Carbon Dioxide Micro-Nano Bubbles Aeration Improves Carbon Fixation Efficiency for Succinic Acid Synthesis by Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. MNB Generator and CO2 Pressurized Fermentation System
2.3. The Emission Regularity of CO2-MNBs in a Simulated Fermentation System
2.4. Anaerobic Fermentation for Succinic Acid Production
2.5. Analytical Methods
2.5.1. Determination of Biomass Concentration and Soluble Component Concentrations
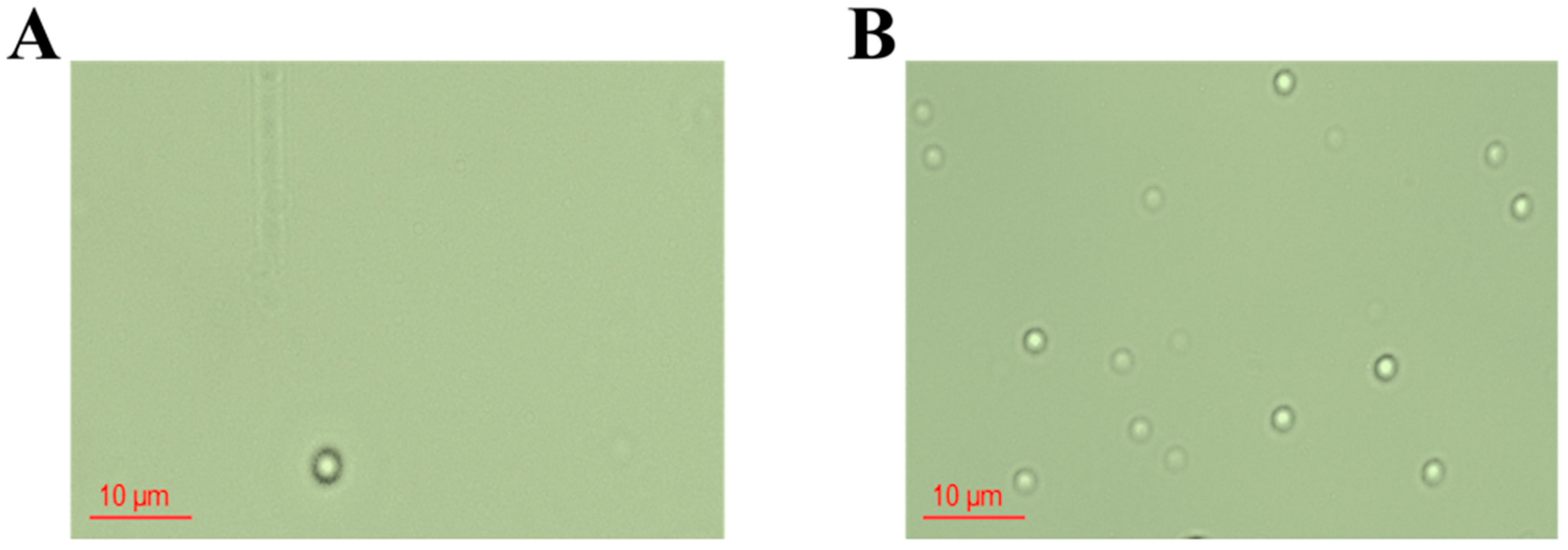
2.5.2. MNB Observation and Counting
2.5.3. Key Enzyme Activity Assay
2.6. Data Analysis
2.7. Statistical Analysis
3. Results and Discussion
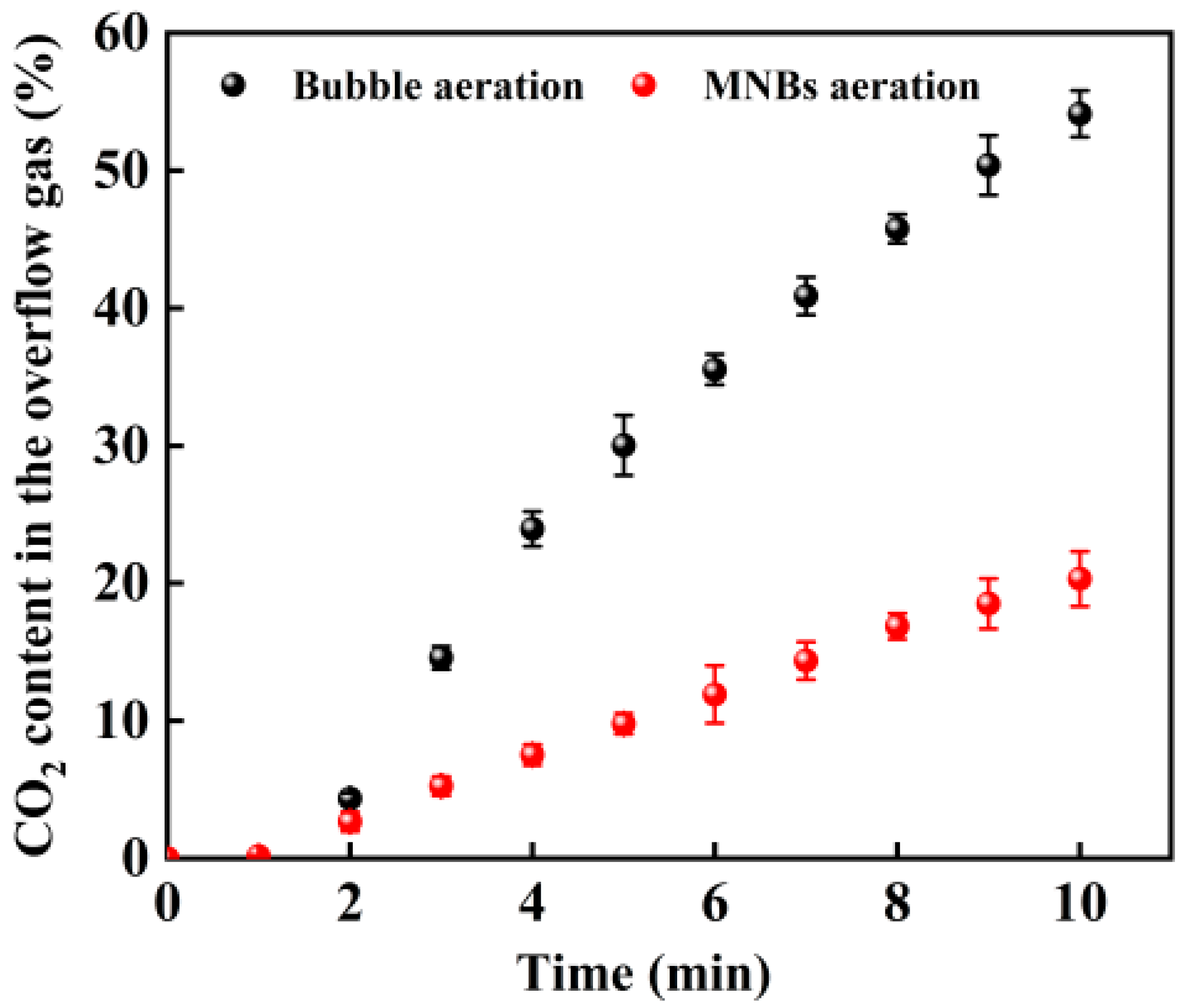
3.1. Comparison of Bubble Aeration and MNB Aeration for CO2 Release in the Simulated Atmospheric Fermentation System
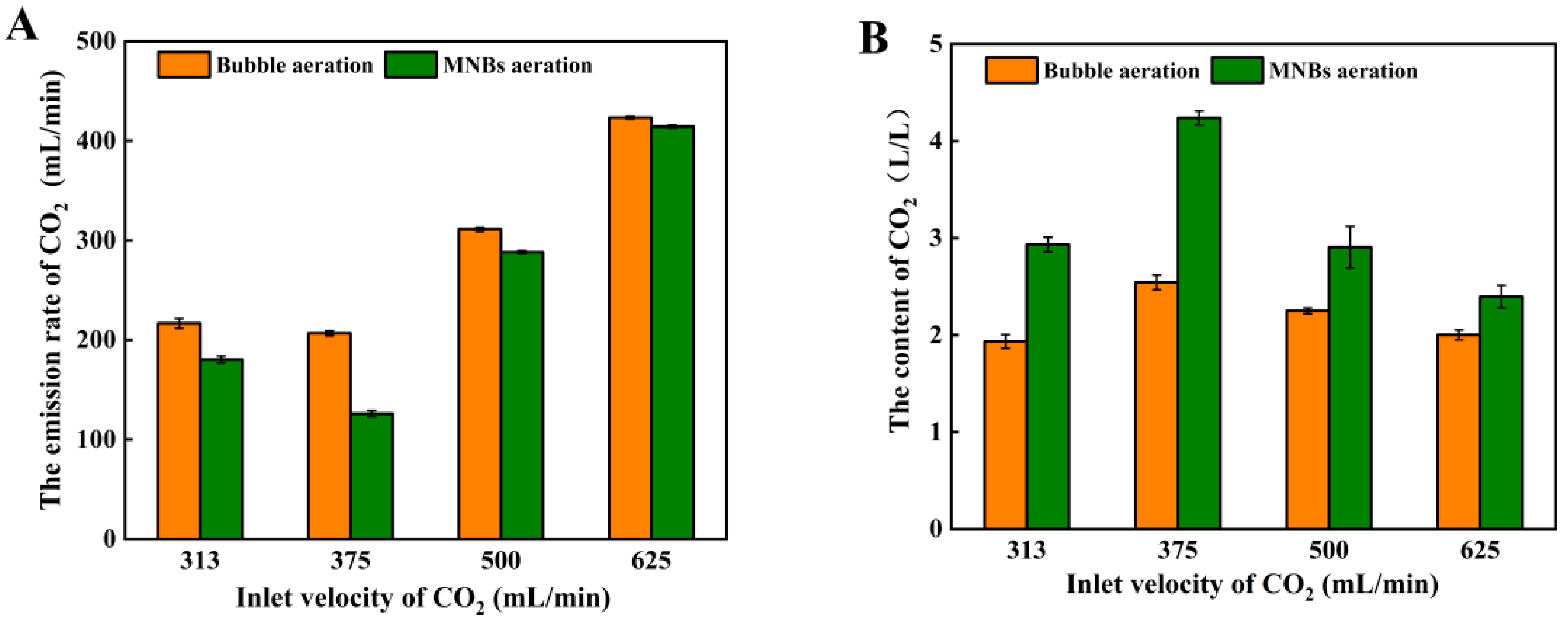
3.2. Effect of Pressure and Inlet Velocity on CO2 Release in the Pressurized Simulated Fermentation System
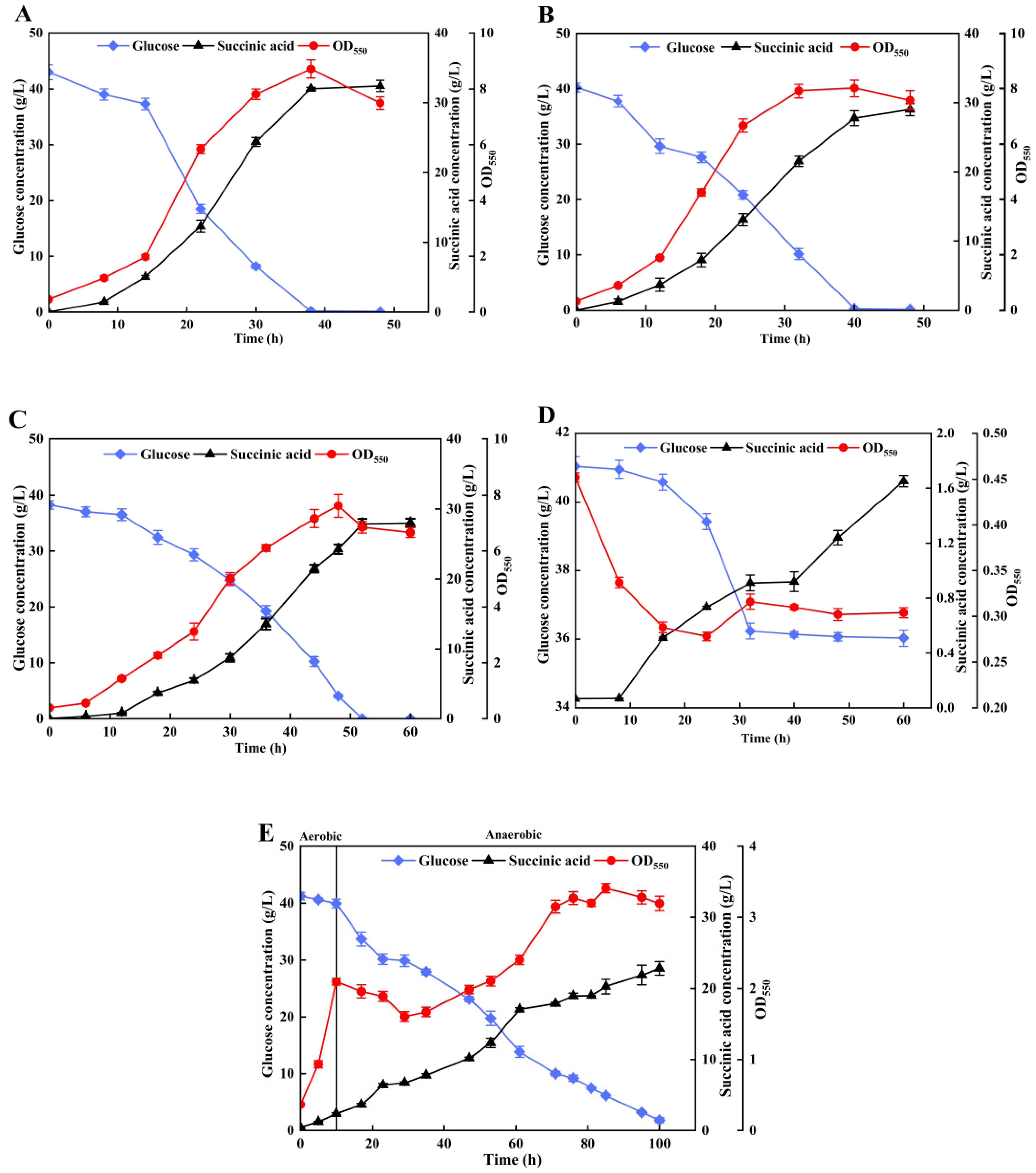
3.3. Effects of MNBs on Anaerobic Synthesis of SA by Escherichia coli
3.4. Reducing Inhibition of SA Production by MNBs Using a Two-Stage Aerobic–Anaerobic Fermentation Strategy
3.5. Synthesis of SA by Biofilm-Enhanced Strains with CO2 MNB Supply
3.6. Metabolic Analysis of SA Synthesis with Different CO2 Supply Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J.H. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Erickson, B.; Nelson, J.E.; Winters, P.C. Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol. J. 2012, 7, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, L.; Xia, S.; Li, H.; Tang, Y.; Liang, X.; Chen, T.; Tang, Y. Combinatorial optimization of CO2 transport and fixation to improve succinate production by promoter engineering. Biotechnol. Bioeng. 2016, 113, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nurhayati; Cheng, C.-L.; Nagarajan, D.; Chang, J.S.; Hu, J.; Lee, D.-J. Carbon capture and utilization of fermentation CO2: Integrated ethanol fermentation and succinic acid production as an efficient platform. Appl. Energy 2017, 206, 364–371. [Google Scholar] [CrossRef]
- Zou, W.; Zhu, L.; Li, H.; Tang, Y. Significance of CO2 donor on the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Microb. Cell Factories 2011, 10, 87. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Vieille, C. 13C-metabolic flux analysis of Actinobacillus succinogenes fermentative metabolism at different NaHCO3 and H2 concentrations. Metab. Eng. 2008, 10, 55–68. [Google Scholar] [CrossRef]
- Amulya, K.; Mohan, S.V. Green hydrogen based succinic acid and biopolymer production in a biorefinery: Adding value to CO2 from acidogenic fermentation. Chem. Eng. J. 2022, 429, 132163. [Google Scholar] [CrossRef]
- Jin, T.; Wang, Y.; Yao, S.; Hu, C.; Ma, T.; Xia, W. Bioconversion of carbon dioxide to succinate by Citrobacter. Chem. Eng. J. 2023, 452, 139668. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Zhou, Y.; Xu, S.; Ren, X.; Wei, Q. Progress on production of succinic acid by Actinobacillus succinogenes-New opportunities for cheap biomass and waste gas utilization. J. Clean. Prod. 2024, 434, 140005. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, K.; Li, J.; Fang, X.; Zheng, X.; Sui, S.; Jiang, M.; Wei, P. Optimization of culture conditions in CO2 fixation for succinic acid production using Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; Senske, G.E. Capture of carbon dioxide from ethanol fermentation by liquid absorption for use in biological production of succinic acid. Appl. Biochem. Biotechnol. 2015, 175, 2104–2113. [Google Scholar] [CrossRef]
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus succinogenes: Advances on succinic acid production and prospects for development of integrated biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.W.; Choi, S.J.; You, J.K.; Hong, W.H.; Lee, S.Y. Effects of dissolved CO2 levels on the growth of Mannheimia succiniciproducens and succinic acid production. Biotechnol. Bioeng. 2007, 98, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.J.; Lopes, R.P.; Simões, M.M.Q.; Delgadillo, I.; Saraiva, J.A. Effect of high pressure on paracoccus denitrificans growth and polyhydroxyalkanoates production from glycerol. Appl. Biochem. Biotechnol. 2019, 188, 810–823. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Effectively converting carbon dioxide into succinic acid under mild pressure with Actinobacillus succinogenes by an integrated fermentation and membrane separation process. Bioresour. Technol. 2018, 266, 26–33. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Li, Z.; Ye, Q. Succinic acid production and CO2 fixation using a metabolically engineered Escherichia coli in a bioreactor equipped with a self-inducing agitator. Bioresour. Technol. 2012, 107, 376–384. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, S.; Ma, J.; Chen, K.; Yu, L.; Yue, F.; Xu, B.; Wei, P. Effect of growth phase feeding strategies on succinate production by metabolically engineered Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Seridou, P.; Kalogerakis, N. Disinfection applications of ozone micro-and nanobubbles. Environ. Sci. Nano 2021, 8, 3493–3510. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, F.; Guo, X.; Qiao, Z.; Zhu, Y.; Jin, N.; Cui, Y.; Yang, W. Research progress on bulk nanobubbles. Particuology 2022, 60, 99–106. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Z.; Zhou, M.; Jie, X.; Wang, L.; Kang, G.; Cao, Y. Removal of CO2 from biogas by membrane contactor using PTFE hollow fibers with smaller diameter. J. Membr. Sci. 2021, 627, 119232. [Google Scholar] [CrossRef]
- Wu, H.; Liao, J.; Chen, X.; Ren, X.; Zhou, J.; Jiang, M. Decarbonization of simulated biogas with microchannel mixer by pressurized water scrubbing. J. Clean. Prod. 2024, 457, 142230. [Google Scholar] [CrossRef]
- Cerrón-Calle, G.A.; Magdaleno, A.L.; Graf, J.C.; Apul, O.G.; Garcia-Segura, S. Elucidating CO2 nanobubble interfacial reactivity and impacts on water chemistry. J. Colloid Interface Sci. 2022, 607, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Abdella, A.A.K.; Xiaonan, S.; Likun, H.; Leidy, M.; Weihua, Q.; Taha, M.; Wen, Z. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef]
- Toyohisa, F.; Hiromi, K.; Zhenyao, H.; Yali, Z.; Hirofumi, M.; Josiane, P.; Gjergj, D.; Chunlin, H.; Yuezou, W. Free radical degradation in aqueous solution by blowing hydrogen and carbon dioxide nanobubbles. Sci. Rep. 2021, 11, 3068. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Cheng, L.; Ma, H.; Gao, X.; Brennan, C.S.; Yan, J. Micro-nano-bubble technology and its applications in food industry: A critical review. Food Rev. Int. 2023, 39, 4213–4235. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Ahmad, R.M.; Kuldeep, G.; Manabendra, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Zhuang, W.; Yang, J.; Wu, J.; Liu, D.; Zhou, J.; Chen, Y.; Ying, H. Extracellular polymer substances and the heterogeneity of Clostridium acetobutylicum biofilm induced tolerance to acetic acid and butanol. RSC Adv. 2016, 6, 33695–33704. [Google Scholar] [CrossRef]
- Gong, C.; He, Y.; Tang, Y.; Hu, R.; Lv, Y.; Zhang, Q.; Tardy, B.L.; Richardson, J.J.; He, Q.; Guo, J.; et al. Biofilms in plant-based fermented foods: Formation mechanisms, benefits and drawbacks on quality and safety, and functionalization strategies. Trends Food Sci. Technol. 2021, 116, 940–953. [Google Scholar] [CrossRef]
- Jiang, M.; Wan, Q.; Zhang, C.; Liang, L.; Cheng, X.; Gou, D.; Liu, R.; Ma, J. Strain Capable of Producing Succinic Acid, Method for Producing Succinic Acid and Application Thereof. C.N.102864113A, 9 January 2013. (In Chinese). [Google Scholar]
- Wu, M.; Zhang, W.; Ji, Y.; Yi, X.; Ma, J.; Wu, H.; Jiang, M. Coupled CO2 fixation from ethylene oxide off-gas with bio-based succinic acid production by engineered recombinant Escherichia coli. Biochem. Eng. J. 2017, 117, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chen, H.; Xiang, J.; Zhang, Y.; Wang, Z.; Zhu, D.; Zhang, Y. Brassinosteroids mediate endogenous phytohormone metabolism to alleviate high temperature injury at panicle initiation stage in rice. Rice Sci. 2023, 30, 70–86. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Ye, R.; Qi, X.; Chen, L.; Liu, X.; Tang, L.; Chen, L.; Chen, H.; Fan, Z. Discovery of novel triazolothiadiazines as fungicidal leads targeting pyruvate kinase. J. Agric. Food Chem. 2022, 70, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, K.; Shen, J.; Wang, Y.; Jeyaraj, A.; Hu, S.; Chen, X.; Ding, Z.; Li, X. Glycine-induced phosphorylation plays a pivotal role in energy metabolism in roots and amino acid metabolism in leaves of tea plant. Foods 2023, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, M.; Zhang, C.; He, A.; Kong, X.; Li, G.; Wei, C.; Jiang, M. Coupled ARTP and ALE strategy to improve anaerobic cell growth and succinic acid production by Escherichia coli. J. Chem. Technol. Biotechnol. 2016, 91, 711–717. [Google Scholar] [CrossRef]
- Capasso, C.; De Luca, V.; Carginale, V.; Cannio, R.; Rossi, M. Biochemical properties of a novel and highly thermostable bacterial α-carbonic anhydrase from Sulfurihydrogenibium yellowstonense YO3AOP1. J. Enzym. Inhib. Med. Chem. 2012, 27, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Eiteman, M.A.; Altman, E. Effect of CO2 on succinate production in dual-phase Escherichia coli fermentations. J. Biotechnol. 2009, 143, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kasiri, S.; Ulrich, A.C.; Prasad, V. Optimization of CO2 fixation by Chlorella kessleri cultivated in a closed raceway photo-bioreactor. Bioresour. Technol. 2015, 194, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.P.; Luthfi, A.A.I.; Manaf, S.F.A.; Wu, T.Y.; Jahim, J.M. Incorporation of CO2 during the production of succinic acid from sustainable oil palm frond juice. J. CO2 Util. 2018, 26, 595–601. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Li, T.; Gao, M.; Hu, J.; Li, J. Effect of micro-nanobubbles with different gas sources on the growth and metabolism of chemoautotrophic microorganisms. Process Biochem. 2023, 126, 117–124. [Google Scholar] [CrossRef]
- Vemuri, G.N.; Eiteman, M.A.; Altman, E. Succinate production in dual-phase Escherichia coli fermentations depends on the time of transition from aerobic to anaerobic conditions. J. Ind. Microbiol. Biotechnol. 2002, 28, 325–332. [Google Scholar] [CrossRef]
- Li, Q.; Huang, B.; He, Q.; Lu, J.; Li, X.; Li, Z.; Wu, H.; Ye, Q. Production of succinate from simply purified crude glycerol by engineered Escherichia coli using two-stage fermentation. Bioresour. Bioprocess. 2018, 5, 41. [Google Scholar] [CrossRef]
- Hammer, N.D.; Schmidt, J.C.; Chapman, M.R. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc. Natl. Acad. Sci. USA 2007, 104, 12494–12499. [Google Scholar] [CrossRef]
- Sheng, H.; Xue, Y.; Zhao, W.; Hovde, C.J.; Minnich, S.A. Escherichia coli O157:H7 curli fimbriae promotes biofilm formation, epithelial cell invasion, and persistence in cattle. Microorganisms 2020, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczańska, B.; Pawłowska, B.; Duda-Madej, A.; Pawlik, K.; Wiśniewski, J.; Grzegrzołka, J.; Turniak, M.; Walczuk, U.; Gamian, A. Effect of amyloid curli fibrils and curli CsgA monomers from Escherichia coli on in vitro model of intestinal epithelial barrier stimulated with cytokines. Int. J. Med. Microbiol. 2019, 309, 274–282. [Google Scholar] [CrossRef]
- Amulya, K.; Kopperi, H.; Mohan, S.V. Tunable production of succinic acid at elevated pressures of CO2 in a high pressure gas fermentation reactor. Bioresour. Technol. 2020, 309, 123327. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhu, X.; Chen, J.; Li, Q.; Zhang, X. Activating phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase in combination for improvement of succinate production. Appl. Environ. Microbiol. 2013, 79, 4838–4844. [Google Scholar] [CrossRef]
- Park, S.J.; Cotter, P.A.; Gunsalus, R.P. Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon, and heme availability. J. Bacteriol. 1995, 177, 6652–6656. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, R.; Ma, J.; Chen, K.; Jiang, M.; Wei, P. Increased production of succinic acid in Escherichia coli by overexpression of malate dehydrogenase. Biotechnol. Lett. 2011, 33, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Lee, S.Y. Metabolic flux analysis for succinic acid production by recombinant Escherichia coli with amplified malic enzyme activity. Biotechnol. Bioeng. 2001, 74, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Razzak, A.; Lee, D.W.; Lee, J.; Hwang, I. Overexpression and Purification of Gracilariopsis chorda Carbonic Anhydrase (GcCAα3) in Nicotiana benthamiana, and its immobilization and use in CO2 Hydration Reactions. Front. Plant Sci. 2020, 11, 563721. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Q.; Li, W.; Xing, J.; Su, Z. Improvement of succinate production by overexpression of a cyanobacterial carbonic anhydrase in Escherichia coli. Enzym. Microb. Technol. 2009, 45, 491–497. [Google Scholar] [CrossRef]



| CO2 Supply Methods | SA (g·L−1) | SA Yield (g·g−1) | CO2 Utilization (%) | Productivity (g·(L·h)−1) |
|---|---|---|---|---|
| AFB | 32.43 ± 0.79 | 0.852 ± 0.02 | 4.85 ± 0.0012 | 0.68 ± 0.02 |
| AFM | 28.98 ± 0.88 | 0.832 ± 0.03 | 11.14 ± 0.0020 | 0.60 ± 0.02 |
| PFB | 27.89 ± 0.72 | 0.863 ± 0.02 | 14.50 ± 0.0037 | 0.54 ± 0.01 |
| PFM | 1.65 ± 0.04 | / | / | / |
| TSF | 22.83 ± 0.95 | 0.657 ± 0.03 | 14.03 ± 0.0003 | 0.23 ± 0.01 |
| CO2 Supply Methods | SA (g·L−1) | SA Yield (g·g−1) | CO2 Utilization (%) | Productivity (g·(L·h)−1) |
|---|---|---|---|---|
| AFB | 30.59 ± 0.72 | 0.846 ± 0.02 | 4.09 ± 0.0012 | 0.58 ± 0.01 |
| PFB | 28.72 ± 0.80 | 0.865 ± 0.03 | 14.20 ± 0.0039 | 0.55 ± 0.02 |
| PFM | 30.40 ± 0.68 | 0.873 ± 0.02 | 30.63 ± 0.0018 | 0.57 ± 0.02 |
| CO2 Supply Methods | Strain | PCK (U·mg−1) | MDH (U·mg−1) | PK (U·mg−1) | CA (U·mL−1) |
|---|---|---|---|---|---|
| AFB | E. coli Suc260 | 10.33 ± 0.10 | 10.9 ± 0.34 | 22.17 ± 2.07 | 0.98 ± 0.05 |
| E. coli Suc260-CsgA | 10.56 ± 0.10 | 12.93 ± 0.09 | 29.59 ± 0.63 | 0.76 ± 0.08 | |
| PFB | E. coli Suc260 | 8.86 ± 0.11 | 8.75 ± 0.12 | 18.72 ± 1.64 | 2.84 ± 0.14 |
| E. coli Suc260-CsgA | 9.71 ± 0.16 | 11.65 ± 0.34 | 29.26 ± 0.74 | 2.06 ± 0.09 | |
| PFM | E. coli Suc260 | 0.25 ± 0.09 | 0.35 ± 0.04 | 1.34 ± 0.96 | 0.02 ± 0.01 |
| E. coli Suc260-CsgA | 9.16 ± 0.25 | 10.82 ± 0.15 | 25.56 ± 1.55 | 3.18 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wu, H.; Huang, Q.; Liao, J.; Wang, L.; Pan, Y.; Xu, A.; Zhang, W.; Jiang, M. Carbon Dioxide Micro-Nano Bubbles Aeration Improves Carbon Fixation Efficiency for Succinic Acid Synthesis by Escherichia coli. Fermentation 2025, 11, 31. https://doi.org/10.3390/fermentation11010031
Chen Y, Wu H, Huang Q, Liao J, Wang L, Pan Y, Xu A, Zhang W, Jiang M. Carbon Dioxide Micro-Nano Bubbles Aeration Improves Carbon Fixation Efficiency for Succinic Acid Synthesis by Escherichia coli. Fermentation. 2025; 11(1):31. https://doi.org/10.3390/fermentation11010031
Chicago/Turabian StyleChen, Ying, Hao Wu, Qianqian Huang, Jingwen Liao, Liuqing Wang, Yue Pan, Anming Xu, Wenming Zhang, and Min Jiang. 2025. "Carbon Dioxide Micro-Nano Bubbles Aeration Improves Carbon Fixation Efficiency for Succinic Acid Synthesis by Escherichia coli" Fermentation 11, no. 1: 31. https://doi.org/10.3390/fermentation11010031
APA StyleChen, Y., Wu, H., Huang, Q., Liao, J., Wang, L., Pan, Y., Xu, A., Zhang, W., & Jiang, M. (2025). Carbon Dioxide Micro-Nano Bubbles Aeration Improves Carbon Fixation Efficiency for Succinic Acid Synthesis by Escherichia coli. Fermentation, 11(1), 31. https://doi.org/10.3390/fermentation11010031







