Abstract
Plastic pollution is an increasingly pressing environmental concern due to its persistence in ecosystems. To address this issue, this study evaluates polyethylene biodegradation and bioelectricity generation using Aspergillus ochraceopetaliformis in microbial fuel cells (MFCs). Single-chamber MFCs were designed (three) with carbon and zinc electrodes, where the fungus was cultivated in a nutrient-rich medium to enhance its metabolic activity. Parameters such as pH, power density, and FTIR spectra were monitored to assess plastic biodegradation. The results demonstrated a significant reduction in polyethylene mass and structure, along with a maximum generation of 0.921 V and 4.441 mA on day 26, with a power density of 0.148 mW/cm2 and a current of 5.847 mA/cm2. The optimal pH for fungal activity in the MFC was recorded at 7.059. Furthermore, FTIR analysis revealed a decrease in peak intensity at 1470 cm−1 and 723 cm−1, indicating structural modifications in the treated plastics. Furthermore, microbial fuel cells connected in series successfully powered an LED bulb, generating a maximum voltage of 2.78 V. These findings confirm the feasibility of using Aspergillus ochraceopetaliformis for biodegradation and bioelectricity generation, although practical applications require further optimization of system conditions and improvements in long-term stability. This research contributes to the development of biotechnological strategies for plastic waste management, sustainable integrating approaches with energy potential.
1. Introduction
The generation of plastic waste has reached alarming levels worldwide. The Organization for Economic Cooperation and Development (OECD) reports that annual plastic waste production has doubled over the last two decades. At the beginning of 2000, about 180 million tons were produced; currently, more than 350 million tons are generated [1,2]. It is estimated that in 2060, plastic waste will almost triple, reaching concerning figures [3]. Currently, only one-fifth of this waste is recycled, while half continues to be deposited in landfills [4]. Approximately 5 trillion plastic bags are used annually, equivalent to almost 10 million per minute [5]. Additionally, up to 8 million tons of plastic are dumped into the oceans yearly, seriously affecting marine life and aquatic ecosystems [6]. Reusing plastic waste is one of the most viable solutions, yet only 20% of plastic waste is recycled [7]. In Peru, for example, around 3 billion plastic bags are generated annually, with an average of 30 kilos of plastic per citizen [8]. These data highlight the urgency of implementing adequate measures to reduce the production and consumption of single-use plastics and improve recycling and waste management systems to mitigate the environmental impact of this waste [9,10].
Plastic biodegradation is a crucial process for reducing the environmental impact of plastic waste [11]. This process involves the breakdown of plastic polymers into simpler compounds through the actions of microorganisms such as bacteria and fungi [12]. Unfortunately, most conventional plastics are designed to be durable and do not degrade quickly; for instance, a plastic bottle can take between 450 and 1000 years to decompose in a landfill [13]. Therefore, utilizing microorganisms capable of degrading plastic presents an innovative and promising solution to the global issue of plastic pollution [14]. This biotechnological approach relies on microorganisms like bacteria and fungi, which can break down plastic polymers by producing specific enzymes. Researchers have identified several strains of fungi that can degrade plastics, such as polyethylene and polyurethane. Under controlled conditions, these microorganisms convert the polymers into simpler compounds, like carbon dioxide and water [15]. Research in this area aims to optimize the conditions for maximizing biodegradation efficiency and better understand the molecular mechanisms involved [16]. Numerous studies have explored the potential of different fungi to degrade plastic. For example, Ferreira et al. (2024) investigated the fungus Penicillium brevicompactum for its degradation potential on circular plastic plates over 28 days, achieving a 15% reduction by the fourteenth day [17]. Similarly, Ibrahim et al. (2024) studied the fungus Fusarium tricinctum for its ability to reduce plastic, attaining a decrease of 8.3% from its initial mass [18]. Their micrographs showed the morphological adaptation of the fungal cells as they colonized the hydrophobic polyurethane.
Recently, the potential of fungi of the species Aspergillus sp. for the biodegradation of plastic, especially polyethylene, has been observed [19]. Studies have identified specific strains of Aspergillus that can decompose synthetic plastics under natural conditions, offering a possible solution to plastic pollution [20]. The capacity of these fungi can be harnessed to break down plastics in biodegradation treatments, contributing to the reduction of plastic waste and the protection of the environment [21]. Notably, the fungus Aspergillus ochraceopetaliformis has demonstrated significant plastic reduction capacity, significantly reducing the weight and tension of polyethylene films. It has been found that the fungus produces a variety of enzymes, such as esterases, lipases, and oxidases, which allow the decomposition of complex plastic polymers into simpler compounds [22,23,24].
On the other hand, microbial fuel cells (MFCs) are bioelectrochemical devices that generate electrical energy based on the interaction between microorganisms and organic matter in an aqueous medium [25]. MFC technology consists of an anode, a cathode, a proton exchange membrane, and an electrical circuit, where microorganisms, usually bacteria, oxidize organic matter at the anode, releasing electrons and protons [26,27]. Recently, this technology has been used to reduce plastic and generate electrical energy simultaneously [28]. Some studies have shown that certain microorganisms present in MFCs can degrade plastics such as polyethylene and polypropylene, converting them into more straightforward and less harmful compounds for the environment [29]. For example, Kalathil et al. (2022) used the bacteria Ideonella sakaiensis to reduce polyethylene terephthalate used as a carbon source in MFC, demonstrating a reduction in the transmittance spectrum by FTIR and generating a current density of 0.6 mA/cm2 [30]. Similarly, the fungus Trichoderma sp. was used as a reducing agent in microbial fuel cells, showing a reduction in the thickness of plastic from 756.87 µm to 446.01 µm while generating an electric current of 5.648 ± 0.093 mA and a voltage of 0.479 ± 0.025 V [31]. The application of different microorganisms for the simultaneous reduction and generation of electricity in microbial fuel cells is still scarce, and the literature lacks information. Hence, using the fungus Aspergillus ochraceopetaliformis as a biodegradable electric current generator will provide novel and alternative methods in this field.
The primary objective of this research is to utilize the fungus Aspergillus ochraceopetaliformis as a plastic biodegrading agent in single-chamber microbial fuel cells. The aim is to observe its potential for generating electrical energy while simultaneously biodegrading plastic over 35 days. The study involves the molecular identification of the fungus and the measurement of various parameters, including power density, voltage, current density, electric current, pH, electrical conductivity, reduction–oxidation potential, electrical resistance, FTIR spectrum, and micrographs of the plastic sample used. Although using microorganisms for plastic biodegradation is still an emerging technology, it holds significant promise for reducing plastic waste and mitigating its environmental impact. With ongoing advancements in research and development of biotechnologies, we may fully harness the potential of these microorganisms in the fight against plastic pollution.
2. Materials and Methods
2.1. Fabrication of Microbial Fuel Cells
The single-chamber microbial fuel cells (MFCs) were constructed using 1 L borosilicate glass (Borosilicate 3.3), ensuring durability and chemical stability. Each MFC was designed with a carbon anode electrode, which had an area of 44 cm2 and a thickness of 0.15 cm, alongside a zinc cathode electrode with an area of 13.52 cm2 and a thickness of 0.08 cm. The electrodes were positioned 10.5 cm apart to optimize electrochemical performance. Each MFC contained a single anodic and cathodic electrode to facilitate electron transfer and enhance energy conversion efficiency. The electrodes were externally connected via a 100 Ω resistor, while an internal Nafion 117 membrane served as the proton exchange medium, ensuring effective ion transport between the compartments. The system configuration is illustrated in Figure 1.
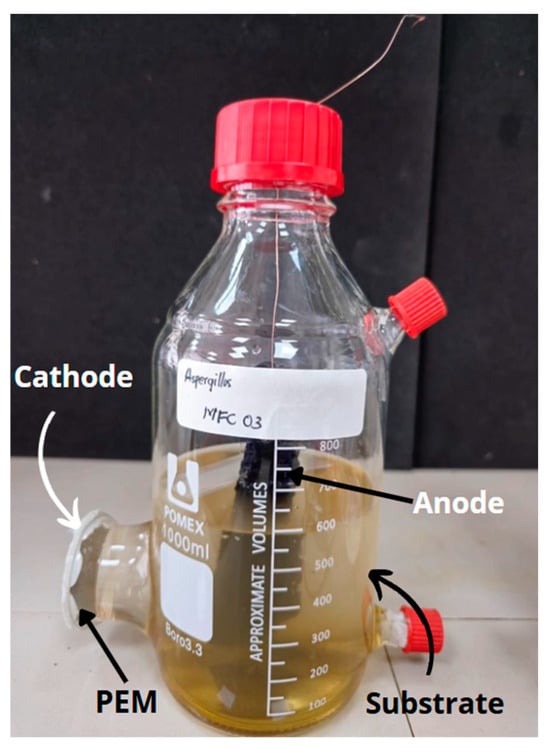
Figure 1.
Schematic of the MFC prototype.
2.2. Characterization of Microbial Fuel Cells
The observed voltage and current profiles were measured with a multimeter (Prasek Premium PR-85), and the pH profiles were recorded using a pH meter (110 Series Oakton) over a period of 30 days. The power density (PD) and current density (CD) profiles were determined based on the methodology described by Segundo et al. (2022) was used with different internal resistance values (0.2 ± 0.1, 5 ± 0.3, 30 ± 2.2, 60 ± 5.5, 110 ± 10.5, 250 ± 13, 360 ± 23.1, 530 ± 40.5, 750 ± 50.2, and 1000± 70.5 Ω) where the power density was found by the formula PD = V × I/A and the current density (CD) = I/A [32]. The resistance and power profiles of the MFCs were measured using an energy sensor (Vernier, ±30 V and ±1000 mA). Micrographs of the plastic samples were obtained through scanning electron microscopy (SEM-EDX, JEOL-JSM, Thermionic, Medellín, Colombia)
2.3. Reactivation of the Aspergillus ochraceopetaliformis Culture and Obtaining the Spore Inoculum
The pure strain of Aspergillus ochraceopetaliformis, isolated at the Institute of Science and Technology of Universidad César Vallejo (Trujillo, Peru), was previously identified through molecular biology techniques. ITS gene analysis revealed 99.78% similarity in BLAST (version 1.48.0) with Aspergillus ochraceopetaliformis, corresponding to accession number PQ764479, via the NCBI portal (https://www.ncbi.nlm.nih.gov/nuccore?term=PQ764479, accessed on 15 December 2024). The culture was reactivated by puncturing inclined Sabouraud agar flasks, which were incubated at 25 °C for 5 to 7 days. After incubation, the flasks showing the highest sporulation were selected. To harvest the spores, 12 mL of sterile distilled water was added to the pure culture and gently resuspended. The spore concentration was subsequently adjusted using a nephelometer reading to 9 × 108 spores/mL.
2.4. Operation of the Microbial Fuel Cell
The single-chamber microbial fuel cell (MFC) was thoroughly disinfected before use to eliminate potential contaminants. This was achieved by sequentially treating the system with 2% hypochlorite followed by 70% alcohol, ensuring a sterile environment for the microbial and electrochemical processes. After disinfection, a spore inoculum of Aspergillus ochraceopetaliformis was introduced at a concentration of 9 × 108 spores/mL in 600 mL of Bushnell–Haas (BH) broth (Bushnell & Haas, 1941), a medium specifically formulated to support microbial growth and enzymatic activity. The BH broth was carefully prepared using precise concentrations of essential nutrients to facilitate microbial metabolism. The composition included KH2PO4 (1 g/L), K2HPO4 (1 g/L), NH4NO3 (1 g/L), MgSO4 (0.2 g/L), FeCl3 (0.05 g/L), and CaCl2 (0.02 g/L), all sourced from Himedia to ensure consistency and quality in the experimental conditions.
To evaluate the microbial degradation of polymeric material, low-density polyethylene sheets measuring 7 × 2 cm2 were incorporated into the system. Each MFC was equipped with three identical polyethylene samples, providing a uniform testing environment to assess microbial activity under controlled conditions. The experiment was conducted in triplicate to ensure reproducibility and statistical validity, allowing for the accurate interpretation of results and minimizing experimental variability.
3. Results and Analysis
3.1. Behavior of Voltage and Electric Current:
The increase in voltage profiles is shown in Figure 2a, where the maximum profile was observed on day 26 (0.921 ± 0.022 V) and then gradually decreased until day 35 (0.842 ± 0.29 V). Microbial fuel cells generate voltage due to the potential difference that occurs at the electrodes (anodic and cathodic); this increase results from electrochemical reactions (redox reactions) that occur on the surfaces of the electrodes [33]. As these reactions decrease, the voltage profiles also decrease [34]. The decrease in voltage and current from day 26 onwards may be linked to the accumulation of metabolites produced by Aspergillus ochraceopetaliformis within the MFC system. This phenomenon has been reported in previous studies on MFCs utilizing fungi and other electrogenic microorganisms [35]. Kižys et al. (2024) used the fungus Saccharomyces cerevisiae in their microbial fuel cells, managing to generate 0.120 V. They noted that the use of metallic nanoparticles in the anodic electrode tends to increase electrochemical reactions, leading to a rise in voltage [36]. Similarly, Moubasher et al. (2024) used the fungus Monodictys castaneae in their microbial fuel cells, achieving a maximum voltage of 0.807 ± 0.002 V. They mentioned that the reduction in voltage profiles is due to a decrease in the metabolic activity rate of the fungus, influenced by factors such as temperature and pH [37].
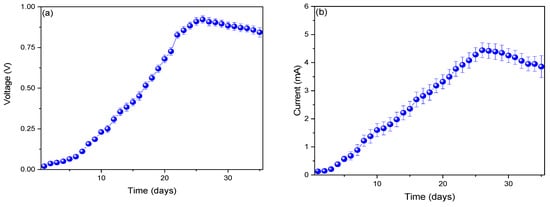
Figure 2.
Monitoring of the profiles of (a) voltage and (b) electric current.
Figure 2b shows an increase in current profiles from the first day (0.127 ± 0.011 mA) to the twenty-sixth day (4.441 ± 0.265 mA), followed by a decrease until the last day (3.857 ± 0.389 mA). The increases in electrical profiles are due to the rise in the concentration of electrons generated during the metabolic process of fungi. The metabolism of fungi occurs as they decompose the substrate used in MFCs [38]. Furthermore, certain fungi have been observed to use mediators (chemical compounds) for electron transfer, while others can perform direct electron transfer to the electrode [39]. Laily et al. (2022) used the fungi A. aculeatus, A. oryzae, and Candida rugosa in microbial fuel cells, managing to generate a maximum current of 51.2 mA on the thirteenth day [40]. They mentioned that fungi transfer electrons directly to the anode through cell membranes, such as cytochromes [40]. Khan et al. (2021) used the fungi Saccharomyces cerevisiae and banana peel in their microbial fuel cells, generating a maximum electric current of 0.97 mA, noting that this fungus metabolizes by degrading the sugars present in the substrate and transfers electrons through mediators [41].
3.2. Monitoring of pH, Conductivity and ORP Profiles
The pH profiles are shown in Figure 3a, depicting an increase from day 1 (5.772 ± 0.275) to day 35 (7.526 ± 0.467), with the optimum pH observed on day 26 (7.059 ± 0.381). Literature reports indicate that an optimal pH is crucial for maximizing electricity production; if the pH is too high or too low, it can inhibit the growth and activity of fungi, reducing the efficiency of the MFC [42,43]. Additionally, pH can affect nutrient solubility and electron availability, impacting cell performance. As eukaryotic organisms, fungi have an optimal pH range for their growth and metabolic activity [44]. Thulasinathan et al. (2021) used the fungus Psathyrella candolleana in their MFCs, which operated at a pH of 5.6, noting that pH profiles influence oxygen reduction reactions in the cathode chamber, thereby affecting the performance of the MFC [45]. Raqba et al. (2022) employed the fungus Trichoderma harzianum in their microbial fuel cells for acetaminophen reduction, initially operating at a pH of 2 before reaching a neutral pH, where the MFC displayed its best profiles [46]. During monitoring, an increase in electrical conductivity profiles was observed from the first day (61.262 ± 0.068 mS/cm) to the twenty-sixth day (254.789 ± 6.957 mS/cm) and then a slight detachment until the last day (254.789 ± 9.871 mS/cm).
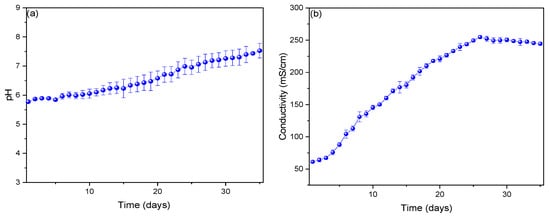
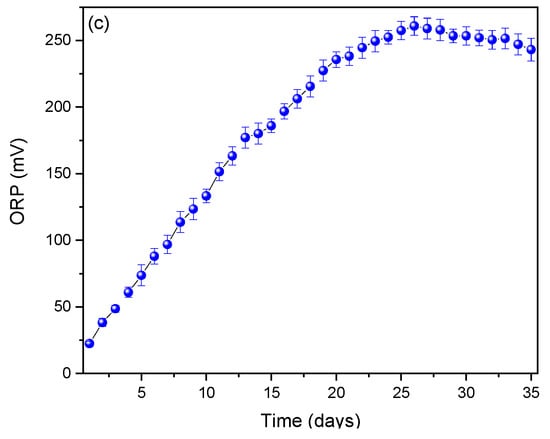
Figure 3.
Monitoring of (a) pH, (b) conductivity and (c) ORP profiles.
Electrical conductivity profiles affect the efficiency with which electrons are transferred between microorganisms and electrodes, influencing the amount of electricity generated. Higher electrical conductivity allows for more efficient electron transfer, which can increase electricity production [47]. Fungi can produce metabolites (such as organic acids) that affect the conductivity of the medium [48]. Arulmani et al. (2021) used Ralstonia sp. as an electrogenic bacteria in their MFCs to generate electricity, achieving 0.610 V and showing an electrical conductivity of 160 mS/cm [49]. They noted that microorganisms can create compounds that alter the conductivity of the electrolyte by influencing its viscosity or ionic composition [49]. The oxidation–reduction potential (ORP) is shown in Figure 3c, where it can be observed that the profiles increase from day 1 (22.488 ± 1.685 mV) to day 26 (260.837 ± 10.857 mV) and then slightly decrease until day 35 (243.095 ± 19.859 mV). Fungi act as biocatalysts, facilitating redox reactions through their metabolic processes, where an appropriate ORP favors electron transport from the fungi to the anode. Conversely, an inadequate ORP can inhibit their metabolic activity [50,51]. Fungi such as Aspergillus niger and Trametes versicolor have been reported to be more effective in environments with specific ORPs due to their extracellular enzymes (e.g., laccases and peroxidases) that participate in redox reactions [52]. Furthermore, an ORP gradient between the anode and the cathode is essential to generate a constant flow of electrons [53].
3.3. Behavior of Power Density as a Function of Current Density and Internal Resistance
The power density profiles as a function of current can be observed in Figure 4a. A maximum power density of 0.148 ± 0.09 mW/cm2 is shown, with a corresponding current density of 5.847 mA/cm2 and a peak voltage of 853.604 ± 25.155 V. Previous studies have indicated that fungi decompose organic substrates and generate electrons that are transferred to the anode. The power density profiles depend on the metabolic capacity of fungi to oxidize organic compounds [44,54]. Additionally, fungi such as Trametes versicolor (laccase producers) or Aspergillus niger are efficient in redox reactions, which favors higher power densities [55]. Environmental conditions, such as pH, temperature, and nutrient availability, also directly influence the activity of fungi and, therefore, the power density [56]. Lin et al. (2021) used the fungus Pleurotus eryngii as a biocatalyst for copper removal in microbial fuel cells, achieving a power density of 41.3 mW/m2, and mentioned that incorporating redox mediators (natural or synthetic) can improve electron transfer [57]. Bashir et al. (2021) used the fungus Saccharomyces cerevisiae as a biocatalyst to generate electrical energy in their microbial fuel cells, noting that electrodes with high electrical conductivity and large surface area, such as those made of carbon nanotubes or graphene, favor a higher power density [58]. Similarly, Votat et al. (2024) used the fungus Trichoderma harzianum as a biocatalyst in their microbial fuel cells, managing to generate 4.1 mW/m2 [59]. They mentioned that in systems with fungi, high power density must be accompanied by stability over time, as factors such as the accumulation of metabolic byproducts, electrolyte degradation, or system contamination can negatively affect stability [59].
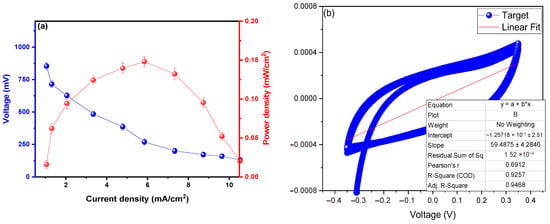
Figure 4.
Profiles of (a) power density as a function of current density and (b) internal resistance.
Figure 4b shows the polarization curve generated by the microbial fuel cells. Applying Ohm’s law, an internal resistance of 59.487 ± 4.254 Ω was observed. The literature reports that high internal resistance reduces current flow, decreasing voltage and power density [60]. Internal resistance is associated with the energy barriers that electrons must overcome during electrochemical reactions at the anode and cathode. The resistance of MFCs with fungi depends on the metabolic activity of fungi and the reactions catalyzed by extracellular enzymes (such as laccases and peroxidases) [61,62]. Meanwhile, Chen et al. (2024) used activated sludge and carbon felts as substrates, generating an internal resistance of 339.25 Ω and a maximum voltage of 0.121 V, noting that in MFCs with fungi, the accumulation of metabolic byproducts (or the lack of nutrients) can increase this resistance [63].
3.4. FTIR Spectrum and SEM Characteristics
The transmittance spectrum of the initial and final state of the samples (plastics) used in microbial fuel cells during the 35 days of monitoring is shown in Figure 5. The vibration bands associated with the asymmetric and symmetric stretching of the C-H bonds in fatty acids were observed between the wavelengths of 2840 cm−1 to 2923 cm−1 [18]. The intensity of the peak at the wavelength of 1470 cm−1 is attributed to the deformation vibrations of bonds related to lipids [64]. Meanwhile, the peak at 723 cm−1 corresponds to specific vibrations of molecules present in the fungus, such as interactions between proteins and carbohydrates [18,64]. The reduction in the intensity of the FTIR spectrum can be attributed to several factors related to chemical and structural modifications in the samples affected by fungi over time. These changes are usually associated with metabolic processes, interactions with the environment, or degradation of cellular components [65,66]. The reduction in the FTIR spectrum of the plastic sample is due to a fungal attack, indicating that the material has undergone chemical modifications resulting from degradation or biodegradation processes. This happens because fungi produce extracellular enzymes that break chemical bonds in polymers, altering their molecular structure [67]. Additionally, it has been observed that enzymatic action breaks the long polymer chains into oligomers, dimers, and monomers, reducing the density of functional bonds in the plastic [59].
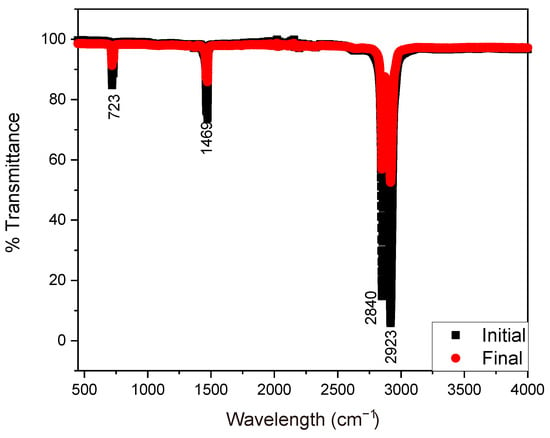
Figure 5.
FTIR spectrum of the plastic samples in initial and final states.
Figure 6 presents micrographs of biodegradable plastic samples treated with Aspergillus ochraceopetaliformis in different configurations of MFCs. In the micrograph corresponding to MFC-1, the surface shows initial irregularities and signs of fragmentation, indicating the beginning of the biodegradation process. The image of MFC-2 reveals more advanced degradation, with loose particles and noticeable alterations in the plastic’s morphology. Finally, MFC-3 exhibits a highly fractured surface with large cracks and disintegrated structures, suggesting significant polymer degradation. The phenomena observed in this process can be explained by the enzymatic activity of A. ochraceopetaliformis, which produces enzymes capable of breaking down the polymer chains of biodegradable plastic [38]. These enzymes decompose polymers into smaller molecules, facilitating their absorption by the fungus as a carbon and energy source [46]. Additionally, the MFC configuration introduces an additional process: microbial activity within the cell generates electrons that can be captured for electricity production. This dual function makes the system a sustainable alternative for plastic degradation with energy-related benefits [51,55]. The presence of cracks in the micrographs indicates an accelerated biodegradation process, potentially enhanced by environmental factors such as humidity, temperature, and the type of plastic used [58]. Figure 6 demonstrates that the integration of fungi into MFC systems could offer an innovative solution for plastic reduction, generating positive impacts both in waste elimination and renewable energy production.
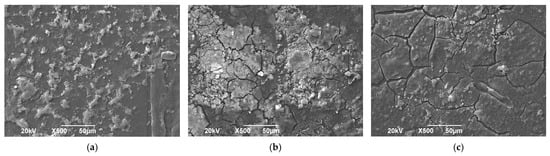
Figure 6.
Micrographs of biodegradable plastic samples by Aspergillus ochraceopetaliformis in (a) MFC-1, (b) MFC2, and (c) MFC-3.
Figure 7 illustrates the process of bioelectricity generation using plastic waste and Aspergillus ochraceopetaliformis in microbial fuel cells (MFCs). The initial accumulation of plastic waste is observed, followed by its treatment with the fungus, which promotes biodegradation. In the next stage, the treated samples are integrated into individual MFCs, generating a voltage of 0.92 V. Subsequently, the series connection of multiple MFCs increases the voltage to 2.78 V, demonstrating the system’s cumulative efficiency. The final section showcases the effective generation of bioelectricity with interconnected cells. This process combines plastic degradation with energy production, leveraging the fungus’s ability to break down polymers while facilitating electrochemical reactions within the MFCs. The research suggests a sustainable approach, with potential applications in waste management and the development of alternative renewable energy sources from plastic waste.
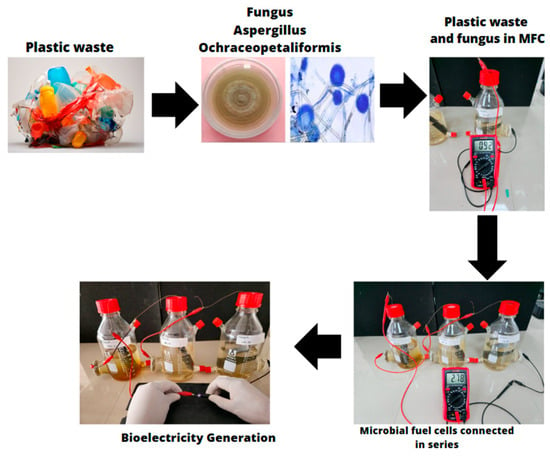
Figure 7.
Bioelectricity generation process.
Current limitations of single-chamber microbial fuel cells (MFCs) utilizing Aspergillus ochraceopetaliformis have shown promising results in generating bioelectricity and degrading plastics [68]. However, several limitations need to be addressed, including scalability, durability, efficiency, environmental dependency, and material issues [32,36]. Current studies are typically conducted on a laboratory scale. Scaling up the process to an industrial level presents challenges, including maintaining efficiency, managing costs, and ensuring consistent performance across larger systems [44,55]. The long-term stability of MFCs can be problematic. Factors such as the accumulation of metabolic byproducts, pH fluctuations, and nutrient depletion can reduce the efficiency of the cells over time. Although MFCs show potential, the overall efficiency of bioelectricity generation and plastic biodegradation needs significant improvement to be commercially viable [28]. The performance of MFCs is highly dependent on environmental conditions such as temperature, pH, and humidity [33]. Variability in these conditions in real-world applications can impact the effectiveness of the cells. The materials used in the construction of MFCs, particularly the electrodes, must be durable and cost-effective. Issues such as electrode degradation over time need to be addressed to ensure long-term operation [64,67].
To overcome these limitations and advance the application of MFCs, future research should focus on areas such as optimization of conditions, long-term stability, efficiency improvement, scalability studies, and integration with other technologies. This requires further research to optimize environmental conditions (pH, temperature, nutrient levels) to maximize the efficiency of MFCs [40,55,59]. This includes understanding the ideal conditions for the metabolic activity of Aspergillus ochraceopetaliformis. Additionally, conducting scalability studies to evaluate the feasibility and performance of MFCs in larger, real-world applications is crucial. This involves addressing challenges related to maintaining efficiency and consistency at a larger scale [39]. Exploring genetic modifications or synthetic biology approaches to enhance the metabolic activity and plastic-degrading capabilities of fungi could involve developing improved strains of Aspergillus ochraceopetaliformis with better performance [49,55]. Investigating the integration of MFCs with other renewable energy technologies to improve overall energy production and sustainability is also essential. This could include hybrid systems that combine MFCs with solar or wind energy [17,47]. A critical aspect that remains to be thoroughly explored is the potential toxicity of the intermediate and final compounds generated during polyethylene degradation by Aspergillus ochraceopetaliformis in MFCs. While FTIR analysis revealed modifications in C–H bonds and the emergence of new functional groups, these findings are purely structural and do not shed light on the nature or hazard of the molecules released. Enzymatic oxidation of the polymer may produce oligomers or chain fragments bearing oxygenated groups (alcohols, carboxylic acids, or ketones) that—at elevated concentrations—could exhibit cytotoxicity or disrupt biological processes in exposed organisms. Likewise, incomplete fragmentation can generate plastic nanoparticles or secondary metabolites that, once released into the environment, could bioaccumulate and trigger oxidative stress in aquatic and terrestrial cells. To mitigate these risks, it is essential to incorporate chromatography coupled with mass spectrometry (GC-MS or LC-MS) to identify and quantify degradation products, along with ecotoxicological assays using cell lines and model organisms (Daphnia magna, algae, or luminescent bacteria). Only through a complementary toxicological evaluation can we ensure that this biotechnological solution does not swap plastic pollution for new chemical threats in the environment.
4. Conclusions
The present study highlights the dual functionality of Aspergillus ochraceopetaliformis in microbial fuel cells (MFCs), demonstrating its ability to biodegrade polyethylene while generating bioelectricity. Over 35 days, the MFCs achieved a peak voltage of 0.921 ± 0.022 V and an electric current of 4.441 ± 0.265 mA, with an optimal pH of 7.059 ± 0.381. These findings confirmed that the fungus effectively decomposes plastic substrates and facilitates electron transfer to the anode, contributing to bioelectricity production. Additionally, a maximum power density of 0.148 ± 0.09 mW/cm2 was observed at a current density of 5.847 mA/cm2, with an internal resistance of 59.487 ± 4.254 Ω, reinforcing the efficiency of the system. The FTIR transmittance spectrum showed a reduction in peaks at 1470 cm−1 and 723 cm−1, indicating changes in lipid and protein structures associated with plastic degradation. Furthermore, MFCs connected in series generated a total voltage of 2.78 V, sufficient to power an LED bulb, demonstrating a potential pathway for renewable energy applications.
While the findings are promising, key challenges remain to enhance the practical viability of this technology. Long-term stability and durability must be improved, as factors such as metabolic byproduct accumulation and pH fluctuations can reduce efficiency over time. The optimization of environmental parameters—including temperature, humidity, and nutrient availability—will be essential to maximize fungal metabolic activity.
Future studies should incorporate comparative analyzes across different experimental conditions, including MFCs with fungal inoculation, fungal biodegradation alone, and control groups without fungi or MFCs. This would allow a more precise assessment of individual contributions to plastic degradation and energy generation. Although these comparisons were not included in the current study, the observed plastic thickness reduction and structural modifications reinforce the biodegradation potential of Aspergillus ochraceopetaliformis in MFC systems. Expanding research to include control-based evaluations will refine these findings, providing deeper insight into the interplay of biotic and abiotic factors in plastic decomposition.
Author Contributions
Conceptualization, N.S.-D. and R.-F.S.; Data curation, R.-F.S. and A.A.-M.; Formal analysis, C.-C.L. and M.D.L.C.-N.; Investigation, M.D.L.C.-N.; Resources, R.-F.S. and C.-C.L.; Software, N.M.O.; Validation, C.-C.L., N.M.O. and N.M.O.; Writing—original draft, R.-F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been financed by the Universidad Cesar Vallejo, project code No P-2023-113.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pilapitiya, P.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haque, R.; Jahirul, M.I.; Rasul, M.G. Pyrolysis of plastic waste for sustainable energy Recovery: Technological advancements and environmental impacts. Energy Convers. Manag. 2025, 326, 119511. [Google Scholar] [CrossRef]
- Mong, G.R.; Tan, H.; Sheng, D.D.C.V.; Kek, H.Y.; Nyakuma, B.B.; Woon, K.S.; Othman, M.H.D.; Kang, H.S.; Goh, P.S.; Wong, K.Y. A review on plastic waste valorisation to advanced materials: Solutions and technologies to curb plastic waste pollution. J. Clean. Prod. 2024, 434, 140180. [Google Scholar] [CrossRef]
- Schade, A.; Melzer, M.; Zimmermann, S.; Schwarz, T.; Stoewe, K.; Kuhn, H. Plastic Waste Recycling—A Chemical Recycling Perspective. ACS Sustain. Chem. Eng. 2024, 12, 12270–12288. [Google Scholar] [CrossRef]
- Vuppaladadiyam, S.S.V.; Vuppaladadiyam, A.K.; Sahoo, A.; Urgunde, A.; Murugavelh, S.; Šrámek, V.; Pohořelý, M.; Trakal, L.; Bhattacharya, S.; Sarmah, A.K.; et al. Waste to energy: Trending key challenges and current technologies in waste plastic management. Sci. Total Environ. 2024, 913, 169436. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.; Gu, J.; Yuan, H.; Chen, Y. Chemical recycling of plastic wastes via homogeneous catalysis: A review. Chem. Eng. J. 2024, 479, 147853. [Google Scholar] [CrossRef]
- Jiao, H.; Ali, S.S.; Alsharbaty, M.H.M.; Elsamahy, T.; Abdelkarim, E.; Schagerl, M.; Al-Tohamy, R.; Sun, J. A critical review on plastic waste life cycle assessment and management: Challenges, research gaps, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 271, 115942. [Google Scholar] [CrossRef]
- Qian, Q.; Ren, J. From plastic waste to potential wealth: Upcycling technologies, process synthesis, assessment and optimization. Sci. Total Environ. 2024, 907, 167897. [Google Scholar] [CrossRef] [PubMed]
- Che, C.A.; Heynderickx, P.M. Hydrothermal carbonization of plastic waste: A review of its potential in alternative energy applications. Fuel Commun. 2024, 18, 100103. [Google Scholar] [CrossRef]
- Praveenkumar, T.R.; Sekar, M.; Pasupuleti, R.R.; Gavurová, B.; Kumar, G.A.; Kumar, M.V. Current technologies for plastic waste treatment for energy recovery, it’s effects on poly aromatic hydrocarbons emission and recycling strategies. Fuel 2024, 357, 129379. [Google Scholar] [CrossRef]
- He, Y.; Deng, X.; Jiang, L.; Hao, L.; Shi, Y.; Lyu, M.; Zhang, L.; Wang, S. Current advances, challenges and strategies for enhancing the biodegradation of plastic waste. Sci. Total Environ. 2024, 906, 167850. [Google Scholar] [CrossRef]
- Dey, S.; Veerendra, G.T.N.; Babu, P.A.; Manoj, A.P.; Nagarjuna, K. Degradation of plastics waste and its effects on biological ecosystems: A scientific analysis and comprehensive review. Biomed. Mater. Devices 2024, 2, 70–112. [Google Scholar] [CrossRef]
- Afshar, S.V.; Boldrin, A.; Astrup, T.F.; Daugaard, A.E.; Hartmann, N.B. Degradation of biodegradable plastics in waste management systems and the open environment: A critical review. J. Clean. Prod. 2024, 434, 140000. [Google Scholar] [CrossRef]
- Crystal Thew, X.E.; Lo, S.C.; Ramanan, R.N.; Tey, B.T.; Huy, N.D.; Chien Wei, O. Enhancing plastic biodegradation process: Strategies and opportunities. Crit. Rev. Biotechnol. 2024, 44, 477–494. [Google Scholar] [CrossRef]
- Rajvanshi, J.; Sogani, M.; Tziouvaras, G.; Kumar, A.; Syed, Z.; Sonu, K.; Gupta, N.S.; Sen, H. An analytical review on revamping plastic waste management: Exploring recycling, biodegradation, and the growing role of biobased plastics. Environ. Sci. Pollut. Res. 2024, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Scieuzo, C.; Salvia, R.; Arias, C.F.; Perez, R.P.; Bertocchini, F.; Falabella, P. Beyond microbial biodegradation: Plastic degradation by Galleria mellonella. J. Polym. Environ. 2024, 32, 2158–2177. [Google Scholar] [CrossRef]
- Ferreira-Filipe, D.A.; Oliveira, L.; Paço, A.; Fernandes, A.J.; Costa, F.M.; Duarte, A.C.; Rocha-Santos, T.; Silva, A.L.P. Biodegradation of e-waste microplastics by Penicillium brevicompactum. Sci. Total Environ. 2024, 935, 173334. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ionescu, D.; Grossart, H.P. Tapping into fungal potential: Biodegradation of plastic and rubber by potent Fungi. Sci. Total Environ. 2024, 934, 173188. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Abdul-Hadi, S.Y.; Darwish, A.G. Aspergillus: Biodiversity, ecological significances, and industrial applications. In Recent Advancement in White Biotechnology Through Fungi: Volume 1: Diversity and Enzymes Perspectives; Springer: Cham, Switzerland, 2019; pp. 121–179. [Google Scholar] [CrossRef]
- Ngo, C.C.; Nguyen, Q.H.; Nguyen, T.H.; Quach, N.T.; Dudhagara, P.; Vu, T.H.N.; Le, T.T.X.; Le, T.T.H.; Do, T.T.H.; Nguyen, V.D.; et al. Identification of fungal community associated with deterioration of optical observation instruments of museums in Northern Vietnam. Appl. Sci. 2021, 11, 5351. [Google Scholar] [CrossRef]
- Kinamot, V.B. Influence of seagrass traits on the diversity of endophytic fungi. Biodivers. J. Biol. Divers. 2024, 25, 1–8. [Google Scholar] [CrossRef]
- Ramdass, A.C.; Rampersad, S.N. Biodiversity and biocatalyst activity of culturable hydrocarbonoclastic fungi isolated from Marac–Moruga mud volcano in South Trinidad. Sci. Rep. 2021, 11, 19466. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, Z.H.; Zeng, E.N.; Mo, L.; Sun, Z.W.; Wang, Y.Z.; Zhang, X.Y. Biodiversity and antimicrobial activity of intestinal fungi from three species of coral reef fishes. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Liao, X.; Yang, J.; Zhou, Z.; Wu, J.; Xu, D.; Yang, Q.; Zhong, S.; Zhang, X. Diversity and antimicrobial activity of intestinal fungi from three species of coral reef fish. J. Fungi 2023, 9, 613. [Google Scholar] [CrossRef]
- Afrin, A.; Pothulapadu, C.A.S. Symphony of light: AIE and MFC in carbazole-based cyanostilbenes. J. Mater. Chem. C 2024, 12, 1923–1944. [Google Scholar] [CrossRef]
- Arun, J.; SundarRajan, P.; Pavithra, K.G.; Priyadharsini, P.; Shyam, S.; Goutham, R.; Le, Q.H.; Pugazhendhi, A. New insights into microbial electrolysis cells (MEC) and microbial fuel cells (MFC) for simultaneous wastewater treatment and green fuel (hydrogen) generation. Fuel 2024, 355, 129530. [Google Scholar] [CrossRef]
- Kothari, R.; Pathak, A.K.; Singh, H.M.; Goria, K.; Sheikh, Z.U.D.; Bharti, A.; Raina, S.; Rachna; Singh, A.; Singh, B.; et al. MFC-mediated wastewater treatment technology and bioelectricity generation: Future perspectives with SDGs 7 & 13. Process Saf. Environ. Prot. 2024, 192, 155–176. [Google Scholar] [CrossRef]
- Yu, Z.X.; Huangfu, L.X.; Yang, Y.L.; Wang, S.S.; Wu, G.H.; Cui, Y.G. Design and control of a novel 3-DOF parallel MFC micromanipulation platform. Eng. Sci. Technol. Int. J. 2025, 61, 101943. [Google Scholar] [CrossRef]
- Geng, J.; O’Dell, J.; Stark, N.; Kitin, P.; Zhang, X.; Zhu, J.Y. Microfibrillated cellulose (MFC) barrier coating for extending banana shelf life. Food Hydrocoll. 2024, 150, 109671. [Google Scholar] [CrossRef]
- Kalathil, S.; Miller, M.; Reisner, E. Microbial fermentation of polyethylene terephthalate (PET) plastic waste for the production of chemicals or electricity. Angew. Chem. Int. Ed. 2022, 61, e202211057. [Google Scholar] [CrossRef]
- Segundo, R.F.; Rocío, P.C.; Luis, C.C.; Angelats Silva, L.M. Potential Use of the Fungus Trichoderma sp. as a Plastic-Reducing Agent and Electricity Generator in Microbial Fuel Cells. Processes 2024, 12, 2904. [Google Scholar] [CrossRef]
- Segundo, R.F.; Magaly, D.L.C.N.; Otiniano, N.M.; Luis, C.C.; Angelats-Silva, L.M. Electricity Generation and Plastic Waste Reduction Using the Fungus Paecilomyces as a Biodegrader in Microbial Fuel Cells. Sustainability 2024, 16, 11137. [Google Scholar] [CrossRef]
- Lozano-Mahecha, R.A.; López-López, K. Isolation and characterization of Colombian endemic bacteria capable of degrading toluene. Rev. Colomb. Biotecnol. 2022, 24, 6–18. [Google Scholar] [CrossRef]
- Hao, D.C.; Wang, F.; Li, C.; Wang, Y.; Xue, J.; Xiao, P.G. Fungal bioaugmentation enhanced herbicide removal via soil microbial fuel cell: Taking Myrothecium verrucaria and haloxyfop-P as an example. Sci. Total Environ. 2025, 958, 178012. [Google Scholar] [CrossRef]
- Sekrecka-Belniak, A.; Toczyłowska-Mamińska, R. Fungi-based microbial fuel cells. Energies 2018, 11, 2827. [Google Scholar] [CrossRef]
- Kižys, K.; Pirštelis, D.; Morkvėnaitė-Vilkončienė, I. Effect of Gold Nanoparticles in Microbial Fuel Cells Based on Polypyrrole-Modified Saccharomyces cerevisiae. Biosensors 2024, 14, 572. [Google Scholar] [CrossRef]
- Moubasher, H.; Tammam, A.; Saleh, M. Enhancing electricity generation using fungal laccase-based microbial fuel cell. J. Microbiol. Biotechnol. Food Sci. 2024, 14, e9703. [Google Scholar] [CrossRef]
- Sukri, A.; Othman, R.; Abd-Wahab, F.; M Noor, N. Self-sustaining bioelectrochemical cell from fungal degradation of lignin-rich agrowaste. Energies 2021, 14, 2098. [Google Scholar] [CrossRef]
- Gorin, M.; Shabani, M.; Votat, S.; Lebrun, L.; Mbokou, S.F.; Pontié, M. Application of fungal-based microbial fuel cells for biodegradation of pharmaceuticals: Comparative study of individual vs. mixed contaminant solutions. Chemosphere 2024, 363, 142849. [Google Scholar] [CrossRef]
- Laily, F.N.; Juliastuti, S.R. Effect of micronutrient addition and development on microbial fuel cells (MFC) from food waste with the help of hydrolytic fungi. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1108, p. 012005. [Google Scholar] [CrossRef]
- Khan, B.; Rathore, A.; Adnan, A.; Yahya, S.; Arshan, M.M.K. Comparative analysis of electric current production by Saccharomyces cerevisiae using a dual chamber microbial fuel cell. Asian J. Adv. Res. 2021, 4, 996–1002. [Google Scholar]
- Tian, Y.; Li, C.; Liang, D.; Xie, T.; He, W.; Li, D.; Feng, Y. Fungus-sourced filament-array anode facilitates Geobacter enrichment and promotes anodic bio-capacitance improvement for efficient power generation in microbial fuel cells. Sci. Total Environ. 2022, 838, 155926. [Google Scholar] [CrossRef]
- Sarma, H.; Bhattacharyya, P.; Jadhav, D.A.; Pawar, P.; Thakare, M.; Pandit, S.; Mathuriya, A.S.; Prasad, R. Fungal-mediated electrochemical system: Prospects, applications and challenges. Curr. Res. Microb. Sci. 2021, 2, 100041. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, S.; Alkhanjaf, A.A.M.; Arora, N.K.; Saxena, B.; Umar, A.; Ibrahim, A.A.; Akhtar, M.S.; Mahajan, A.; Negi, S.; et al. Microbial fuel cells for azo dye degradation: A perspective review. J. Ind. Eng. Chem. 2024, 142, 45–67. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Jayabalan, T.; Sethupathi, M.; Kim, W.; Muniyasamy, S.; Sengottuvelan, N.; Nainamohamed, S.; Ponnuchamy, K.; Alagarsamy, A. Bioelectricity generation by natural microflora of septic tank wastewater (STWW) and biodegradation of persistent petrogenic pollutants by basidiomycetes fungi: An integrated microbial fuel cell system. J. Hazard. Mater. 2021, 412, 125228. [Google Scholar] [CrossRef]
- Raqba, R.; Rafaqat, S.; Ali, N.; Munis, M.F.H. Biodegradation of Reactive Red 195 azo dye and Chlorpyrifos organophosphate along with simultaneous bioelectricity generation through bacterial and fungal based biocathode in microbial fuel cell. J. Water Process Eng. 2022, 50, 103177. [Google Scholar] [CrossRef]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.-E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Pandit, S.; Savla, N.; Sonawane, J.M.; Sani, A.M.; Gupta, P.K.; Mathuriya, A.S.; Rai, A.K.; Jadhav, D.A.; Jung, S.P.; Prasad, R. Agricultural waste and wastewater as feedstock for bioelectricity generation using microbial fuel cells: Recent advances. Fermentation 2021, 7, 169. [Google Scholar] [CrossRef]
- Arulmani, S.R.B.; Gnanamuthu, H.L.; Kandasamy, S.; Govindarajan, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A.; Zhang, H. Sustainable bioelectricity production from Amaranthus viridis and Triticum aestivum mediated plant microbial fuel cells with efficient electrogenic bacteria selections. Process Biochem. 2021, 107, 27–37. [Google Scholar] [CrossRef]
- Hao, D.C.; Li, X.; Wang, Y.; Li, J.; Li, C.; Xiao, P. Xeno-Fungusphere: Fungal-Enhanced Microbial Fuel Cells for Agricultural Remediation with a Focus on Medicinal Plants. Agronomy 2025, 15, 1392. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S. Application of oil palm lignocellulosic derived material as an efficient anode to boost the toxic metal remediation trend and energy generation through microbial fuel cells. J. Clean. Prod. 2021, 314, 128062. [Google Scholar] [CrossRef]
- Idris, M.O.; Kim, H.C.; Yaqoob, A.A.; Ibrahim, M.N.M. Exploring the effectiveness of microbial fuel cell for the degradation of organic pollutants coupled with bio-energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102183. [Google Scholar] [CrossRef]
- Rusyn, I. Role of microbial community and plant species in performance of plant microbial fuel cells. Renew. Sustain. Energy Rev. 2021, 152, 111697. [Google Scholar] [CrossRef]
- Zinovicius, A.; Rozene, J.; Merkelis, T.; Bruzaite, I.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Evaluation of a yeast–polypyrrole biocomposite used in microbial fuel cells. Sensors 2022, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Das, S.; Das, S.; Ghangrekar, M.M. Proficient sanitary wastewater treatment in laboratory and field-scale microbial fuel cell with anti-biofouling Cu0.5Mn0.5Fe2O4 as cathode catalyst. J. Electrochem. Soc. 2021, 168, 054519. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Recent trends in upgrading the performance of yeast as electrode biocatalyst in microbial fuel cells. Chemosphere 2021, 284, 131383. [Google Scholar] [CrossRef]
- Lin, C.W.; Lai, C.Y.; Liu, S.H.; Chen, Y.R.; Alfanti, L.K. Enhancing bioelectricity generation and removal of copper in microbial fuel cells with a laccase-catalyzed biocathode. J. Clean. Prod. 2021, 298, 126726. [Google Scholar] [CrossRef]
- Bashir, S.; Houf, W.; Liu, J.L.; Mulvaney, S.P. 3D Conducting polymeric membrane and scaffold Saccharomyces cerevisiae biofilms to enhance energy conversion in microbial fuel cells. ACS Appl. Mater. Interfaces 2021, 14, 20393–20403. [Google Scholar] [CrossRef]
- Votat, S.; Pontié, M.; Jaspard, E.; Lebrun, L. Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum. Energies 2024, 17, 247. [Google Scholar] [CrossRef]
- Umar, A.; Mubeen, M.; Ali, I.; Iftikhar, Y.; Sohail, M.A.; Sajid, A.; Kumar, A.; Solanki, M.K.; Divvela, P.K.; Zhou, L. Harnessing fungal bio-electricity: A promising path to a cleaner environment. Front. Microbiol. 2024, 14, 1291904. [Google Scholar] [CrossRef]
- Sayed, E.T.; Olabi, A.G.; Mouselly, M.; Alawadhi, H.; Abdelkareem, M.A. Zinc-based metal organic framework on carbon fiber brush as a novel anode of yeast-based microbial fuel cell. Int. J. Hydrogen Energy 2024, 52, 856–864. [Google Scholar] [CrossRef]
- Utami, T.S.; Arbianti, R.; Hidayatullah, I.M.; Yusupandi, F.; Hamdan, M.; Putri, N.F.; Riyadi, F.A.; Boopathy, R. Paracetamol degradation in a dual-chamber rectangular membrane bioreactor using microbial fuel cell system with a microbial consortium from sewage sludge. Case Stud. Chem. Environ. Eng. 2024, 9, 100551. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Li, J. Research on minimizing the MFC internal resistance via a shared electrode MFC-MEC coupling system. Biochem. Eng. J. 2024, 203, 109195. [Google Scholar] [CrossRef]
- Černoša, A.; Cortizas, A.M.; Traoré, M.; Podlogar, M.; Danevčič, T.; Gunde-Cimerman, N.; Gostinčar, C. A screening method for plastic-degrading fungi. Heliyon 2024, 10, e31130. [Google Scholar] [CrossRef]
- Zeghal, E.; Vaksmaa, A.; Vielfaure, H.; Boekhout, T.; Niemann, H. The potential role of marine fungi in plastic degradation–a review. Front. Mar. Sci. 2021, 8, 738877. [Google Scholar] [CrossRef]
- Wu, F.; Guo, Z.; Cui, K.; Dong, D.; Yang, X.; Li, J.; Wu, Z.; Li, L.; Dai, Y.; Pan, T. Insights into characteristics of white rot fungus during environmental plastics adhesion and degradation mechanism of plastics. J. Hazard. Mater. 2023, 448, 130878. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.A.; Ali, A.S. Biodegradation of low density polyethylene (LDPE) by mesophilic fungus ‘Penicillium citrinum’ isolated from soils of plastic waste dump yard, Bhopal, India. Environ. Technol. 2023, 44, 2300–2314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chang, S.H.; Mailhot, G. Emerging Biochemical Conversion for Plastic Waste Management: A Review. Molecules 2025, 30, 1255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).