Taxonomy and Phylogeny of Cystostereaceae (Agaricales, Basidiomycota): A New Genus, Five New Species, and Three New Combinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Morphological Studies
2.3. DNA Extraction and Sequencing
2.4. Phylogenetic Analyses
3. Results
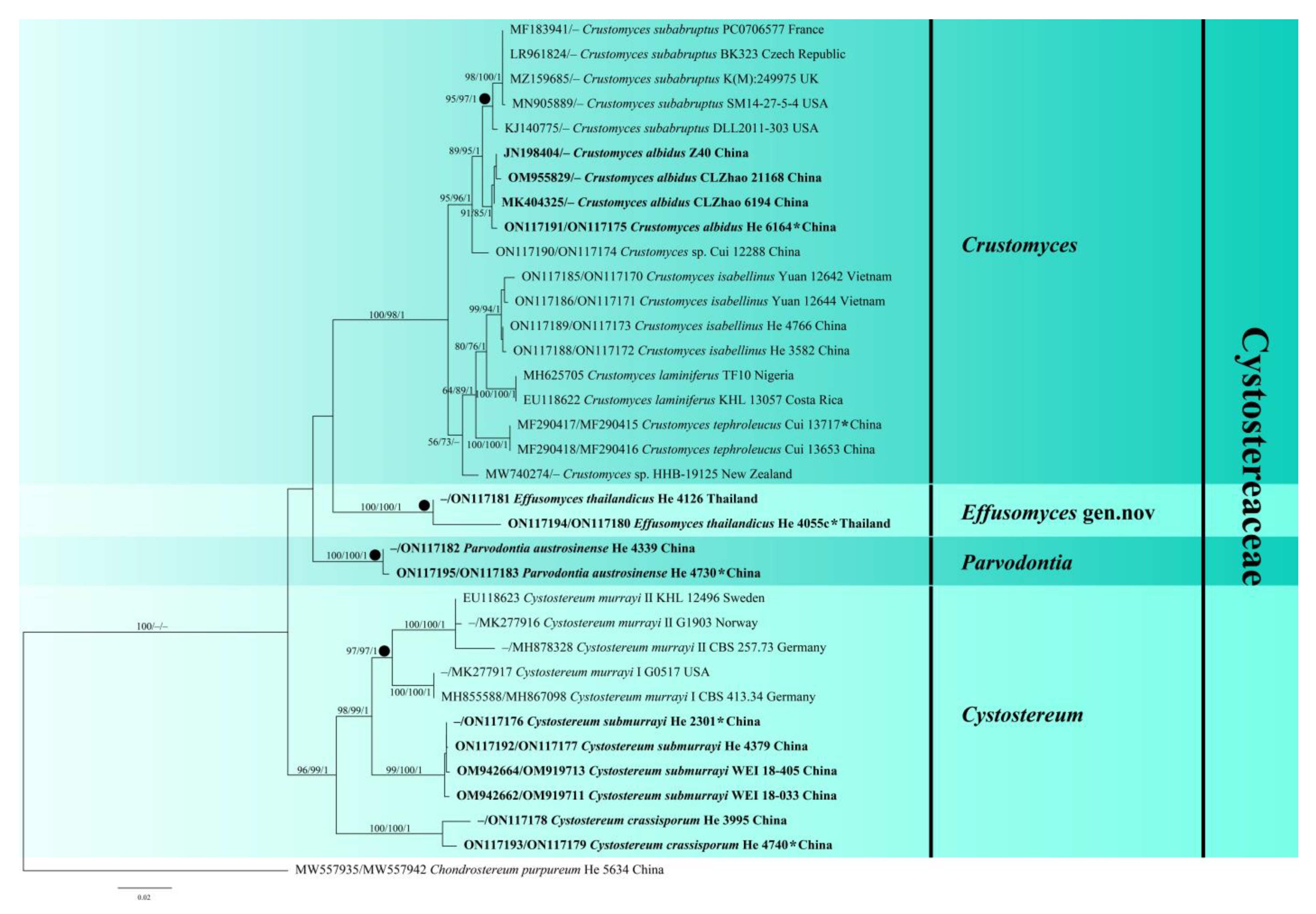
3.1. Phylogenetic Analyses
3.2. Taxonomy
- Crustomyces Jülich Persoonia 10:140. 1978, emended
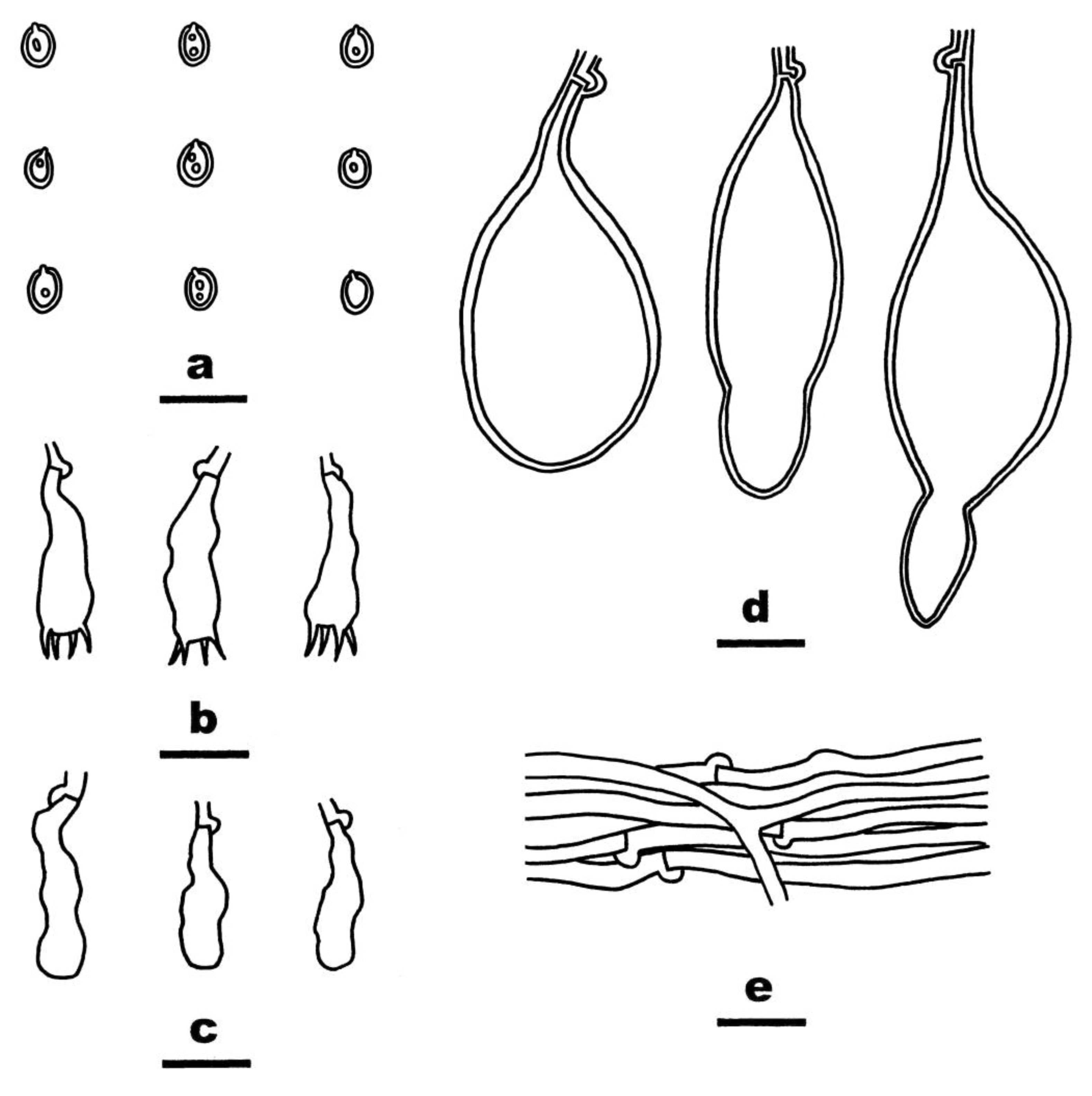
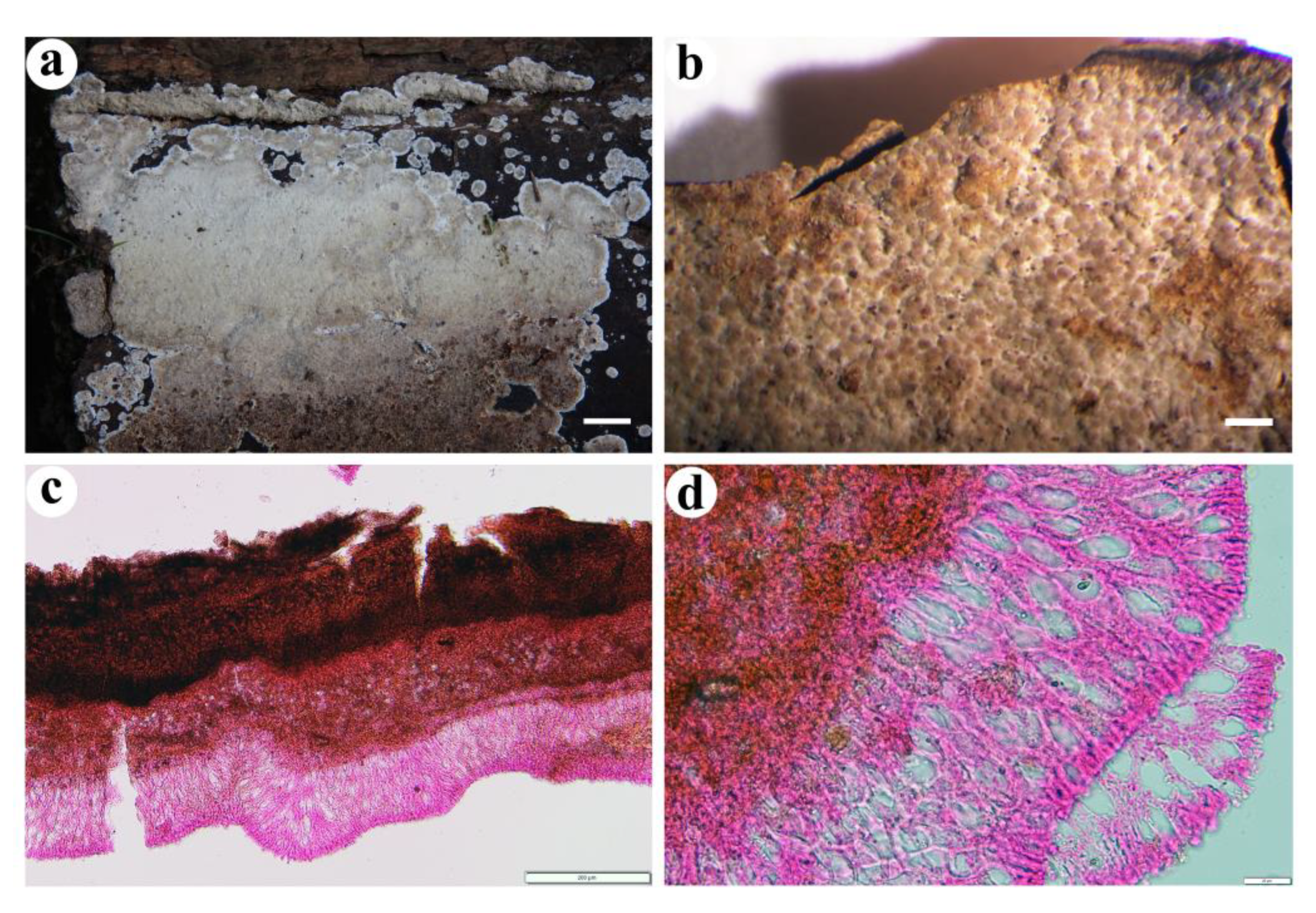
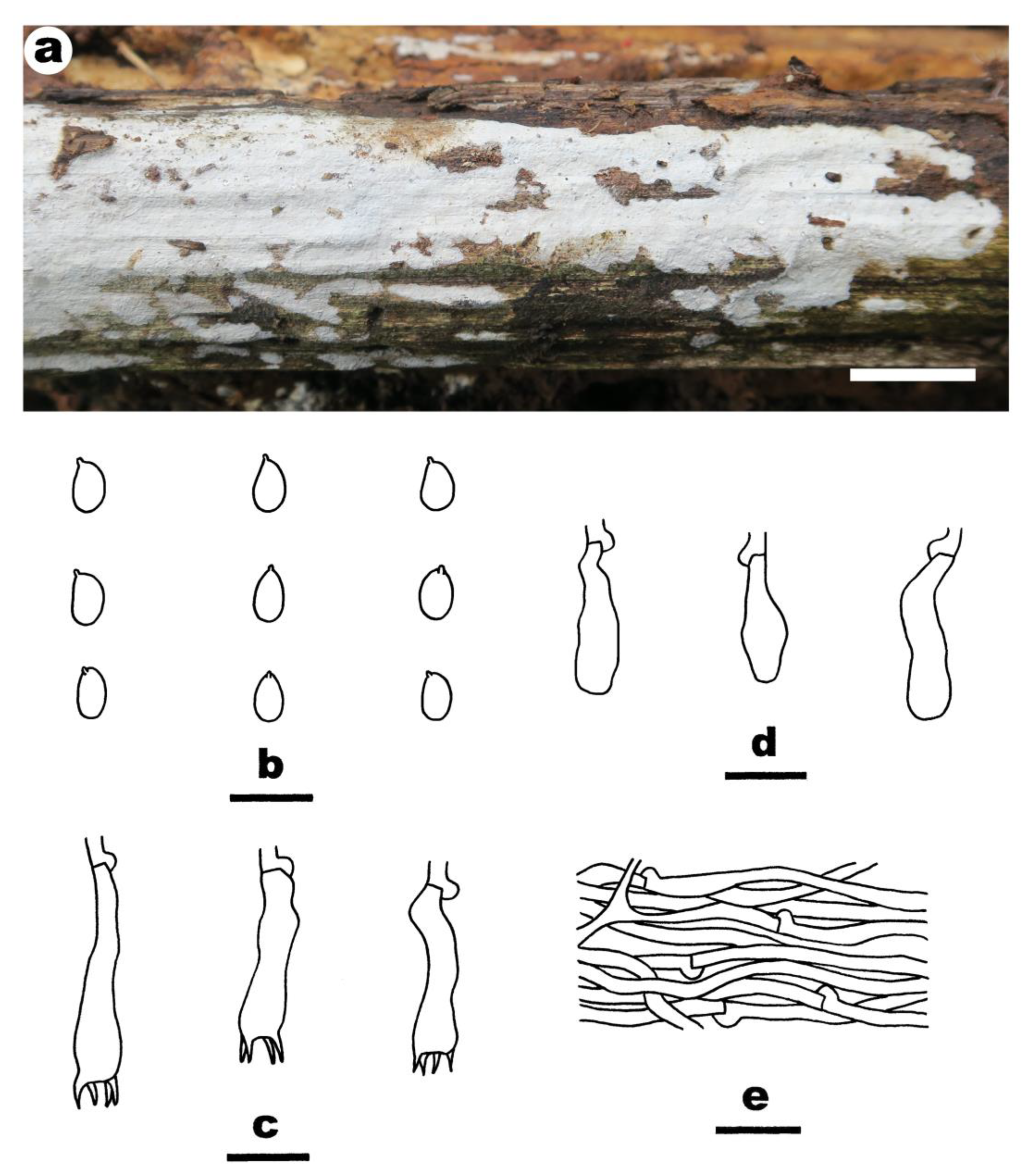
- Crustomyces albidus Yue Li, Nakasone, C.L. Zhao & S.H. He, sp. nov. Figure 3
- Crustomyces isabellinus (Berk. & Broome) Yue Li, Nakasone & S.H. He, combinatio nova Figure 4
- MycoBank: MB844199
- Crustomyces laminiferus (Berk. & M.A. Curtis) Yue Li, Nakasone & S.H. He,
- combinatio nova
- Crustomyces tephroleucus (J. Song, Y.C. Dai & B.K. Cui) Yue Li, Nakasone & S.H. He, combinatio nova
- Effusomyces Yue Li, Nakasone & S.H. He, gen. nov.
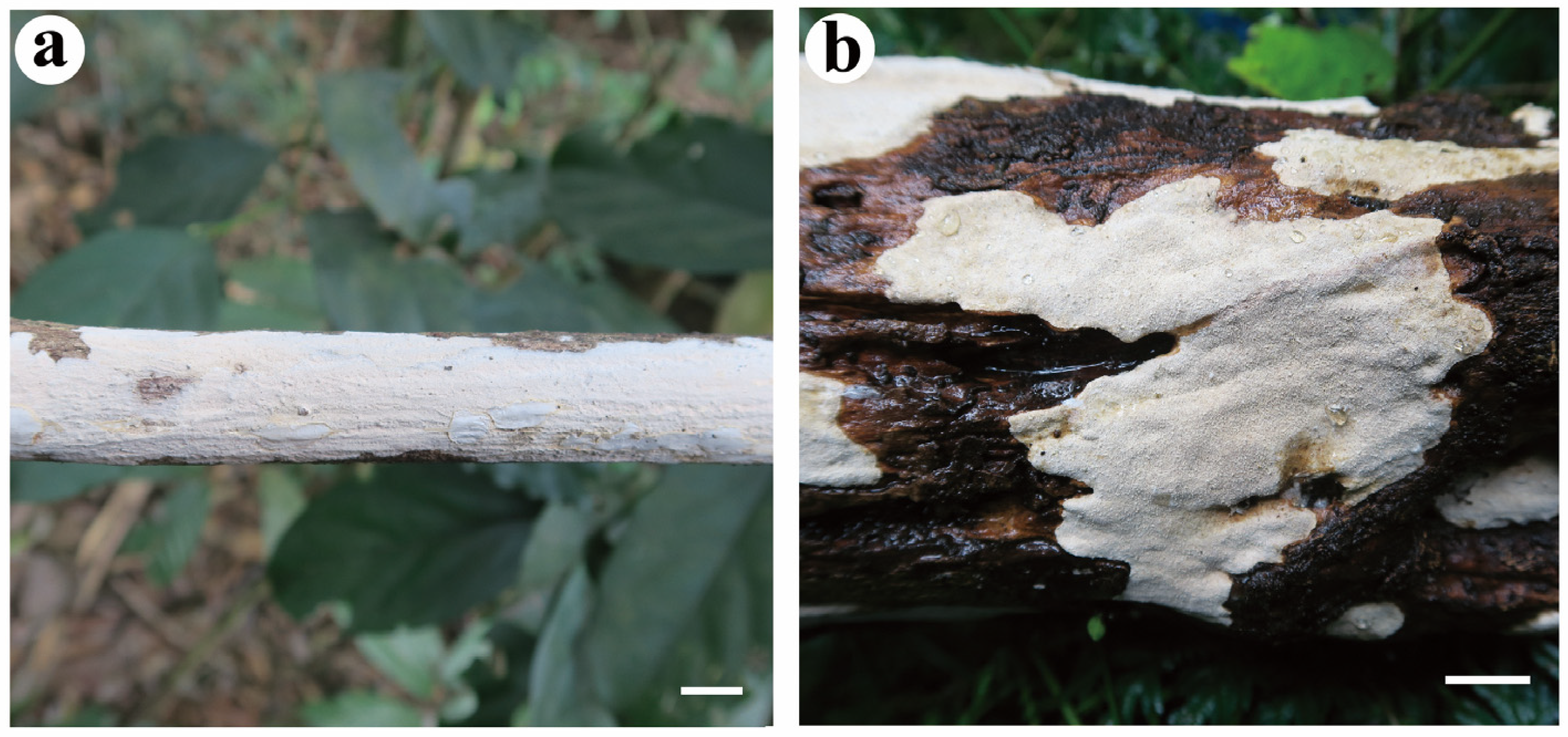
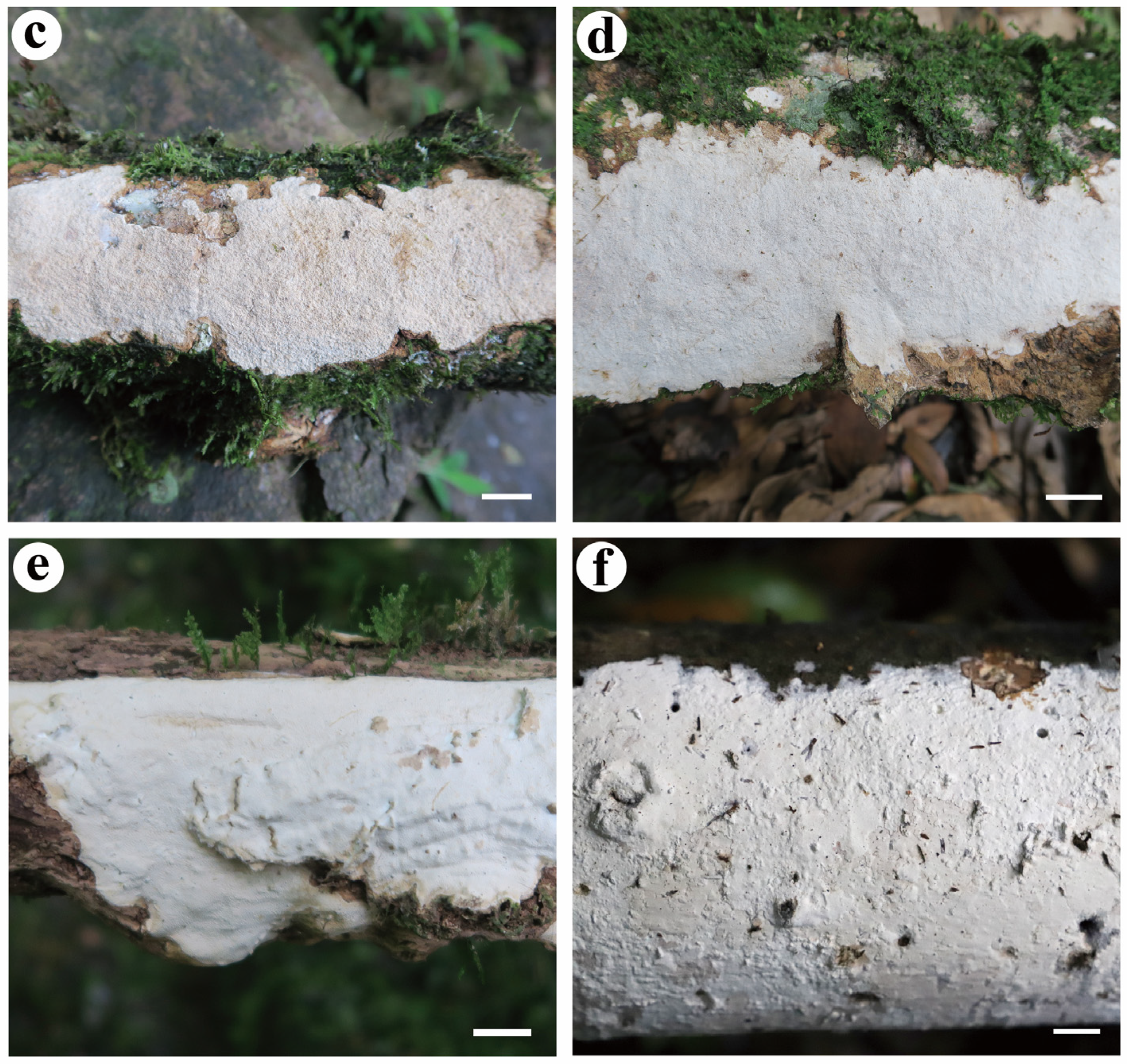
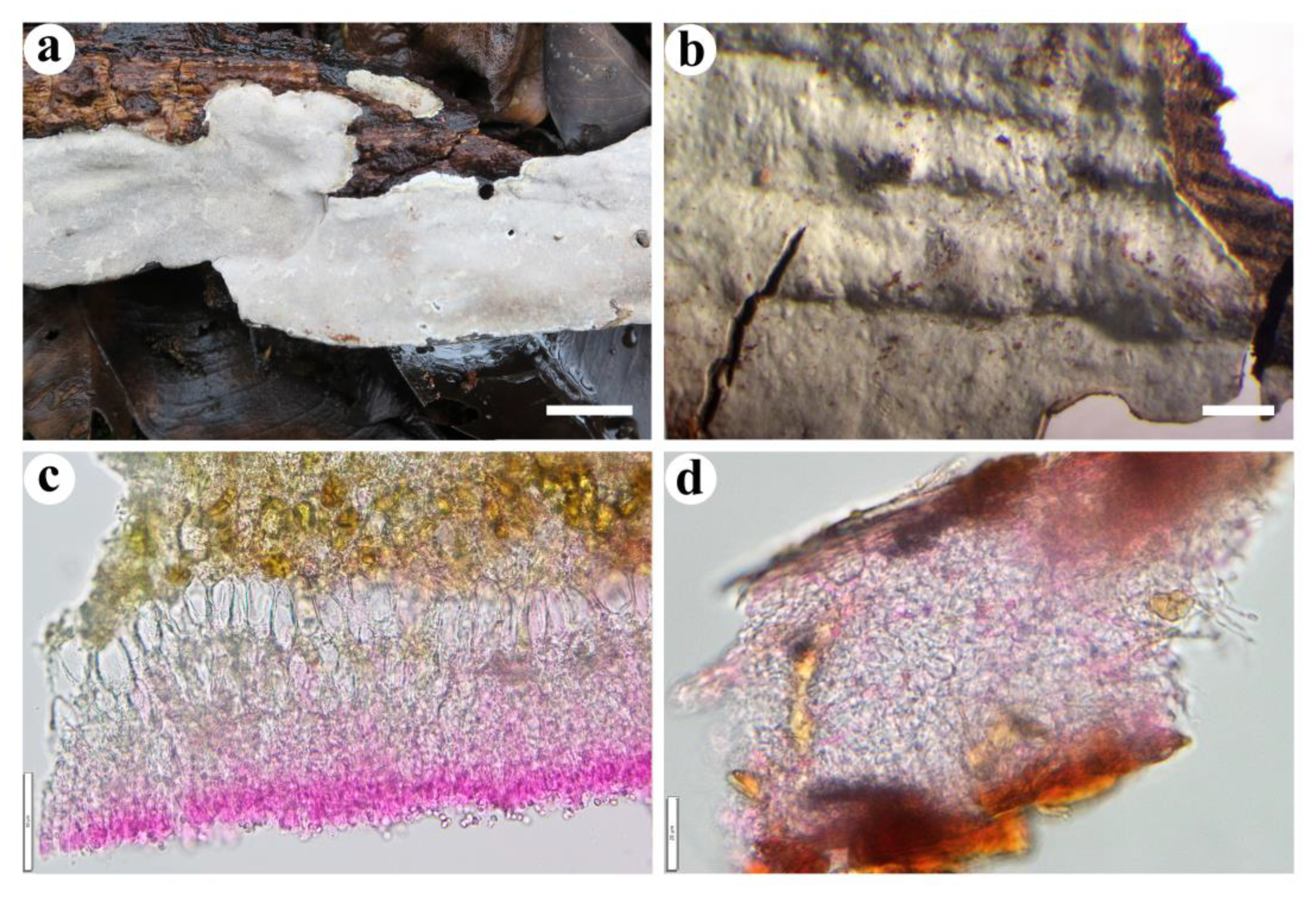
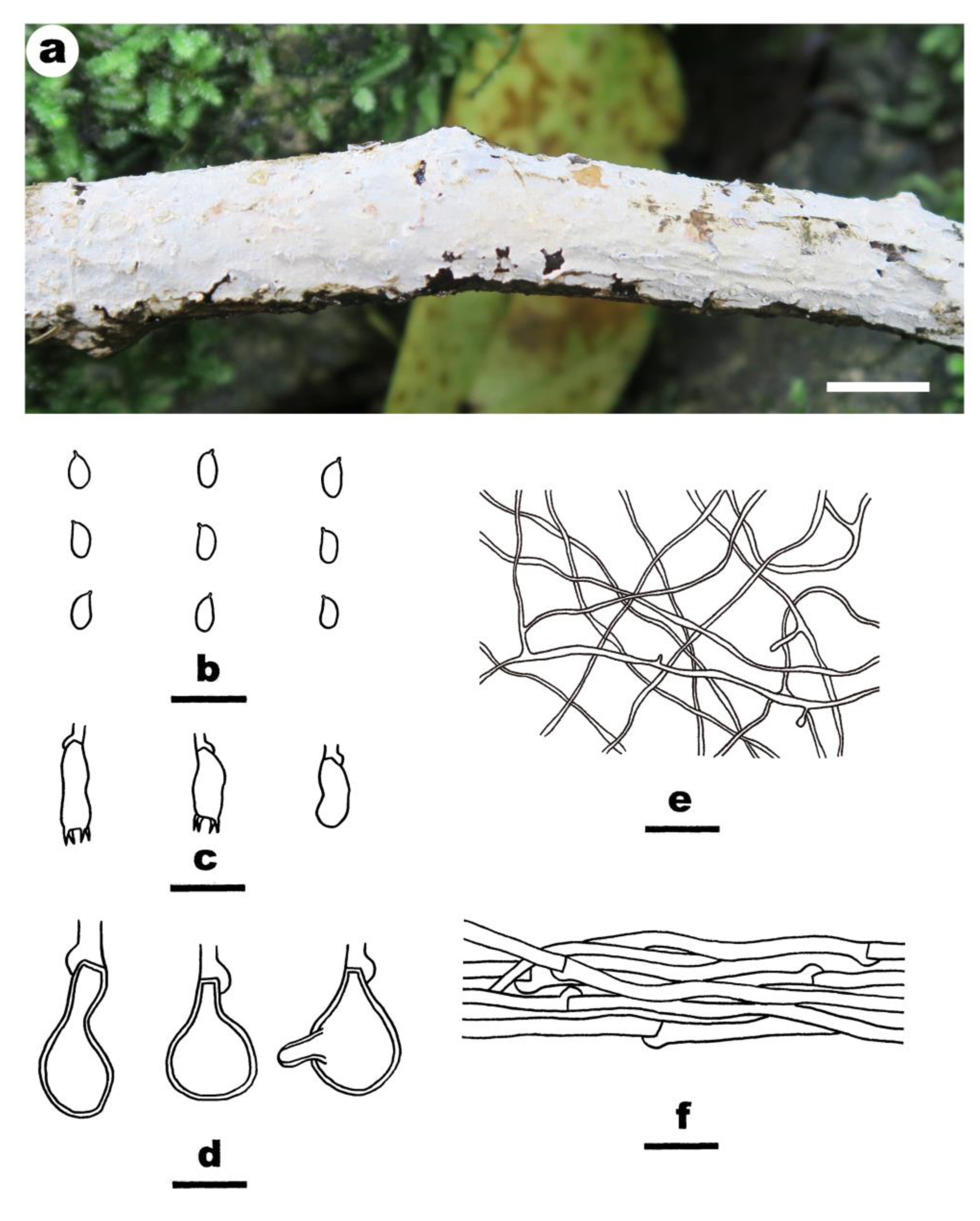
- Effusomyces thailandicus Yue Li, Nakasone & S.H. He, sp. nov. Figure 9
- Parvodontia austrosinensis Yue Li, Nakasone & S.H. He, sp. nov. Figure 10
- A key to genera and species in Cystostereaceae sensu lato
- 1. Gloeocystidia absent………………………………………………………………………………2
- 1. Gloeocystidia present..……………………………………………………………………………3
- 2. Hymenophore poroid....………………………………………………Crustomyces tephroleucus
- 2. Hymenophore smooth..………………………………………………………………Effusomyces
- 3. Microbinding hyphae present...…………………………………………………………………4
- 3. Microbinding hyphae absent, skeletal hyphae present or not........…………………………6
- 4. Gloeocystidia tubular, subulate or subfusiform, encrusted...………………………Cericium
- 4. Gloeocystidia varied shaped, smooth.....……………………………………………....………5
- 5. Gloeocystidia submoniliform...……………………………………………Crustomyces albidus
- 5. Gloeocystidia spherical to ovate with short stalk..………………Parvodontia austrosinensis
- 6. Hymenophore smooth.…..………………………………………………………………………7
- 6. Hymenophore grandinioid, odontoid or hydnoid...…………………………………………8
- 7. Basidiospores thick-walled....………………………………………Cystostereum crassisporum
- 7. Basidiospores thin-walled..………………………………………………………Parvobasidium
- 8. Hyphal system monomitic.….………………………………………………………Parvodontia
- 8. Hyphal system dimitic..…………………………………………………………………………9
- 9. Dendrohyphidia present.…..……....………………………………………………Crustomyces
- 9. Dendrohyphidia absent..…..………………………………………………………Cystostereum
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jülich, W. Higher taxa of Basidiomycetes. Bibl. Mycol. 1981, 85, 485. [Google Scholar]
- Larsson, K.H. Re-thinking the classification of corticioid fungi. Mycol. Res. 2007, 111, 1040–1063. [Google Scholar] [CrossRef] [PubMed]
- Pouzar, Z. New genera of higher fungi III. Ceská Mykol. 1959, 13, 10–19. [Google Scholar]
- Jülich, W. Studies in resupinate Basidiomycetes—V. Persoonia 1978, 10, 137–140. [Google Scholar]
- Song, J.; Xing, J.H.; Ji, X.; Sun, Y.F.; Cui, B.K.; Dai, Y.C. Rigidotubus tephroleucus gen. et sp. nov. (Cystostereaceae, Agaricales) evidenced by morphological characters and phylogenetic analyses. Phytotaxa 2018, 333, 259–266. [Google Scholar] [CrossRef]
- He, M.Q.; Zhao, R.L.; Hyde, K.D.; Begerow, D.; Kemler, M. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; E. Methuen and Co., Ltd.: London, UK, 1978. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017, 20, 1160–1166. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. 2018. Version 3.5.1. Available online: http://www.mesquiteproject.org (accessed on 1 August 2022).
- Swofford, D.L. PAUP *: Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hőhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Larsson, K.H.; Matheny, P.B.; Hibbett, D.S. Amylocorticiales ord. nov. and jaapiales ord. nov.: Early diverging clades of agaricomycetidae dominated by corticioid forms. Mycologia 2010, 102, 865–880. [Google Scholar] [CrossRef]
- Larsson, K.H.; Larsson, E.; Kljalg, U. High phylogenetic diversity among corticioid homobasidiomycetes. Mycol. Res. 2004, 108, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.S.; Han, T.; Li, W.C.; Jia, M.; Xue, L.M.; Rahman, K.; Qin, L.P. Geographic and tissue influences on endophytic fungal communities of taxus chinensis var. mairei in china. Curr. Microbiol. 2013, 66, 40–48. [Google Scholar] [CrossRef]
- Varga, T.; Krizsan, K.; Foldi, C.; Dima, B.; Sanchez-Garcia, M.; Sanchez-Ramirez, S.; Szollosi, G.J.; Szarkandi, J.G.; Papp, V.; Albert, L.; et al. Megaphylogeny resolves global patterns of mushroom evolution. Nat. Ecol. Evol. 2019, 3, 668–678. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; De, V.M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal dna barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2018, 92, 135–154. [Google Scholar] [CrossRef]
- Binder, M.; Hibbett, D.S.; Larsson, K.H.; Larsson, E.; Langer, E.; Langer, G. The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (homobasidiomycetes). Syst. Biodivers. 2005, 3, 113–157. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, H.; He, S.H.; Dai, Y.C. Radulotubus resupinatus gen. et sp. nov. with a poroid hymenophore in pterulaceae (agaricales, basidiomycota). Nova Hedwig. 2016, 103, 265–278. [Google Scholar] [CrossRef]
- Larsson, K.H.; Ryvarden, L. Corticioid Fungi of Europe, Volume 1: Acanthobasidium—Gyrodontium. Synopsis Fungorum; Fungiflora: Oslo, Norway, 2021; Volume 43, pp. 1–266. [Google Scholar]
- Hallenberg, N.; Ryvarden, L. Studies in the Aphyllophorales of Africa 5. Cystostereum artocreas, new to Africa. Mycotaxon 1975, 2, 135–141. [Google Scholar]
- Ryvarden, L. Studies in the Aphyllophorales of Africa 6. Some species from eastern Central Africa. Bull. Jard. Bot. Natl. Belg. 1978, 48, 79–119. [Google Scholar] [CrossRef]
- Niemelä, T.; Renvall, P.; Hjortstam, K. Hagenia abyssinica and its fungal decayers in natural stands. Edinb. J. Bot. 1998, 55, 473–484. [Google Scholar] [CrossRef]
- Wu, S.H. Survey of corticioid fungi in Taiwan, to 2010. Fungal Sci. 2010, 25, 49–60. [Google Scholar]
- Hjortstam, K.; Ryvarden, L. Some new and noteworthy fungi (Aphyllophorales, Basidiomycetes) from Iguazu, Argentina. Mycotaxon 1986, 25, 539–567. [Google Scholar]
- Hjortstam, K. Studies in tropical Corticiaceae (Basidiomycetes). V. Specimens from East Africa collected by L. Ryvarden. Mycotaxon 1983, 17, 555–572. [Google Scholar]
- Hjortstam, K. Corticioid fungi described by M.J. Berkeley II. Species from Cuba. Mycotaxon 1990, 39, 415–423. [Google Scholar]
- Dai, Y.C. A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 2011, 52, 69–79. [Google Scholar] [CrossRef]
- Hjortstam, K. Studies in tropical Corticiaceae (Basidiomycetes) VII. Specimens from East Africa collected by L. Ryvarden. II. Mycotaxon 1987, 28, 19–37. [Google Scholar]
- Kaur, R.; Singh, A.P.; Dhingra, G.S. Cystostereum sirmaurense sp. nov. from India. Mycotaxon 2019, 134, 577–580. [Google Scholar] [CrossRef]
- Hjortstam, K.; Ryvarden, L. Some new tropical genera and species of corticoid fungi (Basidiomycotina, Aphyllophorales). Synop. Fungorum 2004, 18, 20–32. [Google Scholar]
- Baltazar, J.M.; Da Silveira, R.M.B.; Rajchenberg, M. Type studies of J. Rick’s corticioid homobasidiomycetes (Agaricomycetes, Basidiomycota) housed in the Herbarium Anchieta (PACA). Phytotaxa 2016, 255, 101–132. [Google Scholar] [CrossRef]
- Riebeseh, J.; Yurchenko, E.; Nakasone, K.K.; Langer, E. Phylogenetic and morphological studies in Xylodon (Hymenochaetales, Basidiomycota) with the addition of four new species. MycoKeys 2019, 47, 97–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, C.Y.; Wu, S.H. Species diversity, taxonomy and multi-gene phylogeny of phlebioid clade (Phanerochaetaceae, Irpicaceae, Meruliaceae) of Polyporales. Fungal Divers. 2021, 111, 337–442. [Google Scholar] [CrossRef]
- Liu, S.L.; Nakasone, K.K.; Wu, S.H.; He, S.H.; Dai, Y.C. Taxonomy and phylogeny of Lopharia s.s., Dendrodontia, Dentocorticium and Fuscocerrena (Basidiomycota, Polyporales). MycoKeys 2018, 32, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Hjortstam, K.; Ryvarden, L. Lopharia and Porostereum (Corticiaceae); Fungiflora: Oslo, Norway, 1990. [Google Scholar]
- Dai, Y.C. Notes on Lopharia mirabilis (Berk. & Broome) Pat. in China. Fungal Sci. 2002, 17, 31–38. [Google Scholar]
- Deng, C.Y.; Shi, L.Y.; Wang, J.; Xiang, Z.; Li, S.M.; Li, G.J.; Yang, H. Species diversity of the Russula virescens complex “qingtoujun” in southern China. Mycosystema 2020, 39, 1661–1683. [Google Scholar]
- Du, R.; Dai, Y.C. A new species and three new combinations of Skeletocutis (Incrustoporiaceae, Basidiomycota). Mycosystema 2020, 39, 637–644. [Google Scholar]
- Ge, Y.P.; Bau, T. Descriptions of six new species of Crepidotus from China. Mycosystema 2020, 39, 238–255. [Google Scholar]
- Li, G.J.; Deng, C.Y.; Shi, L.Y.; Wang, J.; Meng, Q.F.; Li, S.M. Three new species of Russula subsect. Lactarioideae from China. Mycosystema 2020, 39, 618–636. [Google Scholar]
- Wu, F.; Tohtirjap, A.; Fan, L.F.; Zhou, L.W.; Alvarenga, R.L.M.; Gibertoni, T.B.; Dai, Y.C. Global diversity and updated phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 2021, 7, 933. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, J.J.; Korhonen, K.; Martin, F.; Dai, Y.C. An updated global species diversity and phylogeny in the forest pathogenic genus Heterobasidion (Basidiomycota, Russulales). Front. Microbiol. 2021, 11, 596393. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, L.W.; Vlasák, J.; Dai, Y.C. Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, H.S.; Zhou, L.W.; Yuan, Y.; Cui, B.K.; Dai, Y.C. Polypore diversity in South China. Mycosystema 2020, 39, 653–682. [Google Scholar]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Wu, G.; Yuan, H.S.; Zhou, L.W.; He, S.H.; Ge, Z.W.; Wu, F.; Wei, Y.L.; et al. Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 2021, 40, 770–805. [Google Scholar]
- Ma, H.X.; Si, J.; Dai, Y.C.; Zhu, A.H.; Cui, B.K.; Fan, Y.G.; Yuan, Y.; He, S.H. Diversity of wood-inhabiting macrofungi in Hainan Province, South China. Mycosystema 2022, 41, 695–712. [Google Scholar]



| Taxa | Voucher | Locality | ITS | nrLSU | Reference |
|---|---|---|---|---|---|
| Aphanobasidium pseudotsugae | HHB-822 | USA | — | GU187567 | [17] |
| Athelia pyriformis | Hjm 18581 | Sweden | — | EU118605 | [2] |
| Athelidium aurantiacum | KHL 11068 | Sweden | — | EU118606 | [2] |
| Chondrostereum purpureum | He 5634 | China | MW557935 | MW557942 | Unpublished |
| Clavaria fumosa | KGN98 | Sweden | — | AY586646 | [18] |
| Coniophora puteana | FP-105438 | USA | — | KJ995933 | Unpublished |
| Coniophora olivacea | FP-104386 | USA | — | GU187572 | [17] |
| Coronicium alboglaucum | NH4208 | Sweden | — | AY586650 | [18] |
| Cristinia helvetica | Kristiansen s.n. | Norway | — | EU118620 | [2] |
| Crustomyces albidus | He 6164 * | China | ON117191 | ON117175 | present study |
| Crustomyces albidus | CLZhao 6194 | China | MK404325 | — | Unpublished |
| Crustomyces albidus | CLZhao 21168 | China | OM955829 | — | Unpublished |
| Crustomyces albidus | Z40 | China | JN198404 | — | [19] |
| Crustomyces isabellinus | Yuan 12642 | Vietnam | ON117185 | ON117170 | present study |
| Crustomyces isabellinus | Yuan 12644 | Vietnam | ON117186 | ON117171 | present study |
| Crustomyces isabellinus | He 3582 | China | ON117188 | ON117172 | present study |
| Crustomyces isabellinus | He 4766 | China | ON117189 | ON117173 | present study |
| Crustomyces laminiferus | KHL 13057 | Costa Rica | EU118622 | EU118622 | [2] |
| Crustomyces laminiferus | TF10 | Nigeria | MH625705 | MH625705 | Unpublished |
| Crustomyces sp | Cui 12288 | China | ON117190 | ON117174 | present study |
| Crustomyces sp | HHB-19125 | New Zealand | MW740274 | — | Unpublished |
| Crustomyces subabruptus | DLL2011-303 | USA | KJ140775 | — | Unpublished |
| Crustomyces subabruptus | BK323 | Czech Republic | LR961824 | — | Unpublished |
| Crustomyces subabruptus | K(M):249975 | UK | MZ159685 | — | Unpublished |
| Crustomyces subabruptus | PC0706577 | France | MF183941 | — | Unpublished |
| Crustomyces subabruptus | SM14-27-5-4 | USA | MN905889 | — | Unpublished |
| Crustomyces subabruptus | G0365 | Poland | — | MK277900 | [20] |
| Crustomyces tephroleucus | Cui 13653 | China | MF290418 | MF290416 | [5] |
| Crustomyces tephroleucus | Cui 13717 * | China | MF290417 | MF290415 | [5] |
| Cystostereum murrayiⅠ | CBS 413.34 | Germany | MH855588 | MH867098 | [21] |
| Cystostereum murrayiⅠ | G0517 | USA | — | MK277917 | [20] |
| Cystostereum murrayiⅡ | KHL 12496 | Sweden | EU118623 | EU118623 | [2] |
| Cystostereum murrayiⅡ | G1903 | Norway | — | MK277916 | [20] |
| Cystostereum murrayiⅡ | CBS 257.73 | Germany | — | MH878328 | [21] |
| Cystostereum crassisporum | He 3995 | China | — | ON117178 | present study |
| Cystostereum crassisporum | He 4740 * | China | ON117193 | ON117179 | present study |
| Cystostereum submurrayi | He 2301 * | China | — | ON117176 | present study |
| Cystostereum submurrayi | He 4379 | China | ON117192 | ON117177 | present study |
| Cystostereum submurrayi | WEI 18-033 | China | OM942662 | OM919711 | present study |
| Cystostereum submurrayi | WEI 18-405 | China | OM942664 | OM919713 | present study |
| Dendrothele acerina | GEL5350 | Germany | — | AJ406581 | Unpublished |
| Dendrothele americana | CFMR:101995 | USA | — | NG_071235 | [22] |
| Effusomyces thailandicus | He 4055c * | Thailand | ON117194 | ON117180 | present study |
| Effusomyces thailandicus | He 4126 | Thailand | — | ON117181 | present study |
| Gloeostereum incarnatum | KUC20131022-28 | South Korea | — | KJ668393 | Unpublished |
| Gloeostereum incarnatum | 3332 | Sweden | — | AF141637 | Unpublished |
| Hyphodontiella multiseptata | Ryberg 021022 | Sweden | — | EU118634 | [2] |
| Lichenomphalia umbellifera | Rova 2501 | Sweden | — | EU118645 | [2] |
| Lindtneria trachyspora | KGN 390/00 | Sweden | — | EU118646 | [2] |
| Merulicium fusisporum | Hjm s.n. | Sweden | — | EU118647 | [2] |
| Parvodontia austrosinense | He 4339 | China | — | ON117182 | present study |
| Parvodontia austrosinense | He 4730 * | China | ON117195 | ON117183 | present study |
| Pterula echo | AFTOL-ID 711 | USA | — | AY629315 | Unpublished |
| Radulomyces confluens | Cui 5977 | China | — | KU535669 | [23] |
| Radulomyces confluens | He 2224 | China | — | KU535670 | [23] |
| Radulomyces copelandii | Dai 15061 | China | — | KU535672 | [23] |
| Radulomyces molaris | ML 0499 | Sweden | — | AY586705 | [18] |
| Radulotubus resupinatus | Cui 8383 | China | — | KU535668 | [23] |
| Radulotubus resupinatus | Dai 15315 | China | — | KU535666 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Nakasone, K.K.; Chen, C.-C.; Zhao, C.-L.; Cao, T.; Yuan, H.-S.; He, S.-H. Taxonomy and Phylogeny of Cystostereaceae (Agaricales, Basidiomycota): A New Genus, Five New Species, and Three New Combinations. J. Fungi 2022, 8, 1229. https://doi.org/10.3390/jof8111229
Li Y, Nakasone KK, Chen C-C, Zhao C-L, Cao T, Yuan H-S, He S-H. Taxonomy and Phylogeny of Cystostereaceae (Agaricales, Basidiomycota): A New Genus, Five New Species, and Three New Combinations. Journal of Fungi. 2022; 8(11):1229. https://doi.org/10.3390/jof8111229
Chicago/Turabian StyleLi, Yue, Karen K. Nakasone, Che-Chih Chen, Chang-Lin Zhao, Ting Cao, Hai-Sheng Yuan, and Shuang-Hui He. 2022. "Taxonomy and Phylogeny of Cystostereaceae (Agaricales, Basidiomycota): A New Genus, Five New Species, and Three New Combinations" Journal of Fungi 8, no. 11: 1229. https://doi.org/10.3390/jof8111229
APA StyleLi, Y., Nakasone, K. K., Chen, C.-C., Zhao, C.-L., Cao, T., Yuan, H.-S., & He, S.-H. (2022). Taxonomy and Phylogeny of Cystostereaceae (Agaricales, Basidiomycota): A New Genus, Five New Species, and Three New Combinations. Journal of Fungi, 8(11), 1229. https://doi.org/10.3390/jof8111229







