Abstract
Four new fungi of the family Peniophoraceae, viz., Peniophora roseoalba, P. yunnanensis, Vararia daweishanensis, and V. fragilis are herein proposed, based on a combination of morphological features and molecular evidence. Peniophora roseoalba is characterized by resupinate, membranaceous basidiomata with a rose pink to pale pinkish grey hymenophore, a monomitic hyphal system with clamped generative hyphae, the presence of cystidia, and ellipsoid basidiospores. However, P. yunnanensis differs in being tuberculate, with a pale cream to cream hymenial surface, small lamprocystidia (18–29 × 4.5–7 µm), and subcylindrical basidiospores. Vararia daweishanensis is characterized by resupinate, membranous basidiomata with a pale yellowish hymenial surface, a dimitic hyphal system with clamped generative hyphae, strongly dextrinoid dichohyphae, and allantoid basidiospores; V. fragilis is characterized by resupinate, brittle basidiomata, with a buff to ochraceous hymenial surface and small ellipsoid basidiospores measuring 3.5–5.5 × 2.5–3.5 µm. Sequences of the ITS and nLSU rRNA markers of the studied samples were generated, and phylogenetic analyses were performed with the maximum likelihood, maximum parsimony, and Bayesian inference methods. The nLSU analysis revealed that the four new species can be clustered into the family Peniophoraceae (Russulales), in the genera Peniophora and Vararia. Further studies based on the ITS dataset showed that four fungi of the family Peniophoraceae were new to science.
1. Introduction
The family Peniophoraceae (Russulales) is a large and rather heterogeneous family with seven genera accepted; two genera, Peniophora Cooke and Vararia P. Karst., have the highest number of taxa in this family, in which they play fundamental ecological roles to drive carbon cycling in forest soils, acting as decomposers [1,2].
Peniophora, typified by P. quercina (Pers.) Cooke, is characterized by resupinate, membranaceous to ceraceous basidiomata, with smooth to tuberculate hymenophores having a grey, violaceous, orange, red, or brown hymenial surface, a monomitic hyphal system with clamped generative hyphae; dendrohyphidia, lamprocystidia, and gloeocystidia are present or absent; the basidiospores are ellipsoid, cylindrical to allantoid, smooth, thin-walled, acyanophilous, and without reaction with Melzer [3]. Based on the MycoBank database (http://www.MycoBank.org, accessed on 13 October 2022) and the Index Fungorum (http://www.indexfungorum.org, accessed on 13 October 2022), the genus Peniophora has 637 specific and registered names, but the actual number of species has reached 191 [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Vararia is typified by V. investiens (Schwein.) P. Karst. This genus is characterized by resupinate basidiomata, a dimitic hyphal system with clamped or simple-septate generative hyphae and often, dextrinoid dichohyphae, the presence of gloeocystidia, and variously shaped smooth basidiospores with or without an amyloid reaction [19,20,21]. The MycoBank database (http://www.MycoBank.org, accessed on 13 October 2022) and Index Fungorum (http://www.indexfungorum.org, accessed on 13 October 2022) have registered 96 specific and infraspecific names in Vararia, but the actual number of the species has reached 72, and they occur mainly in the tropical and subtropical areas of the world [22,23,24,25,26,27,28,29,30,31]. However, Vararia is still poorly studied in China [32], from whence eight species, namely, V. amphithallica Boidin, Lanq. & Gilles, V. bispora S.L. Liu & S.H. He, V. breviphysa Boidin & Lanq., V. cinnamomea Boidin, Lanq. & Gilles, V. investiens (Schwein.) P. Karst., V. montana S.L. Liu & S.H. He, V. racemosa (Burt.) D.P. Rogers & H.S. Jacks., and V. sphaericospora Gilb., have been reported in this country [32,33,34].
These pioneering research studies into the Peniophoraceae family were just the prelude to the molecular systematics period. The phylogenetic diversity displayed by corticioid fungal species, based on 5.8S and 28S nuclear rDNA, revealed that the taxa of Peniophoraceae are nested in the russuloid clade, which holds a considerable share of the phylogenetic framework, and include the genera of Peniophora and Vararia [35]. The phylogenetic research about the major clades of mushroom-forming fungi (Homobasidiomycetes) indicated that the largest resupinate forms divided into the polyporoid, russuloid, and hymenochaetoid clades, in which Peniophora grouped with Asterostroma Massee and Scytinostroma Donk [36]. Molecular phylogenetic analyses of nrITS and nrLSU sequences revealed affinities among families with the Peniophorales in the Russulales, in which the presence of distinctive hyphal elements, which are homologous to the defining features of Peniophorales, was consistent with the phylogenetic evidence, and the Varariaceae were grouped closely with the Peniophoraceae [37].
During the investigations into wood-inhabiting fungi in Yunnan Province, China, four new taxa of Peniophoraceae were found that could not be assigned to any described species. Herein, we present the morphological and molecular phylogenetic evidence that supports the recognition of these four new species within the Peniophora and Vararia, based on the internal transcribed spacer (ITS) regions and the large subunit nuclear ribosomal RNA gene (nLSU) sequences.
2. Materials and Methods
2.1. Morphology
Fresh fruiting bodies of the fungi were collected from Chuxiong, Honghe, Puer, and Wenshan of Yunnan Province, in China. The specimens were dried in an electric food dehydrator at 40 °C, then sealed and stored in an envelope bag and deposited in the herbarium of the Southwest Forestry University (SWFC), Kunming, Yunnan Province, China. The macromorphological descriptions are based on field notes and photos captured in the field and laboratory. The color terminology follows the example set by Petersen [38,39,40]. Micromorphological data were obtained from the dried specimens when observed under a light microscope, following the method used by Dai [41]. The following abbreviations were used: KOH = 5% potassium hydroxide water solution, CB = Cotton Blue, CB− = acyanophilous, CB+ = cyanophilous, IKI = Melzer’s reagent, IKI− = both inamyloid and indextrinoid, L = mean spore length (arithmetic average for all spores), W = mean spore width (arithmetic average for all spores), Q = variation in the L/W ratios between the specimens studied, and n = a/b (number of spores (a) measured from a given number (b) of specimens).
2.2. Molecular Phylogeny
The CTAB rapid plant genome extraction kit-DN14 (Aidlab Biotechnologies Co., Ltd., Beijing, China) was used to obtain the genomic DNA from the dried specimens using the manufacturer’s instructions, following a previous study [42]. The nuclear ribosomal ITS region was amplified with the primers ITS5 and ITS4 [43]. The nuclear nLSU region was amplified with the primer pair, LR0R and LR7 (http://lutzonilab.org/nuclear-ribosomal-dna/; accessed on 13 October 2022). The PCR procedure for ITS was as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles at 94 °C for 40 s, 58 °C for 45 s, and 72 °C for 1 min, with a final extension of 72 °C for 10 min. The PCR procedure for nLSU was as follows: initial denaturation at 94 °C for 1 min, followed by 35 cycles at 94 °C for 30 s, 48 °C for 1 min, and 72 °C for 1.5 min, with a final extension of 72 °C for 10 min. The PCR products were purified and sequenced at Kunming Tsingke Biological Technology Company, Limited (Yunnan Province, China). All of the newly generated sequences were deposited in the NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/; accessed on 13 October 2022) (Table 1).
The sequencer, 4.6 (GeneCodes, Ann Arbor, MI, USA), was used to assemble and edit the generated sequence reads. The sequences were aligned in MAFFT 7 (https://mafft.cbrc.jp/alignment/server/; accessed on 13 October 2022), using the “G-INS-i” strategy for the ITS and nLSU dataset, manually adjusted in BioEdit [44]. The sequences of Sistotrema brinkmannii (Bres.) J. Erikss. and S. coronilla (Höhn.) Donk ex. D.P. Rogers, obtained from GenBank, were selected as an outgroup for the phylogenetic analysis of the nLSU phylogenetic tree (Figure 1) [45]; the sequences of Dichostereum durum (Bourdot & Galzin) Pilát and D. effuscatum (Cooke & Ellis) Boidin & Lanq. were selected as an outgroup for phylogenetic analysis of ITS phylogenetic tree (Figure 2) [45]; the sequences of P. incarnata (Pers.) P. Karst. and P. nuda (Fr.) Bres. were selected as an outgroup in the ITS analysis (Figure 3), following the method of a previous study [38].
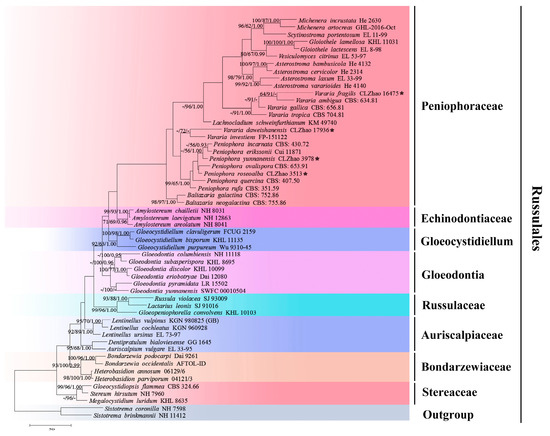
Figure 1.
A maximum parsimony strict consensus tree, illustrating the phylogeny of four new species and related genera in the order Russulales, based on nLSU sequences. The branches are labeled with maximum likelihood bootstrap values of >70%, parsimony bootstrap values of >50%, and Bayesian posterior probabilities of >0.95, respectively. The new species are marked with asterisks.
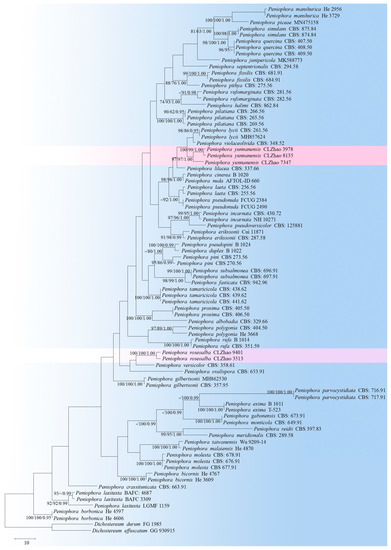
Figure 2.
A maximum parsimony strict consensus tree, illustrating the phylogeny of two new species and the related species in the genus Peniophora, based on the ITS sequences. The branches are labeled with maximum likelihood bootstrap values higher than 70%, parsimony bootstrap proportions that are higher than 50%, and Bayesian posterior probabilities of more than 0.95, respectively.
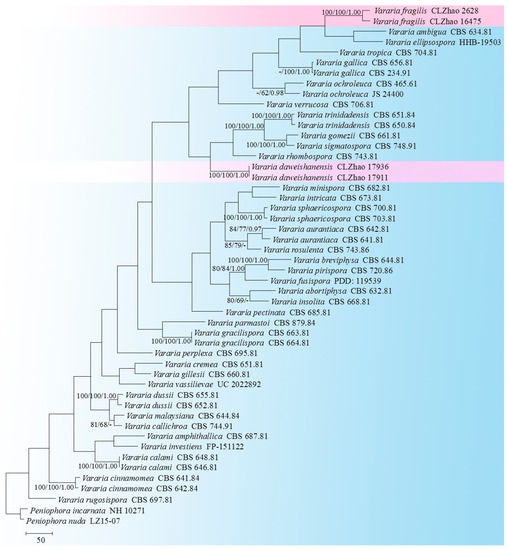
Figure 3.
A maximum parsimony strict consensus tree illustrating the phylogeny of two new species and related species in the genus Vararia, based on ITS sequences. The branches are labeled with a maximum likelihood bootstrap value of >70%, a parsimony bootstrap value of >50%, and Bayesian posterior probabilities of >0.95, respectively.
Maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI) analyses were applied to the three combined datasets, following the technique used in a previous study [42], and the tree construction procedure was performed in PAUP*, version 4.0b10 [46]. All the characters were equally weighted, and gaps were treated as missing data. Trees were inferred using the heuristic search option, with TBR branch swapping and 1000 random sequence additions. The max trees were set to 5000, branches of zero length were collapsed, and all parsimonious trees were saved. Clade robustness was assessed using a bootstrap (BT) analysis with 1000 replicates [47]. The descriptive tree statistics were tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI); these were calculated for each maximum parsimonious tree generated. The multiple sequence alignment was also analyzed using the maximum likelihood (ML) in RAxML-HPC2, through the Cipres Science Gateway (www.phylo.org; accessed on 13 October 2022) [48]. Branch support (BS) for the ML analysis was determined by 1000 bootstrap replicates.

Table 1.
List of species, specimens, and GenBank accession numbers of the sequences used in this study.
Table 1.
List of species, specimens, and GenBank accession numbers of the sequences used in this study.
| Species Name | Specimen No. | GenBank Accession No. | References | Country | |
|---|---|---|---|---|---|
| ITS | nLSU | ||||
| Amylostereum areolatum | NH 8041 | AF506405 | AF506405 | [45] | Sweden |
| A. chailletii | NH 8031 | AF506406 | AF506406 | [45] | Sweden |
| A. laevigatum | NH 12863 | AF506407 | AF506407 | [45] | Sweden |
| Asterostroma bambusicola | He 4132 | KY263871 | this publication | Thailand | |
| A. cervicolor | He 2314 | KY263869 | this publication | China | |
| A. laxum | EL 33-99 | AF506410 | AF506410 | [45] | Sweden |
| A. vararioides | He 4140 | KY263870 | this publication | Thailand | |
| Auriscalpium vulgare | EL 33-95 | AF506375 | AF506375 | [45] | Sweden |
| Baltazaria galactina | CBS: 752.86 | MH873721 | [49] | France | |
| B. neogalactina | CBS: 755.86 | MH873724 | MH873724 | [49] | French |
| B. occidentalis | AFTOL-ID | DQ234539 | [50] | Canada | |
| B. podocarpi | Dai 9261 | KJ583221 | [51] | China | |
| Dentipratulum bialoviesense | GG 1645 | AF506389 | AF506389 | [45] | Sweden |
| Dichostereum durum | FG 1985 | AF506429 | AF506429 | [45] | Sweden |
| D. effuscatum | GG 930915 | AF506390 | AF506390 | [45] | Sweden |
| Gloeocystidiellum bisporum | KHL 11135 | AY048877 | AY048877 | [45] | Sweden |
| G. clavuligerum | FCUG 2159 | AF310088 | AF310088 | [52] | Spain |
| G. purpureum | Wu 9310-45 | AF441338 | AF441338 | [45] | China |
| Gloeocystidiopsis flammea | CBS: 324.66 | AF506437 | AF506437 | [45] | C. African Rep. |
| Gloeodontia columbiensis | NH 11118 | AF506444 | AF506444 | [45] | Spain |
| G. discolor | KHL 10099 | AF506445 | AF506445 | [45] | USA |
| G. eriobotryae | Dai 12080 | JQ349103 | [53] | China | |
| G. pyramidata | LR 15502 | AF506446 | AF506446 | [45] | Colombia |
| G. subasperispora | KHL 8695 | AF506404 | AF506404 | [45] | Norway |
| G. yunnanensis | SWFC 00010504 | MN908254 | [54] | China | |
| Gloeopeniophorella convolvens | KHL 10103 | AF506435 | AF506435 | [45] | USA |
| Gloiothele lactescens | EL 8-98 | AF506453 | AF506453 | [45] | Sweden |
| G. lamellosa | KHL 11031 | AF506454 | AF506454 | [45] | Venezuela |
| Heterobasidion annosum | 06129/6 | KJ583225 | [51] | Russia | |
| H. parviporum | 04121/3 | KJ583226 | [51] | Finland | |
| Lachnocladium schweinfurthianum | KM 49740 | MH260051 | [38] | Cameroon | |
| Lactarius leonis | SJ 91016 | AF506411 | AF506411 | [45] | Sweden |
| Lentinellus cochleatus | KGN 960928 | AF506417 | AF506417 | [45] | Sweden |
| L. ursinus | EL 73-97 | AF506419 | AF506419 | [45] | USA |
| L. vulpinus | KGN 980825 (GB) | AF347097 | AF347097 | [45] | Sweden |
| Megalocystidium luridum | KHL 8635 | AF506422 | AF506422 | [45] | Norway |
| Michenera artocreas | GHL-2016-Oct | MH204692 | [55] | USA | |
| M. incrustata | He 2630 | MH142907 | [55] | China | |
| Peniophora albobadia | CBS: 329.66 | MH858809 | MH858809 | [49] | France |
| P. bicornis | He 3609 | MK588763 | MK588763 | [39] | China |
| P. bicornis | He 4767 | MK588764 | MK588764 | [39] | China |
| P. borbonica | He 4597 | MK588766 | MK588766 | [39] | China |
| P. borbonica | He 4606 | MK588765 | MK588765 | [39] | China |
| P. cinerea | B 1020 | MN475151 | MN475151 | [39] | USA |
| P. crassitunicata | CBS: 663.91 | MH862292 | MH862292 | [49] | France |
| P. duplex | B 1022 | MN475153 | MN475153 | [39] | USA |
| P. erikssonii | CBS: 287.58 | MH857788 | MH857788 | [39] | France |
| P.erikssonii | Cui 11871 | MK588771 | MK588811 | [39] | China |
| P. exima | B 1011 | MN475155 | MN475155 | [39] | USA |
| P. exima | T-523 | MK588772 | MK588772 | [39] | USA |
| P. fasticata | CBS: 942.96 | MH862624 | MH862624 | [39] | Ethiopia |
| P. fissilis | CBS: 681.91 | MH862298 | MH862298 | [39] | France |
| P. fissilis | CBS: 684.91 | MH862299 | MH862299 | [39] | Netherlands |
| P. gabonensis | CBS: 673.91 | MH862293 | MH862293 | [39] | Gabon |
| P. gilbertsonii | CBS: 357.95 | MH862528 | MH862528 | [39] | USA |
| P. gilbertsonii | CBS: 360.95 | MH862530 | MH862530 | [39] | USA |
| P. halimi | CBS: 862.84 | MH861843 | MH861843 | [39] | France |
| P. incarnata | NH 10271 | AF506425 | AF506425 | [45] | Denmark |
| P. incarnata | CBS: 430.72 | MH860518 | MH872230 | [39] | Netherlands |
| P. junipericola | He 2462 | MK588773 | MK588773 | this publication | China |
| P. laeta | CBS: 256.56 | MH857617 | MH857617 | [39] | France |
| P. laeta | CBS: 255.56 | MH857616 | MH857616 | [39] | France |
| P. laxitexta | LGMF 1159 | JX559580 | [39] | Brazil | |
| P. laxitexta | BAFC 3309 | FJ882040 | [39] | Argentina | |
| P. laxitexta | BAFC: 4687 | MN518328 | [39] | Argentina | |
| P. lilacea | CBS: 337.66 | MH858813 | MH858813 | [39] | Armenia |
| P. lycii | CBS: 264.56 | MH857624 | MH857624 | [39] | France |
| P. lycii | CBS: 261.56 | MH857621 | MH857621 | [39] | France |
| P. malaiensis | He 4870 | MK588775 | MK588775 | [39] | China |
| P. manshurica | He 2956 | MK588776 | MK588776 | [39] | China |
| P. manshurica | He 3729 | MK588777 | MK588777 | [39] | China |
| P. meridionalis | CBS: 289.58 | MH857789 | MH857789 | [49] | France |
| P. molesta | CBS: 678.91 | MH862296 | MH862296 | [39] | Cote d’Ivoire |
| P. molesta | CBS: 676.91 | MH862294 | MH862294 | [39] | Gabon |
| P. molesta | CBS: 677.91 | MH862295 | MH862295 | [39] | Gabon |
| P. monticola | CBS: 649.91 | MH862289 | MH862289 | [39] | France |
| P. nuda | AFTOL-ID 660 | DQ411533 | [39] | USA | |
| P. nuda | LZ15-07 | MT859929 | MT859929 | this publication | China |
| P. ovalispora | CBS: 653.91 | MH873971 | [39] | Netherlands | |
| P. ovalispora | CBS: 653.91 | MH862290 | MH862290 | [39] | Netherlands |
| P. parvocystidiata | CBS: 716.91 | MH862305 | MH862305 | [39] | France |
| P. parvocystidiata | CBS: 717.91 | MH862306 | MH862306 | [39] | France |
| P. piceae | B 1010 | MN475158 | MN475158 | this publication | USA |
| P. pilatiana | CBS: 269.56 | MH857627 | MH857627 | [39] | France |
| P. pilatiana | CBS: 265.56 | MH857625 | MH857625 | [39] | France |
| P. pilatiana | CBS: 266.56 | MH857626 | MH857626 | [39] | France |
| P. pini | CBS: 273.56 | MH857631 | MH857631 | [39] | France |
| P. pini | CBS: 270.56 | MH857628 | MH857628 | [39] | France |
| P. pithya | CBS: 275.56 | MH857633 | MH857633 | [49] | France |
| P. polygonia | He 3668 | MH669233 | [56] | China | |
| P. polygonia | CBS: 404.50 | MH856684 | MH856684 | [39] | France |
| P. proxima | CBS: 406.50 | MH856686 | MH856686 | [39] | France |
| P. proxima | CBS: 405.50 | MH856685 | MH856685 | [39] | France |
| P. pseudonuda | FCUG 2384 | GU322866 | this publication | Sweden | |
| P. pseudonuda | FCUG 2390 | GU322865 | this publication | Sweden | |
| P. pseudopini | B 1024 | MN475163 | MN475163 | this publication | USA |
| P. pseudoversicolor | CBS: 125881 | MH864303 | MH864303 | [39] | France |
| P. quercina | CBS: 407.50 | MH856687 | MH868204 | [39] | France |
| P. quercina | CBS: 408.50 | MH856688 | MH856688 | [39] | France |
| P. quercina | CBS: 409.50 | MH856689 | MH856689 | [39] | France |
| P. reidii | CBS: 397.83 | MH861616 | MH861616 | [39] | France |
| P. rosealba | CLZhao 3513 | ON786559 | OP380690 | present study | China |
| P. rosealba | CLZhao 9401 * | ON786560 | present study | China | |
| P. rufa | B 1014 | MN475165 | MN475165 | this publication | USA |
| P. rufa | CBS: 351.59 | MH857891 | MH869432 | [39] | Canada |
| P. rufomarginata | CBS: 281.56 | MH857639 | MH857639 | [39] | France |
| P. rufomarginata | CBS: 282.56 | MH857640 | MH857640 | [39] | France |
| P. septentrionalis | CBS: 294.58 | MH857791 | MH857791 | [39] | Canada |
| P. simulans | CBS: 875.84 | MH861850 | MH861850 | [39] | France |
| P. simulans | CBS: 874.84 | MH861849 | MH861849 | [39] | France |
| P. subsalmonea | CBS: 697.91 | MH862303 | MH862303 | [39] | Netherlands |
| P. subsalmonea | CBS: 696.91 | MH862302 | MH862302 | [39] | Netherlands |
| P. taiwanensis | Wu 9209-14 | MK588794 | MK588794 | [39] | China |
| P. tamaricicola | CBS: 438.62 | MH858203 | MH858203 | [39] | Morocco |
| P. tamaricicola | CBS: 439.62 | MH858204 | MH858204 | [39] | Morocco |
| P. tamaricicola | CBS: 441.62 | MH858205 | MH858205 | [39] | Morocco |
| P. versicolor | CBS: 358.61 | MH858082 | MH858082 | [39] | Morocco |
| P. violaceolivida | CBS: 348.52 | MH857077 | MH857077 | [39] | France |
| P. yunnanensis | CLZhao 3978 | OP380617 | OP380689 | present study | China |
| P. yunnanensis | CLZhao 7347 * | OP380616 | present study | China | |
| P. yunnanensis | CLZhao 8135 | OP380615 | present study | China | |
| Russula violacea | SJ 93009 | AF506465 | AF506465 | [45] | Sweden |
| Scytinostroma portentosum | EL 11-99 | AF506470 | AF506470 | [45] | Sweden |
| Sistotrema brinkmannii | NH 11412 | AF506473 | AF506473 | [45] | Turkey |
| S. coronilla | NH 7598 | AF506475 | AF506475 | [45] | Canada |
| Stereum hirsutum | NH 7960 | AF506479 | AF506479 | [45] | Romania |
| Vararia abortiphysa | CBS: 632.81 | MH861387 | MH861387 | [49] | Gabon |
| V. ambigua | CBS: 634.81 | MH861388 | MH873137 | [49] | France |
| V. amphithallica | CBS: 687.81 | MH861431 | MH861431 | [49] | France |
| V. aurantiaca | CBS: 642.81 | MH861394 | MH861394 | [49] | Gabon |
| V. aurantiaca | CBS: 641.81 | MH861393 | MH861393 | [49] | France |
| V. breviphysa | CBS: 644.81 | MH861396 | MH861396 | [49] | Gabon |
| V. calami | CBS: 646.81 | MH861398 | MH861398 | [49] | France |
| V. calami | CBS: 648.81 | MH861399 | MH861399 | [49] | France |
| V. callichroa | CBS: 744.91 | MH874000 | MH874000 | [49] | France |
| V. cinnamomea | CBS: 642.84 | MH873488 | MH873488 | [49] | Madagascar |
| V. cinnamomea | CBS: 641.84 | MH861794 | MH861794 | [49] | Madagascar |
| V. cremea | CBS: 651.81 | MH873147 | MH873147 | [49] | France |
| V. daweishanensis | CLZhao 17911 | OP380613 | OP615103 | present study | China |
| V. daweishanensis | CLZhao 17936 * | OP380614 | OP380688 | present study | China |
| V. dussii | CBS: 655.81 | MH861405 | MH861405 | [49] | France |
| V. dussii | CBS: 652.81 | MH873148 | MH873148 | [49] | France |
| V. ellipsospora | HHB-19503 | MW740328 | MW740328 | this publication | New Zealand |
| V. fragilis | CLZhao 2628 | OP380611 | present study | China | |
| V. fragilis | CLZhao 16475 * | OP380612 | OP380687 | present study | China |
| V. fusispora | PDD: 119539 | OL709443 | OL709443 | this publication | New Zealand |
| V. gallica | CBS: 234.91 | MH862250 | [49] | Canada | |
| V. gallica | CBS: 656.81 | MH861406 | MH873152 | [49] | France |
| V. gillesii | CBS: 660.81 | MH873153 | MH873153 | [49] | Cote d’Ivoire |
| V. gomezii | CBS: 661.81 | MH873154 | MH873154 | [49] | French |
| V. gracilispora | CBS: 664.81 | MH861412 | MH861412 | [49] | Gabon |
| V. gracilispora | CBS: 663.81 | MH861411 | [49] | Gabon | |
| V. insolita | CBS: 668.81 | MH861413 | MH861413 | [49] | France |
| V. intricata | CBS: 673.81 | MH861418 | MH861418 | [49] | France |
| V. investiens | FP-151122 | MH971976 | MH971977 | [56] | USA |
| V. malaysiana | CBS: 644.84 | MH873490 | MH873490 | [49] | Singapore |
| V. minispora | CBS: 682.81 | MH861426 | MH861426 | [49] | France |
| V. ochroleuca | CBS: 465.61 | MH858109 | MH858109 | [49] | France |
| V. ochroleuca | JS 24400 | AF506485 | AF506485 | [45] | Norway |
| V. parmastoi | CBS: 879.84 | MH861852 | MH861852 | [49] | Uzbekistan |
| V. perplexa | CBS: 695.81 | MH861438 | MH861438 | [49] | France |
| V. pectinata | CBS: 685.81 | MH861429 | [49] | Cote d’Ivoire | |
| V. pirispora | CBS: 720.86 | MH862016 | MH862016 | [49] | France |
| V. rhombospora | CBS: 743.81 | MH861470 | MH861470 | [49] | France |
| V. rosulenta | CBS: 743.86 | MH862028 | [49] | France | |
| V. rugosispora | CBS: 697.81 | MH861440 | MH861440 | [49] | Gabon |
| V. sigmatospora | CBS: 748.91 | MH874001 | MH874001 | [49] | Netherlands |
| V. sphaericospora | CBS: 700.81 | MH873185 | MH873185 | [49] | Gabon |
| V. sphaericospora | CBS: 703.81 | MH861446 | MH861446 | [49] | Gabon |
| V. trinidadensis | CBS: 651.84 | MH861803 | MH861803 | [49] | Madagascar |
| V. trinidadensis | CBS: 650.84 | MH873495 | MH873495 | [49] | Madagascar |
| V. tropica | CBS: 704.81 | MH861447 | MH873189 | [49] | France |
| V. vassilievae | UC2022892 | KP814203 | KP814203 | this publication | USA |
| V. verrucosa | CBS 706.81 | MH861449 | MH861449 | [49] | France |
| Vesiculomyces citrinus | EL 53-97 | AF506486 | AF506486 | [45] | Sweden |
* indicates the holotype.
MrModeltest 2.3 [57] was used to determine the best-fit evolution model for each dataset, using Bayesian inference (BI), which was performed using MrBayes 3.2.7a, with a GTR+I+G model of the DNA substitution and a gamma distribution rate variation across the sites [58]. Four Markov chains were run for 2 runs, beginning from random starting trees for 0.9 million generations for nLSU (Figure 1), for 1.5 million generations for ITS (Figure 2) with trees, and 1 million generations for ITS (Figure 3) with trees, with the parameters sampled every 1000 generations. The first one-quarter of all generations were discarded as the burn-in. The majority rule consensus tree of all the remaining trees was calculated. Branches were considered significantly supported if they received a maximum likelihood bootstrap value (BS) > 70%, a maximum parsimony bootstrap value (BT) > 70%, or Bayesian posterior probabilities (BPP) > 0.95.
3. Results
3.1. Molecular Phylogeny
The nLSU dataset (Figure 1) included sequences from 55 fungal specimens, representing 55 species. The dataset had an aligned length of 1415 characters, of which 923 characters are constant, 152 are variable and parsimony-uninformative, and 340 are parsimony-informative. The maximum parsimony analysis yielded one equally parsimonious tree (TL = 860, CI = 0.3233, HI = 0.6767, RI = 0.6123, RC = 0.1979). The best model for the ITS+nLSU dataset, which was estimated and applied in the Bayesian analysis, was GTR+I+G (lset nst = 6, rates = invgamma; prset statefreqpr = dirichlet (1,1,1,1). The Bayesian analysis and ML analysis resulted in a similar topology to the MP analysis, with an average standard deviation of split frequencies = 0.009575 (BI); the effective sample size (ESS) across the two runs is the double of the average ESS (avg ESS) = 200.5. The phylogeny (Figure 1), based on the combined nLSU sequences, includes six families within the order of Russulales, which indicated that nine genera, comprising Asterostroma Massee, Baltazaria Leal-Dutra, Dentinger & G.W. Griff., Gloiothele Bres., Lachnocladium Lév., Michenera Berk. & M.A. Curtis, Peniophora, Scytinostroma Donk, Vararia, and Vesiculomyces E. Hagstr. could be incorporated into the Peniophoraceae family. Our current four new species can be clustered into the genera of Peniophora and Vararia, respectively.
The ITS-alone dataset of the genus Peniophora (Figure 2) included the sequences from 83 fungal specimens, representing 52 species. The dataset had an aligned length of 607 characters, of which 353 characters were constant, while 64 were variable and parsimony-uninformative, and 190 were parsimony-informative. The maximum parsimony analysis yielded 12 equally parsimonious trees (TL = 1681, CI = 0.3111, HI = 0.6889, RI = 0.4496, RC = 0.1399). The best model for the ITS dataset that was estimated and applied in the Bayesian analysis was GTR+I+G (lset nst = 6, rates = invgamma; prset statefreqpr = dirichlet (1,1,1,1). Bayesian analysis and ML analysis resulted in a similar topology to MP analysis, with an average standard deviation of split frequencies = 0.009599 (BI). The phylogenetic tree indicated that P. roseoalba can be grouped with two close taxa, P. versicolor and P. ovalispora, whereas P. yunnanensis can be grouped with a clade comprising P. lycii and P. violaceolivida.
The ITS-only dataset of the genus Vararia (Figure 3) included sequences from 63 fungal specimens, representing 39 species. The dataset had an aligned length of 1128 characters, of which 511 characters were constant, 133 were variable and parsimony uninformative, and 484 were parsimony informative. Maximum parsimony analysis yielded 6 equally parsimonious trees (TL = 4589, CI = 0.2805, HI = 0.7195, RI = 0.4174, and RC = 0.1171). The best model for the ITS dataset estimated and applied in the Bayesian analysis was GTR+I+G. The Bayesian and ML analyses resulted in a similar topology to that of the MP analysis with split frequencies = 0.0096082 (BI). The phylogram inferred from the ITS sequences (Figure 3) revealed that Vararia daweishanensis could be grouped with four close taxa: V. gomezii, V. rhombospora, V. sigmatospora, and V. trinidadensis, whereas the other species of V. fragilis could be grouped with a clade comprising V. ambigua and V. ellipsospora, with a low level of support.
3.2. Taxonomy

Figure 4.
Basidiomata of Peniophora roseoalba (holotype): the front of the basidiome (A); the characteristic hymenophore (B). Bars: (A) = 1 cm and (B) = 1 mm.
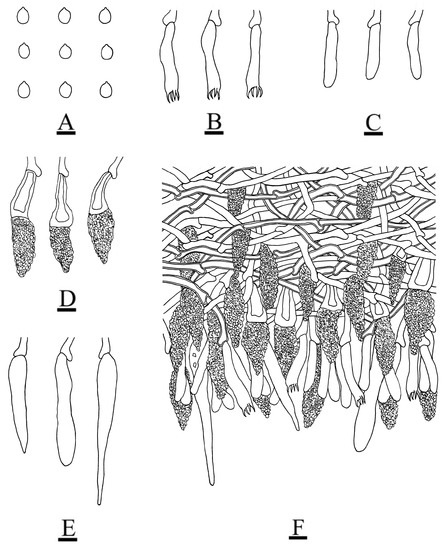
Figure 5.
Microscopic structures of the Peniophora roseoalba (holotype): basidiospores (A); basidia (B); basidioles (C); lamprocystidia (D); subcylindrical to conical gloeocystidia (E); a section of the hymenium (F). Bars: (A–F) = 10 µm.
MycoBank no.: 845758.
Holotype—China, Yunnan Province, Puer, Jingdong county, the Forest of Pineapple, 24°37′ N, 100°45′ E, altitude 2083 m asl., on the fallen branch of an angiosperm, 4 January 2019, CLZhao 9401 (SWFC).
Etymology—Roseoalba (Lat.): referring to the rose to pale pinkish grey color of the hymenial surface of the specimens.
Fruiting body—Basidiomata are annual, resupinate, membranaceous, without odor and taste when fresh, up to 90 mm long, 20 mm wide, 70–100 µm thick. The hymenial surface is smooth, occasionally cracked, and rose to pale pinkish grey. The sterile margin is indistinct and is rose to pinkish grey.
Hyphal system—Monomitic, generative hyphae with clamp connections, colorless, thin- to thick-walled, moderately branched, 1.5–4.5 µm in diameter, CB−, IKI−; tissues unchanged in KOH.
Hymenium—The cystidia are of two types: (1) Gloeocystidia is subcylindrical to conical, smooth, colorless, thin-walled, 31.5–40.5 × 6.5–7.5 µm; (2) Lamprocystidia is abundant in the hymenium, and is conical, thick-walled, encrusted apical part, colorless, 33–42.5 × 7–10.5 µm. The Basidia are subclavate to subcylindrical, slightly constricted in the middle, with four sterigmata and a basal clamp connection, sized 24–39.5 × 3.5–5.5 µm.
Basidiospores—Basidiospores are ellipsoid, colorless, thin-walled, smooth, IKI−, CB−, 4–6.5 × 3–5 µm, L = 5.19 µm, W = 3.8 µm, Q = 1.26–1.48 (n = 60/2).
Additional specimen examined—China, Yunnan Province, Puer, Jingdong County, Wuliangshan National Nature Reserve, 23°57′ N, 100°22′ E, altitude 3376 m asl., found on the fallen branch of an angiosperm, 2 October 2017, CLZhao 3513 (SWFC).
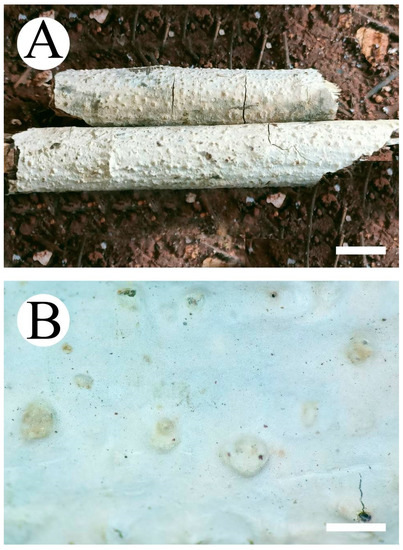
Figure 6.
Basidiomata of Peniophora yunnanensis (holotype): the front of the basidiomata (A); the characteristic hymenophore (B). Bars: (A) = 1 cm and (B) = 1 mm.
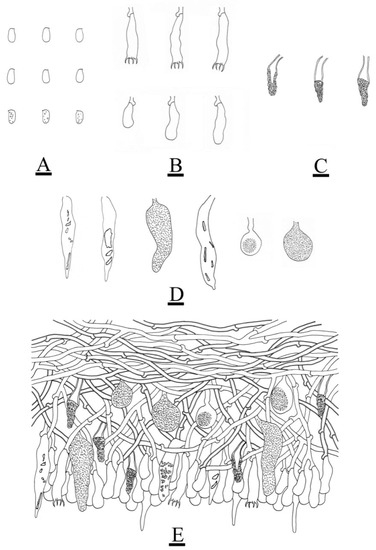
Figure 7.
Microscopic structures of Peniophora yunnanensis (holotype): basidiospores (A); basidia and basidioles (B); lamprocystidia (C); the conical, clavate to fusiform, subglobose gloeocystidia (D); a section of the hymenium (E). Bars: (A–E) = 10 µm.
MycoBank no.: 845760.
Holotype—China, Yunnan Province, Chuxiong, Zixishan Forestry Park, 25°01′ N, 101°24′ E., altitude 2356 m asl., on an angiosperm stump, 2 July 2018, code: CLZhao 7347 (SWFC).
Etymology—Yunnanensis (Lat.): referring to the geographic provenance (Yunnan Province) of the specimens.
Fruiting body—Basidiomata are annual, resupinate, and coriaceous, without odor and taste when fresh, up to 100 mm long, 25 mm wide, and 70–100 µm thick. The hymenial surface is tuberculate and is pale cream to cream. The sterile margin is indistinct and slightly cream-colored.
Hyphal system—Monomitic, generative hyphae with clamp connections, colorless, thin- to thick-walled, moderately branched, 2.5–3.5 µm in diameter, IKI−, CB−; tissues are unchanged in the KOH; the subiculum generative hyphae are dense, with a subparallel arrangement; the subhymenium is composed of strongly agglutinated vertical hyphae.
Hymenium—The cystidia are of two types: (1) Gloeocystidia, which are different in shape, conical, clavate to fusiform, and subglobose, usually containing refractive materials; they are colorless, smooth, thin-walled, and 12.5–58 × 5.5–15.5 µm; (2) Lamprocystidia are abundant in the hymenium, the conical, thick-walled, encrusted apical part, colorless, and 18–29 × 4.5–7 µm. The basidia subclavate changes to subcylindrical, being slightly constricted in the middle to somewhat constricted, with four sterigmata and a basal clamp connection, 22.5–39.5 × 4.5–8 µm.
Basidiospores—The basidiospores are subcylindrical, colorless, thin-walled, and smooth, with oil drops occasionally found inside, IKI−, CB−, (5–) 5.5–10 (–11) × 3–5.5 µm, L = 7.72 µm, W = 4.44 µm, Q = 1.61–1.88 (n = 90/3).
Additional specimens examined (paratypes)—China, Yunnan Province, Puer, Jingdong County, Taizhong Town, Ailaoshan, 24°23′ N, 120°53′ E, altitude 3166 m asl.; found on the fallen branch of an angiosperm, 4 October 2017, CLZhao 3978 (SWFC). Zhenyuan County, Ailaoshan, Jinshan Original Forestry, 24°00′ N, 101°10′ E; altitude 2300 m asl., and found on the fallen branch of an angiosperm, 21 August 2018, CLZhao 8135 (SWFC).
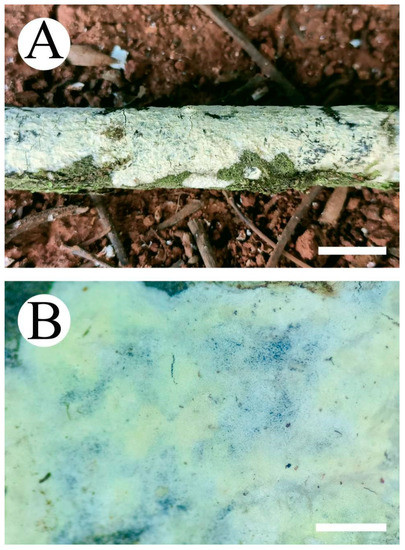
Figure 8.
Basidiomata of the Vararia daweishanensis (holotype): the front of the basidiomata (A); the characteristic hymenophore (B). Bars: (A) = 1 cm and (B) = 1 mm.
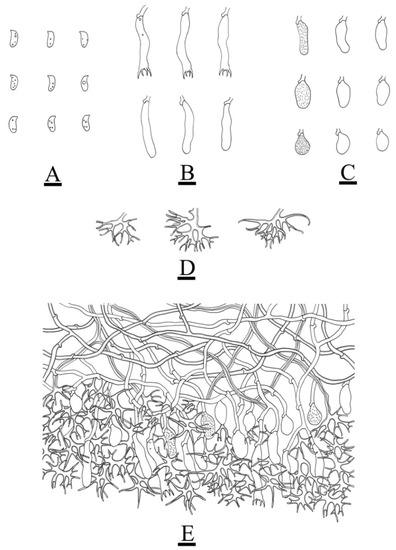
Figure 9.
Microscopic structures of Vararia daweishanensis (holotype): basidiospores (A); basidia and basidioles (B); subcylindrical, elliptical to ovoid gloeocystidia (C); dichohyphae (D); a section of the hymenium (E). Bars: (A–E) = 10 µm.
MycoBank no.: 845761.
Holotype—China, Yunnan Province, Honghe, Pinbian County, Daweishan National Forestry Park, 22°53′ N, 103°35′ E, altitude 1670 m asl., found on a fallen angiosperm branch, 1 August 2019, CLZhao 17936 (SWFC).
Etymology—daweishanensis (Lat.): referring to the provenance (Daweishan) of the specimens.
Fruiting body—Basidiomata are annual, resupinate, membranous, soft, and adnate, up to 80 mm long, 16 mm wide, and 70–150 µm thick. The hymenial surface is smooth and pale yellowish. The sterile margin is distinct, narrow, whitish, and attached.
Hyphal system—Dimitic, generative hyphae with clamp connections, colorless, thin- to thick-walled, occasionally branched, interwoven, 2–4 µm in diameter, IKI−, CB+, tissues are unchanged in KOH; dichohyphae in subhymenium abundant, yellowish, capillary, distinctly thick-walled; dichotomously to irregularly branched, with the main branches up to 4 μm in diameter and with acute tips, moderately dextrinoid when in Melzer’s reagent; more frequently branched with more narrow and shorter branches in the hymenium, with slightly curved tips and a stronger dextrinoid reaction.
Hymenium—The gloeocystidia are empty or are filled with a refractive oil-like matter; they are also subcylindrical. The hymenium is elliptical to ovoid, smooth, colorless, thin-walled, and 9–23 × 7–10.5 µm. The basidia are subcylindrical, with four sterigmata and a basal clamp connection, 26–46 × 5–8 µm.
Basidiospores—The basidiospores are allantoid, colorless, thin-walled, and smooth, with oil droplets inside, IKI−, CB−, (8.5–) 9–13 (–14) × 3.5–5 µm, L = 10.57 µm, W = 4.23 µm, Q = 2.44–2.55 (n = 60/2).
Additional specimens examined (paratypes)—China, Yunnan Province, Honghe, Pinbian County, Daweishan National Forestry Park, 22°53′ N, 103°35′ E, altitude 1670 m asl., found on a fallen angiosperm branch, 1 August 2019, CLZhao 17911 (SWFC).
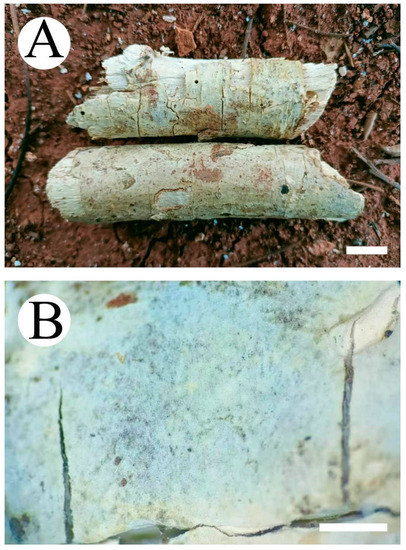
Figure 10.
Basidiomata of Vararia fragilis (holotype): the front of the basidiomata (A); the characteristic hymenophore (B). Bars: (A) = 1 cm and (B) = 1 mm.
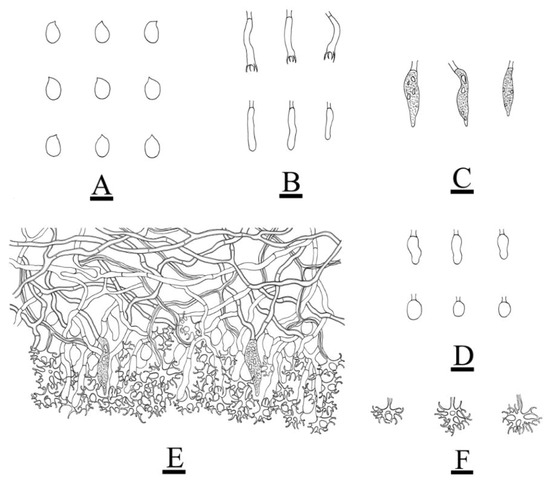
Figure 11.
Microscopic structures of Vararia fragilis (holotype): basidiospores (A); basidia and basidioles (B); fusiform gloeocystidia (C); elliptical to ovoid gloeocystidia (D); a section of the hymenium (E); dichohyphae (F). Bars: (A) = 5 μm, (B–F) = 10 µm.
MycoBank no.: 845763.
Holotype—China, Yunnan Province, Wenshan, Wenshan National Nature Reserve. GPS coordinates: found at 23°22′ N, 104°43′ E, altitude 1500 m asl., found on the fallen branch of an angiosperm, 26 July 2019, CLZhao 16475 (SWFC).
Etymology—fragilis (Lat.): referring to the fragile basidiomata.
Fruiting body—Basidiomata are annual, resupinate, adnate, thin, membranous, and fragile, without odor and taste when fresh, up to 85 mm long, 40 mm wide, and 30–100 µm thick. The hymenial surface is smooth, buff when fresh, buff to ochraceous on drying and cracking. The sterile margin is indistinct, attached, and is cream to buff.
Hyphal system—Dimitic, generative hyphae, bearing simple septa, colorless, thin- to thick-walled, occasionally branched, interwoven, 1.5–3.5 µm in diameter, IKI−, CB+, and with tissues unchanged in KOH; the dichohyphae in the subhymenium are abundant, predominantly yellowish, capillary, distinctly thick-walled, and dichotomously to irregularly branched, with the main branches up to 2 μm in diameter and with acute tips; moderately dextrinoid in Melzer’s reagent; more frequently branched, with more narrow and shorter branches in the subiculum, with slightly curved tips and a stronger dextrinoid reaction.
Hymenium—The gloeocystidia are of two types: (1) elliptical to ovoid, 5.8–16 × 3.5–7 µm; (2) subulate, usually with a constriction at the tip, smooth, colorless, thin-walled, 16.5–27 × 4–7 µm. Basidia subcylindrical, with four sterigmata and a basal simple-septa connection, 13–23.5 × 3–4.5 µm.
Basidiospores—The basidiospores are broad from ellipsoid to ellipsoid, colorless, thin-walled, smooth, IKI−, CB−, 3.5–5.5 (–6) × 2.5–3.5 µm, L = 4.78 µm, W = 3.12 µm, Q = 1.48–1.56 (n = 60/2).
Additional specimen examined (paratype)—China, Yunnan Province, Yuxi, Xiping County, Mopanshan National Forestry Park, 24°07′ N, 101°98′ E, altitude 2614 m asl., on the fallen branch of an angiosperm, 20 August 2017, CLZhao 2628 (SWFC).
4. Discussion
Four genera, Gloiothele, Peniophora, Scytinostroman, and Vararia have been grouped together and clustered within the family Peniophoraceae, as inferred from a dataset with 178 terminal taxa [37]. In the present study, based on the nLSU data (Figure 1), four new species were classified in the family Peniophoraceae and were then classified within the genera of Peniophora and Vararia.
Based on the ITS phylogenetic analysis (Figure 2), two new taxa have been grouped within the genus Peniophora, named P. roseoalba and P. yunnanensis, in which P. roseoalba is grouped with two close taxa, P. versicolor and P. ovalispora; P. yunnanensis was grouped with a clade comprising P. lycii and P. violaceolivida. However, morphologically, Peniophora versicolor differs from P. roseoalba by its dark brown to reddish brown or ochraceous hymenophore, smaller lamprocystidia (10–20 × 8–10 µm), and larger basidiospores (9–11 × 4.5–6 µm) [59]. P. ovalispora is separated from P. roseoalba by having a cream-colored to salmon or brownish hymenophore, with a pruinose margin [13,60]. Peniophora lycii is separated from P. yunnanensis by its even, greyish lilac to bluish violaceous hymenial surface, the presence of the dendrohyphidia and the wider lamprocystidia (22–42 × 14–25 µm) [60]; P. violaceolivida differs in terms of its pale pink, with a violaceous hymenial surface and a fimbriate margin [60].
In the current study, based on the further ITS phylogenetic tree (Figure 3), two new taxa have been grouped within the genus Vararia. These are V. daweishanensis and V. fragilis, in which V. daweishanensis was grouped with four close taxa, namely, V. gomezii, V. rhombospora, V. sigmatospora and V. trinidadensis, while V. fragilis was grouped with a clade comprising V. ambigua and V. ellipsospora. However, morphologically speaking, V. gomezii differs from V. daweishanensis in having a pinkish buff to cream hymenial surface and simple-septate generative hyphae, as well as navicular basidiospores [20]. V. rhombospora is separated from V. daweishanensis by having a fragile basidiomata with a cream to beige gray hymenial surface, with rhomboid and larger basidiospores (15–17 × 5–6 µm) [61]; V. sigmatospora is distinguishable from V. daweishanensis by its simple-septate generative hyphae and fusiform, narrower basidiospores (13–15.2 × 2.5–3 µm) [62]; V. trinidadensis differs in its gray to grayish-white hymenial surface, simple-septate generative hyphae, and fusiform, narrower basidiospores (13–17 × 2.5–3.2 µm) [63]. V. ambigua is distinguishable from V. fragilis by its powdery hymenial surface, as well as by basidia that are swollen at the base and larger basidia (27–40 × 3.5–4 µm) [21]; V. ellipsospora differs from V. fragilis in its fimbriate margin, clamped generative hyphae, wider gloeocystidia (28–48 × 8–11 µm) and larger basidiospores (8–12 × 5.5–6.5 µm) [22].
Morphologically, Peniophora cinerea (Pers.) Cooke, P. laeta (Fr.) Donk, P. laurentii S. Lundell, P. polygonia (Pers.) Bourdot & Galzin, P. rhodocarpa Rehill & B.K. Bakshi are similar to P. roseoalba by having encrusted lamprocystidia. However, P. cinerea differs from P. roseoalba by its smaller lamprocystidia (15–20 × 6–10 µm), and subcylindrical to allantoid basidiospores [60]; P. laeta is separated from P. roseoalba by having a hydnoid to raduloid hymenophore, larger gloeocystidia (60–120 × 8–10 µm) and cylindrical to suballantoid, larger basidiospores (9–15 × 3.5–4.5 µm) [64]; P. laurentii is distinguished from P. roseoalba by tuberculate to plicate or merulioid hymenophore, white margin, simple-septa generative hyphae, as well as longer gloeocystidia (70–150 × 8–12 µm) and larger basidia (50–60 × 6–8 µm) [60]; P. polygonia is separated from P. roseoalba by having bladder like, bigger gloeocystidia (60–100 × 15–25 µm), presence of dendrohyphidia, and cylindrical to allantoid, larger basidiospores (10–14 × 2.5–4 µm) [60]; P. rhodocarpa differs P. roseoalba by having tuberculate, rimose hymenial surface, larger gloeocystidia (50–90 × 12–18 µm) with larger lamprocystidia (60–100 × 12–18 µm), and allantoid, narrower basidiospores (5–8.5 × 1.7–2.2 µm) [60].
Peniophora yunnanensis is similar to P. aurantiaca (Bres.) Höhn. & Litsch., P. bonariensis C.E. Gómez, P. junipericola J. Erikss., P. meridionalis Boidin, P. quercina (Pers.) Cooke, based on having clamped generative hyphae and gloeocystidia. However, Peniophora aurantiaca is distinguished from P. yunnanensis by its orange-red, reddish to reddish grey hymenial surface, larger gloeocystidia (70–150 × 10–20 µm), larger basidia (60–80 × 10–15 µm), and ellipsoid, larger basidiospores (14–20 × 8–12 µm) [60]; P. bonariensis can be delimited from P. yunnanensis by its pinkish grey to greyish violaceous hymenial surface, thick-walled gloeocystidia and larger lamprocystidia (30–50 × 12–25 µm) [60]; P. junipericola differs by having pinkish or greyish red to violaceous hymenial surface, larger lamprocystidia (40–80 × 6–18 µm), and allantoid basidiospores [60]; P. meridionalis differs from P. yunnanensis by its ochraceous grey, yellowish brown hymenial surface, presence of dendrohyphidia, and larger lamprocystidia (35–55 × 8–20 µm) [60]; P. quercina is separated from P. yunnanensis by having the pinkish to pinkish grey or bluish grey to violaceous hymenial surface, and larger lamprocystidia (30–80 × 10–20 µm) [60].
Peniophora yunnanensis resembles P. gilbertsonii Boidin, P. lilacea Bourdot & Galzin, P. limitata (Chaillet ex Fr.) Cooke, P. piceae (Pers.) J. Erikss. and P. rufomarginata (Pers.) Bourdot & Galzin in having a tuberculate hymenial surface. However, Peniophora gilbertsonii is different from P. yunnanensis in having an ochraceous pink to reddish or brown to grey hymenial surface and the presence of dendrohyphidia [60]; P. lilacea can be delimited from P. yunnanensis along its pinkish grey to ochraceous violaceous hymenial surface, along with its thick-walled gloeocystidia in trauma. We recorded the presence of the dendrohyphidia and ellipsoid, wider basidiospores (9–16 × 6.5–10 µm) [60]; P. limitata differs from P. yunnanensis by having pinkish gray or violaceous gray to a dark blue-gray hymenial surface, and wider lamprocystidia (25–60 × 8–12 µm) [19]; P. piceae is distinguished from P. yunnanensis by its reddish grey to grey to a dark violaceous grey hymenial surface, larger lamprocystidia (40–80 × 6–18 µm), and allantoid, narrower basidiospores (6.5–9.5 × 2–2.8 µm) [60]; P. rufomarginata is separated from P. yunnanensis by having a pinkish to pinkish gray or bluish gray hue to the violaceous hymenial surface, along with larger lamprocystidia (30–80 × 10–20 µm) [60].
Vararia amphithallica Boidin, Lanq. & Gilles, V. bispora S.L. Liu & S.H. He, V. montana S.L. Liu & S.H. He, V. ochroleuca (Bourdot & Galzin) Donk and V. rugosispora Boidin, Lanq. & Gilles resembles V. daweishanensis by having a smooth hymenial surface and clavate to cylindrical basidia. However, Vararia amphithallica is distinguished from V. daweishanensis by its fimbriate margin, 2-sterigmata basidia, and ellipsoid to cylindrical basidiospores (9–12 × 4–7 µm) [31]; V. bispora differs in V. daweishanensis by having the thick-walled gloeocystidia, with 2-sterigmata basidia, and larger, fusiform to cylindrical basidiospores (16–24 × 6–8 µm) [31]; V. montana is separated from V. daweishanensis by having the brittle basidiomata, longer gloeocystidia (50–100 × 4–9 µm), and broadly ellipsoid, larger basidiospores (16–24 × 8–14 µm) [31]; V. ochroleuca differs from V. daweishanensis by having cream-colored to pallid ochraceous hymenial surface, slightly thick-walled gloeocystidia, simple-septa generative hyphae, and broadly ellipsoid, to drop-shaped, smaller basidiospores (2.6–3.8 × 2–3.2 µm) [65]. V. rugosispora can be delimited from V. daweishanensis by its simple-septate generative hyphae and longer basidiospores (12–16 × 7–8 µm) [21].
Vararia breviphysa Boidin & Lanq., V. cinnamomea Boidin, Lanq. & Gilles, V. cremea Boidin, Lanq. & Gilles, V. gallica (Bourdot & Galzin) Boidin, V. hauerslevii Boidin, and V. sinapicolor Boidin & Gilles are similar to V. fragilis, based on characteristics such as the thick-walled dichohyphae, and four sterigmata basidia. However, V. breviphysa differs from V. fragilis by having the larger gloeocystidia (50–65 × 6–8.5 µm), fusiform and larger basidiospores (15–22 × 4–6 µm) [20]. V. cinnamomea is distinguished from V. fragilis by its cinnamon hymenial surface, larger basidia (45–65 × 8–10 µm), and larger basidiospores (9–13 × 5–7.2 µm) [25]. V. cremea can be delimited from V. fragilis by the longer gloeocystidia (40–90 × 7–15 µm), and larger basidiospores (15–20 × 2.7–3.5µm) [21]. V. gallica differs from V. fragilis in having a whitish hymenial surface and larger basidiospores (9–12 × 3.5–5 µm) [19]. V. hauerslevii is separated from V. fragilis by its larger gloeocystidia (50–60 × 7–9 µm) and subfusoid, larger basidiospores (10–15 × 3.5–4.5 µm) [66]. V. sphaericospora differs from V. fragilis in having clamped generative hyphae, bigger basidia (33–45 × 6–7 µm), and larger basidiospores (12.5–14 × 5.2–7 µm) [20,21,23].
The taxa of Peniophora and Vararia are typical examples of wood-rotting fungi, which is an extensively studied family [19,67,68,69,70]. So far, several studies on new wood-decaying fungi belonging to the Peniophora and Vararia from China have been reported [34,71,72,73,74,75].
Author Contributions
Conceptualization, C.Z.; methodology, C.Z. and L.Z.; software, C.Z. and L.Z.; validation, C.Z. and L.Z.; formal analysis, C.Z., X.Z., Y.D. and L.Z.; investigation, C.Z. and L.Z.; resources, C.Z.; writing—original draft preparation, C.Z., X.Z. and L.Z.; writing—review and editing, C.Z. and L.Z.; visualization, C.Z.; supervision, C.Z.; project administration, C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the National Natural Science Foundation of China (Project No. 32170004, U2102220) to Changlin Zhao, Yunnan Fundamental Research Project (Grant No. 202001AS070043) to Changlin Zhao, the High-level Talents Program of Yunnan Province (YNQR-QNRC-2018-111) to Changlin Zhao.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: [https://www.ncbi.nlm.nih.gov/; https://www.mycobank.org/page/Simple%20names%20search; http://purl.org/phylo/treebase, submission ID 28664; all accessed on 13 October 2022].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tedersoo, L.; Bahram, M.; Põlme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008; p. 771. [Google Scholar]
- Cooke, M.C. On Peniophora. Grevillea 1879, 8, 17–21. [Google Scholar]
- Burt, E.A. The Thelephoraceae of North America. XIV. Peniophora. Ann. Mo. Bot. Gard. 1925, 12, 213–357. [Google Scholar] [CrossRef]
- Hennings, P. Fungi Africae orientalis III. Bot. Jahrbücher Für Syst. Pflanzengesch. Pflanzengeogr. 1904, 34, 39–57. [Google Scholar]
- Hjortstam, K.; Ryvarden, L. Some new and noteworthy Basidiomycetes (Aphyllophorales) from Nepal. Mycotaxon 1984, 20, 133–151. [Google Scholar]
- Hjortstam, K.; Roberts, P.J.; Spooner, B.M. Corticioid fungi from Brunei Darussalam. Kew Bull. Addit. Ser. 1998, 53, 805–827. [Google Scholar] [CrossRef]
- Bourdot, H. Corticiés nouveaux de la flore mycologique de France III. Rev. Sci. Bourbon. Cent. Fr. 1910, 23, 3–15. [Google Scholar]
- Boidin, J. Hétérobasidiomycètes saprophytes et Homobasidiomycètes résupinés. II. Catalogue raisonné des espèces pyrénéennes de la région de Luchon (Haute-Garonnes). Bull. Société D’histoire Nat. Toulouse 1957, 92, 277–292. [Google Scholar]
- Wu, S.H. Three new species of corticioid fungi from Taiwan. Bot. Stud. 2007, 48, 325–330. [Google Scholar] [CrossRef]
- Ranojevic, N. Zweiter beitrag zur pilzflora serbiens. Ann. Mycol. 1910, 8, 347–402. [Google Scholar]
- Dhingra, G.S. Peniophora hallenbergii sp. nov. from India. Mycotaxon 2013, 126, 235–237. [Google Scholar] [CrossRef]
- Boidin, J.; Lanquetin, P.; Gilles, G. Les Peniophoraceae de la zone intertropicale (Basidiomycetes, Aphyllophorales). Bull. Société Mycol. Fr. 1991, 107, 91–156. [Google Scholar]
- Parmasto, E.; Parmasto, I. Variation in basidiospores in the Hymenomycetes and its significance to their taxonomy. Bibl. Mycol. 1987, 115, 1–168. [Google Scholar]
- Wu, S.H. A study of Peniophora species with simple-septate hyphae occurring in Taiwan. Mycotaxon 2003, 85, 187–199. [Google Scholar]
- Popoff, O.F.; Wright, J.E. Two new corticioid fungi (Aphyllophorales) from NE Argentina and Paraguay. Mycotaxon 1994, 51, 317–324. [Google Scholar]
- Gorjón, S.P.; Jesus, M.A. Some new species and new records of corticioid fungi (Basidiomycota) from the Brazilian Amazon. Phytotaxa 2012, 67, 38–54. [Google Scholar] [CrossRef]
- Jackson, H.S.; Dearden, E.R. Studies of Canadian Thelephoraceae. III. Some new species from British Columbia. Can. J. Res. 1949, 27, 147–156. [Google Scholar] [CrossRef]
- Bernicchia, A.; Gorjón, S.P. Fungi Europaei 12: Corticiaceae s.l.; Edizioni Candusso: Alassio, Italy, 2010. [Google Scholar]
- Boidin, J.; Lanquetin, P. Vararia subgenus Vararia (Basidiomycetes, Lachnocladiaceae): Étude spèciale des espèces d’Afrique intertropicale. Bull. Soc. Mycol. 1975, 91, 457–513. [Google Scholar]
- Boidin, J.; Lanquetin, P.; Gilles, G. Application du concept biologique del’espèce aux Basidiomycètes. Le genre Vararia section Vararia au Gabon. Cryptogam. Mycol. 1980, 1, 265–384. [Google Scholar]
- Cunningham, G.H. Thelephoraceae of New Zealand. Part IV. The genui Vararia. Trans. Roy. Soc. NZ. 1955, 82, 973–985. [Google Scholar]
- Gilbertson, R.L. Some species of Vararia from temperate North America. Pap. Mich. Acad. Sci. 1965, 50, 161–184. [Google Scholar]
- Boidin, J. Basidiomycètes Lachnocladiaceae résupinés de la Republique Centrafricaine. Cah. Maboké 1967, 5, 23–35. [Google Scholar]
- Boidin, J.; Lanquetin, P. Compléments au genre Vararia P. Karst. (Basidiomycètes). Pers. -Mol. Phylogeny Evol. Fungi 1984, 12, 243–262. [Google Scholar]
- Pouzar, Z. Taxonomic studies in resupinate fungi I. Česká Mykol 1982, 36, 141–145. [Google Scholar]
- Boidin, J.; Gilles, G. Contribution à la connaissance du genre Vararia (Basidiomycotina). Bull. Soc. Mycol. 1999, 115, 115–139. [Google Scholar]
- Duhem, B.; Buyck, B. On two new tropical Vararia (Russulales, Basidiomycota) with extremely small, racemose dichohyphidia. Cryptogam. Mycol. 2012, 33, 427–437. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Dhingra, G.S.; Singh, A.P. Vararia longicystidiata sp. nov.(Agaricomycetes) from India. Mycotaxon 2012, 120, 357–360. [Google Scholar] [CrossRef]
- Nakasone, K.K. Taxonomic studies in Chrysoderma, Corneromyces, Dendrophysellum, Hyphoradulum, and Mycobonia. Mycotaxon 2015, 130, 369–397. [Google Scholar] [CrossRef]
- Liu, S.L.; He, S.H. The genus Vararia (Russulales, Basidiomycota) in China. Two new species and two new Chinese records. Nord. J. Bot. 2016, 34, 553–558. [Google Scholar] [CrossRef]
- Dai, Y.C. A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 2011, 52, 69–79. [Google Scholar] [CrossRef]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Wu, G.; Yuan, H.S.; Zhou, L.W.; He, S.H.; Ge, Z.W.; Wu, F.; Wei, Y.L.; et al. Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 2021, 40, 770–805. [Google Scholar] [CrossRef]
- Liu, S.L. Taxonomy and Phylogeny of Vararia and Related Genera in China. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- Larsson, K.H.; Larsson, E.; Kõljalg, U. High phylogenetic diversity among corticioid homobasidiomycetes. Mycol. Res. 2004, 108, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Hibbett, D.S.; Larsson, K.H.; Larsson, E.; Langer, E.; Langer, G. The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Syst. Biodivers. 2005, 3, 113–157. [Google Scholar] [CrossRef]
- Leal-Dutra, C.A.; Neves, M.A.; Griffith, G.W.; Reck, M.A.; Clasen, L.A.; Dentinger, B.T.M. Reclassification of Parapterulicium corner (Pterulaceae, Agaricales), contributions to Lachnocladiaceae and Peniophoraceae (Russulales) and introduction of Baltazaria gen. nov. MycoKeys 2018, 37, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.Y.; Chen, Z.Y.; Zhao, C.L. Phylogenetic and taxonomic analyses of three new wood-inhabiting fungi of Xylodon (Basidiomycota) in a forest ecological system. J. Fungi 2022, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.H. Farvekort. In The Danish Mycological Society’s Colour-Chart; Foreningen til Svampekundskabens Fremme: Greve, Germany, 1996; pp. 1–6. [Google Scholar]
- Qu, M.H.; Wang, D.Q.; Zhao, C.L. A phylogenetic and taxonomic study on Xylodon (Hymenochaetales): Focusing on three new Xylodon species from southern China. J. Fungi 2022, 8, 35. [Google Scholar] [CrossRef]
- Dai, Y.C. Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 2012, 53, 49–80. [Google Scholar] [CrossRef]
- Zhao, C.L.; Wu, Z.Q. Ceriporiopsis kunmingensis sp. nov. (Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Mycol. Prog. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Larsson, E.; Larsson, K.H. Phylogenetic relationships of russuloid basidiomycetes with emphasis on aphyllophoralean taxa. Mycologia 2003, 95, 1037–1065. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP*: Phylogenetic analysis using Parsimony (*and Other Methods); Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES Science Gateway: Enabling high-impact science for phylogenetics researchers with limited resources. Assoc. Comput. Mach. 2012, 39, 1–8. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B.; Wang, Z.; Binder, M.; Curtis, J.M.; Lim, Y.W.; Nilsson, R.H.; Hughes, K.W.; Hofstetter, V.; Ammirati, J.F.; Schoch, C.L.; et al. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 2007, 43, 430–451. [Google Scholar] [CrossRef]
- Chen, J.J.; Shen, L.L. Amylosporus succulentus sp. nov. (Russulales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Cryptogam. Mycol. 2014, 35, 271–282. [Google Scholar] [CrossRef]
- Larsson, E.; Hallenberg, N. Species delimitation in the Gloeocystidiellum porosum-clavuligerum complex inferred from compatibility studies and nuclear rDNA sequence data. Mycologia 2001, 93, 907–914. [Google Scholar] [CrossRef]
- Zhou, L.W.; Dai, Y.C. Taxonomy and phylogeny of wood-inhabiting hydnoid species in Russulales: Two new genera, three new species and two new combinations. Mycologia 2013, 105, 636–649. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Z.J.; Wu, C.H.; Zhao, C.L. Gloeodontia yunnanensis sp. nov. (Russulales, Basidiomycota) from China, evidenced by morphological characters and phylogenetic analyses. Phytotaxa 2020, 43, 111–118. [Google Scholar] [CrossRef]
- Liu, S.L.; Nakasone, K.K.; He, S.H. Michenera incrustata sp. nov. (Peniophoraceae, Russulales) from southern China. Nova Hedwig. 2019, 108, 197–206. [Google Scholar] [CrossRef]
- Liu, S.L.; He, S.H. Taxonomy and phylogeny of Dichostereum (Russulales), with descriptions of three new species from southern China. MycoKeys 2018, 40, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Saccardo, P.A.; Sydow, P. Supplementum universale, Pars V. Sylloge Fungorum 1902, 16, 1–1291. [Google Scholar]
- Andreasen, M.; Hallenberg, N. A taxonomic survey of the Peniophoraceae. Synop Fungorum 2009, 26, 56–119. [Google Scholar]
- Boidin, J.; Lanquetin, P. Les genres Dichostereum et Vararia en Guadeloupe (Basidiomycetes, Lachnocladiaceae). Mycotaxon 1977, 6, 277–336. [Google Scholar]
- Boidin, J.; Gilles, G.; Lanquetin, P. Basidiomycètes Aphyllophorales de l’Île de la Réunion. IX—Les genres Dichostereum Pilat et Vararia Karsten. Bull. Société Mycol. Fr. 1987, 103, 119–135. [Google Scholar]
- Welden, A.L. West Indian species of Vararia with notes on extralimital species. Mycologia 1965, 57, 502–520. [Google Scholar] [CrossRef]
- Donk, M.A. Notes on resupinate Hymenomycetes IV. Fungus 1957, 27, 1–29. [Google Scholar]
- Karasiński, D. Polish resupinate Russulales: The genus Vararia. Acta Mycol. 2010, 45, 45–56. [Google Scholar] [CrossRef]
- Salcedo, I.; Sarrionandia, E.; Olariaga, I. Contribution to the knowledge of the Aphyllophorales (Basidiomycota) of the Basque Country (Spain). V. Nova Hedwig. 2006, 82, 81–90. [Google Scholar] [CrossRef]
- Núñez, M.; Ryvarden, L. East Asian polypores 2. Synop. Fungorum 2001, 14, 165–522. [Google Scholar]
- Dai, Y.C.; Cui, B.K.; Si, J.; He, S.H.; Hyde, K.D.; Yuan, H.S.; Liu, X.Y.; Zhou, L.W. Dynamics of the worldwide number of fungi with emphasis on fungal diversity in China. Mycol. Prog. 2015, 14, 62. [Google Scholar] [CrossRef]
- Dai, Y.C. Two new polypores from tropical China, and renaming two species in Polyporus and Phellinus. Mycoscience 2012, 53, 40–44. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid fungi of Europe. Syn. Fung. 2014, 31, 1–455. [Google Scholar]
- Lin, S.H.; Chen, Z.C. The Corticiaceae and the resupinate Hydnaceae of Taiwan. Taiwania 1990, 35, 69–111. [Google Scholar]
- Wu, S.H. A study of Peniophora species in Taiwan with clamped hyphae. Bot. Bull. Acad. Sin. 2002, 43, 241–250. [Google Scholar]
- Dai, Y.C.; Liu, H.G.; Wu, F.; Cui, B.K.; Si, J.; He, S.H.; Yuan, Y.; Zhou, M.; Zhao, Q.; Liu, S.H.; et al. Resources and Diversity of Wood Decay Fungi in Yunnan, 1st ed.; Science Press: Beijing, China, 2022; pp. 454–455. [Google Scholar]
- Wu, F.; Zhou, L.W.; Vlasák, J.; Dai, Y.C. Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
- Wu, F.; Man, X.W.; Tohtirjap, A.; Dai, Y.C. A comparison of polypore funga and species composition in forest ecosystems of China, North America, and Europe. For. Ecosyst. 2022, 9, 100051. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).