Fermentation of Organic Wastes for Feed Protein Production: Focus on Agricultural Residues and Industrial By-Products Tied to Agriculture
Abstract
1. Introduction
2. Fermentation of Agricultural Organic Wastes into Feed Protein
3. Fermentation of Agri-Industrial By-Products into Feed Protein
4. Others
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting global feed protein demand: Challenge, opportunity, and strategy. Annu. Rev. Anim. Biosci. 2019, 7, 221–243. [Google Scholar] [CrossRef]
- Khanal, P. Use of land-based and aquatic alternative feed resources to establish a circular economy within livestock production. J. Agric. Food Res. 2024, 16, 101087. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, W.; Zhang, S.; Schroyen, M. The role of maternal dietary protein on livestock development, production and health. Anim. Reprod. Sci. 2025, 276, 107835. [Google Scholar] [CrossRef]
- Javourez, U.; Hamelin, L. Technologies turning waste into food and feed have limited environmental benefits. Nat. Sustain. 2025, 8, 334–335. [Google Scholar] [CrossRef]
- Rasool, K.; Hussain, S.; Shahzad, A.; Miran, W.; Mahmoud, K.A.; Ali, N.; Almomani, F. Comprehensive insights into sustainable conversion of agricultural and food waste into microbial protein for animal feed production. Rev. Environ. Sci. Biotechnol. 2023, 22, 527–562. [Google Scholar]
- Areeya, S.; Panakkal, E.J.; Kunmanee, P.; Tawai, A.; Amornraksa, S.; Sriariyanun, M. A review of sugarcane biorefinery: From waste to value-added products. Appl. Sci. Eng. Prog. 2024, 17, 7402. [Google Scholar] [CrossRef]
- Uekert, T.; Bleem, A.C.; Holmes, E.C.; Pal, A.; Johnson, C.W.; Beckham, G.T. Coupling waste feedstocks to microbial protein production in a circular food system. ACS Sustain. Chem. Eng. 2025, 13, 709–724. [Google Scholar]
- Shahzad, H.M.A.; Almomani, F.; Shahzad, A.; Mahmoud, K.A.; Rasool, K. Challenges and opportunities in biogas conversion to microbial protein: A pathway for sustainable resource recovery from organic waste. Process Saf. Environ. Prot. 2024, 185, 644–659. [Google Scholar] [CrossRef]
- Santillan, E.; Yasumaru, F.; Vethathirri, R.S.; Thi, S.S.; Hoon, H.Y.; Sian, D.C.P.; Wuertz, S. Microbial community-based protein from soybean-processing wastewater as a sustainable alternative fish feed ingredient. Sci. Rep. 2024, 14, 2620. [Google Scholar] [CrossRef]
- Ibáñez, M.A.; de Blas, C.; Cámara, L.; Mateos, G.G. Chemical composition, protein quality and nutritive value of commercial soybean meals produced from beans from different countries: A meta-analytical study. Anim. Feed Sci. Technol. 2020, 267, 114531. [Google Scholar]
- Li, J.; Tao, L.; Zhang, R.; Yang, G. Effects of fermented feed on growth performance, nutrient metabolism and cecal microflora of broilers. Anim Biosci. 2022, 35, 596–604. [Google Scholar] [CrossRef]
- Sun, H.; Kang, X.; Tan, H.; Cai, H.; Chen, D. Progress in fermented unconventional feed application in monogastric animal production in China. Fermentation 2023, 9, 947. [Google Scholar] [CrossRef]
- Phiri, R.; Mavinkere Rangappa, S.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Cleaner Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Yu, L.; An, Z.; Xie, D.; Yin, D.; Xie, G.; Gao, X.; Xiao, Y.; Liu, J.; Fang, Z. From waste to protein: A new strategy of converting composted distilled grain wastes into animal feed. Front. Microbiol. 2024, 15, 1405564. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An overview of fermentation in the food industry—Looking back from a new perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Lin, M. Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation 2022, 8, 688. [Google Scholar] [CrossRef]
- Zang, Q.; Chen, X.; Zhang, C.; Lin, M.; Xu, X. Improving crude protein and methionine production, selective lignin degradation and digestibility of wheat straw by Inonotus obliquus using response surface methodology. J. Sci. Food Agric. 2022, 102, 1146–1154. [Google Scholar] [PubMed]
- Wang, Y.; Yu, J.; Li, Q.; Zhang, J.; Naseem, S.; Sun, B.; Tang, L.; Choi, S.; Li, X. Screening the carbon source type in solid-state fermentation with phanerochaete chrysosporium to improve the forage value of corn straw and rice straw. Animals 2023, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, J.; Liu, M.; Zhang, H.; Li, X.; Tian, C.; Chen, Y. Synergistic effect of microorganisms and enzymes on nutritional value of corn stover and wheat straw. Fermentation 2025, 11, 210. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, Z.; Li, J.L.; Yang, Z.J.; Sheng, Q.K.; Liu, X.M.; Zhao, G.D.; Zhao, H.B. Effects of mixing ratio, microbial preparation and packing density on fermentation quality and nutritional value of mixed silage of corn straw and peanut vine. Chin. J. Anim. Nutr. 2024, 36, 2633–2647. [Google Scholar]
- Wu, D.T.; Liang, X.R.; Hu, R.; Wang, Z.S.; Zou, H.W.; Wu, F.L.; Li, H.; Jiang, Y.H.; Peng, Q.H.; Xiao, J.X.; et al. Improvement of nutritional value of cotton residue by solid state fermentation. J. Environ. Chem. Eng. 2025, 13, 117125. [Google Scholar] [CrossRef]
- Sheng, F.B.; Hu, X.; Zeng, J.R.; Tian, X.F.; Wu, Z.Q. Citrus pomace co-fermentation improved its protein and amino acids by Bacillus amyloliquefaciens and Candida utilis. Process Biochem. 2023, 130, 545–554. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Wang, C.P.; Deng, D.; Tian, Z.M.; Lu, H.J.; Li, J.Z.; Yu, M.; Ma, X.Y. Effects of aerobic fermentation with different strains on nutritional value of citrus pomace. Chin. J. Anim. Nutr. 2022, 34, 2030–2040. [Google Scholar]
- Huang, H.B.; Wang, Y.L.; Li, B.H.; Zhang, C.G.; Tang, X.Z.; Tan, B.E.; Yin, Y.L.; Zhang, C.; Jiang, Q. Screening of macadamia green peel fermentation strain and improvement of its feeding value after fermentation. Chin. J. Anim. Nutr. 2024, 36, 1292–1302. [Google Scholar]
- Huang, X.; Xu, Y.; Wu, X.; Ding, Y.; Fan, C.; Xue, Y.; Zhuo, Z.; Cheng, J. Mixed fermentation of Lactiplantibacillus plantarum and Bacillus licheniformis changed the chemical composition, bacterial community, and rumen degradation rate of tea residue. Fermentation 2022, 8, 380. [Google Scholar] [CrossRef]
- Cui, Y.; Peng, S.; Deng, D.; Yu, M.; Tian, Z.; Song, M.; Luo, J.; Ma, X.; Ma, X. Solid-state fermentation improves the quality of chrysanthemum waste as an alternative feed ingredient. J. Environ. Manage. 2023, 330, 117060. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Chen, S.; Wang, X.; Liu, J.; Deng, X.; Cai, H.; Liu, G. Effects of solid-state fermentation on the standardized ileal digestibility of amino acids and apparent metabolizable energy in peanut meal fed to broiler chickens. Fermentation 2023, 9, 346. [Google Scholar] [CrossRef]
- Dong, W.; Dong, S.; Li, Y.; Lei, Y.; Peng, N.; Liang, Y.; Zhao, S.; Ge, X. Comprehensive utilization of palm kernel cake for producing mannose and manno-oligosaccharide mixture and yeast culture. Appl. Microbiol. Biotechnol. 2022, 106, 1045–1056. [Google Scholar] [CrossRef]
- Yu, M.; Wang, P.; Li, F.; Du, J.; Jin, Y.; Zhao, T.; Yi, Q.; Tang, H.; Yuan, B. Fermentation quality and in vitro digestibility of sweet corn processing byproducts silage mixed with millet hull or wheat bran and inoculated with a lactic acid bacteria. Fermentation 2024, 10, 254. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Ding, W.H.; Sun, Y.N.; Ren, H.W.; Li, J.P.; Li, Z.Z. Effects of Lactobacillus plantarum on the ensiling quality of cauliflower wastes at different temperature. J. Basic Eng. 2023, 31, 635–649. [Google Scholar]
- Wang, C.; Li, M.L.; Zhang, S.; Huang, R.C.; Sun, X.Y.; Liu, F.Q.; Wu, J.; Zhang, J.A. Production of biolipid, protein, and carotenoid by R. glutinis As2.703 utilizing a multiple-carbon source system. Ind. Crop. Prod. 2025, 224, 120290. [Google Scholar] [CrossRef]
- Lai, A.Q.; Huang, Y.R.; Luo, H.C.; Jin, Y.D.; Wang, L.Z.; Chen, B.L.; Deng, K.M.; Huang, W.M.; Zhang, Y. Ruminal degradation characteristics of bagasse with different fermentation treatments in the rumen of beef cattle. Anim. Sci. J. 2024, 95, e13937. [Google Scholar] [CrossRef]
- Mittermeier, F.; Fischer, F.; Hauke, S.; Hirschmann, P.; Weuster-Botz, D. Valorization of wheat bran by co-cultivation of fungi with integrated hydrolysis to provide sugars and animal feed. BioTech 2024, 13, 15. [Google Scholar] [CrossRef]
- Chen, T.M.; Li, X.M.; Zhang, G.Y.; Zhao, H.S.; Lu, J.; Cai, G.L. Effect of Aspergillus oryzae fermentation on the feeding quality of cottonseed shells. China Oils Fats 2024, 49, 33–39, 65. [Google Scholar]
- Wu, Y.F.; Xiao, Y.; Okoye, C.O.; Gao, L.; Chen, X.F.; Wang, Y.L.; Jiang, J.X. Fermentation profile and bioactive component retention in honeysuckle residue silages inoculated with lactic acid bacteria: A promising feed additive for sustainable agriculture. Ind. Crop Prod. 2025, 224, 120315. [Google Scholar] [CrossRef]
- Zhang, G.H.; Niu, K.M.; Sheng, P.; Wang, D.S.; Wang, H.X.; He, G.; Wu, B.; Tao, Z.Y.; Zhang, Z.H. Fermented feed from lotus seedpod alleviates the impact of heat stress on beef cattle in summer. Food Biosci. 2025, 65, 106062. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.L.; Zeng, X.D.; Wang, Y.; Qin, H.; Song, P.; Hou, X.Y.; Liu, S.L.; Ma, C.; Huang, Y.; et al. Comprehensive use of distillers’ grains derived from Chinese Baijiu: A review. J. Agr. Food Res. 2024, 18, 101439. [Google Scholar] [CrossRef]
- Hadinoto, K.; Ling, J.K.U.; Pu, S.; Tran, T.T. Effects of alkaline extraction pH on amino acid compositions, protein secondary structures, thermal stability, and functionalities of brewer’s spent grain proteins. Int. J. Mol. Sci. 2024, 25, 6369. [Google Scholar] [CrossRef]
- Yeo, Y.T.; Lim, C.M.; Huaco, A.I.V.; Chen, W.N. Food circular economy and safety considerations in waste management of urban manufacturing side streams. Npj Sci. Food 2024, 8, 65. [Google Scholar] [CrossRef]
- Tsevdou, M.; Ntzimani, A.; Katsouli, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Comparative study of microwave, pulsed electric fields, and high pressure processing on the extraction of antioxidants from olive pomace. Molecules 2024, 29, 2303. [Google Scholar] [CrossRef]
- Hashem, M.; Al-Qahtani, M.S.; Alamri, S.A.; Moustafa, Y.S.; Lyberatos, G.; Ntaikou, I. Valorizing food wastes: Assessment of novel yeast strains for enhanced production of single-cell protein from wasted date molasses. Biomass Convers. Biorefinery 2022, 12, 4491–4502. [Google Scholar] [CrossRef]
- Furlan, O.; de Oliveira, N.S.; de Paula, R.C.; Rosa, R.T.; Michelotto, P.V.; Weber, S.H.; Bianchini, L.F.; Rosa, E.A.R. Pilot scale production of high-content mycoprotein using Rhizopus microsporus var. oligosporus by submerged fermentation and agro-industrial by-products. Bioresour. Technol. 2024, 413, 131515. [Google Scholar]
- Mnisi, C.M.; Kunene, S.I.; Soko, N.N.; Egbu, C.F.; Mlambo, V. Oyster mushroom bioprocessing enhances the nutritional value of olive pomace for ruminants. S. Afr. J. Anim. Sci. 2024, 54, 226–235. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, J.; Zou, G.; Zhang, X.; Shang, H.; Ji, B.; Bai, Y.; Qu, L.; Wei, Y. Enhancing the nutritional quality of defatted cottonseed meal by solid-state fermentation with probiotic microbes. Fermentation 2024, 10, 429. [Google Scholar] [CrossRef]
- Liu, D.D.; Guo, Y.T.; Ma, H.L. Production of value-added peptides from agro-industrial residues by solid-state fermentation with a new thermophilic protease-producing strain. Food Biosci. 2023, 53, 102534. [Google Scholar] [CrossRef]
- Fan, W.W.; Huang, X.H.; Liu, K.H.; Xu, Y.P.; Chi, Z.Y. Advanced upcycling of agro-industrial co-products of corn via different microorganisms. Biomass Bioenergy 2023, 168, 106669. [Google Scholar] [CrossRef]
- Chen, K.; Deng, X.R.; Jiang, D.H.; Qin, L.X.; Lu, M.Q.; Jiang, W.X.; Yang, M.Q.; Zhang, L.L.; Jiang, J.C.; Lu, L.M. Efficient conversion of distillers grains as feed ingredient by synergy of probiotics and enzymes. Front. Microbiol. 2024, 15, 1403011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Wang, Z.; Shen, X.J.; Chen, R.T.; Peng, Y.S.; Cai, Y.F.; Zeng, S.; Liu, D.; Yang, J.P.; Zhuang, W.; et al. Solid-state fermentation through synthetic microbiome: An effective strategy for converting Chinese distillers’ grains into functional protein feed. Int. J. Food Microbiol. 2025, 435, 111154. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Z.; Li, X.Z.; Lu, X.Q.; Liu, G.D.; Qin, Y.Q.; Zhao, J.; Qu, Y.B. Production of single cell protein from brewer’s spent grain through enzymatic saccharification and fermentation enhanced by ammoniation pretreatment. Bioresour. Technol. 2024, 394, 130242. [Google Scholar] [CrossRef]
- Korayem, A.S.M.; Mohammed, F.A.; Abu-Hussien, S.H.; Abosamra, F.M.; El-Dein, S.N.; Rahmy, H.A.F. Statistical optimization of solid-state fermentation by Aspergillus oryzae for valorization of olive cake and its application as a poultry feed. Scientifica 2025, 2025, 4315411. [Google Scholar] [CrossRef]
- Fernandes, H.; Salgado, J.M.; Ferreira, M.; Vrsanska, M.; Fernandes, N.; Castro, C.; Oliva-Teles, A.; Peres, H.; Belo, I. Valorization of brewer’s spent grain using biological treatments and its application in feeds for European seabass (Dicentrarchus labrax). Front. Bioeng. Biotechnol. 2022, 10, 732948. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.W.; Huang, X.H.; Liu, K.H.; Xu, Y.P.; Chi, Z.Y. Symbiosis of Aspergillus niger and Candida utilis for improving nutrition and digestibility in co-fermentation of corn-ethanol co-product and corncob. Biomass Conv. Bioref. 2024, 14, 11963–11975. [Google Scholar] [CrossRef]
- Hang, N.T.B.; Doan, C.C. Improving nutrition facts of cassava and soybean residue through solid-state fermentation by Pleurotus ostreatus mycelium: A pathway to safety animal feed production. Fermentation 2025, 11, 271. [Google Scholar] [CrossRef]
- Du, G.L.; Tisma, M.; He, B.R.; Zhai, X.H.; Yuan, C.Y.; Su, Z.D.; Shi, J.P.; Zhang, B.G. Valorization of the Caragana waste via two-stage bioaugmentation: Optimizing nutrition composition, palatability, and microbial contaminant control. J. Bioresour. Bioprod. 2024, 9, 518–533. [Google Scholar] [CrossRef]
- Du, G.; Shi, J.; Zhang, J.; Ma, Z.; Liu, X.; Yuan, C.; Zhang, B.; Zhang, Z.; Harrison, M.D. Exogenous probiotics improve fermentation quality, microflora phenotypes, and trophic modes of fermented vegetable waste for animal feed. Microorganisms 2021, 9, 644. [Google Scholar] [CrossRef]
- GB 13078-2017; Hygienical Standard for Feeds. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (AQSIQ) and the Standardization Administration of the People’s Republic of China (SAC): Beijing, China, 2017.
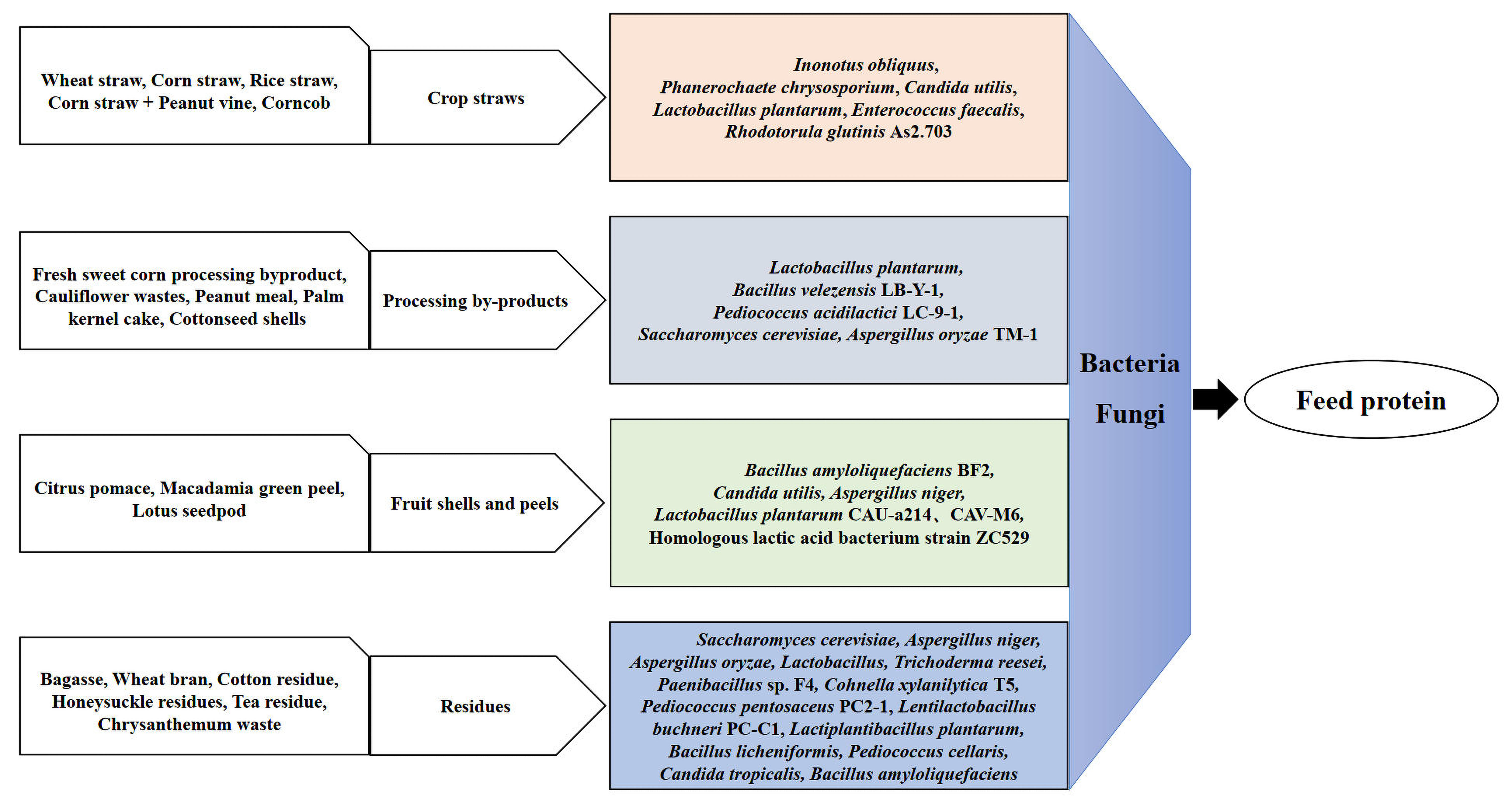
| Agricultural Organic Waste | Fermentation Strain | Fermentation Conditions and Process | Ref. |
|---|---|---|---|
| Wheat straw | Inonotus obliquus | Substrate–moisture ratio 2:1, inoculum 10% (v/w), pH 7.4, (NH4)2SO4 1%, MgSO4·7H2O 0.03%, KH2PO4 0.011%, Tween-80 0.4%, corn starch 10%, 15 d, 26 °C. | [17] |
| Corn straw; rice straw | White rot fungi (Phanerochaete chrysosporium) | Corn straw: 25 g + 15 mL distilled water (3% molasses, 0.1% urea, 10% fungus inoculum, 2 × 108 CFU/mL), 28 °C, 14 d. Rice straw: 25 g + 15 mL distilled water (3% glucose, 0.1% urea, 10% fungus inoculum, 2 × 108 CFU/mL), 28 °C, 14 d. | [18] |
| Corn stover; wheat straw | Candida utilis, Lactobacillus plantarum | Mixed inoculum (25% Candida utilis, 25% Lactobacillus plantarum, 25% cellulase, 25% laccase) at 0.3% (w/w), 25 ± 2 °C, 30 d. | [19] |
| Corn straw and peanut vine | Lactobacillus plantarum, Enterococcus faecalis | Substrate ratio (corn straw:peanut vine) = 3:1; inoculum (5 × 105 CFU/g Lactobacillus plantarum + 5 × 105 CFU/g Enterococcus faecalis), 25 °C, 60 d, 450 to 600 kg/m3. | [20] |
| Cotton residue | Paenibacillus sp. F4, Cohnella xylanilytica T5 | T5 group: 30 °C, 6 d, substrate-to-water ratio 1:0.6, 25% inoculum, 20% corn flour, 1.5% urea. F4 group: 33 °C, 6 days, substrate-to-water ratio 1:0.9, 25% inoculum, 20% corn flour, 1.5% urea addition. | [21] |
| Citrus pomace | Bacillus amyloli-quefaciens BF2, Candida utilis GIM 2.9 | Substrate: 80% citrus pomace + 20% corn gluten (w/w); inoculum: Bacillus amyloli-quefaciens BF2:Candida utilis GIM 2.9 = 1:1, 30 °C, 21% (v/w), 5 d. | [22] |
| Citrus pomace | Aspergillus niger | Substrate: citrus pomace:rice bran = 8:2 (mass), moisture 60%; inoculum: 2% (4.0 × 107 spores), 200 g/substrate flask, aerobic fermentation at 28–30 °C for 7 d. | [23] |
| Macadamia green peel | Homologous lactic acid bacterium strain ZC529 | 0.9% cellulase, 4% fermentation inoculant, anaerobic fermentation at (30 ± 2)°C for 7 d. | [24] |
| Tea residue | Lactiplantibacillus plantarum, Bacillus licheniformis | Substrate: tea residue:wheat bran = 7:3, inoculum: L. plantarum:B. licheniformis = 1:1, 34 °C, 5 d. | [25] |
| Chrysanthemum waste | Pediococcus cellaris, Candida tropicalis, Bacillus amyloliquefaciens | Substrate: chrysanthemum waste:cornmeal = 9:1; inoculum: Pediococcus cellaris:Candida tropicalis:Bacillus amyloliquefaciens = 2:2:1 (6% inoculum), 10 d, 29 ± 0.5 °C. | [26] |
| Peanut meal | Bacillus velezensis LB-Y-1, Pediococcus acidilactici LC-9-1 | Stage 1: Inoculate Bacillus velezensis LB-Y-1 (6.0 × 109 CFU/kg, moisture 37.0%), quasi-aerobic at 38 °C for 54 h (remix q4h). Stage 2: Inoculate Pediococcus acidilactici LC-9-1 (2.0 × 109 CFU/kg moisture 40.0%), quasi-anaerobic in PE bag at 37 °C for 18 h. | [27] |
| Palm kernel cake | Saccharomyces cerevisiae | Enzymolysis: feed–water ratio 1:5, β-mannanase 800 U/g, 55 °C, 72 h, initial pH 4.0. Fermentation: feed–water ratio 1:1.0, inoculation 0.7 × 108 cells/g, 30 °C, complex enzymes 4%, molasses 6%, ammonium sulfate 1%, 48 h. | [28] |
| Fresh sweet corn processing by-product | Lactobacillus plantarum LP1 | Substrate ratio: by-product:wheat bran = 9:1 (SWB), by-product:millet hull = 8:2 (SMH), 45 d, inoculum 5 × 106 CFU/g fresh weight, 20–25 °C. | [29] |
| Cauliflower wastes | Lactobacillus plantarum | 1 kg cauliflower wastes inoculated with 100 mL L. plantarum (6 × 106 cfu/kg), 35 °C, 30 d. | [30] |
| Corncob | Rhodotorula glutinis As2.703 | Pretreatment: 10 g corncob + 30 mL 1.5 wt% H2SO4, 130 °C/1 h; enzymatic hydrolysis: 10% (w/w) cellulase, 50 °C, 180 rpm, 72 h. Fermentation: 10% (v/v) inoculum in 2 L fermenter, 30 °C, pH 6.0, aeration 4 vvm, 400 rpm, 200 μL antifoams at 12 h. | [31] |
| Bagasse | Saccharomyces cerevisiae, Aspergillus niger, Aspergillus oryzae, Lactobacillus | Inoculum ratio (Saccharomyces cerevisiae:Aspergillus niger:Aspergillus oryzae:Lactobacillus = 2:1:1:1 (≥4 × 108 CFU/g, ≥2 × 108 CFU/g, ≥2 × 108 CFU/g, 2 × 108 CFU/g, respectively), 0.1% cellulase, 0.1% xylanase, 0.5% (g/g) urea (DM basis), aerobic followed by anaerobic fermentation, 20–30 °C, 96 h. | [32] |
| Wheat bran | Aspergillus niger, Trichoderma reesei | 1.0 g/L Aspergillus niger, 0.2 g/L Trichoderma reesei, 30 °C, pH 4.5; followed by hydrolysis at 50 °C. | [33] |
| Cottonseed shells | Aspergillus oryzae TM-1 | 20 g cottonseed hulls + 16 mL H2O (pH 7) in 500 mL flask, autoclaved (121 °C, 20 min), inoculated with 10% spore suspension (107 CFU/mL), 30 °C, 4 d | [34] |
| Honeysuckle residues | Lactic acid bacteria (LAB) inoculants: Pediococcus pentosaceus PC2-1(F2), Lentilactobacillus buchneri (formerly Lactobacillus buchneri) PC-C1 | Inoculants (1.0 × 109 CFU/kg fresh material, distilled water-diluted), anaerobic fermented indoors at room temperature for 14 d. | [35] |
| Lotus seedpod | Lactobacillus plantarum CAU-a214, CAV-M6 | Acid degradation: 5% edible vinegar 1:1 (w/v), 32 °C, 7 d. Fermentation: 1% corn flour, 1% molasses, 0.1% cellulase, 0.1% L. plantarum CAU-a214 and CAV-M6 (10 × 109 CFU/g), 10% distilled water, 32 °C, 21 d. | [36] |
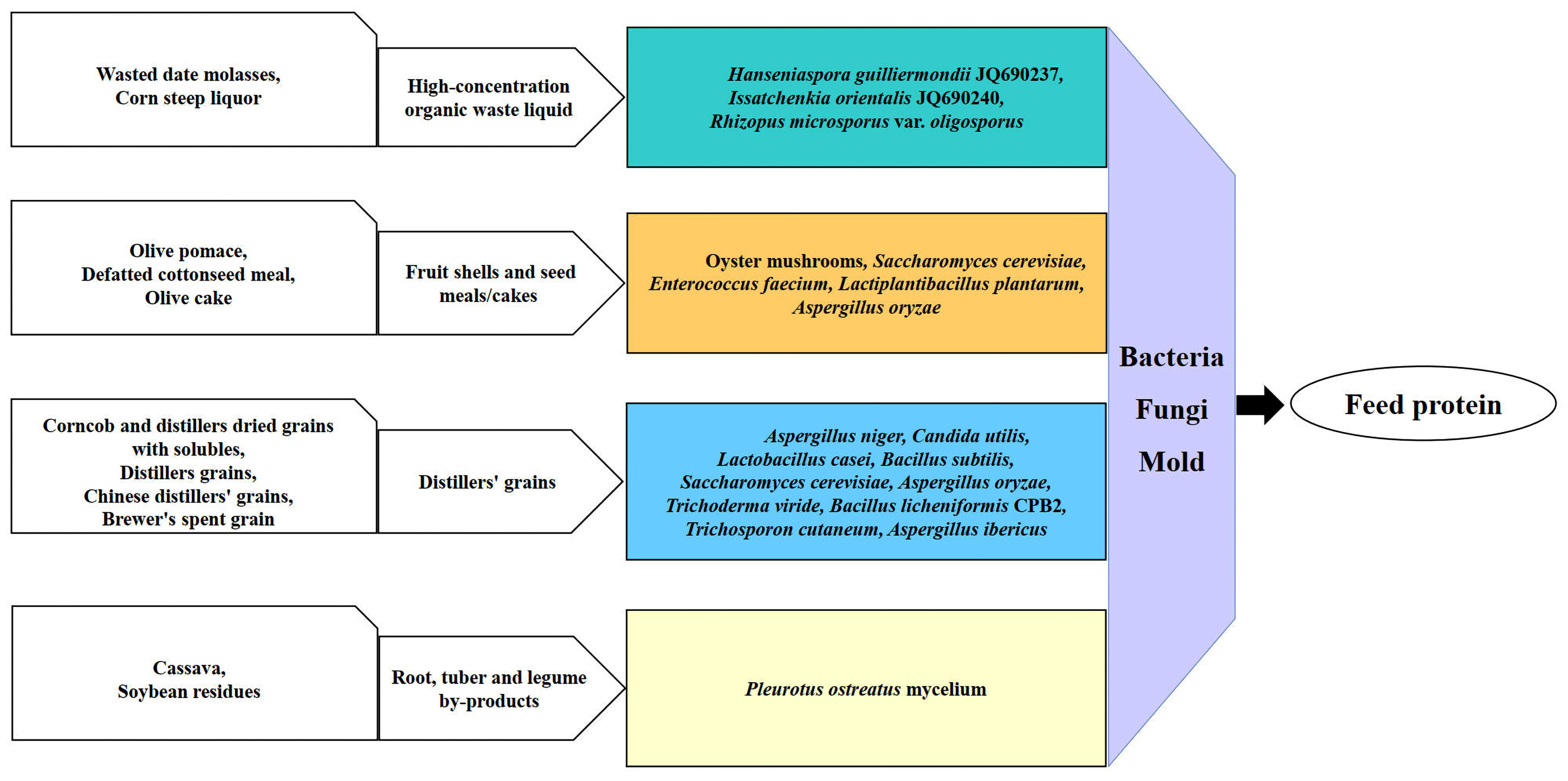
| Agri-Industrial By-Product | Fermentation Strain | Fermentation Conditions and Process | Ref. |
|---|---|---|---|
| Wasted date molasses | Hanseniaspora guilliermondii JQ690237, Issatchenkia orientalis JQ690240 | 100 mL 20% wasted date molasses solution, 5 mL inoculum (108 cell/mL), 25 °C, 150 rpm, 72 h. | [41] |
| Corn steep liquor | Rhizopus microsporus var. oligosporus | 20% (v/v) corn steep liquor dilution, 2 mL inoculum (McFarland scale tube #5), 25 °C, 0.5 vvm, 96 h. | [42] |
| Olive pomace | Oyster mushrooms | 50% inoculum (w/w), 20–25 °C, 35 d | [43] |
| Defatted cottonseed meal | Saccharomyces cerevisiae, Enterococcus faecium, Lactiplantibacillus plantarum | Inoculum: Saccharomyces cerevisiae/Enterococcus faecium/ Lactiplantibacillus plantarum (109 CFU/mL, ratio 1:0.5, v/m), 5 d, 28 °C. | [44] |
| Brewer’s spent grain | Bacillus licheniformis CPB2 | Brewer’s spent grain:soybean meal = 1:1, 12.50% inoculum, 50 °C, 24 h. | [45] |
| Distillers’ dried grains with solubles | Aspergillus niger | 10 g distillers’ dried grains with solubles + 10 g corncob + 40 g water, 10% inoculum (v/w, 107–108 CFU/mL), 30 °C, 7 d. | [46] |
| Distillers’ grains | Lactobacillus casei, Bacillus subtilis, Saccharomyces cerevisiae, Aspergillus oryzae | Inoculum: Aspergillus oryzae:Saccharomyces cerevisiae:Lactobacillus casei:Bacillus subtilis = 1:1:1:1 (10% of substrate), compound enzyme: 0.1% of substrate. Substrate: 45% distillers’ grains, 45% wheat bran, 5% corn, 3% soybean meal, 2% molasses; 31.8 °C, 7 d. | [47] |
| Chinese distillers’ grains | Candida utilis, Trichoderma viride, Bacillus subtilis, Lactobacillus casei | Inoculum: Candida utilis, Bacillus subtilis, Lactobacillus casei (equal volumes, OD600 = 2), Trichoderma viride (equal mycelia suspension), 12% inoculum, 34 °C, 12 d. | [48] |
| Brewer’s spent grain | Trichosporon cutaneum | Ammoniation pretreatment: 11% ammonia dosage (w/w), 63 °C, 26 h. Enzymatic hydrolysis: 10% solid loading (w/v), pH 4.8, 50 °C, 150 rpm; fermentation: 1 mL seed (OD600 = 2) in 20 mL hydrolysate, 30 °C, 200 rpm. | [49] |
| Olive cake | Aspergillus oryzae | Beef extract medium, pH 6, 3% inoculum, 28 °C incubation for 14 d. | [50] |
| Brewer’s spent grain | Aspergillus ibericus | Inoculated with spore suspension (2 × 106 spores/g dry BSG), aerobic fermentation at 25 °C for 7 d. | [51] |
| Corncob and distillers’ dried grains with solubles | Aspergillus niger, Candida utilis | Distillers’ dried grains with solubles:corncob = 8:1, 15.0% inoculum (v/w), 30 °C, material-to-water ratio 0.4, Aspergillus niger:Candida utilis = 1.2:1 (v/v), 10.5 d. | [52] |
| Cassava; soybean residues | Pleurotus ostreatus mycelium | 100 g substrate (80% cassava + 20% soybean residues), 5 mL colony solution, 25–28 °C, 9 d (colony covers plate, 120 mm). | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Cui, C. Fermentation of Organic Wastes for Feed Protein Production: Focus on Agricultural Residues and Industrial By-Products Tied to Agriculture. Fermentation 2025, 11, 528. https://doi.org/10.3390/fermentation11090528
He D, Cui C. Fermentation of Organic Wastes for Feed Protein Production: Focus on Agricultural Residues and Industrial By-Products Tied to Agriculture. Fermentation. 2025; 11(9):528. https://doi.org/10.3390/fermentation11090528
Chicago/Turabian StyleHe, Dan, and Can Cui. 2025. "Fermentation of Organic Wastes for Feed Protein Production: Focus on Agricultural Residues and Industrial By-Products Tied to Agriculture" Fermentation 11, no. 9: 528. https://doi.org/10.3390/fermentation11090528
APA StyleHe, D., & Cui, C. (2025). Fermentation of Organic Wastes for Feed Protein Production: Focus on Agricultural Residues and Industrial By-Products Tied to Agriculture. Fermentation, 11(9), 528. https://doi.org/10.3390/fermentation11090528







