Biotransformation of Acetaminophen by Ganoderma parvulum Ligninolytic Enzymes Immobilized on Chitosan Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cultivation of Ganoderma parvulum for Laccase Production
2.3. Partial Purification and Concentration of Fungal Enzymes
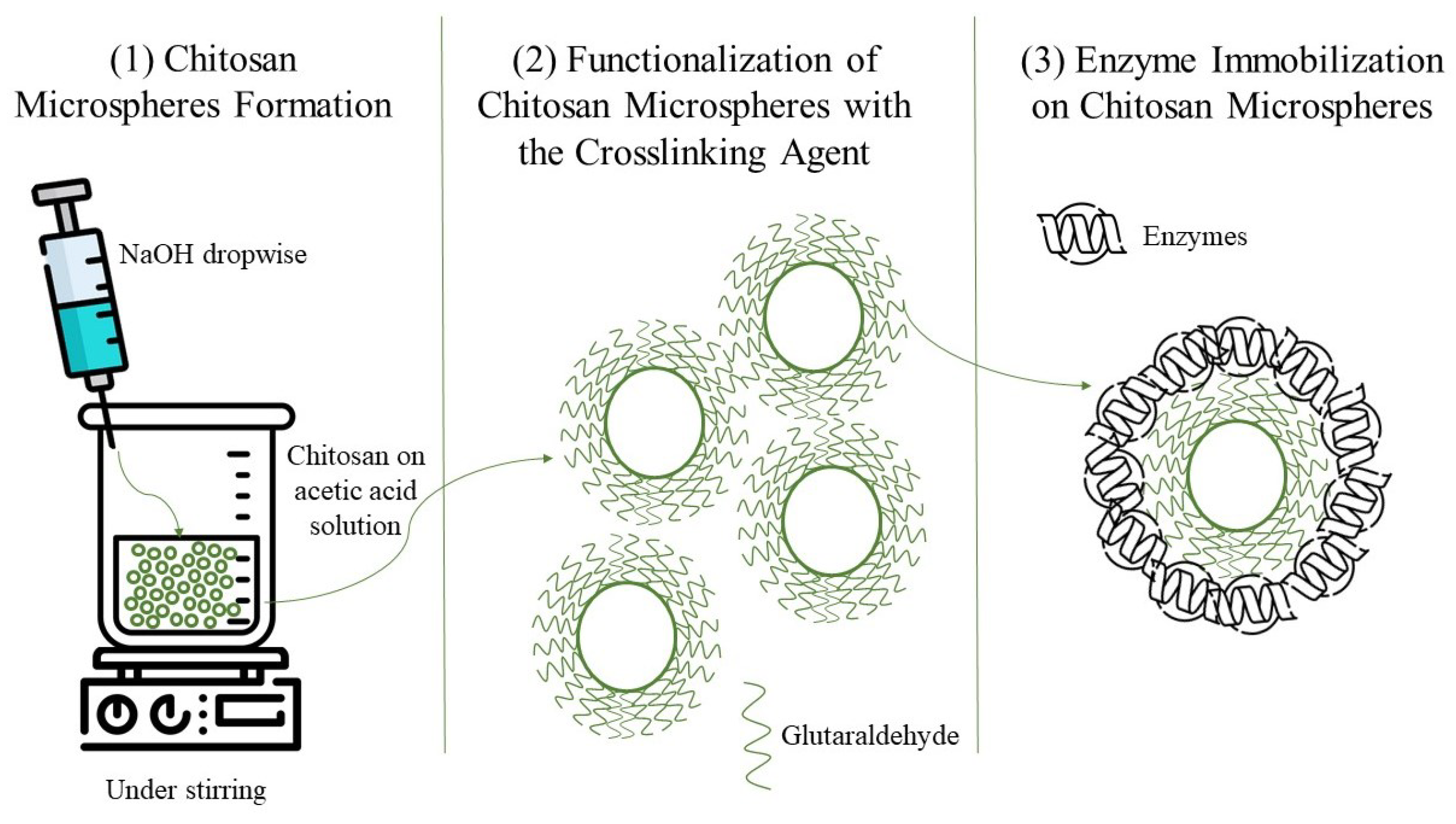
2.4. Covalent Immobilization of Enzymes on Chitosan Microspheres
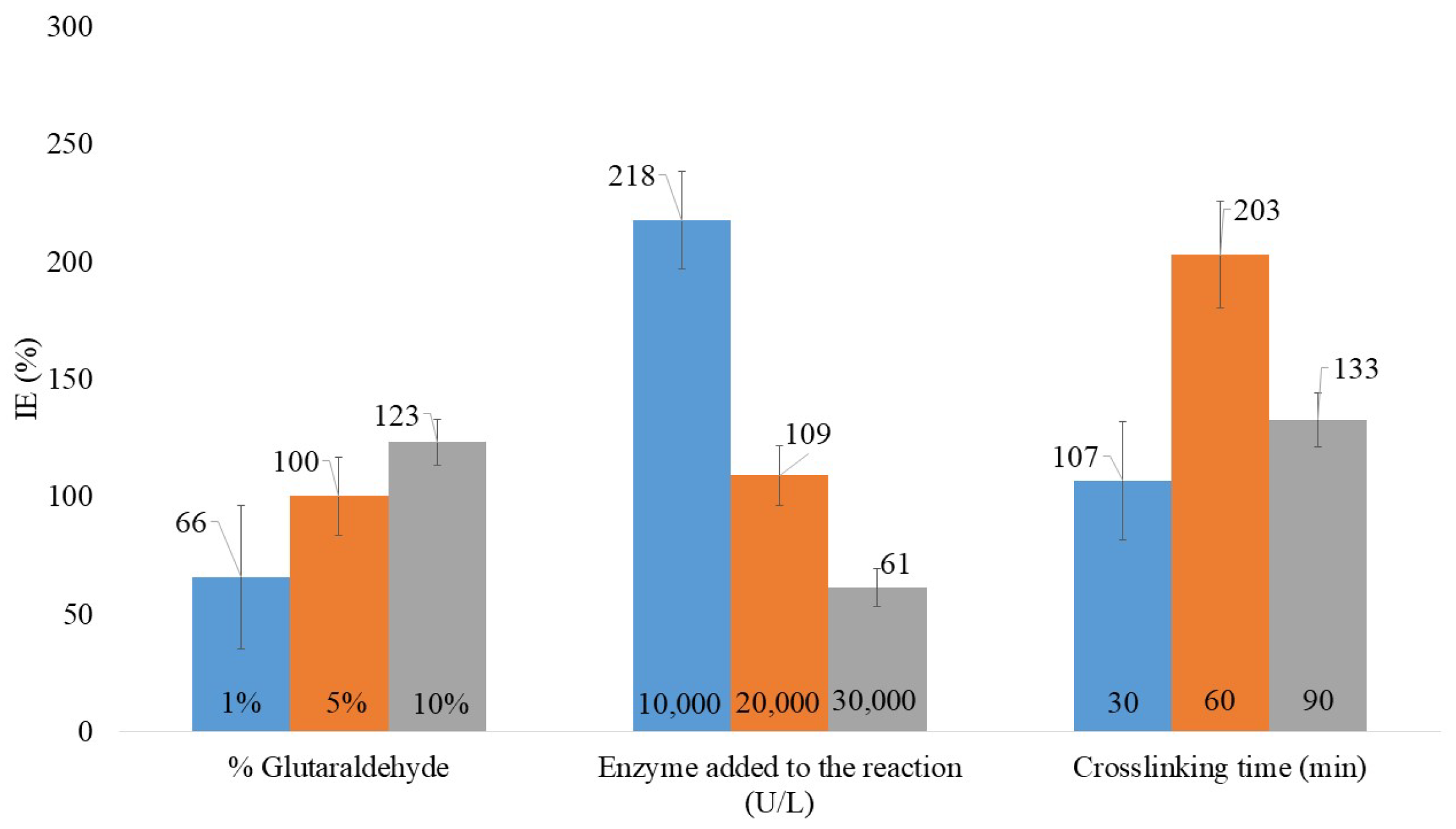
2.5. Evaluation of Laccase Immobilization Efficiency Using a One-Factor-per-Time Approach
2.6. pH and Temperature Stability of Free and Immobilized Laccase
2.7. Acetaminophen Degradation by Ligninolytic Immobilized Enzymes
2.7.1. Acetaminophen Degradation Using Immobilized Laccase
2.7.2. Reusability of Immobilized Laccase for Acetaminophen Transformation
2.8. Analytical Methods
2.8.1. Laccase Activity Determination
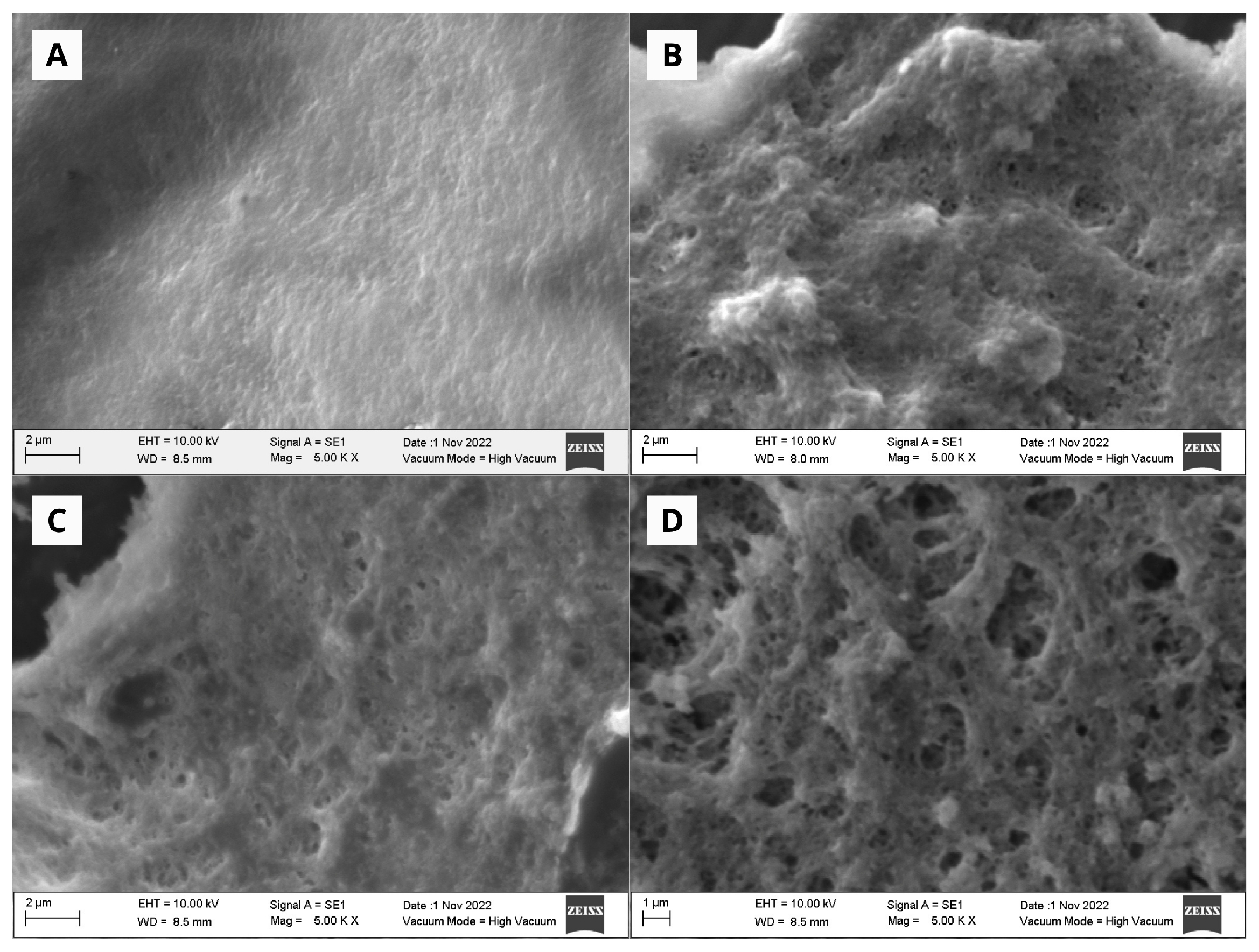
2.8.2. SEM Analysis of Chitosan Microspheres
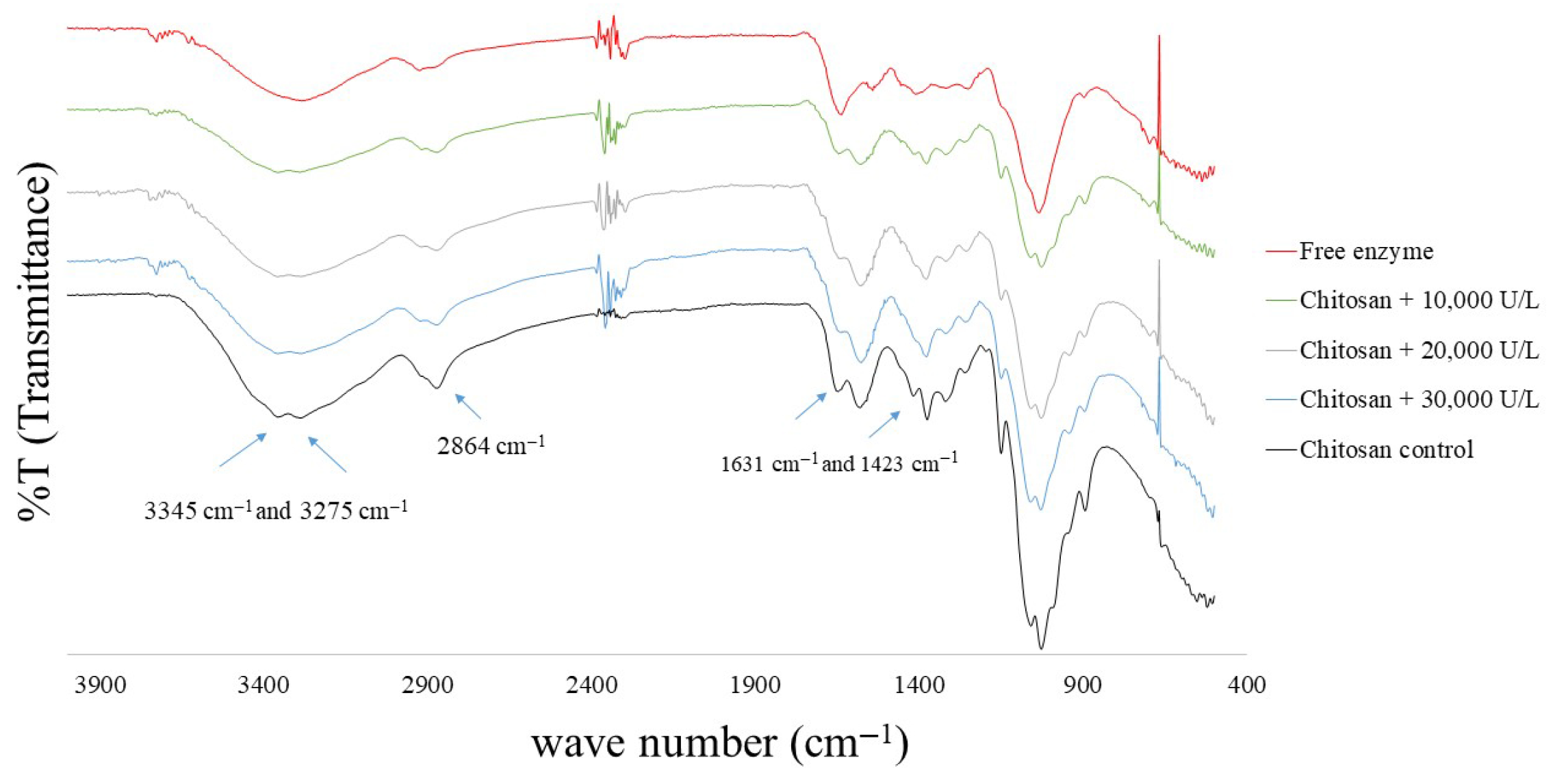
2.8.3. FT-IR Analysis of Chitosan Microspheres
2.8.4. Quantification of Acetaminophen
2.8.5. NMR-Based Analysis of Acetaminophen Degradation by Immobilized Enzymes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Laccase Immobilization Efficiency Using a One-Factor-per-Time Approach
3.2. Chitosan Microsphere Characterization
3.2.1. SEM Analysis
3.2.2. FT-IR Analysis of Chitosan Microspheres with Immobilized Enzymes
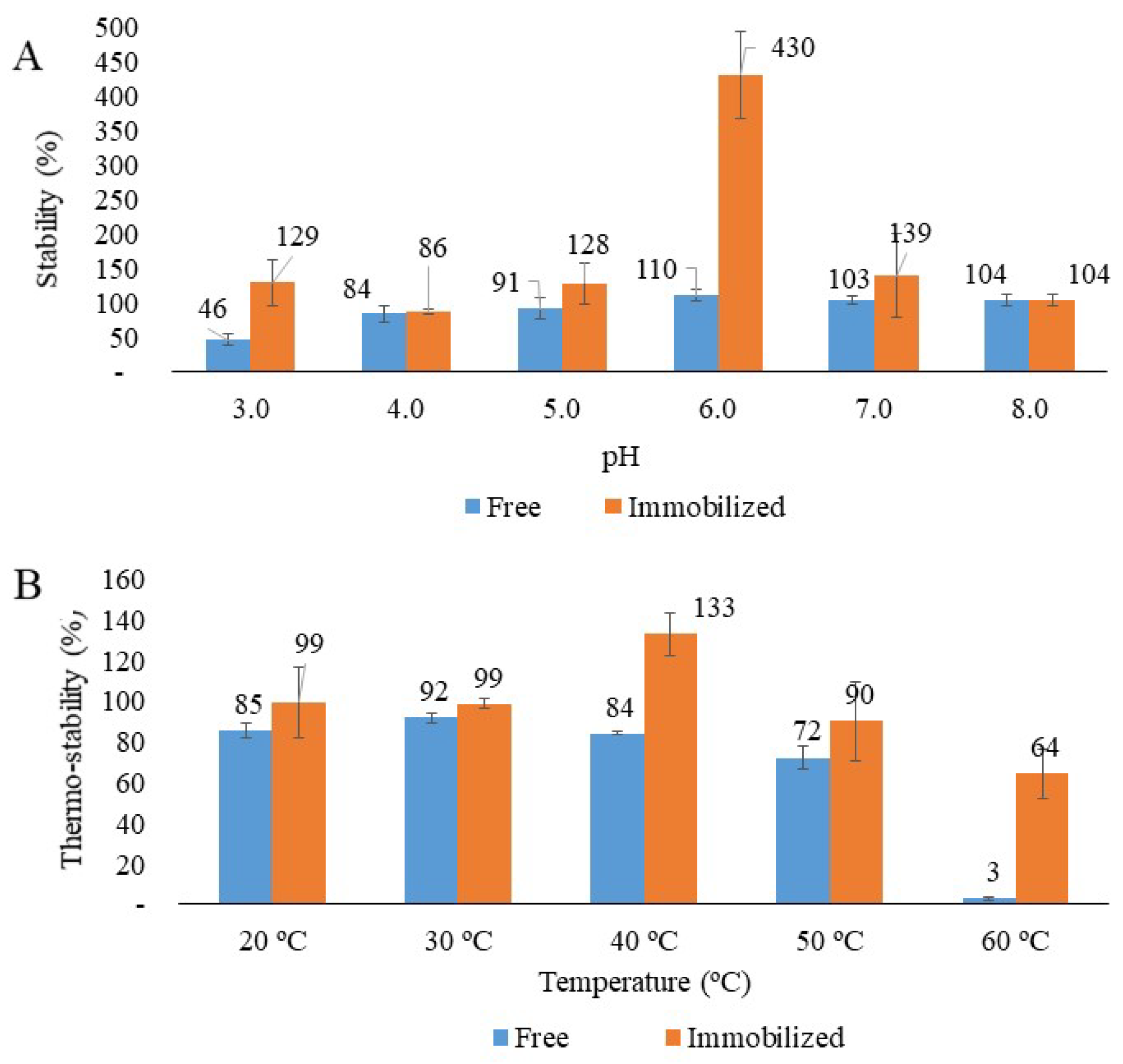
3.3. Stability Assessments of Free and Immobilized Laccase
3.3.1. pH Stability
3.3.2. Temperature Stability
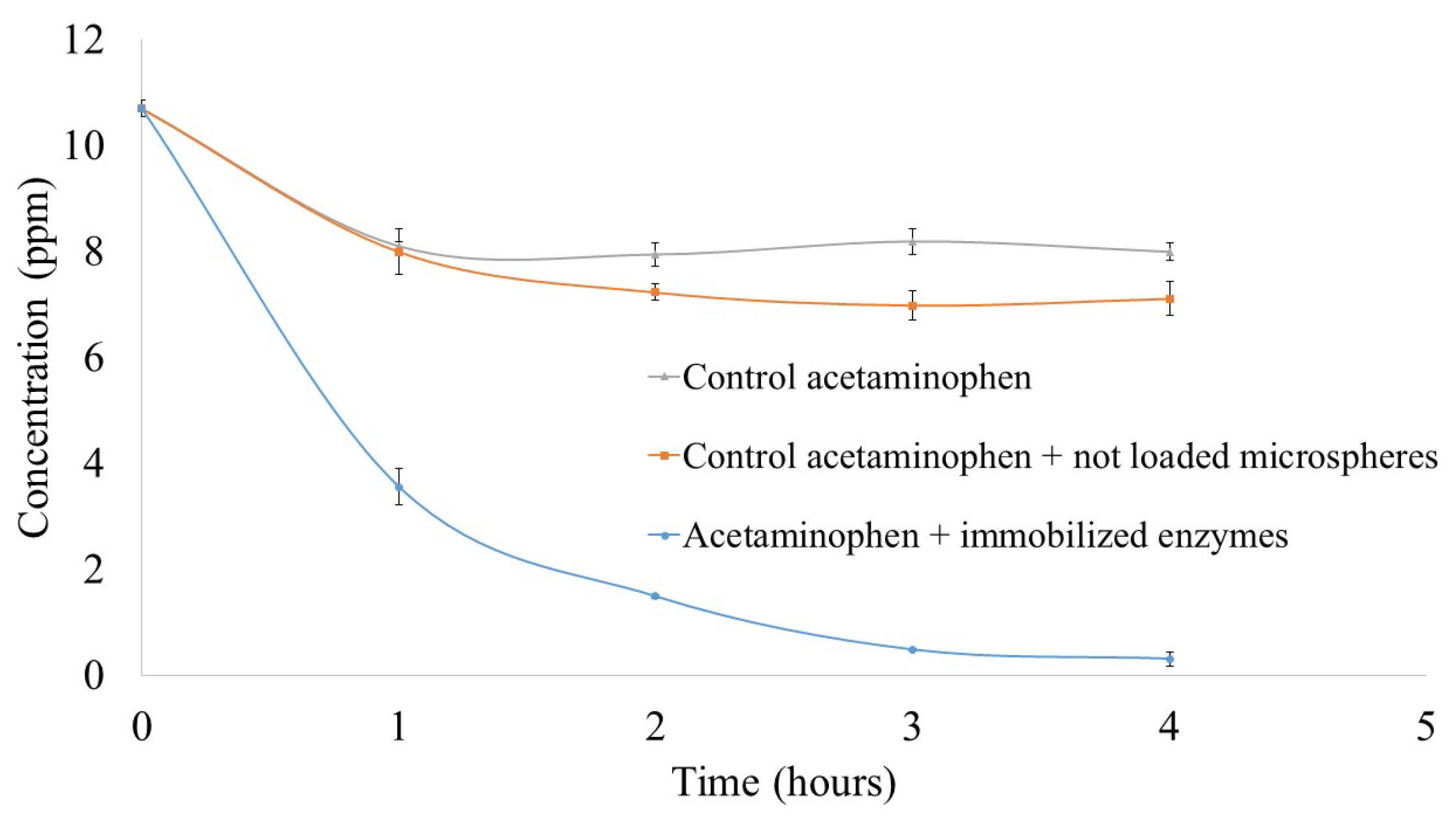
3.4. Acetaminophen Degradation by Ligninolytic Enzymes Immobilized System
3.4.1. Enzymatic Treatment of Acetaminophen
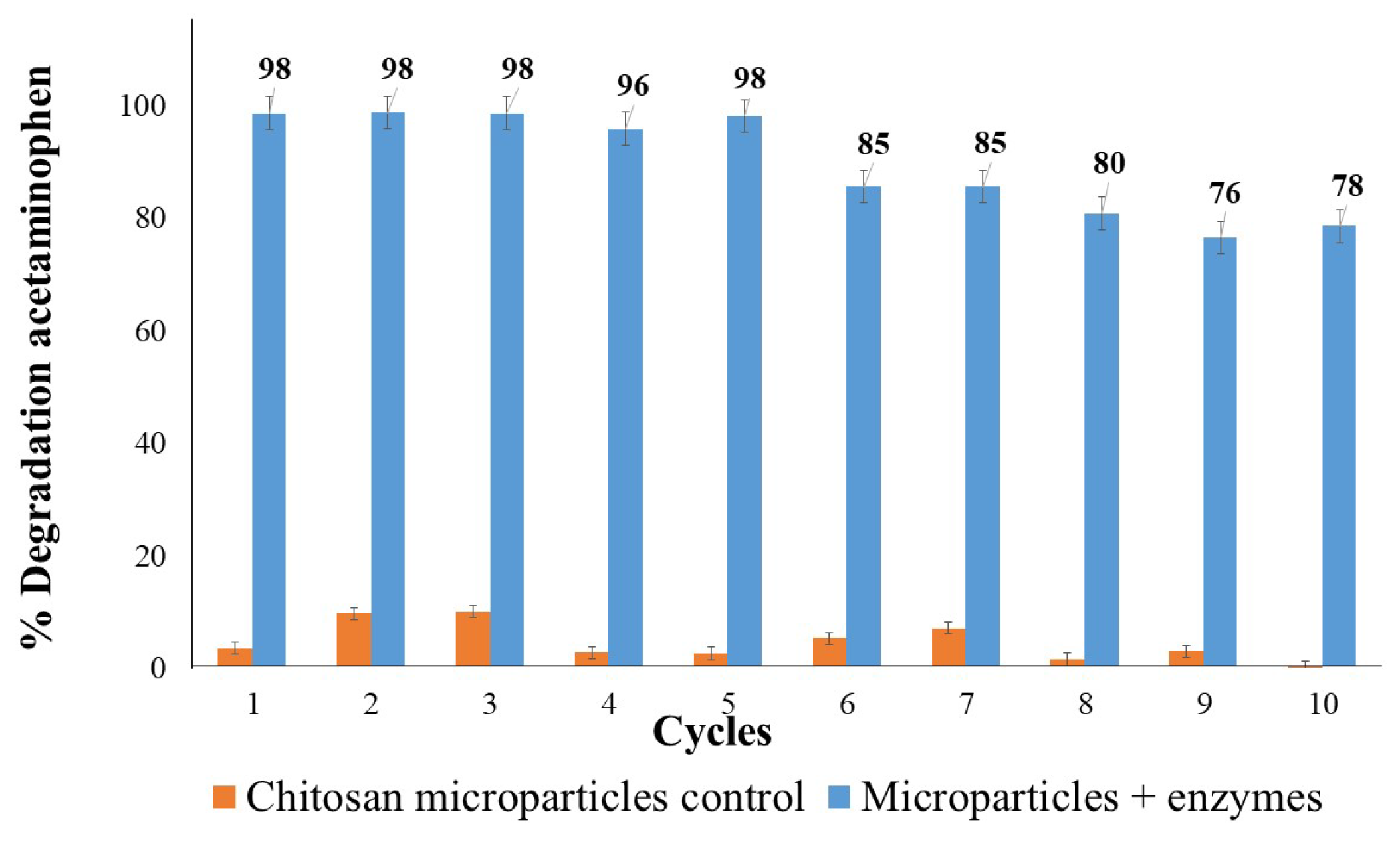
3.4.2. Repeated Use of Immobilized Enzymes in Biotransformation of Acetaminophen
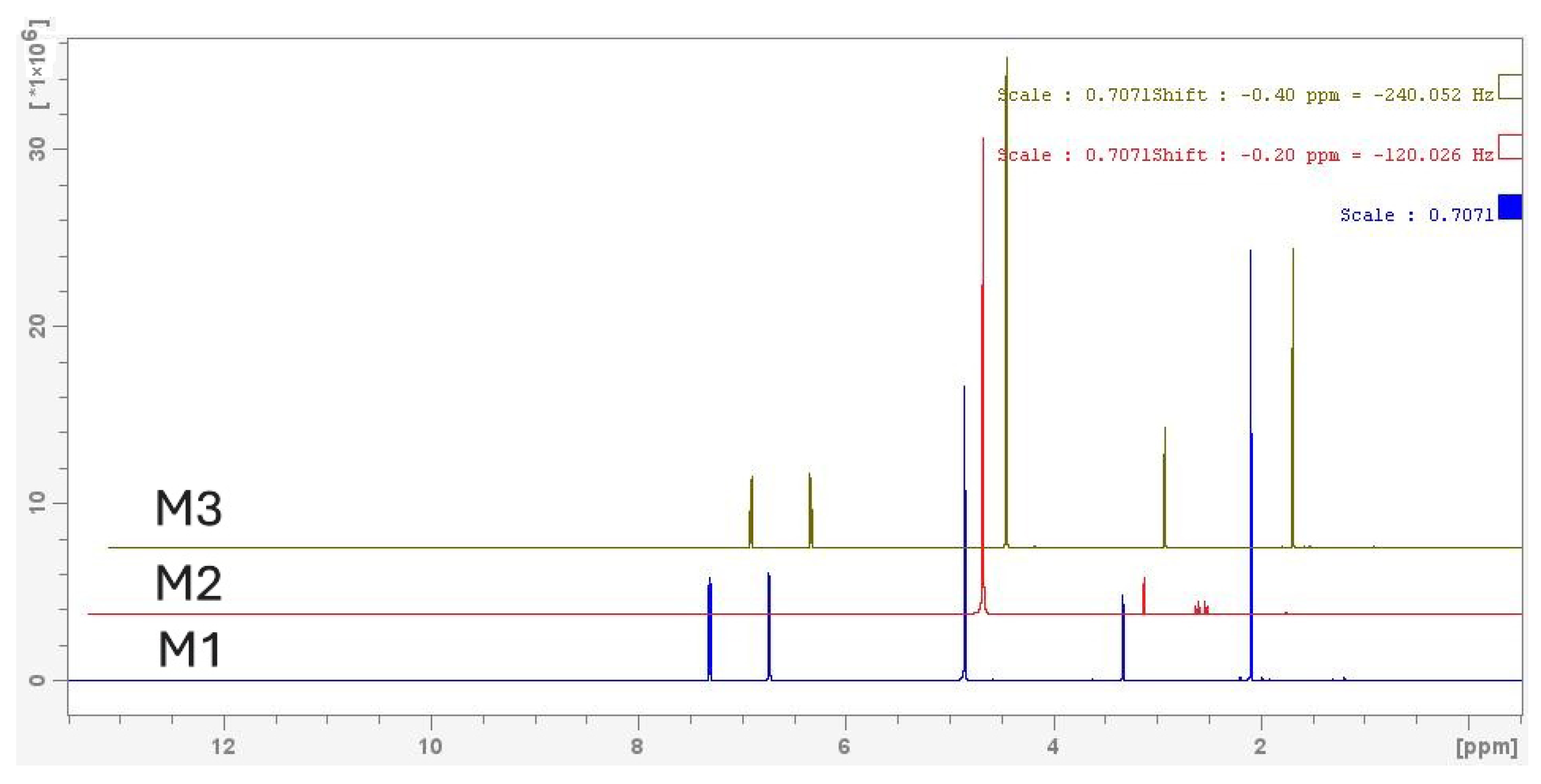
3.4.3. 1H-NMR Analysis of Acetaminophen Biotransformation
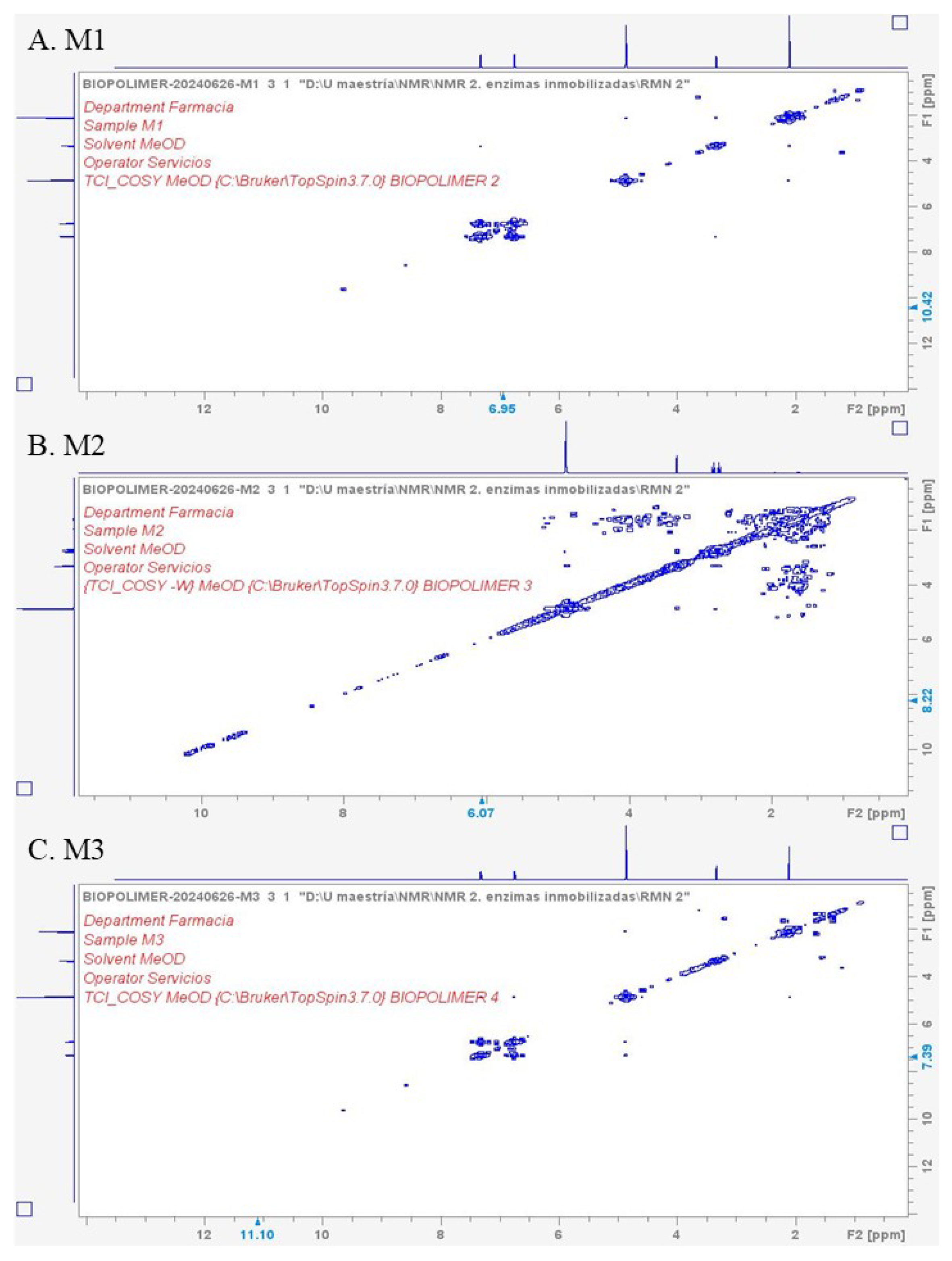
3.4.4. COSY-NMR Analysis of Acetaminophen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. The Sustainable Development Goals Report 2023: Special Edition. 2023. Available online: https://unstats.un.org/sdgs/report/2023/ (accessed on 13 March 2025).
- Hanafiah, Z.M.; Mohtar, W.H.M.W.; El Messaoudi, N.; Miyah, Y.; Maged, A.; Knani, S.; Graba, B.; Gafforov, Y.; Wan-Mohtar, W.A.A.Q.I. Wastewater treatment using Ganoderma Species via Ganoremediation Bioreactor: A Comprehensive Review. Bioresour. Technol. Rep. 2025, 30, 102105. [Google Scholar] [CrossRef]
- Parra Guardado, A.L. Enzymatic Degradation of Recalcitrant Pharmaceutical Micropollutants. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2019. [Google Scholar]
- Barrios-Estrada, C.; de Jesús Rostro-Alanis, M.; Muñoz-Gutiérrez, B.D.; Iqbal, H.M.; Kannan, S.; Parra-Saldívar, R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation—A review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Restrepo, M.A.; López-Legarda, X.; Segura-Sánchez, F. Bioremediation of emerging pharmaceutical pollutants acetaminophen and ibuprofen by white-rot fungi—A review. Sci. Total Environ. 2025, 977, 179379. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Nyaruaba, R.; Ita, R.E.; Okon, S.U.; Addey, C.I.; Ebido, C.C.; Opabunmi, A.O.; Okeke, E.S.; Chukwudozie, K.I. Antibiotic resistance in the aquatic environment: Analytical techniques and interactive impact of emerging contaminants. Environ. Toxicol. Pharmacol. 2022, 96, 103995. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Ratanapongleka, K.; Punbut, S. Removal of acetaminophen in water by laccase immobilized in barium alginate. Environ. Technol. 2017, 39, 336–345. [Google Scholar] [CrossRef]
- UNESCO. Contaminantes Emergentes en la Reutilización de Aguas Residuales en los Países en Desarrollo. 2015. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000235241_spa (accessed on 4 November 2024).
- Chu-Wen, Y.; Yi-En, C.; Bea Ven, C. Microbial Communities Associated with Acetaminophen Biodegradation from Mangrove Sediment. Sustainability 2020, 12, 5410. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen—Toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. Int. 2018, 25, 21498–21524. [Google Scholar] [CrossRef]
- Poddar, K.; Sarkar, D.; Chakraborty, D.; Patil, P.B.; Maity, S.; Sarkar, A. Paracetamol biodegradation by Pseudomonas Strain PrS10 isolated from pharmaceutical effluents. Int. Biodeterior. Biodegrad. 2022, 175, 105490. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Aptitude of oxidative enzymes for treatment of wastewater pollutants: A laccase perspective. Molecules 2019, 24, 2064. [Google Scholar] [CrossRef] [PubMed]
- Gugel, I.; Summa, D.; Costa, S.; Manfredini, S.; Vertuani, S.; Marchetti, F.; Tamburini, E. Mycoremediation of Synthetic Azo Dyes by White-Rot Fungi Grown on Diary Waste: A Step toward Sustainable and Circular Bioeconomy. Fermentation 2024, 10, 80. [Google Scholar] [CrossRef]
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of ligninolytic enzymes from white-rot fungi in cross-linked aggregates. Chemosphere 2018, 202, 694–707. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.A.; Baccar, R.; Caminal, G.; Sarrà, M. Can white-rot fungi be a real wastewater treatment alternative for organic micropollutants removal? A review. Water Res. 2018, 138, 137–151. [Google Scholar] [CrossRef]
- Sun, X.; Lin, X.; Xian, Y.; Zhang, F.; Zhu, L.; Geng, H.; Wang, W.; Zhang, G. Engineering Bacterial Laccase with Improved Catalytic Activity and Thermostability by Rational Design. Appl. Biochem. Biotechnol. 2025, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Geng, A. Construction of CRISPR-Cas9 genome editing platform for white-rot fungus Cerrena Unicolor BBP6 Its effects on extracellular ligninolytic enzyme biosynthesis. Biochem. Eng. J. 2022, 185, 108527. [Google Scholar] [CrossRef]
- GlobeNewswire. Enzymes Global Market Report 2023; GlobeNewswire: Los Angeles, CA, USA, 2023. [Google Scholar]
- Bagewadi, Z.K.; Mulla, S.I.; Ninnekar, H.Z. Purification and immobilization of laccase from Trichoderma Harzianum Strain HZN10 Its Appl. Dye Decolorization. J. Genet. Eng. Biotechnol. 2017, 15, 139–150. [Google Scholar] [CrossRef]
- Gao, W.W.; Zhang, F.X.; Zhang, G.X.; Zhou, C.H. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Geor Malar, C.; Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on surface modification of nanocarriers to overcome diffusion limitations: An enzyme immobilization aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar] [CrossRef]
- López-Legarda, X.; Arboleda-Echavarría, C.; Segura-Sanchez, F. Producción de polisacáridos a partir de Ganoderma Sp., Aisl. En La Región Andin. Rev. Colomb. Biotecnol. 2015, 17, 44–54. [Google Scholar] [CrossRef]
- López-Legarda, X. Producción, Caracterización y Actividad Biológica de Polisacáridos Fúngicos. Doctoral Thesis, Universidad de Antioquia, Medellín, Colombia, 2021. [Google Scholar]
- Kirk, T.K.; Croan, S.; Tien, M.; Murtagh, K.E.; Farrell, R.L. Production of multiple ligninases by Phanerochaete Chrysosporium: Effect of selected growth conditions and use of a mutant strain. Enzym. Microb. Technol. 1986, 8, 27–32. [Google Scholar] [CrossRef]
- Torrez Monroy, G.P. Producción y Purificación de Enzimas Celulolíticas, Lacasas y Manganeso Peroxidasas de Tres Cepas Fúngicas Nativas (IB-105,GL-9B Y co-1). Ph.D. Thesis, Universidad Mayor de San Andres, La Paz, Bolivia, 2015. [Google Scholar]
- Howlader, M.M.; Niroda, A.; Bai, R.G.; Premarathna, A.D.; Tuvikene, R. Fermentation optimization, purification and biochemical characterization of a porphyran degrading enzyme with funoran side-activity from Zobellia uliginosa. Biocatal. Agric. Biotechnol. 2022, 43, 102394. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, S.; Kim, D.; Nguyen, T.V.T.; Nam, Y.; Kim, S.M.; Chang, R.; Kim, Y.H. Enhancing the thermostability of lignin peroxidase: Heme as a keystone cofactor driving stability changes in heme enzymes. Heliyon 2024, 10, e37235. [Google Scholar] [CrossRef] [PubMed]
- Rajeeva, S.; Lele, S.S. Bioprocessing of laccase produced by submerged culture of Ganoderma sp. WR-1. Sep. Purif. Technol. 2010, 76, 110–119. [Google Scholar] [CrossRef]
- Aslam, S.; Asgher, M.; Khan, N.A.; Bilal, M. Immobilization of Pleurotus Nebrodensis WC 850 Laccase Glutaraldehyde Cross-Linked Chitosan Beads for Enhanced Biocatalytic Degradation of Textile Dyes. J. Water Process Eng. 2021, 40, 101971. [Google Scholar] [CrossRef]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Flaticon. Iconos Vectoriales y Stickers—PNG, SVG, EPS, PSD y CSS—Designed by Freepik, Wanicon and Vector Squad from Flaticon. 2025. Available online: https://www.flaticon.com/ (accessed on 18 June 2025).
- Suresh, G.; R, R.; Johney, J. Optimization of laccase production by Pleurotus pulmonarius through solid substrate fermentation of tender coconut fiber: Enhanced laccase production and biomass delignification. Biomass Convers. Biorefinery 2024, 15, 13769–13781. [Google Scholar] [CrossRef]
- Jeong, D.; Choi, K.Y. Biodegradation of Tetracycline Antibiotic by Laccase Biocatalyst Immobilized on Chitosan-Tripolyphosphate Beads. Appl. Biochem. Microbiol. 2020, 56, 306–312. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci. Total Environ. 2017, 584–585, 393–401. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Miao, S.; Suo, H.; Xu, H.; Hu, Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard. Mater. 2021, 401, 123353. [Google Scholar] [CrossRef] [PubMed]
- Salami, F.; Habibi, Z.; Yousefi, M.; Mohammadi, M. Covalent immobilization of laccase by one pot three component reaction and its application in the decolorization of textile dyes. Int. J. Biol. Macromol. 2018, 120, 144–151. [Google Scholar] [CrossRef]
- Arboleda Echavarría, C.; Mejía Gallón, A.I.; Franco Molano, A.E.; Jiménez Tobón, G.A.; Penninckx, M. Autochthonous white rot fungi from the tropical forest of Colombia for dye decolourisation and ligninolytic enzymes production. SYDOWIA Int. J. Mycol. 2008, 60, 165–180. [Google Scholar]
- SDBS Spectral Database for Organic Compounds. SDBS-1H NMR SDBS No.: 3290 Spectral Code: HSP-43-745. 1999. Available online: https://sdbs.db.aist.go.jp/SearchInformation.aspx (accessed on 30 March 2025).
- Bilal, M.; Asgher, M.; Iqbal, M.; Hu, H.; Zhang, X. Chitosan beads immobilized manganese peroxidase catalytic potential for detoxification and decolorization of textile effluent. Int. J. Biol. Macromol. 2016, 89, 181–189. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2018, 37, 790–802. [Google Scholar] [CrossRef]
- Wahba, M.I. Chitosan-glutaraldehyde activated calcium pectinate beads as a covalent immobilization support. Biocatal. Agric. Biotechnol. 2017, 12, 266–274. [Google Scholar] [CrossRef]
- Zheng, F.; Cui, B.K.; Wu, X.J.; Meng, G.; Liu, H.X.; Si, J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodeterior. Biodegrad. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Mehandia, S.; Ahmad, S.; Sharma, S.C.; Arya, S.K. Decolorization and detoxification of textile effluent by immobilized laccase-ACS into chitosan-clay composite beads using a packed bed reactor system: An ecofriendly approach. J. Water Process Eng. 2022, 47, 102662. [Google Scholar] [CrossRef]
- Alvarado Ramírez, L. Inmovilización de Lacasas en Esferas de SiO2 para la Degradación de Rojo Congo. Master’s Thesis, Universidad Autonoma de Nuevo León, Monterrey, Mexico, 2015. [Google Scholar]
- Aricov, L.; Leonties, A.R.; Gîfu, I.C.; Preda, D.; Raducan, A.; Anghel, D.F. Enhancement of laccase immobilization onto wet chitosan microspheres using an iterative protocol and its potential to remove micropollutants. J. Environ. Manag. 2020, 276, 111326. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Fu, C.C.; Juang, R.S. Effective removal of sulfur dyes from water by biosorption and subsequent immobilized laccase degradation on crosslinked chitosan beads. Chem. Eng. J. 2016, 304, 313–324. [Google Scholar] [CrossRef]
- Cen, Q.; Wu, X.; Cao, L.; Lu, Y.; Lu, X.; Chen, J.; Fu, G.; Liu, Y.; Ruan, R. Green production of a yellow laccase by Coriolopsis gallica for phenolic pollutants removal. AMB Express 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Hoinacki Da Silva, C.K.; Polidoro, A.S.; Cabrera Ruschel, P.M.; Thue, P.S.; Jacques, R.A.; Lima, É.C.; Bussamara, R.; Fernandes, A.N. Laccase covalently immobilized on avocado seed biochar: A high-performance biocatalyst for acetaminophen sorption and biotransformation. J. Environ. Chem. Eng. 2022, 10, 107731. [Google Scholar] [CrossRef]
- Merk. NMR Chemical Shifts of Impurities. Available online: https://www.sigmaaldrich.cn/CN/zh/technical-documents/technical-article/analytical-chemistry/nuclear-magnetic-resonance/1h-nmr-and-13c-nmr-chemical-shifts-of-impurities-chart?srsltid=AfmBOops5TheGGQxgV-8_FMKCJU7lkAMAiTJJ2fXmRrUg1Qr7Kc-5aHE (accessed on 6 January 2025).
- Schmiemann, D.; Hohenschon, L.; Bartels, I.; Hermsen, A.; Bachmann, F.; Cordes, A.; Jäger, M.; Gutmann, J.S.; Hoffmann-Jacobsen, K. Enzymatic post-treatment of ozonation: Laccase-mediated removal of the by-products of acetaminophen ozonation. Environ. Sci. Pollut. Res. 2023, 30, 53128–53139. [Google Scholar] [CrossRef] [PubMed]
- Qutob, M.; Hussein, M.A.; Alamry, K.A.; Rafatullah, M. A review on the degradation of acetaminophen by advanced oxidation process: Pathway, by-products, biotoxicity, and density functional theory calculation. RSC Adv. 2022, 12, 18373–18396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flórez-Restrepo, M.A.; López-Legarda, X.; Rostro-Alanis, M.d.J.; Parra-Saldívar, R.; Segura-Sánchez, F. Biotransformation of Acetaminophen by Ganoderma parvulum Ligninolytic Enzymes Immobilized on Chitosan Microspheres. Fermentation 2025, 11, 387. https://doi.org/10.3390/fermentation11070387
Flórez-Restrepo MA, López-Legarda X, Rostro-Alanis MdJ, Parra-Saldívar R, Segura-Sánchez F. Biotransformation of Acetaminophen by Ganoderma parvulum Ligninolytic Enzymes Immobilized on Chitosan Microspheres. Fermentation. 2025; 11(7):387. https://doi.org/10.3390/fermentation11070387
Chicago/Turabian StyleFlórez-Restrepo, María Alejandra, Xiomara López-Legarda, Magdalena de Jesús Rostro-Alanis, Roberto Parra-Saldívar, and Freimar Segura-Sánchez. 2025. "Biotransformation of Acetaminophen by Ganoderma parvulum Ligninolytic Enzymes Immobilized on Chitosan Microspheres" Fermentation 11, no. 7: 387. https://doi.org/10.3390/fermentation11070387
APA StyleFlórez-Restrepo, M. A., López-Legarda, X., Rostro-Alanis, M. d. J., Parra-Saldívar, R., & Segura-Sánchez, F. (2025). Biotransformation of Acetaminophen by Ganoderma parvulum Ligninolytic Enzymes Immobilized on Chitosan Microspheres. Fermentation, 11(7), 387. https://doi.org/10.3390/fermentation11070387





