Abstract
An optimal culture medium and highly stable biometabolites are important in industrial production processes. The response surface methodology with a Box–Behnken design was performed to determine the optimal culture medium of an engineered Aspergillus oryzae strain for cordycepin production by submerged fermentation. The influences of glucose, yeast extract, and adenine concentrations on cordycepin production were explored, and their concentrations were used for experimental design. The results reveal that the optimal culture components involved 30.0 g/L of glucose, 9.8 g/L of yeast extract, and 1.5 g/L of adenine. As predicted, the maximum cordycepin concentration (1724.53 ± 18.30 mg/L) was obtained with a short fermentation time (2 days). A significant increase in cordycepin yield (>50% increase) was observed in the culture grown in the optimized culture medium compared to that grown in the basal medium. A xanthine oxidase inhibitory activity assay demonstrated that the cordycepin product had a pharmacological function. It exhibited strong stability under high thermal and acidic conditions, with over 95% product recovery. The findings of this study are valuable for developing cost-effective processes for producing health-benefiting products.
1. Introduction
With the increasing number of health-conscious consumers in recent years, the market for functional ingredients has expanded annually. Among such functional ingredients, a bioactive compound, cordycepin, exhibits several pharmacological properties, such as anti-inflammatory, anti-diabetic, anti-hyperuricemic, antitumor, antimicrobial, and immune-promoting effects [1]. Due to these properties, it is in strong demand in the healthcare market and the cosmeceutical industry. However, commercial cordycepin products are produced by entomopathogenic fungi (Cordyceps militaris and Cordyceps sinensis), which have low growth rates and cordycepin yields [2,3]. Hence, efforts have been devoted to constructing high-cordycepin-producing strains of C. militaris using proton beam irradiation and by optimizing the cultivation process [4,5]. Although high cordycepin production (8.6–14.3 g/L) has been reported in the mutant C. militaris strain, its cordycepin productivity remains low (<350 mg/L/d) due to the long culture period (30–45 days). To overcome these limitations and meet the increasing market demand, cordycepin production by high-growth-rate microbes has been achieved through synthetic biology and fermentation technologies [6,7,8,9]. Recently, a cordycepin-producing strain of Aspergillus oryzae (AoCordy-T1) was generated by overexpressing metabolic genes involved in cordycepin biosynthesis under the control of constitutive promoters [8]. The engineered A. oryzae strain demonstrated high cordycepin productivity (>500 mg/L/d) with a production period over 10 times shorter compared to Cordyceps spp. Additionally, it showed metabolic diversity in carbon (C5, C6, and C12 sugars) and nitrogen (organic and inorganic) utilization for cordycepin production. Based on these advantages, the engineered A. oryzae strain is of great interest as a powerful candidate for practical production at a larger scale.
The superior strain, optimum culture conditions, and high stability of the targeted product are crucial to developing an efficient production process with economic feasibility. Precision fermentation has been thought to be a powerful strategy for enhancing product yields and overcoming the burden on downstream processing by providing options for cost-effective concentration and purification steps. It has been reported that the type and concentration of carbon and nitrogen sources, along with purine nucleoside precursors in the culture medium, are critical for cordycepin production [3,10,11]. The culture medium used for cordycepin production has been intensively developed for C. militaris and other Cordyceps spp. In the engineered A. oryzae, although glucose, yeast extract, and adenine have been reported to be preferred nutrients for cordycepin production [8], their interactive concentrations on biomass and cordycepin production are not understood. This study investigated optimal glucose, yeast extract, and adenine concentrations to obtain high cordycepin productivity through thesubmerged fermentation (SmF) of the engineered A. oryzae strain. To minimize the total number of experiments, the Box–Behnken design (BBD) and response surface methodology (RSM) were employed to identify the key factors in media composition. The combination of BBD and RSM overcomes the limitations of traditional methods and has been successfully used to optimize culture media to enhance the production of various metabolites [3,10,12]. In addition to optimizing the culture medium, the pharmacological function (xanthine oxidase inhibitory activity) and stability of cordycepin in the cell-free supernatant (CFS) were also evaluated, and its potential application was proposed. The information obtained from this study facilitates the upscaled production of cordycepin using the potent engineered strain of A. oryzae.
2. Materials and Methods
2.1. Fungal Strain and Spore Inoculum Preparation
A cordycepin-producing strain of A. oryzae (AoCordy-T1 or TBRC-BMGC 438) [8] was used throughout this study. The spore inoculum was prepared by cultivating fungal cells on polished rice at 30 °C for 5–7 days. The spores were then resuspended in a 0.01% (v/v) Tween 80 solution and harvested by filtration using Miracloth (MerckMillipore, Darmstadt, Germany). The spore count was determined using a hemacytometer, and the spores were stored at 4 °C.
2.2. Submerged Culture Conditions
SmF of fungal culture was performed in a 250 mL Erlenmeyer flask containing 50 mL of semi-synthetic medium (40 g/L (w/v) glucose, 0.2 g/L NH4Cl, 5 g/L yeast extract, 2.4 g/L KH2PO4, 0.5 g/L MgSO4∙7H2O, 0.1 g/L CaCl2∙2H2O, 15 mg/L FeCl3∙7H2O, 10 mg/L MnSO4∙H2O, 7.5 mg/L ZnSO4∙7H2O, and 1.5 g/L adenine) [8]. A final concentration of 2 × 106 spores/mL was inoculated to the culture medium and incubated at 30 °C with shaking at 200 rpm [8].
2.3. Single-Factor Experiment for Screening Optimal Culture Medium
The effects of glucose (20, 40, 60, and 80 g/L) and yeast extract (2.5, 5, 10, 15, and 20 g/L) concentrations on the growth and cordycepin production of AoCordy-T1 were investigated using a one-factor-at-a-time approach. All experiments were performed in triplicate, and the average and standard deviations of the resulting data were presented. Statistical analysis was performed using the Statistical Package for the Social Sciences version 11.5 software packet with Duncan’s Multiple Range Test. The data with p < 0.05 were considered statistically significant.
2.4. Design of Experiment
The BBD [13] and RSM were applied to determine the optimum substrate concentrations for cordycepin production by AoCordy-T1. The concentrations of glucose (X1), yeast extract (X2), and adenine (X3) were given as the key factors influencing cordycepin production. Each variable was tested at three levels (Table 1): high (+1), intermediate (0), and low (−1). The actual values were based on the results from the screening experiment (Section 2.3) and those from the previous study [8]. A total of 26 experiments were run to identify the significant variables and then measure their responses (Table 2). The results obtained were fitted to a second-order quadratic polynomial equation as follows:

Table 1.
Three independent variables used for Box–Behnken design.

Table 2.
Box–Behnken design and response values.
Y is the cordycepin yield; β0, βj, βjj, and βij are the intercept, linearity, square, and interaction regression coefficients, respectively; and xi and xj are the coded variables.
Design Expert® Software Version 11.0 (Stat-Ease Inc., Minneapolis, MN, USA) was used to analyze the experimental data. A mathematical correlation between three independent variables of cordycepin production was developed. The model coefficients were calculated, and the response surface plots were generated. An analysis of variance (ANOVA) was performed to assess the significance of the model (p < 0.05). Finally, the model and the optimized culture medium were experimentally validated using six replicates.
2.5. Xanthine Oxidase Inhibitory Activity
To measure the xanthine oxidase (XO) inhibitory activity of cordycepin, CFSs of A. oryzae strains (AoCordy-T1 and wild type) grown under the optimal design of experiment (DOE) conditions were freeze-dried for 24–36 h and then dissolved in 0.07 M phosphate buffer (pH 7.5) at concentrations in the range of 1–10 mg/mL. The assay was performed in the same way as in a previous report [14]. The reaction mixture, containing 50 μL of sample, 30 μL of 0.07 M phosphate buffer, and 30 μL of 150 μM XO solution, was incubated at 25 °C for 15 min. Reactions were initiated by adding 60 μL of 150 μM xanthine substrate and incubating at 25 °C for 30 min, and then stopped by adding 25 μL of 1 N hydrochloric acid. Absorbance was measured at 290 nm using a microplate readerBioTek, Synergy H1 (Santa Clara, CA, USA). Phosphate buffer and CFS from the wild-type culture (WT-CFS) were negative controls. Allopurinol was used as a positive control at concentrations ranging from 0 to 100 μg/mL. The XO inhibitory activity was calculated using the following formula:
% Inhibition = 100 × [(A − a) − (B − b)]/(A − a)
A is the enzyme activity without the test sample, and a is the control of A without the test sample and XO enzyme. B and b are the activities of the test samples with and without XO enzyme, respectively. The values of IC50 (half-maximal inhibitory concentration) were calculated from the correlation between % inhibition and sample concentration. The mean values of the test trials were obtained from three replicates (available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 15 December 2024)).
2.6. The Stability of the Cordycepin in the Culture Broth
The impact of temperature and pH on cordycepin stability in the CFS of an engineered A. oryzae strain (AoCordy-T1) was evaluated. Thermal stability was assessed by incubating 5 mL of culture broth (pH 7.0) at different temperatures, namely at 30 (control), 60, 80, and 100 °C for 6 h, as well as under autoclave conditions (15 psi and 121 °C) for 35 min, followed by gradually cooling to 30 °C. The samples were then adjusted to a final volume of 5 mL before analysis by high-performance liquid chromatography (HPLC). The effect of acid–alkali treatment was also examined by adjusting the pH of the CFS to 2, 4, 6, 8, 10, and 12 using 2 mol/L HCl or KOH, followed by incubation at 30 °C for 16 h. Afterward, the CFS was readjusted to pH 7.0 before cordycepin analysis. Cordycepin in the CFS (pH 7.0) was used as a control. The percentage recovery of cordycepin after the treatments was calculated to assess the product’s stability.
2.7. Analytical Procedures
The cell mass and residual sugar concentration in the A. oryzae cultures were determined following the steps in a previous study [8]. The cordycepin production was analyzed using HPLC (Ultimate 3000; Thermo Fisher Scientific, Waltham, MA, USA) equipped with a diode array detector and an Acclaim™ C18 column (5 µm, 4.6 mm × 250 mm) (Thermo Scientific, Waltham, MA, USA). HPLC analysis was conducted at a column temperature of 35 °C, with 15% (v/v) methanol in ultrapure water as a mobile phase at the flow rate of 0.7 mL/min for 15 min. Cordycepin and adenine were identified by comparing their retention times under UV spectra at 260 nm with those of authentic standards (Catalog no. PHL82505; Sigma-Aldrich, St. Louis, MO, USA). The concentrations of cordycepin and adenine in the CFS were quantified by corresponding standard curves in the 0.01–0.5 mg/mL range (R2 > 0.9900).
3. Results and Discussion
3.1. Obtaining Optimal Substrate Variables Using the One-Factor-At-a-Time Screening Approach
Cordycepin production under different concentrations of carbon and nitrogen substrates was examined to define their optimal levels for the DOE analysis. The results show that the initial glucose concentrations ranging from 20 to 80 g/L in the culture medium did not significantly affect cordycepin production in AoCordy-T1 (Figure 1A). As observed in all cultures, the cordycepin concentration rapidly increased during the fast-growing stage (2 d of cultivation) and then slightly increased when the fungal cultures entered the stationary phase, similar to a previous study [8]. Likely, cordycepin was a growth-associated metabolite. High cordycepin productivity (682.09–704.88 mg/L/d) and cordycepin concentrations (1364.18–1409.76 mg/L) were observed in the 40–80 g/L glucose cultures at the fast-growing stage. High glucose concentrations (60 and 80 g/L) did not enhance the cordycepin production of the AoCordy-T1 cultures, and residual glucose concentrations at 25.13 ± 0.11 and 49.59 ± 0.28 g/L, respectively, were detected (Figure 1A). Thus, low glucose concentrations (20–40 g/L) were chosen for further study.
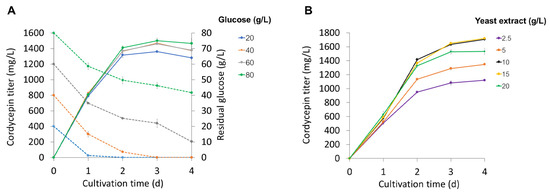
Figure 1.
The effects of glucose (A) and yeast extract (B) concentrations on cordycepin production by the engineered Aspergillus oryzae (AoCordy-T1). The cordycepin titer (solid line) and residual glucose (dash line) concentrations in the culture supernatant are shown. All data are presented as mean values with the standard deviation (SD).
The yeast extract was a preferred nitrogen source for cordycepin synthesis in C. militaris [4]. Focusing on the yeast extract concentration in cordycepin production by A. oryzae, we found that the cordycepin concentration increased with an increase in yeast extract concentrations. However, the highest cordycepin - concentration was found in the 15 g/L yeast extract culture, reaching 1719.40 ± 3.46 mg/L (Figure 1B). A higher concentration of yeast extract (20 g/L) in the culture medium did not promote cordycepin production in the AoCordy-T1 culture. Excess nitrogen source might influence the mycelial growth and cell vitality [15]. However, no significant differences (p > 0.05) were observed in the biomass titers between the cultures using 15 g/L (DCW 22.27 ± 0.70 g/L) and 20 g/L (DCW 22.20 ± 1.49 g/L) of yeast extract. Therefore, 5–15 g/L yeast extract concentrations were used for the DOE analysis.
In addition to carbon and nitrogen sources, purine nucleosides, especially adenine, are important precursors that are directly linked to cordycepin production in various cordycepin producers [4,6,8,16]. However, adenine supplementation is costly in cordycepin production, and the residual adenine in the culture might interfere with product quality. The drawback of high levels of adenine (2.0 g/L) on cordycepin production has been reported [8]. By varying the adenine concentrations in the AoCordy-T1 cultivations, 313.34 ± 11.16 mg/L of residual adenine was detected at the 2-day cultivation time when the culture was supplemented with 2 g/L of adenine. Thus, adenine concentrations in the range of 0.5–1.5 g/L were used for further AoCordy-T1 cultivations.
3.2. Optimization of Cordycepin Production by RSM Using BBD
As glucose, yeast extract, and adenine significantly affected the cordycepin production of A. oryzae, a response surface analysis of these factors at three levels was performed in a submerged culture using the BBD. The design matrices of the three variables and the corresponding experimental results are listed in Table 2. A second-order polynomial equation was established by applying a multiple regression analysis to the experimental data.
An analysis of variance of the model for cordycepin production was significant at p = 0.000, R2 = 0.888, and F-value = 23.13 (Table 3). This indicates that the predicted response values from the model were highly correlated with the experimental values and helped predict cordycepin production by A. oryzae. The statistical analysis revealed that X2, X3, , and X2X3 were highly significant variables for cordycepin production (p < 0.05) (Table 3). Among the variables, adenine (X3) greatly affected cordycepin production (F-value = 126.58; p < 0.05). In addition, an appropriate yeast extract concentration (X2) also impacted cordycepin production, whereas the glucose concentration (X3) did not have a significant effect.

Table 3.
Analysis of variance of cordycepin production using response surface methodology.
The optimal values of the individual variables presented in the 3D response surface plots and 2D contour plots were generated to determine the interaction between the three variables and cordycepin production (Figure 2). The downward opening of the response surface plots indicated that cordycepin production could be enhanced by increasing the concentrations of glucose, yeast extract, and adenine at optimum values (Figure 2). Since the maximum concentration of adenine was limited to 1.5 g/L to prevent residual adenine in the culture, the skewed response surface plot of adenine concentrations on cordycepin production was observed (Figure 2B,C). Notably, high cordycepin production (>1200 mg/L) was consistently observed in the AoCordy-T1 cultures containing a high adenine concentration (1.5 g/L), wherein the concentrations of glucose and yeast extract varied (Table 2 and Figure 2). In contrast, the cultivations using high concentrations of glucose (Figure 2B,E) and yeast extract (Figure 2C,F) led to a decrease in cordycepin production, as indicated by the blue zone. Accordingly, low cordycepin titers (<500 mg/L) were mainly observed in the 15 g/L yeast extract cultures, except for the cultures containing high adenine concentrations (run nos. 1 and 2 in Table 2), where high cordycepin titers (1680.03–1691.05 mg/L) were obtained. These findings suggest that the leverage levels of yeast extract and adenine in the culture medium were critical for cordycepin production by A. oryzae. Based on the predicted model and response surface plots, a maximum response of 1738.67 mg/L of cordycepin yield, without residual adenine, was observed in the medium with 30.0 g/L of glucose, 9.8 g/L of yeast extract, and 1.5 g/L of adenine.
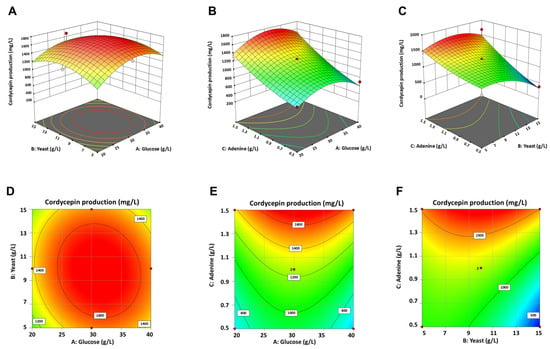
Figure 2.
Three-dimensional response surface plots (A–C) and two-dimensional contour plots (D–F) showing effects of three independent variables (glucose, yeast extract, and adenine concentrations) and their interactions on cordycepin production by engineered Aspergillus oryzae strain.
3.3. Model Verification
To validate the adequacy of the model and optimized culture medium, experiments (six replications) were performed under the optimal conditions (30.0 g/L of glucose, 9.8 g/L of yeast extract, and 1.5 g/L of adenine) with shaking at 200 rpm at 30 °C for two days. High cordycepin concentrations (1724.53 ± 18.30 mg/L) were noted, which concurred with the predicted value of 1738.67 mg/L. This confirmed the reliability of the mathematical model in predicting cordycepin production by SmF of the engineered A. oryzae strain. To date, DOE and RSM have been widely used to optimize the culture medium and conditions for the high production of various metabolites, including cordycepin, of C. militaris [11,17]. Although the cordycepin -concentrations obtained in this study were lower than those achieved with the mutant C. militaris strain (8.6–14.3 g/L) [4,5], the highest cordycepin productivity (862.265 ± 9.15 mg/L/d) with a short production time (2 days) was obtained in this study compared with other cordycepin producers [4,5,6,18]. Cordycepin productivity has been reported to range from 12.47 to 623.23 mg/L/d [8]. Based on chemical and physical factors, several fermentation strategies have been implemented to enhance the cordycepin production of C. militaris and recombinant yeast [4,5,6,18]. To increase the cordycepin titer in the SmF of engineered A. oryzae, optimizing the inoculum size, physical parameters (such as aeration/oxygen supply), and fermentation modes (batch, fed-batch, and repeated batch) could be beneficial. The model and optimized culture medium in this study offer the precise condition for the SmF of the engineered A. oryzae that enhanced cordycepin production with low utility expenses, a major operational cost in the production process.
3.4. In Vitro Xanthine Oxidase Inhibitory Activity of Cordycepin-Containing Cell-Free Supernatant
XO is a key enzyme that is considered a cause of hyperuricemia symptoms. It catalyzes the oxidation of hypoxanthine into xanthine and finally into uric acid [19]. Among the pharmacological properties of the natural cordycepin from C. militaris, its XO inhibitory activity is of interest for the treatment of gout and other arthritic diseases without adverse effects [14,20]. To verify the pharmacological function of recombinant cordycepin in CFS products, the XO inhibitory activity of CFS from AoCordy-T1 culture (COR-CFS) was compared with that of the WT-CFS. An HPLC analysis showed that the freeze-dried COR-CFS sample contained 7.71% cordycepin (77 μg cordycepin/1 mg sample), exhibiting XO inhibitory activity, with an IC50 value of 3.48 ± 0.45 mg/mL (Table 4). Notably, XO inhibitory activity was also observed in the WT-CFS sample; however, a high sample concentration was required for inhibition (IC50 value = 31.68 ± 3.51 mg/mL), which was higher than that of COR-CFS by about 9.10-fold. Possibly, the wild type of A. oryzae might produce purine analogs or other substances that function as XO inhibitors [21]. These results reveal that the recombinant cordycepin in COR-CFS displayed pharmacological functions. However, the purification process is required for using cordycepin as a functional ingredient in therapeutic and nutraceutical products. In addition, other pharmacological functions and product safety should be assessed.

Table 4.
Xanthine oxidase (XO) inhibitory activity of cell-free supernatants (CFSs) from wild-type (WT-CFS) and engineered strain (COR-CFS) of A. oryzae.
3.5. Stability of Cordycepin in Cell-Free Supernatant
The thermal and acid–base tolerance properties of bioactive products are of great interest in production processes (upstream and downstream) and product development. A thermal stability test of cordycepin in CFS showed that incubation at a high temperature (100 °C) for 6 h or under a saturated steam at 121 °C for 35 min did not affect the stability of cordycepin, which was indicated by >95% cordycepin recovery in the crude sample (Figure 3A). Additionally, cordycepin in the crude supernatant was stable under acidic and alkaline conditions (pH 2–10) (Figure 3B). This finding is similar to the previous reports of crude extracts of C. sinensis and C. militaris, which demonstrated that cordycepin could resist high temperatures (90 °C) with 97.9% recovery and was stable under alkaline conditions [22,23,24]. Product concentration and purification are important downstream processes that significantly increase product yield and production costs [25]. Products with high thermostability allow for a wide selection of downstream processes. The high thermal stability of cordycepin facilitates processes using less expensive methods, such as spray drying, instead of freeze-drying, which reduces energy consumption and overall production costs [26,27]. Furthermore, the high stability of cordycepin under steam sterilization will facilitate further refinement of the process for removing recombinant DNA and pathogen contamination from the products without using chemicals or expensive filter membranes [28,29].
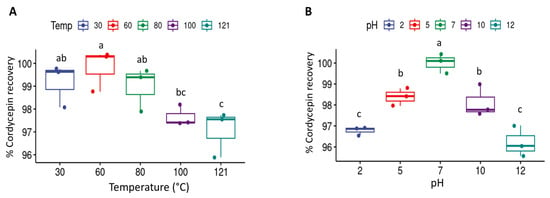
Figure 3.
Thermal stability (A) and acid–base stability (B) of cordycepin product in culture broth. Different superscript letters above box plots for each condition indicate statistically significant difference (p < 0.05) in % cordycepin recovery between CFSs incubated under different conditions.
4. Conclusions
In this study, the effects of major nutrients (glucose, yeast extract, and adenine precursor) on cordycepin production were studied to obtain an optimal culture medium for high cordycepin production by an engineered A. oryzae strain (AoCordy-T1). Based on a response surface analysis using the Box–Behnken design, the optimal concentrations of glucose, yeast extract, and adenine for cordycepin production in A. oryzae were 30.0 g/L, 9.8 g/L, and 1.5 g/L, respectively. The maximum cordycepin concentration (1724.53 ± 18.30 mg/L) was obtained in a short production period (two days of fermentation) and was significantly higher than the culture obtained using a basal medium by 1.53-fold. Moreover, cordycepin in the crude supernatant exhibited pharmacological properties, using xanthine oxidase inhibitory activity as a representative. Furthermore, it had strong stability under high-temperature and acid–base atmospheres. The fundamental information obtained in this study is beneficial for further developing an economically feasible pilot-scale production of cordycepin (upstream and downstream processes).
Author Contributions
Writing the first draft of the manuscript, J.A. and S.J.; conceptualization, S.J. and K.L.; experimental design, experiments, and data analysis, J.A., W.C., S.J., S.P. and N.R.; supervision and project administration, S.J.; revision of the manuscript, S.J. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project is funded by the National Research Council of Thailand (NRCT) and the National Science and Technology Development Agency (NSTDA), Thailand (project number: N42A650392).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data and materials are provided in the main manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SmF | Submerged fermentation |
| BBD | Box–Behnken design |
| RSM | Response surface methodology |
| DOE | Design of experiment |
| AoCordy-T1 | Cordycepin-producing strain of A. oryzae |
| XO | Xanthine oxidase |
| CFS | Cell-free supernatant |
| WT-CFS | CFS from wild-type culture |
| COR-CFS | CFS from AoCordy-T1 culture |
References
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Medicinal Fungus Cordyceps with Its Nutraceutical and Therapeutic Potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, G.; Chai, Z.; Gong, Q.; Guo, J. Synthesis of Cordycepin: Current Scenario and Future Perspectives. Fungal Genet. Biol. 2020, 143, 103431. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, H.; Zeng, B.; Hu, Z. Research Progress on Cordycepin Synthesis and Methods for Enhancement of Cordycepin Production in Cordyceps militaris. Bioengineering 2022, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Das, S.K.; Fujihara, S.; Hatashita, M.; Sakurai, A. Production of Cordycepin by a Repeated Batch Culture of a Cordyceps militaris Mutant Obtained by Proton Beam Irradiation. J. Biosci. Bioeng. 2011, 111, 55–60. [Google Scholar] [CrossRef]
- Masuda, M.; Das, S.K.; Hatashita, M.; Fujihara, S.; Sakurai, A. Efficient Production of Cordycepin by the Cordyceps militaris Mutant G81-3 for Practical Use. Process Biochem. 2014, 49, 181–187. [Google Scholar] [CrossRef]
- Duan, X.-Y.; Tian, Y.; Song, Z.-Q.; Song, L.-P.; Lin, W.-B.; Wang, C.; Yang, H.; Lu, X.-Y.; Ji, X.-J.; Liu, H.-H. High-Level de Novo Biosynthesis of Cordycepin by Systems Metabolic Engineering in Yarrowia lipolytica. Bioresour. Technol. 2022, 363, 127862. [Google Scholar] [CrossRef]
- Huo, C.; Li, H.; Li, Q.; Wang, J.; Li, C.; Wang, L. Construction and Optimization of Cordycepin-Producing Saccharomyces cerevisiae. Shengwu Gongcheng Xuebao/Chin. J. Biotechnol. 2021, 37, 3334–3347. [Google Scholar]
- Jeennor, S.; Anantayanon, J.; Panchanawaporn, S.; Chutrakul, C.; Vongsangnak, W.; Laoteng, K. Efficient de Novo Production of Bioactive Cordycepin by Aspergillus oryzae Using a Food-Grade Expression Platform. Microb. Cell Fact. 2023, 22, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, P.; Xu, L.; Xu, D.; Hu, W.; Cheng, Y.; Yang, S. Construction of Cordycepin High-Production Strain and Optimization of Culture Conditions. Curr. Microbiol. 2022, 80, 12. [Google Scholar] [CrossRef]
- Das, S.K.; Masuda, M.; Hatashita, M.; Sakurai, A.; Sakakibara, M. Optimization of Culture Medium for Cordycepin Production Using Cordyceps militaris Mutant Obtained by Ion Beam Irradiation. Process Biochem. 2010, 45, 129–132. [Google Scholar] [CrossRef]
- Mao, X.-B.; Eksriwong, T.; Chauvatcharin, S.; Zhong, J.-J. Optimization of Carbon Source and Carbon/Nitrogen Ratio for Cordycepin Production by Submerged Cultivation of Medicinal Mushroom Cordyceps militaris. Process Biochem. 2005, 40, 1667–1672. [Google Scholar] [CrossRef]
- Antimanon, S.; Chamkhuy, W.; Sutthiwattanakul, S.; Laoteng, K. Efficient Production of Arachidonic Acid of Mortierella sp. by Solid-State Fermentation Using Combinatorial Medium with Spent Mushroom Substrate. Chem. Pap. 2018, 72, 2899–2908. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Quy, T.N.; Xuan, T.D. Xanthine Oxidase Inhibitory Potential, Antioxidant and Antibacterial Activities of Cordyceps militaris (L.) Link Fruiting Body. Medicines 2019, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Naraian, R.; Sahu, R.K.; Kumar, S.; Garg, S.K.; Singh, C.S.; Kanaujia, R.S. Influence of Different Nitrogen Rich Supplements during Cultivation of Pleurotus florida on Corn Cob Substrate. Environmentalist 2009, 29, 1–7. [Google Scholar] [CrossRef]
- Tan, H.; Wang, L.; Wang, H.; Cheng, Y.; Li, X.; Wan, H.; Liu, C.; Liu, T.; Li, Q. Engineering Komagataella phaffii to Biosynthesize Cordycepin from Methanol Which Drives Global Metabolic Alterations at the Transcription Level. Synth. Syst. Biotechnol. 2023, 8, 242–252. [Google Scholar] [CrossRef]
- Kang, C.; Wen, T.C.; Kang, J.C.; Meng, Z.B.; Li, G.R.; Hyde, K.D. Optimization of Large-Scale Culture Conditions for the Production of Cordycepin with Cordyceps militaris by Liquid Static Culture. Sci. World J. 2014, 2014, 510627. [Google Scholar] [CrossRef]
- Song, Z.; Lin, W.; Duan, X.; Song, L.; Wang, C.; Yang, H.; Lu, X.; Ji, X.; Tian, Y.; Liu, H. Increased Cordycepin Production in Yarrowia lipolytica Using Combinatorial Metabolic Engineering Strategies. ACS Synth. Biol. 2023, 12, 780–787. [Google Scholar] [CrossRef]
- Shoji, A.; Yamanaka, H.; Kamatani, N. A Retrospective Study of the Relationship between Serum Urate Level and Recurrent Attacks of Gouty Arthritis: Evidence for Reduction of Recurrent Gouty Arthritis with Antihyperuricemic Therapy. Arthritis Care Res. 2004, 51, 321. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Zhang, M.; Chen, D.; Shuai, O.; Chen, S.; Su, J.; Jiao, C.; Feng, D.; Xie, Y. Actions of Water Extract from Cordyceps militaris in Hyperuricemic Mice Induced by Potassium Oxonate Combined with Hypoxanthine. J. Ethnopharmacol. 2016, 194, 403–411. [Google Scholar] [CrossRef]
- Sattui, S.E.; Gaffo, A.L. Treatment of Hyperuricemia in Gout: Current Therapeutic Options, Latest Developments and Clinical Implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Chutvirasakul, B.; Jongmeesuk, W.; Tirasomboonsiri, P.; Sansandee, N.; Tadtong, S. Stability Indicating Method to Determine Bioactive Nucleosides in Crude Drugs, Extracts, and Products from Cordyceps sinensis and Cordyceps militaris. Thai J. Pharm. Sci. 2017, 41, 52–60. [Google Scholar] [CrossRef]
- Tang, H.; Chen, C.; Zou, Y.; Lou, H.; Zheng, Q.; Guo, L.; Lin, J.; Ye, Z.; Yun, F. Purification and Structural Characterization of a Novel Natural Pigment: Cordycepene from Edible and Medicinal Mushroom Cordyceps militaris. Appl. Microbiol. Biotechnol. 2019, 103, 7943–7952. [Google Scholar] [CrossRef] [PubMed]
- Marsup, P.; Yeerong, K.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-Anun, C.; Chaiyana, W. Enhancement of Chemical Stability and Dermal Delivery of Cordyceps militaris Extracts by Nanoemulsion. Nanomaterials 2020, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- Shanu, K.; Choudhary, S.; Kumari, S.; Anu, K.; Devi, S. Downstream Processing for Bio-Product Recovery and Purification. In Recent Advances in Bioprocess Engineering and Bioreactor Design; Dhagat, S., Jujjavarapu, S.E., Sampath Kumar, N.S., Mahapatra, C., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 139–169. ISBN 978-981-97-1451-3. [Google Scholar]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Alternative Drying Processes for the Industrial Preservation of Lactic Acid Starter Cultures. Biotechnol. Prog. 2007, 23, 302–315. [Google Scholar] [CrossRef]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing Facts behind Spray Dried Solid Dispersion Technology Used for Solubility Enhancement. Saudi Pharm. J. 2015, 23, 352–365. [Google Scholar] [CrossRef]
- Harikai, N.; Takada, Y.; Saito, M.; Zaima, K.; Shinomiya, K. Relationship Between Amplicon Size and Heat Conditions in Polymerase Chain Reaction Detection of DNA Degraded by Autoclaving. Biopreserv. Biobank. 2024, 22, 268–274. [Google Scholar] [CrossRef]
- Suyama, T.; Kawaharasaki, M. Decomposition of Waste DNA with Extended Autoclaving under Unsaturated Steam. Biotechniques 2013, 55, 296–299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).