacterial chassis offers broad metabolic diversity and remains an essential platform in precision fermentation. Escherichia coli is still the most widely used platform due to its rapid growth and low nutritional cost and is capable of producing both alcohols and intermediates for fuels. Studies consistently show that this bacterium grows considerably faster than native solvent-producing species, reaching maximal specific growth rates of approximately 0.7 to 0.8 h−1 on glucose, whereas organisms such as Clostridium acetobutylicum typically achieve only 0.2 to 0.3 h−1. This disparity results in increased carbon flux and superior biofuel production performance in engineered E. coli strains. Other notable bacterial chassis include Bacillus subtilis, Pseudomonas putida, Clostridium acetobutylicum, and Corynebacterium glutamicum [43,44,45].
2.1.3. Phototrophic Systems and Filamentous Fungi
Microalgae differ from other platforms due to their photosynthetic capacity and high potential for lipid and biofuel production. Although they still face challenges related to industrial-scale cultivation, advances in synthetic biology and metabolic engineering have begun to address these limitations [46]. Chlamydomonas reinhardtii is the primary model organism owing to its robust set of molecular tools and well-annotated genome, and it is widely used in studies of lipid metabolism. Expanding beyond this model, the current status of other promising platforms high-lights the impact of precision engineering: Nannochloropsis oceanica leverages RNA Pol I-based systems to achieve eightfold higher transgene expression for tailored fatty acid production; Synechocystis sp. has reached titers of 230 mg·L−1 of 1-alkenes through CRISPR-mediated strategies; Chlorella sorokiniana is capable of accumulating 13% lutein; and Monoraphidium neglectum offers the unique advantage of sustained growth during nitrogen-limited lipid accumulation. These advancements overcome previous production challenges, positioning these species as effective industrial hosts for a wide range of bioproducts [46].
Although filamentous fungi do not directly produce biofuels, they supply key enzymatic systems used in both 1G (corn-based) ethanol and second-generation (2G) lignocellulosic ethanol production, facilitating polysaccharide hydrolysis and increasing sugar availability for fermentative microorganisms. Among filamentous fungi, Trichoderma reesei and Aspergillus niger stand out for their high secretory potential and enzymatic synthesis capacity, with strains already engineered to produce xylanases, cellulases, and α-L-arabinofuranosidases [27,47]. Thus, their use in microbial consortia can enhance biomass pretreatment and the release of fermentable sugars. This strategy can significantly impact production, as synthetic consortia based on metabolic engineering enable more robust and cooperative processes in which the complementary activities of different species improve the overall performance of fermentation [48].
The enhancement of microorganisms, combined with computational tools, further optimizes bioprocessing performance. Research shows that machine learning techniques such as artificial neural networks (ANNs) and support vector machines (SVMs) can accurately predict (R2 > 0.93) growth, biomass formation, and lipid accumulation during fermentation. This integration of genetic algorithms and SVMs reveals competitive relationships between lipid maximization and biomass production, suggesting important trade-offs in industrial processes [49].

Table 1.
Metabolic engineering strategies of microorganisms for the biosynthesis of alcohol-based biofuels.
Table 1.
Metabolic engineering strategies of microorganisms for the biosynthesis of alcohol-based biofuels.
| Microorganism | Type of Biofuel | Metabolic Engineering Strategy | Results | References |
|---|---|---|---|---|
| E. coli | Ethanol | Co-culture of two modified strains: one uses only glucose (xylR deletion) and the other only xylose (xylR mutation + deletions in ptsI, ptsG, galP, glk) | Production of 0.49 g/L/h and 46 g/L ethanol | [50] |
| Two-phase, two-temperature strategy with temperature-inducible promoters controlling glucose pathways; insertion of pdc and adhB from Zymomonas mobilis | Production of 127 g of ethanol from 260.9 g of sugars; productivity 4.06 g/L | [51] | ||
| Isoprenoid alcohols | The strategies included overexpression of the nudB and IspA genes via a heterologous MVA pathway; increased production through AphA phosphatase overexpression and idi modulation; use of an IspA mutant to redirect C10 alcohols synthesis | Production of 2027 mg/L of isoprenoid alcohols (C5–C15) and derivatives, with composition modulation by idi expression | [52] | |
| Isobutanol | Use of Entner-Doudoroff (ED) pathway to balance redox, inactivation of acid byproduct genes, expression to tuning to increase isobutanol flux | Production of 15.0 g/L of isobutanol, yield of 0.37 g isobutanol/g glucose | [53] | |
| Modified E. coli W (ldhA, adhE, pta, frdA deletions) + constitutive promoter library for isobutanol pathway | Production of up to 20 g/L of isobutanol using cheese whey as substrate | [54] | ||
| S. cerevisiae | Isobutanol | Overexpression of ZNF1, and deletion/reduction in BUD21, insertion of genes for isobutanol pathway + xylose metabolism (XR-XDH, xylose transporter, xylulokinase) | Production of 14.8 g/L of isobutanol, equivalent to 155.9 mg/g of xylose consumed (264.75% increase vs. control) | [55] |
| Elimination of competing pathways (LPD1 deletion) + overexpression of pyruvate carboxylase, malate dehydrogenase, and malic enzyme, activating an alternative transhydrogenase-like pathway | Production of 1.62 g/L of isobutanol from 100 g/L of glucose, with a yield of 0.016 g/g of glucose consumed in 24 h | [56] | ||
| Ethanol | Sequential gene deletion (GPD1, ALD6, NDE1/NDE2) + heterologous SeEutE expression for acetic acid tolerance and hypoxic fermentation | E5 strain showed a 200% increase in fermentation rate, consuming 25% of acetic acid | [57] | |
| Heterologous xylA expression from Burkholderia cenocepacia + adaptive evolutionary engineering via sequential batch culture with xylose | Ethanol yield and productivity 13% (0.51 × 0.45 g/g) and 120% (0.42 × 0.19 g/g cells·h) higher than the non-evolved strain, in addition to eliminating xylitol accumulation | [58] | ||
| Isoprenol | Insertion of mevalonate pathway for isoprenol biosynthesis; IPP (isopentenyl diphosphate) bypass construction; deletion of promiscuous kinase that diverted metabolic flux; bottlenecks identification via metabolomics + overexpression of promiscuous alkaline phosphatase | Production of 383.1 mg/L of isoprenol | [59] | |
| Octanol | Chain-length engineered FAS1 (Fas1R1834K/Fas2) → octanoyl-CoA → hydrolysis to octanoic acid → heterologous CAR expression (Mycobacterium marinum) + Sfp (B. subtilis) → reduction to 1-octanol → Ahr (Escherichia coli) overexpression | Achieved 26.0 ± 3.6 mg/L 1-octanol after 72 h; Ahr over-expression reached 49.5 ± 0.8 mg/L; also accumulated ~90 mg/L octanoic acid, identified growth toxicity of even ~50 mg/L 1-octanol for yeast | [19] | |
| Propanol | Artificial pathway: pyruvate → citramalate (cimA) → 2 KB; tdcB overexpression; enhanced thrA/B/C; GLY1 deletion | High density anaerobic fermentation produced 180 mg/L 1-propanol from glucose in the engineered strain | [20] | |
| Clostridium sp. | Butanol | Deletion of six histidine kinase (HK) genes via CRISPR-Cas9. | Butanol production increased by 40.8% (13.8 g/L) and 17.3% (11.5 g/L) compared to the wild strain; productivity increased by 40% and 20%, respectively | [21] |
| Push-pull engineering: ter overexpression to direct the flow of acetyl-CoA → butyryl-CoA; acid reassimilation pathway decoupled from acetone production to redirect carbon from butyrate and acetate to butyryl-CoA; deletion of xylR and araR + overexpression of xylT | The modified strain produced 4.96 g/L of n-butanol from corn cob, demonstrating a 235-fold increase compared to the wild strain | [22] | ||
| Overexpression of adhE2 and Ck hbd, coupling NADP+/NADPH metabolism to butanol biosynthesis to increase acetyl-CoA flux and the supply of reducing equivalents | A 50–60% increase in butanol production (0.24–0.28 vs. 0.15–0.18 g/g) and up to 4.5 times the butanol/ethanol and alcohol/acid ratios | [23] | ||
| Ethanol | Inactivation of adhE and deletion of aor1 and aor2, via ClosTron and allelic exchange mutagenesis, redirecting the metabolic flow of the acetogenic pathway towards ethanol formation | The inactivation of adhE led to an increase of up to 180% in autotrophic ethanol production | [24] | |
| Hexanol e Butanol | Insertion of gene clusters from C. kluyveri, C. acetobutylicum and Clostridium carboxidivorans via CRISPR-Cas9, expanding the biosynthesis pathway of hexanol and butanol alcohols | Production of 1075 mg/L of butanol and 133 mg/L of hexanol from fructose; 174 mg/L of butanol and 15 mg/L of hexanol from gas (CO2/H2); after further integration, 158 mg/L of butanol and 251 mg/L of hexanol | [25] |
Metabolic engineering strategies of microorganisms for the biosynthesis of alcohol-based biofuels. Summary of studies employing genetic and metabolic modifications in bacteria and yeasts to enhance alcohol production (ethanol, isobutanol, propanol, octanol, among others). Gene names are italicized to distinguish them from protein names.
2.2. Engineering Strategies for Microbial Chassis in PF
Innovation in PF is fundamentally driven by the rational engineering of microbial chassis, supported by advanced metabolic engineering and synthetic biology tools designed to reprogram metabolic pathways for the efficient production of target compounds. Genome editing strategies, including targeted mutagenesis and CRISPR-Cas systems, have been widely applied to optimize microbial performance by increasing the yields of both native metabolites and recombinant products. Synthetic biology and pathway engineering extend these approaches by enabling the construction and refinement of heterologous pathways, improving substrate utilization efficiency and allowing the use of low-cost and renewable feedstocks [33].
2.2.1. Genome Editing Technologies
CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats associated with Cas proteins) is a system derived from the adaptive immune machinery of bacteria and archaea that has been repurposed as a highly precise and programmable genome editing tool. It introduces double-strand breaks at specific genomic loci guided by a single-guide RNA (sgRNA), which recognizes the target DNA sequence in conjunction with a protospacer adjacent motif (PAM), typically 5′-NGG-3′ in the case of Streptococcus pyogenes Cas9 (SpCas9). The Cas9 nuclease is composed of two catalytic domains, the histidine–asparagine–histidine (HNH) and RuvC-like (RuvC) endonuclease domains, organized into a recognition (REC) lobe and a nuclease (NUC) lobe, which together cleave both DNA strands several nucleotides upstream of the PAM site [60].
Following DNA cleavage, the resulting double-strand breaks are repaired by microbial cells through either non-homologous end joining (NHEJ) or homology-directed repair (HDR), enabling controlled deletions, insertions, or nucleotide substitutions. These repair outcomes provide a mechanistic foundation for the application of CRISPR–Cas9 in metabolic engineering, where precise genome modifications are required to redirect metabolic fluxes, enhance product yields, and improve cellular robustness. Accordingly, in fermentation biotechnology, CRISPR–Cas9 has become the platform of choice for microbial engineering, in both prokaryotic and eukaryotic, due to its high specificity, efficiency, and multiplexing capability, facilitating the redesign of metabolic pathways and the development of high-performance industrial microorganisms, including chassis such as Escherichia coli and Clostridium spp. [60,61].
Bibliometric analyses indicate that CRISPR-Cas9 has played a pivotal role in accelerating the development of fourth-generation (4G) biofuels. The precise engineering of microorganisms has enabled enhanced lipid accumulation, optimization of biomass-to-fuel conversion pathways, and improved tolerance to environmental stresses, inhibitory compounds, and the biofuels themselves. By integrating genome editing with metabolic engineering, this approach helps overcome the classical cost and yield limitations associated with earlier biofuel generations, enabling the use of residual feedstocks and crops cultivated on marginal soils. Moreover, CRISPR-Cas9-mediated modification of lignin composition in agricultural residues simplifies biomass pretreatment and reduces processing costs in bioethanol production, positioning advanced biofuels as a strategy aligned with the Sustainable Development Goals related to clean energy and climate action, while opening new avenues for synthetic biology-driven pathway redesign and systems-level metabolic reengineering [62].
More recently, retron-mediated recombineering has emerged as a powerful genome editing strategy that exploits single-stranded DNA (ssDNA) donors generated in vivo by bacterial retrons during DNA replication, enabling the continuous and homology-dependent introduction of precise genetic modifications. In this system, a retron cassette is programmed to produce a multicopy ssDNA molecule carrying the desired mutation, flanked by sequences homologous to the upstream and downstream regions of the target locus. This ssDNA is synthesized by the retron-encoded reverse transcriptase from a non-coding msr/msd RNA (multicopy single-stranded RNA/multicopy single-stranded DNA), a retron-specific RNA structure in which the msr region acts as the RNA scaffold for reverse transcription, while the msd region encodes the single-stranded DNA donor. Incorporation occurs preferentially at the replication fork, particularly on the lagging strand, with the assistance of a single-stranded DNA annealing protein (SSAP), which promotes homologous pairing and homology-directed repair. As a result, the engineered mutation is progressively fixed in daughter strands over successive cell generations, enabling scalable and multiplexed genome editing [63].
This method has stood out due to its higher editing efficiency and the removal of the necessity for multiple cycles of external oligonucleotide introduction. The characteristics of this methodology enable editing in various phylogenetically distant bacterial species, including those where CRISPR is inefficient or unfeasible, making it a valuable alternative for industrial and environmental chassis that are underexplored in PF. Its effectiveness has already been demonstrated in bacterial chassis, including E. coli, Citrobacter freundii, Klebsiella pneumoniae, Vibrio natriegens, Aeromonas hydrophila, Shewanella oneidensis, Erwinia amylovora, Pseudomonas aeruginosa, P. putida, Pseudomonas syringae, Acinetobacter baylyi, Limosilactobacillus reuteri, Streptococcus suis, Mycobacterium smegmatis, and Cutibacterium acnes [64].
2.2.2. Synthetic Biology and Pathway Engineering
Synthetic biology is broadly defined as the systematic application of engineering principles to design new biological systems or to redesign existing ones by controlling genetic and metabolic components to perform specific functions, such as increasing efficiency, productivity, or enabling novel functionalities in microorganisms, including the production of next-generation biofuels. In this context, pathway engineering refers to the deliberate design and modification of metabolic routes within host organisms through strategies such as the construction of synthetic pathways, overexpression of rate-limiting enzymes, and deletion of competing pathways. These approaches aim to redirect metabolic fluxes toward the biosynthesis of advanced biofuels and are often implemented through the integrated use of genome editing tools and metabolic engineering frameworks [65].
Within the framework of advanced biofuel production, synthetic biology plays a central role by enabling the design of novel metabolic pathways and the construction of platform microorganisms capable of synthesizing molecules beyond ethanol and biodiesel, such as terpenoids and fusel alcohols, which can be further upgraded into fuels with higher energy density. In this context, microbial chassis such as P. putida, extensively engineered to valorize lignin-derived compounds into high-value intermediates, and Clostridium species modified to redirect acetyl-CoA from the Wood–Ljungdahl pathway (WLP) toward the production of longer-chain alcohols, including hexanol, exemplify how synthetic biology and metabolic engineering strategies are broadening the portfolio and improving the yields of advanced biofuels [66].
Pathway engineering is a core strategy for constructing platform microorganisms capable of producing biofuels and biochemicals at near-industrial levels by fine-tuning metabolic fluxes and mitigating the accumulation of toxic intermediates. Multilevel modulation strategies, including gene copy number, promoter and ribosome-binding site strength, enzyme scaffolding, and pathway compartmentalization, enable precise control over enzyme expression and localization, thereby maximizing TRY of key precursors such as fatty acids, alcohols, and terpenoids. Notably, the integration of dynamic sensing and regulatory elements has emerged as a critical advance, allowing real-time adjustment of source and sink pathways in response to intracellular metabolite levels. These developments underscore the transition from static pathway optimization toward dynamic metabolic control, which forms the foundation of regulatory circuit engineering [67].
2.2.3. Regulatory Circuits and Dynamic Metabolic Control
Gene regulatory circuits are defined as network-based representations of the interactions among the components of a gene regulation system, such as transcription factors, genes, and proteins, as well as the underlying regulatory logic that governs how information is processed and propagated through these interactions. This flow of regulatory information gives rise to dynamic system-level behaviors, including adaptive responses, oscillations, and multiple stable states, which can be exploited in biotechnological applications, such as the dynamic optimization of metabolic pathways for biofuel production [68].
In yeast systems, these regulatory principles can be implemented through the modular combination of cis-regulatory elements, such as hybrid promoters containing specific binding sites, and trans-acting activation or repression modules coupled to external signal sensors, enabling tight ON/OFF control of gene expression throughout the fermentation process. In S. cerevisiae, this approach includes the use of GAL-based promoters and orthogonal regulatory systems, such as Tet-ON/Tet-OFF circuits or temperature-responsive transcription factors, to activate or repress metabolic pathways in response to defined environmental or chemical cues. Such designs allow controlled transitions between growth phases with low metabolic burden and production phases characterized by high expression of target pathways. Overall, these synthetic regulatory architectures demonstrate how gene regulatory circuits can be rationally engineered to minimize cellular burden, enhance expression robustness across diverse cultivation conditions, and enable the dynamic modulation of complex metabolic routes, including those directed toward biofuel production [69].
Dynamic metabolic control in biofuel bioprocesses relies on synthetic regulatory circuits that adjust, in real time, the expression of pathway enzymes in response to intracellular or population-level signals, such as metabolite concentrations, cell density, or growth state, rather than maintaining static overexpression throughout fermentation. This strategy enables temporal redirection of metabolic fluxes, for instance by channeling acetyl-CoA from the Tricarboxylic Acid (TCA) cycle toward isopropanol production once a threshold cell density is reached through quorum-sensing systems that repress growth-associated genes while activating product-forming pathways. In parallel, metabolite-responsive regulators sensing toxic intermediates, such as acyl-CoA or farnesyl pyrophosphate (FPP), can dynamically repress upstream modules, including the mevalonate pathway, while activating downstream product synthesis, such as amorphadiene or biodiesel-like esters, thereby mitigating toxicity and improving yields. Additionally, circuits coupled to glycolytic flux or metabolites like acetyl phosphate have been used to trigger rate-limiting steps of production pathways only during stationary phase, reducing metabolic burden during growth and reallocating cellular resources toward high productivities in the production phase, a critical requirement for large-scale biofuel bioreactors [70].
In practical biofuel bioprocesses, these concepts have been implemented primarily in model and industrial microorganisms such as E. coli and yeasts, in which biosensor-regulator circuits dynamically adjust the expression of fuel-producing pathways in response to cues. For instance, in biodiesel and fatty acid-producing E. coli, feedback control systems have been engineered to sense the accumulation of toxic intermediates such as malonyl-CoA or acyl-CoA and accordingly up- or downregulate pathway genes, maintaining precursor levels within safe ranges while improving genetic stability and product titers. Another reported strategy involves oxygen-responsive promoters in aerobic systems, where oxygen limitation in large-scale bioreactors triggers the modulation of glucose transporter expression, reducing substrate uptake, preventing excessive acetate formation, and alleviating energetic stress during the production of advanced biofuels [71].
Modular regulatory platforms capable of sensing multiple classes of signals enable adaptive reprogramming of microbial chassis metabolism, allowing optimized responses to fluctuating bioreactor conditions and coordinated transitions between distinct fermentation stages [72]. Collectively, these advances underpin the emergence of highly customizable, intelligent, and increasingly automated fermentation processes, substantially enhancing operational flexibility, process robustness, and industrial applicability [73].
2.3. Process Monitoring and Control (Omics, Sensors, Artificial Intelligence)
PF requires rigorous monitoring and control that extends beyond the core fermentation stage. This phase is crucial for ensuring reproducibility and maintaining optimal bioprocess conditions, as optimized workflows generate large volumes of multi-omics data (genomic, transcriptomic, proteomic, and metabolomic), which are essential for assessing reproducibility and supporting the optimized conditions fundamental to PF [74]. To enable such intensive data collection, it becomes imperative to transition from conventional bioprocessing to smart biomanufacturing, which employs sensors for continuous data monitoring and integrates analytical algorithms, including AI-based methods capable of extracting information from massive and multidimensional datasets [75].
The integration of AI in PF has evolved from simple statistical correlations to a transversal tool for yield prediction and real-time kinetic control. ANNs, particularly Multilayer Perceptrons (MLP), have become the predominant architecture, utilized in approximately 90% of studies concerning lignocellulosic ethanol due to their superior ability to map the non-linearities of enzymatic hydrolysis and fermentation. Comparative studies indicate that MLP models consistently outperform Response Surface Methodology (RSM), achieving predictive accuracies of R2 ≈ 0.997 in glucose-to-ethanol conversions [76,77,78,79].
Beyond standard ANNs, a diverse array of algorithms has demonstrated exceptional robustness in complex substrates:
- Ensemble Learning: Methods such as Random Forest (RF), XGBoost, and CatBoost utilize variables like Chemical Oxygen Demand (COD) and volatile acid profiles to identify critical operational triggers via SHAP (SHapley Additive exPlanations) analysis [80,81,82].
- Deep Learning: Architectures including Recurrent Neural Networks (RNN), Long Short-Term Memory (LSTM), Gated Recurrent Units (GRU), and Transformers are increasingly applied to process temporal bioprocess data [83,84].
- Hybrid Systems: Adaptive Neuro-Fuzzy Inference Systems (ANFIS) provide a balance between the predictive power of neural networks and the interpretability of fuzzy logic, enabling early fault detection and process stabilization [85].
Traditionally, bioprocess monitoring relies on hardware-based sensors; however, these devices present limitations such as high cost, measurement delays, and the need for frequent maintenance, which can hinder operation under certain environmental conditions. In this context, soft sensors, also referred to as virtual or inferential sensors, integrate data-driven mathematical models with physical principles to estimate quality parameters that are difficult to measure directly. There are developed through three distinct approaches: (I) model-driven, which uses first-principles or physicochemical models; (II) data-driven, which uses statistical modeling or AI/machine learning; and (III) hybrid (gray-box), which combines data-driven and first-principles modeling. AI algorithms such as ANNs, deep neural networks (including recurrent neural networks, RNNs; long short-term memory networks, LSTMs; gated recurrent units, GRUs; and Transformers), fuzzy systems, support vector regression (SVR), principal component analysis (PCA), and evolutionary algorithms have been widely applied to soft sensin [86].
The frontier of PF lies in the deployment of Digital Twins—virtual replicas that synchronize mechanistic kinetic models with real-time data streams. A pivotal advancement is the use of Soft Sensors integrated with Raman or Near-Infrared (NIR) spectroscopy. For instance, the application of Spatio-Temporal Convolutional Neural Networks (STC-CNN) to sequential Raman spectra has enabled the automated control of glucose feeding in fed-batch systems, reducing glycerol byproduct formation by 6.7% and increasing maximum ethanol concentrations [87,88,89].
AI has also revolutionized microbial strain design and biosynthetic pathways. AutoCRISPR speeds up development cycles by using convolutional neural networks (CNNs) to guess what will happen when editing goes wrong. This cuts the time it takes from months to weeks [90]. Furthermore, the synergy between Machine Learning and Constraint-Based Modeling (CBM) allows for high-level optimization; utilizing XGBoost to optimize promoter combinations has achieved yield increases of 7.4% at elevated temperatures (40 °C) in S. cerevisiae [91].
These computational architectures, coupled with evolutionary algorithms like Genetic Algorithms (GA) and Particle Swarm Optimization (PSO), have led to typical productivity gains of 5–20%, positioning AI as an indispensable driver for the economic viability of advanced biofuels [78,92]. However, as a relatively new and rapidly evolving field, existing challenges include the lack of data standardization, the limited interpretability of AI models (essential in regulatory contexts), and the need for interdisciplinary collaboration to ensure robust, safe, and sustainable applications [93].
3. Application in Biofuel Production
3.1. Bioethanol and Advanced Alcohols
Global bioethanol and biodiesel production between 2005 and 2018 surged from 49.9 billion to 167.9 billion liters. Bioethanol accounted for approximately 65% of this total, while biodiesel constituted around 35%. The countries with the highest bioethanol production (>80%) are the United States and Brazil, whereas the European Union leads biodiesel production. In 2022, global biofuel production was estimated at approximately 1.914 million barrels of oil equivalent per day, with a projected growth of 38 billion liters between 2023 and 2028. The primary feedstocks utilized in this process are predominantly corn grain (16.1%), followed by vegetable oil (13.5%), other feed grains (3.3%), and wheat grain (1.7%) [94,95].
Liquid biofuel production is largely dominated by alcohols, with bioethanol being the primary product, accounting for approximately 65% of global output, supported by its cleaner combustion and higher thermal efficiency [96]. Bioethanol is classified into four generations, highlighting the need to transition from 1G production (based on food crops and associated with sustainability concerns) to 2G and subsequent pathways [10,97]. Lignocellulosic biomass (2G) emerged as an abundant but uncompetitive raw material due to its inedible nature (unlike 1G materials) and recalcitrant structural matrix, which requires expensive pretreatment and enzymatic hydrolysis steps before fermentation. While 1G biofuels rely on readily available sugars or starches, 2G platforms utilize the carbon retained in cellulose and hemicellulose polymers from agricultural and forestry residues [11,98].
Beyond these feedstock challenges, industrial efficiency in ethanol production is invariably lower than the theoretical yield of 51% (w/w), meaning 1 g of glucose can yield a maximum of 0.51 g of ethanol due to process losses and limitations [99]. The standard industrial host, S. cerevisiae, is highly tolerant of ethanol but unable to metabolize xylose (C5), the second most abundant sugar in lignocellulose. Other microorganisms, such as Z. mobilis, also fail to metabolize C5 sugars and exhibit sensitivity to bioprocess stresses [9]. These values fluctuate depending on the feedstock, technology, generation, and operational conditions. Furthermore, conventional microbial fermentation is limited by the inefficiency of wild-type microorganisms.
To provide a more holistic overview of the current bioenergy landscape, this study expands the comparative analysis beyond traditional 1G and 2G technologies by incorporating third-generation (3G) and 4G biofuels. While 3G biofuels utilize algal biomass to overcome land use competition, 4G technologies represent the frontier of the field, employing genetically engineered microorganisms and carbon capture strategies to directly convert CO2 into drop-in fuels. This comprehensive classification, summarized in Table 2, evaluates each generation not only by their feedstock and metabolic pathways but also through standardized metrics of industrial yield (g/g) and final product titer (g/L), highlighting the technical bottlenecks that remain for the large-scale deployment of advanced generations.

Table 2.
Comparison of 1G to 4G ethanol industrial yields versus theoretical.
To overcome these limitations in yield and purity, PF and metabolic engineering are essential. Current strategies focus on cultivating strains that can (1) simultaneously con-vert C5 and C6 sugars [110]; (2) increase tolerance to inhibitors and to ethanol [111]; and (3) improve carbon flux toward the target product via genetic modifications, including gene insertion, deletion, or regulation [112]. The main strategies are provided in detail in Table 1, listed in Section 2.1.
In this context, the yeast S. cerevisiae is the principal industrial chassis for 2nd generation (2G) ethanol production. However, it lacks the native capability to consume pentoses from lignocellulosic hydrolysates, necessitating genetic/metabolic modifications. Optimization initiates with the co-fermentation of hexoses and pentoses, enabled by the insertion of heterologous pathways (such as genes from Pichia stipitis). Subsequent modifications include the modulation of stress response gene expression (e.g., TPS1) to increase tolerance to multiple inhibitors, enhancement of thermotolerance, and process intensification strategies like cell recycling and in situ ethanol removal [113,114].
More advanced strategies involve systems-level approaches, such as combinatorial promoter optimization to modulate key genes in the fermentative pathway (e.g., PDC1, ADH1). In addition to engineered S. cerevisiae, non-conventional yeasts are also utilized as alternative platforms. These include Scheffersomyces stipitis and Spathaspora passalidarum (which are naturally pentose-fermenting) and K. marxianus and Ogataea polymorpha (which are thermo-tolerant) [91,113,114].
The term “advanced alcohols” (2G, 3G, and 4G) refers to alcohols produced via technological routes, from feedstocks, and possessing chemical properties that differ from, and are more sustainable than, conventional (1G) ethanol. PF is equally crucial for the biosynthesis of advanced alcohols (isobutanol, propanol, pentanol, and octanol), representing a high-performance alternative to ethanol due to its lower corrosiveness, higher calorific value, and lower hygroscopicity [115,116]. Although isobutanol production is predominantly fossil-based, metabolic engineering in hosts such as S. cerevisiae and E. coli has been applied to optimize synthesis (Figure 3) by introducing heterologous pathways (bacteria) or enhancing native pathways and coupling with xylose assimilation (yeasts) [117,118].
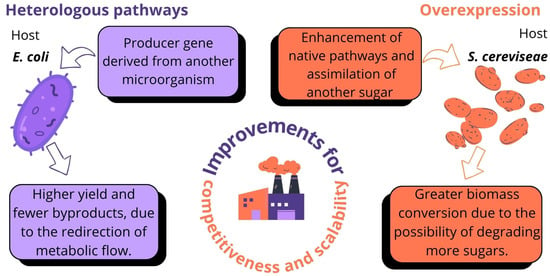
Figure 3.
Metabolic engineering for the production of advanced alcohols.
The integration of PF is, therefore, the way to optimize the conversion of lignocellulosic substrates into advanced bioalcohols, enabling competitiveness and industrial scaling. A comparison between unmodified (wild-type) and GM microorganisms reveals that GM strains exhibit 10- to 100-fold higher yields, reduced fermentation times, and enhanced conversion efficiency of lignocellulosic sugars in the production of advanced alcohols, as listed in Table 3.

Table 3.
Yields of wild-type and genetically modified microorganisms in the production of ethanol, isobutanol, and n-butanol.
3.2. Drop-In Hydrocarbons and Lipid-Based Biofuels
Despite the advances in studies involving the production of biofuels, most are not fully compatible with current fossil fuel-based engines and supply systems, requiring blends with nonrenewable fuel sources, such as gasoline, or even adaptations in the engines [119,120]. Given this issue, various studies aim to produce fuels with properties similar to those currently used, such as gasoline, diesel, and aviation kerosene [121,122,123].
These fuels, known as “drop-in” hydrocarbons, are derived from renewable sources like plant biomass and possess identical physical and chemical properties to petroleum derivatives, enabling them to directly substitute conventional fuels without necessitating modifications to engines or refueling infrastructure. The biomass utilized for fuel production can be transformed via thermochemical methods (gasification, pyrolysis, hydrothermal liquefaction) or biochemical processes, wherein microorganisms ferment sugars and lipids to generate compounds that replace fossil fuels [124]. Among these microorganisms, those that produce enzymes like acyl-ACP reductase (AAR) and aldehyde-deformylating oxygenase (ADO) are notable for their ability to transform fatty acids into alkanes and alkenes [125,126].
The metabolic pathways for generating drop-in fuels differ based on the microorganism utilized and encompass: (1) the genetic modification of yeasts such as Y. lipolytica to transform biomass-derived sugars into liquid alkanes and alkenes [71,72]; (2) the cultivation of oleaginous algae abundant in lipids (triacylglycerols—TAGs and fatty acids), which can be converted into biodiesel or bio-oil via transesterification and hydrothermal liquefaction [127,128,129,130,131]; and (3) the engineering of lignocellulolytic bacteria for the synthesis of short- and long-chain hydrocarbons [132,133].
In this context, the yeast Y. lipolytica is notable for its metabolic versatility and capacity for lipid fuel synthesis [134]. Cyanobacteria are also promising for producing alkanes and alkenes naturally via the AAR/ADO pathway, with genetic engineering aimed at optimizing these pathways [125]. Oilseed algae represent a group with significant potential for “drop-in” biofuel production, as they accumulate neutral lipids and hydrocarbons capable of substituting biodiesel, aviation kerosene, and green diesel [135,136].
Metabolic customization plays a central role in this process, enabling the adjustment of chain length, branching degree, and saturation level of carbon chains, which define the physicochemical properties of the resulting fuels. For instance, short-chain and branched biofuels (C4–C8), known for their enhanced combustion efficiency, are derived from α-ketoacids via the Ehrlich pathway [137,138,139]. The modification of genes linked to this pathway has been a major focus for increasing production yields [137,138,140]. Metabolic engineering approaches in S. cerevisiae have been utilized to augment the production of branched alcohols (e.g., isobutanol) via the Ehrlich pathway and subsequently transform them into short-chain branched esters by overexpression of alcohol acyltransferases [141,142]. The Ehrlich pathway has been engineered in hosts such as E. coli, B. subtilis, C. glutamicum, Brevibacterium flavum, Ralstonia eutropha, and Synechococcus elongatus 7942 [143,144].
Several specific pathways are targets for this engineering. Engineering of the Ehrlich pathway for advanced alcohols focuses on three enzymatic steps and their corresponding genes. First, Transaminases (such as BAT1 and BAT2) initiate the process by converting amino acids into α-keto acids. Subsequently, α-Keto Acid Decarboxylases (KDCs), such as ARO10 or the heterologous kivd gene, remove carbon dioxide (CO2) to form an aldehyde. Finally, Alcohol Dehydrogenases (ADHs) reduce this aldehyde to the desired alcohol, with the overexpression of precursor pathway genes (such as ILV) also being a common strategy to maximize metabolic flux [145,146].
Conversely, long-chain and branched hydrocarbons (C15–C20) exhibit improved fluidity and lower freezing points, making them ideal for aviation applications [143,147]. These molecules are produced by Gram-positive bacteria such as B. subtilis, using α-ketoacid dehydrogenase (BKD) and the FASII system to elongate α-ketoacids. Genes from this pathway, including the bkd operon and fabH2, are inserted to synthesize long-chain branched fatty acids [143,148,149].
Another determining factor is the saturation level of the molecules, which affects fluidity, thermal stability, and freezing point [150]. Unsaturation lowers the melting point, which is good for aviation biofuels [151]. On the other hand, higher saturation levels improve thermal and oxidative stability, which is good for marine and automotive fuels [152]. The engineering of pathways that control the activity of desaturases and reductases makes it possible to change these properties. The overexpression of desaturases (Ole1 or Fad2) in microorganisms such as S. cerevisiae and Y. lipolytica aims to increase the production of unsaturated fatty acids, whereas deletion of these enzymes redirects metabolism toward more saturated and stable compounds [153,154,155,156,157,158]. Thus, advances in metabolic engineering and synthetic biology have enabled the improvement of microorganisms capable of synthesizing drop-in hydrocarbons with tunable properties, accelerating the commercial feasibility of lipid-based biofuels derived from renewable sources. However, despite these advances, a substantial gap still exists between the production of sustainable aviation fuel (SAF) and the global demand for aviation fuel. Current SAF supply accounts for only about 3% of total renewable fuel consumption, reflecting both limited production capacity and constraints associated with the availability of biomass on a large scale [159]. Some uses and types of advanced biofuels produced in PF are listed in Figure 4 and metabolic engineering strategies employed are listed in Table 4.
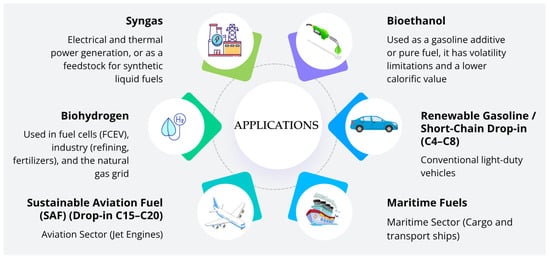
Figure 4.
Uses and types of advanced biofuels produced in PF.
For other products, the Fatty Acid Synthesis (lipogenesis) pathway employed by GM Yarrowia lipolytica involves the conversion of biomass-derived sugars into acetyl-CoA, subsequently into fatty acids/lipids, and finally into liquid alkanes/alkenes, utilizing metabolic engineering to optimize each stage of the process. In this process, the AAR/ADO pathway functions as a shunt branching from primary fatty acid (lipid) synthesis. The route initiates when the first enzyme, AAR, utilizes an Acyl-ACP molecule (an activated fatty acid) and converts it into a fatty aldehyde. Concurrently, the second enzyme, ADO, acts upon this aldehyde, removing the carbon from the aldehyde group (deformylation) to generate a terminal alkane. The production of alkenes (containing double bonds) follows the exact same pathway, occurring simply when the initial Acyl-ACP substrate is already unsaturated [160,161].

Table 4.
Metabolic engineering approaches microorganisms to produce “drop-in” and lipid based.
Table 4.
Metabolic engineering approaches microorganisms to produce “drop-in” and lipid based.
| Species | Target Product | WT Yield | GM Yield | WT vs. GM Improvement | Conditions | Genetic Strategy (GM) | References |
|---|---|---|---|---|---|---|---|
| E. coli | Isobutanol | ∼0 g/L | 22 g/L | N/A (WT = 0) | Glucose (10%), fed-batch, 30 °C, 48 h | Insertion of isobutanol pathway: overexpression of alsS (BS), ilvC/D (Ec), kivd (Lc), adhA (Ec) | [162] |
| S. cerevisiae | Isobutanol | ∼0.008 g/L | 1.6 g/L | ∼200-fold increase | Glucose (20 g/L), batch, 30 °C, 72 h | Overexpression of ILV2, ILV5, ILV3 (valine pathway), ARO10 (KDC), and ADH2 (ADH) | [163] |
| E. coli | n-Butanol | ∼0 g/L | 15 g/L (∼0.19 g/g glucose) | N/A (WT = 0) | Glucose (80 g/L), fed-batch, 37 °C, anaerobic, 72 h | Modified Clostridial pathway (CoA-dependent): optimized thl, hbd, crt, bcd, etfAB, adhE2 | [164] |
| S. elongatus PCC 7942 | Isobutanol (secreted) | ∼0 g/L | 0.45 g/L | N/A (WT = 0) | Phototrophic culture (CO2), 30 °C, 6 days | Insertion of isobutanol pathway (as in E. coli): alsS (BS), ilvC/D (Ec), kivd (Lc), adhA (Ec) | [165] |
| E. coli | Alkanes (C13–C17) | ∼0 g/L | 0.58 g/L | N/A (WT = 0) | Glucose (20 g/L) + Oleic Acid (0.1%), 30 °C, 48 h | Insertion of Acyl-ACP Reductase (AAR) and Aldehyde-Deformylating Oxygenase (ADO) from cyanobacteria | [166] |
| Y. lipolytica | Lipids (TAGs) | ∼5 g/L | ∼15 g/L | ∼3-fold increase | Glucose (150 g/L), fed-batch, high C/N ratio, 96 h | Overexpression of DGA1 and DGA2 (TAG synthesis) and ACC1 (malonyl-CoA synthesis) | [167] |
| Y. lipolytica | Lipids (TAGs) | ∼7.5 g/L | ∼35 g/L | ∼4.6-fold increase | Glucose, fed-batch, high C/N ratio, 120 h | Deletion of all 6 POX genes (β-oxidation) + overexpression of DGA1 and DGA2 | [168] |
| Rhodosporidium toruloides | Lipids (TAGs) | ∼0.41 × X g/L | ∼0.65 × X g/L | ∼1.6-fold increase | Glucose (70 g/L), C/N = 100, 144 h | Overexpression of ACC1 (Acetyl-CoA carboxylase) using CRISPR-Cas9 | [169] |
| Saccharomyces cerevisiae | Farnesene | ∼0 g/L | ∼25 g/L | N/A (WT = 0) | Sugarcane molasses, fed-batch, 30 °C, ∼120 h | Overexpression of the Mevalonate (MVA) pathway (e.g., tHMG1), deletion of ERG9, insertion of Farnesene Synthase | [170] |
| Escherichia coli | Free Fatty Acids (FFAs) | ∼0 g/L (secreted) | ∼4.5 g/L | N/A (WT = 0) | Glucose (20 g/L), batch, 37 °C, 24 h | Deletion of fadD (prevents re-import/degradation) + overexpression of Thioesterase (TesA) | [14] |
Overview of microbial engineering strategies for the generation of hydrocarbon-like biofuels, including diesel, biojet fuel, and gasoline analogs. The table compiles microorganisms, target compounds, and performance outcomes reported in the literature. N/A = Not applicable, WT = Wild type, GM = Genetically modified. Gene names are italicized to distinguish them from protein names.
3.3. Syngas Fermentation
An emerging technological route for the sustainable synthesis of energy intermediates is synthesis gas (syngas) fermentation. In this process, anaerobic bacteria (particularly those of the Clostridium genus) are employed to convert industrial waste gases, such as CO2, carbon monoxide (CO), using hydrogen (H2) as a critical electron donor, into industrial products, including butanol, ethanol, and hexanol. The central biological mechanism in acetogenic bacteria is the WLP, also known as the reductive acetyl-CoA pathway, which enables acetogens to convert carbon from gaseous substrates into the metabolic intermediate acetyl-CoA. This intermediate is subsequently converted into biomass and acetate, which is further reduced to generate the desired alcohols (Figure 5) [15,16,171].
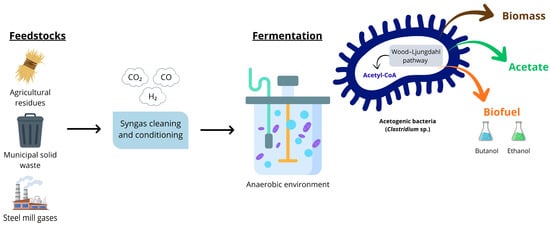
Figure 5.
Biological Conversion of Syngas into acetate, biomass, and biofuels via the Wood–Ljungdahl Pathway.
However, process efficiency and stability are variable and influenced by factors such as microbial strain, nutrient medium, and gas composition. A critical factor is oxygen (O2), which acts as a strong inhibitor and can completely suppress alcohol production at elevated concentrations [172]. A practical study demonstrated the conversion potential of biogenic syngas (gas generated by gasification) using the acetogenic bacteria Clostridium carboxidivorans and Clostridium autoethanogenum. Initially, O2 represents a critical component with a suppressive effect: untreated bi-ogenic syngas, containing 2459 ppm of O2, resulted in the complete inhibition of C. carboxidivorans’s metabolism, preventing growth, gas uptake, and alcohol production. The pre-treatment with a Pd catalyst and the subsequent reduction in the O2 content to 293 ppm was the decisive factor, resulting in an efficiency comparable to the reference artificial syngas, with biomass, acetate, and ethanol concentrations in the same order of magnitude and a carbon balance of 89.7–93.2%, similar to the 93.9% of the con-trol. The use of biogenic syngas (with reduced O2) in C. autoethanogenum resulted in gains in efficiency and selectivity relative to the reference gas. Process optimization was demonstrated by a 31% increase in the final ethanol concentration (from 2.74 g/L to 3.60 g/L) and a significant 104% increase in the final 2,3-butanediol (2,3-BDO) concentration (from 0.52 g/L to 1.06 g/L) [172].
Metabolic engineering and synthetic biology can overcome limitations in productivity and selectivity. Given the limited energy availability in chassis like C. autoethanogenum, computational (in silico) modeling aids in enhancing selectivity by identifying bottlenecks in branching pathways that produce undesirable by-products [173]. A recent study employed in silico stoichiometric and kinetic modeling for a systematic analysis of C. autoethanogenum metabolic network, identifying the most effective single, double, and triple interventions for the overproduction of 2,3-BDO with minimal carbon loss. Quantitative analysis revealed that the overexpression of the pyruvate–ferredoxin oxidoreductase (PFOR) enzyme constituted the most effective single intervention on the 2,3-BDO production rate, leading to a 2.6-fold increase (reaching 0.10 ± 0.023 compared to 0.039 ± 0.0091 of the WT). The most promising double intervention was the combined overexpression of PFOR and Acetolactate Synthase (ACLS), which elevated production to 0.14 ± 0.030, representing a 3.59-fold increase relative to the WT and a 1.4-fold gain over the single PFOR intervention. Furthermore, the triple intervention involving the overexpression of PFOR, ACLS, and Acetolactate Decarboxylase (ACLDC) achieved a flux value of 0.15 ± 0.056, corresponding to a fourfold increase compared to the WT and a 7% enhancement relative to the combined positive intervention of PFOR and ACLS [174].
Validated genetic approaches encompass the inactivation of the adhE gene in C. autoethanogenum, yielding a 180% enhancement in ethanol production, and a reduction of up to 38% in the acetate byproduct, alongside the creation of CO-responsive transcriptional modules in Eubacterium limosum, which, when integrated with CRISPR–Cas9, redirect carbon flux towards target products such as 2,3-BDO [24,175].
3.4. Biohydrogen as a Strategic Energy Vector
Hydrogen is recognized as a fundamental energy vector due to its clean combustion (which results solely in water vapor) and its high mass energy density. It exhibits a higher heating value (HHV) in the range of 120–142 MJ/kg, which is approximately three times the energy content of gasoline (whose fractions range between 41.1 and 44.6 MJ/kg) [176,177,178]. Hydrogen is considered an essential energy vector for a future carbon-neutral system. Although low-carbon alternatives exist, such as blue hydrogen (with Carbon Capture and Storage—CCS) and green hydrogen (produced via electrolysis), global production is overwhelmingly dominated by fossil routes. Currently, over 95% of the world’s hydrogen originates from non-renewable sources (primarily steam methane reforming—SMR), with green hydrogen (derived from 100% renewable electricity) restricted to only 1% of the total output [176,179,180].
In this context, biohydrogen production emerges as a decentralized and resilient alternative. Biohydrogen is a high-performance, non-polluting energy carrier that relies on biological systems or thermochemical treatments to convert organic waste or biomass. This route is particularly attractive because it employs low-cost renewable feedstocks, offering a more flexible mechanism to overcome the cost and infrastructure barriers faced by other low-emission hydrogen pathways [17].
3.4.1. Biological Pathways and Yield Engineering
Biohydrogen production predominantly transpires via dark fermentation (DF) and photosynthetic processes (photofermentation and biophotolysis), with yield engineering as the principal approach to enhance its purity and productivity. During DF, microorganisms function in anaerobic environments to transform organic substrates into alcohols, organic acids (such as acetic and butyric acids), and gases, including H2. This light-independent process is predominantly executed by bacteria from the Clostridium and Enterobacter genera. The efficiency is contingent upon hydrogenase activity and the corresponding electron transfer mechanisms [100,181].
The formate–hydrogen lyase pathway, characteristic of Enterobacter, converts pyruvate into formic acid, which is subsequently cleaved to release H2 and CO2. In contrast, in the ferredoxin pathway (Clostridium), the PFOR enzyme oxidizes pyruvate. The maximum theoretical yield (4 mol H2 per mol of glucose) is achieved when the fate of acetyl-CoA is directed toward acetate formation, but it is reduced by half when acetyl-CoA is diverted to butyrate production [100,181]. For Enterobacter, several studies have directly compared wild-type and genetically engineered strains. For example, the overexpression of the fhlA gene in Enterobacter cloacae WL1318 increased cumulative hydrogen production by 188% and improved the H2/S ratio by 37% compared with the wild-type strain [182].
In contrast, photofermentation and biophotolysis constitute phototrophic hydrogen-producing pathways, functioning as complementary routes to DF in integrated biohydrogen systems. In photofermentation, photosynthetic bacteria such as Rhodobacter use light to oxidize organic acids, often generated during DF, and convert them into hydrogen through the combined activity of hydrogenases and nitrogenases [183,184,185,186]. The overall reaction can be represented as (Equation (1)):
Recent studies have demonstrated the applicability of this route using real effluents; for example, Rhodobacter capsulatus B10 produced up to 126.5 mL H2/g of VFA from effluents derived from orange peel, with the highest yields obtained at a 25% effluent concentration and reduced performance at higher concentrations due to increased opacity of the medium and inhibition of the substrate [187]. The efficiency of this process depends on key parameters such as light intensity, optimization of the carbon-to-nitrogen ratio, and the incorporation of metal cofactors [183,184,185,186].
Building on this phototrophic framework, biophotolysis represents another light-driven route for hydrogen formation but relies directly on water photolysis rather than organic acid oxidation. In biophotolysis, solar energy captured by photosystem II promotes the cleavage of water molecules, releasing oxygen, electrons, and protons. The reduced electrons generated in this process are subsequently directed toward hydrogen production via proton reduction, catalyzed by hydrogenase or nitrogenase enzymes. This pathway can occur as either direct or indirect biophotolysis [188].
In direct biophotolysis, solar energy captured by photosystem II drives water splitting, releasing electrons that are transferred to photosystem I, where they reduce protons through the action of hydrogenases. This results in hydrogen formation without greenhouse gas emissions, while oxygen is simultaneously released into the atmosphere [188]. The overall reaction is shown in Equation (2):
In indirect biophotolysis, electrons generated during photosynthesis are first used to fix CO2 and synthesize carbohydrates (Equations (3) and (4)), which are subsequently degraded, in the absence of light, to produce hydrogen [186]:
While phototrophic routes rely on light as the primary energy source, DF constitutes a fully anaerobic and light-independent biological process, representing a complementary strategy for hydrogen production. DF converts organic residues into hydrogen and volatile fatty acids (VFAs) and is considered a truncated form of anaerobic digestion involving three main microbial stages. In the first stage, hydrolysis, hydrolytic bacteria degrade complex biopolymers into sugars, amino acids, and lipids. In the subsequent acidogenesis step, these molecules are fermented by acidogenic bacteria, producing VFAs such as acetic, propionic, butyric, lactic, and valeric acids, in addition to alcohols and carbonic acid. Hydrogen yield depends on the dominant acidogenic pathway: the acetate pathway produces 4 mol H2 per mol glucose (Equation (5)), while the butyrate pathway yields only 2 mol H2 per mol glucose (Equation (6)) [189]. Thus, the microbial composition and predominant metabolic route determine the overall efficiency of hydrogen production.
Linking DF to practical applications, the DF of food waste is particularly efficient due to its high carbohydrate content. In batch tests, various combinations of Clostridium butyricum, Clostridium beijerinckii, Lactobacillus plantarum, and Lactobacillus pentosus were evaluated, with the most effective formulation producing 46.0 ± 0.7 mL H2 g VS−1. When employed in a bioaugmentation strategy with sterile and non-sterile food waste, this combination achieved 89.6 ± 1.0 mL H2 g VS−1 and 76.7 ± 2.6 mL H2 g VS−1, respectively. The resulting microbial community was dominated by C. butyricum sensu stricto I, while Enterobacter contributed to lower yields in non-sterile conditions [190].
To overcome the yield and purity limitations of natural pathways, biohydrogen pro-duction can be enhanced through PF and metabolic engineering. In DF, genetic engineering can employ tools such as CRISPR-Cas to modify electron flow, redirecting reduced ferredoxin specifically to H2 production, minimizing the formation of competing byproducts. This result can be achieved by overexpressing hydrogenases and cofactor recycling enzymes, such as ferredoxin-NADH oxidoreductases and PFOR, while simultaneously suppressing competing metabolic pathways. These modifications can lead to significant improvements, enabling a 100% yield increase compared to wild-type strains, approaching or exceeding the theoretical maximum of 4 mol of H2 per mol of glucose used [100,191,192].
In biophotolysis, genetic engineering operates at two levels: structural and enzymatic. Structurally, it is possible to modify microalgae, such as C. reinhardtii, by reducing the size of their light-harvesting antennae and diminishing the chlorophyll content per unit cell volume. This leads to increased light penetration and transmittance, thereby enhancing photosynthetic performance, which is critical for boosting biomass and lipid production [193]. Structural light optimization is a crucial factor for gains in H2 productivity in high-density cultivation systems. In assays under saturating light intensity (285 μE m−2 s−1), the truncated antenna mutant (tla1) achieved a maximum specific H2 photoproduction rate of approximately 3.0 μmol mg−1Chlh−1, exceeding the parental strain (approximately 0.75 μmol mg−1Chlh−1) by fourfold. Furthermore, this modification conferred upon the mutant an efficiency of light energy conversion to H2 up to 8.5 times higher than the wild-type under saturating light [194].
Enzymatically, protein engineering overcomes enzyme sensitivity to O2 by creating transgenic mutant enzymes, for instance, with single and double mutations, in which the enzyme gas access channel is narrowed. This design allows for the preferential H2 egress while simultaneously restricting O2 ingress. In in vitro assays with 5% O2, the mutant produced up to 22 µL mL−1 of H2, a productivity approximately 7 times greater than the wild-type (3 µL mL−1). In long-term in vivo trials, the H2 concentration in the mutant reached 70 µL mL−1, a value approximately 30 times superior to the wild-type [195].
3.4.2. Barriers, Scalability, and Hybrid Biorefineries
Biohydrogen production encounters substantial obstacles that hinder its commercial feasibility, notably the low efficiency and yield of biological processes on an industrial scale. Various intrinsic and environmental factors serve as constraints, including the organism utilized, substrate type and concentration, pH, light availability, temperature, by-product inhibition, and oxygen sensitivity [196,197,198].
Moreover, biohydrogen is produced as a mixture with other gases, requiring rigorous purification techniques (such as pressure swing adsorption or selective membranes) to achieve the 99.9% purity necessary for fuel cell applications [199]. Storage and transportation present another major logistical and economic challenge due to its low energy density, necessitating costly and complex compression or liquefaction processes, as well as specialized infrastructure and safety considerations [200]. Bioreactor design, including systems such as the dynamic membrane bioreactor (DMBR), is also critical for achieving scalability [201].
To overcome the economic infeasibility of standalone production, the concept of hybrid biorefinery has gained prominence. This model focuses on the coproduction of multiple products, such as in co-fermentation systems using C. acetobutylicum and Enterobacter aerogenes to generate H2, ethanol, butanol, and acetone, while solid residues are digested to produce biomethane [202]. Other ways to coproduce include making organic acids and biopolymers from DF effluents and using genetic innovation to improve the coproduction of H2 and ethanol [196,203].
One particularly efficient reactor strategy is coupling DF with microbial electrolysis cells (MECs), which are bioelectrochemical systems in which electroactive microorganisms provide oxidized volatile fatty acids (VFAs) and other carboxylates at the anode, converting them to H2 at the cathode [204,205,206]. In this way, byproducts such as acetate, butyrate, and propionate, which limit the H2 yield in DF, are converted into gas and increase energy production from the substrate [207,208]. In integrated DF-MEC systems, the H2 yield can be increased by 40–300% compared to isolated DF, depending on the type of substrate and process [205,207,209]. DF provides the MECs with an effluent with good conductivity and a VFA profile that improves charge transfer, mitigating one of the main kinetic limitations of MECs [204,206,210]. This integration recovers substrate value, reduces costs, and demonstrates that the systems of co-production processes are more sustainable than stand-alone processes [195,198]. Despite promising performance, challenges such as cost, energy expenditure, and methanogenesis still limit large-scale application, requiring process optimization, pretreatments, and methanogenesis reduction strategies [204,206,210].
4. Advantages of Precision Fermentation for Biofuels
4.1. High Product Selectivity
High product selectivity in PF for biofuels is a central advantage, resulting from the synergy between genetic engineering and stringent bioprocess control [112,211,212]. Practical examples highlight this precision: deletion of the ldh gene in E. aerogenes increased production of the precursor 2,3-BDO by 71.2% [213]; metabolic manipulations in S. cerevisiae for alcohol coproduction raised the purity of 3-methyl-1-butanol from 42% to 71% [214]; and a coordinated strategy in S. cerevisiae (combining deletion of competing genes and cofactor optimization) enabled the selective production of FAMEs (Fatty Acid Methyl Esters) [154].
For genetic potential to be expressed at an industrial scale, advanced metabolic control is essential. Bioprocess strategies such as electrofermentation (EF) overcome conventional limitations by using electrodes to regulate the redox balance, increasing yields and minimizing by-product formation [215,216]. Similarly, optimizations such as enhanced Simultaneous Saccharification and Fermentation (eSSF), employing cellulosic emulsions, enable rapid and efficient hydrolysis of complex substrates (e.g., isobutanol production within one day at 30 °C) [217]. Optimization of operational conditions (O2 availability, pH, and temperature) is a fundamental metabolic strategy capable of boosting yields (e.g., from 65% to 85% for 2,3-BDO) and achieving high selectivity by eliminating by-products and ensuring large-scale feasibility [218].
4.2. Substrate Flexibility
The flexibility of the substrate is a significant key advantage of PF, facilitating the exploration of sustainable alternatives to fossil fuels and promoting the advancement of a circular economy. PF facilitates the utilization of diverse low-cost, non-food carbon sources, overcoming resource competition and enhancing the value of the substantial quantities of residual lignocellulosic biomass produced by global agriculture, thereby preventing additional waste generation and reducing environmental impact [219,220].
Overcoming biomass recalcitrance can be achieved through optimized pretreatment. For example, pretreating reed straw residues with L-glutamic acid increased the yield of fermentable sugars by more than fivefold [221]. Additional strategies such as hydrolysis, hybrid fermentation, microbial co-interactions, and cocultures can also be employed to enhance process efficiency and convert residues into multiple biofuels, including bioethanol, biobutanol, and biomethane [221,222]. Advanced strategies such as PF are likewise used to expand the capacity to process diverse wastes, maximizing production and improving overall performance [216]. Furthermore, PF broadens substrate utilization to include gaseous sources such as syngas [223].
4.3. Modular Scalability and Synergistic Integration in Biorefineries
The strategy of decoupling from the traditional model of industrial scalability makes PF economically advantageous. Its perspective contrasts with the reliance on single, slowly constructed, and high-cost facilities.
4.3.1. The ‘Scale-Out’ vs. ‘Scale-Up’ Model
PF employs a scale-out strategy, utilizing multiple standardized, modular fermentation units, rather than the conventional scale-up method that focuses on enlarging a single large reactor. This transition offers economic benefits via consistent and foreseeable growth; it enables enterprises to initiate with smaller units and augment capacity in response to rising demand; and it enhances deployment efficiency, as prefabricated or standardized modules can be swiftly installed [29,224].
4.3.2. Synergistic Integration in Biorefineries
The modular nature of PF allows a unit to function as a plug-and-play module that can be retrofitted into an existing facility, for example, a pulp mill, a first-generation ethanol plant, or a biodiesel facility.
This integration enables three levels of synergy:
- I.
- Feedstock Synergy: The PF module can be designed to consume by-products or waste streams from the host facility. For example, it can use biogenic CO2 released from 1G ethanol fermentation, crude glycerol from a biodiesel plant, or hydrolysate from a pulp mill, transforming low-value residues into feedstocks for advanced biofuel production [225,226,227].
- II.
- Utility and Infrastructure Synergy: A new PF unit can share existing infrastructure, such as steam and electricity generation, inbound and outbound logistics, and wastewater treatment systems. This reduces costs associated with Capital Expenditure (CAPEX) and Operational Expenditure (OPEX) for new processes [226,227].
- III.
- Portfolio Synergy: The integration of new modules enables diversification of the product portfolio with higher value-added compounds. In this way, an ethanol-producing facility can begin producing SAF, thereby gaining access to additional markets [228,229,230].
4.4. Potential for Low-Carbon and Carbon-Negative Processes
PF for biofuels is strategically positioned within the broader context of Negative Emission Technologies (NETs), which are essential for atmospheric CO2 removal and global warming mitigation [166]. NETs encompass a portfolio of biological and engineering approaches, including Direct Air Capture (DAC), BECCS (Bioenergy with Carbon Capture and Storage), and biochar (carbon sequestration via pyrolyzed biomass), with mitigation potentials ranging from 0.013 MtCO2/year (current DAC systems) to 1.8 GtCO2/year (biochar) [231,232]. To put these figures into perspective, global energy-related CO2 emissions reached a record 37.4 Gt in 2023 [159]. Thus, while DAC technologies are still in an incipient stage of development, the projected capacity of biochar to mitigate approximately 4.8% of annual global emissions underscores that a diversified portfolio of NETs is vital for climate stabilization.
The industrial viability of these processes, such as DAC depends on the optimization of key performance indicators (KPIs), including carbon removal efficiency (CRE) and carbon removal rate (CRR) [233]. PF functions as a complementary technology focused on optimizing biological CO2-conversion routes. Processes such as gas fermentation, EF, and waste valorization enable carbon capture and conversion. Pressurized Electrofermentation (PEF) has demonstrated the ability to overcome mass-transfer limitations, optimizing CO2 conversion into value-added products, and its carbon-negative potential has been validated through Life Cycle Assessment (LCA) [234].
Despite these strengths, precision fermentation presents inherent weaknesses that must be overcome to ensure industrial feasibility. A key limitation is the metabolic burden imposed on engineered strains: redirecting carbon flux toward biofuel synthesis often slows growth and reduces overall robustness compared with wild-type organisms [32]. In addition, the genetic instability of high-performing recombinant strains during long-term continuous fermentation remains a critical weakness, frequently leading to the loss of engineered traits [235,236]. From an operational standpoint, the need for highly sterilized environments and precise process control substantially increases both capital and operating expenditures relative to traditional open-pond or less controlled fermentation systems [29,210]. Collectively, these limitations demand rigorous optimization of both the microbial chassis and the bioreactor design, as further detailed in the challenges discussed in Section 5.
5. Key Challenges
5.1. Production Costs and Industrial Scale-Up
Despite the expansion of the biofuel market, technological and environmental challenges continue to limit large-scale production. This context identifies reducing production costs and achieving commercial viability as key determinants for the advancement of this sector [237,238]. To allow for a more comprehensive assessment, Table 5 includes the production costs and technological routes of the main biofuels, as well as the estimated production costs of conventional fossil fuels, enabling a direct comparison between renewable and fossil energy sources.

Table 5.
Costs of major biofuels from precision fermentation.
Although production cost is a critical parameter, industrial scale and market availability remain decisive factors for the effective replacement of fossil fuels. In 2023, global ethanol production reached 112 billion liters, representing an increase of approximately 5% compared to 2022, with Brazil and the United States accounting for 81% of worldwide production of this biofuel [245]. In contrast, global gasoline consumption remains high, with the United States leading at 8809.9 thousand barrels per day in 2022, followed by China and Brazil, which rank among the three largest global consumers of this fossil energy source [240].
A similar disparity is observed for emerging higher alcohols. In terms of market scale and energy production, propanol still represents a small fraction compared to conventional fossil fuels. The global propanol market reached approximately USD 3.76 billion in 2023, with a projected compound annual growth rate of about 6.6% between 2023 and 2030 [241]. However, fossil fuels, such as coal, continue to dominate the global energy supply, with countries like China, India, Indonesia, and the United States producing millions of tons annually. Notably, China alone produced approximately 4,577,545.5 thousand tons in 2022 [242]. This contrast highlights the substantial challenge faced by biofuels in achieving large-scale substitution of fossil energy carriers.
The growing interest in renewable feedstocks makes it essential to overcome challenges associated with industrial scale-up, including the continuous supply of raw materials and the optimization of cost–benefit relationships [237,238]. The need for a more robust supply chain and storage systems increases the risk of feedstock degradation and raises investment costs. However, this bottleneck can be mitigated through the adoption of pretreatment techniques and by adapting production processes to enable the blending of different raw materials [243]. In parallel, the integration of genetic engineering approaches has emerged as a strategy to optimize efficiency and enhance production yields. Overcoming the global energy crisis also depends on international cooperation to ensure the effective integration of biofuels, which will drive technological progress and strengthen the sector’s sustainability [246]. In this context, as discussed in Section 7.1 and Section 7.3, overcoming these cost-related barriers relies on the integration of digitalization and the adoption of biorefinery models to valorize side-streams.
5.2. Genetic Stability of Strains
The genetic stability of strains is one of the key challenges in the biotechnological production of biofuels. Some industrial strains exhibit metabolic limitations, such as carbon catabolite repression, which hinders the efficient co-utilization of different sugars and restricts the utilization of lignocellulosic substrates. Additionally, exposure to toxic compounds in hydrolysates (furan derivatives, phenolics, organic acids) compromises cell growth and productivity. Therefore, enhancing tolerance to inhibitors, intermediates, and products through rational or evolutionary engineering strategies is essential for developing robust strains and ensuring high yields. Another obstacle is genetic instability arising from metabolic imbalances (nutrient depletion, metabolite accumulation, stress), which can cause undesirable mutations. To mitigate these effects, the development of strains with sensor-regulatory systems capable of adjusting cellular metabolism to environmental fluctuations is crucial [112,247].
5.3. Microbial Tolerance to Solvents and Produced Fuels
Microbial tolerance to solvents and produced fuels represents a critical challenge in the biotechnological production of biofuels, as many of the compounds formed exhibit significant toxicity to the producer cells. During the process, the product and metabolic intermediates can inhibit microbial growth, affecting viability and yield. In this context, hydrocarbon-based biofuels, such as pinene demonstrate toxicity to model microorganisms, like E. coli, even at low concentrations (2%). This toxicity is related to the partitioning of the compounds into the cell membrane, compromising its integrity and essential physiological functions [248,249].
To address this challenge, targeted approaches utilize the modulation of specific mechanisms, including chaperones, cell wall components, transporter proteins, and regulators of cellular functions. For example, gene modulation using deactivated CRISPR (dCRISPR) has increased microbial tolerance, as targeted perturbations in E. coli gene expression resulted in greater bacterial resistance to prolonged exposure to n-butanol and n-hexane [250,251].
Future strategies to mitigate the lack of strain stability and the intolerance to solvents and fuels include AI-driven genetic design and cell-free systems, which bypass cellular maintenance requirements (Section 7.1 and Section 7.3).
5.4. Regulatory and Biosafety Bottlenecks
The implementation of biofuels, although fundamental for sustainability and global energy integration, is intrinsically dependent on a complex policy framework, including fiscal incentives, mandates, and carbon pricing. However, the effectiveness and sustainability of these policies face significant bottlenecks. Brazil, despite being a pioneer (e.g., Proálcool and National Program of Production and Use of Biodiesel—PNPB), has demonstrated that while diversification of the energy matrix is achievable, these programs may exhibit limited socio-environmental inclusion and a strong dependence on pre-existing institutional frameworks [252,253].
The primary challenges are financial and regulatory. Budgetary constraints and competing development priorities limit the allocation of resources for clean energy [254]. The high initial costs of infrastructure and technology, coupled with the perception of risk in unstable economies, hinder access to private capital [255]. Paradoxically, global GHG mitigation mechanisms themselves, such as carbon taxation (UN, 2021), act as an indirect bottleneck by increasing production costs and requiring administrative and technological infrastructure that remains unavailable to many nations [252].
Parallel to these socioeconomic hurdles, the environmental viability of emerging platforms, particularly PF, requires a rigorous analysis regarding the utilization of GM microorganisms. A critical vulnerability lies in genomic instability and the risk of horizontal gene transfer (HGT). This phenomenon enables the transmission of transgenic DNA sequences to native microbial populations, potentially propagating virulence factors or antibiotic resistance within the ecosystem [109,256]. Furthermore, life cycle sustainability is challenged by waste management; extraction byproducts and wastewater may harbor residual genetic material, necessitating stringent biological inactivation protocols to mitigate risks of environmental contamination [109]. In this context, the development of biocontainment strategies, such as conditional kill-switches and the use of auxotrophic strains, is essential to align biotechnological advancement with ecological safety, serving as a cornerstone for regulatory validation [256,257].
5.5. Competition with Biochemical and Thermochemical Routes
Biofuel production places PF (a biochemical route) in direct competition with other conversion platforms, namely thermochemical ones. Biochemical routes, which include fermentation, anaerobic digestion, and transesterification, utilize microorganisms and enzymes. In contrast, thermochemical routes are gaining prominence due to their flexibility in processing diverse biomass feedstocks, generating a portfolio of energy products (bio-oil, syngas, biochar) through heat [8,258,259].
The thermochemical platform is diversified, with processes classified by their operational conditions. It includes gasification (conversion > 700 °C into syngas), pyrolysis (decomposition that prioritizes bio-oil or biochar depending on the heating rate), and torrefaction (a pre-treatment to increase calorific value and hydrophobicity). This route also offers solutions for specific substrates, such as direct combustion (for any type of biomass, with <50% moisture content) and hydrothermal carbonization (efficient for wet biomass at temperatures < 350 °C) [260,261,262,263,264].
Both biochemical and thermochemical platforms share technical and economic challenges that impact their viability. Key technical challenges include feedstock heterogeneity, high moisture content, and contaminants. Economically, high pre-treatment, capital, and operational costs, combined with regulatory and social acceptance barriers, are limiting factors. Given the complexity of these challenges, the strategic integration of thermochemical and biochemical routes is proposed as an alternative to optimize biomass utilization and maximize overall biorefinery efficiency [261,264].
6. Integration into Biorefineries
6.1. Integrated Biorefinery Models: Co-Production of Fuels, Green Chemicals, and Bioplastics
Integrated biorefinery models are strategic pillars of the circular bioeconomy, combining biochemical and thermochemical routes for the full valorization of biomass, enabling the co-production of biofuels, green chemicals, and bioplastics [73,265]. This approach relies on a flexible product portfolio that mitigates economic risks, responds to market fluctuations, and maximizes substrate utilization across multiple value chains [266,267,268]. In this context, the availability and composition of lignocellulosic biomass become fundamental, since agricultural residues typically contain about 40 to 50% cellulose, 20 to 30% hemicellulose, and 10 to 30% lignin, representing an abundant and underutilized carbon source for bioprocesses [269].
PF is a core technology within these integrated systems, particularly for the production of advanced drop-in biofuels such as isobutanol and farnesene. These molecules are chemically compatible with existing fossil infrastructures and exhibit superior physicochemical properties compared to ethanol. Overcoming biological limitations—including product toxicity and low metabolic flux—in microbial hosts (e.g., E. coli, S. cerevisiae) is increasingly feasible through advanced metabolic engineering, adaptive evolution, and cell-free or modular biocatalytic systems, enabling commercial scaling as demonstrated by Gevo and Amyris [250,270,271].
Integration also expands the use of carbon sources beyond C6 sugars, focusing on the valorization of C5-rich side streams (xylose, arabinose) to generate platform chemicals such as succinic acid and 3-HP [272,273]. Optimizing wastewater treatment processes can also reduce capital investment requirements, lowering market entry barriers for lignocellulosic biorefineries and enhancing their economic feasibility. In addition, wastewater streams generated during lignocellulosic pretreatment and hydrolysis may present organic loads of up to 260 kg COD per ton of biomass processed and approximately 44 kg of inorganic species per ton, reflecting the high chemical and nutrient burden associated with these processes [274].
Similarly, integrated biorefinery models must account for the CO2 fluxes associated with lignocellulosic processing. Estimates indicate that biomass conversion systems can release approximately 200–470 kg of CO2 equivalent per ton of processed biomass, at temperatures between 400 and 700 °C [275]. In a Brazilian study, agroforestry residues estimated at ~276 Tg C would have a conversion potential corresponding to ~1014 Tg of CO2 [276].
PF is equally applied in the synthesis of bioplastics, either through the production of high-purity monomers (lactic acid for PLA) or by the direct intracellular accumulation of polymers (PHAs) under nutritional stress, utilizing waste or C1 gases as feedstock [277,278,279]. In this context, the integration of lignocellulosic biomass streams, wastewater reuse, and CO2-derived carbon sources establishes an efficient multi-input biorefinery architecture designed to maximize mass and energy recovery while minimizing environmental impacts.
6.2. Synergy Between Microbial, Thermochemical, and Enzymatic Pathways
The integration of enzymatic, microbial, and thermochemical routes is crucial for maximizing the conversion of lignocellulosic biomass into biofuels and bioproducts. Chemical pre-treatment is necessary to disrupt the recalcitrant structure of the biomass, partially removing lignin and exposing the cellulose and hemicellulose fibers. Subsequently, the fibers are converted into fermentable sugars by enzymatic cocktails composed of cellulases, hemicellulases, and accessory enzymes. This process occurs under elevated temperature and pH, as summarized in Figure 6 [280,281,282].
Figure 6.
Diagram showing a biorefinery concept using thermo-microbial hybridization to convert biomass into biofuels, bioplastics, and chemicals.
At the end of the treatment, cellulose is converted into glucose (C6), while hemicellulose is converted into xylose, arabinose, and other C5 sugars. Conversion efficiency can exceed 85–90% under optimized conditions, and all produced sugars are utilized in precision fermentation for bioproduct production. Current processes utilize optimized enzymatic cocktails, surfactants, enzyme immobilization, enzyme engineering, and process integration, such as fed-batch (in which biomass is gradually incorporated), always seeking to improve efficiency [283,284].
The microbial route, PF itself, is responsible for consuming the sugars (C5 and C6) derived from the enzymatic hydrolysis of biomass and converting them into target molecules such as biofuels (ethanol, butanol, isobutanol, fatty acids, esters), platform chemicals (succinic, itaconic, lactic acid, GABA, amino acids, monomers for bioplastics), aromatic compounds, terpenes, higher alcohols, and even nanomaterials. This conversion is performed by native or GM microorganisms, utilizing natural, heterologous, or fully synthetic metabolic pathways. The choice of pathway depends on the target product, the microbial chassis, and precursor availability [285,286,287,288].
Frequently, metabolic pathways are optimized through metabolic engineering, synthetic biology, and computational modeling tools. This allows for redirecting carbon flux toward the desired product, eliminating competing pathways, modulating gene expression, and optimizing cofactor utilization. Strategies such as pathway modularization; glucose and xylose (C6 and C5) co-utilization, which increases yield and productivity; microbial consortia, which enable metabolic division of labor, greater robustness, and complex substrate conversion; and dynamic metabolic regulation have made it possible to overcome physiological limitations and enhance process efficiency, specificity, and robustness [286,287,289,290].
At the end of enzymatic hydrolysis, the remaining lignin is the primary residue of lignocellulosic biomass, and this residue is generally destined for combustion for energy cogeneration. However, thermochemical routes, such as pyrolysis and gasification, can be used to convert residual lignin into new products. Pyrolysis generates bio-oil, phenolics, aromatics, and combustible gases, whereas gasification converts lignin into syngas rich in CO, H2, and CO2. This gaseous mixture can be utilized as a substrate in gas fermentation by microorganisms such as C. autoethanogenum, enabling the production of ethanol, organic acids, and other bioproducts [291,292,293].
These two listed routes can be divided into synergy 1 (lignin valorization), in which the combustion of lignin for energy allows the conversion into aromatic compounds, and synergy 2 (hybridization), employed as a substrate in fermentation, and utilized by acetogenic organisms. Technologies such as LanzaTech’s already operate at an industrial scale; gas fermentation converts carbon waste streams into sustainable fuels (ethanol), textiles, and chemicals [294,295,296].
6.3. Emerging Industrial Examples
Techno-Economic Assessment (TEA) studies are widely used to analyze the viability of PF, as they estimate CAPEX, OPEX, and mass and energy balances, and calculate indicators such as Minimum Selling Price (MSP), Net Present Value (NPV), and Internal Rate of Return (IRR) of the process. These studies infer that CAPEX and OPEX are high due to specialized equipment, complex downstream processing, intensive energy consumption, and limitations in TRY parameters. Furthermore, the feedstock cost, which can account for up to 35% of the total cost, is the primary factor impacting the MSP. Therefore, improvements in these areas have a direct impact on economic viability [29,297,298,299,300].
In addition to economic analysis, LCA is employed to quantify the environmental impact of precision fermentation biofuels, analyzing impacts from raw material production to end-use and disposal. LCA considers direct and indirect land use change (iLUC) and accounts for GHG emissions, water, and energy consumption at all fermentation stages. The synergy between LCA and the biorefinery concept reduces the carbon footprint and makes the biofuel significantly “greener”, as scenarios with greater waste valorization and co-production exhibit lower emissions and environmental costs [261,301].
6.4. TEA and LCA
LanzaTech employs the bioconversion of CO-rich waste gases from steel mills, directing them into bioreactors where C. autoethanogenum, via the WLP pathway, performs the conversion into ethanol and other chemicals. The produced ethanol can be used directly as a biofuel or converted into SAF through additional processes, such as the Alcohol-to-Jet (ATJ) route. The operations take place in industrial settings, leveraging gaseous residues from processes like steel production, thereby reducing emissions and promoting the circular economy [302,303,304].
The companies Gevo and Butamax have developed PF technologies designed to be integrated (“bolt-on”) with the corn milling, hydrolysis, and corn ethanol fermentation steps already present in 1G plants, leveraging existing infrastructure. In this process, GM microorganisms are introduced that block competing pathways (the ethanol pathway) and redirect the conversion of sugars toward isobutanol [270,305,306,307].
7. Future Perspectives
The future of Precision Fermentation (PF) is directly linked to its ability to address the technical and economic bottlenecks identified in Section 5. Although PF is already established in both research and industrial biofuel production, there is significant potential to be explored through the convergence of technological and biological innovations. Current perspectives point toward a scenario where the integration of advanced tools, such as AI, Machine Learning and Digital Twins, will be fundamental in the discovery and modification of strains and metabolic pathways. These technologies, alongside process optimization, allow for the precise prediction of parameter effects, offering robust solutions to the challenges of efficiency and scalability [308,309,310,311].
Other points to be explored include the continued adoption of non-conventional feedstocks, such as industrial CO2, urban waste, and effluents, applying circular bioeconomy and carbon-negative practices focused on sustainability implementation. Coupled with these, the use of microbial consortia and biohydrogen as a clean energy vector shows a trend toward using PF processes not merely as an alternative, but as an indispensable tool for sustainable and economically viable production scale-up [312,313,314,315,316].
In alignment with the challenges previously identified, Table 6 summarizes the correspondence between the main bottlenecks in precision fermentation and the future technological perspectives discussed in Section 7, linking the challenges to the opportunities offered by the tools to be explored.

Table 6.
Correspondence between identified challenges and future perspectives in precision fermentation for biofuels.
7.1. Optimization Through Technology: AI, Automation, and Digital Twins
The integration of AI tools can accelerate the modification and optimization of strains focused on biofuel production. AI has the potential to become fundamental in genetic design, assisting in the processing of large volumes of omics data, which can help predict the genetic modifications necessary to increase the production of the desired biofuel and productivity itself. This process can be achieved through protein engineering and the identification of novel metabolic engineering target pathways unlikely to be determined by traditional methods. By applying AI, it is possible to predict a strain’s response to different stress conditions even before these conditions occur [310,317,318].
Another possibility includes bioprospecting, which allows for the cultivation, monitoring, and evaluation of a large number of discovered or GM strains. With automation and the use of models, alongside advanced sensors and real-time image analysis, faster identification of more productive and resistant strains is possible. This also allows for greater precision regarding optimal parameters and reproducibility, as many tests are still conducted at laboratory scale and may face reproducibility issues, needing to be scaled up to an industrial level [319,320,321].
In industrial scale-up, tools like Digital Twins can be very useful for process improvement. As real-time virtual models of physical bioreactors, these twins can simulate interactions, kinetics, and changes in essential cultivation parameters, such as agitation rate and aeration, before implementing them in the actual bioreactor. This can help reduce waste, shorten optimization time, and ensure greater productivity and stability [308,322].
7.2. Sustainability-Aligned Feedstocks: Utilization of Industrial CO2 Emissions, Municipal Solid Waste, and Effluents
Within the scope of scale-up aligned with sustainable practices, the trend is toward integrating biofuel production with the circular economy. The use of feedstocks that are not only abundant but also low-cost and pose low pollution risks will be the target for biofuels under development [323].
In the pursuit of a carbon-neutral or carbon-negative balance, the utilization of GM or promising microorganisms to convert industrial CO2 emissions into biofuels stands out. This process can be achieved through metabolic engineering, which can enhance strain tolerance (thereby increasing biofuel production), or through the discovery of more resilient strains via bioprospecting. Furthermore, the search for future feedstocks will focus on using low-cost carbon and nitrogen sources, such as agro-industrial residues, municipal solid waste (composed of organic components), and wastewater effluents. This strategy aims to reduce production costs and mitigate problems related to the disposal of these wastes [313,324,325,326].
7.3. Biological Advancements: Bioprospecting, Microbial Consortia, Cell-Free Systems, and Biorefineries
Addressing both the challenges in biofuel production and its future opportunities and prospects, processes that aid in the manipulation of specific metabolic pathways, as well as the use of advanced biological systems, emerge as promising avenues in the quest for efficiency.
From a biological standpoint, one of the first steps that can aid future advancements in the field is bioprospecting in extreme environments. This can lead to the discovery of enzymes exhibiting greater tolerance to factors like temperature and pH, and which are also capable of degrading the utilized feedstocks more easily [324,327]. Based on what is found in nature, it is possible, through genetic engineering, to create even more effective enzymes, thereby reducing production costs.
Another biological possibility may arise from the use of microbial consortia, in which different species can perform each function required for biofuel production, addressing stages such as hydrolysis, sugar conversion, and biofuel synthesis. This consortium approach can thereby enhance the tolerance and efficiency of the strains [315].
A third promising avenue is the use of Cell-Free Systems (CFS) in the future of biofuel production. In these systems, enzymes are extracted from the microorganisms and introduced into the system, eliminating the need for microbial maintenance and regulation, thereby removing concerns about toxic components and accelerating production [326].
The biorefinery possibilities included in this production are also promising, allowing not only for the production of biofuels but also for the simultaneous production of other high-value products simultaneously. The polymer, food ingredient, and pharmaceutical sectors could be contemplated to reduce costs and enable biofuels to compete with the petroleum industry [328,329].
7.4. Biohydrogen: Optimization and Integration
Low-carbon hydrogen plays a role in decarbonizing emissions from other processes and in storing renewable energy. Furthermore, it exhibits low energy consumption and high scalability potential, positioning it as a key focus for future biofuel research. In this regard, the perspectives for biohydrogen point toward the integration of different fermentation forms—whether in the absence of light or in the presence of light energy—and toward the use of photosynthetic microorganisms. Such processes can not only enhance the yield of this biofuel but also generate other value-added products, aligning with biorefinery and circular economy practices [330,331].
7.5. Carbon Negative or Neutral
A critical consideration is the pursuit of carbon-neutral or carbon-negative targets. Specifically in the biofuels sector, priority must be given to the valorization of waste streams and CO2 captured from industrial emissions, not only reducing current emissions but also offsetting those already made. With the scale-up of biofuel production and the utilization of abundant CO2, there is significant potential to be harnessed, especially in a scenario dependent on fossil fuels, which are finite resources, thereby contributing to energy security and ensuring a clean energy source [332,333].
8. Conclusions
Precision fermentation has already established itself as one of the most promising strategies for overcoming the historical limitations of biotechnological biofuel production. Modern metabolic engineering approaches including dynamic regulatory circuits, high-accuracy genome editing, and multi-omics integration have enabled unprecedented advances in the productivity, selectivity, and robustness of industrial microorganisms. These advances broaden the frontier of advanced biofuels, from second-generation alcohols to drop-in hydrocarbons, achieving chemical properties comparable to fossil fuels and expanding the potential for decarbonization on a global scale. However, structural challenges remain, especially in genetic stability, solvent tolerance, and the technical complexities of scale-up, which lead to high process costs, as well as regulatory barriers for products derived from genetically modified microorganisms. Overcoming these bottlenecks requires a combination of PF with automation, artificial intelligence, and its integration into modular biorefineries capable of valorizing different carbon streams. It is concluded that PF not only improves the biochemical performance of processes but also lays the groundwork for carbon-neutral or carbon-negative pathways, positioning itself as a central element in the transition to sustainable energy systems.
Author Contributions
Conceptualization: W.J.M.-B.; investigation, data curation and writing—original draft preparation: D.B.P., G.L.-S., L.B.d.N.S., L.V.B.d.A., A.d.S.V. and V.A.P.; writing—review and editing: W.J.M.-B., L.R.C., R.P. and J.L.S.; visualization: D.B.P., G.L.-S., V.A.P., L.B.d.N.S., A.d.S.V. and L.V.B.d.A.; supervision: C.S.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (Process number 88887.994959/2024-00, 88887.941186/2024-00 and 88887.151528/2025-00), Fundação de Amparo à Pesquisa do Estado do Amazonas (Resolution No. 002/2023—CD/FAPEAM and EDITAL N. 014/2024—BIO CT&I/FAPEAM 01.02.016301.00737/2025-22), Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Process number 141036/2022-2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
To the National Institute for Amazonian Research (INPA) and the Edible Fungi Cultivation Laboratory (LCFC). During the preparation of this manuscript, the authors used ChatGPT 5, QuillBot and Gemini for the purposes of assisting with English translation and text revision. Canvas tools were used for the creation and refinement of the figures presented in this article. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2,3-BDO | 2,3-butanediol |
| AAR | Acyl-ACP Reductase |
| ACLDC | Acetolactate Decarboxylase |
| ACLS | Acetolactate Synthase |
| ADHs | Alcohol Dehydrogenases |
| ADO | Aldehyde-Deformylating Oxygenase |
| AI | Artificial Intelligence |
| ANFIS | Adaptive Neuro-Fuzzy Inference Systems |
| ANNs | Artificial Neural Networks |
| ATJ | Alcohol-to-Jet |
| BECCS | Bioenergy with Carbon Capture and Storage |
| BKD | α-Ketoacid Dehydrogenase |
| CAPEX | Capital Expenditure |
| CBM | Constraint-Based Modeling |
| CCS | CCSCarbon Capture and Storage |
| CFS | Cell-Free Systems |
| CNNs | Convolutional Neural Networks |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| COD | Chemical Oxygen Demand |
| CRE | Carbon Removal Efficiency |
| CRISPR-Cas | Clustered Regularly Interspaced Short Palindromic Repeats associated with Cas proteins |
| CRR | Carbon Removal Rate |
| DAC | Direct Air Capture |
| DF | Dark Fermentation |
| DMBR | Dynamic Membrane Bioreactor |
| ED | Entner-Doudoroff |
| EF | Electrofermentation |
| eSSF | Enhanced Simultaneous Saccharification and Fermentation |
| FAMEs | Fatty Acid Methyl Esters |
| FFAs | Free Fatty Acids |
| FPP | Farnesyl Pyrophosphate |
| GA | Genetic Algorithms |
| GHG | Greenhouse Gases |
| GM | Genetically Modified |
| GRAS | Generally Recognized as Safe |
| GRU | Gated Recurrent Units |
| H2 | Hydrogen |
| HDR | Homology-Directed Repair |
| HHV | Higher Heating Value |
| HNH | Histidine–Asparagine–Histidine |
| iLUC | Indirect Land Use Change |
| IRR | Internal Rate of Return |
| KDCs | α-Keto Acid Decarboxylases |
| KPIs | Key Performance Indicators |
| LCA | Life Cycle Assessment |
| LSTM | Long Short-Term Memory |
| MAGE | Multiplex Automated Genome Engineering |
| MECs | Microbial Electrolysis Cells |
| MLP | Multilayer Perceptrons |
| msd | Multicopy Single-Stranded DNA |
| MSP | Minimum Selling Price |
| msr | multicopy single-stranded RNA |
| NETs | Negative Emissions Technologies |
| NHEJ | Non-Homologous End Joining |
| NIR | Near-Infrared |
| NPV | Net Present Value |
| NUC | Nuclease |
| O2 | Oxygen |
| OPEX | Operational Expenditure |
| PAM | Protospacer Adjacent Motif |
| PCA | Principal Component Analysis |
| PEF | Pressurized Electrofermentation |
| PF | Precision Fermentation |
| PFOR | Pyruvate:Ferredoxin Oxidoreductase |
| PSO | Particle Swarm Optimization |
| REC | Recognition |
| RF | Random Forest |
| RSM | Response Surface Methodology |
| RuvC | RuvC-like Nuclease Domain |
| SAF | Sustainable Aviation Fuel |
| sgRNA | Single-guide RNA |
| SHAP | SHapley Additive exPlanations |
| SMR | Steam Methane Reforming |
| SSAP | Single-stranded DNA Annealing Protein |
| ssDNA | Single-stranded DNA |
| STC-CNN | Spatio-Temporal Convolutional Neural Networks |
| SVMs | Support Vector Machines |
| SVR | Support Vector Regression |
| Syngas | Synthesis Gas |
| TAGs | Triacylglycerols |
| TCA | Tricarboxylic Acid |
| TEA | Techno-Economic Analysis |
| TRY | Titers, Rates, and Yields |
| VFAs | Volatile Fatty Acids |
| WLP | Wood-Ljungdahl Pathway |
| WMO | World Meteorological Organization |
References
- Khan, N.; Sudhakar, K.; Mamat, R. Role of Biofuels in Energy Transition, Green Economy and Carbon Neutrality. Sustainability 2021, 13, 12374. [Google Scholar] [CrossRef]
- United Nations Human, Economic, Environmental Toll of Climate Change on the Rise: WMO|UN News. Available online: https://news.un.org/en/story/2023/04/1135852 (accessed on 1 November 2025).
- Yasmeen, R.; Shah, W.U.H. Energy Uncertainty, Geopolitical Conflict, and Militarization Matters for Renewable and Non-Renewable Energy Development: Perspectives from G7 Economies. Energy 2024, 306, 132480. [Google Scholar] [CrossRef]
- Kazmi, A.; Sultana, T.; Ali, A.; Nijabat, A.; Li, G.; Hou, H. Innovations in Bioethanol Production: A Comprehensive Review of Feedstock Generations and Technology Advances. Energy Strategy Rev. 2025, 57, 101634. [Google Scholar] [CrossRef]
- Center for Climate and Energy Solutions (C2ES). Renewable Energy. Center for Climate and Energy Solutions. Available online: https://www.c2es.org/content/renewable-energy/ (accessed on 1 November 2025).
- Global Biofuel Production by Country. 2024. Available online: https://www.statista.com/statistics/274168/biofuel-production-in-leading-countries-in-oil-equivalent/ (accessed on 27 December 2025).
- Kiehbadroudinezhad, M.; Merabet, A.; Ghenai, C.; Abo-Khalil, A.G.; Salameh, T. The Role of Biofuels for Sustainable MicrogridsF: A Path towards Carbon Neutrality and the Green Economy. Heliyon 2023, 9, e13407. [Google Scholar] [CrossRef]
- Rial, R.C. Biofuels versus Climate Change: Exploring Potentials and Challenges in the Energy Transition. Renew. Sustain. Energy Rev. 2024, 196, 114369. [Google Scholar] [CrossRef]
- Hefner, M.; Marland, G.; Oda, T. The Changing Mix of Fossil Fuels Used and the Related Evolution of CO2 Emissions. Mitig. Adapt. Strateg. Glob. Change 2024, 29, 56. [Google Scholar] [CrossRef]
- Joyia, M.A.K.; Ahmad, M.; Chen, Y.-F.; Mustaqeem, M.; Ali, A.; Abbas, A.; Gondal, M.A. Trends and Advances in Sustainable Bioethanol Production Technologies from First to Fourth Generation: A Critical Review. Energy Convers. Manag. 2024, 321, 119037. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated Lignocellulosic Value Chains in a Growing Bioeconomy: Status Quo and Perspectives. Gcb Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Arshad, N.; Panakkal, E.J.; Kalivarathan, P.B.; Tawai, A.; Chuetor, S.; Wanmolee, W.; Kirdponpattara, S.; Chantarasiri, A.; Rakesh, S.; Septevani, A.A.; et al. Emerging Technologies in Pretreatment and Hydrolysis for High-Solid-Loading Bioethanol Production from Lignocellulosic Biomass. Fermentation 2025, 11, 613. [Google Scholar] [CrossRef]
- Joshi, A.; Verma, K.K.; DRajput, V.; Minkina, T.; Arora, J. Recent Advances in Metabolic Engineering of Microorganisms for Advancing Lignocellulose-Derived Biofuels. Bioengineered 2022, 13, 8135–8163. [Google Scholar] [CrossRef]
- Lu, X.; Vora, H.; Khosla, C. Overproduction of Free Fatty Acids in E. coli: Implications for Biodiesel Production. Metab. Eng. 2008, 10, 333–339. [Google Scholar] [CrossRef]
- Perret, L.; Lacerda, B.; Delgado, K.H.; Zevaco, T.A.; Neumann, A.; Sauer, J. COx Fixation to Elementary Building Blocks: Anaerobic Syngas Fermentation vs. Chemical Catalysis. Chem. Ing. Tech. 2022, 94, 1667–1687. [Google Scholar] [CrossRef]
- Owoade, A.; Alshami, A.S.; Levin, D.; Onaizi, S.; Malaibari, Z.O. Progress and Development of Syngas Fermentation Processes toward Commercial Bioethanol Production. Biofuels Bioprod. Biorefining 2023, 17, 1328–1342. [Google Scholar] [CrossRef]
- Samrot, A.V.; Rajalakshmi, D.; Sathiyasree, M.; Saigeetha, S.; Kasipandian, K.; Valli, N.; Jayshree, N.; Prakash, P.; Shobana, N. A Review on Biohydrogen Sources, Production Routes, and Its Application as a Fuel Cell. Sustainability 2023, 15, 12641. [Google Scholar] [CrossRef]
- Joshi, S.; Mishra, S. Recent Advances in Biofuel Production through Metabolic Engineering. Bioresour. Technol. 2022, 352, 127037. [Google Scholar] [CrossRef]
- Henritzi, S.; Fischer, M.; Grininger, M.; Oreb, M.; Boles, E. An Engineered Fatty Acid Synthase Combined with a Carboxylic Acid Reductase Enables de Novo Production of 1-Octanol in Saccharomyces Cerevisiae. Biotechnol. Biofuels 2018, 11, 150. [Google Scholar] [CrossRef]
- Nishimura, Y.; Matsui, T.; Ishii, J.; Kondo, A. Metabolic Engineering of the 2-Ketobutyrate Biosynthetic Pathway for 1-Propanol Production in Saccharomyces cerevisiae. Microb. Cell Factories 2018, 17, 38. [Google Scholar] [CrossRef]
- Xin, X.; Cheng, C.; Du, G.; Chen, L.; Xue, C. Metabolic Engineering of Histidine Kinases in Clostridium beijerinckii for Enhanced Butanol Production. Front. Bioeng. Biotechnol. 2020, 8, 214. [Google Scholar] [CrossRef]
- Wen, Z.; Ledesma-Amaro, R.; Lu, M.; Jin, M.; Yang, S. Metabolic Engineering of Clostridium cellulovorans to Improve Butanol Production by Consolidated Bioprocessing. ACS Synth. Biol. 2020, 9, 304–315. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Q.; Guo, X.; Hu, J.; Wang, G.; Lu, L.; Qin, Z.; Fu, H.; Wang, J.; Yang, S.-T. Metabolic Engineering of Clostridium tyrobutyricum for High-Yield n-Butanol Production by Increasing Intracellular Reducing Equivalent with NADPH-Dependent 3-Hydroxybutyryl-CoA Dehydrogenase. ACS Synth. Biol. 2025, 14, 2341–2353. [Google Scholar] [CrossRef]
- Liew, F.; Henstra, A.M.; Kӧpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic Engineering of Clostridium autoethanogenum for Selective Alcohol Production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lauer, I.; Philipps, G.; Jennewein, S. Metabolic Engineering of Clostridium ljungdahlii for the Production of Hexanol and Butanol from CO2 and H2. Microb. Cell Factories 2022, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chaffin, T.A.; Ahkami, A.H.; Blumwald, E.; Stewart, C.N. Plant Synthetic Biology Innovations for Biofuels and Bioproducts. Trends Biotechnol. 2022, 40, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Chai, K.F.; Ng, K.R.; Samarasiri, M.; Chen, W.N. Precision Fermentation to Advance Fungal Food Fermentations. Curr. Opin. Food Sci. 2022, 47, 100881. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Nielsen, J. Yeast Synthetic Biology Advances Biofuel Production. Curr. Opin. Microbiol. 2022, 65, 33–39. [Google Scholar] [CrossRef]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for Future Food Systems. EMBO Rep. 2021, 22, EMBR202152680. [Google Scholar] [CrossRef]
- Silva, S.; Bautista-Hérnandez, I.; Gomez-García, R.; Costa, E.M.; Machado, M. Precision Fermentation as a Tool for Sustainable Cosmetic Ingredient Production. Appl. Sci. 2025, 15, 9246. [Google Scholar] [CrossRef]
- Knychala, M.M.; Boing, L.A.; Ienczak, J.L.; Trichez, D.; Stambuk, B.U. Precision Fermentation as an Alternative to Animal Protein, a Review. Fermentation 2024, 10, 315. [Google Scholar] [CrossRef]
- Pereira, A.A.; Yaverino-Gutierrez, M.A.; Monteiro, M.C.; Souza, B.A.; Bachheti, R.K.; Chandel, A.K. Precision Fermentation in the Realm of Microbial Protein Production: State-of-the-Art and Future Insights. Food Res. Int. 2024, 200, 115527. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, H.R.; Lee, S.Y. Comprehensive Evaluation of the Capacities of Microbial Cell Factories. Nat. Commun. 2025, 16, 2869. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-S.; Chong, H.-Y.; Kim, J.-H.; Kim, J.-Y. Method for Mass Production of Primary Metabolites, Strain for Mass Production of Primary Metabolites, and Method for Preparation Thereof. WO2007094646A1, 23 August 2007. [Google Scholar]
- Qiao, K.; Imam Abidi, S.H.; Liu, H.; Zhang, H.; Chakraborty, S.; Watson, N.; Kumaran Ajikumar, P.; Stephanopoulos, G. Engineering Lipid Overproduction in the Oleaginous Yeast Yarrowia lipolytica. Metab. Eng. 2015, 29, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhong, L.; Sun, Y.; Sun, N.; Zhang, L.; Liu, W.; Qu, Y.; Zhong, Y. Enhancement of Cellulase Production in Trichoderma reesei via Disruption of Multiple Protease Genes Identified by Comparative Secretomics. Front. Microbiol. 2019, 10, 2784. [Google Scholar] [CrossRef] [PubMed]
- Dagariya, S.; Bhatankar, J.; Chand, D.T.; Ram, G.B.; Giudici, P. Metabolic and Evolutionary Engineering of Food Yeasts. Processes 2025, 13, 1852. [Google Scholar] [CrossRef]
- Beato, F.B.; Bergdahl, B.; Rosa, C.A.; Forster, J.; Gombert, A.K. Physiology of Saccharomyces cerevisiae Strains Isolated from Brazilian Biomes: New Insights into Biodiversity and Industrial Applications. FEMS Yeast Res. 2016, 16, fow076. [Google Scholar] [CrossRef]
- Ciani, M.; Picciotti, G. The Growth Kinetics and Fermentation Behaviour of Some Non-Saccharomyces Yeasts Associated with Wine-Making. Biotechnol. Lett. 1995, 17, 1247–1250. [Google Scholar] [CrossRef]
- Moon, S.Y.; An, N.-Y.; Lee, J.Y. Transforming Non-Conventional Yeasts into Key Players in Biotechnology: Advances in Synthetic Biology Applications. Front. Microbiol. 2025, 16, 1600187. [Google Scholar] [CrossRef]
- Zheng, X.; Ledesma-Amaro, R.; Zhao, Z.K.; Wang, Y.; Zhao, X.-Q.; Yang, X. Rhodotorula Yeasts as Potential Chassis for Sustainable Food Biotechnology. J. Agric. Food Chem. 2025, 73, 19157–19173. [Google Scholar] [CrossRef] [PubMed]
- Calero, P.; Nikel, P.I. Chasing Bacterial Chassis for Metabolic Engineering: A Perspective Review from Classical to Non-traditional Microorganisms. Microb. Biotechnol. 2018, 12, 98–124. [Google Scholar] [CrossRef]
- Buendia-Kandia, F.; Rondags, E.; Framboisier, X.; Mauviel, G.; Dufour, A.; Guedon, E. Diauxic Growth of Clostridium acetobutylicum ATCC 824 When Grown on Mixtures of Glucose and Cellobiose. AMB Express 2018, 8, 85. [Google Scholar] [CrossRef]
- Gombert, A.K.; Kilikian, B.V. A Simple Way of Achieving a High Cell Concentration in Recombinant Escherichia coli Cultivation. Braz. J. Chem. Eng. 1997, 14, 185–190. [Google Scholar] [CrossRef]
- Lu, X.; Hagemann, M.; Liu, J.; Shukla, P.; Tan, X. Engineering Microalgal Chassis Cells. Front. Microbiol. 2023, 14, 1237999. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, F.L.; Contesini, F.J.; Fanchini, R.; Gerhardt, J.A.; Corrêa, A.B.; Antoniel, E.P.; Wassano, N.S.; Levassor, L.; Rabelo, S.C.; Franco, T.T.; et al. Engineering the Secretome of Aspergillus niger for Cellooligosaccharides Production from Plant Biomass. Microb. Cell Factories 2024, 23, 323. [Google Scholar] [CrossRef] [PubMed]
- Duncker, K.E.; Holmes, Z.A.; You, L. Engineered Microbial Consortia: Strategies and Applications. Microb. Cell Factories 2021, 20, 211. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhao, X.; He, C.; Zhang, X. Improving Lipid Production by Rhodotorula glutinis for Renewable Fuel Production Based on Machine Learning. Front. Chem. Sci. Eng. 2024, 18, 51. [Google Scholar] [CrossRef]
- Flores, A.D.; Ayla, E.Z.; Nielsen, D.R.; Wang, X. Engineering a Synthetic, Catabolically Orthogonal Coculture System for Enhanced Conversion of Lignocellulose-Derived Sugars to Ethanol. ACS Synth. Biol. 2019, 8, 1089–1099. [Google Scholar] [CrossRef]
- Sun, J.; Tian, K.; Wang, J.; Dong, Z.; Liu, X.; Permaul, K.; Singh, S.; Prior, B.A.; Wang, Z. Improved Ethanol Productivity from Lignocellulosic Hydrolysates by Escherichia coli with Regulated Glucose Utilization. Microb. Cell Factories 2018, 17, 66. [Google Scholar] [CrossRef]
- Zada, B.; Wang, C.; Park, J.-W.; Jeong, S.; Park, J.-E.; Singh, H.H.; Kim, S.-W. Metabolic Engineering of Escherichia coli for Production of Mixed Isoprenoid Alcohols and Their Derivatives. Biotechnol. Biofuels 2018, 11, 210. [Google Scholar] [CrossRef]
- Noda, S.; Mori, Y.; Oyama, S.; Kondo, A.; Araki, M.; Shirai, T. Reconstruction of Metabolic Pathway for Isobutanol Production in Escherichia coli. Microb. Cell Factories 2019, 18, 124. [Google Scholar] [CrossRef]
- Novak, K.; Baar, J.; Freitag, P.; Pflügl, S. Metabolic Engineering of Escherichia coli W for Isobutanol Production on Chemically Defined Medium and Cheese Whey as Alternative Raw Material. J. Ind. Microbiol. Biotechnol. 2020, 47, 1117–1132. [Google Scholar] [CrossRef]
- Songdech, P.; Butkinaree, C.; Yingchutrakul, Y.; Promdonkoy, P.; Runguphan, W.; Soontorngun, N. Increased Production of Isobutanol from Xylose through Metabolic Engineering of Saccharomyces cerevisiae Overexpressing Transcription Factor Znf1 and Exogenous Genes. FEMS Yeast Res. 2024, 24, foae006. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Ishii, J.; Kondo, T.; Ida, K.; Tezuka, H.; Kondo, A. Increased Isobutanol Production in Saccharomyces cerevisiae by Eliminating Competing Pathways and Resolving Cofactor Imbalance. Microb. Cell Factories 2013, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Rezk, M.; Mohammed, M.O.; Nagata, T.; Watanabe, T.; Katahira, M. Engineering a Hypoxia-Tolerant Saccharomyces cerevisiae for Rapid Ethanol Production via Co-Utilization of Glucose and Acetic Acid and Redox-Enhanced Flocculation. Sustain. Energy Fuels 2025, 9, 3838–3852. [Google Scholar] [CrossRef]
- Vilela, L.D.F.; de Araujo, V.P.G.; Paredes, R.D.S.; Bon, E.P.D.S.; Torres, F.A.G.; Neves, B.C.; Eleutherio, E.C.A. Enhanced Xylose Fermentation and Ethanol Production by Engineered Saccharomyces cerevisiae Strain. AMB Express 2015, 5, 16. [Google Scholar] [CrossRef]
- Kim, J.; Baidoo, E.E.K.; Amer, B.; Mukhopadhyay, A.; Adams, P.D.; Simmons, B.A.; Lee, T.S. Engineering Saccharomyces cerevisiae for Isoprenol Production. Metab. Eng. 2021, 64, 154–166. [Google Scholar] [CrossRef]
- Dudeja, C.; Mishra, A.; Ali, A.; Singh, P.P.; Jaiswal, A.K. Microbial Genome Editing with CRISPR–Cas9: Recent Advances and Emerging Applications Across Sectors. Fermentation 2025, 11, 410. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, T.; Qiao, M. Current Application and Future Prospects of CRISPR-Cas in Lactic Acid Bacteria: A Review. Food Res. Int. 2025, 209, 116315. [Google Scholar] [CrossRef]
- Verdezoto-Prado, J.; Chicaiza-Ortiz, C.; Mejía-Pérez, A.B.; Freire-Torres, C.; Viteri-Yánez, M.; Deng, L.; Barba-Ostria, C.; Guamán, L.P. Advances in Environmental Biotechnology with CRISPR/Cas9: Bibliometric Review and Cutting-Edge Applications. Discov. Appl. Sci. 2025, 7, 167. [Google Scholar] [CrossRef]
- Kaur, N.; Pati, P.K. Retron Library Recombineering: Next Powerful Tool for Genome Editing after CRISPR/Cas. ACS Synth. Biol. 2024, 13, 1019–1025. [Google Scholar] [CrossRef]
- González-Delgado, A.; Bonillo-Lopez, L.; Johnson, M.S.; Knödlseder, N.; Ko, C.-C.; Lekbach, Y.; Oh, J.-H.; Selvakumar, H.; Wold, M.C.; Yu, Z.; et al. Genome Editing of Phylogenetically Distinct Bacteria Using Portable Retron-Mediated Recombineering. bioRxiv 2025. [Google Scholar] [CrossRef]
- Yadav, J.; Marwah, H.; Kumar, C. Synthetic Biology and Metabolic Engineering Paving the Way for Sustainable Next-Gen Biofuels: A Comprehensive Review. Energy Adv. 2025, 4, 1209–1228. [Google Scholar] [CrossRef]
- Pfleger, B.F.; Takors, R. Recent Progress in the Synthesis of Advanced Biofuel and Bioproducts. Curr. Opin. Biotechnol. 2023, 80, 102913. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Toparlak, Ö.D.; Koffas, M.A. Metabolic Pathway Balancing and Its Role in the Production of Biofuels and Chemicals. Curr. Opin. Biotechnol. 2015, 33, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Schiavon, M.; Montejano-Montelongo, I.; Orozco-Ruiz, F.S.; Sotomayor-Vivas, C. The Art of Modeling Gene Regulatory Circuits. npj Syst. Biol. Appl. 2024, 10, 60. [Google Scholar] [CrossRef]
- Peng, B.; Bandari, N.C.; Lu, Z.; Howard, C.B.; Scott, C.; Trau, M.; Dumsday, G.; Vickers, C.E. Engineering Eukaryote-like Regulatory Circuits to Expand Artificial Control Mechanisms for Metabolic Engineering in Saccharomyces cerevisiae. Commun. Biol. 2022, 5, 135. [Google Scholar] [CrossRef]
- Liu, D.; Mannan, A.A.; Han, Y.; Oyarzún, D.A.; Zhang, F. Dynamic Metabolic Control: Towards Precision Engineering of Metabolism. J. Ind. Microbiol. Biotechnol. 2018, 45, 535–543. [Google Scholar] [CrossRef]
- Hollinshead, W.; He, L.; Tang, Y.J. Biofuel Production: An Odyssey from Metabolic Engineering to Fermentation Scale-Up. Front. Microbiol. 2014, 5, 344. [Google Scholar] [CrossRef]
- Müller, M.M.; Arndt, K.M.; Hoffmann, S.A. Genetic Circuits in Synthetic Biology: Broadening the Toolbox of Regulatory Devices. Front. Synth. Biol. 2025, 3, 1548572. [Google Scholar] [CrossRef]
- Liu, L.; Ding, D.; Wang, H.; Ren, X.; Lee, S.Y.; Zhang, D. Balancing Cell Growth and Product Synthesis for Efficient Microbial Cell Factories. Adv. Sci. 2025, 12, e10649. [Google Scholar] [CrossRef]
- Donati, S.; Mattanovich, M.; Hjort, P.; Jacobsen, A.B.S.; Blomquist, S.D.; Mangaard, D.; Gurdo, N.; Pastor, F.P.; Maury, J.; Hanke, R.; et al. An Automated Workflow for Multi-Omics Screening of Microbial Model Organisms. npj Syst. Biol. Appl. 2023, 9, 14. [Google Scholar] [CrossRef]
- Espinel-Ríos, S.; López, J.M.; Avalos, J.L. Omics-Driven Hybrid Dynamic Modeling of Bioprocesses with Uncertainty Estimation. Biochem. Eng. J. 2025, 2, 109637. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Musonge, P. Comparative Evaluation of Bioethanol Fermentation Process Parameters Using RSM, ANN and ANFIS. Biofuels Bioprod. Biorefining 2023, 17, 961–975. [Google Scholar] [CrossRef]
- Smuga-Kogut, M.; Kogut, T.; Markiewicz, R.; Słowik, A. Use of Machine Learning Methods for Predicting Amount of Bioethanol Obtained from Lignocellulosic Biomass with the Use of Ionic Liquids for Pretreatment. Energies 2021, 14, 243. [Google Scholar] [CrossRef]
- Owusu, W.A.; Marfo, S.A. Artificial Intelligence Application in Bioethanol Production. Int. J. Energy Res. 2023, 2023, 7844835. [Google Scholar] [CrossRef]
- Coşgun, A.; Günay, M.E.; Yıldırım, R. A Critical Review of Machine Learning for Lignocellulosic Ethanol Production via Fermentation Route. Biofuel Res. J. 2023, 10, 1859–1875. [Google Scholar] [CrossRef]
- Shomope, I.; Tawalbeh, M.; Al-Othman, A.; Almomani, F. Predicting Biohydrogen Production from Dark Fermentation of Organic Waste Biomass Using Multilayer Perceptron Artificial Neural Network (MLP–ANN). Comput. Chem. Eng. 2025, 192, 108900. [Google Scholar] [CrossRef]
- Fahimi Bandpey, A.; Abdi, J.; Firozjaee, T.T. Improved Estimation of Dark Fermentation Biohydrogen Production Utilizing a Robust Categorical Boosting Machine-Learning Algorithm. Int. J. Hydrogen Energy 2024, 52, 190–199. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Altaee, A.; Li, D. Machine Learning Modeling and Analysis of Biohydrogen Production from Wastewater by Dark Fermentation Process. Bioresour. Technol. 2022, 343, 126111. [Google Scholar] [CrossRef]
- Mienye, I.D.; Swart, T.G.; Obaido, G. Recurrent Neural Networks: A Comprehensive Review of Architectures, Variants, and Applications. Information 2024, 15, 517. [Google Scholar] [CrossRef]
- Ghojogh, B.; Ghodsi, A. Recurrent Neural Networks and Long Short-Term Memory Networks: Tutorial and Survey. arXiv 2023, arXiv:2304.11461. [Google Scholar] [CrossRef]
- Walia, N.; Singh, H.; Sharma, A. ANFIS: Adaptive Neuro-Fuzzy Inference System—A Survey. Int. J. Comput. Appl. 2015, 123, 32–38. [Google Scholar] [CrossRef]
- Perera, Y.S.; Ratnaweera, D.A.A.C.; Dasanayaka, C.H.; Abeykoon, C. The Role of Artificial Intelligence-Driven Soft Sensors in Advanced Sustainable Process Industries: A Critical Review. Eng. Appl. Artif. Intell. 2023, 121, 105988. [Google Scholar] [CrossRef]
- Ji, K.; Yu, X.; Chen, L.; Wang, Y.; Guo, Z.; Chen, B.; Li, Q.; Li, Z.; Zhang, H.; Wang, G.; et al. Data-Augmented Deep Learning Algorithm for Accurate Control of Bioethanol Fermentation Using an Online Raman Analyzer. Biotechnol. Bioeng. 2025, 122, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Medeiros Garcia Alcântara, J.; Iannacci, F.; Morbidelli, M.; Sponchioni, M. Soft Sensor Based on Raman Spectroscopy for the In-Line Monitoring of Metabolites and Polymer Quality in the Biomanufacturing of Polyhydroxyalkanoates. J. Biotechnol. 2023, 377, 23–33. [Google Scholar] [CrossRef]
- Brunner, V.; Siegl, M.; Geier, D.; Becker, T. Challenges in the Development of Soft Sensors for Bioprocesses: A Critical Review. Front. Bioeng. Biotechnol. 2021, 9, 722202. [Google Scholar] [CrossRef]
- Priyadharshini, D.; Muthuvel, I.; Saraswathy, S.; Kavitha, P.S.; Jegadeeswari, V. Precision to Plate: AI-Driven Innovations in Fermentation and Hyper-Personalized Diets. Front. Nutr. 2025, 12, 1659511. [Google Scholar] [CrossRef]
- Khamwachirapithak, P.; Sae-Tang, K.; Mhuantong, W.; Tanapongpipat, S.; Zhao, X.-Q.; Liu, C.-G.; Wei, D.-Q.; Champreda, V.; Runguphan, W. Optimizing Ethanol Production in Saccharomyces cerevisiae at Ambient and Elevated Temperatures through Machine Learning-Guided Combinatorial Promoter Modifications. ACS Synth. Biol. 2023, 12, 2897–2908. [Google Scholar] [CrossRef]
- Ezzatzadegan, L.; Yusof, R.; Morad, N.A.; Shabanzadeh, P.; Muda, N.S.; Borhani, T.N. Experimental and Artificial Intelligence Modelling Study of Oil Palm Trunk Sap Fermentation. Energies 2021, 14, 2137. [Google Scholar] [CrossRef]
- Butean, A.; Cutean, I.; Barbero, R.; Enriquez, J.; Matei, A. A Review of Artificial Intelligence Applications for Biorefineries and Bioprocessing: From Data-Driven Processes to Optimization Strategies and Real-Time Control. Processes 2025, 13, 2544. [Google Scholar] [CrossRef]
- Kurowska, K.; Marks-Bielska, R.; Bielski, S.; Kryszk, H.; Jasinskas, A. Food Security in the Context of Liquid Biofuels Production. Energies 2020, 13, 6247. [Google Scholar] [CrossRef]
- Hyrchenko, Y.; Skibina, T.; Us, Y.; Veckalne, R. World Market of Liquid Biofuels: Trends, Policy and Challenges. E3S Web Conf. 2021, 280, 05005. [Google Scholar] [CrossRef]
- Serrano, V.A.; Alberto, C.; Castro, K.T. A Review on Biobutanol: Eco-Friendly Fuel of the Future, History, Current Advances, and Trends. Fuels 2024, 6, 55. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Božidar Šantek, B.; Šantek, M.I. The Production of Bioethanol from Lignocellulosic Biomass: Pretreatment Methods, Fermentation, and Downstream Processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Novia, N.; Melwita, E.; Jannah, A.M.; Selpiana, S.; Yandriani, Y.; Afrah, B.D.; Rendana, M. Current Advances in Bioethanol Synthesis from Lignocellulosic Biomass: Sustainable Methods, Technological Developments, and Challenges. J. Umm Al-Qura Univ. Appl. Sci. 2025. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; de Medeiros Dantas, J.M.; Lavoie, J.-M. Optimization of Yeast, Sugar and Nutrient Concentrations for High Ethanol Production Rate Using Industrial Sugar Beet Molasses and Response Surface Methodology. Fermentation 2021, 7, 86. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production. Appl. Sci. 2024, 14, 10789. [Google Scholar] [CrossRef]
- Silva, R.F.; do Socorro Mascarenhas, M.; Batistote, M. Biomass: Bioethanol Transformation And Production Process. Rev. De Agric. Neotrop. 2022, 9, e7089. [Google Scholar] [CrossRef]
- Lozano, E.d.V.; Nogueira, L.C.; Alcântara, G.U.; Costa, G.H.G. Híbridos de Milho Afetam a Quantidade de Etanol Produzida No Cerrado Do Centro-Oeste Paulista. Front. J. Soc. Technol. Environ. Sci. 2020, 9, 424–438. [Google Scholar] [CrossRef]
- Woźniak, A.; Kuligowski, K.; Świerczek, L.; Cenian, A. Review of Lignocellulosic Biomass Pretreatment Using Physical, Thermal and Chemical Methods for Higher Yields in Bioethanol Production. Sustainability 2025, 17, 287. [Google Scholar] [CrossRef]
- Secches, T.O.; Santos Viera, C.F.; Pereira, T.K.; Santos, V.T.; Ribeirodos Santos, J.; Pereira, G.A.; Carazzolle, M.F. Brazilian Industrial Yeasts Show High Fermentative Performance in High Solids Content for Corn Ethanol Process. Bioresour. Bioprocess. 2022, 9, 97. [Google Scholar] [CrossRef]
- Krajang, M.; Malairuang, K.; Sukna, J.; Rattanapradit, K.; Chamsart, S. Single-Step Ethanol Production from Raw Cassava Starch Using a Combination of Raw Starch Hydrolysis and Fermentation, Scale-up from 5-L Laboratory and 200-L Pilot Plant to 3000-L Industrial Fermenters. Biotechnol. Biofuels 2021, 14, 68. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Rajan, K.; Djioleu, A.; Rocha, T.M.; Brumano, L.P.; de Souza Melo, Y.C.; dos Santos, J.C.; Rosa, C.A.; Carrier, D.J.; da Silva, S.S. Sustainable Second-Generation Ethanol Production from Switchgrass Biomass via Co-Fermentation of Pentoses and Hexoses Using Novel Wild Yeasts. Bioenerg. Res. 2022, 15, 1157–1168. [Google Scholar] [CrossRef]
- Soares, L.B.; Santana, M.B.; da Silveira, J.M.; do Nascimento, L.L.; de Meirelles, M.Y.; Henriques, R.O.; Zanella, E.; Araujo, M.F.; Stambuk, B.U.; da Costa, A.C.; et al. Evaluating the Production of Second-Generation Ethanol by Spathaspora passalidarum Immobilized on Sugarcane Bagasse. Bioenerg. Res. 2023, 16, 2022–2035. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb Cell Fact 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, B.; Syed Muhammad, S.A.F.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth Generation Biofuel: A Review on Risks and Mitigation Strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Lou, J.; Wang, J.; Yang, Y.; Yang, Q.; Li, R.; Hu, M.; He, Q.; Du, J.; Wang, X.; Li, M.; et al. Development and Characterization of Efficient Xylose Utilization Strains of Zymomonas mobilis. Biotechnol. Biofuels 2021, 14, 231. [Google Scholar] [CrossRef]
- Jilani, S.B.; Olson, D.G. Mechanism of Furfural Toxicity and Metabolic Strategies to Engineer Tolerance in Microbial Strains. Microb. Cell Factories 2023, 22, 221. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Ojuederie, O.B.; Talia, P.M.; Babalola, O.O. Bioprospecting of Microbial Strains for Biofuel Production: Metabolic Engineering, Applications, and Challenges. Biotechnol. Biofuels 2021, 14, 5. [Google Scholar] [CrossRef]
- Cunha, J.T.; Soares, P.O.; Baptista, S.L.; Costa, C.E.; Domingues, L. Engineered Saccharomyces cerevisiae for Lignocellulosic Valorization: A Review and Perspectives on Bioethanol Production. Bioengineered 2020, 11, 883–903. [Google Scholar] [CrossRef]
- Vasylyshyn, R.; Ruchala, J.; Dmytruk, K.; Sibirny, A. Recent Progress in Engineering Yeast Producers of Cellulosic Ethanol. FEMS Yeast Res. 2025, 25, foaf035. [Google Scholar] [CrossRef]
- Olson, A.L.; Tunér, M.; Verhelst, S. A Review of Isobutanol as a Fuel for Internal Combustion Engines. Energies 2023, 16, 7470. [Google Scholar] [CrossRef]
- Dedov, A.G.; Karavaev, A.A.; Loktev, A.S.; Osipov, A.K. Bioisobutanol as a Promising Feedstock for Production of “Green” Hydrocarbons and Petrochemicals (A Review). Pet. Chem. 2021, 61, 1139–1157. [Google Scholar] [CrossRef]
- Nawab, S.; Zhang, Y.; Ullah, M.W.; Lodhi, A.F.; Shah, S.B.; Rahman, M.U.; Yong, Y.-C. Microbial Host Engineering for Sustainable Isobutanol Production from Renewable Resources. Appl. Microbiol. Biotechnol. 2024, 108, 33. [Google Scholar] [CrossRef]
- Chen, X.; Nielsen, K.F.; Borodina, I.; Kielland-Brandt, M.C.; Karhumaa, K. Increased Isobutanol Production in Saccharomyces Cerevisiae by Overexpression of Genes in Valine Metabolism. Biotechnol. Biofuels 2011, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Duan, Y.; Liang, D.; Li, T.; Luo, H.; Chen, R. Biofuels and Their Blends—A Review of the Effect of Low Carbon Fuels on Engine Performance. Sustainability 2024, 16, 10300. [Google Scholar] [CrossRef]
- Wu, G.; Ge, J.C.; Choi, N.J. A Comprehensive Review of the Application Characteristics of Biodiesel Blends in Diesel Engines. Appl. Sci. 2020, 10, 8015. [Google Scholar] [CrossRef]
- Dupuis, D.P.; Grim, R.G.; Nelson, E.D.; Tan, E.C.D.; Ruddy, D.A.; Hernández, S.; Westover, T.L.; Hensley, J.E.; Carpenter, D. High-Octane Gasoline from Biomass: Experimental, Economic, and Environmental Assessment. Appl. Energy 2019, 241, 25–33. [Google Scholar] [CrossRef]
- Meyers, J.; Mensah, J.B.; Holzhäuser, F.J.; Omari, A.; Blesken, C.C.; Tiso, T.; Palkovits, S.; Blank, L.M.; Pischinger, S.; Palkovits, R. Electrochemical Conversion of a Bio-Derivable Hydroxy Acid to a Drop-in Oxygenate Diesel Fuel. Energy Environ. Sci. 2019, 12, 2406–2411. [Google Scholar] [CrossRef]
- Xu, J.; Long, F.; Jiang, J.; Li, F.; Zhai, Q.; Wang, F.; Liu, P.; Li, J. Integrated Catalytic Conversion of Waste Triglycerides to Liquid Hydrocarbons for Aviation Biofuels. J. Clean. Prod. 2019, 222, 784–792. [Google Scholar] [CrossRef]
- Kargbo, H.; Harris, J.S.; Phan, A.N. “Drop-in” Fuel Production from Biomass: Critical Review on Techno-Economic Feasibility and Sustainability. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Hayashi, Y.; Arai, M. Recent Advances in the Improvement of Cyanobacterial Enzymes for Bioalkane Production. Microb. Cell Factories 2022, 21, 256. [Google Scholar] [CrossRef]
- Bruder, S.; Moldenhauer, E.J.; Lemke, R.D.; Ledesma-Amaro, R.; Kabisch, J. Drop-in Biofuel Production Using Fatty Acid Photodecarboxylase from Chlorella Variabilis in the Oleaginous Yeast Yarrowia lipolytica. Biotechnol. Biofuels 2019, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, Y.J.; Kang, M.-K.; Krivoruchko, A.; Buijs, N.A.; Nielsen, J. Enabling the Synthesis of Medium Chain Alkanes and 1-Alkenes in Yeast. Metab. Eng. 2017, 44, 81–88. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, Q.; Dai, J.; Zhao, H.; Shi, S. Engineering Oleaginous Yeast Rhodotorula toruloides for Production of Alkanes and Alkenes. Metab. Eng. 2025, 91, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Ghedini, E.; Taghavi, S.; Menegazzo, F.; Signoretto, M. A Review on the Efficient Catalysts for Algae Transesterification to Biodiesel. Sustainability 2021, 13, 10479. [Google Scholar] [CrossRef]
- Gautam, R.; Vinu, R. Reaction Engineering and Kinetics of Algae Conversion to Biofuels and Chemicals via Pyrolysis and Hydrothermal Liquefaction. React. Chem. Eng. 2020, 5, 1320–1373. [Google Scholar] [CrossRef]
- Scopel, L.; Marcantonio, V. Gasification of Chlorella Vulgaris for Syngas Production and Energy Generation Through Gas Turbine. Energies 2024, 17, 6085. [Google Scholar] [CrossRef]
- Carruthers, D.N.; Kim, J.-H.; Mendez-Perez, D.; Monroe, E.; Myllenbeck, N.; Liu, Y.; Davis, R.W.; Sundstrom, E.; Lee, T.S. Microbial Production of High Octane and High Sensitivity Olefinic Ester Biofuels. Biotechnol. Biofuels Bioprod. 2023, 16, 60. [Google Scholar] [CrossRef]
- Lehtinen, T.; Virtanen, H.; Santala, S.; Santala, V. Production of Alkanes from CO2 by Engineered Bacteria. Biotechnol. Biofuels 2018, 11, 228. [Google Scholar] [CrossRef]
- Park, Y.-K.; Ledesma-Amaro, R. What Makes Yarrowia lipolytica Well Suited for Industry? Trends Biotechnol. 2022, 41, 242–254. [Google Scholar] [CrossRef]
- Xu, Y. Biochemistry and Biotechnology of Lipid Accumulation in the Microalga Nannochloropsis oceanica. J. Agric. Food Chem. 2022, 70, 11500–11509. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Gupta, R.K. Salinity-Induced Physiochemical Alterations to Enhance Lipid Content in Oleaginous Microalgae Scenedesmus sp. BHU1 via Two-Stage Cultivation for Biodiesel Feedstock. Microorganisms 2023, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, S.; Tan, X.; Guo, L.; Liu, W.; Tian, W.; Zhang, H.; Jiang, T.; Meng, W.; Liu, Y.; et al. Production of α-Ketoisovalerate with Whey Powder by Systemic Metabolic Engineering of Klebsiella oxytoca. Microb. Cell Factories 2024, 23, 264. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Begum, A.; Gunn, L.H.; Lindblad, P. Directed Evolution of α-Ketoisovalerate Decarboxylase for Improved Isobutanol and 3-Methyl-1-Butanol Production in Cyanobacteria. Biotechnol. Biofuels Bioprod. 2025, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Wu, P.; Chen, H.; Chen, Y.; Zhang, Y.; Yuan, J. Microbial Synthesis of Branched-Chain β,γ-Diols from Amino Acid Metabolism. Nat. Commun. 2025, 16, 4568. [Google Scholar] [CrossRef]
- Pastore, A.E.L.; Coplien, J.; Anthony, L.C.; Sato, T.K.; Zhang, Y.; Karlen, S.D.; Hittinger, C.T.; Maravelias, C.T. On the Synthesis of Biorefineries for High-Yield Isobutanol Production: From Biomass-to-Alcohol Experiments to System Level Analysis. RSC Sustain. 2024, 2, 2532–2540. [Google Scholar] [CrossRef]
- Zago, M.; Branduardi, P.; Serra, I. Towards Biotechnological Production of Bio-Based Low Molecular Weight Esters: A Patent Review. RSC Adv. 2024, 14, 29472–29489. [Google Scholar] [CrossRef]
- Bai, W.; Geng, W.; Wang, S.; Zhang, F. Biosynthesis, Regulation, and Engineering of Microbially Produced Branched Biofuels. Biotechnol. Biofuels 2019, 12, 84. [Google Scholar] [CrossRef]
- Werner, F.; Schwardmann, L.S.; Siebert, D.; Rückert-Reed, C.; Kalinowski, J.; Wirth, M.-T.; Hofer, K.; Takors, R.; Wendisch, V.F.; Blombach, B. Metabolic Engineering of Corynebacterium glutamicum for Fatty Alcohol Production from Glucose and Wheat Straw Hydrolysate. Biotechnol. Biofuels Bioprod. 2023, 16, 116. [Google Scholar] [CrossRef]
- Wang, G.; Wang, M.; Yang, J.; Li, Q.; Zhu, N.; Liu, L.; Hu, X.; Yang, X. De Novo Synthesis of 2-Phenylethanol from Glucose by Metabolically Engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac026. [Google Scholar] [CrossRef]
- Koonthongkaew, J.; Ploysongsri, N.; Toyokawa, Y.; Ruangpornvisuti, V.; Takagi, H. Improvement of Fusel Alcohol Production by Engineering of the Yeast Branched-Chain Amino Acid Aminotransaminase. Appl. Environ. Microbiol. 2022, 88, e00557-22. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Makertihartha, I.G.B.N.; Indarto, A.; Prakoso, T.; Soerawidjaja, T.H. A Review of Biodiesel Cold Flow Properties and Its Improvement Methods: Towards Sustainable Biodiesel Application. Energies 2024, 17, 4543. [Google Scholar] [CrossRef]
- Bentley, G.J.; Jiang, W.; Guaman, L.P.; Xiao, Y.; Zhang, F. Engineering Escherichia coli to Produce Branched-Chain Fatty Acids in High Percentages. Metab. Eng. 2016, 38, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Kurniasih, N.; Foo, J.L.; Su, S.; Chang, M.W. Metabolic Engineering of Saccharomyces cerevisiae for the Overproduction of Short Branched-Chain Fatty Acids. Metab. Eng. 2016, 34, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Hossain, A.K.; Duraisamy, G. Experimental Investigation of Neat Biodiesels’ Saturation Level on Combustion and Emission Characteristics in a CI Engine. Energies 2021, 14, 5203. [Google Scholar] [CrossRef]
- Zhu, L.; Cheung, C.S.; Huang, Z. Impact of Chemical Structure of Individual Fatty Acid Esters on Combustion and Emission Characteristics of Diesel Engine. Energy 2016, 107, 305–320. [Google Scholar] [CrossRef]
- Bharti, R.K.; Kaushal, C.; Singh, A.; Dhar, D.W.; Babu, R.; Kaushik, A. Evaluation of Fuel Properties for Possible Biodiesel Output Based on the Fatty Acid Composition of Oleaginous Plants and Microalgae. Sci. Total Environ. 2024, 918, 170448. [Google Scholar] [CrossRef]
- Konzock, O.; Matsushita, Y.; Zaghen, S.; Sako, A.; Norbeck, J. Altering the Fatty Acid Profile of Yarrowia lipolytica to Mimic Cocoa Butter by Genetic Engineering of Desaturases. Microb. Cell Factories 2022, 21, 25. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Meng, Q.; Yang, C.; Hu, Y.; Wang, C.; Wu, P.; Ruan, C.; Li, W.; Cheng, S.; et al. Saccharomyces cerevisiae Cellular Engineering for the Production of FAME Biodiesel. AMB Express 2024, 14, 42. [Google Scholar] [CrossRef]
- Kobalter, S.; Wriessnegger, T.; Pichler, H. Engineering Yeast for Tailored Fatty Acid Profiles. Appl. Microbiol. Biotechnol. 2025, 109, 101. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Yu, Q.; Mao, J.; Zhang, B.; Xing, L.; Li, M. Deletion of Genes Encoding Fatty Acid Desaturases Leads to Alterations in Stress Sensitivity in Pichia pastoris. FEMS Yeast Res. 2015, 15, fov020. [Google Scholar] [CrossRef] [PubMed]
- Tsakraklides, V.; Kamineni, A.; Consiglio, A.L.; MacEwen, K.; Friedlander, J.; Blitzblau, H.G.; Hamilton, M.A.; Crabtree, D.V.; Su, A.; Afshar, J.; et al. High-Oleate Yeast Oil without Polyunsaturated Fatty Acids. Biotechnol. Biofuels 2018, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mei, C.; Yap, S.A.; Cai, L.; Ji, L. Understanding and Exploiting the Fatty Acid Desaturation System in Rhodotorula Toruloides. Biotechnol. Biofuels 2021, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- International Air Transport Association CO2 Emissions in 2023. Available online: https://www.iea.org/reports/co2-emissions-in-2023 (accessed on 20 December 2025).
- Parveen, H.; Yazdani, S.S. Insights into Cyanobacterial Alkane Biosynthesis. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab075. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Fan, M.; Jia, C.; Shi, L.; Pan, X.; Cao, P.; Zhao, X.; Chang, W.; Li, M. Structural Insights into Catalytic Mechanism and Product Delivery of Cyanobacterial Acyl-Acyl Carrier Protein Reductase. Nat. Commun. 2020, 11, 1525. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-Fermentative Pathways for Synthesis of Branched-Chain Higher Alcohols as Biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef]
- Branduardi, P.; Longo, V.; Berterame, N.M.; Rossi, G.; Porro, D. A Novel Pathway to Produce Butanol and Isobutanol in Saccharomyces cerevisiae. Biotechnol. Biofuels 2013, 6, 68. [Google Scholar] [CrossRef]
- Shen, C.R.; Lan, E.I.; Dekishima, Y.; Baez, A.; Cho, K.M.; Liao, J.C. Driving Forces Enable High-Titer Anaerobic 1-Butanol Synthesis in Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 2905–2915. [Google Scholar] [CrossRef]
- Atsumi, S.; Higashide, W.; Liao, J.C. Direct Photosynthetic Recycling of Carbon Dioxide to Isobutyraldehyde. Nat. Biotechnol 2009, 27, 1177–1180. [Google Scholar] [CrossRef]
- Schirmer, A.; Rude, M.A.; Li, X.; Popova, E.; del Cardayre, S.B. Microbial Biosynthesis of Alkanes. Science 2010, 329, 559–562. [Google Scholar] [CrossRef]
- Blazeck, J.; Hill, A.; Liu, L.; Knight, R.; Miller, J.; Pan, A.; Otoupal, P.; Alper, H.S. Harnessing Yarrowia lipolytica Lipogenesis to Create a Platform for Lipid and Biofuel Production. Nat. Commun. 2014, 5, 3131. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Wasylenko, T.M.; Zhou, K.; Xu, P.; Stephanopoulos, G. Lipid Production in Yarrowia lipolytica Is Maximized by Engineering Cytosolic Redox Metabolism. Nat. Biotechnol. 2017, 35, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Honda, K.; Kazuhito, F. Current Advances in Alteration of Fatty Acid Profile in Rhodotorula toruloides: A Mini-Review. World J. Microbiol. Biotechnol. 2023, 39, 234. [Google Scholar] [CrossRef] [PubMed]
- Westfall, P.J.; Pitera, D.J.; Lenihan, J.R.; Eng, D.; Woolard, F.X.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.J.; Owens, A.; et al. Production of Amorphadiene in Yeast, and Its Conversion to Dihydroartemisinic Acid, Precursor to the Antimalarial Agent Artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118. [Google Scholar] [CrossRef]
- Bourgade, B.; Islam, M.A. Progresses and Challenges of Engineering Thermophilic Acetogenic Cell Factories. Front. Microbiol. 2024, 15, 1476253. [Google Scholar] [CrossRef]
- Rückel, A.; Oppelt, A.; Leuter, P.; Johne, P.; Fendt, S.; Weuster-Botz, D. Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum. Fermentation 2022, 8, 465. [Google Scholar] [CrossRef]
- Wan, S.; Lai, M.; Gao, X.; Zhou, M.; Yang, S.; Li, Q.; Li, F.; Xia, L.; Tan, Y. Recent Progress in Engineering Clostridium autoethanogenum to Synthesize the Biochemicals and Biocommodities. Synth. Syst. Biotechnol. 2024, 9, 19–25. [Google Scholar] [CrossRef]
- Ghadermazi, P.; Re, A.; Ricci, L.; Chan, S.H.J. Metabolic Engineering Interventions for Sustainable 2,3-Butanediol Production in Gas-Fermenting Clostridium autoethanogenum. mSystems 2022, 7, e01111-21. [Google Scholar] [CrossRef]
- Jin, S.; Ganesh, I.; Bae, J.; Lee, D.; Kang, S.; Lee, H.; Lee, J.W.; Cho, B.-K. Metabolic Engineering of Acetogenic Bacteria Using CO Gas-Sensing Transcriptional ON/OFF Modules. Metab. Eng. 2025, 91, 290–301. [Google Scholar] [CrossRef]
- Aravindan, M.; Kumar, V.M.; Hariharan, V.S.; Narahari, T.; Kumar, P.A.; Madhesh, K.; Kumar, G.P.; Prabakaran, R. Fuelling the Future: A Review of Non-Renewable Hydrogen Production and Storage Techniques. Renew. Sustain. Energy Rev. 2023, 188, 113791. [Google Scholar] [CrossRef]
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The Future of Hydrogen: Challenges on Production, Storage and Applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Faisal, F.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Dexter, R.B. Automobile Fuels (Diesel and Petrol) from Plastic Pyrolysis Oil—Production and Characterisation. Energy Rep. 2022, 8, 730–735. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the Role of Hydrogen in the 21st Century Energy Transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Smarte, M.; Tetteh, E.K.; Amune, U.O.; Mustapha, S.I.; Kiambi, S.L. Hydrogen as a Clean Energy Carrier: Advancements, Challenges, and Its Role in a Sustainable Energy Future. Clean Energy 2025, 9, 52–88. [Google Scholar] [CrossRef]
- Simão, G.F.; Luzia, C.; Moreira, E.S.; Soares, H.; Micoli, L.; Valderez, M. Hydrogen Production by Enterobacter cloacae via Dark Fermentation Using an Agroindustrial Residue, Cashew Apple Bagasse. Biofuels Bioprod. Biorefining 2025, 19, 1207–1218. [Google Scholar] [CrossRef]
- Zhang, Q.; You, S.; Li, Y.; Qu, X.; Jiang, H. Enhanced Biohydrogen Production from Cotton Stalk Hydrolysate of Enterobacter cloacae WL1318 by Overexpression of the Formate Hydrogen Lyase Activator Gene. Biotechnol. Biofuels 2020, 13, 94. [Google Scholar] [CrossRef]
- Deseure, J.; Obeid, J.; Willison, J.C.; Magnin, J.-P. Reliable Determination of the Growth and Hydrogen Production Parameters of the Photosynthetic Bacterium Rhodobacter capsulatus in Fed Batch Culture Using a Combination of the Gompertz Function and the Luedeking-Piret Model. Heliyon 2021, 7, e07394. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.-S.; Ho, S.-H. Biohydrogen Production from Microalgae for Environmental Sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef]
- Ivanenko, A.A.; Laikova, A.A.; Zhuravleva, E.A.; Shekhurdina, S.V.; Vishnyakova, A.V.; Kovalev, A.A.; Kovalev, D.A.; Trchounian, K.A.; Litti, Y.V. Biological Production of Hydrogen: From Basic Principles to the Latest Advances in Process Improvement. Int. J. Hydrogen Energy 2024, 55, 740–755. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, D.; Wang, C.; Fang, D.; Cao, L.; Gong, C. Genetic Engineering for Biohydrogen Production from Microalgae. iScience 2023, 26, 107255. [Google Scholar] [CrossRef]
- López-Hernández, B.N.; Escamilla-Alvarado, C.; Albalate-Ramírez, A.; Rivas-García, P.; Amézquita-García, H.J.; Rodríguez-Valderrama, S.; Paredes, M.G. Photofermentative Hydrogen Production from Real Dark Fermentation Effluents: A Sequential Valorization of Orange Peel Waste. Fermentation 2025, 11, 504. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen Production From Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Sneha, N.P.; Rafi, S.M.; Sarkar, O. Dark Fermentative Hydrogen Production: Potential of Food Waste as Future Energy Needs. Sci. Total Environ. 2023, 888, 163801. [Google Scholar] [CrossRef] [PubMed]
- Díez, M.P.; Villanueva-Galindo, E.; Moreno-Andrade, I.; Díaz, E.; Rubia, M.A.; Mohedano, A.F.; Perez-Rangel, M. Enhanced Hydrogen Production from Food Waste via Bioaugmentation with Clostridium and Lactobacillus. Biomass Convers. Biorefinery 2025, 15, 27501–27513. [Google Scholar] [CrossRef]
- Husaini, C.U.N.; Roslan, R.; Ramzi, A.B.; Luthfi, A.; Tan, J.P.; Lim, S.S.; Ding, G.T.; Jahim, J.M.; Abdul, P.M. The CRISPR Technology: A Promising Strategy for Improving Dark Fermentative Biohydrogen Production Using Clostridium spp. Int. J. Hydrogen Energy 2023, 48, 23498–23515. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pescarolo, S.; Gilli, G.; Gilardi, G.; Valetti, F. Hydrogen Production Pathways in Clostridia and Their Improvement by Metabolic Engineering. Biotechnol. Adv. 2024, 73, 108379. [Google Scholar] [CrossRef]
- Vani, M.V.; Basha, P.O.; Chandrasekhar, T.; Riazunnisa, K. Development and Characterization of Chlamydomonas reinhardtii Low Chlorophyll Mutants to Improve Photosynthetic Efficiency and Biomass. Rev. Bras. Botânica 2023, 46, 307–318. [Google Scholar] [CrossRef]
- Kosourov, S.N.; Ghirardi, M.L.; Seibert, M. A Truncated Antenna Mutant of Chlamydomonas reinhardtii Can Produce More Hydrogen than the Parental Strain. Int. J. Hydrogen Energy 2011, 36, 2044–2048. [Google Scholar] [CrossRef]
- Yang, D.-W.; Syn, J.-W.; Hsieh, C.-H.; Huang, C.-C.; Chien, L.-F. Genetically Engineered Hydrogenases Promote Biophotocatalysis-Mediated H2 Production in the Green Alga Chlorella sp. DT. Int. J. Hydrogen Energy 2019, 44, 2533–2545. [Google Scholar] [CrossRef]
- Usman, M.; Kavitha, S.; Kannah, Y.; Yogalakshmi, K.N.; Sivashanmugam, P.; Bhatnagar, A.; Kumar, G. A Critical Review on Limitations and Enhancement Strategies Associated with Biohydrogen Production. Int. J. Hydrogen Energy 2021, 46, 16565–16590. [Google Scholar] [CrossRef]
- Goria, K.; Singh, H.M.; Singh, A.; Kothari, R.; Tyagi, V.V. Insights into Biohydrogen Production from Algal Biomass: Challenges, Recent Advancements and Future Directions. Int. J. Hydrogen Energy 2023, 52, 127–151. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Muin, N.A.A.; Isah, A.N.; Asli, U.A.; Sadikin, A.N.; Norazahar, N.; Kamaruddin, M.J.; Hassim, M.H.; Wai Shin, H.; Azman, N.R. A Short Review on Various Purification Techniques Suitable for Biohydrogen-Mixed Gases. J. Energy Saf. Technol. (JEST) 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Bari, H.E.; Lahboubi, N.; Habchi, S.; Rachidi, S.; Bayssi, O.; Nabil, N.; Mortezaei, Y.; Villa, R. Biohydrogen Production from Fermentation of Organic Waste, Storage and Applications. Clean. Waste Syst. 2022, 3, 100043. [Google Scholar] [CrossRef]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current Strategies and Future Perspectives in Biological Hydrogen Production: A Review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Denayer, J.F.M.; Karimi, K. Efficient Coproduction of Butanol, Ethanol, and Biohydrogen from Municipal Solid Waste through a Cocultivated Biorefinery. Energy Convers. Manag. 2022, 255, 115303. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.; Magaña, G.; Rodríguez, F.; Léon-Rodríguez, A.; Sánchez, A. Co-Production of Ethanol-Hydrogen by Genetically Engineered Escherichia Coli in Sustainable Biorefineries for Lignocellulosic Ethanol Production. Chem. Eng. J. 2021, 406, 126829. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial Electrohydrogenesis Cell and Dark Fermentation Integrated System Enhances Biohydrogen Production from Lignocellulosic Agricultural Wastes: Substrate Pretreatment towards Optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Marone, A.; Ayala-Campos, O.R.; Trably, E.; Carmona-Martínez, A.A.; Moscoviz, R.; Latrille, E.; Steyer, J.-P.; Alcaraz-Gonzalez, V.; Bernet, N. Coupling Dark Fermentation and Microbial Electrolysis to Enhance Bio-Hydrogen Production from Agro-Industrial Wastewaters and by-Products in a Bio-Refinery Framework. Int. J. Hydrogen Energy 2017, 42, 1609–1621. [Google Scholar] [CrossRef]
- Srivastava, P.; García-Quismondo, E.; Palma, J.; González-Fernández, C. Coupling Dark Fermentation and Microbial Electrolysis Cells for Higher Hydrogen Yield: Technological Competitiveness and Challenges. Int. J. Hydrogen Energy 2024, 52, 223–239. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Pérez-Bernal, M.F.; Bernet, N.; Trably, E. Sequential Dark Fermentation and Microbial Electrolysis Cells for Hydrogen Production: Volatile Fatty Acids Influence and Energy Considerations. Bioresour Technol 2023, 374, 128803. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, D.; Choi, H.; Cha, J.; Baek, G.; Lee, C. Comparative Study of Exoelectrogenic Utilization Preferences and Hydrogen Conversion among Major Fermentation Products in Microbial Electrolysis Cells. Bioresour. Technol. 2024, 393, 130032. [Google Scholar] [CrossRef] [PubMed]
- Khongkliang, P.; Kongjan, P.; Utarapichat, B.; Reungsang, A.; O-Thong, S. Continuous Hydrogen Production from Cassava Starch Processing Wastewater by Two-Stage Thermophilic Dark Fermentation and Microbial Electrolysis. Int. J. Hydrogen Energy 2017, 42, 27584–27592. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, H.U.; Na, J.-G.; Ko, Y.-S.; Cho, J.S.; Lee, S.Y. Factors Affecting the Competitiveness of Bacterial Fermentation. Trends Biotechnol 2023, 41, 798–816. [Google Scholar] [CrossRef]
- Zulaiha, S. Genetic Engineering of Microalgae Lipid Biosynthesis for Sustainable Biodiesel Production. World J. Adv. Res. Rev. 2021, 11, 072–077. [Google Scholar] [CrossRef]
- Peña-Castro, J.M.; Muñoz-Páez, K.M.; Robledo-Narváez, P.N.; Vázquez-Núñez, E. Engineering the Metabolic Landscape of Microorganisms for Lignocellulosic Conversion. Microorganisms 2023, 11, 2197. [Google Scholar] [CrossRef]
- Wu, Y.; Chu, W.; Yang, J.; Xu, Y.; Shen, Q.; Yang, H.; Xu, F.; Liu, Y.; Lu, P.; Jiang, K.; et al. Metabolic Engineering of Enterobacter Aerogenes for Improved 2,3-Butanediol Production by Manipulating NADH Levels and Overexpressing the Small RNA RyhB. Front. Microbiol. 2021, 12, 754306. [Google Scholar] [CrossRef]
- Yogiswara, S.; Rombout, J.; Micharikopoulos, G.; Craemer, S.D.; Herrera-Malaver, B.; van Landschoot, L.; Mannaerts, S.; do Amaral, M.; Voordeckers, K.; Spaepen, S.; et al. Metabolic Engineering of Saccharomyces cerevisiae for Co-Production of Ethanol and 3-Methyl-1-Butanol from Sugarcane Molasses. Biotechnol. Biofuels Bioprod. 2025, 18, 86. [Google Scholar] [CrossRef]
- Pilania, P.; Bhushan, K.; Phutela, U.G. Electro-Fermentation for Biofuel and Biochemical Production. Fermentation 2025, 11, 219. [Google Scholar] [CrossRef]
- Salar-García, M.J.; Ortiz-Martínez, V.M.; Sánchez-Segado, S.; Valero, R.S.; López, A.S.; Lozano-Blanco, L.J.; Godínez-Seoane, C. Sustainable Production of Biofuels and Biochemicals via Electro-Fermentation Technology. Mol./Mol. Online/Mol. Annu. 2024, 29, 834. [Google Scholar] [CrossRef]
- Hoffman, S.M.; Alvarez, M.; Alfassi, G.; Rein, D.M.; Garcia-Echauri, S.; Cohen, Y.; Avalos, J.L. Cellulosic Biofuel Production Using Emulsified Simultaneous Saccharification and Fermentation (eSSF) with Conventional and Thermotolerant Yeasts. Biotechnol. Biofuels 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Tinôco, D.; Seldin, L.; Coutinho, P.L.d.A.; Freire, D.M.G. Optimization of Fermentation Conditions as a Metabolic Strategy for the High-Yield and High-Selectivity Bio-Based 2,3-Butanediol Production. J. Ind. Eng. Chem. 2023, 125, 345–359. [Google Scholar] [CrossRef]
- Sarma, J.B.; Mahanta, S.; Tanti, B. Maximizing Microbial Activity and Synergistic Interaction to Boost Biofuel Production from Lignocellulosic Biomass. Arch. Microbiol. 2024, 206, 448. [Google Scholar] [CrossRef] [PubMed]
- Faria, D.J.; Paula, A.; Conte-Junior, C.A. Fermentation of Biomass and Residues from Brazilian Agriculture for 2G Bioethanol Production. ACS Omega 2024, 9, 40298–40314. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Shao, Y.; Zhang, C.; You, X.; Yang, Q.; Xie, F.; Yang, R.; Luo, H. Efficient Pretreatment of Phragmites Australis Biomass Using Glutamic Acid for Bioethanol Production by a Hybrid Hydrolysis and Fermentation Strategy. Bioprocess Biosyst. Eng. 2025, 48, 1133–1146. [Google Scholar] [CrossRef]
- Singh, S.; Nara, R.; Yadav, M.; Sharma, C.; Agrawal, S.; Kumar, A. Oil Palm Biomass: A Potential Feedstock for Lignocellulolytic Enzymes and Biofuels Production. Environ. Sci. Pollut. Res. 2025, 32, 11791–11814. [Google Scholar] [CrossRef]
- Neto, A.S.; Wainaina, S.; Chandolias, K.; Piatek, P.; Taherzadeh, M.J. Exploring the Potential of Syngas Fermentation for Recovery of High-Value Resources: A Comprehensive Review. Curr. Pollut. Rep. 2024, 11, 7. [Google Scholar] [CrossRef]
- Albino, M.; Gargalo, C.L.; Nadal-Rey, G.; Albæk, M.O.; Krühne, U.; Gernaey, K.V. Hybrid Modeling for On-Line Fermentation Optimization and Scale-Up: A Review. Processes 2024, 12, 1635. [Google Scholar] [CrossRef]
- Naresh Kumar, A.; Sarkar, O.; Chandrasekhar, K.; Raj, T.; Narisetty, V.; Mohan, S.V.; Pandey, A.; Varjani, S.; Kumar, S.; Sharma, P.; et al. Upgrading the Value of Anaerobic Fermentation via Renewable Chemicals Production: A Sustainable Integration for Circular Bioeconomy. Sci. Total Environ. 2022, 806, 150312. [Google Scholar] [CrossRef]
- Mongkhonsiri, G.; Charoensuppanimit, P.; Anantpinijwatna, A.; Gani, R.; Assabumrungrat, S. Process Development of Sustainable Biorefinery System Integrated into the Existing Pulping Process. J. Clean. Prod. 2020, 255, 120278. [Google Scholar] [CrossRef]
- Perpelea, A.; Wijaya, A.W.; Martins, L.C.; Rippert, D.; Klein, M.; Angelov, A.; Peltonen, K.; Teleki, A.; Liebl, W.; Richard, P.; et al. Towards Valorization of Pectin-Rich Agro-Industrial Residues: Engineering of Saccharomyces Cerevisiae for Co-Fermentation of d-Galacturonic Acid and Glycerol. Metab. Eng. 2022, 69, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumpas, D.-S.; Sagani, A.; Hull, A.; Hull, A.; Karellas, S.; Grammelis, P. Life Cycle Assessment of an Innovative Alcohol-to-Jet Process: The Case for Retrofitting a Bioethanol Plant for Sustainable Aviation Fuel Production. Renew. Energy 2024, 228, 120512. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.; Tyndall, S. Innovation in Precision Fermentation for Food Ingredients. Crit. Rev. Food Sci. Nutr. 2023, 64, 6218–6238. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.; Kwon, H.; Wu, M.; Wang, M. Retrospective Analysis of the U.S. Corn Ethanol Industry for 2005–2019: Implications for Greenhouse Gas Emission Reductions. Biofuels Bioprod. Biorefining 2021, 15, 1318–1331. [Google Scholar] [CrossRef]
- Shahbaz, M.; Alherbawi, M.; Okonkwo, E.C.; Al-Ansari, T. Evaluating Negative Emission Technologies in a Circular Carbon Economy: A Holistic Evaluation of Direct Air Capture, Bioenergy Carbon Capture and Storage and Biochar. J. Clean. Prod. 2024, 466, 142800. [Google Scholar] [CrossRef]
- Santos, F.M.; Gonçalves, A.L.; Pires, J.C.M. Negative Emission Technologies. In Bioenergy with Carbon Capture and Storage: Using Natural Resources for Sustainable Development; Magalhães Pires, J.C., Cunha Gonçalves, A.L.D., Eds.; Academic Press: London, UK, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Postweiler, P.; Engelpracht, M.; Rezo, D.; Gibelhaus, A.; Assen, N. von der Environmental Process Optimisation of an Adsorption-Based Direct Air Carbon Capture and Storage System. Energy Environ. Sci. 2024, 17, 3004–3020. [Google Scholar] [CrossRef]
- Tharak, A.; Mohan, S.V. Electrode-Assisted Pressurized CO2 Fermentation for Acetic Acid and Ethanol Production: Enhanced Carbon Fixation, Metabolic Efficiency, and Sustainability in Carbon-Negative Bioprocesses. ACS Sustain. Chem. Eng. 2024, 13, 352–373. [Google Scholar] [CrossRef]
- Duperray, M.; Delvenne, M.; François, J.M.; Delvigne, F.; Capp, J.-P. Genomic and Metabolic Instability during Long-Term Fermentation of an Industrial Saccharomyces Cerevisiae Strain Engineered for C5 Sugar Utilization. Front. Bioeng. Biotechnol. 2024, 12, 1357671. [Google Scholar] [CrossRef]
- De Mol, M.L.; Marcoen, V.; Maryns, I.; Snoeck, N.; Beauprez, J.J.; De Maeseneire, S.L.; Soetaert, W.K. Evaluation of Long-Term Fermentation Performance with Engineered Saccharomyces Cerevisiae Strains. Fermentation 2023, 9, 721. [Google Scholar] [CrossRef]
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K. (Ken) Emerging Technologies for Biodiesel Production: Processes, Challenges, and Opportunities. Biomass Bioenergy 2022, 163, 106521. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal Biomass Valorization for Biofuel Production and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Okolie, J.A.; Tabat, M.E.; Gunes, B.; Epelle, E.I.; Mukherjee, A.; Nanda, S.; Dalai, A.K. A Techno-Economic Assessment of Biomethane and Bioethanol Production from Crude Glycerol through Integrated Hydrothermal Gasification, Syngas Fermentation and Biomethanation. Energy Convers. Manag. X 2021, 12, 100131. [Google Scholar] [CrossRef]
- Gasoline Prices Around the World, 15 December 2025. Available online: https://www.globalpetrolprices.com/gasoline_prices/ (accessed on 20 December 2025).
- Sun, C.; Yang, L.; Zhao, C.; Li, H.; Deng, Z.; Liu, G.; Wu, C. Propanol Production through Microbial Fermentation of Biomass. Bioresour. Bioprocess 2025, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Diesel Prices Around the World, 15 December 2025. Available online: https://www.globalpetrolprices.com/diesel_prices/ (accessed on 20 December 2025).
- Sandesh, K.; Ujwal, P. Trends and Perspectives of Liquid Biofuel—Process and Industrial Viability. Energy Convers. Manag. X 2021, 10, 100075. [Google Scholar] [CrossRef]
- Kerosene Prices Around the World, 22 December 2025. Available online: https://www.globalpetrolprices.com/kerosene_prices/ (accessed on 26 December 2025).
- Energy Research Office (EPE). Biofuels Outlook Analysis–Year 2023. Technical Note EPE/DPG/SDB/2024/03; Energy Research Office: Rio de Janeiro, Brazil, 2024. Available online: https://www.epe.gov.br (accessed on 1 November 2025).
- Malik, K.; Capareda, S.C.; Kamboj, B.R.; Malik, S.; Singh, K.; Arya, S.; Bishnoi, D.K. Biofuels Production: A Review on Sustainable Alternatives to Traditional Fuels and Energy Sources. Fuels 2024, 5, 157–175. [Google Scholar] [CrossRef]
- Xu, P. Production of Chemicals Using Dynamic Control of Metabolic Fluxes. Curr. Opin. Biotechnol. 2018, 53, 12–19. [Google Scholar] [CrossRef]
- Mukhopadhyay, A. Tolerance Engineering in Bacteria for the Production of Advanced Biofuels and Chemicals. Trends Microbiol. 2015, 23, 498–508. [Google Scholar] [CrossRef]
- Niu, F.-X.; He, X.; Wu, Y.; Liu, J. Enhancing Production of Pinene in Escherichia Coli by Using a Combination of Tolerance, Evolution, and Modular Co-Culture Engineering. Front. Microbiol. 2018, 9, 01623. [Google Scholar] [CrossRef]
- Keasling, J.; Garcia Martin, H.; Lee, T.S.; Mukhopadhyay, A.; Singer, S.W.; Sundstrom, E. Microbial Production of Advanced Biofuels. Nat. Reviews. Microbiol. 2021, 19, 701–715. [Google Scholar] [CrossRef]
- Otoupal, P.B.; Chatterjee, A. CRISPR Gene Perturbations Provide Insights for Improving Bacterial Biofuel Tolerance. Front. Bioeng. Biotechnol. 2018, 6, 00122. [Google Scholar] [CrossRef]
- Suali, E.; Suali, L. Impact Assessment of Global Biofuel Regulations and Policies on Biodiversity. In Environmental Sustainability of Biofuels; Hakeem, K.R., Bandh, S.A., Malla, F.A., Mehmood, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–161. [Google Scholar]
- Grangeia, C.; Santos, L.; Lazaro, L.L.B. The Brazilian Biofuel Policy (RenovaBio) and Its Uncertainties: An Assessment of Technical, Socioeconomic and Institutional Aspects. Energy Convers. Manag. X 2022, 13, 100156. [Google Scholar] [CrossRef]
- Salvarli, M.S.; Salvarli, H. For Sustainable Development: Future Trends in Renewable Energy and Enabling Technologies. In Renewable Energy—Resources, Challenges and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Falcone, P.M. Sustainable Energy Policies in Developing Countries: A Review of Challenges and Opportunities. Energies 2023, 18, 6682. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I. Engineered Bacteria as Living Therapeutics: Next-Generation Precision Tools for Health, Industry, Environment, and Agriculture. AIMS Microbiol. 2025, 11, 946–962. [Google Scholar] [CrossRef]
- Shams, A.; Fischer, A.; Bodnar, A.; Kliegman, M. Perspectives on Genetically Engineered Microorganisms and Their Regulation in the United States. ACS Synth. Biol. 2024, 13, 1412–1423. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging Technologies for Biofuel Production: A Critical Review on Recent Progress, Challenges and Perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Achaw, O.-W. Bioenergy and Biofuel Production from Biomass Using Thermochemical Conversions Technologies—A Review. AIMS Energy 2022, 10, 585–647. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Hammad, A.; El-Sherif, D.M.; Abouzid, M.; Gaballah, M.S.; Elwakeel, K.Z. Thermochemical Conversion Strategies of Biomass to Biofuels, Techno-Economic and Bibliometric Analysis: A Conceptual Review. J. Environ. Chem. Eng. 2021, 9, 106503. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of Biomass to Biofuels and Life Cycle Assessment: A Review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sar, T.; Gowd, S.C.; Rajendran, K.; Kumar, V.; Sarsaiya, S.; Li, Y.; Sindhu, R.; Binod, P.; Zhang, Z.; et al. A Comprehensive Review on Thermochemical, and Biochemical Conversion Methods of Lignocellulosic Biomass into Valuable End Product. Fuel 2023, 342, 127790. [Google Scholar] [CrossRef]
- Lanjekar, P.R.; Panwar, N.L.; Patel, M.R.; Divyangkumar, N. Exploring Sustainable Energy: An Overview of Biochemical and Thermochemical Conversion of Dairy and Food Waste. Environ. Pollut. Manag. 2024, 1, 152–166. [Google Scholar] [CrossRef]
- Wang, S.; Wan, Z.; Han, Y.; Jiao, Y.; Li, Z.; Fu, P.; Li, N.; Zhang, A.; Yi, W. A Review on Lignin Waste Valorization by Catalytic Pyrolysis: Catalyst, Reaction System, and Industrial Symbiosis Mode. J. Environ. Chem. Eng. 2023, 11, 109113. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Martin-Martinez, F.J. Biorefineries: Achievements and Challenges for a Bio-Based Economy. Front. Chem. 2022, 10, 973417. [Google Scholar] [CrossRef] [PubMed]
- Schröder, T.; Lauven, L.-P.; Sowlati, T.; Geldermann, J. Strategic Planning of a Multi-Product Wood-Biorefinery Production System. J. Clean. Prod. 2019, 211, 1502–1516. [Google Scholar] [CrossRef]
- Shibukawa, V.P.; Ramos, L.; Cruz-Santos, M.M.; Prado, C.A.; Jofre, F.M.; Arruda, G.; Silva, S.; Mussatto, S.I.; Santos, J. Impact of Product Diversification on the Economic Sustainability of Second-Generation Ethanol Biorefineries: A Critical Review. Energies 2023, 16, 6384. [Google Scholar] [CrossRef]
- Nair, L.G.; Verma, P. Harnessing Carbon Potential of Lignocellulosic Biomass: Advances in Pretreatments, Applications, and the Transformative Role of Machine Learning in Biorefineries. Bioresour. Bioprocess. 2025, 12, 97. [Google Scholar] [CrossRef]
- Qiu, M.; Shen, W.; Yan, X.; He, Q.; Cai, D.; Chen, S.; Wei, H.; Knoshaug, E.P.; Zhang, M.; Himmel, M.E.; et al. Metabolic Engineering of Zymomonas Mobilis for Anaerobic Isobutanol Production. Biotechnol. Biofuels 2020, 13, 15. [Google Scholar] [CrossRef]
- Bumrungtham, P.; Promdonkoy, P.; Prabmark, K.; Bunterngsook, B.; Boonyapakron, K.; Tanapongpipat, S.; Champreda, V.; Runguphan, W. Engineered Production of Isobutanol from Sugarcane Trash Hydrolysates in Pichia Pastoris. J. Fungi 2022, 8, 767. [Google Scholar] [CrossRef]
- Baptista, S.L.; Costa, C.E.; Cunha, J.T.; Soares, P.O.; Domingues, L. Metabolic Engineering of Saccharomyces Cerevisiae for the Production of Top Value Chemicals from Biorefinery Carbohydrates. Biotechnol. Adv. 2021, 47, 107697. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, G.; Sun, S.; Fan, C.; Feng, X.; Xiong, P. Biosynthetic Pathway and Metabolic Engineering of Succinic Acid. Front. Bioeng. Biotechnol. 2022, 10, 843887. [Google Scholar] [CrossRef]
- Tobin, T.; Gustafson, R.; Bura, R.; Gough, H.L. Integration of Wastewater Treatment into Process Design of Lignocellulosic Biorefineries for Improved Economic Viability. Biotechnol. Biofuels 2020, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Luo, H.; Colosi, L.M. Slow Pyrolysis as a Platform for Negative Emissions Technology: An Integration of Machine Learning Models, Life Cycle Assessment, and Economic Analysis. Energy Convers. Manag. 2020, 223, 113258. [Google Scholar] [CrossRef]
- Rocha, P.; Salcedo-Puerto, O.; Nuncira, J.; Emebu, S.; Mendoza-Martinez, C. Renewable Energy Potential and CO2 Performance of Main Biomasses Used in Brazil. Energies 2023, 16, 3959. [Google Scholar] [CrossRef]
- Balasubramanian, V.K.; Muthuramalingam, J.B.; Chen, Y.-P.; Chou, J.-Y. Recent Trends in Lactic Acid-Producing Microorganisms through Microbial Fermentation for the Synthesis of Polylactic Acid. Arch. Microbiol. 2023, 206, 31. [Google Scholar] [CrossRef]
- Nduko, J.M.; Taguchi, S. Microbial Production of Biodegradable Lactate-Based Polymers and Oligomeric Building Blocks From Renewable and Waste Resources. Front. Bioeng. Biotechnol. 2020, 8, 618077. [Google Scholar] [CrossRef]
- Obruča, S.; Dvořák, P.; Sedláček, P.; Koller, M.; Sedlář, K.; Pernicová, I.; Šafránek, D. Polyhydroxyalkanoates Synthesis by Halophiles and Thermophiles: Towards Sustainable Production of Microbial Bioplastics. Biotechnol. Adv. 2022, 58, 107906. [Google Scholar] [CrossRef]
- Basera, P.; Chakraborty, S.; Sharma, N. Lignocellulosic Biomass: Insights into Enzymatic Hydrolysis, Influential Factors, and Economic Viability. Discov. Sustain. 2024, 5, 311. [Google Scholar] [CrossRef]
- Olivieri, G.; Wijffels, R.H.; Marzocchella, A.; Russo, M.E. Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass. Catalysts 2021, 11, 680. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Silva, A.S.; Espinheira, R.P.; Teixeira, R.S.S.; Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P.S. Constraints and Advances in High-Solids Enzymatic Hydrolysis of Lignocellulosic Biomass: A Critical Review. Biotechnol. Biofuels 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Nawaz, A.; Fouillaud, M.; Dufossé, L.; Haq, I.U.; Mukhtar, H. Microbial Cell Factories: Biodiversity, Pathway Construction, Robustness, and Industrial Applicability. Microbiol. Res. 2024, 15, 247–272. [Google Scholar] [CrossRef]
- Cho, J.S.; Kim, G.B.; Eun, H.; Moon, C.W.; Lee, S.Y. Designing Microbial Cell Factories for the Production of Chemicals. JACS Au 2022, 2, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Francois, J.M.; Alkim, C.; Morin, N. Engineering Microbial Pathways for Production of Bio-Based Chemicals from Lignocellulosic Sugars: Current Status and Perspectives. Biotechnol. Biofuels 2020, 13, 118. [Google Scholar] [CrossRef]
- Fujiwara, R.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic Engineering of Escherichia Coli for Shikimate Pathway Derivative Production from Glucose–Xylose Co-Substrate. Nat. Commun. 2020, 11, 279. [Google Scholar] [CrossRef]
- Nonaka, D.; Kishida, M.; Hirata, Y.; Mori, A.; Kondo, A.; Mori, Y.; Noda, S.; Tanaka, T. Modular Pathway Engineering for Enhanced Production of Para -Aminobenzoic Acid and 4-Amino-Phenylalanine in Escherichia Coli via Glucose/Xylose Co-Utilization. Appl. Environ. Microbiol. 2025, 91, e0246824. [Google Scholar] [CrossRef]
- Sgobba, E.; Wendisch, V.F. Synthetic Microbial Consortia for Small Molecule Production. Curr. Opin. Biotechnol. 2019, 62, 72–79. [Google Scholar] [CrossRef]
- Qian, Q.; Luo, Z.; Sun, H.; Wei, Q.; Shi, J.; Li, L.; Wang, K.; Zhou, J. Process Evaluation of Simulated Novel Cellulosic Ethanol Biorefineries Coupled with Lignin Thermochemical Conversion. Renew. Energy 2024, 231, 120965. [Google Scholar] [CrossRef]
- Saraeian, A.; Aui, A.; Gao, Y.; Wright, M.M.; Foston, M.; Shanks, B.H. Evaluating Lignin Valorization via Pyrolysis and Vapor-Phase Hydrodeoxygenation for Production of Aromatics and Alkenes. Green Chem. 2020, 22, 2513–2525. [Google Scholar] [CrossRef]
- Wang, Y.; Baral, N.R.; Yang, M.; Scown, C.D. Co-Processing Agricultural Residues and Wet Organic Waste Can Produce Lower-Cost Carbon-Negative Fuels and Bioplastics. Environ. Sci. Technol. 2023, 57, 2958–2969. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; You, S.; Park, Y.-K. Bioenergy Generation from Thermochemical Conversion of Lignocellulosic Biomass-Based Integrated Renewable Energy Systems. Renew. Sustain. Energy Rev. 2023, 178, 113240. [Google Scholar] [CrossRef]
- Hidalgo, D.; Urueña, A.; Martín-Marroquín, J.M.; Díez, D. Integrated Approach for Biomass Conversion Using Thermochemical Routes with Anaerobic Digestion and Syngas Fermentation. Sustainability 2025, 17, 3615. [Google Scholar] [CrossRef]
- Liakakou, E.; Infantes, A.; Vreugdenhil, B.; Neumann, A. Connecting Lignin Gasification with Syngas Fermentation. engrXiv 2020. [Google Scholar] [CrossRef]
- Arias, A.; Costa, C.E.; Moreira, M.T.; Feijoo, G.; Domingues, L. Environmental and Techno-Economic Assessment on the Valorization of Vine-Side Streams to Produce Resveratrol. J. Clean. Prod. 2023, 429, 139622. [Google Scholar] [CrossRef]
- Salas-Villalobos, U.A.; Torres-Acosta, M.A.; Aguilar, O. Techno-Economic Analysis as a Valuable Tool for the Selection of High-Potential Extractive Fermentation Approaches for Large-Scale Prodigiosin Production. Chem. Eng. J. 2024, 481, 148279. [Google Scholar] [CrossRef]
- Sosa-Martínez, J.D.; Morales-Oyervides, L.; Montañez, J.; Contreras-Esquivel, J.C.; Balagurusamy, N.; Gadi, S.K.; Salmerón, I. Sustainable Co-Production of Xylanase, Cellulase, and Pectinase through Agroindustrial Residue Valorization Using Solid-State Fermentation: A Techno-Economic Assessment. Sustainability 2024, 16, 1564. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Tom-James, A.; Falowo, O.A.; Okoji, A.; Adeyi, O.; Olalere, A.O.; Eloka-Eboka, A. Techno-Economic Analysis of Cellulase Production by Trichoderma Reesei in Submerged Fermentation Processes Using a Process Simulator. S. Afr. J. Chem. Eng. 2022, 42, 98–105. [Google Scholar] [CrossRef]
- Manakas, P.; Balafoutis, A.T.; Kottaridi, C.; Vlysidis, A. Sustainability Assessment of Orange Peel Waste Valorization Pathways from Juice Industries. Biomass Convers. Biorefinery 2024, 15, 6525–6544. [Google Scholar] [CrossRef]
- Gunes, B. A Critical Review on Biofilm-Based Reactor Systems for Enhanced Syngas Fermentation Processes. Renew. Sustain. Energy Rev. 2021, 143, 110950. [Google Scholar] [CrossRef]
- Heffernan, J.K.; Valgepea, K.; Lemgruber, R.d.S.P.; Casini, I.; Plan, M.; Tappel, R.; Simpson, S.D.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-Valorization Using Clostridium Autoethanogenum for Sustainable Fuel and Chemicals Production. Front. Bioeng. Biotechnol. 2020, 8, 204. [Google Scholar] [CrossRef]
- Valgepea, K.; Talbo, G.; Takemori, N.; Takemori, A.; Ludwig, C.; Mahamkali, V.; Mueller, A.P.; Tappel, R.; Köpke, M.; Simpson, S.D.; et al. Absolute Proteome Quantification in the Gas-Fermenting Acetogen Clostridium autoethanogenum. mSystems 2022, 7, e00026-22. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, N.M.; Binod, P.; Sindhu, R.; Awasthi, M.K.; Pandey, A. Microbial Engineering for the Production of Isobutanol: Current Status and Future Directions. Bioengineered 2021, 12, 12308–12321. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-Y.; Lo, Y.-C.; Dong, C.-D.; Nagarajan, D.; Chang, J.-S.; Lee, D.-J. Biobutanol Production from Lignocellulosic Biomass Using Immobilized Clostridium Acetobutylicum. Appl. Energy 2020, 277, 115531. [Google Scholar] [CrossRef]
- Zhan, Y.; Xu, Y.; Lu, X.; Zhou, F.; Zheng, P.; Wang, D.; Cai, D.; Yang, S.; Chen, S. Metabolic Engineering of Bacillus Licheniformis for Sustainable Production of Isobutanol. ACS Sustain. Chem. Eng. 2021, 9, 17254–17265. [Google Scholar] [CrossRef]
- Moretta, F.; Fedeli, M.; Manenti, F.; Bozzano, G. Conceptual Design of Digital Twin for Bio-Methanol Production from Microalgae. Chem. Eng. Trans. 2022, 92, 253–258. [Google Scholar] [CrossRef]
- Bruzaite, I.; Rozene, J.; Morkvenaite-Vilkonciene, I.; Ramanavicius, A. Towards Microorganism-Based Biofuel Cells: The Viability of Saccharomyces Cerevisiae Modified by Multiwalled Carbon Nanotubes. Nanomaterials 2020, 10, 954. [Google Scholar] [CrossRef]
- Ching, P.M.L.; Mayol, A.P.; Lois, J.; Marvin, A.; Richard, H.Y.S.; Sy, C.; Ubando, A.T.; Culaba, A.B. AI Methods for Modeling the Vacuum Drying Characteristics of Chlorococcum Infusionum for Algal Biofuel Production. Process Integr. Optim. Sustain. 2021, 5, 247–256. [Google Scholar] [CrossRef]
- Bamisaye, A.; Ige, A.R.; Adegoke, K.A.; Adegoke, I.A.; Bamidele, M.O.; Alli, Y.A.; Adeleke, O.; Idowu, M.A. Amaranthus Hybridus Waste Solid Biofuel: Comparative and Machine Learning Studies. RSC Adv. 2024, 14, 11541–11556. [Google Scholar] [CrossRef]
- Oke, D.; Dunn, J.B.; Hawkins, T.R. Reducing Economy-Wide Greenhouse Gas Emissions with Electrofuels and Biofuels as the Grid Decarbonizes. Energy Fuels 2024, 38, 6048–6061. [Google Scholar] [CrossRef]
- Ashour, M.; Alprol, A.E.; Heneash, A.M.M.; Saleh, H.; Abualnaja, K.M.; Alhashmialameer, D.; Mansour, A.T. Ammonia Bioremediation from Aquaculture Wastewater Effluents Using Arthrospira Platensis NIOF17/003: Impact of Biodiesel Residue and Potential of Ammonia-Loaded Biomass as Rotifer Feed. Materials 2021, 14, 5460. [Google Scholar] [CrossRef]
- Byun, J.; Kwon, O.; Kim, J.; Han, J. Carbon-Negative Food Waste-Derived Bioethanol: A Hybrid Model of Life Cycle Assessment and Optimization. ACS Sustain. Chem. Eng. 2022, 10, 4512–4521. [Google Scholar] [CrossRef]
- Gomez, J.A.; Höffner, K.; Barton, P.I. Production of Biofuels from Sunlight and Lignocellulosic Sugars Using Microbial Consortia. Chem. Eng. Sci. 2021, 239, 116615. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Karimi, K.; Angelidaki, I. Coproduction of Hydrogen, Butanol, Butanediol, Ethanol, and Biogas from the Organic Fraction of Municipal Solid Waste Using Bacterial Cocultivation Followed by Anaerobic Digestion. Renew. Energy 2022, 194, 552–560. [Google Scholar] [CrossRef]
- Aniza, R.; Chen, W.-H.; Yang, F.-C.; Pugazhendh, A.; Singh, Y. Integrating Taguchi Method and Artificial Neural Network for Predicting and Maximizing Biofuel Production via Torrefaction and Pyrolysis. Bioresour. Technol. 2022, 343, 126140. [Google Scholar] [CrossRef] [PubMed]
- Arshad, I.; Ahsan, M.; Zafar, I.; Sajid, M.; Sehgal, S.A.; Yousaf, W.; Noor, A.; Rashid, S.; Garai, S.; Moovendhan, M.; et al. Bioinformatics Approaches in Upgrading Microalgal Oil for Advanced Biofuel Production through Hybrid ORF Protein Construction. Biomass Convers. Biorefinery 2023, 15, 27185–27205. [Google Scholar] [CrossRef]
- Lockwood, K.S.; Ahmed, S.F.; Huq, N.A.; Stutzman, S.C.; Foust, T.D.; Labbe, N.J. Advances in Predictive Chemistry Enable a Multi-Scale Rational Design Approach for Biofuels with Advantaged Properties. Sustain. Energy Fuels 2022, 6, 5371–5383. [Google Scholar] [CrossRef]
- Kim, S.; Tariq, S.; Heo, S.; Moosazadeh, M.; Yoo, C. Multi-Objective Pathway Selection for Sustainable Biorefinery Process with Harvest Scheduling under Time-Varying Climate: Techno-Economic Analysis and Life Cycle Assessment Approach. Energy Convers. Manag. 2025, 343, 120247. [Google Scholar] [CrossRef]
- Rozene, J.; Morkvenaite-Vilkonciene, I.; Bruzaite, I.; Dzedzickis, A.; Ramanavicius, A. Yeast-Based Microbial Biofuel Cell Mediated by 9,10-Phenantrenequinone. Electrochim. Acta 2021, 373, 137918. [Google Scholar] [CrossRef]
- Amirkhanov, B.; Kunelbayev, M.; Issa, S.; Amirkhanova, G.; Nurgazy, T.; Zhumasheva, A.; Alipbeki, O. Enhancing Sustainable Biogas Generation Through a Real-Time Digital Twin of a Modular Bioreactor. J. Appl. Data Sci. 2025, 6, 2312–2331. [Google Scholar] [CrossRef]
- Sitthikitpanya, N.; Khamtib, S.; Sittijunda, S.; Imai, T.; Reungsang, A. Valorization of Sugarcane Leaves and Co-Digestion with Microalgal Biomass to Produce Biofuels and Value-Added Products under the Circular Economy and Zero-Waste Concepts. Energy Convers. Manag. 2024, 299, 117854. [Google Scholar] [CrossRef]
- Premaratne, M.; Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Nimarshana, P.H.V.; Ariyadasa, T.U. Nitrogen-Limited Cultivation of Locally Isolated Desmodesmus Sp. for Sequestration of CO2 from Simulated Cement Flue Gas and Generation of Feedstock for Biofuel Production. J. Environ. Chem. Eng. 2021, 9, 105765. [Google Scholar] [CrossRef]
- Araújo-Gomes, E.R.; Moreira, G.D.S.; Ishihara, J.H. Uso de Biorreatores Para Tratamento Do Efluente Industrial Do Dendê: Produção de Biofertilizante e Biogás. Rev. Bras. De Gest. Ambient. E Sustentabilidade 2024, 11, 735–746. [Google Scholar]
- Lu, F.; Smith, P.R.; Mehta, K.; Swartz, J.R. Development of a Synthetic Pathway to Convert Glucose to Hydrogen Using Cell Free Extracts. Int. J. Hydrogen Energy 2015, 40, 9113–9124. [Google Scholar] [CrossRef]
- Cao, B.; Hu, S.; Zhu, K.; Pan, C.; Marrakchi, F.; Ni, J.; Yuan, C.; Qian, L.; Chen, H.; Yuan, J.; et al. Response Surface Optimization of Product Yields and Biofuel Quality during Fast Hydrothermal Liquefaction of a Highly CO2-Tolerant Microalgae. Sci. Total Environ. 2022, 860, 160541. [Google Scholar] [CrossRef]
- Madadi, M.; Elsayed, M.; Song, G.; Razieh, S.-A.; Denayer, J.F.; Kargaran, E.; Azad, S.A.; Karimi, K.; Sun, F.; Gupta, V.K. Transformative Biorefinery Model for Biomass Valorization into Biofuel and Renewable Platform Chemicals. J. Energy Chem. 2025, 110, 109–123. [Google Scholar] [CrossRef]
- Sbarciog, M.; Buck, V.D.; Akkermans, S.; Bhonsale, S.; Polanska, M.; Van Impe, J.F.M. Design, Implementation and Simulation of a Small-Scale Biorefinery Model. Processes 2022, 10, 829. [Google Scholar] [CrossRef]
- Hassan, G.K.; Al-Sayed, A.; Afify, A.A.; El-Liethy, M.A.; Elagroudy, S.; El-Gohary, F.A. Production of Biofuels (H2&CH4) from Food Leftovers via Dual-Stage Anaerobic Digestion: Enhancement of Bioenergy Production and Determination of Metabolic Fingerprinting of Microbial Communities. Egypt. J. Chem. 2021, 64, 4105–4115. [Google Scholar]
- Ribeiro, A.R.; Junior, J.A.F.; Devens, K.U.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Optimization of the Bio-H2 and Lactate Production from Vinasse and Molasses: An Experimental Approach for the Bioenergy Development in Brazil. Environ. Technol. 2025, 46, 5241–5258. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, G.; Liu, L.; Zhang, H.; Mu, L.; Lu, X.; Zhu, J. A Negative-Carbon Footprint Process with Mixed Biomass Feedstock Maximizes Conversion Efficiency, Product Value and CO2 Mitigation. Bioresour. Technol. 2022, 351, 127004. [Google Scholar] [CrossRef]
- Yoo, E.; Lee, U.; Wang, M. Life-Cycle Greenhouse Gas Emissions of Sustainable Aviation Fuel through a Net-Zero Carbon Biofuel Plant Design. ACS Sustain. Chem. Eng. 2022, 10, 8725–8732. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.