Abstract
Extra energy consumption, inefficient nitrogen removal, and excessive sludge production are major challenges faced by wastewater treatment plants (WWTPs) that rely on the traditional activated sludge process. Fermentation of wasted activated sludge (WAS) and novel nitrogen removal technologies based on partial nitrification (PN) have emerged as promising solutions to these issues. Recent studies have revealed an innovative strategy that integrates these two processes by supplementing fermentation liquid into activated sludge to induce PN. This review summarizes the research progress on PN establishment induced by the fermentation process. The microbiology and establishment methods of PN are briefly introduced, followed by a detailed discussion on the process, influencing factors, and product characteristics of WAS fermentation. The core section focuses on the side-stream and main-stream approaches of fermentation-induced PN, comparing their performance and application prospects. The potential mechanisms are explored, with an emphasis on the roles of free ammonia for the side-stream approach and the high tolerance of ammonium oxidizers to in-site fermentation stress for the main-stream approach. Finally, the limitations of the current research and future perspectives are discussed, highlighting the need for further investigation into microbial ecology, process optimization, and long-term stability. This review aims to provide insights into the synergistic integration of WAS fermentation and PN for sustainable and energy-efficient wastewater treatment.
1. Introduction
Due to a range of human activities, such as chemical fertilizer application, livestock farming, and fossil fuel burning, tons of nitrogen contaminants, mainly as ammonia, have been discharged into natural and artificial water bodies [1,2]. Nitrogen contaminants have exceeded the load of nitrogen transformation by indigenous microorganisms, resulting in an imbalance in the nitrogen cycle and causing a series of environmental problems [3]. Nitrogen pollution in water leads to eutrophication of water bodies and algal blooms, eventually causing poor water quality and affecting drinking water safety [4,5]. Methods for treating nitrogen pollution are multiple, of which activated sludge is one of the most popular and practical methods in global wastewater treatment plants [6,7]. Activated sludge, a mixture of complex microbial community and diverse compounds, is able to remove nitrogen contaminants from wastewater via nitrification and denitrification microorganisms [8]. However, two issues, excessive wasted activated sludge (WAS) production and energy consumption, handicap the sustainability and efficiency of nitrogen pollution treatment using activated sludge.
WAS generated during wastewater treatment via activated sludge contains a large amount of organic matter, nutrients, and even toxic matter [9]. To treat abandoned sludge safely and efficiently, WAS has been utilized as substances for fermentation, which recycles organics stored in WAS [10]. Sludge fermentation refers to the microbial transformation of organic compounds, including extracellular polymeric substances (EPSs) and intracellular organics, into volatile fatty acids (VFAs), which can be utilized to synthesize polyhydroxyalkanoates and prepare microbial fuel cells [11]. However, the majority of organics in WAS are resistant to being degraded and utilized by fermenting microorganisms. These non-biodegradable organics generally need to be hydrolyzed into smaller compounds which can be directly used by fermenting microorganisms. The hydrolysis stage is time consuming and is the rate-limiting stage of fermentation [12]. To promote hydrolysis performance, sludge pretreatment technologies, such as thermal heating, alkalization, ultrasound, and free nitrous acid, are applied to increase hydrolysis rates [13,14,15,16]. Rapid hydrolysis can provide more substrates for the fermentation reaction. But, accompanying WAS fermentation, fermentation liquid containing by-products such as organics and contaminants is inevitably generated [17]. These by-products, on the one hand, can be recycled back into the main stream for further treatment; on the other hand, they have been applied to enhance the denitrification rate by increasing influent organic substances [18].
In order to solve the second issue of activated sludge application for extra energy and organic matter consumption, a novel nitrogen removal technology called partial nitrification (PN) has been rapidly developed based on two-step nitrification (NH4+→NO2−→NO3−) [19,20]. The PN process can realize nitrite accumulation (NH4+→NO2−) by eliminating nitrite-oxidizing bacteria (NOB) activity and maintaining ammonia-oxidizing bacteria (AOB) activity simultaneously [21]. Then, denitrifiers directly reduce nitrite instead of nitrate to generate nitrogen gas. Compared with two-step nitrification and denitrification, PN and denitritation can save 25% of oxygen consumption, 40% of organic carbon consumption, and 20% of carbon dioxide emissions [22]. At the same time, nitrogen removal efficiency is greatly improved over shortened reaction steps. Given the coexistence of nitrite and ammonia in PN effluent, a PN-triggered anaerobic ammonia oxidation process has been repeatedly constructed, and this coupling can greatly reduce the operating cost and secondary pollution (e.g., wasted sludge) during nitrogen removal [21,23]. In summary, PN-based technologies have great application value and potential in the field of wastewater treatment, but the independent methods to achieve PN, such as manipulating environmental factors of sludge or treating sludge with chemicals, may be inefficient for achieving the PN trait in some cases, at the cost of additional resources [24,25]. Thus, sustainable and low-investment methods for PN establishment are required urgently.
Interestingly, recent studies have revealed a novel strategy that combines these two processes by using anaerobic WAS fermentation liquid to induce PN via main-stream and side-stream ways [26,27]. These methods managed to reuse the fermentation by-products and reduced the cost of PN establishment synchronously, and contributed to solving the abovementioned major issues in the fields of wastewater treatment. Despite the promising potential of this integrated approach, its application is still in its infancy, and the underlying mechanisms remain largely unknown. Moreover, the existing literature on this topic is scattered and lacks a systematic review. Therefore, this paper aims to provide a comprehensive overview of the research progress on establishing PN using WAS fermentation liquid with a focus on the potential mechanisms and implications for sustainable wastewater treatment. The review begins with a brief introduction to the microbiology and conventional establishment methods of PN, followed by a detailed discussion on the characteristics of WAS fermentation and its by-products. The core section focuses on the mechanisms and applications of fermentation-liquid-induced PN in both side-stream and mainstream treatment configurations. Finally, the limitations of current research are discussed, and future research directions are proposed to guide the development of this integrated approach towards full-scale applications.
2. Microbial Basis and Establishment Methods of Partial Nitrification
2.1. Microbial Basis of Partial Nitrification
The fundament of PN transition from two-step nitrification is selective inhibition of NOB instead of AOB using multiple methods such as low DO, free nitrous acid (FNA), and free ammonia (FA), as demonstrated in Figure 1. Thus, the core microbes for PN establishment are AOB and NOB. Two steps of nitrification contain initial ammonium oxidation and subsequent nitrite oxidation. Firstly, ammonium is oxidized to nitrite using oxygen as the electron acceptor [28]. The ammonium-oxidizing reaction is catalyzed by ammonia monooxygenase encoded by the amoA (NH4+→NH2OH) and hydroxylamine oxidoreductase coded by HAO (NH2OH→NO2−). The gene amoA is normally a characteristic gene for identifying AOB in a microbial community [29]. AOB belong to the Nitrobacteriaceae family in the Proteobacteria phylum. General AOB include Nitrosomonas, Nitrosospira, and Nitrosococcus, among others [30]. The second step of nitrification is nitrite oxidation, which also requires oxygen as the electron acceptor to further convert nitrite into nitrate under the catalysis of nitrite oxidoreductase (NXR). Bacteria functioning with oxidizing nitrite, i.e., NOB, are distributed in the Nitrobacteriaceae family too, and mainly include Nitrospira, Nitrobacter, Nitrococcus, and Nitrospina. Dueholm et al. analyzed the microbial community structure of activated sludge in 740 wastewater treatment plants (WWTPs) located in 31 countries in 2022 and found that Nitrosomonas and Nitrospira were the highest abundant AOB and NOB genera, respectively, in activated sludge of wastewater treatment plants [31]. Our previously research and that carried out by peers indicated that selective depression of NOB activity during PN establishment could be attributed to Nitrospira abundance reduction [32,33,34]. Hence, Nitrospira may be the vital nitrifier for PN transition. The dynamics of NOB repression during PN transition have been captured in mathematical models [35]. However, the mathematical model can only explain the NOB repression dynamic in the corresponding system and not the dynamic in other systems. Thus, it may be necessary to develop a generalized model to explain the NOB activity fluctuation during biological nitrogen removal.
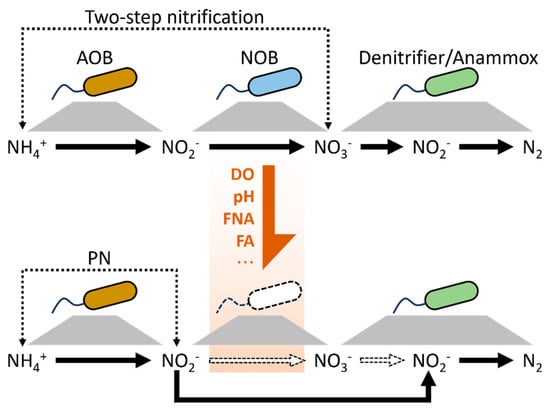
Figure 1.
Microbial nitrogen removal via PN-based processes.
AOB and NOB share many analogous traits, including Gram-negative, aerobic, and chemoautotrophic traits, resulting in a high degree of niche overlap between the two in sludge microbiota [36]. In WWTPs, the nitrification process is usually carried out in an oxic tank, where the O unit of A2O is processed. By providing sufficient oxygen and suitable conditions, the reproduction and activity of AOB and NOB can be promoted, thereby achieving effective ammonia reduction. Thus, the sludges for PN investigation are generally taken from the oxic tank of WWTPs [24]. Additionally, it has also been found that special microorganisms can independently complete the entire nitrification process. That is, both ammonia oxidation and nitrite oxidation are completed by the same microorganism, which is named the Complete Ammonia Oxidizer (Comamox) [37]. In 2015, three Nitrospira isolations with complete ammonia oxidation ability were reported, namely, Candidatus Nitrospira inopinata, Candidatus Nitrospira Nitrosa, and Candidatus Nitrospira nitificans [38,39]. The abundance of Comamox in activated sludge ranged from 0.28% to 0.64%, as reported in [40]. The contribution of Comamox to ammonia removal may be considerable, but the coexistence of Comamox with AOB and NOB in sludge microbiota could be an obstacle for achieving PN. Because, theoretically, Comamox possesses nitrite oxidation capability and should be washed out, the ammonia oxidation capability would be undermined at the same time by losing Comamox. Therefore, a sludge with abundant Comamox is inappropriate to be applied to achieving the PN process.
2.2. Partial Nitrification Establishment Methods
Based on the differences in physiological and ecological characteristics between AOB and NOB, researchers have developed a variety of methods for establishing PN. These methods can be broadly categorized into manipulating environmental factors and using selective inhibitors. This section will systematically discuss the principles and application effects of these methods.
2.2.1. Environmental Factor Manipulation
Temperature, dissolved oxygen (DO), and pH are the key environmental factors that can be manipulated to establish PN by exploiting the physiological differences between AOB and NOB. These factors play crucial roles in selecting AOB over NOB, as the two groups of nitrifying bacteria exhibit different sensitivities and optimal ranges for growth and activity. The manipulation of these environmental parameters can create selective pressures that favor the growth and activity of AOB while suppressing NOB, thus promoting the accumulation of nitrite and the establishment of PN.
- Temperature
Temperature is a critical factor that affects the growth rates, enzyme kinetics, and substrate affinities of nitrifying bacteria. AOB and NOB have different optimal temperature ranges and temperature sensitivities. Camilla et al. demonstrated that the optimal temperatures for Nitrosomonas and Nitrobacter growth were 35 °C and 38 °C, respectively [41]. Hellinga et al. (1998) proposed typical growth curves for AOB and NOB, indicating that the minimum sludge age of AOB under low-temperature conditions exceeded that of NOB, while the opposite was true under high-temperature conditions [42]. Therefore, PN can be achieved under high-temperature conditions by adjusting the sludge age. However, for sewage at room temperature or low temperature, an economic feasibility analysis is required if heating is used to achieve PN. The opinions on optimal temperature for PN establishment differed among other studies. The optimal temperature for achieving PN was stated to be 22–27 °C and 25 °C, which is far from the critical temperature of 35 °C proposed in the SHARON process [43,44,45]. Despite these differences, many studies have confirmed that stable and efficient PN can be achieved under normal or low-temperature conditions by applying other methods in combination with appropriate temperature control [32,46,47];
- 2.
- Dissolved Oxygen
Because nitrifying bacteria are aerobic autotrophic bacteria, DO concentration is able to affect their activity [48]. The effect of DO on AOB and NOB is normally quantitatively simulated using the Monod equation with the oxygen half-saturation constant (KO) characterizing oxygen affinity [49]. Reported KO,AOB and KO,NOB values, as shown in Figure 2, indicated that AOB had a higher oxygen affinity than NOB [50,51,52,53,54]. When DO concentration was below 1.0 mg/L, the rate of AOB growth was 2.6 times that of NOB [55], leading to nitrite accumulation [56]. Although the theory that low DO concentration selectively favors AOB growth has been widely accepted and cited in relation to PN establishment by controlling DO, several opposite results have also been reported. Both Refs. [57,58] observed that KO,AOB exceeded KO,NOB in activated sludge, hindering nitrite accumulation. Therefore, the DO controlling strategy may be not available for all nitrogen-removal sludges.
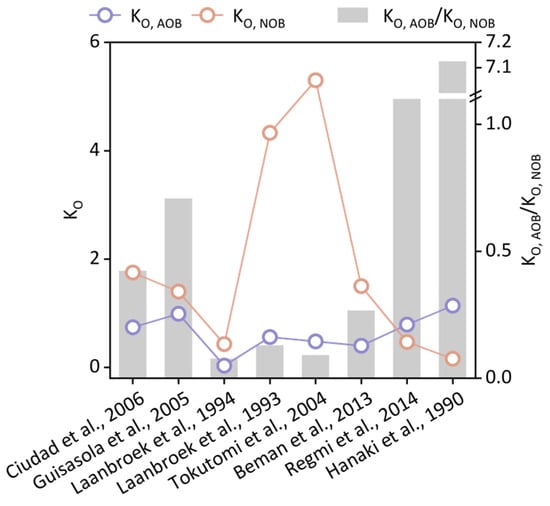
Figure 2.
Experimental KO,AOB and KO,NOB values reported in the literature [1,2,3,4,5,6,7,8].
Although many researchers have reported that low DO concentration facilitated PN establishment by depressing NOB activity and growth, the optimal DO range for PN establishment may vary depending on the reactor configuration and wastewater characteristics. Hanaki et al. reported that, when DO was controlled under 0.5 mg/L, the AOB growth rate nearly doubled, while the NOB growth rate did not increase [59]. Guo et al. (2008) suggested that AOB and nitrite accumulation peaked at a DO concentration of 1.5 mg/L [60]. Ruiz et al. (2003) achieved 65% nitrite accumulation and 98% ammonia removal by controlling DO at 0.7 mg/L [61]. Despite the differences in reported critical DO values, PN traits were generally obtained with DO under 1.5 mg/L. More attention should be paid to the multiple issues induced by low DO, including nitrification efficiency reduction, sludge disintegration, and filamentous bacteria expansion [62]. Hence, online methods are now used for real-time DO control to avoid high DO concentrations and excessive aeration;
- 3.
- pH
The pH of the wastewater can influence the growth and activity of AOB and NOB, as well as the speciation of ammonia and nitrite. AOB have a slightly higher optimal pH range (7.0–8.5) compared to NOB (6.0–7.5) [63]. When pH dropped to 6.6, the PN reaction basically stopped [24]. Actually, recent works thought the influence of pH on AOB and NOB activities mainly depended on the formation of free ammonia (FA) and free nitrous acid (FNA) in sludge [64]. FA and FNA are both nitrification inhibitors but have different impacts on AOB and NOB. Their roles in PN establishment are recapped hereinafter.
2.2.2. Selective Inhibitors
Selective inhibitors that preferentially suppress NOB activity have emerged as a promising approach to establish PN. These inhibitors include free ammonia (FA), free nitrous acid (FNA), and various chemical compounds.
- Free Ammonia (FA)
FA refers to free ammonia, the unionized form of ammonium (i.e., NH3), whose concentration positively correlates with NH4+ content and alkalinity. Although NH4+ is the substance of nitrification, high levels of FA can simultaneously inhibit AOB and NOB activities, and NOB are more sensitive to FA [65]. The FA inhibition threshold of AOB ranged from 10 to 165 mg N/L, while, for NOB, an FA concentration of 0.1–5 mg N/L could clearly inhibit its activity [66]. Theoretically, when FA levels range between 5 and 10 mg N/L, AOB can normally grow and oxidize ammonia, but NOB activity is inhibited. Hence, several pieces of research established PN by using FA whose content was located within this range [67,68]. Sui et al. established PN with 10.61 mg N/L of FA and gained 84% of the nitrite accumulation rate (NAR) [47]. The mechanisms of FA inhibition on AOB and NOB are complicated. First of all, FA is able to inhibit the activity of enzymes related to nitrification reactions. Electron transfer and proton transfer can be disturbed by FA. Yang et al. showed that FA had a specific inhibitory effect on nitrite oxidoreductase, which is the key enzyme catalyzing nitrite to nitrate in NOB [69]. This specific inhibitory effect depresses NOB ability and also explains why NOB are more sensitive to FA than AOB. In addition, FA can inhibit nitrifier activity by affecting the intracellular pH. FA at an inhibitory level passively diffuses through the cell membrane to the cytoplasm, causing proton imbalance or potassium deficiency [70]. When FA enters into intracellular cytoplasm, protons can be neutralized, causing alkalization elevation. Then, the cell must maintain proton balance and pump proton inside by consuming energy for the initiation of the potassium pump. Due to extracellular alkalization, this process, in turn, accelerates the inward transport of NH3. At the same time, excess potassium ions have to be pumped out to balance the potential difference. Overall, this will increase the energy demand for maintenance as it requires maintaining ion gradients on the cytoplasmic membrane and may lead to inhibition of specific enzyme reactions;
- 2.
- Free Nitrous Acid (FNA)
FNA, as protonated nitrite, is a broad-spectrum antibacterial agent, but it can selectively inhibit NOB activity, thereby efficiently establishing PN [71]. In 2014, Yuan et al. from the University of Queensland in Australia firstly discovered that, under the same treatment conditions, FNA had a higher inhibitory effect on NOB activity than AOB activity [34]. They treated the activated sludge with an FNA solution with a concentration of 1.35 mg N/L for 24 h and found that AOB activity remained at 50% after treatment, while NOB activity was almost undetectable. Then, the treated sludge was placed in SBR for continuous operation, achieving PN with an NAR of 80%. The preliminary work of our research group also confirmed the feasibility of establishing PN through FNA treatment. After treating sludge with 1.2 mg N/L of FNA for 18 h, NOB activity was completely inhibited, while the activity of AOB remained around 57% after treatment. By using this method, a stable PN trait was successfully established with a maximum NAR of over 90% and displayed considerable resistance to high dissolved oxygen levels and low temperature stress [32]. Subsequently, multiple research teams worldwide successfully established PN in different reactors using the principle of FNA selective inhibition of NOB activity [72,73]. For the first time, Jiang et al. achieved PN using the FNA method in an A2O reactor, which officially promotes FNA-induced PN for continuous flow treatment processes with higher market share [33]. This achievement further confirms the feasibility and potential application of FNA in the field of sewage treatment.
At present, exploration of the inhibitory mechanism of FNA on microbial cells mainly focuses on its effects on bacterial metabolism, substrate transmembrane transport, oxygen acquisition, and oxidative phosphorylation processes. FNA can act as an uncoupling agent to disrupt the electron transfer process during the ATP generation reaction, affecting energy production and inhibiting cellular metabolic activity [74]. As a proton carrier, FNA can increase the ability of protons to pass through cells. Therefore, in order to maintain the balance of intracellular and extracellular potentials, cells need to expel excess free protons from the cell to the extracellular space. This process has to consume additional ATP, leading to insufficient ATP to maintain normal cellular metabolism. In addition, FNA carries nitrite and causes certain oxidative damage to various enzymes that catalyze metabolism, including nitrogen-form-conversion-related enzymes and enzymes involved in substance decomposition and synthetic metabolism [75]. Meanwhile, FNA may also produce oxidizable reactive oxygen species (ROS) during the disruption of electron transfer. Then, another antibacterial pathway for FNA produces oxidants that destroy cellular substances and structures.
The differences in FNA inhibition of AOB and NOB activities are reported to be attributable to their regulatory abilities in FNA antibacterial pathways. As shown in Figure 3, the uncovered FNA detoxification pathways of AOB and NOB mainly focus on nitrite conversion ways [34,76]. A shared nitrite conversion way between AOB and NOB is the nitrite reductase encoded by nirK, which can convert nitrite into nitric oxide. In addition to the nirK pathway, NOB have two additional nitrite conversion pathways compared to AOB, namely, nirB, encoding nitrite reductase, and norA/B, encoding nitrite reductase [76,77,78]. In theory, NOB individuals have more FNA detoxification mechanisms, and their FNA sensitivity should be lower than that of AOB. However, in reality, AOB exhibit lower FNA sensitivity in the sludge microbial community. Researchers have utilized metagenomics and proteomics methods to elucidate the metabolic pathways by which AOB cells alleviate FNA biotoxicity [76]. Except nirK, AOB cells were also capable of attenuating FNA stress by upregulating the synthesis of oxidative stress repair enzymes and enhancing energy generation. However, due to the low detection level of NOB-related proteins in the experiment, the molecular biological response mechanism of NOB to FNA stress has not been elucidated. In summary, the metabolic perspective of individual response to FNA stress seems unlikely to reveal the selective inhibition of NOB activity by FNA. Therefore, in order to clarify how FNA triggers PN, collective regulation relying on cell communication, such as quorum sensing, should be introduced from the perspective of microbial population regulation.
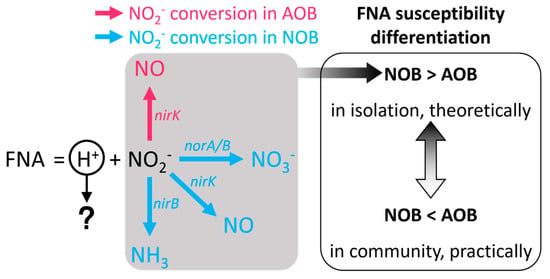
Figure 3.
FNA detoxification pathways in AOB and NOB.
- 3.
- Other Nitrite-Oxidizing Bacteria Inhibitors
Sulfides, heavy metals, benzethonium chloride (BZC), and other NOB inhibitors have also been applied to establish the PN trait. Erguder et al. increased NAR from less than 13% to 75% by adding sulfides at 45 mg S/L [79]. Sulfides could inhibit both AOB and NOB, with nitrite oxidation being the most affected [80]. Sulfides have also been combined with other methods, such as autotrophic denitrification, to achieve high total nitrogen removal [81]. The inhibitory effect of heavy metals on NOB depends on sludge type, exposure duration, and temperature [82]. Nickel toxicity was more significant for NOB than AOB at 23 °C and 35 °C, while it mainly targeted AOB at 10 °C [83]. BZC, a quaternary ammonium compound, can affect nitrification in biological denitrification systems. Cui et al. showed that 70 mg/L BZC initiated nitrification and maintained stable PN for 91 cycles [84]. Wu et al. established PN and maintained it for 125 days by continuously pumping low levels of BZC (0.2–1 mg/L). BZC may promote stable PN by increasing EPS secretion, changing protein secondary structure, and inhibiting Nitrospira [85].
In summary, more and more NOB inhibitors are being discovered and utilized to establish PN. However, these NOB inhibitors, such as heavy metal, sulfide, and certain antibacterial agents, inevitably cost additional resources and introduce emerging pollutants into the PN system. Recently, an innovative PN establishment method has been proposed, which is initiating PN using sludge fermentation. This method can efficiently reuse fermentation by-products and reduce the cost and risk of PN establishment.
3. Fermentation of Waste Activated Sludge
3.1. Process and Influencing Factors of Fermentation
Fermentation can properly reuse the wasted organics in excessive sludge by transforming them into available organic forms with microbial activities [86]. Available organics, mainly as volatile fatty acids (VFAs), are able to be utilized in the fields of bioresource production and bioenergy [87], but the majority of organics in WAS are polymeric organic compounds, such as EPS, with non-biodegradability. Thus, the polymeric organics are firstly hydrolyzed into simpler compounds like amino acids, sugars, and long-chain fatty acids, as depicted in Figure 4. Acidogenic bacteria, serving as important fermenting microbes, can subsequently convert these simpler compounds into VFAs, including acetic acid, propionic acid, and butyric acid [14]. Then, VFAs can be further utilized to generate methane and hydrogen through acetogenesis and methanogenesis processes.
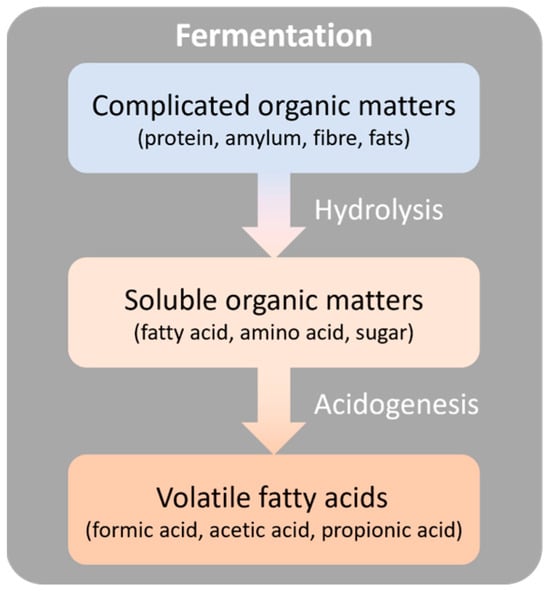
Figure 4.
The general process of WAS fermentation.
Fermentation is a process involving multiple microorganisms, and the acidogenesis process is usually accompanied by competition between acid-producing bacteria and methane-producing bacteria [88]. In order to limit the growth and reproduction of methane-producing bacteria, fermentation conditions can be optimized. By optimizing factors such as temperature, pH, and retention time during fermentation, VFA production can be promoted. For instance, mesophilic temperatures (30–38 °C) are commonly used for WAS fermentation as they support a diverse microbial community and stable fermentation process [9]. The pH is a critical parameter affecting the fermentation pathway and product distribution. A pH range of 4.0–6.5 favors the production of VFAs, while a near-neutral pH (6.5–7.5) is optimal for methanogenesis [89]. In other research, the use of potassium ferrate under alkaline conditions could also facilitate VFA production by promoting cell wall destruction [90]. At the same time, enriched bacteria under alkaline conditions are capable of producing acid or secreting hydrolytic enzymes, increasing the activity of enzymes related to hydrolysis and acidification. Retention time determines the extent of sludge degradation and the composition of fermentation products. Typical retention times for WAS fermentation range from a few days to several weeks, depending on the desired products [91]. C/N reflects the nutritional level of sludge as a fermentation substrate and is one of the important factors affecting fermentation efficiency. Over improving C/N, VFA production increased first and then decreased [92]. In fact, the decreased production with high C/N was ascribed to the organic overloading, i.e., the loading beyond the design capacity. This depression in acidogenic bacteria may be ascribed to the accumulation of fatty acids with a high concentration of organic substances for further methanogenesis. Thus, too high a C/N could depress VFA production. Although VFA production from fermentation of wastage sludge could be relatively considerable with relatively low C/N, it will be further improved by increasing C/N with external carbon addition [93]. It was generally believed that the C/N ratio for fermentation should be between 20 and 35, with 25 being optimal [93].
Fermentation microbes normally prefer to utilize soluble and biodegradable organic matter instead of complex matter [94]. The fermentation can be fast, and hydrolysis is very slow because of the occurrence of high molecular polymers such as EPS excreted by microbes during biological treatment and particulate organic matter [95]. Thus, as is known to all, hydrolysis limits fermentation efficiency, causing long retention time and large land occupation. Another reason hindering fermentation rate is that the majority of biodegradable organic matter in WAS exists within microbial cells [96]. However, fermenting bacteria have no access to this organic matter due to the protection of the cellular wall and the membrane separating them from the extracellular solution. This fact makes the disintegration of the cellular wall and membrane essential for releasing intracellular organic matters, but a cellular wall possessing a semi-rigid structure is a refractory inert substance which is difficult to destroy merely with microorganisms. Therefore, sludge pretreatments aiming to destruct cell walls and release intracellular organic matters have been applied before WAS enters into the anaerobic reaction tank [97]. The pretreatments mainly include thermal, ultrasound, microwave, advanced oxidization, and enzyme pretreatments. Table 1 recaps the effects of various pretreatments on fermentation performances. As reported, pretreatments could enhance the soluble chemical oxygen demand (SCOD) concentrations by 32.6% to 20.1 folds, and reduced volatile solid concentrations by 24.6% to 79.4%. At the same time, other objectives, such as eliminating pathogens and increasing dewaterability, can also be achieved during sludge pretreatments [98,99]. These effects may reduce subsequent consumption for WAS disposal after fermentation. For instance, pathogen elimination can reduce disinfectant doses. High dewaterability decreases the difficulty in sludge dewatering and leads to convenient storage and transport [100].

Table 1.
Effects of various sludge pretreatments on fermentation performance.
3.2. Characteristics of Wasted Activated Sludge Fermentation Products
WAS fermentation liquid contains a complex mixture of compounds, including VFAs, ammonium, phosphate, and soluble microbial products (SMPs) [86]. VFAs, such as acetic acid, propionic acid, and butyric acid, are the main products of acidogenesis and serve as substrates for subsequent methanogenesis. The concentration and composition of VFAs in fermentation liquid depend on various factors, such as the type of sludge, fermentation conditions, and pretreatment methods [110]. Feng et al. (2009) [85] reported that acetic acid and propionic acid were the dominant VFAs in WAS fermentation liquid, accounting for 50–70% of the total VFAs.
Ammonium is released during the fermentation of WAS due to the degradation of nitrogenous compounds, such as proteins and urea. The concentration of ammonium in fermentation liquid can range from a few hundred to two thousand milligrams per liter, depending on the nitrogen content of the sludge and the extent of fermentation [111,112,113]. High levels of ammonium can inhibit methanogenesis and cause toxicity to microorganisms [114]. However, ammonium is also an essential nutrient for microbial growth and can be beneficial for downstream processes, such as PN. Phosphate is another important component in WAS fermentation liquid, originating from the degradation of phosphorus-containing compounds, such as phospholipids and nucleic acids. The concentration of phosphate in fermentation liquid is generally lower than that of ammonium and VFAs, ranging from a few to several hundred milligrams per liter [115]. Phosphate can precipitate with metal ions, such as calcium and magnesium, forming insoluble compounds that may cause scaling and clogging issues in downstream processes. SMPs are a diverse group of organic compounds produced by microorganisms during the fermentation process, including enzymes, EPS, and humic-like substances [116]. SMPs can serve as substrates for microbial growth, but they can also contribute to the formation of recalcitrant organic matter and cause fouling in downstream processes. The concentration and composition of SMPs in fermentation liquid depend on the microbial community structure and the fermentation conditions [117]. In addition to the abovementioned components, WAS fermentation liquid may also contain trace amounts of heavy metals, antibiotics, and other micropollutants, depending on the source of the sludge and the wastewater treatment processes [118]. These contaminants can have inhibitory effects on microbial activity and may require additional treatment steps before the fermentation liquid can be used for downstream applications.
4. Combination of Partial Nitrification and Sludge Fermentation
4.1. Contribution of Sludge Fermentation to Nitrogen Removal
Wasted sludge compositions are extremely complex, with dual attributes of “resource” and “pollution” [119]. Resources are rich in organic matter (such as carbohydrates, proteins, and nutrients (nitrogen, phosphorus, potassium, etc.)), and belong to the category of high-yield reusable resources. Pollution is caused by the presence of many toxic and harmful substances such as heavy metals, polycyclic aromatic hydrocarbons, pathogenic microorganisms, antibiotics, and parasite eggs, which cause environmental pollution and disrupt ecological balance [120]. Fermentation of sludge can degrade the non-biodegradable organic matter in sludge to produce available organic matter. At the same time, fermentation may kill part of pathogenic microorganisms and decrease sludge mass, reducing the cost of WAS disposition and risk. Hence, it has become a development trend for fulfilling the strategic goals of carbon peak and carbon neutrality worldwide [121].
The recycled matter during sludge fermentation is normally recognized as VFAs, which can be applied for bioenergy and biomatter synthesis [122], but the recovery of VFAs like resources in fermentation liquid can be tough and has a high cost. To reuse the VFAs in fermentation liquid feasibly and efficiently, fermentation liquids can be added into activated sludge as a denitrification carbon source by replacing general external carbon sources like acetic acid and propionic acid [123]. For example, Tong et al. found that, under the condition of pH 10, excess sludge achieved great hydrolysis and fermentation performance, with a significant increase in VFA production [111]. The carbon source in this fermentation liquid was retained and added to the biological denitrification and phosphorus removal reactor. Then, denitrification efficiency was enhanced, and the total nitrogen removal efficiency increased from 44.0% to 92.9%. Ji et al. also stated that the denitrification performance with fermentation liquid as the sole external carbon exceeded the performance with acetic acid as the sole external carbon [124]. Sun et al. fermented sludge pretreated by combining CaO2 with a low temperature, and used the produced VFAs for nitrogen removal in wastewater [125]. When 0.2 g/g of CaO2-pretreated sludge acid was added as an external denitrification carbon source at 70 °C, the total nitrogen removal rate of wastewater increased by 57.3%. Liu et al. established a full-scale process for anaerobic alkaline fermentation of sewage sludge to produce VFAs [126], and they found that the fermentation broth with VFAs showed similar effects to acetic acid in phosphorus and nitrogen removal, with nitrogen and phosphorus removal rates reaching 72.39% and 89.65%, respectively.
4.2. Coupling of Sludge Fermentation with Partial Nitrification
The above section indicates that fermentation liquid addition into a biological nitrogen removal system can promote nitrogen removal by providing an external carbon substrate for denitrification. At the same time, fermentation can also improve the process of biological nitrogen removal by transforming nitrification into nitritation, i.e., the PN trait. The strategies for obtaining the fermentation-triggered PN trait include main-stream and side-stream pathways, as recapped in Figure 5. The main-stream fermentation strategy was conducted in a reactor where activated sludge removed nitrogen pollutants via two-step nitrification [27]. After this, activated sludge underwent starvation without feeding, i.e., in-site fermentation, for long or short terms, and activities of both AOB and NOB could be depressed [127]. Subsequently, through the reactivation period, inactivated AOB could be revivified to the same AOB activity degree as before in-site fermentation. However, NOB activity still remained undetectable, leading to the PN trait. Josep et al. conducted in-site fermentation separately in aerobic and anoxic conditions and found that the decay rate of AOB after aerobic fermentation exceeded the decay after anoxic fermentation [128]. After the recovery of the wastewater system, the NAR reached 98%. Liu et al. also established the PN trait with an NAR of 95% through 14 days of aerobic starvation [129]. The genetic abundance of AOB and NOB cells remained stable during in-site fermentation, but the AOB population gradually outnumbered the NOB population in the reactivation period. In fact, the duration of in-site fermentation required to achieve PN could be short, one or two days [130]. PN coupling with in-site fermentation also facilitated the reduction in sludge production markedly, which has been proven in laboratory and pilot-scale studies [128,131]. In sum, although in-site fermentation can depress both AOB and NOB activities in sludge microbiota, the recovery of AOB activity in the reactivation period exceeds the recovery of NOB activity, leading to nitrite accumulation. Comparing microbiota compositions before in-site fermentation and after reactivation, the abundance of dominant NOB genera, Nitrospira, decreased by 99.72%, while that of dominant AOB genera, Nitrosomonas, increased by 1.91 times [27]. However, the higher tolerance of AOB to in-site fermentation than that of NOB has not been properly elucidated yet.
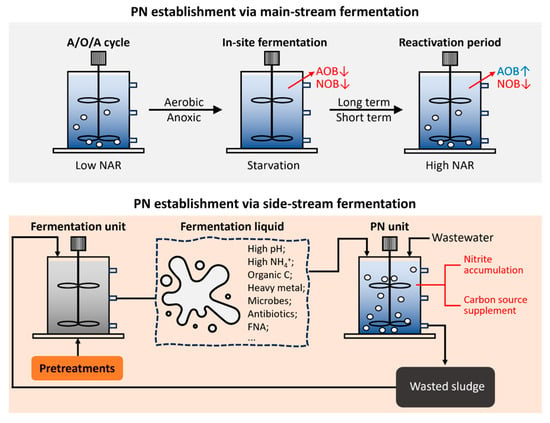
Figure 5.
Two strategies of PN establishment via fermentation process.
Side-stream strategy refers to PN induction by adding fermentation liquid into the reactor for biological nitrogen removal via activated sludge. Theoretically, the addition of fermentation liquid could increase the ammonium load in the main stream and remove the extra ammonium from fermentation at the same time. Liu et al. added exogenous fermentation liquid into activated sludge and found that NOB activity was inhibited more severely than AOB activity [132]. After long-term running, the NAR reached above 90%. The addition of sludge fermentation products not only promoted the NAR to 50–90%, but also enriched anammox bacteria abundance [26,133]. The side-stream strategy could reduce excess sludge production by recycling excess sludge discharged from the PN reactor to the fermentation unit. The main mechanisms of PN establishment via the side-stream strategy, i.e., fermentation liquid addition, may correlate with the composition of fermentation products. Alkalinity and NH4+ could be produced during sludge fermentation, thus forming a high concentration of FA in the fermentation liquid. Additionally, heavy metal in sludge could not be efficiently degraded during fermentation. Other research also proved that sludge fermentation could generate FNA, which was applied to induce PN [134]. FA, heavy metal, and FNA can all facilitate PN establishment, as previously reported [67,71]. Hence, the performance of PN via the side-stream fermentation strategy may be improved by increasing the concentrations of the PN inducer in fermentation liquid. In fact, FA concentration could be elevated by increasing ammonium concentration or the alkalinity in fermentation liquid after the accompanying sludge pretreatments. For instance, ultrasound pretreatment may increase FA concentration by releasing more ammonium, and alkaline chemical pretreatment can obtain high FA by strengthening alkalinity [135]. However, excessive high FA may depress the activities of fermentation microorganisms and undermine fermentation performance [135]. Hence, the modification of fermentation products by combining sludge pretreatments should be carefully considered for balancing the efficiencies of fermentation and PN induction. Additionally, the side-stream strategy of adding fermentation liquid into activated sludge may also enrich anammox bacteria and facilitate the system of PN–anammox [127].
5. Implications
PN-based nitrogen removal processes could save energy and substrate consumptions, promote nitrogen removal performance, and reduce wasted sludge production. PN establishment methods, such as low DO, high temperature, FA or FNA, and inhibitors, normally take a long time, beyond 30 days, to convert two-step nitrification into stable and efficient PN [50,79]. However, main- or side-stream fermentation strategies could rapidly establish the PN trait within half a month or even a few days [130]. Thus, fermentation methods outcompeted other PN methods in terms of establishment efficiency. The side-stream strategy could degrade inorganic and organic carbon, ammonium, phosphonium, and other pollutants in fermentation liquid, which was a new pathway for treating and recycling the non-resource by-products of sludge fermentation. Additionally, the addition of exogenous fermentation liquid into activated sludge can contribute to PN transition and supplement the external carbon source for denitrification at the same time. Unexpectedly, supplementary fermentation liquid could also enrich anammox bacteria in anaerobic tanks. Therefore, sludge fermentation can promote nitrogen removal from multiple aspects.
The combination of sludge fermentation with the PN-based nitrogen removal process provided an innovative strategy to reduce wasted sludge production and synchronously establish short-cut nitrogen removal within WWTPs (Figure 6). Conventionally, A2O tanks removing most carbon and nitrogen pollutants discharge excessive wasted sludge which is fermented subsequently for producing biogas, including H2 and CH4 [122]. Although part of the resources in wasted sludge was recycled and stored in biogas, the recycling efficiency was low, and the majority of resources and toxic matter remained in fermentation liquid. By reusing the products in the fermentation liquid to initiate the PN-based nitrogen removal process, the remaining resources can be further recycled, and the contaminant degree of the fermentation liquid will be attenuated. The proper combination of the fermentation and PN processes may realize zero pollutant emissions within WWTPs and generate economic products at the same time.
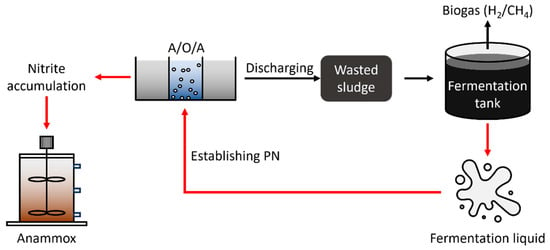
Figure 6.
An innovative strategy for reducing wasted sludge and synchronously establishing short-cut nitrogen removal within WWTPs.
Author Contributions
Conceptualization, X.W. and C.J.; resources, D.W., Y.Y., L.F., J.P., X.Z. (Xinyuan Zhang) and T.Y.; writing—original draft preparation, X.W.; writing—review and editing, C.J. and X.Z. (Xuliang Zhuang); supervision, X.Z. (Xuliang Zhuang); funding acquisition, X.Z. (Xuliang Zhuang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (nos. 42177099 and 42230411), the Provincial Science and Technology Innovative Program for Carbon Peak and Carbon Neutrality of Jiangsu of China, the “Leading Goose” R&D Program of Zhejiang (no. 2023C03131 and no. 2023C03132), and the Key Technology and Equipment System for Intelligent Control of the Water Supply Network (no. 2022YFC3203800).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Gu, B. Urban rivers as hotspots of regional nitrogen pollution. Environ. Pollut. 2015, 205, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.D.; Bishop, C.A.; Struger, J. Nitrogen pollution: An assessment of its threat to amphibian survival. Environ. Health Perspect. 1999, 107, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Currell, M.J.; Cao, G. Deep challenges for China’s war on water pollution. Environ. Pollut. 2016, 218, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.; Ramakrishnan, V. Nitrogen sources and cycling in the ecosystem and its role in air, water and soil pollution: A critical review. J. Pollut. Eff. Control 2015, 3, 1–26. [Google Scholar]
- Liu, Y.; Tay, J.-H. State of the art of biogranulation technology for wastewater treatment. Biotechnol. Adv. 2004, 22, 533–563. [Google Scholar] [CrossRef] [PubMed]
- Gernaey, K.V.; van Loosdrecht, M.C.; Henze, M.; Lind, M.; Jørgensen, S.B. Activated sludge wastewater treatment plant modelling and simulation: State of the art. Environ. Model. Softw. 2004, 19, 763–783. [Google Scholar] [CrossRef]
- Dold, P.; Ekama, G. A general model for the activated sludge process. In Water Pollution Research and Development; Elsevier: Amsterdam, The Netherlands, 1981; pp. 47–77. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Lehne, G.; Müller, A.; Schwedes, J. Mechanical disintegration of sewage sludge. Water Sci. Technol. 2001, 43, 19–26. [Google Scholar] [CrossRef]
- Ramirez, I.; Volcke, E.I.; Rajinikanth, R.; Steyer, J.-P. Modeling microbial diversity in anaerobic digestion through an extended ADM1 model. Water Res. 2009, 43, 2787–2800. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Zhou, Q.; Gu, G. Biological short-chain fatty acids (SCFAs) production from waste-activated sludge affected by surfactant. Water Res. 2007, 41, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, H.W.; Chin, C.T.-L.; Hong, E.; Philip, L.; Suraishkumar, G.; Sen, T.K.; Khiadani, M. Effect of hybrid (microwave-H2O2) feed sludge pretreatment on single and two-stage anaerobic digestion efficiency of real mixed sewage sludge. Process Saf. Environ. Prot. 2020, 136, 194–202. [Google Scholar] [CrossRef]
- Biswal, B.K.; Huang, H.; Dai, J.; Chen, G.-H.; Wu, D. Impact of low-thermal pretreatment on physicochemical properties of saline waste activated sludge, hydrolysis of organics and methane yield in anaerobic digestion. Bioresour. Technol. 2020, 297, 122423. [Google Scholar]
- Choi, J.-M.; Han, S.-K.; Lee, C.-Y. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Hendriks, A.; van Lier, J.; de Kreuk, M. Pre-treatments to enhance the biodegradability of waste activated sludge: Elucidating the rate limiting step. Biotechnol. Adv. 2018, 36, 1434–1469. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Liu, J.; Zhang, L.; Zhang, Q.; Peng, Y. Sludge fermentation liquid addition attained advanced nitrogen removal in low C/N ratio municipal wastewater through short-cut nitrification-denitrification and partial anammox. Front. Environ. Sci. Eng. 2020, 15, 26. [Google Scholar] [CrossRef]
- Gao, D.; Peng, Y.; Li, B.; Liang, H. Shortcut nitrification–denitrification by real-time control strategies. Bioresour. Technol. 2009, 100, 2298–2300. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xia, L.; Ma, T.; Zhang, Y.; Zhou, Y.; He, X. Achieving short-cut nitrification and denitrification in modified intermittently aerated constructed wetland. Bioresour. Technol. 2017, 232, 10–17. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, T. A potential new process for improving nitrogen removal in constructed wetlands—Promoting coexistence of partial-nitrification and ANAMMOX. Ecol. Eng. 2007, 31, 69–78. [Google Scholar] [CrossRef]
- Peng, Y.Z.; Zhu, G.B. Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl. Microbiol. Biotechnol. 2006, 73, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Su, S.-H.; Huang, Y.-T.; Chen, S.-S.; Sung, S.; Lin, J.-G. Partial nitrification and anammox process: A method for high strength optoelectronic industrial wastewater treatment. Water Res. 2013, 47, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Annachhatre, A.P. Annachhatre, Partial nitrification—Operational parameters and microorganisms involved. Rev. Environ. Sci. Bio/Technol. 2007, 6, 285–313. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Peng, Y.; Li, X.; Zhang, Q. Realization of partial nitrification and in-situ anammox in continuous-flow anaerobic/aerobic/anoxic process with side-stream sludge fermentation for real sewage. Bioresour. Technol. 2022, 346, 126520. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Zeng, W.; Peng, X.; Ma, C.; Peng, Y. Rapid achievement of partial nitrification in domestic sewage treatment by in-site fermentation coupled with sludge discharge: Performance and mechanisms. J. Water Process. Eng. 2023, 55, 104192. [Google Scholar] [CrossRef]
- Alexander, M. Nitrification. Soil Nitrogen 1965, 10, 307–343. [Google Scholar]
- Gerardi, M.H. Nitrification and Denitrification in the Activated Sludge Process; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Prosser, J.I. The ecology of nitrifying bacteria. In Biology of the Nitrogen Cycle; Elsevier: Amsterdam, The Netherlands, 2007; pp. 223–243. [Google Scholar]
- Dueholm MK, D.; Nierychlo, M.; Andersen, K.S.; Rudkjøbing, V.; Knutsson, S.; Albertsen, M.; Nielsen, P.H. MiDAS 4: A global catalogue of full-length 16S rRNA gene sequences and taxonomy for studies of bacterial communities in wastewater treatment plants. Nat. Commun. 2022, 13, 1908. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xu, S.; Wang, R.; Zhou, S.; Wu, S.; Zeng, X.; Bai, Z.; Zhuang, G.; Zhuang, X. Comprehensive assessment of free nitrous acid-based technology to establish partial nitrification. Environ. Sci. Water Res. Technol. 2018, 4, 2113–2124. [Google Scholar] [CrossRef]
- Jiang, C.; Xu, S.; Wang, R.; Feng, S.; Zhou, S.; Wu, S.; Zeng, X.; Wu, S.; Bai, Z.; Zhuang, G.; et al. Achieving efficient nitrogen removal from real sewage via nitrite pathway in a continuous nitrogen removal process by combining free nitrous acid sludge treatment and DO control. Water Res. 2019, 161, 590–600. [Google Scholar] [CrossRef]
- Wang, Q.L.; Ye, L.; Jiang, G.; Hu, S.; Yuan, Z. Side-stream sludge treatment using free nitrous acid selectively eliminates nitrite oxidizing bacteria and achieves the nitrite pathway. Water Res. 2014, 55, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kirim, G.; McCullough, K.; Bressani-Ribeiro, T.; Domingo-Félez, C.; Duan, H.; Al-Omari, A.; De Clippeleir, H.; Jimenez, J.; Klaus, S.; Ladipo-Obasa, M.; et al. Mainstream short-cut N removal modelling: Current status and perspectives. Water Sci. Technol. 2022, 85, 2539–2564. [Google Scholar] [CrossRef] [PubMed]
- Lücker, S.; Wagner, M.; Maixner, F.; Pelletier, E.; Koch, H.; Vacherie, B.; Rattei, T.; Damsté, J.S.S.; Spieck, E.; Le Paslier, D.; et al. A metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 13479–13484. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Annavajhala, M.K.; Kapoor, V.; Santo-Domingo, J.; Chandran, K. Comammox functionality identified in diverse engineered biological wastewater treatment systems. Environ. Sci. Technol. Lett. 2018, 5, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Grunditz, C.; Dalhammar, G. Development of nitrification inhibition assays using pure cultures of nitrosomonas and nitrobacter. Water Res. 2001, 35, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Hellinga, C.; Schellen, A.A.J.C.; Mulder, J.W.; van Loosdrecht, M.C.M.; Heijnen, J.J. The SHARON process: An innovative method for nitrogen removal from ammonium-rich waste water. Water Sci. Technol. 1998, 37, 135–142. [Google Scholar] [CrossRef]
- Yoo, H.; Ahn, K.H.; Lee, H.J.; Lee, K.H.; Kwak, Y.J.; Song, K.G. Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res. 1999, 33, 145–154. [Google Scholar] [CrossRef]
- Balmelle, B.; Nguyen, K.M.; Capdeville, B.; Cornier, J.C.; Deguin, A. Study of Factors Controlling Nitrite Buildup in Biological Processes for Water Nitrification. Water Sci. Technol. 1992, 26, 1017–1025. [Google Scholar] [CrossRef]
- Mulder, J.W.; van Loosdrecht, M.C.M.; Hellinga, C.; van Kempen, R. Full-scale application of the SHARON process for treatment of rejection water of digested sludge dewatering. Water Sci. Technol. 2001, 43, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Tian, M.; Wang, R.R.; Liu, F. Application of pH, DO and OUR control for short-cut nitrification. Renew. Sustain. Energy 2012, 347–353, 2112–2116. [Google Scholar] [CrossRef]
- Sui, Q.W.; Liu, C.; Zhang, J.; Dong, H.; Zhu, Z.; Wang, Y. Response of nitrite accumulation and microbial community to free ammonia and dissolved oxygen treatment of high ammonium wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 4177–4187. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, R.; Malinska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Gong, B.; Zhou, J.; He, Q.; Qing, X. Efficient simultaneous partial nitrification, anammox and denitrification (SNAD) system equipped with a real-time dissolved oxygen (DO) intelligent control system and microbial community shifts of different substrate concentrations. Water Res. 2017, 119, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Blackburne, R.; Yuan, Z.G.; Keller, J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 2008, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, G.; Werner, A.; Bornhardt, C.; Muñoz, C.; Antileo, C. Differential kinetics of ammonia- and nitrite-oxidizing bacteria: A simple kinetic study based on oxygen affinity and proton release during nitrification. Process. Biochem. 2006, 41, 1764–1772. [Google Scholar] [CrossRef]
- Guisasola, A.; Jubany, I.; Baeza, J.A.; Carrera, J.; Lafuente, J. Respirometric estimation of the oxygen affinity constants for biological ammonium and nitrite oxidation. J. Chem. Technol. Biotechnol. 2005, 80, 388–396. [Google Scholar] [CrossRef]
- Laanbroek, H.J.; Bodelier, P.L.E.; Gerards, S. Oxygen-Consumption Kinetics of Nitrosomonas-Europaea and Nitrobacter-Hamburgensis Grown in Mixed Continuous Cultures at Different Oxygen Concentrations. Arch. Microbiol. 1994, 161, 156–162. [Google Scholar] [CrossRef]
- Laanbroek, H.J.; Gerards, S. Competition for Limiting Amounts of Oxygen between Nitrosomonas-Europaea and Nitrobacter-Winogradskyi Grown in Mixed Continuous Cultures. Arch. Microbiol. 1993, 159, 453–459. [Google Scholar] [CrossRef]
- Tokutomi, T. Operation of a nitrite-type airlift reactor at low DO concentration. Water Sci. Technol. 2004, 49, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Beman, J.M.; Shih, J.L.; Popp, B.N. Nitrite oxidation in the upper water column and oxygen minimum zone of the eastern tropical North Pacific Ocean. ISME J. 2013, 7, 2192–2205. [Google Scholar] [CrossRef] [PubMed]
- Manser, R.; Gujer, W.; Siegrist, H. Consequences of mass transfer effects on the kinetics of nitrifiers. Water Res. 2005, 39, 4633–4642. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Miller, M.W.; Holgate, B.; Bunce, R.; Park, H.; Chandran, K.; Wett, B.; Murthy, S.; Bott, C.B. Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Res. 2014, 57, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, K.; Wantawin, C.; Ohgaki, S. Nitrification at Low-Levels of Dissolved-Oxygen with and without Organic Loading in a Suspended-Growth Reactor. Water Res. 1990, 24, 297–302. [Google Scholar] [CrossRef]
- Guo, X.; Kim, J.H.; Behera, S.K.; Park, H.S. Influence of dissolved oxygen concentration and aeration time on nitrite accumulation in partial nitrification process. Int. J. Environ. Sci. Technol. 2008, 5, 527–534. [Google Scholar] [CrossRef]
- Ruiz, G.; Jeison, D.; Chamy, R. Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration. Water Res. 2003, 37, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Lackner, G.; Peters, E.E.; Helfrich, E.J.N.; Piel, J. Insights into the lifestyle of uncultured bacterial natural product factories associated with marine sponges. Proc. Natl. Acad. Sci. USA 2017, 114, E347–E356. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bae, W.; Chung, J.; Baek, S.-C. Empirical model of the pH dependence of the maximum specific nitrification rate. Process. Biochem. 2007, 42, 1671–1676. [Google Scholar] [CrossRef]
- Lu, X.Y.; Duan, H.; Oehmen, A.; Carvalho, G.; Yuan, Z.; Ye, L. Achieving combined biological short-cut nitrogen and phosphorus removal in a one sludge system with side-stream sludge treatment. Water Res. 2021, 203, 117563. [Google Scholar] [CrossRef]
- Ganigué, R.; López, H.; Balaguer, M.D.; Colprim, J. Partial ammonium oxidation to nitrite of high ammonium content urban land fill leachates. Water Res. 2007, 41, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.; Baek, S.; Chung, J.; Lee, Y. Optimal operational factors for nitrite accumulation in batch reactors. Biodegradation 2001, 12, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Shim, H.; Park, S.-J.; Kim, S.-J.; Bae, W. Optimization of free ammonia concentration for nitrite accumulation in shortcut biological nitrogen removal process. Bioprocess Biosyst. Eng. 2006, 28, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Duan, H.; Wei, W.; Ni, B.-J.; Laloo, A.; Yuan, Z. Achieving stable mainstream nitrogen removal via the nitrite pathway by sludge treatment using free ammonia. Environ. Sci. Technol. 2017, 51, 9800–9807. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Alleman, J.E. Investigation of Batchwise Nitrite Buildup by an Enriched Nitrification Culture. Water Sci. Technol. 1992, 26, 997–1005. [Google Scholar] [CrossRef]
- Kayhanian, M. Ammonia inhibition in high-solids biogasification: An overview and practical solutions. Environ. Technol. 1999, 20, 355–365. [Google Scholar] [CrossRef]
- Duan, H.; Gao, S.; Li, X.; Ab Hamid, N.H.; Jiang, G.; Zheng, M.; Bai, X.; Bond, P.L.; Lu, X.; Chislett, M.M.; et al. Improving wastewater management using free nitrous acid (FNA). Water Res. 2020, 171, 115382. [Google Scholar] [CrossRef] [PubMed]
- Pedrouso, A.; del Río, Á.V.; Morales, N.; Vázquez-Padín, J.R.; Campos, J.L.; Méndez, R.; Mosquera-Corral, A. Nitrite oxidizing bacteria suppression based on in-situ free nitrous acid production at mainstream conditions. Sep. Purif. Technol. 2017, 186, 55–62. [Google Scholar] [CrossRef]
- Nan, X.; Ma, B.; Qian, W.; Zhu, H.; Li, X.; Zhang, Q.; Peng, Y. Achieving nitritation by treating sludge with free nitrous acid: The effect of starvation. Bioresour. Technol. 2019, 271, 159–165. [Google Scholar] [CrossRef]
- Zhou, Y.; Oehmen, A.; Lim, M.; Vadivelu, V.; Ng, W.J. The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res. 2011, 45, 4672–4682. [Google Scholar] [CrossRef]
- Chislett, M.; Yu, Z.; Donose, B.C.; Guo, J.; Yuan, Z. Understanding the Effect of Free Nitrous Acid on Biofilms. Environ. Sci. Technol. 2022, 56, 11625–11634. [Google Scholar] [CrossRef] [PubMed]
- Laloo, A.E.E.; Wei, J.; Wang, D.; Narayanasamy, S.; VanWonterghem, I.; Waite, D.; Steen, J.; Kaysen, A.; Heintz-Buschart, A.; Wang, Q.; et al. Mechanisms of Persistence of the Ammonia-Oxidizing Bacteria Nitrosomonas to the Biocide Free Nitrous Acid. Environ. Sci. Technol. 2018, 52, 5386–5397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Gunsalus, R.P. The nrfA and nirB nitrite reductase operons in Escherichia coli are expressed differently in response to nitrate than to nitrite. J. Bacteriol. 2000, 182, 5813–5822. [Google Scholar] [CrossRef] [PubMed]
- Cantera, J.J.L.; Stein, L.Y. Molecular diversity of nitrite reductase genes (nirK) in nitrifying bacteria. Environ. Microbiol. 2007, 9, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Erguder, T.H.; Boon, N.; Vlaeminck, S.E.; Verstraete, W. Partial Nitrification Achieved by Pulse Sulfide Doses in a Sequential Batch Reactor. Environ. Sci. Technol. 2008, 42, 8715–8720. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.I.B.; Thalasso, F.; López, F.d.M.C.; Texier, A. Inhibitory effect of sulfide on the nitrifying respiratory process. J. Chem. Technol. Biotechnol. 2013, 88, 1344–1349. [Google Scholar] [CrossRef]
- Vela, J.D.; Dick, G.J.; Love, N.G. Sulfide inhibition of nitrite oxidation in activated sludge depends on microbial community composition. Water Res. 2018, 138, 241–249. [Google Scholar] [CrossRef]
- Li, X.; Kapoor, V.; Impelliteri, C.; Chandran, K.; Domingo, J.W.S. Measuring nitrification inhibition by metals in wastewater treatment systems: Current state of science and fundamental research needs. Crit. Rev. Environ. Sci. Technol. 2016, 46, 249–289. [Google Scholar] [CrossRef]
- Randall, C.W.; Buth, D. Nitrite Buildup in Activated-Sludge Resulting from Combined Temperature and Toxicity Effects. J. Water Pollut. Control. Fed. 1984, 56, 1045–1049. [Google Scholar]
- Cui, Y.; Gao, J.; Zhang, D.; Zhao, Y.; Wang, Y. Rapid start-up of partial nitrification process using benzethonium chloride—A novel nitrite oxidation inhibitor. Bioresour. Technol. 2020, 315, 123860. [Google Scholar] [CrossRef]
- Wu, Z.J.; Gao, J.; Cui, Y.; Wang, Z.; Zhao, Y.; Zhang, H.; Guo, Y.; Li, Z. Feeding low-level benzethonium chloride can promote the start-up, fast recovery and long-term stable maintenance of partial nitrification for low-ammonium wastewater. Bioresour. Technol. 2022, 353, 127152. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Zheng, X. Enhancement of Waste Activated Sludge Protein Conversion and Volatile Fatty Acids Accumulation during Waste Activated Sludge Anaerobic Fermentation by Carbohydrate Substrate Addition: The Effect of pH. Environ. Sci. Technol. 2009, 43, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.D.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.-H.; Pandey, A. Dark fermentation: Production and utilization of volatile fatty acid from different wastes—A review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef] [PubMed]
- She, Y.C.; Hong, J.; Zhang, Q.; Chen, B.-Y.; Wei, W.; Xin, X. Revealing microbial mechanism associated with volatile fatty acids production in anaerobic acidogenesis of waste activated sludge enhanced by freezing/thawing pretreatment. Bioresour. Technol. 2020, 302, 122869. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Yang, H.; Zhang, H.; Liu, Y.; Su, H.; Shen, M. Improving methane productivity of waste activated sludge by ultrasound and alkali pretreatment in microbial electrolysis cell and anaerobic digestion coupled system. Environ. Res. 2020, 180, 108863. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, J.; Wang, M.; Xin, X.; Xu, J.; Zhang, J. Efficient Volatile Fatty Acids Production from Waste Activated Sludge after Ferrate Pretreatment with Alkaline Environment and the Responding Microbial Community Shift. ACS Sustain. Chem. Eng. 2018, 6, 16819–16827. [Google Scholar] [CrossRef]
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced biomethanation of kitchen waste by different pre-treatments. Bioresour. Technol. 2011, 102, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Brune, D.E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Horan, N.J.; Anaman, K. Optimisation of C:N Ratio for Co-Digested Processed Industrial Food Waste and Sewage Sludge Using the BMP Test. Int. J. Chem. React. Eng. 2011, 9. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.-P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Gao, Q.; Li, L.; Zhang, Y.; Zhou, H.; Jiang, J.; Wei, L.; Wang, G.; Ding, J.; Zhao, Q. Advanced oxidation processes (AOPs)-based sludge pretreatment techniques for enhanced short-chain fatty acids production: A critical review. Chem. Eng. J. 2024, 489, 151496. [Google Scholar] [CrossRef]
- He, D.; Xiao, J.; Wang, D.; Liu, X.; Fu, Q.; Li, Y.; Du, M.; Yang, Q.; Liu, Y.; Wang, Q.; et al. Digestion liquid based alkaline pretreatment of waste activated sludge promotes methane production from anaerobic digestion. Water Res. 2021, 199, 117198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, D.; Zhang, C.; Ping, Q.; Wang, L.; Li, Y. Comparison of different sewage sludge pretreatment technologies for improving sludge solubilization and anaerobic digestion efficiency: A comprehensive review. Sci. Total Environ. 2024, 921, 171175. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Li, Z.; Wang, D.; Ohlsen, T.; Dong, H. Performance of thermal pretreatment and mesophilic fermentation system on pathogen inactivation and biogas production of faecal sludge: Initial laboratory results. Biosyst. Eng. 2016, 151, 171–177. [Google Scholar] [CrossRef]
- Alqaralleh, R.M.; Kennedy, K.; Delatolla, R. Microwave vs. alkaline-microwave pretreatment for enhancing Thickened Waste Activated Sludge and fat, oil, and grease solubilization, degradation and biogas production. J. Environ. Manag. 2019, 233, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Doğan, I.; Sanin, F.D. Alkaline solubilization and microwave irradiation as a combined sludge disintegration and minimization method. Water Res. 2009, 43, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Mehrdadi, N.; Kootenaei, F.G. An investigation on effect of ultrasound waves on sludge treatment. Energy Procedia 2018, 153, 325–329. [Google Scholar] [CrossRef]
- Alagöz, B.A.; Yenigün, O.; Erdinçler, A. Ultrasound assisted biogas production from co-digestion of wastewater sludges and agricultural wastes: Comparison with microwave pre-treatment. Ultrason. Sonochemistry 2018, 40, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, E.B.; Aksoy, A.; Sanin, F.D. Maximizing the energy potential of urban sludge treatment: An experimental study and a scenario-based energy analysis focusing on anaerobic digestion with ultrasound pretreatment and sludge combustion. Energy 2021, 221, 119876. [Google Scholar] [CrossRef]
- Han, Y.; Zhuo, Y.; Peng, D.; Yao, Q.; Li, H.; Qu, Q. Influence of thermal hydrolysis pretreatment on organic transformation characteristics of high solid anaerobic digestion. Bioresour. Technol. 2017, 244 Pt 1, 836–843. [Google Scholar] [CrossRef]
- Zou, X.; Yang, R.; Zhou, X.; Cao, G.; Zhu, R.; Ouyang, F. Effects of mixed alkali-thermal pretreatment on anaerobic digestion performance of waste activated sludge. J. Clean. Prod. 2020, 259, 120940. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, H.; Ye, L.; Liu, L.; Batstone, D.J.; Yuan, Z. Increasing capacity of an anaerobic sludge digester through FNA pre-treatment of thickened waste activated sludge. Water Res. 2019, 149, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Pirlou, M.; Ebrahimi-Nik, M.; Khojastehpour, M.; Ebrahimi, S.H. Mesophilic co-digestion of municipal solid waste and sewage sludge: Effect of mixing ratio, total solids, and alkaline pretreatment. Int. Biodeterior. Biodegrad. 2017, 125, 97–104. [Google Scholar] [CrossRef]
- Xu, X.-J.; Wang, W.-Q.; Chen, C.; Xie, P.; Liu, W.-Z.; Zhou, X.; Wang, X.-T.; Yuan, Y.; Wang, A.-J.; Lee, D.-J.; et al. Bioelectrochemical system for the enhancement of methane production by anaerobic digestion of alkaline pretreated sludge. Bioresour. Technol. 2020, 304, 123000. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; More, T.; Yan, S.; Tyagi, R.; Surampalli, R. Fenton pre-treatment of secondary sludge to enhance anaerobic digestion: Energy balance and greenhouse gas emissions. Chem. Eng. J. 2016, 283, 285–292. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, J.; Wen, K.; Liu, Z.; Wang, G.; Liu, W.; Wang, A.; Yue, X. What could the entire cornstover contribute to the enhancement of waste activated sludge acidification? Performance assessment and microbial community analysis. Biotechnol. Biofuels 2016, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Chen, Y.G. Recovery of nitrogen and phosphorus from alkaline fermentation liquid of waste activated sludge and application of the fermentation liquid to promote biological municipal wastewater treatment. Water Res. 2009, 43, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Wang, Y.; Lian, Y.; Wang, Q.; Yang, Q.; Wang, D.; Xie, G.-J.; Zeng, G.; Sun, Y.; et al. Clarifying the Role of Free Ammonia in the Production of Short-Chain Fatty Acids from Waste Activated Sludge Anaerobic Fermentation. ACS Sustain. Chem. Eng. 2018, 6, 14104–14113. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Q.; Wang, D.; Tang, L.; Xia, J.; Wang, Q.; Zeng, G.; Yang, Q.; Li, X. Free ammonia-based sludge treatment reduces sludge production in the wastewater treatment process. Chemosphere 2018, 205, 484–492. [Google Scholar] [CrossRef]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Low pH anaerobic digestion of waste activated sludge for enhanced phosphorous release. Water Res. 2015, 81, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.-J.; Rittmann, B.E.; Yu, H.-Q. Soluble microbial products and their implications in mixed culture biotechnology. Trends Biotechnol. 2011, 29, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Wei, W.; Gong, Y.; Yu, Q.; Li, Q.; Sun, J.; Yuan, Z. Technologies for reducing sludge production in wastewater treatment plants: State of the art. Sci. Total Environ. 2017, 587, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Chipasa, K.B. Accumulation and fate of selected heavy metals in a biological wastewater treatment system. Waste Manag. 2003, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, S.; Dennehy, C.; Lawlor, P.; Hu, Z.; Wu, G.; Zhan, X.; Gardiner, G. Inactivation of pathogens in anaerobic digestion systems for converting biowastes to bioenergy: A review. Renew. Sustain. Energy Rev. 2020, 120, 109654. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Zheng, L.; Gong, H.; Dai, L.; Dai, X. An overview of removing heavy metals from sewage sludge: Achievements and perspectives. Environ. Pollut. 2020, 266, 115375. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, M.; Li, J.; Yao, Y.; Tang, J.; Niu, Q. The dosage-effect of biochar on anaerobic digestion under the suppression of oily sludge: Performance variation, microbial community succession and potential detoxification mechanisms. J. Hazard. Mater. 2022, 421, 126819. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.H.; Duan, N.; Dong, B.; Dai, L. High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: Stability and performance. Waste Manag. 2013, 33, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Z.; Peng, Y.; Liu, Z.; Liu, Y.; Zhao, L. Development of a novel partial nitrification, fermentation-based double denitrification bioprocess (PN-F-Double/DN) to simultaneous treatment of mature landfill leachate and waste activated sludge. Water Res. 2021, 203, 117540. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Chen, Y.G. Using Sludge Fermentation Liquid To Improve Wastewater Short-Cut Nitrification-Denitrification and Denitrifying Phosphorus Removal via Nitrite. Environ. Sci. Technol. 2010, 44, 8957–8963. [Google Scholar] [CrossRef]
- Sun, J.; Song, J.; Fang, W.; Cao, H. Enhanced nitrogen removal upon the addition of volatile fatty acids from activated sludge by combining calcium peroxide and low-thermal pretreatments. J. Environ. Sci. 2021, 108, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hao, X.; Jiang, T.; Li, X.; Yang, J.; Wang, B. Feasibility of in-situ sludge fermentation coupled with partial denitrification: Key roles of initial organic matters and alkaline pH. Bioresour. Technol. 2024, 401, 130730. [Google Scholar] [CrossRef]
- Torà, J.A.; Lafuente, J.; Baeza, J.A.; Carrera, J. Long-term starvation and subsequent reactivation of a high-rate partial nitrification activated sludge pilot plant. Bioresour. Technol. 2011, 102, 9870–9875. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Q.; Ma, B.; Li, J.; Ma, L.; Wang, S.; Peng, Y. Rapid achievement of nitritation using aerobic starvation. Environ. Sci. Technol. 2017, 51, 4001–4008. [Google Scholar] [CrossRef]
- Ye, L.H.; Li, D.; Zhang, J.; Zeng, H. Start-up and performance of partial nitritation process using short-term starvation. Bioresour. Technol. 2019, 276, 190–198. [Google Scholar] [CrossRef]
- Wang, B.; Qiao, X.; Hou, F.; Liu, T.; Pang, H.; Guo, Y.; Guo, J.; Peng, Y. Pilot-scale demonstration of a novel process integrating Partial Nitritation with simultaneous Anammox, Denitrification and Sludge Fermentation (PN plus ADSF) for nitrogen removal and sludge reduction. Sci. Total Environ. 2022, 815, 152835. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, L.; Qiu, S.; He, Q.; Zhang, Q.; Peng, Y.; Peng, Y. Insight into the mechanism of nitritation establishment through external fermented sludge addition. Bioresour. Technol. 2021, 341, 125763. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, Q.; Wang, S.; Li, X.; Wang, R.; Peng, Y. Superior nitrogen removal and efficient sludge reduction via partial nitrification-anammox driven by addition of sludge fermentation products for real sewage treatment. Bioresour. Technol. 2023, 372, 128689. [Google Scholar] [CrossRef]
- Law, Y.Y.; Ye, L.; Wang, Q.; Hu, S.; Pijuan, M.; Yuan, Z. Producing free nitrous acid—A green and renewable biocidal agent—From anaerobic digester liquor. Chem. Eng. J. 2015, 259, 62–69. [Google Scholar] [CrossRef]
- Shen, Y.; Qiu, S.; Chen, Z.; Zhang, Y.; Trent, J.; Ge, S. Free ammonia is the primary stress factor rather than total ammonium to Chlorella sorokiniana in simulated sludge fermentation liquor. Chem. Eng. J. 2020, 397, 125490. [Google Scholar] [CrossRef]
- Geng, Y.-K.; Yuan, L.; Liu, T.; Li, Z.-H.; Zheng, X.; Sheng, G.-P. In-situ alkaline pretreatment of waste activated sludge in microbial fuel cell enhanced power production. J. Power Sources 2021, 491, 229616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).