Abstract
2-phenylethanol (2-PE) is a valuable aromatic alcohol with diverse applications in cosmetics, food, beverages, and pharmaceutical industries. Currently, 2-PE is produced either through chemical synthesis or by extraction from plant materials. However, both conventional production methods have their own limitations. Therefore, there is a need for more eco-friendly and cost-effective approaches to produce natural 2-PE. Biotechnological routes, particularly microbial fermentations, hold promise for natural 2-PE production, especially when using low-cost substrates. In this study, 2-PE was produced by de novo synthesis via the shikimate pathway, using the yeast Yarrowia lipolytica in a medium composed of sugar beet molasses (SBM) and yeast extract (YE) as carbon and nitrogen sources, respectively. A genetically engineered strain was generated, in which the SUC2 gene was transformed, expressing the invertase enzyme, enabling Y. lipolytica to efficiently utilize SBM as a cost-effective substrate. A central composite design allowed for the optimization of the concentrations of the carbon and nitrogen sources, resulting in approximately 0.71 g(2-PE)/L(culture medium). The results obtained highlight the potential of utilizing SBM as a low-cost substrate for 2-PE production, advancing biotechnological approaches in fragrance synthesis.
1. Introduction
2-Phenylethanol (2-PE) is an aromatic alcohol, known for its profound rosy scent. Its pleasant odor makes it highly valued in various industrial sectors, including cosmetics, pharmaceuticals, and food and beverages. For instance, it is utilized as an aroma additive in perfumes, shampoos, and soaps [1,2]. Its antibacterial and antifungal properties make it a valuable additive, as it not only imparts an attractive fragrance, but it also protects the products from microbial contamination, thereby preventing their rapid deterioration [3]. 2-PE displays remarkable market value. In 2021, 2-PE’s global annual output exceeded 10,000 tons [4].
Currently, most of the 2-PE is produced via chemical synthesis, with a price of around 5 USD/kg [5]. This synthesis involves three distinct processes: first, a Friedel–Crafts reaction of ethylene oxide with benzene in the presence of aluminum chloride; second, the hydrogenation of styrene oxide with sodium hydroxide and Raney nickel as a catalyst; and third, the oxidation of propylene with 2-phenylethyl hydroperoxide [6]. However, these methods have multiple drawbacks, requiring high temperatures (above 300 °C) and high pressure, using carcinogenic and toxic chemicals such as benzene and styrene oxide, and generating harmful by-products. Consequently, this synthesis is environmentally unfriendly and lowers the quality of the product and its range of applications [7].
On the other hand, 2-PE is present in nature and is traditionally extracted from the essential oils of many flowers, including jasmine, hyacinth, lily, and roses. However, the low concentration of 2-PE in the flowers makes the extraction process expensive and complex, alongside the negative environmental impact due to waste disposal [8]. Furthermore, additional challenges arise regarding the availability of 2-PE in flowers, such as seasonal fluctuations and susceptibility to diseases and pests, thereby further constraining the supply of natural 2-PE [9]. All of the previously mentioned factors contribute to the increased price of natural 2-PE, which typically reaches around 1000 USD/kg and is susceptible to further increase with the rising demand [10].
Since fermentation products are considered natural by the European and American legislation when obtained using natural substrates, biotechnological methods, particularly microbial fermentations, emerge as promising alternatives for the production of natural 2-PE from plants [11]. The production of value-added bio-products using fermentations of either fungi or bacteria has been a trending headline in the biotechnological sector. Particularly, utilizing agro-industrial by-products as substrates in these biotechnological processes has been a focal point of interest [12,13]. The estimated price of 2-PE produced through the biotechnological route is around 220 USD/kg [9]. In fact, 2-PE can be synthesized by several yeast species, including Yarrowia lipolytica, Saccharomyces cerevisiae, Kluyveromyces marxianus, Pichia fermentans, Candida glycerinogenes, and Zygosaccharomyces rouxii [1,14]. Among the various 2-PE producers, Y. lipolytica possesses many advantages making it a promising candidate for industrial applications [15,16]. It has been used to produce different metabolites such as lipids, organic acids, polyols, amino acids, aromatic compounds, and many others [17]. This organism has been granted the Generally Recognized as Safe (GRAS) status by the US Food and Drug Administration (FDA) [18]. Y. lipolytica can consume a wide range of substrates, including glucose, fructose, and glycerol, but not sucrose [19]. One major advantage of this organism is that it is Crabtree-negative, meaning it can achieve high cell density without accumulating ethanol, under aerobic conditions [20].
In yeasts, 2-PE can be synthesized as a result of the amino acid catabolism through two pathways. First, through the bioconversion of L-phenylalanine (L-Phe) via the Ehrlich pathway. The conversion is carried out through a three-step enzymatic reaction, starting with the transamination of L-Phe to phenylpyruvate, which is then decarboxylated to phenylacetaldehyde, and finally reduced to 2-PE by dehydrogenation [21]. Second, 2-PE can be biosynthesized by de novo synthesis starting from monomers (e.g., monosaccharides), via the shikimate pathway. Phosphoenol pyruvate and erythrose-4-phosphate, intermediates of glycolysis and the pentose phosphate pathway, respectively, are converted into 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP). DAHP undergoes several reactions to form chorismate, which then leads to the formation of phenylpyruvate. Phenylpyruvate is subsequently converted into 2-PE [22]. It is important to note the high cost of the precursor L-Phe and its availability are among the limiting factors for large-scale production of 2-PE using the Ehrlich pathway, making its de novo biosynthesis a more economical alternative [23]. Genetic engineering strategies were applied to improve the efficiency of the de novo production of 2-PE by increasing the metabolic fluxes through the shikimate pathway [24]. The ability of Y. lipolytica to produce 2-PE was first reported by Celińska et al. (2013), using a genetically modified strain (NCYC3825) [25]. The authors reported a 2-PE production of 1.98 g/L after 168 h fermentation, in a culture medium containing glucose as a carbon source. The medium was supplemented with L-Phe and the production of 2-PE occurred via the Ehrlich pathway.
To make the microbial synthesis route more attractive for industrialization, the use of affordable raw materials is essential [4]. An emerging practice involves the valorization of agro-industrial by-products derived from the processing of agricultural and sideline products [3]. Using these residues offers a wide range of advantages, including the reduction of pollution, as these by-products are produced in large volumes, and their disposal is usually associated with environmental issues. Additionally, their low cost and rich composition, containing carbohydrates, proteins, lipids, and minerals, make their valorization appealing, providing a suitable medium for microbial growth [26,27]. Genetic engineering methods are also employed to enhance yeast’s ability to fully consume various substrates [28].
One important by-product of the sugar refining process is sugar beet molasses (SBM). The European Union is the world’s largest producer of beet sugar, with an annual production of around 17 million tons [29]. SBM has a wide range of applications. Primarily, it is used in cattle feed. However, it is also utilized as a fermentation feedstock for the production of baker’s yeast, as well as for manufacturing amino acids and antibiotics in pharmaceutical industries [29,30,31]. SBM is rich in sucrose, making it a suitable carbon source for microbial growth. In addition to sucrose, SBM contains traces of nitrogen compounds and micronutrients including vitamins and minerals, such as calcium, magnesium, sodium, and potassium [32,33]. Currently, there is increasing interest in producing high-value compounds through yeast fermentation in a culture medium with SBM as a major carbon source.
The aim of this study was therefore to optimize the de novo production of 2-PE by fermentation using Y. lipolytica. Necessary genetic modifications of the yeast were performed prior to fermentation. The culture media were composed of SBM and yeast extract (YE), used as carbon and nitrogen sources, respectively, and the production of 2-PE was optimized through a central composite design.
2. Materials and Methods
2.1. Synthetic Media and Growth Conditions
Escherichia coli strains were cultivated in Luria-Bertani (LB) medium with constant shaking, 180 rpm, at 37 °C (Stuart SI500, orbital incubator, Cole-Palmer, Villepinte, France). LB medium is composed of 10 g/L tryptone, 5 g/L YE, and 10 g/L NaCl. Kanamycin (50 µg/mL) or ampicillin (100 µg/mL) antibiotics were added to the medium for plasmid selection.
Y. lipolytica strains were grown in either a rich yeast extract peptone dextrose (YPD) medium for yeast inoculum preparation or in a minimal yeast nitrogen base (YNB) medium for the selection of transformants. YPD is composed of 10 g/L glucose, 10 g/L peptone, and 10 g/L YE. When necessary, YPD was supplemented with 0.25 g/L hygromycin. The YNB–based medium consisted of 1.7 g/L YNB (without amino acids and ammonium sulfate, BD, Le Pont de Claix, France), 5 g/L NH4Cl, 50 mmol/L phosphate buffer (pH 6.8), and 20 g/L substrate (glucose or sucrose). For the selection of leucine or uracil auxotrophs, after marker excision, transformants were plated on a YNB–based medium supplemented with 0.1 g/L leucine (YNBLeu) or 0.1 g/L uracil (YNBUra), respectively.
Solid agar media for E. coli and Y. lipolytica were prepared by adding 15 g/L agar (Invitrogen, Saint-Aubin, France) to the liquid media.
Stock cell cultures, for long-term conservation of the Y. lipolytica strains, were prepared in a 0.5 L baffled Erlenmeyer flask. Rich YPD broth (0.1 L) was prepared and inoculated with an isolated colony grown on a YPD agar plate. The culture was incubated in a shaking incubator for 24 h at 28 °C and 170 rpm agitation. Sterile glycerol was added to the culture to a final concentration of 25% and then stored in cryotubes at −80 °C.
Evaluation of invertase production was performed in microtiter plates containing minimal YNB–based medium and sucrose. Strains were pre-cultivated for 24 h in YPD broth. Cells were centrifuged and washed twice with distilled water. The cells were then suspended in a YNB–based medium supplemented with sucrose (1%) and uracil (0.01%). Inoculation with the different pre-cultures was performed in 96-well microplates to obtain a final OD600 of 0.1. The growth analysis was performed at 28 °C under constant agitation using a Biotek Synergy MX microtiter plate reader (Biotek Instruments, Colmar, France). The growth of the Y. lipolytica strains was monitored by measuring the OD at 600 nm every 30 min for 120 h.
2.2. Strains and Plasmid Construction
The Y. lipolytica JMY8032 was used as the chassis strain in this study. It was derived from the Po1d strain, which was itself derived from the wild-type W29 (ATCC20460) strain. JMY8032 overexpresses key enzymes in the aromatic amino acid (AAA) pathway, such as YlARO1, YlARO2, ScARO4K229L, ScARO7T226I, ARO8, ARO10, and TKL1, driving the production of 2-PE [34]. The strain was further engineered, in this study, to be able to metabolize SBM.
2.2.1. Marker Recovery
Initially, the selective markers already present in JMY8032 strain were excised through the Cre-lox system. The replicative plasmid JME547 (pUB4-CRE) was transformed into JMY8032 [35]. This generated the auxotrophic JMY9390 strain, in which three selection markers, namely URA3, LEU2, and NAT (Nourseothricin), were recovered.
To ensure that during the recombination event, no imprecise excisions occurred, and there was no loss of either the cassette or any other gene expressed within it, colony PCR was performed for each construction previously inserted. PCR amplifications were carried out in an Applied Biosystems 2720 thermal cycler with GoTaq DNA polymerase (Promega, Madison, WI, USA). Plasmids from three E. coli reference strains (listed in Table S1), GGE192, JME4940, and JME4786, expressing ARO1-ARO2, ARO8-ARO10, and ScARO4K229L-ScARO7T226I, were used as positive controls for PCR amplification [34]. All the primers used in this study are listed in Table S3. All plasmids were extracted using the QIAprep Spin Miniprep Kit (Qiagen, Hilden, Germany). All of the reactions were performed according to the manufacturer’s instructions. PCR was followed by gel electrophoresis in a 0.8% agarose gel with ethidium bromide, and the fragment size profile obtained was analyzed.
2.2.2. Vector Construction through Golden Gate Assembly
Plasmid containing overexpression cassettes of the amyloglucosidase (AMG), the ScARO3K222L genes, and the α-amylase was constructed using the golden gate (GG) method and the toolkit developed for Y. lipolytica [36].
The GG approach enables the assembly of multiple and interchangeable DNA fragments in a single reaction through the implementation of digestion–ligation cycles. To construct a functional integrative expression cassette, it should include, at least, one of each of the following: a promoter, gene, terminator (which together form one transcription unit), along with a selection marker, and genome insertion sequences Up and Down, all of which are cloned into a donor vector. The GG system relies on the BsaI type IIs restriction enzyme, which allows the ligation of the different bricks in a predefined order.
The plasmids from E. coli of the acceptor (GGE114) and the donor vectors (GGE147, JME5727, GGE014, GGE152, JME4802, GGE015, GGE317, JME5735, and GGE080) (Table S1) [36], were extracted using the QIAprep Spin Miniprep kit, according to the manufacturer’s instructions. Equimolar quantities of each vector (50 pmoles of each DNA fragment to be assembled) were mixed and then assembled through digestion and ligation cycles with BsaI restriction enzyme (New England Biolabs, Ipswich, England) and T4 ligase. The reaction was performed in a thermocycler under the following thermal program conditions: [37 °C for 5 min; 16 °C for 5 min] × 30, 55 °C for 10 min, 80 °C for 10 min, and 15 °C ∞.
Subsequently, the GG–assembled vector (10 µL) was transformed into E. coli DH5α competent cells (80 µL). Transformants were plated on LB agar medium containing 100 μg/mL ampicillin. After an overnight incubation (≈24 h) at 37 °C, white colonies were selected and verified by PCR. Specific oligonucleotides were used to amplify the vector from the amyloglucosidase gene and back from the α-amylase gene (Table S3). PCR was followed by gel electrophoresis and analysis of the fragments’ size profile. Finally, the generated E. coli strain JME5742 was stored at −80 °C in glycerol stock.
2.2.3. Transformations of Y. lipolytica
The JMY9390 Y. lipolytica strain was transformed with the pTEF-SUC2-LEU2ex cassette present in the JME1657 plasmid (S. cerevisiae SUC2), resulting in the JMY9392 strain. The plasmid of interest was previously digested with NotI restriction enzyme (New England Biolabs, Ipswich, England) to recover the expression cassette, and transformed into Y. lipolytica according to the lithium acetate method [37]. Transformants were selected on YNBUra medium. The insertion of the cassette was confirmed by PCR with primers pairing with pTEF and SUC2 (Table S3). PCR was followed by gel electrophoresis and analysis of the fragments’ size profile.
The JMY9392 Y. lipolytica was transformed with the JME5742 plasmid previously digested by NotI, generating the JMY9398 strain. Transformants were selected on YNB medium. The insertion of the cassette was confirmed by PCR with primers pairing AMG and α-amylase. PCR was followed by gel electrophoresis and analysis of the fragments’ size profile.
All of the strains used in this study are listed in Table 1. The overall process of JMY9398 strain construction is summarized in Figure 1.

Table 1.
Main plasmids and strains used in this study.

Figure 1.
Summary of JMY9398 strain construction.
2.3. Fermentation Conditions
2.3.1. Growth Media Composition and Culture Conditions
Fermentations were conducted in SBM-based media. In particular, the fermentation media were composed of SBM, as the main carbon substrate, and were supplemented with different concentrations of YE, as an organic nitrogen substrate. The initial concentration of sucrose in the SBM used in this study was equivalent to 800 g/L, previously determined by high performance liquid chromatography (HPLC) [13]. Dilutions of the SBM were carried out in distilled water. All the culture media and glassware used were priorly sterilized at 121 °C for 20 min using an HMC 110 V autoclave (HMC Europe GmbH, Tüßling, Germany).
To inoculate the fermentation media, a preculture was prepared and incubated for 24 h before starting the fermentation. In a 0.5 L Erlenmeyer flask, 0.1 L of YPD broth was prepared and inoculated by adding 2 mL of Y. lipolytica stock cell culture. The pre-culture was incubated in a shaking incubator (New Brunswick Scientific, C24 Incubator Shaker, Edison, NJ, USA) for 24 h at 28 °C and 170 rpm agitation speed.
Cultures were performed in 0.5 L Erlenmeyer flasks containing 0.1 L of culture media. The preliminary study consisted of 9 different culture media compositions in which the concentrations of SBM (5, 10, 20, 30, and 40 g/L) and YE (1, 3, 5, and 10 g/L) were varied (Table 2). Na2HPO4 (0.05 mol/L) and KH2PO4 (0.05 mol/L) buffers were added to all fermentation media to adjust the pH to 6. Culture media were inoculated with the previously prepared overnight preculture to reach an initial optical density of 0.2 at 600 nm (OD600). The flasks were then placed in the shaking incubator at 28 °C, 170 rpm, for 168 h. During the incubation period, a 1 mL sample was withdrawn aseptically every 24 h from each flask, and the cell growth was monitored as described in Section 2.4. After measuring the OD600, the samples were centrifuged for 15 min at 13,000 rpm using a microcentrifuge (Micro Star 12, VWR, Rosny-Sous-Bois, France), and the resulting supernatant was separated from the pellet and stored at −20 °C for further analysis.

Table 2.
Composition of the different culture media tested during the preliminary study.
2.3.2. Optimization of Culture Media Composition: Design of Experiment
Response surface methodology (RSM) was implemented using a central composite design to optimize the production of 2-PE in an SBM–based medium. RSM was used to assess, in this study, the impact of the two factors, SBM (20–40 g/L) and YE (3–5 g/L) concentrations, and the interaction between them on the 2-PE accumulation. The central composite design (22 + star points) resulted in 19 experiments, for 9 different experimental conditions, including four factorial design points (repeated twice) coded (−1 and +1); four star points (repeated twice) coded (−α and +α), where α = 1.41; and three repetitions at the center point (0,0), in addition to two test points. The ranges of concentrations of the two independent factors were set according to the results of the preliminary experiments presented in Table 2. The response studied for this experimental design was the concentration of 2-PE produced (g2-PE/Lculture medium).
The design of all experiments and the results were analyzed using Statgraphics Centurion XVII-X64.
2.4. Analytical Methods: OD, 2-PE, Sugar Consumption
Cell growth of flask cultures was monitored by measuring the optical density of cultures at 600 nm (OD600) using a spectrophotometer (Thermo Electron Corporation, BioMate 3, Madison, WI, USA). Withdrawn fermentation samples were diluted 33.3 times, using a sterile non-inoculated medium, and their corresponding ODs were measured. Sterile media were used as a blank of the spectrophotometer.
HPLC (Agilent Technologies 1260 infinity II, Santa Clara, CA, USA) coupled with a refractive index (RI) detector was used to determine the concentration of 2-PE and sugars in the fermentation supernatants.
For the quantification of 2-PE, the HPLC was equipped with a reversed phase column, XBridgeTM C18 column (Waters, Milford, MA, USA) with a length of 250 mm, an internal diameter of 4.6 mm, and a film thickness of 5 µm. A mobile phase of 80% water and 20% acetonitrile was used at a flow rate of 1.2 mL/min for 20 min. The temperature of the column was set at 35 °C. Samples from the fermentation supernatants were filtered using 0.45 µm filters and then diluted 1.5 times with 80% water and 20% acetonitrile. The injection volume was set to 20 µL. Quantification was carried out using a standard curve, which was drawn using pure 2-PE (ACROS Organics, Thermo Scientific Chemicals, Geel, Belgium, 99% purity). For each condition, all of the withdrawn samples were injected and the 2-PE production over time throughout the fermentation was drawn.
For the sugar consumption analysis, the concentrations of sucrose, glucose, and fructose (the latter two being liberated into the medium due to sucrose hydrolysis by invertase) were determined using HPLC-RI equipped with an Aminex HPX-87H column (BIO-RAD, Hercules, CA, USA). The mobile phase was composed of ultrapure water and the flow rate was adjusted to 0.6 mL/min. The temperature of the column was set at 35 °C. The injection volume was set to 20 µL. Samples of the fermentation supernatants were diluted 50 times with water. Identification and quantification were conducted by employing a standard curve specific to each substrate.
2.5. Elemental Analysis
Elemental analysis of the SBM and YE used in this study was performed, by SynBioN, to quantify the carbon and nitrogen in both components. Thermo Scientific FlashSmart analyzer was used to perform this analysis, which operates according to the dynamic flash combustion of the sample. After weighing the samples, they were introduced separately into the combustion reactor, where they were combusted for 15 s at 950 °C in the presence of copper oxide and under an oxygen stream. The entire system was purged with helium. The produced gases were then swept through a GC column and detected by a Thermal Conductivity Detector (TCD). Results were recorded and analyzed using EagerSmart Data Handling Software (version 1.0). Each sample was analyzed in duplicate.
2.6. Statistical Analysis
All of the fermentations were performed in duplicate. The obtained results were the average of two biological replicates ± standard deviations (SD). One-way analysis of variance (ANOVA) was used to determine the significant differences (at 95% confidence level) using Excel software (Microsoft Excel 2016 MSO 16.0.17328.20124).
3. Results and Discussion
3.1. Strain Construction and Validation of Invertase Activity
Since the ultimate objective of this study was to valorize agro-industrial by-products for the production of 2-PE, generating a strain capable of consuming various complex sugars present in different industrial by-products (which naturally lacks the ability to do so) is crucial. It is noteworthy that using low-cost substrates (e.g., industrial by-products) may reduce the production cost of 2-PE, and thereby accelerate its industrialization.
Y. lipolytica lacks the ability to naturally metabolize sucrose, due to the absence of the invertase enzyme [39]. To enable Y. lipolytica to utilize the SBM substrate as a carbon source, it was imperative to introduce an efficient heterologous invertase gene, which allows it to break down sucrose into glucose and fructose. The chassis strain used in this study (JMY8032) already expresses a former SUC2 gene (SUC2-302), but the expressed invertase has a low secretion efficiency [40]. After successively transforming the expression cassette from the JME1657 plasmid, containing the full S. cerevisiae SUC2 sequence, with an optimized secretion signal sequence and the strong Y. lipolytica promoter pTEF (SUC2-1462), which previously showed increased extracellular activity [40], the growth of the newly generated strain JMY9392 was evaluated in a minimal medium supplemented with 1% sucrose (as a unique carbon source). The growth of JMY9392 was compared with other Y. lipolytica strains expressing different versions of SUC2 (listed in Table S2). The growth curves of the different strains are presented in Figure 2.
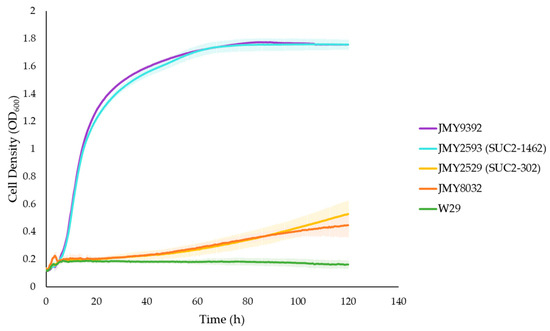
Figure 2.
Growth curves in 96-well plates of multiple Y. lipolytica strains in a minimal media supplemented with 1% sucrose and 0.01% uracil.
As depicted in Figure 2, the W29 strain was unable to grow in the minimal medium, thus serving as a negative control. The JMY2529 and JMY8032 strains express only one copy of a weakly secreted SUC2 gene (SUC2-302), which only allows a slow growth in a minimal medium supplemented with sucrose, as previously reported [40]. Both strains showed similar slow growth rates. On the other hand, the JMY2593 strain, which was generated in the aforementioned study, expressing a new cassette (SUC2-1462) with an optimal expression of the invertase enzyme, showed an optimal growth in sucrose-based media and thus served as a positive control [40]. The newly generated JMY9392 strain showed a similar growth rate to the JMY2593 strain, reaching a maximum growth rate on sucrose. Therefore, unlike the chassis strain JMY8032, the JMY9392 strain has the ability to efficiently assimilate the disaccharide sucrose present in any culture media.
The ARO3 gene codes for the first step of the shikimate pathway but this gene was not yet overexpressed in the JMY8032 chassis strain. Therefore a mutated version of this gene, the ScARO3K222L gene, which is insensitive to feedback inhibition, was introduced [34], aiming to increase the flux into the shikimate pathway. Taking advantage of the GG modular cloning system, other genes were introduced in the same construction, which will be useful for the hydrolysis and consumption of not only sucrose-based by-products but also starch-based industrial by-products in the future. Therefore, a cassette for overexpressing the amyloglucosidase (AMG, from Aspergillus niger), the α-amylase (from rice), and the ScARO3K222L was generated. This cassette was transformed into the JMY9392 strain (expressing the SUC2 gene), generating the JMY9398 strain, that can be used to produce 2-PE from a much larger scope of by-products, and which was used in this study.
3.2. Impact of SBM and YE on Y. lipolytica’s Growth and 2-PE Production
After successfully generating the strain JMY9398 expressing the invertase enzyme and thus hydrolyzing the sucrose present in the SBM, fermentations were carried out to optimize the production of 2-PE in an SBM-based medium. To prepare the culture media for cultivating Y. lipolytica, SBM was used as the carbon source and supplemented with YE as the nitrogen source. The influence of both factors on cellular growth and on 2-PE production was investigated by varying the concentrations of SBM and YE from 5 to 40 g/L and from 0 to 10 g/L, respectively (Figure 3).
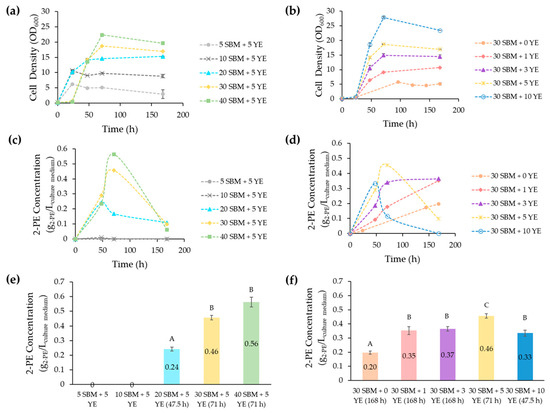
Figure 3.
The profile of the JMY9398 strain growth and of 2-PE production as a function of time in media composed of varying concentrations of SBM supplemented with different YE concentrations. (a,b) Yeast growth over time using cell density (OD600); (c,d) 2-PE production over time in the different media formulations; (e,f) maximal production of 2-PE in each medium. Error bars represent the standard deviation of biological replicates for each condition. Different uppercase letters indicate significant differences between the 2-PE titers in the different culture media (p < 0.05). SBM: sugar beet molasses; YE: yeast extract; 2-PE: 2-phenylethanol.
The results obtained showed that the maximal cell density (OD600nm) reached during the stationary phase was significantly different when the concentration of sucrose in SBM increased from 5, to 10, to 20, to 30, and finally to 40 g/L. The cell density was continuously increasing while increasing the concentration of sucrose in the SBM; however, at higher concentrations, specifically 30 and 40 g/L, an increase in the duration of the lag (adaptation) phase was observed (Figure 3a). A longer time was needed to reach a maximal cell density. Similar results were reported in a previous study, where the lag phase duration was prolonged significantly in the fermentation of Clostridium butyricum W5, as the concentration of SBM increased from 20 to 120 g/L, which was probably due to the elevated osmotic pressure exerted by the concentrated SBM within the medium [41]. By analyzing the cellular growth profile for JMY9398 in the different media, it was observed that the maximum biomass concentration was obtained 72 h after starting the fermentation in a medium composed of 30 g/L SBM and 10 g/L YE (Figure 3b). However, the highest growth rate of JMY9398 did not correspond to the highest titer of 2-PE, which was 0.56 g/L of 2-PE in a medium composed of 40 g/L SBM and 5 g/L YE (Figure 3c,e). Increasing the concentration of YE would increase cell density rather than 2-PE production, since YE is a nitrogen source known to favor cellular proliferation [42].
Figure 3c–f depict the 2-PE production of Y. lipolytica in the different compositions of the SBM–based media, varying either the SBM concentration (Figure 3c,e) or the YE concentration (Figure 3d,f). As shown in Figure 3c,e, the 2-PE production increased gradually with the concentration of SBM. A significant difference in 2-PE production (p < 0.05) was observed when the concentration of SBM was increased from 20 to 30 g/L and further from 30 to 40 g/L. However, due to the increased osmotic pressure proportional to the SBM added to the medium, the cellular growth of yeast might be inhibited beyond a certain concentration [43]. Additionally, SBM might contain elevated concentrations of certain inhibitory compounds generated during sugar processing, which could potentially impede yeast performance [44]. Higher SBM concentrations are associated with increased levels of toxic compounds, namely phenolic compounds, which may present an antimicrobial effect [45]. It should be highlighted that SBM not only serves as a carbon source but also as a rich feedstock of various micronutrients including magnesium, iron, and potassium [46].
Nitrogen concentration is a factor that had a great effect on the synthesis of multiple volatile compounds [47]. The effect of supplementing the medium with YE was investigated, and as shown in Figure 3d,f, the addition of 1 g/L YE has significantly improved the production of 2-PE. Moreover, the addition of 5 g/L YE improved the production of 2-PE to 0.46 g/L which was the highest recorded titer between the different supplementations of the 30 g/L SBM with YE. It was previously reported that the nature of the nitrogen source present in the medium has a huge effect on inducing or repressing important genes involved in the AAA synthesis, and hence either driving the AAA pathway or repressing it. In a recent study, it was shown that adding ammonia to the culture medium led to increased activation of genes related to the synthesis of amino acids, whereas adding amino acids primed the cells for protein synthesis [48]. In addition, a shorter fermentation time was observed when the initial nitrogen content was increased [47], which concurs with the results of the current study. As seen in Figure 3d, the peak of production of 2-PE for the medium containing 10 g/L YE was at 48 h after starting the fermentation, whereas the peak of 2-PE production in the medium containing 1 g/L YE was at 168 h. It was also reported that nitrogen has a quadratic effect on 2-PE production [47], which was also seen when the concentration of YE was increased to 10 g/L and the 2-PE concentration started to significantly decrease (p < 0.05) compared to the medium containing 5 g/L of the YE. It is noteworthy to mention that there was no significant difference (p > 0.05) in the production of 2-PE between the media containing 1 and 3 g/L YE.
These preliminary fermentations allowed us to set the experimental domain of each factor to optimize the production of 2-PE in an SBM–based culture medium.
3.3. Optimization of the SBM-Based Culture Media Composition
Response surface methodology using a central composite design was used to study the impact of the two factors, SBM and YE, on maximizing the concentration of 2-PE in the culture medium. Table 3 presents the experimental conditions and the measured responses for the 19 fermentations performed within the experimental design.

Table 3.
Central composite design for the independent variables and the corresponding response related to 2-PE concentration (g2-PE/Lculture medium). SD denotes standard deviation.
Figure 4a presents the JMY9398 yeast growth throughout the fermentation in the 19 experiments performed, and Figure 4b shows the production of 2-PE throughout the 168 h fermentation, alongside the maximal 2-PE concentrations (g2-PE/Lculture medium) in each of the culture media compositions, respectively.
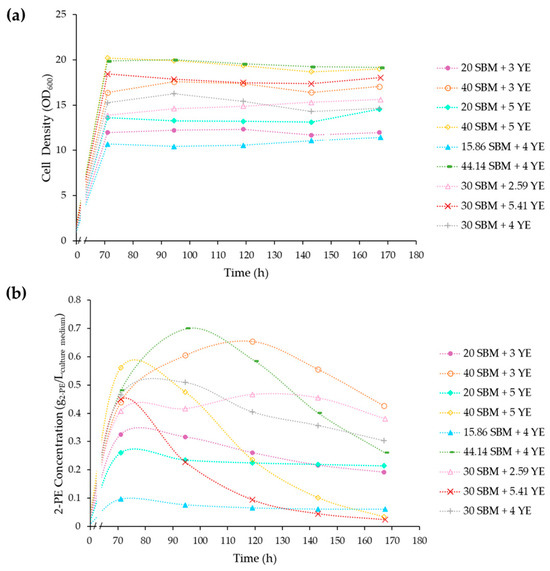
Figure 4.
Cell growth and 2-PE production in the culture conditions of the experimental design. (a) Yeast growth over time using cell density (OD600); (b) 2-PE Production in the different media formulations. Error bars represent the standard deviation of biological replicates for each condition. Different uppercase letters indicate significant differences (p < 0.05). SBM: sugar beet molasses; YE: yeast extract; 2-PE: 2-phenylethanol.
The highest production of 2-PE was observed in the medium containing 44.14 g/L SBM and 4 g/L YE, resulting in a concentration of 0.7 g/L 2-PE. The medium composed of 40 g/L SBM and 3 g/L YE yielded 0.65 g/L 2-PE, which was not significantly different in titer (p > 0.05) but utilized a lower supply of YE. There was a significant difference in yeast growth (p < 0.05), with higher cell density, in the medium containing higher concentrations of SBM and YE. In both media, despite the yeast reaching the stationary phase and ceasing growing, the bioconversion of 2-PE continued, with its concentrations increasing in the culture media, which is consistent with the previous findings [49].
The lowest production of 2-PE was observed in the medium containing 15.86 g/L SBM and 4 g/L YE, producing 0.1 g/L 2-PE. This concentration is significantly lower, by a factor of seven, compared to the highest recorded titer, indicating the substantial impact of SBM on 2-PE production.
The influence of YE concentration on fermentation duration and the timing of the 2-PE production peak was also observed. In media with 30 g/L SBM, one supplemented with 2.59 g/L YE and the other with 5.41 g/L YE, there was no significant difference in 2-PE production (approximately 0.46 ± 0.1 g/L for both). However, the production peak occurred earlier in the medium with 5.41 g/L YE at 71 h compared to the medium with lower YE concentration at 119 h.
The fermentations outlined in the experimental design (Table 3) were validated using test points. The first test point condition, using 23.88 g/L sucrose in SBM and 3.65 g/L YE, produced 0.379 g/L 2-PE. The second test point condition, using 30 g/L sucrose in SBM and 4.71 g/L YE, produced 0.445 g/L 2-PE. Analysis of variance indicated that the empirical model was statistically validated, with significant regression (p < 0.05), and non-significant lack of fit (p > 0.05). The R2 was 95.71% indicating a good model fit. The second-degree model necessary to predict the 2-PE response value is given in Equation (1).
2PE(g/L) = −0.82 + 0.0441 × SBM(g/L) + 0.2145 × YE(g/L) − 0.00035 × (SBM(g/L))2 − 0.0011 × SBM(g/L) × YE(g/L) − 0.0253 × (YE(g/L))2
The Pareto Chart (Figure 5a) illustrates the significance of the assessed factors on the 2-PE concentration (g2-PE/Lculture medium). Bars extending beyond the vertical line indicate statistical significance with a confidence level exceeding 95%. The chart shows a positive linear effect and a slightly negative quadratic effect for SBM, whereas YE exhibits negative linear and quadratic effects, both of which are marginally significant. There was no significant interaction effect between SBM and YE, indicating that 2-PE concentration varied independently of the second factor. The inserts in Figure 5a show the variation of 2-PE according to one factor, ranging from a low (−1) to a high level (+1), with the other variable fixed at the central level (0). Increasing the SBM concentration from 20 to 40 g/L significantly increased 2-PE production. Conversely, increasing the YE concentration from 3 to 5 g/L resulted in a slight decrease in 2-PE production. The interaction plot suggests that a 3 g/L YE concentration slightly increases 2-PE production compared to 5 g/L YE.
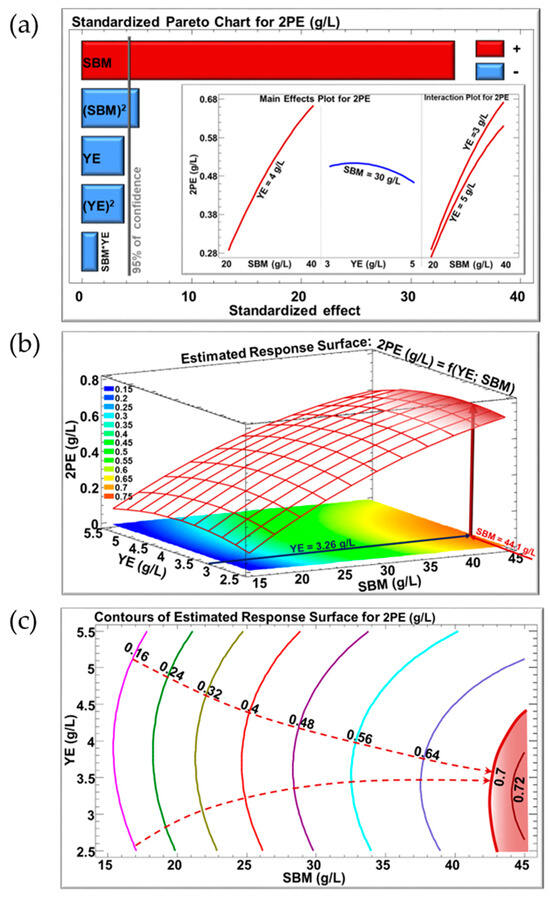
Figure 5.
(a) Pareto chart for 2-PE production, where the insert shows the evolution of 2-PE as a function of the following: SBM (YE = 4 g/L), YE (SBM = 30 g/L), and SBM (YE = 3 or 5 g/L); (b) predicted response surface for 2-PE as a function of SBM concentration and YE concentration; (c) contour plot of the estimated response surface for 2-PE. SBM: sugar beet molasses; YE: yeast extract; 2PE: 2-phenylethanol.
Figure 5b illustrates the response surface of 2-PE as a function of SBM and YE concentrations simultaneously. This three-dimensional model shows the optimal zone, particularly the maximal 2-PE titer, which is colored in reddish orange, where any concentration of SBM or YE will produce approximately 0.7 g/L 2-PE. The suggested optimal composition of the culture medium to maximize 2-PE production was 44.14 g/L SBM and 3.26 g/L YE, yielding an optimum 2-PE production of 0.72 g/L.
The contour plot of the estimated response surface for 2-PE as a function of SBM and YE concentrations is shown in Figure 5c. This plot shows that increasing the concentration of SBM in the medium while decreasing the concentration of YE resulted in an increase in the 2-PE titer from 0.16 g/L to 0.72 g/L. Each point on the iso-response curve, with x representing the SBM concentration and y representing the YE concentration, produces the same concentration of 2-PE. For example, the iso-response curve in cyan corresponds to the production of 0.56 g/L 2-PE. This concentration could be produced in a medium containing either 40 g/L SBM and 5.5 g/L YE or 34 g/L SBM with 2.6 g/L YE. Such information can assist in maximizing 2-PE production while optimizing the most cost-effective conditions. Consequently, it allows the optimization of other response parameters, such as the production cost of the target molecule. To validate the predictive capability of the model, fermentations were conducted under the expected optimal conditions.
3.4. Fermentations under the Optimal Culture Conditions
Based on the results of Section 3.2 and Section 3.3, the optimal culture media composition was tested to verify the predictions of the design. Fermentations in 44.14 g/L SBM and 3.2 g/L YE were performed using the JMY9398 strain. As shown in Figure 6, 0.71 ± 0.02 g/L of 2-PE were produced after 146 h of fermentation. The obtained value was not significantly different (p > 0.05) from the predicted one provided by Statgraphics (0.72 g/L 2-PE). The obtained 2-PE titer was 26.8% higher than the initial value before the optimization process (0.56 g/L versus 0.71 g/L 2-PE).
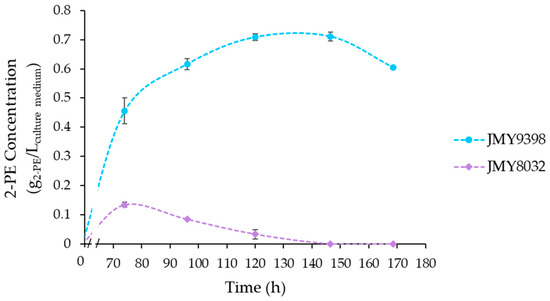
Figure 6.
2-PE production (g2-PE/Lculture medium) under the optimal media composition, using the JMY8032 and JMY9398 strains. Error bars represent the standard deviation of biological replicates for each condition.
Fermentation under the optimal medium composition was also carried out using the JMY8032 chassis strain, which does not overexpress the optimized SUC2 and ARO3 genes. As illustrated in Figure 6, JMY8032 produced 0.13 g/L of 2-PE after 74 h of fermentation. This highlights the significance of transforming the SUC2 gene to enable Y. lipolytica to efficiently utilize sucrose in SBM as a carbon source.
Upon evaluating sugar consumption in the culture medium for JMY9398, it was observed that sucrose was completely consumed within 24 to 48 h after initiating fermentation. As sucrose was being degraded, the concentrations of glucose and fructose increased in the medium. Subsequently, once sucrose was exhausted, glucose was rapidly consumed, followed by fructose (Figure 7), which is coherent with previous findings [40]. A complete depletion of sugars in the media was observed by the end of the fermentation.
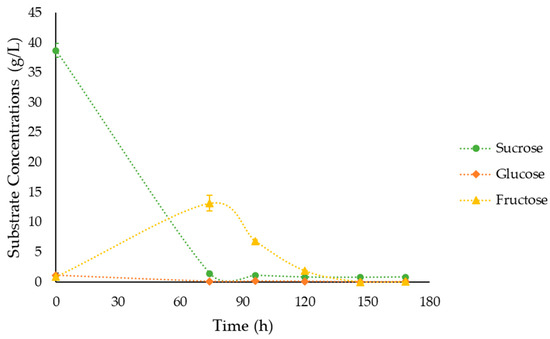
Figure 7.
Substrate consumption during the fermentation of JMY9398 in 44.14 g/L sucrose in SBM and 3.2 g/L YE. Error bars represent the standard deviation of biological replicates for each condition.
Regarding the impact of the C/N ratio, since complex components, such as SBM and YE, were used in the culture media, elemental analysis was performed to determine the carbon and nitrogen content in each medium. Results showed that the used SBM contains 29.45 ± 0.32% carbon and 1.16 ± 0% nitrogen, whereas the YE contains 39.73 ± 0.17% carbon and 10.97 ± 0.04% nitrogen. Therefore, the estimated C/N ratio for the optimal medium composition was around 16.53.
Many studies are currently being conducted on the bioproduction of 2-PE through microbial fermentations of yeast, utilizing various agro-industrial by-products. However, most studies focus on 2-PE synthesis through the Ehrlich pathway used the costly precursor L-Phe. A summary of multiple studies conducted on the production of 2-PE in synthetic media and agro-industrial waste and by-products, with and without the supplementation of L-Phe, was given in a recent review article [24]. In fact, so far, few studies have been conducted on the production of 2-PE using the yeast Y. lipolytica, as summarized in Table 4. To our knowledge, none of these studies have explored the de novo synthesis of 2-PE. A maximal production of 3.2 g/L 2-PE, using Y. lipolytica, was reported in a medium composed of crude glycerol as the carbon source and supplemented with L-Phe [9]. Focusing on the de novo biosynthesis of 2-PE through the fermentation of yeasts on agro-industrial by-products, it has been shown that K. marxianus, ITD00262 strain, has the capacity to produce 0.96 g/L, 0.7 g/L, and 0.47 g/L of 2-PE after 24-h fermentations in sweet whey, acid whey, and curd whey, respectively, in the absence of L-Phe [50]. In another study, it has been reported that S. cerevisiae has the ability to produce 1.55 g/L of 2-PE in a medium composed of 39.28 g/L tobacco waste, in the absence of L-Phe [51]. It is important to note that various factors can affect the concentration of 2-PE produced, including the species and strain of yeast used, the genetic modifications made to the yeast, the composition of the culture medium (whether synthetic or derived from agro-industrial by-products), the nitrogen source added to the culture medium (the presence or absence of L-Phe), and the fermentation conditions [24].
Subsequently, 2-PE production through de novo biosynthesis seems promising. Several strategies can be applied to improve the 2-PE titer. These include (i) further engineering the Y. lipolytica strain to better drive the biosynthesis pathway of 2-PE; (ii) optimizing the fermentation process, particularly aeration, considering that Y. lipolytica is a strictly aerobic yeast; (iii) optimizing the medium composition by supplementing it with additional carbon sources, organic or inorganic nitrogen sources, minerals, vitamins, cofactors, and organic acids; (iv) additionally enhancing the yeast’s tolerance to 2-PE; and finally (v) implementing in situ product removal (ISPR) techniques to separate 2-PE from the fermentation medium immediately after its biosynthesis. The significant impact of ISPR on 2-PE production during fermentation has been previously demonstrated [52]. The authors showed that by using oleyl alcohol for in situ product removal, the 2-PE production of K. marxianus CBS 600 increased from 0.84 g/L to 3.06 g/L 2-PE, representing a 3.6-fold improvement in production. On the other hand, utilizing ISPR techniques helps maintain the 2-PE produced throughout the fermentation by removing it from the media, thereby avoiding any decrease in 2-PE towards the end of the fermentation. This decrease in 2-PE concentration could be related to the bioconversion of 2-PE to other metabolites, namely 2-phenethyl acetate [53,54].

Table 4.
Summary of 2-PE production capacity of different Y. lipolytica strains. GMO: Genetically modified organism; SBM: sugar beet molasses.
Table 4.
Summary of 2-PE production capacity of different Y. lipolytica strains. GMO: Genetically modified organism; SBM: sugar beet molasses.
| Strain | Carbon Source | Supplementation with L-Phe | 2-PE Production | Reference |
|---|---|---|---|---|
| NCYC3825 (GMO) | 40 g/L Glucose | 7 g/L (Added after 73 h of cultivation) | 1.98 g/L | [25] |
| po1fk7P (GMO) | 40 g/L Glucose | 4 g/L | 2.67 g/L | [55] |
| CH 1/5 (non-GMO) | 40 g/L Crude Glycerol | 8 g/L (at t = 0 h), with an additional 4 g/L added after 80 h of fermentation. | 3.2 g/L | [9] |
| JMY9398 (GMO) | 44.14 g/L SBM | 0 g/L | 0.71 g/L | This study |
4. Conclusions
In this study, a Y. lipolytica strain was successfully engineered for the de novo production of 2-PE, utilizing an SBM medium supplemented with YE. After optimizing the culture medium, the highest titer of 2-PE reached was 0.71 g/L. The findings obtained underscore the feasibility of a sustainable and economically viable approach for 2-PE synthesis. To further improve the production process, exploring various sucrose-rich and starch-rich agro-industrial by-products holds promise. Additionally, identifying a natural and low-cost nitrogen source as a substitute for YE could significantly reduce production costs. Lastly, optimizing the extraction and recovery methods of 2-PE is pivotal for the following steps towards scale-up and industrialization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation10060290/s1; Table S1: complementary E. coli strains used in this study; Table S2: complementary Y. lipolytica strains used in this study; Table S3: primers used in this study. Reference [56] is cited in the supplementary materials.
Author Contributions
Conceptualization, M.K. and N.L.; methodology, S.M. and T.R.; software, N.L. and M.K.; validation, N.L. and M.K.; formal analysis, N.L., M.K., T.R. and S.M.; investigation, S.M., N.L., T.R. and M.K.; resources, M.K.; data curation, N.L.; writing—original draft preparation, S.M. and T.R.; writing—review and editing, N.L., M.K., T.R., R.G.M. and S.M.; visualization, S.M., N.L. and M.K.; supervision, M.K., R.G.M., T.R. and N.L.; project administration, M.K. and N.L.; funding acquisition, M.K., R.G.M., N.L. and T.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education, Research and Innovation (France), by The Research Council of the Saint Joseph University of Beirut, FS-165 (Lebanon), and by Ecole Supérieure de Chimie Organique et Minérale (Compiègne, France).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We extend our gratitude to Sandrine Adach from SynBioN—L2CM (University of Lorraine-CNRS—http://synbion.univ-lorraine.fr/accueil/ (accessed on 28 May 2024)) for her valuable contribution to this study by conducting the elemental analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, R.; Song, Q.; Xia, H.; Song, N.; Yang, Q.; Zhang, X.; Yao, L.; Yang, S.; Dai, J.; Chen, X. Isolation and Identification of Non-Saccharomyces Yeast Producing 2-Phenylethanol and Study of the Ehrlich Pathway and Shikimate Pathway. J. Fungi 2023, 9, 878. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Phenylethyl Alcohol. Food Chem. Toxicol. 2012, 50, S224–S239. [Google Scholar] [CrossRef] [PubMed]
- Drężek, K.; Kozłowska, J.; Detman, A.; Mierzejewska, J. Development of a Continuous System for 2-Phenylethanol Bioproduction by Yeast on Whey Permeate-Based Medium. Molecules 2021, 26, 7388. [Google Scholar] [CrossRef] [PubMed]
- Lukito, B.R.; Basri, N.; Thong, A.; Hermansen, C.; Weingarten, M.; Peterson, E.C. Co-Culture of Kluyveromyces Marxianus and Meyerozyma Guilliermondii with In Situ Product Recovery of 2-Phenylethanol. J. Agric. Food Chem. 2023, 23, 8991–8997. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Hu, X.; Liu, X.; Zhao, Y.; Xu, Y. Production of 2-Phenylethanol by Microbial Mixed Cultures Allows Resource Recovery of Cane Molasses Wastewater. Fresenius Environ. Bull. 2014, 23, 1356–1365. [Google Scholar]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological Production of 2-Phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef]
- Holyavkin, C.; Turanlı-Yıldız, B.; Yılmaz, Ü.; Alkım, C.; Arslan, M.; Topaloğlu, A.; Kısakesen, H.İ.; de Billerbeck, G.; François, J.M.; Çakar, Z.P. Genomic, Transcriptomic, and Metabolic Characterization of 2-Phenylethanol-Resistant Saccharomyces Cerevisiae Obtained by Evolutionary Engineering. Front. Microbiol. 2023, 14, 1148065. [Google Scholar] [CrossRef]
- Zhu, N.; Xia, W.; Wang, G.; Song, Y.; Gao, X.; Liang, J.; Wang, Y. Engineering Corynebacterium Glutamicum for de Novo Production of 2-Phenylethanol from Lignocellulosic Biomass Hydrolysate. Biotechnol. Biofuels Bioprod. 2023, 16, 75. [Google Scholar] [CrossRef]
- Braga, A.; Freitas, B.; Cordeiro, A.; Belo, I. Valorization of Crude Glycerol as Carbon Source for the Bioconversion of L-Phenylamine to 2-Phenylethanol by Yarrowia Species. J. Chem. Technol. Biotechnol. 2021, 96, 2940–2949. [Google Scholar] [CrossRef]
- Alonso-Vargas, M.; Téllez-Jurado, A.; Gómez-Aldapa, C.A.; Ramírez-Vargas, M.D.R.; Conde-Báez, L.; Castro-Rosas, J.; Cadena-Ramírez, A. Optimization of 2-Phenylethanol Production from Sweet Whey Fermentation Using Kluyveromyces Marxianus. Fermentation 2022, 8, 39. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-Phenylethanol and 2-Phenylethyl Acetate Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef] [PubMed]
- Mitri, S.; Salameh, S.J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- El Kantar, S.; Koubaa, M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 284. [Google Scholar] [CrossRef]
- Gao, H.; Wang, H.; Zhang, Y.; Wang, Y.; Liu, G.; Zhao, Q.; Yu, Z.; Xin, F.; Zhang, W. Design and Optimization of Artificial Light-Driven Microbial Consortia for the Sustainable Growth and Biosynthesis of 2-Phenylethanol. Chem. Eng. J. 2023, 466, 143050. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Dulermo, T.; Nicaud, J.M. Engineering Yarrowia lipolytica to Produce Biodiesel from Raw Starch. Biotechnol. Biofuels 2015, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology; Springer: Berlin/Heidelberg, Germany, 1996; pp. 313–388. [Google Scholar]
- Eszterbauer, E.; Németh, Á. Investigations for a Yarrowia-Based Biorefinery: In Vitro Proof-of-Concept for Manufacturing Sweetener, Cosmetic Ingredient, and Bioemulsifier. Fermentation 2023, 9, 793. [Google Scholar] [CrossRef]
- Carsanba, E.; Agirman, B.; Papanikolaou, S.; Fickers, P.; Erten, H. Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation 2023, 9, 687. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R. What Makes Yarrowia lipolytica Well Suited for Industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Mesquita, D.P.; Cordeiro, A.; Belo, I.; Ferreira, E.C.; Amaral, A.L. Monitoring Biotechnological Processes through Quantitative Image Analysis: Application to 2-Phenylethanol Production by Yarrowia lipolytica. Process Biochem. 2023, 129, 221–229. [Google Scholar] [CrossRef]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Bioproduction of 2-Phenylethanol and 2-Phenethyl Acetate by Kluyveromyces Marxianus through the Solid-State Fermentation of Sugarcane Bagasse. Appl. Microbiol. Biotechnol. 2018, 102, 4703–4716. [Google Scholar] [CrossRef]
- Carlquist, M.; Gibson, B.; Karagul Yuceer, Y.; Paraskevopoulou, A.; Sandell, M.; Angelov, A.I.; Gotcheva, V.; Angelov, A.D.; Etschmann, M.; de Billerbeck, G.M.; et al. Process Engineering for Bioflavour Production with Metabolically Active Yeasts—A Mini-Review. Yeast 2015, 32, 123–143. [Google Scholar] [CrossRef]
- Kong, S.; Pan, H.; Liu, X.; Li, X.; Guo, D. De Novo Biosynthesis of 2-Phenylethanol in Engineered Pichia Pastoris. Enzym. Microb. Technol. 2020, 133, 109459. [Google Scholar] [CrossRef]
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods 2022, 11, 109. [Google Scholar] [CrossRef]
- Celińska, E.; Kubiak, P.; Białas, W.; Dziadas, M.; Grajek, W. Yarrowia lipolytica: The Novel and Promising 2-Phenylethanol Producer. J. Ind. Microbiol. Biotechnol. 2013, 40, 389–392. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia Kudriavzevii through Solid-State Fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Lazar, Z.; Dulermo, T.; Neuvéglise, C.; Crutz-Le Coq, A.M.; Nicaud, J.M. Hexokinase—A Limiting Factor in Lipid Production from Fructose in Yarrowia lipolytica. Metab. Eng. 2014, 26, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.T.; Song, H.; Raschbauer, M.; Emerstorfer, F.; Omann, M.; Stelzer, F.; Neureiter, M. Utilization of Desugarized Sugar Beet Molasses for the Production of Poly(3-Hydroxybutyrate) by Halophilic Bacillus megaterium Uyuni S29. Process Biochem. 2019, 86, 9–15. [Google Scholar] [CrossRef]
- Saric, L.; Filipcev, B.; Simurina, O.; Plavsic, D.; Saric, B.; Lazarevic, J.; Milovanovic, I. Sugar Beet Molasses: Properties and Applications in Osmotic Dehydration of Fruits and Vegetables. Food Feed Res. 2016, 43, 135–144. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Beigbeder, J.B.; de Medeiros Dantas, J.M.; Lavoie, J.M. Optimization of Yeast, Sugar and Nutrient Concentrations for High Ethanol Production Rate Using Industrial Sugar Beet Molasses and Response Surface Methodology. Fermentation 2021, 7, 86. [Google Scholar] [CrossRef]
- Grigs, O.; Didrihsone, E.; Bolmanis, E. Investigation of a Broad-Bean Based Low-Cost Medium Formulation for Bacillus Subtilis MSCL 897 Spore Production. Fermentation 2023, 9, 390. [Google Scholar] [CrossRef]
- Larroude, M.; Nicaud, J.M.; Rossignol, T. Yarrowia lipolytica Chassis Strains Engineered to Produce Aromatic Amino Acids via the Shikimate Pathway. Microb. Biotechnol. 2021, 14, 2420–2434. [Google Scholar] [CrossRef]
- Fickers, P.; Le Dall, M.T.; Gaillardin, C.; Thonart, P.; Nicaud, J.M. New Disruption Cassettes for Rapid Gene Disruption and Marker Rescue in the Yeast Yarrowia lipolytica. J. Microbiol. Methods 2003, 55, 727–737. [Google Scholar] [CrossRef]
- Larroude, M.; Park, Y.K.; Soudier, P.; Kubiak, M.; Nicaud, J.M.; Rossignol, T. A Modular Golden Gate Toolkit for Yarrowia lipolytica Synthetic Biology. Microb. Biotechnol. 2019, 12, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.C.; Beckerich, J.M.; Gaillardin, C. One-Step Transformation of the Dimorphic Yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 1997, 48, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Gajdoš, P.; Nicaud, J.M.; Rossignol, T.; Čertík, M. Single Cell Oil Production on Molasses by Yarrowia lipolytica Strains Overexpressing DGA2 in Multicopy. Appl. Microbiol. Biotechnol. 2015, 99, 8065–8074. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Hussain, M.S.; Gambill, L.; Blenner, M. Alternative Substrate Metabolism in Yarrowia lipolytica. Front. Microbiol. 2018, 9, 320134. [Google Scholar] [CrossRef]
- Lazar, Z.; Rossignol, T.; Verbeke, J.; Crutz-Le Coq, A.M.; Nicaud, J.M.; Robak, M. Optimized Invertase Expression and Secretion Cassette for Improving Yarrowia lipolytica Growth on Sucrose for Industrial Applications. J. Ind. Microbiol. Biotechnol. 2013, 40, 1273–1283. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B. Process Optimization of Biological Hydrogen Production from Molasses by a Newly Isolated Clostridium Butyricum W5. J. Biosci. Bioeng. 2009, 107, 138–144. [Google Scholar] [CrossRef]
- Al Sahyouni, W.; El Kantar, S.; Khelfa, A.; Park, Y.K.; Nicaud, J.M.; Louka, N.; Koubaa, M. Optimization of Cis-9-Heptadecenoic Acid Production from the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 245. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y.; Ke, C.; Bai, Y.; Liu, X.; Li, S. Production of Welan Gum from Cane Molasses by Sphingomonas Sp. FM01. Carbohydr. Polym. 2020, 244, 116485. [Google Scholar] [CrossRef]
- Eliodório, K.P.; de Gois e Cunha, G.C.; de Oliveira Lino, F.S.; Sommer, M.O.A.; Gombert, A.K.; Giudici, R.; Basso, T.O. Physiology of Saccharomyces Cerevisiae during Growth on Industrial Sugar Cane Molasses Can Be Reproduced in a Tailor-Made Defined Synthetic Medium. Sci. Rep. 2023, 13, 10567. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.; Ortucu, S.; Aydogan, M.N.; Arslan, N.P. Lipid Production from Sugar Beet Molasses under Non-Aseptic Culture Conditions Using the Oleaginous Yeast Rhodotorula Glutinis TR29. Renew. Energy 2016, 99, 198–204. [Google Scholar] [CrossRef]
- Olbrich, H. The Molasses. Princ. Sugar Technol. 1963, 3, 511–697. [Google Scholar]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Mouret, J.R. Combined Effects of Nutrients and Temperature on the Production of Fermentative Aromas by Saccharomyces Cerevisiae during Wine Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- de Jesús Rodríguez-Romero, J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-Phenylethylacetate Production by Nonconventional Yeasts Using Tequila Vinasses as a Substrate. Biotechnol. Rep. 2020, 25, e00420. [Google Scholar] [CrossRef]
- Wittmann, C.; Hans, M.; Bluemke, W. Metabolic Physiology of Aroma-Producing Kluyveromyces Marxianus. Yeast 2002, 19, 1351–1363. [Google Scholar] [CrossRef]
- Conde-Báez, L.; Castro-Rosas, J.; Villagómez-Ibarra, J.R.; Páez-Lerma, J.B.; Gómez-Aldapa, C. Evaluation of Waste of the Cheese Industry for the Production of Aroma of Roses (Phenylethyl Alcohol). Waste Biomass Valorization 2017, 8, 1343–1350. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; Jin, Y.; Liu, H.; Zhang, H.; Sun, Y.; Liu, G. Biosynthesis of 2-Phenylethanol Using Tobacco Waste as Feedstock. Biocatal. Biotransform. 2013, 31, 292–298. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Sell, D.; Schrader, J. Screening of Yeasts for the Production of the Aroma Compound 2-Phenylethanol in a Molasses-Based Medium. Biotechnol. Lett. 2003, 25, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, P. Recent Advances in Biotechnological Production of 2-Phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Arias, S.; Olivera, E.R.; Arcos, M.; Naharro, G.; Luengo, J.M. Genetic Analyses and Molecular Characterization of the Pathways Involved in the Conversion of 2-Phenylethylamine and 2-Phenylethanol into Phenylacetic Acid in Pseudomonas Putida U. Environ. Microbiol. 2008, 10, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ma, J.; Zhu, Y.; Xu, P. Refactoring Ehrlich Pathway for High-Yield 2-Phenylethanol Production in Yarrowia lipolytica. ACS Synth. Biol. 2020, 9, 623–633. [Google Scholar] [CrossRef]
- Celińska, E.; Ledesma-Amaro, R.; Larroude, M.; Rossignol, T.; Pauthenier, C.; Nicaud, J.M. Golden Gate Assembly System Dedicated to Complex Pathway Manipulation in Yarrowia lipolytica. Microb. Biotechnol. 2017, 10, 450–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).