Abstract
The continuous usage years of mud pits significantly impact the formation of volatile flavor compounds (VFCs) in Wuliangye Baijiu fermentation, yet their role in regulating initial microbial diversity and VFCs during fermentation remains unclear. This research analyzed the dynamic relationships among microorganisms, physicochemical characteristics, and VFCs from two different aged mud pits (continuous usage for 20 years: JC20 and 40 years: JC40). As fermentation advanced, VFCs increased, with JC40 showing a greater increment than JC20. Furthermore, the predominated microorganisms during fermentation of two pits were significantly different, resulting in distinct microbial successions. And the microbial diversity in JC40 was higher than JC20 at the fermentation anaphase from different layers of zaopei samples. Microbes were identified that highly correlated with physicochemical properties and VFCs, and the important microbial species differed between the two pits, proving the importance of mud pit continuous usage years for Wuliangye Baijiu production.
1. Introduction
As the leading enterprise, Wuliangye Baijiu ranks among the most favored Baijiu varieties in China with sales reaching CNY 89.1 billion in 2024. Renowned for its distinctive fermentation process and characteristic flavor profiles [1,2], it enjoys widespread consumption. The most notable feature of Wuliangye Baijiu lies in its fermentation within a specialized mud pit (also called JiaoChi), which created an ideal habitat for microbial proliferation [3]. The Fresh zaopei, meticulously crafted from a blend of sorghum, rice, corn, glutinous rice, wheat, rice husks, and baobaoqu powder, undergoes a 70-day fermentation within the sealed pit, a crucial process for developing its signature flavor compounds (Figure 1) [4,5]. Based on production experience, the higher-quality Wuliangye Baijiu is typically distilled from the down level zaopei from older pits. This observation underscores the fact that the continuous usage duration of mud pits has a substantial impact on the fermentation of zaopei at different layers [6,7,8,9]. It is commonly accepted that the longer the mud pit has been in use, the better the quality of Wuliangye Baijiu will be [10,11]. Consequently, it is essential to elucidate the relationship between the quality of Wuliangye Baijiu and the continuous used years of mud pit.
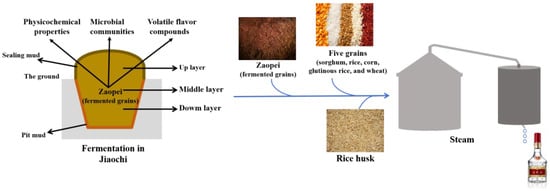
Figure 1.
A brief schematic diagram of Wuliangye fermentation.
As previous research showed, older pits harbor a diverse consortium of functional microorganisms, including Clostridium, Caproiciproducens, Methanobrevibacter, Aspergillus, and Candida, while Lactobacillus dominates in younger mud pits [10,12,13]. These microbes actively participate in zaopei fermentation, shaping the composition of bacterial and fungal communities in complex ways [3,14,15].
A further study reported that Monascus and Trichosporon represent the exclusive dominant functional fungal species in the older mud pits. Moreover, eight core functional microbial genera have been identified, which are associated with the changes in volatile metabolites during the fermentation process [6]. A comprehensive body of research has delved into the intricate microbial diversity during strong flavor baijiu fermentation, as evidenced by the studies [16,17,18]. Notably, Bacilli emerge as the predominant microbial community during the early cultivation phase. Their relative abundance steadily escalates from approximately 65% to around 95% over the process of fermentation, trailed by Bacteroidetes and Clostridium. At the genus level, Lactobacillus exerts significant dominance, constituting 60–90% of the total bacterial population [3,19]. Another study has identified three bacterial communities—Bacillus, Lactobacillus, and Acetobacter—and six fungal genera—Issatchenkia, Pichia, Candida, Aspergillus, Monascus, and Rhizopus—as the key microbial components in zaopei [20]. Indeed, starch and other macromolecules are degraded and utilized with the help of microorganisms during fermentation, resulting in the accumulation of more than 3000 compounds in zaopei. However, the dynamic succession process during the fermentation of Wuliangye between compounds and microorganisms, as well as between microorganisms, still remains unclear.
So far, numerous studies have explored the VFCs in strong flavor baijiu. Analytical techniques such as gas chromatography/olfactometry have been employed to identify key VFCs in renowned baijiu brands, including Wuliangye, Yanghe, Jiananchun, and Gujinggong [21,22,23]. The VFCs of Wuliangye Baijiu were further analyzed by LiChrolut ENSPE fractionation coupled with comprehensive 2D gas chromatography and time-of-flight mass spectrometry (GC×GC-TOFMS), identifying about 500 compounds from over 3000 peaks [24]. In total, 75 aroma components were identified in Wuliangye-flavor raw liquor by headspace solid phase microextraction, liquid–liquid microextraction combined gas chromatography-mass spectrometry (HS-SPME/LLME-GC-MS) [25]. Despite these advancements, no report has been conducted to investigate the dynamic change in microorganisms and its effects on VFCs formation in the Wuliangye zaopei fermentation of mud pits with different continuously used years.
This study aimed to assess how continuous usage years of mud pits affect microbial communities and VFCs during zaopei fermentation. Zaopei samples from mud pits with two different continuous usage years were selected as research subjects. Physicochemical properties, VFCs, and microbial community structures during Wuliangye Baijiu fermentation were monitored dynamically. Fermentation feature tests, headspace solid phase microextraction (HS-SPME) coupled with gas chromatography mass spectrometry (GC/MS) analysis, and metagenomic sequencing were used. Our results revealed that different continuous usage years of mud pits significantly influenced the microbial succession patterns and VFC profiles, thereby providing a more holistic understanding of microbial dynamics, as well as the changes in physicochemical characteristics and VFCs during Wuliangye fermentation.
2. Materials and Methods
2.1. Sample Collection
Two mud pits (4.3 m (L) × 3.3 m (W) × 2.3 m (H)) with 20 years (named JC20) and 40 years (named JC40) of continuous use from Wuliangye Yibin Co., Ltd. (Yibin, China), a representative China-based producer of strong-flavor baijiu, were selected for the fermentation of Wuliangye Baijiu. Zaopei samples were obtained from the two pits on the 0th, 5th, 15th, 20th, 27th, 34th, 41st, 48th, 55th, and 69th day of fermentation. Zaopei samples were obtained from three points (each point was regarded as a biological replication) in the upper, middle, and bottom layers of each pit using a hollow cylindrical sampler. All collected samples were pooled and thoroughly mixed in sterile plastic sampling bags immediately after collection to ensure uniformity. And then they were promptly transported to the laboratory and stored at −80 °C until they were needed for study.
2.2. Physiochemical Properties of Zaopei
A total of 5 key parameters (fermentation temperature, titratable acidity, moisture, total starch, and titratable reducing sugar) were detected to investigate the dynamic change in the two pits. The moisture percentage of zaopei was assessed via the gravimetric method, where samples were dried at 105 °C for a minimum of 3 h. The titratable acidity in zaopei was detected through titration with 0.1 M NaOH, using phenolphthalein as an indicator (endpoint at pH 8.2). Starch and titratable reducing sugar contents were analyzed using Fehling’s reagent.
2.3. Analysis of VFCs in Zaopei
A total of 1 g of zaopei sample was taken into the headspace bottle and then the bottle cap was closed and immediately tightened. Volatile compounds were analyzed using an Agilent 7890A gas chromatograph interfaced with an Agilent 5977B inert mass spectrometer operating in electron ionization (EI) mode (Agilent, Santa Clara, CA, USA). Volatile components were extracted by a 2 cm 50/30 μm DVB/CAR/PDMS fiber (Supelco, Bellefonte, PA, USA). Prior to extraction, samples were thermally equilibrated at 50 °C for 5 min, followed by 45 min of extraction at the same temperature.
The SPME fiber was then thermally desorbed in the GC injection port (250 °C for 5 min, splitless mode). Compound separation was achieved using an HP-INNOWAX column (60 m length × 0.32 mm internal diameter, 0.5 µm stationary phase thickness) (Agilent, Santa Clara, CA, USA). The GC method employed helium as carrier gas (1 mL/min constant flow) with the following temperature program: initial hold at 40 °C for 3 min, followed by a 8 °C/min ramp to 250 °C, and a final isothermal hold for 5 min. Mass spectrometric detection was performed in electron impact ionization mode (70 eV) with the ion source maintained at 250 °C. A full-scan analysis covered 35–350 amu for compound characterization. Identification used NIST library matching (score ≥ 85), retention indices from n-alkanes, and comparison with reference standards. 4-Octanol (Sigma-Aldrich, St. Louis, MO, USA) at ~100 ppm served as the internal standard. Semi-quantification was based on the volatile compounds’ relative peak areas to 4-octanol.
2.4. DNA Extraction
The PowerSoil® DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA, USA) was used for DNA extraction from 0.5 g homogeneous frozen samples following the manufacturer’s protocols. The purity and concentration of DNA were evaluated with a NanoDrop 2000 UV spectrophotometer (Thermo Scientific, Waltham, MA, USA). All DNA samples were stored at −80 °C until for study.
2.5. Statistical Analysis
Data were presented as the mean ± standard deviation (SD). R language were used for date analysis. The Pearson correlation coefficient r2 was calculated using R language and the heat map of r2 was drawn by R 3.5.1. Differences were considered to be statistically significant at p < 0.05.
3. Results and Discussion
3.1. Dynamics of Fermentation Properties During Wuliangye Baijiu Making Process
We systematically monitored the dynamic variations in fermentation temperature, moisture content, total starch, titratable reducing sugar, and titratable acidity throughout the fermentation processes of JC20 and JC40 (Figure 2). Initially, as Baijiu is produced in an open environment, the temperatures of JC20 and JC40 were comparable. As fermentation progressed, the temperature increased rapidly and gradually declined until the end. Notably, in both mud pits, the temperature gradient was consistent with traditional practices, where the lower layer zaopei registered the highest temperature, while the upper layer zaopei exhibited the lowest (Figure 2A). Regarding moisture content, a consistent upward trend was observed in both mud pits throughout the fermentation period. However, the lower layer zaopei of JC20 demonstrated a more pronounced increase, rising from 55.70% ± 0.14 to 72.26% ± 1.00, compared to JC40’s range of 56.55% ± 0.92 to 69.49% ± 0.02 (Figure 2B). The total titratable acids steadily accumulated during fermentation in both samples, reaching their peak concentrations at the end of the process (Figure 2C). Across all three layers, the zaopei from JC20 exhibited higher acidity levels than those from JC40, with the lower layer zaopei of both mud pits having the most significant acidity values: 4.56 ± 0.07 for JC20 and 5.25 ± 0.05 for JC40. The total starch content started high at the beginning of fermentation and steadily decreased until the final sampling point in both JC20 and JC40 (Figure 2D). Specifically, the middle and lower layers of zaopei in JC20 showed greater starch consumption compared to their counterparts in JC40. For the middle layers, the starch content decreased from 21.7% ± 0.84 to 12.35% ± 0.25 in JC20, contrasting with 22.92% ± 0.29 to 15.00% ± 0.13 in JC40; for the lower layers, the reduction was from 24.30% ± 0.34 to 9.50% ± 0.10 in JC20 versus 21.59% ± 0.22 to 13.58% ± 0.13 in JC40. The titratable reducing sugar content in JC40’s zaopei across all three layers underwent a rapid increase followed by a sharp decline during fermentation. In contrast, the changes in JC20 were more subdued, characterized by a modest rise and subsequent decrease (Figure 2E).
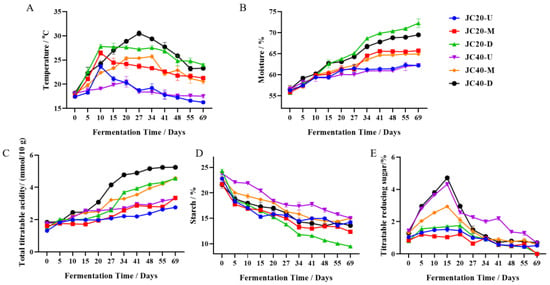
Figure 2.
Dynamics of zaopei fermentation properties during Wuliangye Baijiu fermentation. (A) Temperature, (B) moisture, (C) total titratable acidity, (D) starch, (E) titratable reducing sugar. The different color curve represent different layer zaopei samples (blue, red, green, purple, yellow, and black represent JC20-up, JC20-middle, JC20-down, JC40-up, JC40-middle, and JC40-down samples, respectively). Each data point is the mean of three measurements ± SD.
3.2. Variation of Main VFCs in Fermentation Process
To explore the impact of mud pit continuous usage years on VFCs, HS-SPME/GC-MS was used to analyze zaopei from JC20 and JC40. A total of 29 main VFCs were semi quantified, including 19 esters, 3 alcohols, 5 acids, and 2 others (score > 85). Heat map analysis (Figure 3) showed that metabolite profiles increased during fermentation, with greater variation in JC40 than in JC20. However, these flavor substances had distinct dynamic changes during fermentation.
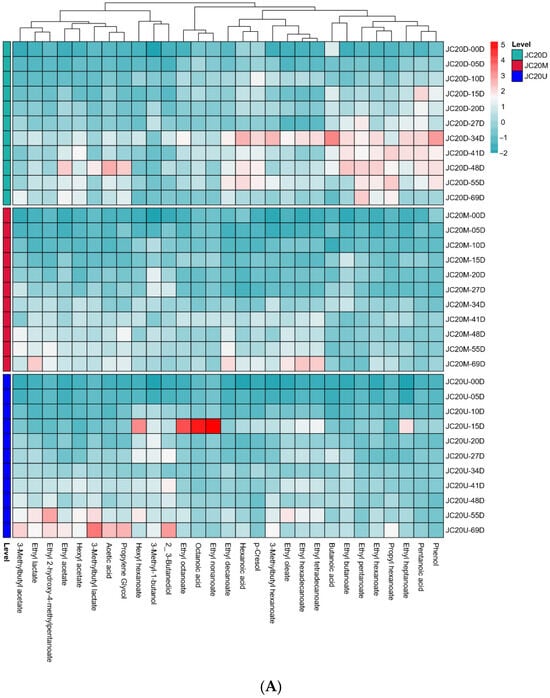
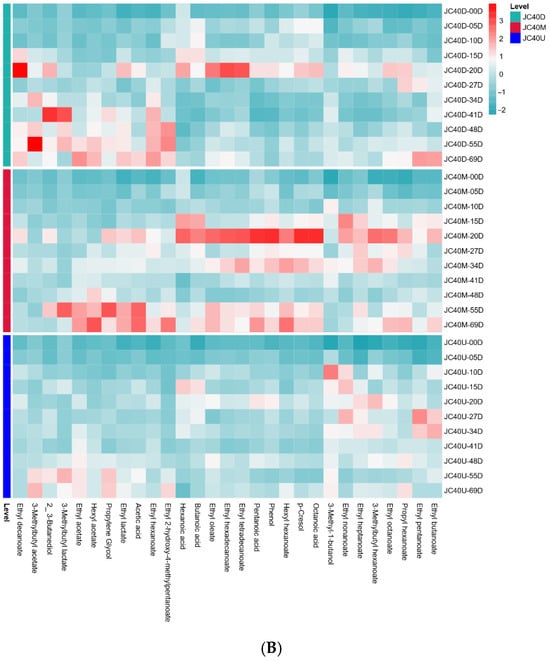
Figure 3.
Heat map of changes in differential VFCs during the fermentation of JC20 (A) and JC40 (B).
Esters, crucial for the fruity aroma of Wuliangye Baijiu, were the most abundant VFCs in all zaopei samples. Notably, esters constituted the largest proportion of VFCs in all zaopei samples, accounting for 70.10–78.92% of the total VFCs at the end of fermentation (Table S1). Our results demonstrated that the concentrations of major esters steadily increased during fermentation, reaching their peak values at the conclusion of the process (Figure 3). Both the total ester content and most individual ester concentrations in JC40’s three-layer zaopei samples were significantly higher than those in JC20. Among them, ethyl hexanoate, ethyl acetate, and ethyl lactate dominated the ester composition in all zaopei samples (Table S1). As a key determinant of Wuliangye Baijiu’s quality, ethyl hexanoate content in the three-layer zaopei of JC40 at the end of fermentation was superior to that of JC20, following the order: JC40-D > JC40-M > JC40-U > JC20-D > JC20-M > JC20-U (Table S1). These results aligned with previous research [6], suggesting that longer mud pit continuous usage years lead to better quality Wuliangye Baijiu production.
Volatile acids are essential components among the VFCs of Wuliangye Baijiu, playing a pivotal role in its aroma profile. An optimal concentration of these acids endows the liquor with a harmonious and enduring pleasantness [26]. For example, hexanoic and acetic acids are major acids in strong flavor baijiu; suitable amounts enhance liquor aroma, but excessive levels disrupt its style [25]. In this study, five main volatile organic acids were identified in both JC20 and JC40 (Figure 3). Their concentrations exhibited an initial increase during the early fermentation phase, followed by either stabilization or a decline. This trend can likely be attributed to their involvement in esterification reactions, where they serve as key precursors for the synthesis of corresponding esters (Figure 3). Notably, as the primary precursors for ethyl hexanoate, hexanoic acid levels in all three layers of JC40 zaopei were significantly higher than those in JC20 (p < 0.05, Table S1). This disparity in hexanoic acid content may well account for the elevated ethyl hexanoate levels observed in the zaopei of JC40 compared to JC20.
Alcohols, formed through glucose metabolism and the dehydrodecarboxylation of amino acids, act as essential precursors for ester formation. Higher alcohols are one of the important flavor compounds in Wuliangye Baijiu, yet excessive concentrations are unpalatable. In this study, three main volatile alcohols were identified (Figure 3). Notably, 3-methyl-1-butanol, a key constituent of higher alcohols, exhibited significantly higher concentrations in the three-layer zaopei samples of JC20 compared to JC40 at the end of fermentation (p < 0.05, Table S1).
3.3. Diversity and Differences in Microbial Community Composition During Fermentation
To explore the composition of microbial communities in different ages of mud pits, the fermentation process was monitored over a 70-day period. After quality control, a total of 1,179,351,053 high quality sequences were successfully retrieved for the bacterial communities across all samples. The number of sequences per sample ranged from 1,839,808 to 35,301,333. For eukaryote sequences, 23,505,524 high-quality reads were obtained, with the read counts per sample spanning from 10,582 to 3,541,662.
Subsequently, high-throughput sequencing was performed to assess microbial community succession during fermentation, with taxonomic classification conducted at both the phylum and genus levels. Analysis revealed the presence of four dominant bacterial phyla and four predominant fungal phyla, all exhibiting relative abundances above 0.1% (Figure S1). Among the bacterial, Firmicutes, Actinobacteria, Proteobacter, and Bacteroidetes dominated in the early stages of fermentation in both mud pits. Their abundances then decreased while Firmicutes increased during fermentation with a relative abundance of more than 95.74% at the end of fermentation of JC40 and JC20 except the upper layer zaopei sample of JC20, which was 56.98% (Figure S1A,B). For fungi, Ascomycota was identified to be the predominant organism throughout the entire fermentation process. Moreover, at the end of fermentation, all zaopei samples had a relative abundance of Ascomycota exceeding 88.76% (Figure S1C,D).
At the genus level, the succession of microbial communities during the fermentation of JC20 and JC40 exhibited distinct characteristics. The distribution of microbial genera in JC40 was more balanced than that in JC20, potentially explaining why high quality Wuliangye Baijiu is commonly produced in older mud pits such as JC40. Initially, Pantoea and Kosakonia emerged as the predominant genera, while Cronobacter was dominant later. As fermentation advanced, the relative abundances of Pantoe, Kosakonia, and Cronobacter decreased, possibly due to the significant enrichment of lactic acid bacteria towards the end of the fermentation period.
Bacteria, a key functional microorganism, secretes numerous hydrolytic enzymes that aid in the formation of flavor compounds during Wuliangye Baijiu fermentation. In JC20, Acinetobacter was a major genus from day 5–15 but its proportion dropped rapidly as fermentation continued. Apilactobacillus became dominant on day 20 and remained so until the end of fermentation in both JC20 and JC40 (Figure 4A,B). Moreover, a notable divergence was observed in the dominant microbial communities of zaopei samples between JC40 and JC20 during the early fermentation stage (Figure 4B), which might be due to the differences in mud pit microorganisms and environmental microorganisms.
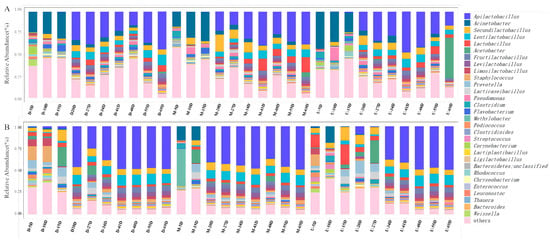
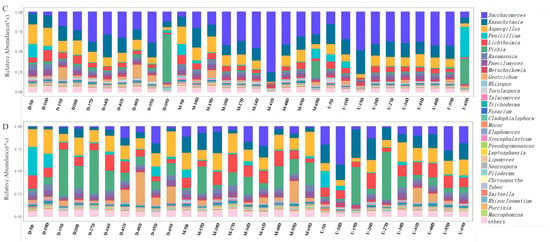
Figure 4.
Bacterial (A,B) and fungal (C,D) community structure succession at the genus level. A and C (B,D) represent the results of JC20 (JC40).
Fungi play a crucial role in Wuliangye Baijiu fermentation, secreting diverse enzymes that drive the biosynthesis of flavor compounds [27]. Among them, Saccharomyces stands out as a pivotal microorganism for alcohol production. It demonstrates remarkable efficiency in sugar conversion, predominantly driving ethanol fermentation during the brewing of Wuliangye Baijiu. Our results indicate that in the upper and middle layer zaopei of JC20 and JC40, the relative abundance of Saccharomyces rose rapidly in the early and middle fermentation stages, consistent with the fact that the upper and middle layer zaopei have a higher yield than the lower layers. Kazachstania and Aspergillus also exhibited abundance at the beginning fermentation of JC20 and maintained relative stability as fermentation progressed (Figure 4C). For JC40, apart from the significant increase in Saccharomyces, the proportion of Pichia was also found to be increased during the fermentation (Figure 4D). Additionally, the relative abundances of Aspergillus and Lichtheimia maintained relative stability in each samples, highlighting their stable contribution to the fermentation ecosystem (Figure 4D).
3.4. Correlation Analyses Revealed the Relationships Between Different Microbes of Different Pits
Microbial interactions play a pivotal role in shaping the microbial composition, as they are intricately linked to fluctuations in microbial populations and have a direct impact on microbial metabolism during the fermentation process [28,29]. To elucidate these complex relationships, Pearson’s correlation coefficients and p values were computed for the top 30 bacterial and fungal genera from JC20 and JC40 (Figure 5). The correlation analysis results revealed that in JC20, 19 bacterial and 24 fungal genera exhibited correlated behavior (Figure 5A), while in JC40, 22 bacterial and 29 fungal genera showed similar patterns of correlation (Figure 5B). Notably, across both JC20 and JC40, bacterial communities generally displayed a negative correlation with fungal communities. Conversely, positive associations were observed among different fungal genera within each sample.
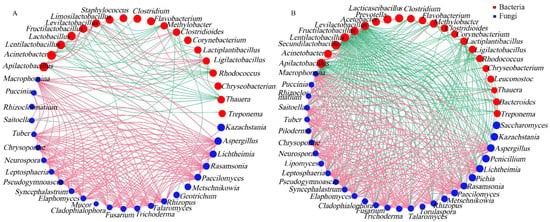
Figure 5.
Microbial association networks were constructed for samples JC20 (A) and JC40 (B). Bacterial and fungal nodes are represented by red and blue circles, respectively. Significant positive correlations (r > 0.7, p < 0.05) are denoted by red lines. Significant negative correlations (r < −0.7, p < 0.05) are indicated by green lines.
Delving deeper into the findings, in JC20, Levilactobacillus, Fructilactobacillus, Apilactobacillus, and Clostridioides were strongly positively correlated (|r| > 0.9, p < 0.05). In contrast, Clostridioides negatively correlated with Methylobacter, Acinetobacter, and several other genera (|r| > 0.7, p < 0.05). Multiple lactobacillus related genera were negatively correlated with Methylobacter (|r| > 0.7, p < 0.05), except for Rhodococcus, which showed a strong positive correlation with it (|r| > 0.9, p < 0.05) (Figure 5A). Regarding JC40, Clostridioides negatively correlated with Methylobacter, Flavobacterium, Acinetobacter, Lipomyces, Thauera, and Rhodococcus (|r| > 0.7, p < 0.05), but no genera showed a strong positive correlation with Clostridioides (|r| > 0.9, p < 0.05). Seven bacterial genera (Levilactobacillus, Fructilactobacillus, Lentilactobacillus, Apilactobacillus, Ligilactobacillus, Lactiplantibacillus, and Clostridioides) displayed significant negative correlation with Methylobacter (|r| > 0.7, p < 0.05), while Acinetobacter and Clostridium were positively correlated with Methylobacter (|r| > 0.9, p < 0.05) (Figure 5B). For fungi, in JC20, Aspergillus correlated positively with 16 fungal genera and Rhizopus with 17 (|r| > 0.9, p < 0.05) (Figure 5A). For JC40, Aspergillus positively correlated with 15 fungi (|r| > 0.9, p < 0.05) but negatively with five bacteria (|r| > 0.7, p < 0.05). Similarly, Rhizopus had positive links with 15 fungi and negative ones with five bacteria under the same significance. Saccharomyces and Pichia were positively correlated with Torulaspora and Piloderma (|r| > 0.9, p < 0.05), respectively (Figure 5B). Overall, bacteria–fungi correlations were mostly negative (|r| > 0.7, p < 0.05) (Figure 5).
The results of high throughput sequencing revealed that the dominant microbial genera in JC20 and JC40 samples evolved with fermentation time and environmental changes. This suggests that certain genera, such as Secundilactobacillus, Acinetobacter, Limosilactobacillus, and Flavobacterium, may struggle to adapt to rising ethanol concentrations and increased acidity. These environmental shifts result from the selective pressure exerted by Apilactobacillus and Saccharomyces. Moreover, there were three bacteria (Limosilactobacillus, Lactobacillus, Staphylococcus) and one fungi (Geotrichum) that showed correlated behavior only in JC20, while six bacteria (Bacteroides, Leuconostoc, Prevotella, Acetobacter, Secundilactobacillus, and Lacticaseibacillus) and six fungi (Pichia, Penicillium, Saccharomyces, Talaromyces, Piloderma, and Lipomyces) showed correlated behavior only in JC40. Correlation analyses indicated that microbial community interactions in JC40 were more diverse and stronger than in JC20, which was in line with the typical characteristics of the brewing process of “the older the pit, the better the baijiu” [30]. Additionally, as dominant fungal genera, Rhizopus and Saccharomyces genera demonstrate robust fermentation, decomposition capabilities, and resistance to acid and ethanol [29,31,32].
3.5. Correlations Between Microorganisms with Physiochemical Properties
Environmental factors govern the changes in microbial community structures. To elucidate the relationships between microbial communities and physicochemical properties during the fermentation of JC20 and JC40, RDA was conducted (Figure 6). The results demonstrated that the microbial community dynamics were significantly influenced by variables such as total starch content, titratable acid levels, moisture, titratable reducing sugar, and fermented temperature. In terms of bacterial communities, in JC20 fermentation, Acinetobacter, Clostridium, and Corynebacterium were associated with total starch and titratable reducing sugar and Lactococcus, Acetobacter, Staphylococcus, and Pediococcus had significantly positive correlation with titratable acids of JC20 (Figure 6A). As for JC40, Acinetobacter, Clostridium, Methylobacter, Pediococcus, and Staphylococcus were only correlated with total starch over the fermentation of JC40 (Figure 6B). And Fructilactobacillus and Apilactobacillus had significantly positive correlation with titratable acids (Figure 6B). According to previous studies, these bacteria can produce acids by using carbohydrates [33,34,35].
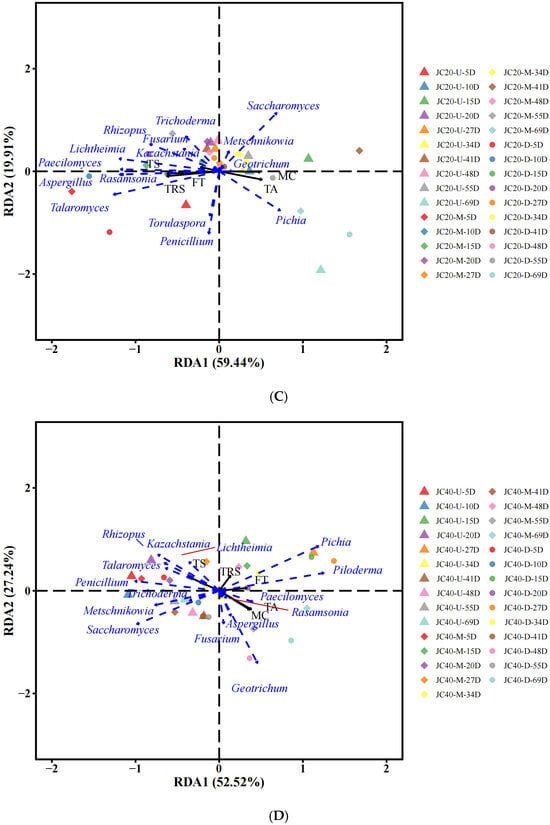
Figure 6.
Microbial RDA analysis (top 15 species) and fermentation characteristics. Correlation analysis between fermentation parameters and bacterial genera (A,B) or fungal genera (C,D). (A,C) represent the results of JC20; (B,D) represent the results of JC40. TS: total sugar, TA: titratable acid, MC: moisture content, TRS: titratable reducing sugar, FT: fermented temperature.
For fungi, Aspergillus plays a central role in the saccharification, fermentation, and esterification processes that are fundamental to Chinese Baijiu production [36,37,38]. In this study, in JC20, Aspergillus was linked to titratable acids (Figure 6C), while in JC40, it correlated with titratable reducing sugar and temperature (Figure 6D). Furthermore, Rhizopus and Kazachstanic were related to the total starch content in both JC20 and JC40. Rhizopus is known for its strong amylase-producing ability during Wuliangye Baijiu fermentation [29,39], and can break down starch into fermentable sugars [40], consistent with our findings. Moreover, Pichia exhibited significant positive associations with titratable reducing sugar and temperature, highlighting its potential role in responding to key environmental factors during the fermentation process.
3.6. Correlations Between Microorganisms with VFCs
In the production of Chinese Baijiu, understanding the interactions between microorganisms and VFCs during fermentation is crucial, given the complexity of the Baijiu fermentation system, which involves numerous microorganisms and chemical compounds [38,41]. Therefore, Pearson’s correlation coefficient was used to reveal the interaction between microorganisms (top 10 bacteria and top 10 fungi) and 15 main VFCs in this study (Figure 7).
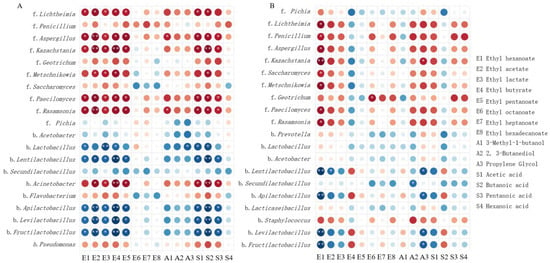
Figure 7.
Correlation analysis of microbiota and VFCs during JC20 (A) and JC40 (B) fermentation. Pearson’s rank correlations between microorganisms (top 10 bacteria and top 10 fungi) and 19 main VFCs. Circle size represents correlation strength (large circles for strong, small for weak), and the color scale indicates correlation type: dark blue for perfect positive correlation (1), dark red for perfect negative (−1). Significant correlations are marked: * for |r| > 0.7 (p < 0.01), ** for |r| > 0.9 (p < 0.01).
The correlation analysis result revealed that nearly all top 10 fungal species in both JC20 and JC40 fermentation pits played pivotal roles in synthesizing main VFCs, including ethyl hexanoate [E1], ethyl acetate [E2], and ethyl lactate [E3]. These fungi also contributed to the formation of 2,3-butanediol [A2], along with organic acids such as acetic acid [S1] and pentanoic acid [S3]. To the best of our knowledge, Aspergillus facilitates esterase production [38,42], and Paecilomyces, Rasamsonia, and Kazachstania produce glucoamylase to break down glucose and generate various VFCs such as acids, esters, and alcohols [33]. Our findings in Figure 7 supported these observations. However, differences emerged in the production of other VFCs. For JC20, the synthesis of ethyl butyrate [E4], ethyl valerate [E5], 3-methyl-1-butanol [A1], and butanoic acid [S2] positively correlated with most fungal species (Figure 7A), while for JC40, ethyl caprylate [E6], ethyl palmitate [E8], and hexanoic acid [S4] were positive correlated with most fungal microorganisms (Figure 7B).
In contrast, most bacteria showed weak relation to the formation of VFCs during the fermentation process, and some bacteria such as Acetobacter, Lactobacillus, Lentilactobacillus, Secundilactobacillus, Apilactobacillus, Levilactobacillus, and Fructilactobacillus showed negative correlation with VFCs synthesis of JC20 (Figure 7A). Similarly, in JC40, Lentilactobacillus, Secundilactobacillus, Apilactobacillus, Levilactobacillus, and Fructilactobacillus showed negative correlations with the production of the majority of VFCs (Figure 7B). As Figure 5 showed, it might be because bacteria showed a negative correlation with fungi.
The correlation analysis between VFCs and microorganisms offers valuable insights into the microbial species driving VFC biosynthesis. This knowledge can be leveraged to precisely regulate VFC production during Wuliangye Baijiu fermentation. However, it is crucial to acknowledge that fermentation is a multifaceted process influenced by numerous factors. For instance, introducing or increasing the abundance of specific microbial strains may have far-reaching effects on the entire fermentation ecosystem. Only by taking a holistic approach and considering all variables involved in fermentation can we achieve consistent VFC production, uphold the quality of zaopei, and ultimately produce superior Wuliangye Baijiu.
4. Conclusions
This investigation examined microbial succession patterns and associated biochemical transformations in fermentation pits with distinct operational histories (20-year [JC20] vs. 40-year [JC40] continuous use) from the Wuliangye production system.
In this study, two different mud pits (JC20 and JC40), that were used continuously for many years, from Wuliangye were selected to monitor the dynamic changes and the relation among the microbial and physiochemical properties and VCFs. The results showed a positive association between different fungi genera while it presenting a generally negative correlation between different bacteria genera or between fungi and bacteria genera from both two pits. Moreover, fungal microorganisms (such as Aspergillus, Paecilomyces, Rasamsonia, and Kazachstania) were found to make greater contributions to the synthesis of main VFCs during the fermentation process of both two pits. Additionally, the diversity of microorganisms successfully identified of three different layers zaopei samples from JC40 at the fermentation anaphase were significantly higher than that of JC20, which also resulted in the production of higher quality Wuliangye from JC40. The results offer key insights into microbiota that drive the generation of VFCs in Wuliangye production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11070388/s1, Table S1: Detection results of VFCs at the end of fermentation. Figure S1: Bacterial (A,B) and fungal (C,D) community structure succession at the phylum level. A and C (B and D) represent the results of JC20 (JC40).
Author Contributions
Y.L. (Yanping Lu): Conceptualization, Methodology, Validation, Data curation, Visualization, Project administration, and Writing—Original draft preparation. Y.L. (Yalan Li): Methodology, Validation, and Investigation. B.W.: Methodology and Validation. Y.L. (Yuzhu Li): Methodology, J.S.: Resources. J.Z.: Conceptualization and Writing—Reviewing and Editing. D.Z.: Supervision. Y.X.: Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Yanping Lu, Yalan Li, Bing Wan, Yuzhu Li, Jian Su, Jia Zheng, and Dong Zhao were employed by the company “Wuliangye Yibin Co., Ltd”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, H.L.; Sun, B.G. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yoshizaki, Y.; Ikenaga, M.; Han, X.L.; Okutsu, K.; Futagami, T.; Tamaki, H.; Takamine, K. Manufactural impact of the solid-state saccharification process in rice-flavor baijiu production. J. Biosci. Bioeng. 2020, 129, 315–321. [Google Scholar] [CrossRef]
- Qian, W.; Lu, Z.M.; Chai, L.J.; Zhang, X.J.; Li, Q.; Wang, S.T.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Cooperation within the microbial consortia of fermented grains and pit mud drives organic acid synthesis in strong-flavor Baijiu production. Food Res. Int. 2021, 147, 110449. [Google Scholar] [CrossRef]
- Wu, Q.; Kong, Y.; Xu, Y. Flavor Profile of Chinese Liquor Is Altered by Interactions of Intrinsic and Extrinsic Microbes. Appl. Environ. Microbiol. 2015, 82, 422–430. [Google Scholar] [CrossRef]
- Liu, M.K.; Tang, Y.M.; Guo, X.J.; Zhao, K.; Penttinen, P.; Tian, X.H.; Zhang, X.Y.; Ren, D.Q.; Zhang, X.P. Structural and Functional Changes in Prokaryotic Communities in Artificial Pit Mud during Chinese Baijiu Production. mSystems 2020, 5, e00829-00819. [Google Scholar] [CrossRef]
- Xu, S.S.; Zhang, M.Z.; Xu, B.Y.; Liu, L.H.; Sun, W.; Mu, D.D.; Wu, X.F.; Li, X.J. Microbial communities and flavor formation in the fermentation of Chinese strong-flavor Baijiu produced from old and new Zaopei. Food Res. Int. 2022, 156, 111162. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, J.B.; Rui, J.P.; Xu, Z.C.; Zhou, Y.; Hu, X.H.; Wang, X.; Liu, M.H.; Li, D.P.; Li, X.Z. Prokaryotic Communities in Pit Mud from Different-Aged Cellars Used for the Production of Chinese Strong-Flavored Liquor. Appl. Environ. Microbiol. 2014, 80, 2254–2260. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, N.; Tang, H. Analysis of microbial community diversity and physicochemical factors in pit mud of different ages based on high-throughput sequencing. Can. J. Microbiol. 2022, 68, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.B.; Jia, Y.L.; Zhang, Q.; Pu, S.C.; Shi, C. Bacterial diversity associated with volatile compound accumulation in pit mud of Chinese strong-flavor baijiu pit. AMB Express 2023, 13, 3. [Google Scholar]
- Zhang, H.M.; Meng, Y.J.; Wang, Y.L.; Zhou, Q.W.; Li, A.J.; Liu, G.Y.; Li, J.X.; Xing, X.H. Prokaryotic communities in multidimensional bottom-pit-mud from old and young pits used for the production of Chinese Strong-Flavor Baijiu. Food Chem. 2020, 312, 126084. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, B.R.; Wang, Y.B.; Zhang, Q.P.; He, X.X.; Yang, P.; Zhou, J.; Guan, X.; Huang, X.H. Proteomic and high-throughput analysis of protein expression and microbial diversity of microbes from 30- and 300-year pit muds of Chinese Luzhou-flavor liquor. Food Res. Int. 2015, 75, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.K.; Tang, Y.M.; Zhao, K.; Liu, Y.; Guo, X.J.; Ren, D.Q.; Yao, W.C.; Tian, X.H.; Gu, Y.F.; Yi, B.; et al. Determination of the fungal community of pit mud in fermentation cellars for Chinese strong-flavor liquor, using DGGE and Illumina MiSeq sequencing. Food Res. Int. 2017, 91, 80–87. [Google Scholar] [CrossRef]
- Liu, M.-K.; Tang, Y.-M.; Guo, X.-J.; Zhao, K.; Tian, X.-H.; Liu, Y.; Yao, W.-C.; Deng, B.; Ren, D.-Q.; Zhang, X.-P. Deep sequencing reveals high bacterial diversity and phylogenetic novelty in pit mud from Luzhou Laojiao cellars for Chinese strong-flavor Baijiu. Food Res. Int. 2017, 102, 68–76. [Google Scholar] [CrossRef]
- Wang, X.S.; Du, H.; Zhang, Y.; Xu, Y. Environmental Microbiota Drives Microbial Succession and Metabolic Profiles during Chinese Liquor Fermentation. Appl. Environ. Microbiol. 2018, 84, e02369-17. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.W.; Zeng, Y.L.; Ma, J.Y.; Cai, K.Y.; Guo, T.T.; Tan, G.X.; Yu, X.; Hu, Y.L.; Peng, N.; Zhao, S.M. Characteristics and Functions of Dominant Yeasts Together with Their Applications during Strong-Flavor Baijiu Brewing. Foods 2024, 13, 2409. [Google Scholar] [CrossRef]
- Hong, J.X.; Huang, H.; Zhao, D.R.; Sun, J.Y.; Huang, M.Q.; Sun, X.T.; Sun, B.G. Investigation on the key factors associated with flavor quality in northern strong aroma type of Baijiu by flavor matrix. Food Chem. 2023, 426, 136576. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, J.; He, G.Q. Effect of microbial interaction on flavor quality in Chinese baijiu fermentation. Front. Nutr. 2022, 9, 960712. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Wu, M.Q.; Zhao, D.; Zheng, J.; Dai, M.Q.; Li, X.T.; Li, W.W.; Zhang, C.N.; Sun, B.G. Simulated Fermentation of Strong-Flavor Baijiu through Functional Microbial Combination to Realize the Stable Synthesis of Important Flavor Chemicals. Foods 2023, 12, 644. [Google Scholar] [CrossRef]
- Chai, L.J.; Lu, Z.M.; Zhang, X.J.; Ma, J.; Xu, P.X.; Qian, W.; Xiao, C.; Wang, S.T.; Shen, C.H.; Shi, J.S.; et al. Zooming in on Butyrate-Producing Clostridial Consortia in the Fermented Grains of Baijiu via Gene Sequence-Guided Microbial Isolation. Front. Microbiol. 2019, 10, 01397. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, C.Q.; Luo, H.B. Diversity and Function of Microbial Community in Chinese Strong-Flavor Baijiu Ecosystem: A Review. Front. Microbiol. 2018, 9, 671. [Google Scholar] [CrossRef]
- Fan, W.L.; Qian, M.C. Identification of aroma compounds in Chinese ‘Yanghe Daqu’ liquor by normal phase chromatography fractionation followed by gas chromatography [sol] olfactometry. Flavour Frag. J. 2006, 21, 333–342. [Google Scholar] [CrossRef]
- Fan, W.L.; Qian, M.C. Characterization of Aroma Compounds of Chinese “Wuliangye” and “Jiannanchun” Liquors by Aroma Extrac. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef]
- Zhao, D.R.; Shi, D.M.; Sun, J.Y.; Li, A.J.; Sun, B.G.; Zhao, M.M.; Chen, F.; Sun, X.T.; Li, H.H.; Huang, M.Q.; et al. Characterization of key aroma compounds in Gujinggong Chinese Baijiu by gas chromatography-olfactometry, quantitative measurements, and sensory evaluation. Food Res. Int. 2018, 105, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, Z.L.; Yang, K.Z.; Liu, Z.P.; Zhao, D.; Qian, M.C. Volatile Analysis of Wuliangye Baijiu by LiChrolut EN SPE Fractionation Coupled with Comprehensive GC × GC-TOFMS. Molecules 2022, 27, 1318. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.Y.; Liu, L.L.; Zheng, J.; Chen, T.; Chen, F.; Li, P.P.; Zhang, M.; Shen, X.J. Correlation analysis between aroma components and microbial communities in Wuliangye-flavor raw liquor based on HS-SPME/LLME-GC-MS and PLFA. Food Res. Int. 2021, 140, 109995. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liang, R.; Wu, C.D.; Zhou, R.Q.; Liao, X.P. Discrimination of different kinds of Luzhou-flavor raw liquors based on their volatile features. Food Res. Int. 2014, 56, 77–84. [Google Scholar] [CrossRef]
- Zhang, H.X.; Tan, Y.W.; Wei, J.L.; Du, H.; Xu, Y. Fungal Interactions Strengthen the Diversity-Functioning Relationship of Solid-State Fermentation Systems. mSystems 2022, 7, e0040122. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Sandrine, R.; Raphaëlle, T.M.; Mohand, S.; Régis, G.; Philippe, S.K.; Hervé, A. Wine microbiome: A dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 856–873. [Google Scholar] [CrossRef]
- Huang, Z.R.; Hong, J.L.; Xu, J.X.; Li, L.; Guo, W.L.; Pan, Y.Y.; Chen, S.J.; Bai, W.D.; Rao, P.F.; Ni, L.; et al. Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol. 2018, 76, 487–496. [Google Scholar] [CrossRef]
- Yan, G.L.; Duan, L.L.; Liu, P.T.; Duan, C.Q. Transcriptional Comparison Investigating the Influence of the Addition of Unsaturated Fatty Acids on Aroma Compounds During Alcoholic Fermentation. Front. Microbiol. 2019, 10, 1115. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Cardoso, R.; Custódio, V.; Fernandes, J.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Wine fermentation microbiome: A landscape from different Portuguese wine appellations. Front. Microbiol. 2015, 1, 905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jin, B.; Kelly, J.M. Production of lactic acid and byproducts from waste potato starch by Rhizopus arrhizus: Role of nitrogen sources. World J. Microbiol. Biotechnol. 2007, 23, 229–236. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Tian, H.X.; Ai, L.Z.; Yu, H.Y. Metagenomic analysis reveals the impact of JIUYAO microbial diversity on fermentation and the volatile profile of Shaoxing-jiu. Food Microbiol. 2020, 86, 103326. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, L.P.; Wu, H.J.; Wang, Y.S.; Wang, Z.W.; Zheng, F.P.; Lu, X.; Xu, J.L. The changes of microbial community and flavor compound in the fermentation process of Chinese rice wine using Fagopyrum tataricum grain as feedstock. Sci. Rep. 2019, 9, 3365. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, L.P.; Sun, Z.B.; Liu, Q.S.; Lu, X.; Li, Z.P.; Xu, J.L. Bacterial succession and the dynamics of flavor compounds in the Huangjiu fermented from corn. Arch. Microbiol. 2020, 202, 299–308. [Google Scholar] [CrossRef]
- Liang, H.P.; Luo, Q.C.; Zhang, A.; Wu, Z.Y.; Zhang, W.X. Comparison of bacterial community in matured and degenerated pit mud from Chinese Luzhou-flavour liquor distillery in different regions. J. Inst. Brew. 2016, 122, 48–54. [Google Scholar] [CrossRef]
- Jing, Z.B.; Cheng, J.M.; Jin, J.W.; Su, J.S.; Bai, Y. Revegetation as an efficient means of improving the diversity and abundance of soil eukaryotes in the Loess Plateau of China. Ecol. Eng. 2014, 70, 169–174. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Huang, H.Q.; Lu, H.Y.; Wu, M.Q.; Lin, M.W.; Zhang, C.S.; Zhao, Z.G.; Li, W.W.; Zhang, C.N.; Li, X.T.; et al. Characterization of an Aspergillus niger for Efficient Fatty Acid Ethyl Ester Synthesis in Aqueous Phase and the Molecular Mechanism. Front. Microbiol. 2022, 12, 820380. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.C.; Lin, X.Z.; He, Z.G.; Su, H.; Li, W.X.; Guo, Q.Q. Comparison of microbial communities and amino acid metabolites in different traditional fermentation starters used during the fermentation of Hong Qu glutinous rice wine. Food Res. Int. 2020, 136, 109329. [Google Scholar] [CrossRef]
- Jiang, L.; Su, W.; Mu, Y.C.; Mu, Y. Major Metabolites and Microbial Community of Fermented Black Glutinous Rice Wine with Different Starters. Front. Microbiol. 2020, 11, 593. [Google Scholar] [CrossRef]
- Li, J.Y.; Ding, Z.; Dong, W.Q.; Li, W.W.; Wu, Y.F.; Zhu, L.N.; Ma, H.F.; Sun, B.G.; Li, X.T. Analysis of differences in microorganisms and aroma profiles between normal and off-flavor pit mud in Chinese strong-flavor Baijiu. J. Biosci. Bioeng. 2024, 137, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, R.; Converti, A.; Pirozzi, D.; Molinari, F. Efficient and selective microbial esterification with dry mycelium of Rhizopus oryzae. J. Biotechnol. 2001, 92, 21–26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).