Abstract
Silage preservation is critical for livestock’s stable forage supply during cold seasons and fostering the recycling of agricultural byproducts. We assessed the effects of adding previously fermented juices (PFJ) and different proportions of Sorghum Sudanense (Piper) Stapf (Sudan grass) on the silage quality of Medicago sativa L. (alfalfa). A 50% alfalfa and 50% Sudan grass (M50) mixture exhibited superior performance in sensory evaluation and fermentation quality. The addition of PFJ (PFJI group) further enhanced quality, and increasing the proportion of alfalfa improved the nutritional quality. However, PFJI had no significant effect on the degradation rates of these nutritional indicators (p > 0.05). As alfalfa proportion increased, the disappearance rates of dry matter, crude protein, neutral detergent fiber, and acid detergent fiber in silage increased. Principal component, correlation, and membership function analyses revealed that treatment with M50 without PFJ (PFJ0+M50) had the best effect, followed by treatment with 25% alfalfa and 75% Sudan grass (PFJ0+M25) and 100% alfalfa (PFJ0+M0). Therefore, the mixing ratio of alfalfa and Sudan grass should be maintained between 25% and 50% to optimize the nutritional and fermentation qualities of silage. These findings offer crucial guidance for alfalfa silage preparation, promoting enhanced livestock industry productivity and sustainable agricultural development.
1. Introduction
The livestock industry is the cornerstone of agriculture and rural economies worldwide [], and its continuous growth has caused an increasing demand for high-quality feed []. In the northern regions of China, forage production faces seasonal challenges, causing an excess of forage resources during the growing season and a shortage during the non-growing season, threatening the effective management of feed and nutritional supply for animals []. Therefore, using forage with high protein content has become increasingly essential.
Medicago sativa is rich in proteins and vitamins and is an essential high-quality forage for livestock production. Known as the “king of forage”, it is recognized worldwide for its adaptability, high yield, and nutritional value []. It is a major source of forage for ruminants in China [] and is primarily used for green feed, silage, and hay preparation. However, its haymaking process encounters challenges such as leaf loss and significant nutritional depletion during the harvest period owing to concurrent rainy and hot weather conditions. Therefore, the production of alfalfa silage in China has recently increased rapidly. Compared with haymaking, silage is less affected by weather conditions, retains most nutrients, facilitates large-scale and long-term storage, and improves palatability, compensating for the limitations of alfalfa hay [].
Although alfalfa is high in protein, its low soluble carbohydrate and dry matter contents can cause severe protein breakdown by lactic acid bacteria, acetic acid bacteria, or butyric acid bacteria during silage fermentation, making it challenging to rapidly lower the pH, affecting the success rate of silage []. To reduce Clostridium growth, protein hydrolysis, and heterotrophic fermentation, and improve silage palatability [], exploring suitable silage methods for alfalfa is essential to enhance its silage quality. Current alfalfa silage methods used to overcome these challenges include semi-dry, stretch-film-wrapped, mixed, and additive silages. Semi-dry silage technology has already been applied, and mixed silage and adding microbial starter silages represent the future development directions for alfalfa silage [].
Although there are many options for raw silage materials, the nutritional value of silage is relatively simple, and the nutrients are unbalanced. Mixed or combined silage, tailored to specific livestock nutrient requirements, fosters an optimal fermentation environment, ensuring comprehensive nutrition and good palatability []. Sudan grass is an annual high-yield forage crop of Gramineae suitable for green feeding, hay modulation, and fermented silage []. Its fermentation process is relatively straightforward []. Mixing Sudan grass and leguminous forage [] or adding PFJ broth (two days before making silage, mow the clean top parts of alfalfa and Sudan grass, respectively; cut them into 2–5 cm sections with scissors; mix them evenly; weigh out 200 g; add the same amount of distilled water; use a home beater to make pulp; filter and juice with two layers of gauze; add 2% sugar; and pour them into brown bottles for anaerobic fermentation in the dark for two days)[] can improve Sudan grass silage quality. High-quality silage additives can also enhance leguminous forage silage quality while reducing fermentation time. Some lactic acid bacteria (LAB) additives have positive effects; however, their production costs are high. PFJ is rich in various lactic acid bacteria (such as Lactococcus and Lactobacillus), which are primarily derived from the microbial communities that are epiphytic on plant surfaces. These microorganisms naturally occur during the fermentation process, eliminating the need for additional costly cultivation steps. As a result, PFJ is a natural, economically viable, and environmentally friendly alternative [].
To address the limitations of alfalfa silage, we aimed to assess the potential enhancement effects of combining previously fermented juice (PFJ) with Sudan grass on alfalfa silage quality of M. sativa. Sudan grass, with its simple fermentation characteristics and high soluble sugar content, is poised to offset alfalfa’s sugar deficiency during silage preparation, is expected to compensate for the insufficient sugar in alfalfa during silage preparation. Combined with the natural live bacterial characteristics of PFJ, we hypothesized that this mixed strategy could promote fermentation during silage preparation, thereby improving the nutritional value and palatability of silage feed. The findings from this study may provide guidance and a practical basis for optimizing alfalfa silage and promoting the sustainable development of the livestock industry.
2. Materials and Methods
2.1. Experimental Materials
This planting experiment was conducted in Ashili Township, Changji City, Xinjiang, on the middle section of the northern slope of the Tianshan Mountains (43°59′–43°60′ N, 86°67′–86°71′ E) at an altitude of 2200–2400 m []. The area has climatic conditions and soil environments ideal for growing forage grass. The Sudan grass variety selected for this planting was “Xinsu No.2 Sudan Grass” (Sorghum sudanense.cv.Xinsu No.2), characterized by high yield and good resistance and suitability for the local climatic conditions. The Sudan grass was sown on 1 May 2021. Adequate soil preparation and seed treatment were performed before sowing to ensure sowing quality. During cutting, the Sudan grass was in the heading stage of the first crop and had grown well without pests or diseases. The alfalfa variety chosen for this planting was “Xinmu No.1 Alfalfa” (Medicago varia Martin.cv.Xinmu No.1), known for its high protein content and yield and wide adaptability, making it a suitable high-quality forage crop. Alfalfa was sown on 20 April 2021, and soil preparation and seed treatments were conducted before sowing. During harvest, the alfalfa was in the early flowering stage of the first crop and thrived without pests or diseases. Sudan grass and alfalfa were harvested simultaneously on 4 July 2022. The harvested forage was subsequently processed. The silage vacuum packaging bags utilized in this experiment are the Deli brand model 14913, with dimensions of 28 cm wide by 35 cm, and are constructed from food-grade PA+PE material. The experimental setup employed these bags to simulate bagged silage conditions, providing a controlled environment for the fermentation process. Given the controlled fermentation environment and the specific design of the bags, our findings are particularly relevant to bagged silage practices. The results are expected to contribute to the optimization of PFJ addition strategies in bagged silage methods, potentially enhancing the overall quality of silage feed.
2.2. Experimental Design
A two-factor interactive experimental design was used to clarify the effects of PFJ addition and the alfalfa-to-Sudan grass mixing ratio on silage quality. The first factor, PFJ addition, comprised two treatments:
- PFJI: adding PFJ at a concentration of 1%;
- PFJ0: no PFJ added; an equal amount of distilled water was added instead (control).
The second factor was the mixing ratio of alfalfa (represented by the letter M) to Sudan grass (represented by the letter S). Five treatments were established based on the fresh weight ratio of alfalfa-to-Sudan grass:
- M100: 100% alfalfa and 0% Sudan grass;
- M75: 75% alfalfa and 25% Sudan grass;
- M50: 50% alfalfa and 50% Sudan grass;
- M25: 25% alfalfa and 75% Sudan grass;
- M0: 0% alfalfa and 100% Sudan grass.
By combining the first and second factors, 10 treatments were developed: PFJI+M100, PFJI+M75, PFJI+M50, PFJI+M25, PFJI+M0, PFJ0+M100, PFJ0+M75, PFJ0+M50, PFJ0+M25, and PFJ0+M0. Each treatment was replicated thrice to ensure the accuracy and reliability of the experimental results.
2.3. Experimental Methods
2.3.1. Silage Sample Preparation
After harvesting the raw silage materials, they were dried to a moisture content of 60–70%, crushed, mixed evenly following the experimental design, weighed to 300 g, placed in a silage bag, vacuum-packed, sealed using a vacuum packaging machine (de li 14891), purchased from Deli group Co., Ltd. (Ningbo, Zhejiang, China), and stored at 20–25 °C (room temperature) for 60 days.
2.3.2. Fermented Green Juice Preparation
Following the method of Wang Peng [], 200 g of alfalfa at the early flowering stage was weighed, and 400 mL of distilled water was added. The mixture was blended using a mini plant sample crusher (BJ-800A, Baijie, China). The plant residue was filtered through two layers of gauze, and 2 g of glucose was added to the filtrate. After full dissolution, the mixture was diluted to 1000 mL and placed in a water bath of 30 °C constant temperature (HSWX-420BS, XingMiao, Shanghai, China) for anaerobic fermentation for 48 h before use.
2.3.3. Silage Quality Analysis
Measurement indicators and methods encompass sensory evaluation, fermentation quality, nutritional composition, and the degradation rate of nutritional components.
- Sensory evaluation: The German Agricultural Association silage sensory quality scoring method was used for the comprehensive sensory evaluation of silage quality based on odor, texture, and color [].
- Fermentation quality assessment: After the silage period expired, 20 g of fresh samples were added to 180 mL of deionized water, stirred well, and refrigerated at 4 °C for 24 h. The pH of the extract was directly measured using a LeiCi brand pHS-3C acidity meter; the ammonia nitrogen (AN) content was determined using the phenol–sodium hypochlorite colorimetric method [], and the ratio of AN to total nitrogen (TN) was calculated. Following the method described by Cao et al., the lactic acid (LA), acetic acid (AA), propionic acid (PA), and butyric acid (BA) contents in the extract were analyzed using Shimadzu high-performance liquid chromatography [].
- Nutritional component analysis: After the silage matured, fresh samples were taken from the silage tank, oven-dried at 120 °C for 20 min to eliminate the greenness, further dried to constant weight at 65 °C, and the initial moisture content was determined. The air-dried sample was crushed using a crusher, passed through a 40-mesh sieve, and stored in a sealed plastic bag for later use. The dry matter (DM) content was determined using the drying method []; the crude protein (CP) content was determined using the Kjeldahl method []; the neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were determined using the Van Soest method []; and the crude ash (Ash) content was determined using the high-temperature incineration method []. The ether extraction (EE) content was determined using the Soxhlet fat extraction method [], and the water-soluble carbohydrates (WSCs) were determined using the sulfuric acid–anthrone colorimetric method []. The relative feeding value (RFV) was calculated using the formula []:
RFV = [(88.9 − 0.779 × ADF) × 120/NDF]/1.29]
- 4.
- Degradation rate of nutritional components: All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Animal Welfare and Ethics Committee of Xinjiang Agricultural University, Urumqi, Xinjiang, China (project identification code: 2023032). The research sheep were purchased from farmers in Aerxiategou, Hejing County and Hejing Town, Bazhou, Xinjiang. The experiment involved four healthy and disease-free Chinese Merino sheep (Xinjiang type) with permanent rumen fistulas, aged 1.5 years, and with similar body weights (average weight 35 ± 1.48 kg). Their daily diet during the experimental period comprised corn stalks as roughage and a mixed concentrate purchased from Xinjiang Tiankang Biological Co. Ltd. (Urumqi, Xinjiang, China) The mixed culture solution was prepared and pretreated before cultivation following the method described by Menke et al. []. Before starting the experiment, the collected rumen fluid was filtered through a four-layer gauze and mixed with an anaerobic buffer at a ratio of 1:2. Subsequently, it was placed in a water bath at 39 °C and shaken until use. Each batch of samples was processed within 30 min before and after the treatment []. We accurately weighed the fermented samples required for the experimental design, prepared three replicates for each test sample, and recorded them in detail. A nylon screen mesh with a pore size of 300 mesh was used to make a nylon bag with a unified size of 12 cm × 6 cm (height × width). The three sides of the bag were sewn with a fine polyester thread to prevent fraying, and the loose edges were singed with a candle. The bags were cleaned, dried, and weighed before use. The fermented sample was poured into the nylon bag, rinsed with water, and gently flushed until the water was completely clear. Next, it was placed in an oven at 65 °C to dry to a constant weight. The degradation rates of DM, CP, NDF, and ADF were measured at 72 h. The degradation rate of nutrients was calculated using the following formula: nutrient degradation rate (%) = (the DM weight of the sample put into the nylon bag × the nutrient content of the original sample (%)—the DM weight remaining after digestion × the nutrient content of the digested sample (%)/(the DM weight of the sample put into the nylon bag × the nutrient content of the original sample) × 100% [].
2.4. Statistical Analysis
After summarizing and organizing the basic data using Excel 2016, statistical analyses were performed using SPSS 26.0, including Duncan’s multiple comparisons. The effects of additives, mixing ratios, and their interactions on silage quality were analyzed using a two-factor analysis of variance (ANOVA). Principal component extraction was performed for the measured quality-related indicators. Graphs and correlation analyses were generated using OriginLab OriginPro 2021 software, and the experimental data were expressed as mean ± standard deviation. A comprehensive evaluation was conducted using the membership function method in Fzuuy mathematics []. Indicators that positively correlated with silage quality were calculated using Formula (1), and those that negatively correlated with silage quality were calculated using Formula (2).
where Fij represents the membership degree of the jth indicator for the ith treatment; Xij represents the measured value of the jth indicator for the ith treatment; and Xmax and Xmin represent the maximum and minimum values of the jth indicator among all test subjects, respectively.
Fij+ = (Xij − Xmin)/(Xmax − Xmin)
Fij− = 1 − (Xij − Xmin)/(Xmax − Xmin)
3. Results
3.1. Nutritional Composition Analysis of Silage Raw Materials
Table 1 presents the nutritional composition analysis of raw silage materials, demonstrating that alfalfa exhibited significantly higher DM, CP, EE, Ash, and RFV (p < 0.05) levels than those of Sudan grass, with a CP content of 15.76%. However, the NDF, ADF, and WSC contents of alfalfa were lower than those of Sudan grass, with WSC at only 3.75%. Therefore, mixed silage can provide nutrient complementation.

Table 1.
Nutrient contents of raw material.
3.2. Effects of Additives and Mixing Ratios on the Sensory Indicators of Alfalfa Silage
The analysis of silage sensory indicators (Table 2) showed that in the PFJ0 group, the M50 treatment appeared yellow-green after silage preparation, with a slightly pungent smell and a loose and non-sticky texture and was the highest-scoring group with a silage grade of “excellent”. The silage grades for the other mixing ratio treatments were “good”. This indicated that M50 was the best mixing ratio for alfalfa and Sudan grass mixed silage.

Table 2.
Sensory evaluation of alfalfa and Sudan grass mixed silage under different treatments.
In the PFJI group, the M0–M50 treatments appeared yellow-green after silage preparation and had a slightly pungent smell and a loose and non-sticky texture. The silage grades for these treatments all reached “excellent”, while the silage grades for the other mixing ratio treatments were “good”. This shows that adding PFJ can produce “excellent” silage feed based on the M0–M50 mixing ratio.
The sensory scores of the PFJI group were comparable to or even higher than those of the PFJ0 group, suggesting that PFJ addition is essential for improving the sensory quality of mixed silage feed.
3.3. Effects of PFJ and Mixing Ratio on the Fermentation Quality of Alfalfa Silage
The analysis of the primary effects of different factors showed that PFJ addition had a highly significant effect (p < 0.01; Figure 1) on the AA and PA contents and a significant effect (p < 0.05; Figure 1) on the BA contents of the mixed silage feed. However, it had no significant effect on pH, AN/TN ratio, or LA content (p > 0.05; Figure 1). The mixing ratio factor and the interaction effect had highly significant effects on the fermentation quality indicators of the mixed silage feed (p < 0.01; Figure 1). Among them, the mixing ratio was the primary factor affecting the fermentation quality of alfalfa silage feed.
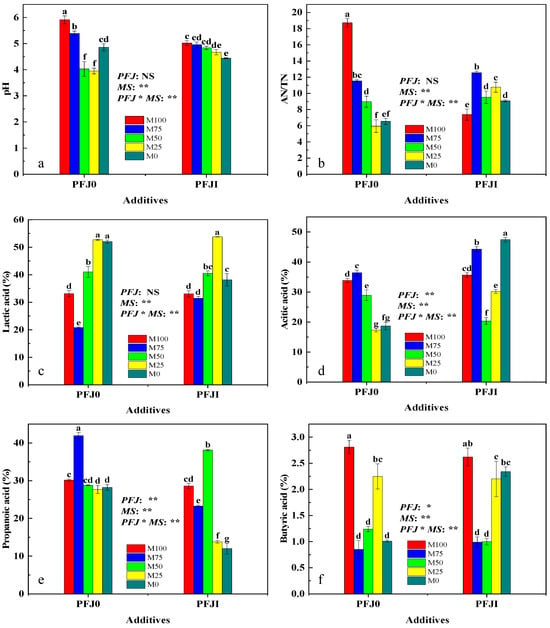
Figure 1.
Effects of additives and mixing ratios on the fermentation quality of mixed silage of alfalfa and Sudan grass. (a) Effects of additives and mixing ratio on the pH of mixed silage of alfalfa and Sudan grass. (b) Effects of additives and mixing ratio on the ammonia nitrogen/total nitrogen (AN/TN) of mixed silage of alfalfa and Sudan grass. (c) Effects of additives and mixing ratios on lactic acid content in mixed silage of alfalfa and Sudan grass. (d) Effects of additives and mixing ratios on acetic acid content in mixed silage of alfalfa and Sudan grass. (e) Effects of additives and mixing ratios on propionic acid content in mixed silage of alfalfa and Sudan grass. (f) Effects of additives and mixing ratios on butyric acid content in mixed silage of alfalfa and Sudan grass. Note: “**” indicates a highly significant difference (p < 0.01), “*” and different lower letters marked on the bars indicates a significant difference (p < 0.05), and “NS” indicates no significant difference (p > 0.05). Abbreviations: MS, mixing ratio; PFJ, previously fermented juice.
As the alfalfa concentration decreased, the pH and AN/TN ratio decreased (p < 0.05; Figure 1a,b). The pH and AN/TN ratio in the PFJI group were lower than those in the PFJ0 group (p < 0.05; Figure 1a,b). Similar trends were observed for LA and BA contents (Figure 1c,f). Changes in the mixing ratio led to fluctuations in LA and BA contents (decreased, increased, and decreased again). LA content peaked when the alfalfa proportion was 25%. However, when the alfalfa proportion increased to 50% and 75%, the BA content decreased to its lowest level. The LA content in most mix treatment ratios of the PFJI group was higher than that of the PFJ0 group, whereas the BA content was relatively low; however, there were no significant differences between most treatments (p > 0.05). As the mixing ratio changed, the AA content initially increased, subsequently decreased, and finally increased again. The AA content in the PFJI group was higher than that in the PFJ0 group, and the differences between most treatments were significant (p < 0.05; Figure 1d). With a decreased alfalfa proportion, the PA content increased, decreased, and finally stabilized. At the same PFJ addition level, the PA content reached its highest value under the M75 and M50 treatments, which was significantly different from that of the other treatments (p < 0.05). The PA content in most treatments of the PFJI group was significantly lower than that in the PFJ0 group (p < 0.05; Figure 1e).
The fermentation quality was better when the mixing ratio of alfalfa was 25–50%, particularly for the M50 treatment, where the BA content was relatively low. This indicates that different additives can increase or decrease the content of certain components in silage feed. Different PFJ concentrations had varying effects on fermentation indicators. At most mixing ratios, the LA and AA contents in the silage feed increased; however, this caused decreased pH, AN/TN ratio, and PA and BA contents.
3.4. Effects of Additives and Mixing Ratio on the Nutritional Composition of Alfalfa Silage
The analysis of the primary effects of different factors (Table 3) showed that PFJ addition had a highly significant effect on DM, NDF, and WSC contents (p < 0.01); a significant effect on RFV contents (p < 0.05); and no significant effect on other indicators (p > 0.05). The mixing ratio had a highly significant effect on CP, NDF, ADF, Ash, WSC, and RFV (p < 0.01) as well as EE (p < 0.05) contents and no significant effect on DM (p > 0.05). The interaction effect had a highly significant effect on NDF and WSC contents (p < 0.01) and no significant effect on other indicators (p > 0.05). Therefore, the mixing ratio was the primary factor affecting the nutritional quality of alfalfa and Sudan grass mixed silage.

Table 3.
Nutritional quality of mixed silage of alfalfa and Sudan grass under different treatments.
The NDF, ADF, and WSC contents of M100 (alfalfa single silage) were significantly lower than those of M0 (Sudan grass single silage) (p < 0.05), while there was no significant difference in the DM content (p > 0.05). The RFV and CP, Ash, and EE contents of M100 were significantly higher than those of M0 (p < 0.05). Under the mixing ratios of M75, M50, and M25, the RFV and DM, CP, NDF, ADF, Ash, EE, and WSC contents were between M100 and M0. Among them, CP, Ash, EE, and RFV contents decreased with decreasing alfalfa proportion, while NDF, ADF, and WSC contents increased. There was no regular change in the DM content.
With an increased alfalfa proportion, the nutritional quality of the silage feed increased. The WSC content in PFJ0 group was significantly higher than that in PFJ0 group (p < 0.05), and the differences between other indexes did not reach a significant level (p > 0.05).
3.5. Effects of Additives and Mixing Ratio on the Nutrient Degradation Rate of Alfalfa Silage
The analysis of the primary effects of different factors (Figure 2) showed that PFJ addition and the interaction effect had highly significant effects on natural detergent fiber disappearance (NDFD) and acid detergent fiber disappearance (ADFD) (p < 0.01) and had no significant effect on the dry matter disappearance (DMD) and crude protein disappearance (CPD) (p > 0.05). The mixing ratio had a highly significant effect on the DMD, CPD, NDFD, and ADFD contents (p < 0.01). Therefore, the mixing ratio is the primary factor affecting the nutrient degradation rate of alfalfa silage.
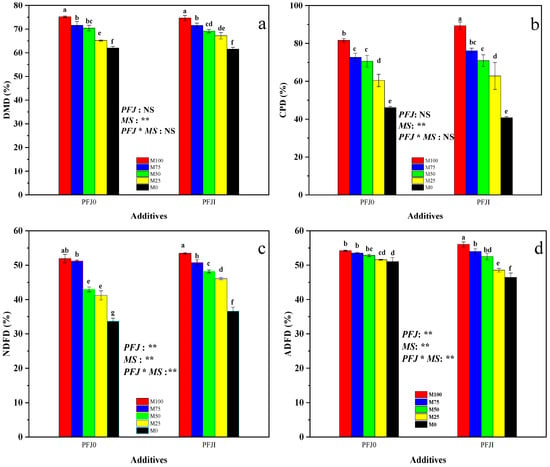
Figure 2.
Degradation rates of nutritional components in mixed silage of alfalfa and Sudan grass with different treatments. (a) Effects of additives and mixing ratios on DMD of mixed silage of alfalfa and Sudan grass. (b) Effects of additives and mixing ratios on CPD of mixed silage of alfalfa and Sudan grass. (c) Effects of additives and mixing ratios on DNFD of mixed silage of alfalfa and Sudan grass. (d) Effects of additives and mixing ratios on ADFD of mixed silage of alfalfa and Sudan grass. Note: “**” indicates a highly significant difference (p < 0.01), “*” and different lowercase letters marked on the bars indicates a significant difference (p < 0.05), and “NS” indicates no significant difference (p > 0.05). Abbreviations: MS, mixing ratio; PFJ, previously fermented juice.
Decreasing alfalfa proportion correlated with decreasing trends in DMD, CPD, NDFD, and ADFD, and most of the differences between treatments were significant (p < 0.05). This indicates a positive correlation between the nutrient degradation rate and alfalfa proportion in the mixed silage.
There was no significant difference in DMD between the PFJI group and the PFJ0 group (p > 0.05; Figure 2a). The CPD of the PFJI+M100 treatment group was significantly higher than that of the PFJ0+M100 treatment group (p < 0.05; Figure 2b). In the PFJI group, when the proportion of alfalfa was 0–50%, the NDFD was significantly higher than that in the PFJ0 group (p < 0.05; Figure 2c). The ADFD of the PFJI+M100 treatment group was significantly higher than that of the PFJ0+M100 treatment group (p < 0.05; Figure 2d). However, there were no significant differences among the other treatments (p > 0.05, Figure 2). This suggests that adding PFJ does not significantly improve the nutrient degradation rate of the mixed silage of alfalfa and Sudan grass.
3.6. Principal Component Analysis (PCA) of the Quality of Alfalfa Silage with Different Treatments
PCA provided a comprehensive overview of silage quality indicators. First, we examined the correlations among 13 quality indicators, including DM, CP, NDF, ADF, EE, Ash, WSC, pH, LA, AA, PA, AN/TN, ratio, and RFV, of mixed silage with different treatments (Figure 3).
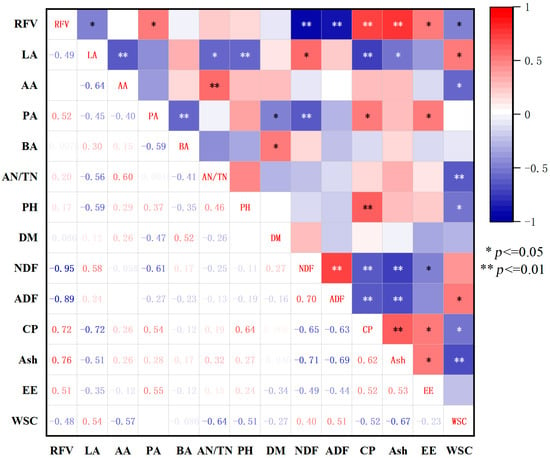
Figure 3.
Correlation coefficients of main quality traits of mixed silage of alfalfa and Sudan grass with different treatments. Note: The darker the red color, the stronger the positive correlation; the darker the blue color, the stronger the negative correlation; no symbol indicates no significant correlation.
Fifty-three pairs of indicators were not correlated (p > 0.05); sixteen pairs were positively correlated, with six being highly significantly positively correlated (p < 0.01) and ten significantly positively correlated (p < 0.05). Moreover, 22 pairs were negatively correlated, with 13 being highly significantly negatively correlated (p < 0.01) and 9 significantly negatively correlated (p < 0.05).
RFV was positively correlated with CP, Ash, and EE contents, with a highly significant correlation with CP and Ash contents (p < 0.01), indicating that the higher the CP and Ash contents, the higher the feeding value of mixed silage; RFV was negatively correlated with NDF, ADF, and WSC contents, and the correlation with NDF and ADF contents was highly significant (p < 0.01), indicating that the higher the NDF and ADF contents, the greater the risk of reducing the nutritional quality of mixed silage. Regarding the fermentation quality of mixed silage, RFV was significantly positively correlated with the PA content (p < 0.05), indicating that the higher the PA content, the better the fermentation quality of mixed silage. RFV was significantly negatively correlated with LA content (p < 0.05). This shows that additives that increase the LA content may not necessarily improve the RFV of the mixed alfalfa and Sudan grass silages.
To eliminate the misleading effects of overlapping information, PCA was performed on the 13 primary quality indicators of mixed silage under different treatments (Table 4 and Table 5).

Table 4.
Extraction analysis result and cumulative contribution rates of the principal components.

Table 5.
Characteristic values of each factor.
Furthermore, principal components were selected based on the principle of a cumulative contribution rate ≥ 85% (Table 4). The results showed that the cumulative contribution rate of the first four factors reached 87.761%, with an information loss of only 12.239%. Therefore, they represented the original 13 indicators.
Among the four principal components, PC1 had an eigenvalue of 6.181 and a contribution rate of 36.04%. The indicators with a higher correlation with PC1 in the corresponding eigenvector included ADF, NDF, CP, and Ash. PC2 had an eigenvalue of 2.505 and a contribution rate of 20.20%. The indicators with a higher correlation with PC2 in the corresponding eigenvector included AA, LA, and WSC. PC3 had an eigenvalue of 1.62 and a contribution rate of 16.00%. The indicators with a higher correlation with PC3 in the corresponding eigenvector included BA and PA. PC4 had an eigenvalue of 1.11 and a contribution rate of 15.54%. The indicators with a higher correlation with PC4 in the corresponding eigenvector included pH, the AN/TN ratio, and DM (Table 5).
3.7. Comprehensive Evaluation Using the Fuzzy Mathematical Membership Function Method
Owing to variations in the fermentation quality and the nutritional composition of alfalfa silage with different treatments, evaluating which treatment is more effective is challenging. The fuzzy membership function is an essential statistical evaluation method; therefore, it was adopted for a comprehensive evaluation of the various indicators (Table 6). Based on the PCA and the correlation analysis of various indicators, ADF was selected for PC1, WSC for PC2, BA for PC3, and pH for PC4.

Table 6.
The value of membership function and comprehensive evaluation of principal component.
Of all treatments, PFJ0+M50 performed best, with an average membership function value of 0.70, followed by PFJ0+M25 and PFJ0+M0. PFJ0+M75 and PFJI+M50 had average membership function values of 0.62 and 0.55, respectively, indicating poor performance. The average membership function values of the remaining treatments ranged from 0.29–0.51, indicating average performance.
4. Discussion
4.1. Sensory Evaluation Analysis of Different Treatments of Alfalfa Silage
In this experiment, the silage treated with PFJ0+M50, PFJI+M0, PFJI+25M, and PFJI+M50 appeared yellow-green, slightly pungent, loose, and non-sticky, and was rated as “Grade 1 Excellent”, while other treatments were rated as “Grade 2 Fair”. The sensory scores of the PFJI group were comparable to or even higher than those of the PFJ0 group. Studies have shown that mixing different raw materials can help create good fermentation conditions for silage. Moreover, minimizing residual air in the silage bag after vacuum packaging can prevent a prolonged respiration period, thereby reducing sugar wastage and favoring the proliferation of the dominant flora [,]. Similarly, the addition of LAB and cellulase compounds improved the odor, texture, and color of corn stalk silage [], consistent with our findings. Therefore, mixing high-sugar forage, such as alfalfa, with other forages or using PFJ during the silage process can effectively improve feed texture, thereby enhancing sensory evaluation. However, while sensory evaluation provides a quick and intuitive means of assessing silage quality, it is subjective [,]. Therefore, we conducted an in-depth analysis of the nutritional content and fermentation quality of silage.
4.2. Fermentation Quality Analysis of Different Treatments of Alfalfa Silage
In this study, the mixing ratio factors and interactions significantly affected the fermentation quality indicators of the mixed silage (p < 0.01). The additive factors significantly influenced the AA, PA, and BA contents of the mixed silage (p < 0.01) and had no significant effect on the pH, AN/TN, and LA content (p > 0.05). These findings align with those of Liang et al. [] and Hu et al. []. However, the influence of various factors on silage fermentation quality varies, which may be related to the different raw materials and additives used.
High-quality silage typically exhibits higher LA content, lower BA and AN contents, and a lower pH. pH is a key indicator for evaluating silage quality; most microbial growth is inhibited at pH < 4.2, meeting the criteria for high-quality silage. When the pH is 4.2–4.5, it is considered medium quality, and if it is > 4.5, it indicates poor silage quality []. Studies have found that the pH of alfalfa and Sudan grass silages decreases significantly after adding PFJ (p < 0.01) []. We found that when alfalfa constituted 25–50% of the mix, silage pH was <4.2, meeting high-quality standards. Conversely, the pH of the other treatment groups did not meet this criterion, possibly because decreasing alfalfa ratios allow naturally occurring LAB in the mixed raw materials to meet silage fermentation needs.
The AN/TN ratio is the primary indicator of silage protein degradation; lower ratios signify better the silage quality. The AN/TN ratio in high-quality silage should be <100 g/kg []. We found that the AN/TN ratio in alfalfa silage met these requirements only when the proportion of alfalfa did not exceed 50%. This indicates that adding Sudan grass or PFJ considerably reduces the AN/TN ratio in alfalfa silage.
Organic acid content and composition reflect the quality of the silage fermentation process, the most significant of which are LA, AA, and BA. High-quality silage typically has a higher proportion of LA and BA content < 0.1% []. In this study, when the proportion of alfalfa was between 25–50%, the LA content reached the highest value, and the AA content dropped to the lowest value. Moreover, when the proportion of alfalfa was between 50–75%, the BA content dropped to the lowest value. When the proportion of alfalfa was between 0–25%, the PA content decreased to its lowest value. Decreasing alfalfa proportions correlated with decreasing pH, LA, AA, PA, and AN/TN ratio, and increasing BA content. The results of this study align with those of a mixed silage experiment on Italian ryegrass and alfalfa by Aiyou et al. []. For most mixing ratios of the PFJ treatment group, the LA content was higher than that of the PFJ0 group, while the BA content was relatively low. This shows that PFJ treatment is beneficial for improving the fermentation quality of silage.
4.3. Analysis of the Nutritional Composition in the Mixed Silage of Alfalfa and Sudan Grass
The moisture and WSC contents of raw silage materials are crucial factors for producing high-quality silage. Theoretically, the minimum WSC requirement for high-quality raw silage materials is 6.0–7.0% (of DM), with a moisture content of 65–75% []. WSC is a fermentation substrate directly utilized by LAB; the higher the WSC content, the more LA produced and the lower the AA content, resulting in improved silage fermentation quality []. CP content is crucial for evaluating forage feeding quality and directly affects animal nutrition. ADF and NDF are the most effective indicators for evaluating fiber quality, and their contents are inversely correlated with livestock digestibility []. Alfalfa and Sudan grass exhibited significant differences in their nutritional characteristics. The former is characterized by a high CP and low WSC, and the latter is characterized by a low CP and high WSC. Adding Sudan grass to alfalfa can increase the total CP content of the raw materials to varying degrees while reducing the NDF and ADF contents, thereby optimizing the overall nutritional quality of the raw materials. However, this addition also reduces total WSC and DM contents, which may affect the silage fermentation process. These findings suggest that, when formulating silage, it is necessary to comprehensively consider the potential impact of adding different grass species on feed nutrients and their fermentation characteristics.
Therefore, controlling the moisture content of raw materials and adjusting the appropriate addition ratio is essential to ensure successful alfalfa silage preparation and obtain silage with high nutritional value. In this study, after harvesting fresh raw silage materials, the moisture content was controlled between 63.37–69.08% through an appropriate drying treatment. When alfalfa addition does not exceed 50%, it can significantly improve the WSC level of silage, effectively overcoming the challenge of high buffering capacity, thereby ensuring the smooth progress of the silage process and obtaining a higher nutritional value. The analysis of the primary effects of different factors showed that the mixing ratio was the primary factor affecting the nutritional quality of alfalfa silage. As the proportion of alfalfa increases, the nutritional quality of the silage increases, as determined by the inherent characteristics of the raw material. The contribution of PFJ to improving the nutritional value of alfalfa silage was relatively small, aligning with the results of Jian et al. []. At the same mixing ratio, the WSC content in the PFJI group was significantly reduced (p < 0.05), and there were no significant changes in other nutrient contents (p > 0.05). A possible reason is that microorganisms in the PFJI group use WSC as their energy source or that certain components in the PFJI group may promote the WSC decomposition process.
4.4. Analysis of Nutrient Degradation Rate in Mixed Silage of Alfalfa and Sudan Grass
The DMD, CPD, NDFD, and ADFD of M100 were significantly higher than those of M0 (p < 0.05). As the alfalfa proportion decreased, the DMD, CPD, NDFD, and ADFD of the silage showed a downward trend, and most treatments showed significant differences (p < 0.05). This trend may be attributed to the high degree of lignification of Sudan grass, where cellulose and hemicellulose in the cell wall are encased in lignin, strengthening the rigid structure of the Sudan grass cell wall and limiting its digestibility by microorganisms. The results of this study are consistent with those of Jintao et al., who observed similar effects when adding LAB and molasses to mixed silage of whole-plant paper mulberry and straw [].
A study showed that adding LAB to whole-plant corn silage can improve the digestion of DM, organic matter (OA), ADF, NDF, and CP in steers []. Another study showed that adding PFJ from alfalfa and red clover to alfalfa and red clover silage improved in vitro DM digestibility (p < 0.05) []. In the present study, the effect of PFJ addition on the degradation rate of various nutrients under different mixing ratios of alfalfa and Sudan grass varied. PFJ significantly improved the nutrient degradation rate of the mixed silage of alfalfa and Sudan grass. The results of this study are consistent with these previous studies.
5. Conclusions
Mixing alfalfa and Sudan grass for silage is an effective feed production method that can achieve nutrient complementarity and improve silage nutritional value and fermentation quality. By controlling the moisture content of the raw materials and adjusting the mixing ratio, the silage quality can be further optimized. In addition, using PFJ can improve the sensory and fermentation qualities and RFV of silage significantly. This study is significant for enhancing the economic benefits of animal husbandry and promoting sustainable development. In this experiment, we utilized silage bags made of food-grade PA+PE material, a design that significantly aids in the effective management of the fermentation environment. Based on the research findings obtained under the current experimental conditions, we believe that this approach is particularly suitable for application in the practice of wrapped silage feed. The plastic film used for wrapped silage, due to its specially designed permeability, not only optimizes the control of the fermentation process but also greatly enhances the reproducibility and scalability of the positive effects observed in our experiments. This conclusion aims to provide a solid scientific basis for further optimizing PFJ (previously fermented juice) addition strategies in various silage methods in the future, ultimately striving to comprehensively elevate the overall quality and production efficiency of silage feed.
Author Contributions
Y.D. contributed to the project idea, design, and execution of the study. Z.C., P.G. and J.G. were in charge of laboratory analyses. Y.D. and Z.K. were responsible for writing the manuscript. Y.D. and A.Y. were responsible for scientific editing and finalizing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Backbone Talent Training Project for the “Three Rural Issues” in the Xinjiang Uygur Autonomous Region of China, grant number 2022SNGGNT073, and the Special Project on Basic Business Expenses of Public Welfare Research Institutes in Xinjiang Uygur Autonomous Region of China, grant number KY2023031.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Welfare and Ethics Committee of Xinjiang Agricultural University, Urumqi, Xinjiang, China (protocol code 2023032; approval was granted on 17 January 2023), for studies involving animals.
Informed Consent Statement
Not applicable for studies not involving humans.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the technical assistance of Kaisaer Sadike, Animal Husbandry and Veterinary Station, Yingjisha County.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, L.; Bao, X.; Guo, G.; Huo, W.; Xu, Q.; Wang, C.; Li, Q.; Liu, Q. Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa Silage Fermentation Characteristics and In Vitro Ruminal Methane Production, Fermentation Patterns, and Microbiota. Animals 2021, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhao, H.; Liu, G.; You, Y.; Ma, L.; Liu, N.; Zhang, Y. Effects of nitrogen and maize plant density on forage yield and nitrogen uptake in an alfalfa–silage maize relay intercropping system in the North China Plain. Field Crops Res. 2021, 263, 108068. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Ge, Q.; Yang, B.; Li, S. Effects of harvest period and mixed ratio on the characteristic and quality of mixed silage of alfalfa and maize. Anim. Feed Sci. Technol. 2023, 306, 115796. [Google Scholar] [CrossRef]
- Bao, J.; Sun, Z.; Lu, J.; Li, Y.; Liu, G.; Yu, Z. Effects of Different Mixing Ratio and Silage Time on the Silage Quality of Alfalfa and Sweet Sorghum. Siliao Gongye 2021, 42, 43–47. [Google Scholar]
- Wen, A.; Yuan, X.; Wang, J.; Desta, S.T.; Shao, T. Effects of four short-chain fatty acids or salts on dynamics of fermentation and microbial characteristics of alfalfa silage. Anim. Feed Sci. Technol. 2017, 223, 141–148. [Google Scholar] [CrossRef]
- Jin, C. Effect of Different Additives on Mixed Silage of Corn and Alfalfa; Northwest A&F University: Xianyang, China, 2021. [Google Scholar]
- Fijałkowska, M.; Pysera, B.; Lipiński, K.; Strusińska, D. Changes of nitrogen compounds during ensiling of high protein herbages—A review. Ann. Anim. Sci. 2015, 15, 289–305. [Google Scholar] [CrossRef]
- Jones, D.J.C. The biochemistry of silage. J. Agric. Sci. 1991, 117, 386. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.-Q.; Wen, A.-Y.; Shao, T. Effects of molasses addition on the fermentation quality of broccoli residue, rice straw and alfalfa mixed silage. Actapratac. Sin. 2014, 23, 248. [Google Scholar]
- Sufuer, B.; Aishan, A.; Sha-zhou, A.N.; Yimiti, Y.; Ashan, S. Effects of Adding Formaldehyde Additives on Fermented Half-Dry Silage of Sorghum Sudanense Stapf for Nutrients Change Rule. Xinjiang Agric. Sci. 2014, 51, 1900–1906. [Google Scholar]
- Qin, L.G.; Xu, Q.F.; Wang, B.P.; Yu, Z.; Sun, Q.-Z. Effects of Formic Acid or Sucrose on Sorghum sudanense (Piper) Stapf Silage. Chin. J. Grassl. 2010, 32, 76–80. [Google Scholar]
- Hasiyati, T.; Halidai, R.; Zuerdong, R.; Aibibula, Y. Study on Fermentation Quality of Sudangrass and Sweet Clover Mix Silage. Grass-Feed. Livest. 2013, 4, 62–64. [Google Scholar]
- Aibibula, Y.; Reshalaitihan, M.; Liu, C. Effect Addition of Previously Fermented Juice on Fermentation Quality of Alfalfa and Sudan Grass Silages. Grass-Feed. Livest. 2012, 1, 60–65. [Google Scholar]
- Pu, W. Effects of Fermented Green Juices on Sainfoin and Alfalfa Silage Quality and Its Mechanism; Lanzhou University: Lanzhou, China, 2022. [Google Scholar]
- Jiang, K.W.; Zhang, Q.Q.; Wang, Y.F.; Li, H.; Yang, Q.; Tursunnayi, R. Differences in soil bacterial communities of desert grasslands in Tianshan under different grazing disturbances. Pratac. Sci. 2023, 40, 1243–1257. [Google Scholar]
- Wang, P.; Souma, K.; Ishii, N.; Okada, S.; Uchimura, T.; Ohshima, M.; Masuko, T. Effects of Adding Fermented Juice of Epiphytic Lactic Acid Bacteria on Fermentation Quality and Flora of Lactic Acid Bacteria in Grass Silage. Jpn J. Grassl. Sci. 2009, 55, 141–147. [Google Scholar]
- Xu, C.; Yang, F.; Zhang, J.G. Silage Science and Technology; Science Press: Beijing, China, 2013. [Google Scholar]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, Y.M.; Hirakubo, T. Fermentation characteristics and microorganism composition of total mixed ration silage with local food by-products indifferent seasons. Anim. Sci. J. 2011, 82, 259–266. [Google Scholar] [CrossRef]
- Filya, I.; Ashbell, G.; Hen, Y.; Weinberg, Z.G. The effect of bacterial inoculants on the fermentation and aerobic stability of whole crop wheat silage. Anim. Feed Sci. Technol. 2000, 88, 39–46. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Muscato, T.V.; Sniffen, C.J.; Van Soest, P.J. Nitrogen fractions in selected feed stuffs. J. Dairy Sci. 1982, 65, 217–225. [Google Scholar] [CrossRef]
- Vansoest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non starch polysaccharides in relation nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zhang, L.Y. Feed Analysis and Feed Quality Detection Technology, 2nd ed.; China Agricultural University Press: Beijing, China, 2007. [Google Scholar]
- Owens, V.N.; Albrecht, K.A.; Muck, R.E.; Duke, S.H. Protein degradation and fermentation characteristics of red clover and alfalfa silage harvested with varying levels of total nonstructural carbohydrates. Crop Sci. 1999, 39, 1873–1880. [Google Scholar] [CrossRef]
- Undersander, D.J.; Moore, E.; Schneider, N. Relative Forage Quality. Focus on Forage. 2010. Available online: https://www.foragelab.com (accessed on 16 October 2023).
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Pei, C.X. Effect of Different Harvest Time and Drying Methods on Nutrient Like WSC of Herbage; Shanxi Agricultural University: Jinzhong, China, 2001. [Google Scholar]
- Wang, X.-Z. Effect of Compaction and Harvest Stage on Quality and Mycotoxin of Whole-Plant Corn Silage. Ph.D. Thesis, Shihezi University, Shihezi, China, 2019. [Google Scholar]
- Ge, J.; Yang, C.J.; Yang, Z.M.; Bai, X.M.; Zhao, H.X.; Liu, G.H. Quality of mixed naked oats (Avena nuda) and alfalfa (Medicago sativa) silage. Acta Pratac. Sin. 2015, 24, 104–113. [Google Scholar]
- Huang, X.H. Sophora alopecuroides and Corn Straw Mixed Silaging and Quality Evaluation of Silage; Lanzhou University: Lanzhou, China, 2014. [Google Scholar]
- Xingjun, X.; Lujia, H.; Shin-ichiro, H.A.A.; Kazuhisa, N.A.A.A. Effects of lactobacillus and cellulase on the quality of corn stover silage. J. China Agric. Univ. 2003, 8, 21–24. [Google Scholar]
- Haiwei, R.; Cong, W.; Junwei, D.; Zhizhong, L.; Jinping, L.; Yongming, S. Mixed ensiling quality of maize straw with waste cabbage and biogas production potential analysis. Trans. Chin. Soc. Agric. Eng. 2016, 32, 187–193. [Google Scholar]
- Gu, Y.J.; Zhan, J.S.; Sha, W.F.; Zhu, J.; Zhan, K.; Lin, M.; Zhang, W.Y. Comparative Analysis of Fermentation Quality and Nutrient Content of Mixed Storage of Soybean Straw and Corn Stover at Different Proportions. China Feed 2016, 6, 21–24. [Google Scholar]
- Liang, X.-Y.; Ji, Y.; Yi, J.; Fu, M.-Z.; Hu, Y.-B. Effects of mixing ratio and additives on ensilage eficieney of mixed chicory and silage maize. Acta Pratac. Sin. 2018, 27, 173–181. [Google Scholar]
- Hu, Y.B.; Liang, X.Y.; Yi, J.; Guan, H.; Zhang, J.; Zhang, J.M.; Meng, Y.H.; Ji, Y. Effects of mixing ratio and additives on the quality of napier grass and whole-plant corn mixed silage. Pratac. Sci. 2022, 39, 778–786. [Google Scholar]
- Zhang, J.G.; Kawamoto, H.; Cai, Y. Relationships between the addition rates of cellulose or glucose and silage fermentation at different temperatures. Anim. Sci. J. 2010, 81, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Jin, Y.M.; Sun, Q.Z.; Wang, L.; Zhang, H.J. Effect of Additives on Fermentation Quality of Hempleaf Nettle (Urtica cannabina L.) Silage. Acta Agrestia Sin. 2010, 18, 291–296. [Google Scholar]
- Wen, A.-Y.; Yuan, X.-J.; Wang, J.; Wang, J.; Shao, T. Study on Fermentation Quality of Mixed Silage of Alfalfa and Italian Ryegrass. J. Anhui Sci. Technol. Univ. 2011, 25, 10–14. [Google Scholar]
- Dong, K.H.; Shen, Y.X. Forage Production; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Li, X.-L.; Zhang, X.-Y.; Tang, Y.-G.; He, F.; Zhang, J.-Z. Effect of concentrate-forage ratio in diet on liveweight gain of stall fed goats. Acta Pratac. Sin. 2008, 17, 85–91. [Google Scholar]
- Fu, J.-T.; Wang, X.-K.; Ni, K.-K.; Yang, F.-Y. The effects of adding lactic acid bacteria and molasses on termentation of Broussonetia papyrifera and rice straw mixed silage. Acta Pratac. Sin. 2020, 29, 121–128. [Google Scholar]
- Muck, R.E.; Pitt, R.E. Aerobic deterioration in corn silage relative to the silo face. Trans. ASAE 1994, 37, 735–743. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Y.; Ling, Q.; Na, N.; Xu, H.; Vyas, D.; Adesogan, A.T.; Xue, Y. Effects of Adding Pre-Fermented Fluid Prepared from Lucerne or Red Clover on Fermentation Quality and In Vitro Digestibility of the Ensiled Wilting-Forages. Agriculture 2021, 11, 454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).