A Deep Analytical Investigation of the Aroma Chemistry of Incrocio Bruni 54 and Its Differentiation from Italian White Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Standards
2.2. Samples
| Producer | Sample | Vintage | Closure | Altitude | Province, Country |
|---|---|---|---|---|---|
| I 3 filari | IB_1 | 2019 | Cork | 200 m | Macerata, Italy |
| Cantina Polenta | IB_2 | 2019 | Synthetic | 130 m | Ancona, Italy |
| Terracruda | IB_3 | 2019 | Synthetic | 400 m | Pesaro Urbino, Italy |
| Strologo | IB_4 | 2019 | Synthetic | 230 m | Ancona, Italy |
| Terrargillosa | IB_5 | 2019 | Synthetic | 150 m | Ascoli Piceno, Italy |
| Fontezoppa | IB_6 | 2019 | Cork | 150 m | Macerata, Italy |
| Conventino | IB_7 | 2018 | Synthetic | 270 m | Pesaro Urbino, Italy |
| La Montata | IB_8 | 2019 | Synthetic | 400 m | Pesaro Urbino, Italy |
| Bruscia | IB_9 | 2019 | Synthetic | 200 m | Pesaro Urbino, Italy |
| Villa Lazzarini | IB_10 | 2019 | Cork | 180 m | Macerata, Italy |
| Podere Santa Lucia | IB_11 | 2018 | Cork | 140 m | Ancona, Italy |
| Cantina Mezzanotte | IB_12 | 2019 | Cork | 40 m | Ancona, Italy |
| Finocchi | IB_13 | 2019 | Cork | 450 m | Ancona, Italy |
| Muraro | IB_14 | 2019 | Cork | 170 m | Pesaro Urbino, Italy |
| Ca’ Le Suore | IB_15 | 2019 | Synthetic | 300 m | Pesaro Urbino, Italy |
| Tenuta Santi Giacomo e Filippo | IB_16 | 2018 | Cork | 110 m | Pesaro Urbino, Italy |
| Vignamato | IB_17 | 2019 | Cork | 250 m | Ancona, Italy |
2.3. Analysis of Major Aroma Compounds
2.4. Analysis of Volatile Thiols
2.5. Analysis of Volatile Carbonyl Compounds
2.6. Analysis of Methyl Salicylate Glycosides
2.7. Comparison with a Reference Dataset of Italian White Wines
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IB | Incrocio Bruni 54 |
| VT | Volatile thiol |
| VOC | Volatile organic compound |
| MeSA | Methyl salicylate |
| 4MMP | 4-mercapto-4-methylpentan-2-one |
| 3MH | 3-mercaptohexanol |
| 3MHA | 3-mercaptohexyl acetate |
| TDN | 1,1,6-Trimethyl-1,2-dihydronaphthalene |
| ODT | Odor detection threshold |
| OAV | Odor activity value |
References
- Crespan, M.; Migliaro, D.; Larger, S.; Pindo, M.; Palmisano, M.; Manni, A.; Manni, E.; Polidori, E.; Sbaffi, F.; Silvestri, Q.; et al. Grapevine (Vitis vinifera L.) varietal assortment and evolution in the Marche region (central Italy). OENO One 2021, 3, 17–37. [Google Scholar] [CrossRef]
- Papi, R. Incrocio Bruni 54. Un Vitigno Marchigiano; Youcanprint: Tricase, Italy, 2022. [Google Scholar]
- Carlin, S.; Lotti, C.; Correggi, L.; Mattivi, F.; Arapitsas, P.; Vrhovšek, U. Measurement of the effect of accelerated aging on the aromatic compounds of Gewürztraminer and Teroldego wines, using a SPE-GC-MS/MS protocol. Metabolites 2022, 12, 180. [Google Scholar] [CrossRef]
- Zanoni, G.; Giglini Tassotti, L.; Vrhovšek, U.; Carlin, S. Insight on Lugana wines flavor with a new LC-MS method for the detection of polyfunctional thiols. J. Food Compos. Anal. 2025, 142, 107458. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Carlin, S.; Lotti, C.; Vrhovšek, U.; Mattivi, F. Development of a fully automated method HS-SPME-GC-MS/MS for the determination of odor-active carbonyls in wines: A “green” approach to improve robustness and productivity in the oenological analytical chemistry. J. Agric. Food Chem. 2024, 72, 1995–2007. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Luzzini, G.; Samaniego Solis, J.; Forte, F.; Ugliano, M. Two sides to one story—Aroma chemical and sensory signature of Lugana and Verdicchio wines. Molecules 2021, 26, 2127. [Google Scholar] [CrossRef] [PubMed]
- Carlin, S.; Piergiovanni, M.; Pittari, E.; Lisanti, M.T.; Moio, L.; Piombino, P.; Marangon, M.; Curioni, A.; Rolle, L.; Río Segade, S.; et al. The contribution of varietal thiols in the diverse aroma of Italian monovarietal white wines. Food Res. Int. 2022, 157, 111404. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Termopoli, V. Derivatization strategies in flavor analysis: An overview over the wine and beer scenario. Chemistry 2022, 4, 1679–1695. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Masuero, D.; Carlin, S.; Luzzini, G.; Furlan, N.; Slaghenaufi, D.; Ugliano, M.; Rolle, L.; Río Segade, S.; Piombino, P.; et al. Free methyl salicylate and its glycosides mapping in monovarietal Italian white wines. OENO One 2023, 57, 115–127. [Google Scholar] [CrossRef]
- Wang, J.; Gambetta, J.M.; Jeffery, D.W. Comprehensive study of volatile compounds in two Australian rosé wines: Aroma extract dilution analysis (AEDA) of extracts prepared using solvent-assisted flavor evaporation (SAFE) or headspace solid-phase extraction (HS-SPE). J. Agric. Food Chem. 2016, 64, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Oellig, C.; Zhang, Y.; Liu, Y.; Chen, Y.; Zhang, Y. Characterization of the key odorants in goji wines in three levels of sweetness by applications of sensomics approach. Food Chem. 2024, 461, 140803. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of some volatile constituents of bell peppers. J. Agric. Food Chem. 1969, 17, 1322–1327. [Google Scholar] [CrossRef]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Boonbumrung, S.; Tamura, H.; Mookdasanit, J.; Nakamoto, H.; Ishihara, M.; Yoshizawa, T.; Varanyanond, W. Characteristic aroma components of the volatile oil of yellow keaw mango fruits determined by limited odor unit method. Food Sci. Technol. Res. 2001, 7, 200–206. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.; Elizondo, A.; Moya, E. A study of carbonyl compounds in beer. Part II. Flavor and flavor thresholds of aldehydes and ketones added to beer. Tech. Q. Master Brew. Assoc. Am. 1970, 7, 143–149. [Google Scholar]
- Darriet, P.; Pons, M.; Henry, R.; Dumont, O.; Findeling, V.; Cartolaro, P.; Calonnec, A.; Dubourdieu, D. Impact odorants contributing to the fungus type aroma from grape berries contaminated by powdery mildew (Uncinula necator); incidence of enzymatic activities of the yeast Saccharomyces cerevisiae. J. Agric. Food Chem. 2002, 50, 3277–3282. [Google Scholar] [CrossRef]
- Culleré, L.; Cacho, J.; Ferreira, V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. J. Agric. Food Chem. 2007, 55, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Culleré, L.; Ferreira, V.; Cacho, J. Analysis, occurrence and potential sensory significance of aliphatic aldehydes in white wines. Food Chem. 2011, 127, 1397–1403. [Google Scholar] [CrossRef]
- Buttery, R.G.; Acree, T.E.; Teranishi, R. (Eds.) Quantitative and sensory aspects of flavor of tomato and other vegetables and fruits. In Flavor Science: Sensible Principles and Techniques; ACS Professional Reference Book; American Chemical Society: Washington, DC, USA, 1993; pp. 259–286. [Google Scholar]
- Ferreira, V.; Ardanuy, M.; López, R.; Cacho, J.F. Relationship between flavor dilution values and odor unit values in hydroalcoholic solutions: Role of volatility and a practical rule for its estimation. J. Agric. Food Chem. 1998, 46, 4341–4346. [Google Scholar] [CrossRef]
- Falcao, L.D.; Lytra, G.; Darriet, P.; Barbe, J.C. Identification of ethyl 2-hydroxy-4-methylpentanoate in red wines, a compound involved in blackberry aroma. Food Chem. 2012, 132, 230–236. [Google Scholar] [CrossRef]
- Tat, L.; Comuzzo, P.; Battistutta, F.; Zironi, R. Sweet-like off-flavor in Aglianico del Vulture wine: Ethyl phenylacetate as the mainly involved compound. J. Agric. Food Chem. 2007, 55, 5205–5212. [Google Scholar] [CrossRef]
- Tominaga, T.; Blanchard, L.; Darriet, P.; Dubourdieu, D. A powerful aromatic volatile thiol, 2-furanmethanethiol, exhibiting roast coffee aroma in wines made from several Vitis vinifera grape varieties. J. Agric. Food Chem. 2000, 48, 1799–1802. [Google Scholar] [CrossRef]
- Chatonnet, P.; Lavigne, V.; Boidron, J.N.; Dubourdieu, D. Identification and analysis of heavy volatile sulfur compounds in wines by gas chromatography and flame photometry. Sci. Aliment. 1992, 12, 513–532. [Google Scholar]
- Saliba, A.J.; Bullock, J.; Hardie, W.J. Consumer rejection threshold for 1,8-cineole (eucalyptol) in Australian red wine. Food Qual. Prefer. 2009, 20, 500–504. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Tarasov, A.; Giuliani, N.; Dobrydnev, A.; Schuessler, C.; Volovenko, Y.; Rauhut, D.; Jung, R. 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) sensory thresholds in Riesling wine. Foods 2020, 9, 606. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Antalick, G.; Tempère, S.; Šuklje, K.; Blackman, J.W.; Deloire, A.; de Revel, G.; Schmidtke, L.M. Investigation and sensory characterization of 1,4-cineole: A potential aromatic marker of Australian Cabernet Sauvignon wine. J. Agric. Food Chem. 2015, 63, 9103–9111. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar]
- Raymond, J.L.G.; Schumaker, M.A.; Langenheim, J.H. Variation in chemical and physical properties during leaf development in California bay tree (Umbellularia californica): Predictions regarding palatability for deer. Biochem. Syst. Ecol. 1996, 24, 93–103. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Tominaga, T.; Masneuf, I.; Peyrot des Gachons, C.; Murat, M.L. The role of yeasts in grape flavor development during fermentation: The example of Sauvignon Blanc. AJEV 2006, 57, 81–88. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef]
- Carlin, S.; Masuero, D.; Guella, G.; Vrhovsek, U.; Mattivi, F. Methyl salicylate glycosides in some Italian varietal wines. Molecules 2019, 24, 3260. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Lavigne, V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. Food Agric. 1993, 62, 191–202. [Google Scholar] [CrossRef]
- Ferreira, V.; de la Fuente, A.; Sáenz-Navajas, M.P. Wine aroma vectors and sensory attributes. In Managing Wine Quality, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 3–39. [Google Scholar]
- Mateo-Vivaracho, L.; Zapata, J.; Cacho, J.; Ferreira, V. Analysis, occurrence, and potential sensory significance of five polyfunctional mercaptans in white wines. J. Agric. Food Chem. 2010, 58, 10184–10194. [Google Scholar] [CrossRef]
- Fox, D.J.; Harbertson, J.F. Comparison of pre-fermentation and post-fermentations alcohol adjustments on aromatic chemistry and sensory composition of Sauvignon blanc wine. Food Chem. 2025, 460, 140757. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Dubourdieu, D. Study of the formation mechanisms of some volatile compounds during the aging of sweet fortified wines. J. Agric. Food Chem. 1999, 47, 2837–2846. [Google Scholar] [CrossRef]
- Muhl, J.R.; Derycke, M.; Pilkington, L.I.; Fedrizzi, B.; Deed, R.C. A green liquid chromatography-tandem mass spectrometry method for the simultaneous analysis of volatile thiols and their precursors in oenological samples. J. Chrom. A 2023, 1707, 464273. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Hydrolysis and formation of volatile esters in New Zealand Sauvignon blanc wine. Food Chem. 2012, 135, 486–493. [Google Scholar] [CrossRef]
- Oliveira Santana, G.R.; Crepalde, L.T.; Cardoso Hernandes, K.; Diogo Silveira, R.; Padilha, G.; Reitenbach, A.F.; Kulkamp de Souza, A.L.; Welke, J.E.; Caliari, V.; Burin, V.M. Impact of skin contact on the volatile composition and sensory properties of white wines from resistant varieties. Food Chem. 2025, 489, 144967. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]

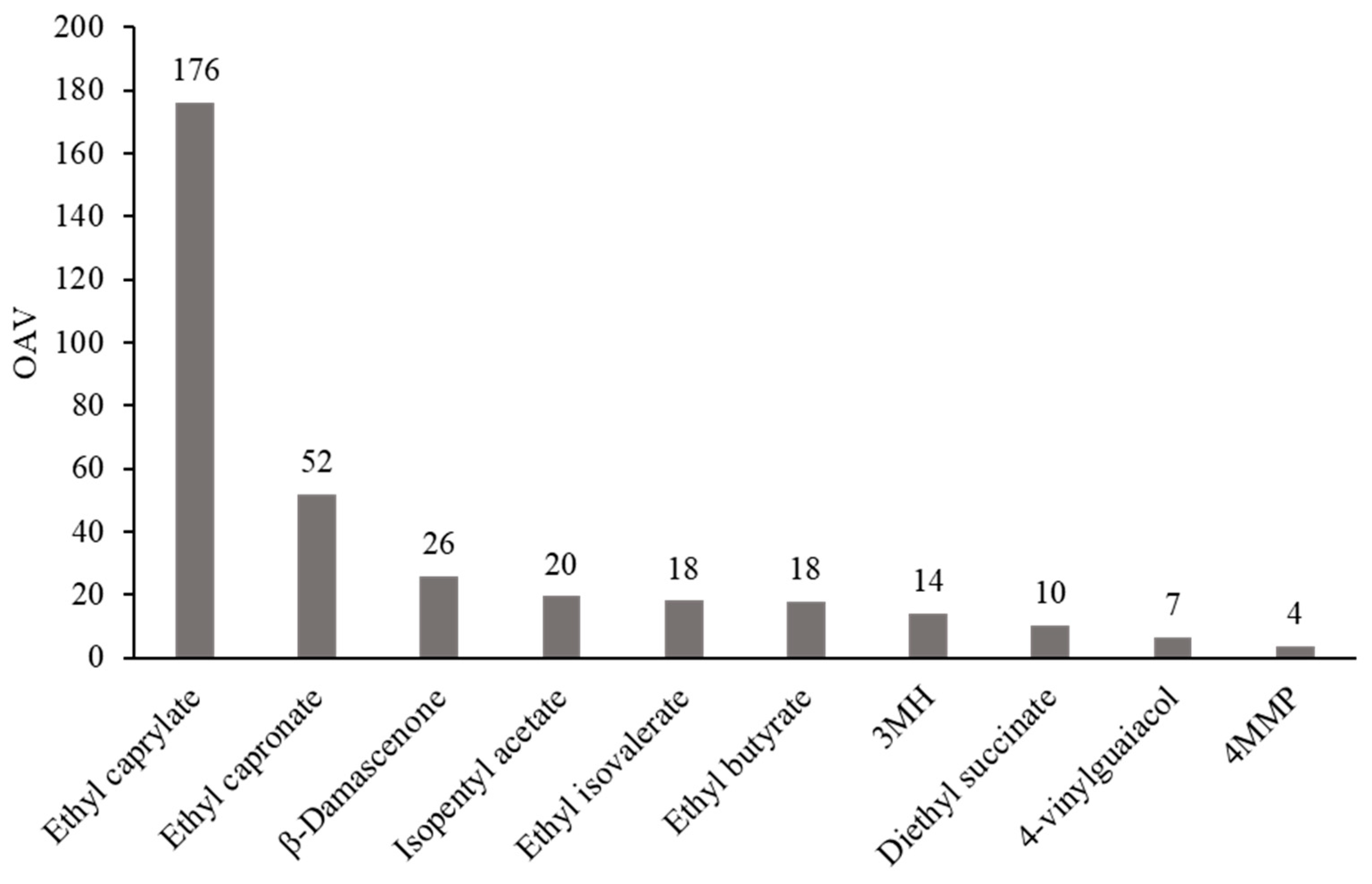
| VOC | Mean | Min | Max | ODT | OAV |
|---|---|---|---|---|---|
| Acid (µg/L) | |||||
| Decanoic acid | 2200 | 1110 | 4240 | 10,000 [10] | 0.22 |
| Octanoic acid | 3940 | 3170 | 4930 | 3000 [10] | 1.31 |
| Valeric acid | 17.7 | 11.6 | 33.0 | 11,000 [11] | 0.001 |
| Alcohol (µg/L) | |||||
| 1-hexanol | 617 | 190 | 1170 | 2500 [12] | 0.08 |
| cis-3-hexen-1-ol | 60.4 | 24.9 | 130 | 70 [13] | 0.86 |
| trans-3-hexen-1-ol | 35.1 | 3.43 | 84.5 | 600 [14] | 0.06 |
| Aldehyde (µg/L) | |||||
| 2-furfural | 194 | 49.5 | 877 | 14,100 [10] | 0.01 |
| 2-methylpentanal | 0.10 | 0.05 | 0.39 | ||
| 2-propenal | 7.14 | 3.41 | 17.1 | ||
| 3-methyl-2-butenal | 0.59 | 0.55 | 0.69 | ||
| 5-methyl-2-furfural | 6.77 | 2.28 | 24.0 | 1100 [15] | 0.01 |
| Benzaldehyde | 8.35 | 0.01 | 107 | 2000 [16] | 0.004 |
| Butanal | 7.48 | 1.60 | 16.5 | 9.00 [17] | 0.83 |
| Decanal | 8.22 | 3.02 | 28.8 | 5.00 [18] | 1.34 |
| trans-2-butenal | 1.77 | 1.52 | 2.11 | ||
| trans-2-decenal | 2.03 | 1.97 | 2.13 | 1.00 [17] | 2.03 |
| trans-2-heptenal | 0.11 | 0.09 | 0.32 | 4.6 [18] | 0.02 |
| trans-2-hexenal | n.d. | n.d. | n.d. | 4.00 [18] | |
| trans-2-nonenal | n.d. | n.d. | n.d. | 0.60 [18] | |
| trans-2-octenal | n.d. | n.d. | n.d. | 3.00 [18] | |
| trans-2-pentenal | 0.19 | 0.18 | 0.23 | 1500 [19] | 0.00 |
| Heptanal | 1.28 | 0.54 | 4.10 | 3.00 [12] | 0.43 |
| Hexanal | 2.10 | 0.02 | 16.1 | 4.50 [13] | 0.42 |
| Nonanal | 7.97 | 1.39 | 69.3 | 15.0 [17] | 0.53 |
| Octanal | 1.34 | 0.76 | 5.10 | 0.70 [17] | 1.91 |
| Pentanal | 1.37 | 0.41 | 6.15 | 12.0 [20] | 0.11 |
| Propanal | 25.1 | 7.06 | 72.6 | 9.50 [20] | 2.64 |
| Undecanal | 7.69 | 3.53 | 23.5 | 5.00 [20] | 1.54 |
| Benzenoid (µg/L) | |||||
| Benzyl alcohol | 161 | 53.9 | 334 | 200,000 [21] | 0.00 |
| Methyl salicylate | 16.9 | 1.53 | 41.9 | 38 [9] | 0.42 |
| Ester (µg/L) | |||||
| Butyl acetate | 0.57 | 0.01 | 3.61 | 1800 [21] | 0.00 |
| Diethyl succinate | 2040 | 1560 | 2490 | 200 [10] | 10.2 |
| Ethyl 2-methylbutyrate | 29.5 | 10.9 | 70.6 | 18.0 [10] | 1.64 |
| Ethyl butyrate | 357 | 186 | 629 | 20.0 [10] | 17.9 |
| Ethyl caprate | 269 | 84.5 | 596 | 200 [10] | 1.35 |
| Ethyl capronate | 724 | 427 | 1290 | 14.0 [10] | 51.7 |
| Ethyl caprylate | 881 | 384 | 1450 | 5.00 [21] | 176 |
| Ethyl cinnamate | 0.55 | 0.15 | 1.48 | 1.10 [21] | 0.50 |
| Ethyl heptanoate | 0.52 | 0.33 | 0.71 | 18.0 [22] | 0.03 |
| Ethyl isovalerate | 54.4 | 25.9 | 95.8 | 3.00 [22] | 18.1 |
| Ethyl leucate | 117 | 55.8 | 209 | 400 [23] | 0.29 |
| Ethyl phenylacetate | 5.10 | 3.48 | 7.40 | 73.0 [24] | 0.07 |
| Ethyl valerate | 1.16 | 0.44 | 2.28 | ||
| Hexyl acetate | 14.3 | 0.60 | 59.1 | 1500 [10] | 0.01 |
| Isobutyl acetate | 22.2 | 9.00 | 39.2 | 1600 [21] | 0.01 |
| Isopentyl acetate | 585 | 113 | 2112 | 30.0 [10] | 19.5 |
| Phenylethyl acetate | 89.7 | 15.50 | 337 | 250 [10] | 0.36 |
| Furan (µg/L) | |||||
| Furfurylthiol | n.d. | n.d. | n.d. | 0.002 [25] | |
| Heterocycle (µg/L) | |||||
| Benzothiazole | 0.75 | n.d. | 2.11 | 50.0 [26] | 0.02 |
| Ketone (µg/L) | |||||
| 2-aminoacetophenone | 0.12 | 0.07 | 0.17 | 0.50 [27] | 0.23 |
| 2-butanone | 14.2 | 0.26 | 102 | 50,000 [5] | 0.00 |
| 2-cyclohexen-1-one | 0.69 | n.d. | 1.34 | ||
| 2-decanone | 0.11 | 0.09 | 0.19 | ||
| 2-heptanone | 1.67 | 0.30 | 4.42 | ||
| 2-hexanone | 0.16 | 0.11 | 0.23 | ||
| 2-methyl-3-pentanone | 0.31 | 0.24 | 0.60 | ||
| 2-nonanone | 2.02 | 1.38 | 3.32 | 5.00 [5] | 0.40 |
| 2-octanone | 0.72 | 0.68 | 0.78 | 50.0 [5] | 0.01 |
| 2-pentanone | n.d. | n.d. | 2.86 | 1.38 [15] | |
| 2-undecanone | 1.51 | 1.33 | 2.74 | 7.00 [5] | 0.22 |
| 3-hexanone | n.d. | n.d. | n.d. | ||
| 3-methyl-2-butanone | 1.07 | 0.03 | 2.31 | ||
| 3-methylthio-2-butanone | 0.31 | n.d. | 0.36 | ||
| 3-pentanone | 2.54 | 1.01 | 8.45 | ||
| 4-methylthio-2-butanone | 0.10 | n.d. | 0.34 | ||
| 4-methyl-2-pentanone | 0.59 | 0.50 | 0.98 | ||
| 4-methyl-4-methylthio-2-pentan | 11.36 | 3.23 | 28.0 | ||
| 6-methyl-5-hepten-2-one | 0.09 | n.d. | 0.96 | 50.0 [5] | 0.00 |
| Lactone (µg/L) | |||||
| cis-whiskey lactone | n.d. | n.d. | n.d. | 67.0 [21] | |
| δ-decalactone | 8.48 | 4.25 | 11.6 | 386 [10] | 0.02 |
| γ-decalactone | 1.08 | 0.56 | 2.17 | 10.0 [28] | 0.11 |
| γ-dodecalactone | n.d. | n.d. | n.d. | ||
| γ-nonalactone | 2.54 | 1.18 | 4.61 | 30.0 [16] | 0.08 |
| γ-octalactone | 0.50 | n.d. | 0.97 | ||
| Menthalactone | n.d. | n.d. | n.d. | ||
| trans-whiskey lactone | 0.05 | n.d. | 0.51 | 67.0 [12] | 0.00 |
| Methoxypyrazine (µg/L) | |||||
| 2-sec-butyl-3-methoxypyrazine | n.d. | n.d. | n.d. | ||
| Norisoprenoid (µg/L) | |||||
| β-damascenone | 1.29 | 0.37 | 2.83 | 0.05 [21] | 25.7 |
| β-damascone | n.d. | n.d. | n.d. | ||
| β-ionone | n.d. | n.d. | n.d. | 0.03 [12] | |
| Safranal | 0.23 | 0.16 | 0.34 | ||
| TDN | 5.88 | 2.24 | 9.96 | 2.00 [29] | 2.94 |
| Phenol (µg/L) | |||||
| 4-ethylguaiacol | 32.7 | n.d. | 183 | 33.0 [30] | 1.00 |
| 4-vinylguaiacol | 66.1 | 22.9 | 164 | 10.0 [30] | 6.61 |
| Eugenol | 1.25 | 0.30 | 3.49 | 6.00 [30] | 0.21 |
| Guaiacol | 0.36 | 0.22 | 0.87 | 10.0 [30] | 0.03 |
| Zingerone | 3.51 | 0.99 | 7.49 | ||
| Terpenes (µg/L) | |||||
| 1,4-cineole | 0.48 | 0.25 | 1.54 | 0.63 [31] | 0.76 |
| 1,8-cineole | 0.04 | n.d. | 0.45 | 1.10 [27] | 0.04 |
| α-terpineol | 7.01 | 2.96 | 14.5 | 250 [16] | 0.03 |
| β-citronellol | 1.02 | n.d. | 3.38 | 100 [21] | 0.01 |
| Geranic acid | 16.4 | 12.2 | 33.8 | ||
| Geraniol | 1.10 | n.d. | 3.46 | 30.0 [30] | 0.04 |
| Linalool | 3.46 | 0.86 | 10.4 | 25.2 [12] | 0.01 |
| Linalool oxide A | 3.71 | 1.12 | 10.1 | ||
| Linalool oxide B | 1.96 | 0.77 | 4.52 | ||
| Nerol | n.d. | n.d. | n.d. | 400 [32] | |
| cis-rose oxide | 0.07 | 0.05 | 0.16 | 0.20 [32] | 0.37 |
| trans-rose oxide | n.d. | n.d. | 0.02 | ||
| Terpinen-4-ol | 1.79 | 1.00 | 2.88 | 340 [33] | 0.01 |
| trans-terpin | 4.36 | 2.20 | 14.7 | ||
| Thiols (ng/L) | |||||
| Benzylmercaptan | 0.87 | n.d. | 2.00 | ||
| 3MH | 845 | n.d. | 2330 | 60.0 [34] | 14.1 |
| 3MHA | 3.60 | 0.37 | 20.6 | 4.00 [34] | 0.90 |
| 4MMP | 2.84 | 0.19 | 21.1 | 0.80 [34] | 3.54 |
| Methyl salicylate glycosides (µg/L) | |||||
| MeSAG | 82.3 | 10.7 | 233 | ||
| MeSA-primeveroside | 10.2 | 1.33 | 30.6 | ||
| MeSA-violutoside | 47.8 | 12.6 | 110 | ||
| MeSA-canthoside A | 6.37 | n.d. | 57.3 | ||
| MeSA-rutinoside | 33.8 | 4.41 | 87.2 | ||
| MeSA-gentiobioside | 179 | 7.08 | 599 | ||
| MSTG-A | n.d. | n.d. | n.d. |
| Compound | Incrocio Bruni 54 | Italian Whites | Verdicchio | Lugana | |||
|---|---|---|---|---|---|---|---|
| Parameter | Conc. | Conc. | p-Value | Conc. | p-Value | Conc. | p-Value |
| 4MMP * | 2.84 | 2.32 | 0.3445 | 1.16 | 0.1072 | 2.45 | 0.3865 |
| 3MH * | 846 | 448 | 0.0011 | 355 | 0.0093 | 399 | 0.0114 |
| 3MHA * | 3.60 | 3.04 | 0.3247 | 1.49 | 0.0663 | 2.64 | 0.2488 |
| Methyl salicylate | 16.9 | 1.80 | 0.0000 | 5.26 | 0.0021 | 4.96 | 0.0017 |
| MeSAG | 82.3 | 108 | 0.0991 | 452 | 0.0000 | 504 | 0.0000 |
| MeSA-primeveroside | 10.2 | 9.02 | 0.3511 | 31.3 | 0.0014 | 34.5 | 0.0000 |
| MeSA-violutoside | 47.8 | 47.3 | 0.4843 | 115 | 0.0005 | 145 | 0.0000 |
| MeSA-canthoside A | 6.37 | 2.05 | 0.1341 | 4.75 | 0.3446 | 2.73 | 0.1801 |
| MeSA-rutinoside | 33.9 | 10.1 | 2.9038 | 36.8 | 0.3848 | 38.6 | 0.3189 |
| MeSA-gentiobioside | 179 | 115 | 0.1271 | 461 | 0.0262 | 561 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piergiovanni, M.; Moretton, M.; Masuero, D.; Carlin, S. A Deep Analytical Investigation of the Aroma Chemistry of Incrocio Bruni 54 and Its Differentiation from Italian White Varieties. Fermentation 2025, 11, 590. https://doi.org/10.3390/fermentation11100590
Piergiovanni M, Moretton M, Masuero D, Carlin S. A Deep Analytical Investigation of the Aroma Chemistry of Incrocio Bruni 54 and Its Differentiation from Italian White Varieties. Fermentation. 2025; 11(10):590. https://doi.org/10.3390/fermentation11100590
Chicago/Turabian StylePiergiovanni, Maurizio, Martina Moretton, Domenico Masuero, and Silvia Carlin. 2025. "A Deep Analytical Investigation of the Aroma Chemistry of Incrocio Bruni 54 and Its Differentiation from Italian White Varieties" Fermentation 11, no. 10: 590. https://doi.org/10.3390/fermentation11100590
APA StylePiergiovanni, M., Moretton, M., Masuero, D., & Carlin, S. (2025). A Deep Analytical Investigation of the Aroma Chemistry of Incrocio Bruni 54 and Its Differentiation from Italian White Varieties. Fermentation, 11(10), 590. https://doi.org/10.3390/fermentation11100590









