Abstract
Floccularia luteovirens is a rare edible and medicinal fungus endemic to the Qinghai–Tibet Plateau, prized for its abundance of high-value bioactive metabolites such as polysaccharides, terpenoids, and ergothioneine, which exhibit a variety of biological activities including immunomodulatory, antioxidant, and antitumor effects. Due to the current lack of successful domestication and limited wild resources, liquid fermentation technology has become an important strategy for the large-scale production of its mycelium and bioactive components. This review systematically summarizes the biological characteristics of F. luteovirens, the diversity of its metabolites, biosynthetic pathways, regulatory mechanisms influenced by environmental factors, and the application of multi-omics technologies in related research. It is suggested that future studies should integrate multi-omics approaches to elucidate its stress response and metabolic regulatory networks, and achieve high-value utilization of this resource through stress-resistant breeding and optimization of fermentation processes.
1. Introduction
Rare edible and medicinal fungi represent a significant component of the edible mushroom industry, possessing considerable economic value and serving as a key focus of scientific research. Globally, approximately 150,000 fungal species have been identified, of which over 2000 are edible and widely distributed across Asia, Europe, the Americas, Africa, and Oceania [,]. To date, more than 100 species of edible and medicinal fungi have been successfully cultivated artificially. According to FAO statistics from 2018, the global annual production of mushrooms has reached tens of millions of tons, with Asia remaining the dominant producing region []. China, with its vast territory and rich fungal resources, has seen an increasing number of rare edible and medicinal species identified and developed in recent years, driven by technological progress, indicating promising industrial prospects. Among these, Floccularia luteovirens (Alb. & Schwein.) Pouzar, a rare edible and medicinal fungus endemic to the alpine meadows of the Qinghai–Tibet Plateau, is of particular research and development value due to its abundance of bioactive compounds such as polysaccharides, terpenoids, and flavonoids [].
However, the utilization of F. luteovirens faces multiple challenges. The species has not yet been successfully domesticated, and reliance on limited wild resources is insufficient to meet demand []. From a scientific perspective, although existing studies have revealed the potential of certain bioactive components (e.g., polysaccharides and terpenoids) in immunomodulation, antibacterial, antioxidant, and antitumor activities in vitro [,], there remains a lack of systematic in vivo functional validation, metabolic pathway analysis, and detailed interpretation of structure–activity relationships []. Moreover, while liquid fermentation is regarded as an effective approach for the large-scale production of mycelium and active metabolites (such as proteins, polysaccharides, and terpenoids) [], issues such as low target metabolite yields and unclear metabolic regulation mechanisms still hinder its efficient development. Therefore, it is imperative to strengthen fundamental biological studies to decipher the molecular regulatory networks underlying its growth and secondary metabolism. Concurrently, liquid fermentation processes should be optimized, and metabolic engineering strategies should be employed to enhance the production of target compounds. Systematic pharmacological evaluation and safety assessments are also needed to facilitate the translation of laboratory findings into functional products.
To address these challenges, this review systematically outlines the research trajectory of F. luteovirens, from its ecological distribution to comprehensive development, focusing on the following aspects: assessing ecological characteristics and resource status, employing multi-omics analyses to reveal its secondary metabolic potential, elucidating the biosynthetic pathways and regulatory mechanisms of key active ingredients, and guiding the optimization of fermentation and extraction processes—including nutrient and environmental parameter modulation, fermentation process intensification, and improvements in extraction and drying strategies. Ultimately, this work aims to promote the application of F. luteovirens in functional foods and drug lead compounds, providing a reference for the high-value development of rare edible fungi. Therefore, this review aims to systematically consolidate the current knowledge on the biosynthesis, regulation, and utilization of metabolites in F. luteovirens to bridge the gap between its fundamental biology and industrial application. Specifically, we seek to (1) summarize the diversity and bioactivities of key metabolites, such as polysaccharides and terpenoids; (2) elucidate their biosynthetic pathways and the molecular and environmental mechanisms regulating their production; (3) evaluate advances and challenges in artificial cultivation and liquid fermentation for scalable production; and (4) identify critical research gaps and propose future directions for its high-value exploitation. By integrating research from ecological distribution to process optimization, this work provides a comprehensive framework to advance the use of F. luteovirens in functional foods and pharmaceutical leads.
2. Methodology
2.1. Search Strategies and Number of References
To ensure the systematicity, transparency, and reproducibility of this review, a rigorous literature search and screening process was implemented. The specific strategies and criteria are detailed below.
2.2. Screening Criteria and Process
The literature screening followed a structured process to minimize bias: (1) Initial Screening: Duplicate articles removed using reference management software—Zotero 7.0.30. (2) Title and Abstract Screening: The remaining articles were screened based on their titles and abstracts against the inclusion and exclusion criteria. Irrelevant studies were excluded at this stage. (3) Full-Text Assessment: The full texts of the potentially relevant articles were retrieved and thoroughly evaluated to determine their final eligibility.
2.3. Search the Database
The search was performed in the following electronic databases to ensure comprehensive coverage: Web of Science core collection, PubMed, Science Direct, CNKI (China National Knowledge Infrastructure), Wanfang Data.
2.4. Search Keywords
The search strategy employed a combination of keywords and their synonyms related to the organism and its metabolites. Key English terms included: “Floccularia luteovirens”, “Armillaria luteo-virens” (Former name), “yellow mushroom”, “polysaccharide”, “terpenoid”, “biosynthesis”, “fermentation”, “metabolite”, and “genome”. Corresponding Chinese keywords (e.g., “黄绿卷毛菇”, “黄绿蜜环菌”, “多糖”, “萜类”, “发酵”) were used for Chinese databases. Boolean operators (AND, OR) were applied to combine these terms effectively.
2.5. Time Range
The literature search covered the period from the inception of each respective database up to September 2025, to include both foundational historical research and the most recent advances in the field.
3. Biological Characteristics and Ecological Adaptations of F. luteovirens
3.1. Geographical Distribution and Habitat
F. luteovirens is a valuable edible fungus endemic to high-altitude areas in China, with its core geographical distribution centered on the Qinghai–Tibet Plateau and its surrounding areas. This range specifically encompasses Qinghai Province, Tibet Autonomous Region, and the Garzê Tibetan Autonomous Prefecture in Sichuan Province, spanning latitudes from 29°33′ to 38°09′ N and longitudes from 90°04′ to 102°01′ E []. In terms of its habitat, this species relies on high-altitude grassland and alpine meadow ecosystems, with a vertical distribution range of 3200~4800 m, and the unique plateau soil texture and climatic conditions together constitute its ideal habitat []. Specific climatic parameters show that in terms of temperature, it is highly adaptable and can tolerate the cold environment of the plateau, with the average temperature of the coldest month (January) ranges from −17.4–3.8 °C, and the average temperature of the summer and autumn seasons (June to September), the average temperature is between 6.2 °C and 15.9 °C. Regarding humidity, it experiences distinct moisture conditions: the mean relative humidity during the growing season is 41% to 74%, annual precipitation ranges from 344 to 574 mm, mean annual evaporation is 1393.8 to 2441.4 mm, and the humidity coefficient ranges from 0.42 to 0.78 []. These moisture characteristics, defined by “low precipitation and high evaporation” align well with the arid high-altitude environment.
3.2. Adaptation Mechanisms
3.2.1. Environmental Adaptability of Macro Growth Cycle
The habitat specificity of high-altitude grassland on the Tibetan Plateau imposes compound extreme environmental pressure on F. luteovirens: in addition to strong ultraviolet radiation, it is subject to multiple limiting factors such as low-temperature stress, sharp diurnal temperature differences (up to 15–20 °C), and low soil organic matter content (resulting in nutrient-poor conditions), with synergies existing between these factors. This multifaceted stress regime acts as a potent selective filter across its entire life cycle, from mycelial colonization and primordium initiation to fruiting body maturation. In response, F. luteovirens has evolved a suite of targeted adaptive strategies, integrating eco-physiological adjustments in growth phenology with molecular-level mechanisms—such as enhanced antioxidant defense and stress-responsive gene expression—to successfully colonize this challenging ecological niche.
The growth cycle of F. luteovirens exhibits strict seasonality and environmental dependence: fruiting bodies appear only briefly from late summer to early autumn (June to September), a period that coincides with rising temperatures on the plateau and relatively concentrated rainfall. Its growth process is jointly regulated by soil temperature and humidity—only when soil temperature stabilizes between 6 and 16 °C and humidity remains at 40–70% can the mycelium rapidly expand and differentiate to form fruiting bodies. As temperatures drop (after October), the fungus enters dormancy, shortening its growth window to avoid unfavorable conditions [].
3.2.2. Adaptation Mechanisms at the Molecular Level
Currently, multi-omics technologies—including genome, transcriptome, metabolome, proteome analyses—have become an important tool to analyze the adaptive mechanisms of biological environments—by analyzing multi-dimensional genomics data under droughts, low temperatures, salinity and alkalinity, the adaptive strategies of organisms in the areas of gene expression regulation, metabolic network remodeling and functional protein synthesis can be systematically revealed [,,,]. As summarized in Table 1, recent molecular omics studies have advanced our understanding of environmental adaptation in F. luteovirens. However, research on its molecular mechanisms underlying environmental adaptation remains preliminary. Current studies have predominantly focused on transcriptomic responses to single-factor UV stress, leading to the identification of several differentially expressed genes associated with pigment synthesis and antioxidant responses—such as those involved in riboflavin biosynthesis []. Yet these findings fail to capture the integrated regulatory network activated under the combined stresses of low temperature, high UV radiation, and nutrient deficiency that characterize its natural alpine habitat. Furthermore, metabolic characteristics across different developmental stages—mycelial, primordium, and fruiting body phases—and their dynamic coupling with environmental factors remain to be fully elucidated.

Table 1.
Molecular Omics Study on the Environmental Adaptation and Response of F. luteovirens.
A key area warranting in-depth exploration is whether the adaptation of F. luteovirens to plateau extremes relies on an integrated signal perception and transduction system, potentially centered on the crosstalk and amplification of multiple stress signals at the second messenger level. One critical scientific question arises: under combined stress conditions, does F. luteovirens employ a signaling hub composed of secondary messengers such as ROS, Ca2+, and NO—similar to the mechanism identified in Ganoderma species as described in Section 4.2.2? If so, how does this network dynamically integrate upstream signals—UV radiation, low temperature, and nutrient scarcity—to coordinately regulate downstream physiological processes including pigment synthesis, antioxidant defense, and energy metabolism? A systematic dissection of these questions will provide a comprehensive understanding of the alpine adaptation mechanisms of F. luteovirens from an integrative biology perspective, and establish a theoretical foundation for its artificial domestication and targeted metabolic regulation.
4. Bioactive Metabolites of F. luteovirens
4.1. Diversity of Bioactive Metabolites
4.1.1. Metabolome Characteristics and Product Diversity of Mycelium
F. luteovirens is rich in proteins, amino acids, sugars, alkaloids, and small amounts of organic acids, flavonoids, cardiac glycosides, steroidal triterpenoids, glycosides, and saponins []. In edible fungi research, the application of metabolomics helps to systematically analyze macrofungal metabolites, elucidate metabolic patterns [], screen bioactive substances, and optimize cultivation conditions [], offering important technological support for the in-depth exploration of the metabolic potential of mycelium. However, metabolomic studies on F. luteovirens are mainly limited to comparative analyses of the composition of wild fruiting bodies, while the metabolite profiles of the mycelial stage remain less studied. As metabolites in the mycelium serve as the basis for the synthesis and accumulation of bioactive substances, and given that metabolomics, as a comprehensive analytical method, is capable of systematically analyzing low-molecular-weight metabolites in organisms, it directly reflecting metabolic changes under different conditions []. The study by Liu et al. provided a 27 Mb reference-level genome of F. luteovirens, containing 7068 protein-coding genes, and predicted the genome composition and gene functions []. Genome enrichment and pathway analysis indicated the potential biosynthesis of terpenoids, polyketides, and polysaccharides in F. luteovirens. Previous studies have analyzed the enzyme kinetics and metabolic profiling of the liquid culture system of F. luteovirens; untargeted metabolomics identified more than 3569 metabolites, including 148 terpenoids, 26 alkaloids, and 31 flavonoids in liquid cultured mycelia over a 20-day culture cycle, with differential metabolites focusing mainly on carboxylic acid derivatives and lipids [].
4.1.2. Key Signature Metabolites
Recent studies have shown that geographic differences in the antioxidant activity of F. luteovirens are mainly associated with the regulation of its phenylalanine biosynthesis and metabolic pathways. Targeted metabolomics analysis revealed that vanillic acid may be a key metabolite affecting its antioxidant capacity []. Further results showed that vanillic acid content varied significantly among samples from different geographical sources and exhibited a significant positive correlation with total flavonoid content, glutathione level, and hydroxyl radical scavenging capacity (Figure 1). Consequently, vanillic acid was identified as a key marker for distinguishing antioxidant activity. On the other hand, the unique yellow phenotype of this species was mainly attributed to the accumulation of riboflavin, whose content directly determines the color expression of the fruiting bodies, and the expression of the riboflavin transporter protein gene FIMCH5 was closely associated with the formation of this phenotype []. Based on the above findings, vanillic acid and riboflavin can be used as important chemical markers for origin identification, authenticity recognition, and quality assessment of F. luteovirens. The riboflavin content is utilized for morphology-assisted identification, while the vanillic acid level is used for an effective assessment of antioxidant quality.
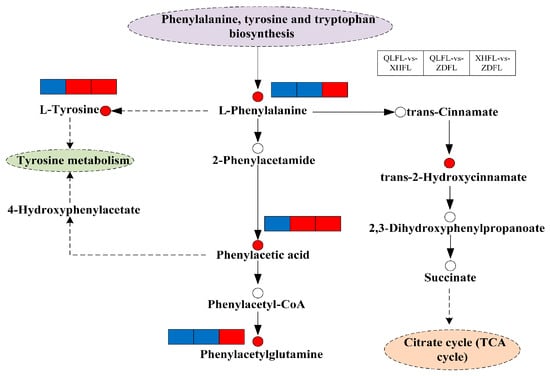
Figure 1.
Overview of metabolic pathways mapped to possible regulation of key metabolites in pairwise comparisons of F. luteovirens from different regions. Note: Small red circles indicate significant upregulation of metabolites; small empty circles indicate undetected metabolites; small red rectangles indicate significant upregulation of metabolites between groups; small blue rectangles indicate significant downregulation of metabolites between groups; solid arrows represent facilitation, and dotted arrows represent indirect facilitation (Tang et al., 2024 []).
4.2. Biological Function of Key Active Ingredients
4.2.1. Polysaccharides
Current research on the key bioactive components of F. luteovirens is summarized in Table 2. The polysaccharides exhibit remarkable antioxidant and antitumor activities, along with hygroscopic and moisturizing properties, demonstrating their broad application potential in health foods and cosmetics [,,]. Specifically, the F. luteovirens polysaccharide (FLP1) from the fruiting bodies significantly enhances intestinal immune responses via the MAPK/Nrf2 signaling pathway, improves gut microbiota structure, and effectively alleviates immunosuppression [,]. It is important to note, however, that these compelling findings are predominantly derived from mouse models. Their relevance to human physiology remains to be established, and the exact structural motifs of FLP1 responsible for these effects are yet to be conclusively identified. Multiple studies have shown that both F. luteovirens polysaccharides (FLPs) and exopolysaccharides (FLEP) display significant scavenging capacities against DPPH and ABTS free radicals, inhibit cancer cell proliferation in vitro, and exert protective effects against hydrogen peroxide-induced oxidative damage in cell models [,]. While these results are promising, the direct correlation between simple chemical antioxidant assays and complex in vivo health benefits is often weak, representing a common challenge in the field of functional food research. Furthermore, owing to their excellent moisture absorption and retention properties, FLPs show promising cosmetic applications. Notably, the exopolysaccharide exhibits both antibacterial and tyrosinase inhibitory activities, making it suitable for preserving aquatic products such as Pacific white shrimp and significantly extending their shelf life [,]. Recent studies also indicate that edible coatings formulated with FLPs and chitosan can be effectively applied in the preservation of aquatic products like tuna [].

Table 2.
Biological Functions of Key Bioactive Constituents from F. luteovirens.
4.2.2. Terpenoids
Terpenoids represent a major class of bioactive natural products in fungi, classified based on the number of isoprene units into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30), and tetraterpenes (C40). Their biosynthesis is initiated from the universal precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) [,]. Fungi serve as a prominent source of diverse terpenoids. Advances in sequencing technologies and bioinformatics have established genome mining as one of the most powerful strategies for discovering novel terpenoids from fungal genomes []. To date, numerous terpene cyclases have been identified, including canonical class I and class II terpene cyclases, emerging UbiA-type membrane-bound terpene cyclases, as well as various tailoring enzymes such as cytochrome P450 monooxygenases, flavin-dependent monooxygenases, and acyltransferases []. Among terpenoids, triterpenes are predominantly distributed in certain basidiomycetes. Lanosterol serves as the key biosynthetic precursor for ergosterol and other triterpenoid metabolites, which exhibit a range of pharmacological activities including antitumor and immunomodulatory effects []. Meanwhile, sesquiterpenoids also demonstrate notable bioactivities. For instance, protoilludane-type sesquiterpene aryl esters extracted from F. luteovirens showed significantly improved yield following process optimization. These compounds exhibit remarkable antioxidant activity, with DPPH and hydroxyl radical scavenging rates reaching (62.60 ± 1.88)% and (95.99 ± 1.74)%, respectively. Furthermore, they displayed potent inhibitory effects against Escherichia coli, Staphylococcus aureus, and Bacillus cereus, highlighting their potential as natural food antioxidants and preservatives []. However, the evidence is currently limited to in vitro assays. Future research must address critical aspects such as efficacy in real food matrices, sensory impact, and in vivo safety to substantiate their practical application.
4.2.3. Other High-Value Compounds
Studies have shown that F. luteovirens is rich in a variety of highly bioactive compounds, exhibiting significant development potential. Using hydrophilic interaction chromatography (HILIC) coupled with online HPLC-DPPH activity screening, high-purity L-(+)-ergothioneine was efficiently isolated from its methanol extract. This compound is a rare amino acid-derived antioxidant, with a content as high as 5.36 mg/g in the fruiting bodies. It can accumulate within cells via specific transporters, efficiently scavenge free radicals, and is hailed as the “longevity vitamin,” making it an excellent source for functional foods and health products []. Furthermore, the low molecular weight fraction (<6 kDa) obtained by hollow fiber membrane fractionation significantly induced apoptosis in non-small cell lung cancer cells by activating the Caspase-3 pathway. The active constituents include various amino acids, nucleosides, terpenoids, and alkaloids, providing a new direction for anti-tumor drug development []. Simultaneously, the F. luteovirens water extract (FLW), as a multi-component complex system, demonstrated comprehensive efficacy in a type II diabetes model by activating the Nrf2/HO-1 pathway and inhibiting the mTOR/GSK-3β pathway, thereby improving glucose and lipid metabolism, and alleviating oxidative stress and inflammatory responses. In a migraine model, it also modulated neurotransmitter levels, effectively alleviating pain and inflammation, showcasing multi-target regulatory therapeutic characteristics and broad application prospects in the prevention and adjuvant treatment of metabolic and neurological diseases [,]. Additionally, lipids extracted via supercritical CO2 extraction were analyzed by GC-MS, identifying 25 fatty acids, including linoleic acid (relative content 10.6%) and other unsaturated fatty acids, further clarifying the material basis for its nutritional and functional components [].
4.3. Major Biosynthetic Pathways
4.3.1. Polysaccharide Biosynthetic Pathway
Research on the polysaccharide structure of F. luteovirens remains at an early stage. Liu et al. isolated and purified a major polysaccharide fraction, GLP, using Sepharose CL-6B gel column chromatography []. Structural characterization by infrared spectroscopy and nuclear magnetic resonance (NMR) revealed that GLP is an arabinoxylan composed of α-D-xylopyranose and β-D-arabinopyranose residues, featuring a backbone of 1 → 4 linkages with 1 → 6 branched chains. In another study, Liu Yan characterized the molecular structures of ALP-1, ALP-2, and ALP-3 using an integrated approach combining partial degradation, methylation analysis, NMR spectroscopy, and FT-IR []. All three polysaccharides were identified as glucans. ALP-1 primarily contains T-, 1,3-, and 1,3,6-linked glucopyranosyl residues, representing a β-1,3-glucan backbone branched at the C6 position. In contrast, ALP-2 and ALP-3 are characterized by α-1,4-linked backbones, classifying them as glycogen-like or amylose-like glucans.
Our group has elucidated the dynamic process of polysaccharide biosynthesis in F. luteovirens at the physiological level by analyzing enzyme activities and metabolite profiles across different culture periods. Polysaccharide production peaked on day 20, with intracellular polysaccharides reaching 23.78 ± 0.90 mg/g and extracellular polysaccharides at 0.93 ± 0.11 mg/mL. This period also coincided with maximal activities of hydrolytic enzymes such as amylase and cellulase, which degrade macromolecular carbon sources into small sugars, thereby supplying essential precursors like UDP-glucose and UDP-xylose for polysaccharide assembly []. Metabolomic analysis further indicated continuous accumulation of amino acids—including L-glutamine, L-histidine, L-tyrosine, and L-proline—throughout the cultivation, with significant enrichment of metabolic pathways such as alanine, aspartate, and glutamate metabolism. This active amino acid metabolism not only furnishes energy (ATP) and reducing equivalents (NADPH) but also provides amino donors—particularly glutamine—for the biosynthesis of amino sugars (e.g., glucosamine, a component of chitin and certain polysaccharides) [].
Ultimately, polysaccharide biosynthesis is tightly regulated at the genetic level. Although no gene clusters directly involved in polysaccharide synthesis have been reported in F. luteovirens, studies on other edible macro-fungi suggest that the synthesis of different polysaccharide types relies on specific glycosyltransferase (GT) gene families [,]. The expression of these genes is finely tuned by cultural conditions, nutrient availability, and metabolic cues—including the aforementioned amino acids—thereby governing the efficiency, chain length, and branching pattern of polysaccharide synthesis. We thus hypothesize that supplementing the culture medium with specific amino acids may not only boost polysaccharide yield by supplying precursors and energy but also modulate the expression of key biosynthetic genes via upstream signaling pathways, leading to more efficient polysaccharide production. Future work should focus on identifying these key genes and elucidating their regulatory mechanisms, thereby establishing a foundation for the targeted synthetic biological engineering of FLPs.
4.3.2. Triterpenoid Biosynthetic Pathways
Fungi within the Ascomycota and Basidiomycota phyla are prolific producers of structurally diverse terpenoids. While the biosynthesis of several sesquiterpenoid mycotoxins and bioactive diterpenoids in ascomycetes has been extensively characterized, the molecular mechanisms underlying terpenoid biosynthesis in basidiomycetes—including many macrofungi—remain largely unexplored, despite their capacity to synthesize a wide array of terpenoid natural products [].
Among metabolites, triterpenoids represent core pharmacologically active constituents, and their biosynthetic pathways along with associated environmental regulation are increasingly becoming a research focus. The mevalonate (MVA) pathway is widely recognized as the primary route for triterpenoid biosynthesis: acetyl-CoA is sequentially converted by a series of enzymes, including the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), to farnesyl pyrophosphate (FPP) []. FPP is then processed by squalene synthase (SQS) and squalene epoxidase (SE), culminating in cyclization by lanosterol synthase (LS) to form the triterpenoid skeleton. Subsequent modifications by cytochrome P450 monooxygenases contribute to the structural diversity of triterpenoids [,]. HMGR serves as the key rate-limiting enzyme governing the efficiency of this pathway.
Advances in genomics have enabled the use of bioinformatics tools to mine biosynthetic gene clusters (BGCs), providing a powerful approach to elucidate secondary metabolite biosynthesis []. For instance, in the basidiomycete Hypholoma sublaterium—a producer of the antitumor compound clavicoric acid—oxidosqualene synthase and squalene epoxidase genes have been identified as likely key players in its biosynthesis []. Genomic sequencing (27 Mb) and functional annotation of F. luteovirens by Liu et al. further revealed considerable potential for the synthesis of metabolites such as terpenoids, polyketides, and polysaccharides in this species [].
Environmental regulation is increasingly regarded as an effective strategy for activating silent BGCs and optimizing the production of target metabolites. Studies have shown that variations in culture conditions—such as carbon and nitrogen sources, pH, and aeration—as well as stress factors including light, temperature, and reactive oxygen species, can markedly influence terpenoid output and profile [,,,,]. These effects are often mediated through transcriptional regulation of key genes such as terpene synthases, across developmental stages including mycelia, primordia, and fruiting bodies. For example, exogenous methyl jasmonate (MeJA) was shown to enhance triterpenoid accumulation in G. lucidum and upregulate key triterpenoid biosynthetic genes (e.g., HMGR, FPS, LS). Coupled with gene overexpression and silencing techniques, several candidate genes involved in triterpenoid biosynthesis and regulation have been identified []. Omics analyses further revealed that MeJA induces metabolic reprogramming in G. lucidum, suppressing primary metabolic processes such as glucose metabolism and protein synthesis while favoring the production of secondary metabolites like triterpenoids []. Thus, rational optimization of environmental conditions offers a viable means to efficiently induce triterpenoid biosynthesis without the need for complex genetic engineering [,].
In summary, although progress has been made in identifying key enzymes, gene clusters, and environmental regulators of triterpenoid biosynthesis in basidiomycetes—with species such as F. luteovirens showing promising metabolic potential—the complete elucidation of terpenoid pathways, including BGC-based biosynthetic mechanisms and multi-level regulatory networks, remains an important goal. Future efforts should integrate multi-omics and synthetic biology strategies to advance our understanding of triterpenoid biosynthesis in basidiomycetes and provide a theoretical foundation for their efficient exploitation.
5. Genetic and Environmental Regulation of Bioactive Metabolites Synthesis in F. luteovirens
5.1. Molecular Basis and Genetic Regulation of Synthesis
Natural products have consistently served as a pivotal source for the discovery and development of therapeutics for human use, as well as products for animal health and crop protection [,,]. Microbial genome mining emerged as an important strategy for revitalizing drug discovery in the early 2000s; at that time, researchers discovered that the number of secondary metabolite BGCs encoded in newly sequenced actinomycete genomes far exceeded the sizes predicted based on known metabolites [,]. In recent years, with the continuous advancement of genome mining technology, an increasing number of novel natural products have been identified and characterized []. Moreover, genome mining provides synthetic biology with a growing repository of genetic building blocks and tools. This advancement facilitates the design of novel bioactive secondary metabolite derivatives, such as antibiotics, through combinatorial biosynthesis strategies [].
In this context, genome analysis of specific microorganisms has become an important way to exploit their medicinal potential. For example, a high-quality genome assembly of Ophiocordyceps sinensis revealed a genome size of approximately 110.8 Mb and 63 secondary metabolite gene clusters, including multiple polyketide synthases, non-ribosomal peptide synthetases, and others []. Similarly, genome sequencing of Morchella esculenta annotated a total of 9550 protein-coding genes and predicted the possible structures of its metabolites []. The value of this resource in natural product development was further highlighted by the genome study of F. luteovirens: its genomes was approximately 27–28.8 Mb, encoding 7000–8333 protein-coding genes, and phylogenetic analyses confirmed its belonging to the genus Floccularia. The study identified 400 Carbohydrate-Active enZyme (CAZyme) genes and 357 species-specific gene families, providing a genetic basis for its polysaccharide metabolism and formation of unique medicinal components []. More notably, the study also predicted 16 secondary metabolite BGCs, including clusters known to produce guadinomine with antimicrobial activity and melleolides with both entomotoxic and antimicrobial activity []. In addition, the genome encodes 400 CAZyme genes and 357 species-specific gene families [], providing the molecular basis for the synthesis of complex polysaccharides and specific metabolites. The key transporter protein gene FlMCH5 was specifically highly expressed in yellow mushrooms, and functional experiments confirmed that its overexpression in tobacco significantly increased riboflavin content (up to 36.94 μg/g) [], suggesting that this gene plays a central regulatory role in riboflavin accumulation. Additionally, the study identified a variety of metabolites including 148 terpenes and phenolics, such as vanillic acid [,], further confirming the potential of this species in synthesizing high-value natural products such as riboflavin, terpenoids, and phenolic acids at the level of genetic regulation. These studies have systematically revealed the genetic basis and regulatory mechanism of secondary metabolite synthesis in F. luteovirens, highlighting its important value in the development of medicinal active ingredients.
A series of key functional genes and their regulatory mechanisms have been revealed in F. luteovirens. The specific high expression of the core transporter protein gene FlMCH5 was shown to be the key genetic basis for riboflavin accumulation and the formation of the yellow phenotype of the fruiting bodies []. In addition, this species possesses a strong environmental stress response capacity, with 225 differentially expressed genes (DEGs) induced by strong UV radiation, which are significantly enriched in the pathways of environmental signal transduction, DNA damage repair, and pigment metabolism, underscoring its transcriptional regulatory potential to cope with extreme environments []. The reliability of these functional gene studies is supported by a stable system of internal reference genes—studies have shown that genes such as H3, ACT, EF-Tu, etc. among others, are stably expressed under a variety of abiotic stresses, such as salt, drought, oxidation, heat, extreme pH, cadmium stress, etc., thus providing key technical support for the precise quantification and functional analysis of the related gene expression [].
5.2. Regulatory Role and Mechanisms of Environmental Factors
5.2.1. Regulation by Abiotic Stresses
Beyond genetic background and culture medium composition, the growth environment constitutes a pivotal factor influencing the formation of fruiting bodies in edible and medicinal fungi, directly determining essential agronomic traits including morphology, yield, flavor profile, and stress resistance. Deciphering the regulatory mechanisms through which environmental factors—such as temperature, humidity, light intensity and quality, and carbon dioxide concentration—govern the growth and metabolism of edible and medicinal fungi is instrumental for optimizing cultivation environment management strategies, thereby enhancing both the yield and quality of germplasm resources [].
Abiotic stress represents a crucial strategy for modulating secondary metabolism in fungi. In edible fungi, diverse physical, chemical, and nutritional signals can function as effective stress stimuli, significantly influencing the biosynthesis of target metabolites through activation of specific signaling pathways—such as calcium signaling and reactive oxygen species (ROS) signaling—and upregulation of key enzymatic genes. In G. lucidum, for instance, various individual chemical or biological elicitors—including small molecules like MeJA, salicylic acid, and acetic acid, as well as metal ions such as Na+ and Cu2+ [,,,]—physical treatments (e.g., heat stress, pH modulation) [,], and nutritional conditions (e.g., carbon source, nitrogen source, and their ratio) have been demonstrated to effectively upregulate genes involved in ganoderic acid (GA) biosynthesis [,]. These responses are mediated through multiple mechanisms, including the calcium/calcineurin signaling pathway and alterations in membrane fluidity, among others.
Specifically, regarding F. luteovirens, studies have indicated that fruiting bodies from different geographical origins exhibit substantial regional disparities in metabolite abundance, confirming the decisive role of geographic provenance—with its associated environmental and climatic conditions—in determining quality and bioactivity []. Our team’s previous research revealed that triterpenoid accumulation in liquid cultures of F. luteovirens reached its peak at 20 days, synchronizing with the maximal activities of enzymes such as amylase and superoxide dismutase (SOD) []. We hypothesize that triterpenoid biosynthesis is linked to nutrient utilization and cellular redox balance. This “enzyme activity–metabolite accumulation” relationship is a common phenomenon in secondary metabolism [], analogous to mechanisms reported in various fungi, such as the upregulation of genes like HMGR under nitrogen limitation [,].
Regarding temperature effects, heat stress may influence triterpenoid synthesis by modifying membrane fluidity. For example, studies on other fungi have shown that heat stress increases membrane fluidity and upregulates the expression of genes including HMGR and SQS [], suggesting that a comparable mechanism may operate in F. luteovirens. In terms of light regulation, research on medicinal fungi has demonstrated that white light irradiation—particularly a three-stage strategy (2 days of dark culture → 6 days under 0.94 W/m2 white light → subsequent exposure to 4.70 W/m2 white light)—significantly enhances triterpenoid production, an effect attributed to the redirected metabolic flux toward triterpenoid biosynthesis []. Meanwhile, blue light has also been shown to promote triterpenoid accumulation in certain fungal species []. These findings provide valuable references for investigating light regulation in F. luteovirens. However, related experiments have not yet been initiated, and there remains a notable lack of analysis regarding the effects of different light intensities and phase-specific combinations on triterpenoid synthesis in this species.
5.2.2. Cross-Talk of Stress Signals
The regulation of fungal secondary metabolism by abiotic stress involves a complex intracellular signaling network, characterized by notable “cross-talk” among different stress signaling pathways. As a basidiomycete known for producing bioactive metabolites, some edible and medicinal fungi has emerged as a potential model system for investigating how environmental factors regulate metabolism in basidiomycetes []. For instance, heat stress activates both nitric oxide (NO) and calcium ion (Ca2+) signaling pathways. These pathways exhibit upstream-downstream relationships and engage in cross-talk to modulate the biosynthesis of GA []. Further mechanistic studies reveal that heat stress triggers Ca2+ signaling via the activation of specific phospholipid signaling pathways involving PI-4-P and PIP2 []. In contrast, under Cu2+ stress, signaling cross-talk is manifested as a bidirectional interaction between ROS and Ca2+: Cu2+ induces ROS accumulation, leading to elevated Ca2+ levels, which in turn feedback-regulate ROS production. This interplay forms a finely tuned feedback loop that coordinates mycelial growth and GA synthesis []. Collectively, these studies illustrate from multiple perspectives that the synergy and cross-talk among multiple second messengers—such as NO, Ca2+, ROS, and phospholipid molecules—constitute a central mechanism enabling precise regulation of secondary metabolism in fungi in response to environmental stresses.
6. Biotechnological Utilization: From Mycelial Production to Functional Products
6.1. Strategies for Enhanced Metabolite Production
6.1.1. Fermentation Process Optimization
Given the challenges in the artificial cultivation of F. luteovirens fruiting bodies, biotechnology, particularly liquid fermentation, has emerged as a sustainable and efficient strategy for the large-scale production of its mycelium and bioactive metabolites. The production of polysaccharides and other metabolites can be significantly enhanced through precise optimization of fermentation conditions. Lessons from other fungi, such as Ganoderma lucidum, demonstrate the profound impact of carbon and nitrogen source manipulation. For instance, optimizing the carbon-to-nitrogen (C/N) ratio to 20:10 was crucial for maximizing both biomass and GA yield []. Advanced strategies, like the synergistic use of exogenous elicitors with a variable-temperature regime in certain edible fungi, such as Agaricus bitorquis, have boosted mycelial polysaccharide yield by over 65% [], underscoring the efficacy of integrated physical and chemical induction. For F. luteovirens, research on fermentation optimization remains nascent but promising. Initial studies have employed orthogonal design to refine medium composition [] and introduced novel approaches like microparticles and surfactants to enhance extracellular polysaccharide (FLEP) yield []. Previous research has identified culture duration as a critical factor, with polysaccharide accumulation peaking at a specific fermentation time, beyond which degradation may occur []. However, a significant knowledge gap exists regarding the systematic optimization of key parameters (e.g., C/N ratio, dissolved oxygen, specific elicitors) for triterpenoid production. Future research must leverage insights from regulatory mechanisms (Section 5.2) to design intelligent fermentation strategies that deliberately induce stress responses to activate silent BGCs and enhance the yield of high-value terpenoids.
6.1.2. Artificial Culture and Condition Optimization
The foundation for cultivating F. luteovirens was laid by early studies on carbon and nitrogen source utilization []. Subsequent work has systematically explored nutritional and environmental factors, including pH, temperature, and light, using single-factor and orthogonal designs [,,]. A detailed investigation into light quality (white, red, yellow, blue) found no significant promotive effect on mycelial growth compared to dark conditions []. While this suggests light is not a primary requirement for vegetative growth, it does not preclude its potential role, observed in other fungi like Monascus purpureus and Hypsizygus marmoreus [,], in regulating secondary metabolism. Therefore, future studies should shift focus from mycelial biomass to the specific effects of light quality, photoperiod, and their interaction with other factors on the synthesis of pigments (e.g., riboflavin) and other valuable metabolites.
6.2. Downstream Processing for Product Development
6.2.1. Extraction Process Innovation
The objective of downstream processing is the efficient, economical, and green recovery of bioactive compounds. For F. luteovirens, extraction strategies must be tailored to the target metabolite’s properties. The classical hot-water extraction, optimized via Response Surface Methodology (RSM), is effective for fruiting body polysaccharides (FLPs) []. In contrast, lipophilic compounds like protoilludane-type sesquiterpene aryl esters are more efficiently extracted with organic solvents (e.g., ethyl acetate) assisted by ultrasound (UAE), which significantly improves yield and bioactivity []. A notable advancement is the integration of in situ extraction during fermentation, where adding microparticles and surfactants increased FLEP yield by 1.5-fold []. The future lies in the flexible combination and optimization of modern techniques (UAE, MAE, SFE) based on the polarity, scale, and economic constraints of the target product.
6.2.2. Influence of Post-Treatment (Drying) Methods
The drying process is a critical determinant of final product quality, directly influencing the preservation of heat-sensitive metabolites. Consistent evidence from F. luteovirens confirms that vacuum freeze-drying (VFD) best preserves antioxidant activity, metabolite integrity, and microstructure []. Metabolomic studies on F. luteovirens further identify amino acid metabolism as a core pathway responding to drying stress. However, the high cost of VFD limits its industrial application. Although thermal drying techniques such as microwave drying are more practical, they may lead to structural collapse and the loss of bioactive metabolites []. Notably, the optimal drying strategy is compound-dependent. Thus, the choice of drying method should be guided by the properties of target metabolites, and research on cost-effective hybrid technologies (e.g., hybrid hot air–microwave vacuum drying) should be prioritized to facilitate industrial application.
6.3. Cultivation Mode Comparison and Scale-Up Potential
A comparative analysis clearly positions liquid fermentation as the most viable near-term strategy for utilizing F. luteovirens, particularly when contrasted with the ongoing challenges of fruiting body cultivation, which remains unsuccessful and relies on limited, ecologically unsustainable wild harvests. In contrast, liquid fermentation of the mycelium offers a compelling alternative, as it is nutritionally similar to the fruiting body [] and serves as a rich source of diverse bioactivities, including immunomodulatory polysaccharides and antioxidant terpenoids []. The feasibility of scaling up this process to a 70 L fermenter, supported by established kinetic models for growth and substrate consumption, lays a solid foundation for industrial production []. The paramount advantages of liquid fermentation lie in its sustainability and controllability, providing a year-round, reliable supply of uniform biomass independent of seasonal and environmental constraints, while also facilitating easier extraction and achieving higher economic efficiency compared to processing solid fruiting bodies [,]. Therefore, future commercialization efforts should prioritize the refinement of liquid fermentation processes, even as research toward the breakthrough of fruiting body domestication retains its scientific importance.
7. Conclusions and Outlook
7.1. Conclusions
As a rare edible and medicinal fungus endemic to the Tibetan Plateau, F. luteovirens has gained significant attention in health industry development and basic research owing to its abundant bioactive metabolites and distinctive environmental adaptability. This review demonstrates that the integrated application of multi-omics technologies serves as a central driving force for deciphering its biological processes. Genomic studies have uncovered its compact genome architecture and abundant BGCs, providing a genetic basis for the biosynthesis of pharmacologically active compounds such as guadinomine and melleolides. Integrated transcriptomic and metabolomic analyses have successfully elucidated the key mechanisms underlying riboflavin accumulation and yellow pigment formation, while functional genomics has established a methodological foundation for precise gene expression profiling under diverse stress conditions.
Notably, aiming to address the core scientific questions raised earlier regarding its alpine adaptation mechanisms, current research has yielded preliminary but critical insights: First, regarding whether F. luteovirens relies on an integrated signal perception and transduction system to adapt to plateau extremes, genomic annotation has identified 205 environmental adaptation genes, and transcriptomic analysis under UV stress has detected 225 DEGs significantly enriched in pathways of environmental signal transduction, DNA damage repair, and pigment metabolism—collectively confirming the existence of a multi-dimensional integrated signal perception and transduction system that provides a genetic basis for coping with complex extreme environments. Second, for the question of whether a signaling hub composed of secondary messengers (e.g., ROS, Ca2+, NO) exists under combined stress, metabolomic studies have identified vanillic acid as a key antioxidant marker, and the dynamic correlation between SOD) activity and triterpenoid accumulation further supports the core role of ROS in stress response. Combined with its synergistic adaptation to low temperature, high UV radiation, and nutrient scarcity, it is reasonable to infer that F. luteovirens may employ a secondary messenger crosstalk mechanism to integrate stress signals, though the specific molecular interaction network requires further experimental validation. Third, in terms of how upstream signals regulate downstream physiological processes, multi-omics data show that the specific high expression of the riboflavin transporter gene FlMCH5 mediates pigment synthesis (yellow phenotype formation); the phenylalanine biosynthesis and metabolism pathway promotes vanillic acid accumulation to enhance antioxidant defense; and the synchronous dynamic changes in amylase/cellulase activity and polysaccharide synthesis optimize energy metabolism—forming a regulatory chain of “stress signal → gene expression → metabolic reprogramming → physiological adaptation”.
Overall, current research has preliminarily outlined the diversity of metabolites, bioactivities, and response patterns to environmental factors in F. luteovirens, affirming its considerable potential for applications in functional foods, pharmaceuticals, and cosmetics. Nevertheless, this field still confronts serious challenges: First, fundamental biological questions remain unresolved—artificial domestication has not yet been achieved, and the precise regulatory network governing secondary metabolism, particularly the signal integration mechanisms in response to combined stresses, remains poorly understood. Second, technical bottlenecks are prominent—large-scale production strategies such as liquid fermentation still face obstacles such as low yields of active products and unclear regulatory mechanisms. Third, research depth is inadequate—studies on the chemical structures, structure-activity relationships, and in vivo mechanisms of action of bioactive components remain relatively limited, resulting in insufficient deep processing of derived products and limited value addition.
7.2. Prospects
Future research on F. luteovirens should aim to achieve breakthroughs spanning mechanistic understanding, technological innovation, and industrial application. At the fundamental research level, it is essential to further deepen integrated multi-omics analyses, with a focus on deciphering the regulatory networks linking composite stress signals—such as low temperature, high solar radiation, and low oxygen—with secondary metabolite synthesis, taking into account habitat conditions. Attention should also be given to the role of epigenetic regulation, such as DNA methylation and histone modifications, in these processes. Concurrently, systematic comparisons of metabolic profiles across different developmental stages—including mycelia, primordia, and fruiting bodies—should be conducted.
At the technological development level, efforts should be intensified to promote the application of modern biotechnological tools. This includes leveraging gene editing technologies such as CRISPR-Cas9 in combination with conventional mutagenesis approaches for targeted breeding of stress-tolerant and high-yielding strains. Based on an in-depth understanding of the regulatory principles governing environmental factors, precision fermentation parameters—such as carbon and nitrogen sources, light quality, and temperature strategies—should be optimized. Additionally, innovative, efficient, and environmentally friendly extraction and post-processing technologies—such as ultrasound-assisted extraction and combined drying methods—should be developed to maximize the retention of bioactive constituents. The ultimate objective is to realize high-value and sustainable utilization of resources. Future work should focus on developing high value-added products derived from its active ingredients—such as terpenoids, polysaccharides, and L-(+)-ergothioneine—for instance, by preparing nanoparticles to enhance oral bioavailability, or by expanding their applications in functional foods, pharmaceuticals, and cosmetics. Through such systematic research, it is anticipated that the challenges of artificial domestication and industrial production of F. luteovirens will ultimately be overcome, enabling its transition from a wild resource to an industrially cultivated species, thereby providing a valuable reference for the conservation and utilization of rare edible fungal resources.
Author Contributions
Conceptualization, X.Z. and L.T.; investigation, S.G. and W.L.; resources, C.X. and S.G.; data curation, T.S. and L.T.; writing—original draft preparation, T.S.; writing—review and editing, T.S.; project administration, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Tianfu Emei Plan Young Talent Project (Sichuan Emei No. 2688), Chengdu City Major Talent Plan: Rong Piao Talent Project (Cheng special appointment No. 1312), Tianfu New District Talent Plan in Sichuan (Certificate number: 2025-0093) and Zhuoni County Strong Science and Technology Subsidy Project (2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article, as no new data was created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hibbett, D.; Nagy, L.G.; Nilsson, R.H. Fungal Diversity, Evolution, and Classification. Curr. Biol. 2025, 35, R463–R469. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Menolli, N.; Ye, L.; Karunarathna, S.C.; Perez-Moreno, J.; Rahman, M.M.; Rashid, M.H.; Phengsintham, P.; Rizal, L.; et al. Reviewing the World’s Edible Mushroom Species: A New Evidence-Based Classification System. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1982–2014. [Google Scholar] [CrossRef]
- Sangeeta; Sharma, D.; Ramniwas, S.; Mugabi, R.; Uddin, J.; Nayik, G.A. Revolutionizing Mushroom Processing: Innovative Techniques and Technologies. Food Chem. X 2024, 23, 101774. [Google Scholar] [CrossRef]
- Ni, Y.; Liao, Q.; Gou, S.; Shi, T.; Li, W.; Feng, R.; Zhao, Z.; Zhao, X. Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens. J. Fungi 2024, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dia, Z.M. Study on the nutritive composition in Armillaria luteo-virens and sustainable utilization of Qing Hai province. J. Shaanxi Norm. Univ. (Nat. Sci. Ed.) 2008, 36, 93–98. (In Chinese) [Google Scholar] [CrossRef]
- Taji, S.; Yamada, T.; Wada, S.; Tokuda, H.; Sakuma, K.; Tanaka, R. Lanostane-Type Triterpenoids from the Sclerotia of Inonotus obliquus Possessing Anti-Tumor Promoting Activity. Eur. J. Med. Chem. 2008, 43, 2373–2379. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically Active Polysaccharide from Edible Mushrooms: A Review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Yang, Y.L.; Ren, J.L.; Zhang, H. Research Progress of Terpenoids and Bioactivities in Edible Mushroom. Sci. Technol. Food Ind. 2019, 40, 305–310. (In Chinese) [Google Scholar] [CrossRef]
- Mao, X.; Zhao, M.; Li, T.; Liu, Y.; Kong, X.H.; Ye, Y. Research progress in liquid fermentation technology and application of bioactive components in edible fungi. J. Food Saf. Qual. 2025, 16, 195–202. (In Chinese) [Google Scholar] [CrossRef]
- Xie, Z.L.; Zhao, L.Z.; Li, N.; Lei, J.Q.; Zhang, F.M. The correlation of geographic distribution and ecological environment of endemic species Floccularia luteovirens on Qinghai-Tibet Plateau. Acta Ecol. Sin. 2016, 36, 2851–2857. (In Chinese) [Google Scholar]
- Zhao, L.Z. An overview of the study of Armillaria luteo-virens on the Tibetan Plateau. Edible Fungi 2015, 37, 1–3. (In Chinese) [Google Scholar]
- Chen, Q.L.; Diao, Z.M.; Han, Y. The Economic Value and Sustainable Utilization of Armillaria luteo-virens. World Notes Antibiot. 2011, 32, 161–164, 173. (In Chinese) [Google Scholar] [CrossRef]
- Wang, S.; He, J.; Hu, B.; Deng, M.; Li, W.; Guo, J.; Song, Y.; Zheng, Q.; Song, X.; Ma, F.; et al. An Integrative Multi-Omics Analysis of Histone Modifications and DNA Methylation Reveals the Epigenomic Landscape in Apple under Drought Stress. Plant Biotechnol. J. 2025, 23, 4440–4460. [Google Scholar] [CrossRef]
- Xu, C.; Gui, Z.; Huang, Y.; Yang, H.; Luo, J.; Zeng, X. Integrated Transcriptomics and Metabolomics Analyses Provide Insights into Qingke in Response to Cold Stress. J. Agric. Food Chem. 2023, 71, 18345–18358. [Google Scholar] [CrossRef]
- Yang, F.-S.; Liu, M.; Guo, X.; Xu, C.; Jiang, J.; Mu, W.; Fang, D.; Xu, Y.-C.; Zhang, F.-M.; Wang, Y.-H.; et al. Signatures of Adaptation and Purifying Selection in Highland Populations of Dasiphora fruticosa. Mol. Biol. Evol. 2024, 41, msae099. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Liu, J.; Wang, L.; Liu, P.; Yin, Z.; Guo, S.; Ma, J.; Lu, Z.; Wang, T.; et al. Proteomic Discovery of H2O2 Response in Roots and Functional Characterization of PutGLP Gene from Alkaligrass. Planta 2018, 248, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xie, Z.; Jiang, H.; Xu, H.; Liu, B.; Meng, Q.; Peng, Q.; Tang, Y.; Duan, Y. The Molecular Mechanism of Yellow Mushroom (Floccularia luteovirens) Response to Strong Ultraviolet Radiation on the Qinghai-Tibet Plateau. Front. Microbiol. 2022, 13, 918491. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhang, Q.; Li, W.; Cao, L.; Feng, R.; Zhao, Z.; Zhao, X. Selection and Validation of Reference Genes for Normalization of Gene Expression in Floccularia luteovirens. Fungal Biol. 2024, 128, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Gao, Q.; Zhang, F.; Fu, P.; Wang, J.; Yan, H.; Chen, S. Genetic Variation and Phylogenetic Relationships of the Ectomycorrhizal Floccularia luteovirens on the Qinghai-Tibet Plateau. J. Microbiol. 2017, 55, 600–606. [Google Scholar] [CrossRef]
- Gan, X.; Bao, X.; Liu, B.; Li, Y.; Cao, D.; Zhang, H.; Zong, Y. Chemical Constituents and Molecular Mechanism of the Yellow Phenotype of Yellow Mushroom (Floccularia luteovirens). J. Fungi 2022, 8, 314. [Google Scholar] [CrossRef]
- Xing, R.; Yan, H.; Gao, Q.; Zhang, F.; Wang, J.; Chen, S. Microbial Communities Inhabiting the Fairy Ring of Floccularia luteovirens and Isolation of Potential Mycorrhiza Helper Bacteria. J. Basic. Microbiol. 2018, 58, 554–563. [Google Scholar] [CrossRef]
- Tang, C.; Fan, Y.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants 2024, 13, 620. [Google Scholar] [CrossRef]
- Xie, Z.L.; Xu, H.Y.; Guo, J.; Meng, Q.; Dai, D.R.; Mao, Y.J. Study on Nutritional Types of Floccularia luteovirens and Identification of Genes Related to Environmental Adaptation; Qinghai University: Qinghai, China, 2021. (In Chinese) [Google Scholar]
- Xie, Z.L.; Guo, J.; Meng, Q. Analysis of genes related to the environmental adaptation of Floccularia luteovirens. In Proceedings of the 2018 Annual Academic Conference of the Mycological Society of China, Tai’an, China, 11 August 2018; p. 62. (In Chinese). [Google Scholar]
- Bai, S.J.; Bao, J.Y. Qualitative analysis on active ingredients of Armillaria luteo-virens. North. Hortic. 2012, 3, 161–163. (In Chinese) [Google Scholar]
- Fan, T.; Ren, R.; Tang, S.; Zhou, Y.; Cai, M.; Zhao, W.; He, Y.; Xu, J. Transcriptomics Combined with Metabolomics Unveiled the Key Genes and Metabolites of Mycelium Growth in Morchella importuna. Front. Microbiol. 2023, 14, 1079353. [Google Scholar] [CrossRef]
- Zhao, X.; Hengchao, E.; Dong, H.; Zhang, Y.; Qiu, J.; Qian, Y.; Zhou, C. Combination of Untargeted Metabolomics Approach and Molecular Networking Analysis to Identify Unique Natural Components in Wild Morchella sp. by UPLC-Q-TOF-MS. Food Chem. 2022, 366, 130642. [Google Scholar] [CrossRef]
- Wu, I.-W.; Liao, Y.-C.; Tsai, T.-H.; Lin, C.-H.; Shen, Z.-Q.; Chan, Y.-H.; Tu, C.-W.; Chou, Y.-J.; Lo, C.-J.; Yeh, C.-H.; et al. Machine-Learning Assisted Discovery Unveils Novel Interplay between Gut Microbiota and Host Metabolic Disturbance in Diabetic Kidney Disease. Gut Microbes 2025, 17, 2473506. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, H.; Zhang, X.; Chen, Q. The Genomic and Transcriptomic Analyses of Floccularia luteovirens, a Rare Edible Fungus in the Qinghai–Tibet Plateau, Provide Insights into the Taxonomy Placement and Fruiting Body Formation. J. Fungi 2021, 7, 887. [Google Scholar] [CrossRef]
- Liu, Y. The Molecular Staructure, Conformation and Immunomodulatory Activity of Glucans from Floccularia luteovirens. Ph.D. Thesis, Tianjin University of Science and Technology, Tianjin, China, 2025. (In Chinese). [Google Scholar]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical Characterization, Antioxidant Properties and Anticancer Activity of Exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Xu, H.M.; Zhang, X.; Zhu, Q.Y.; Chen, Y.J.; Chen, Q.H.; Liu, Z.J. Optimization of Fermentation Conditions and Biological Activities of Exopolysaccharides from Floccularia luteovirens. J. Nucl. Agric. Sci. 2023, 37, 1598–1608. (In Chinese) [Google Scholar]
- Ma, H.; Mueed, A.; Liu, D.; Ali, A.; Wang, T.; Ibrahim, M.; Su, L.; Wang, Q. Polysaccharides of Floccularia luteovirens Regulate Intestinal Immune Response, and Oxidative Stress Activity through MAPK/Nrf2/Keap1 Signaling Pathway in Immunosuppressive Mice. Int. J. Biol. Macromol. 2024, 277, 134140. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Mueed, A.; Ma, Y.; Ibrahim, M.; Su, L.; Wang, Q. Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice. Foods 2024, 13, 3881. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Wang, S.; Li, C.; Chen, C.; Wan, X.; Li, D.; Li, Y. Polysaccharides of Floccularia luteovirens Alleviate Oxidative Damage and Inflammatory Parameters of Diabetic Nephropathy in Db/Db Mice. Front. Biosci.-Landmark 2023, 28, 82. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhang, X.; Zhu, Q.Y.; Wu, M.Y.; Zhang, L.Z.; Chen, Q.H.; Liu, Z.J. Effect of Extracellular Polysaccharides of Floccularia luteovirens on the Quality of Pacific White Shrimp during Refrigeration. Food Res. Dev. 2024, 45, 44–51+66. (In Chinese) [Google Scholar]
- Yang, S.; Xu, H.M.; Yin, X.L.; Ma, M.Z.; Miao, W.H.; Chen, Q.H.; Liu, Z.J.; Zhao, Y.D. Effect of polysaccharides of Floccularia luteovirens composite freshness coating on the storage quality of Tuna. Food Ferment. Ind. 2025. (In Chinese) [Google Scholar] [CrossRef]
- Schmidt-Dannert, C. Biosynthesis of Terpenoid Natural Products in Fungi. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–61. ISBN 978-3-319-20107-8. [Google Scholar]
- Zhang, X.; Zhu, Q.Y.; Xu, H.M.; Wu, M.Y.; Chen, X.E.; Chen, Q.H.; Liu, Z.J. Optimization of extraction process and activity of protoilludane sesquiterpene aryl esters from Floccularia luteovirens. J. Zhejiang Univ. (Agric. Life Sci.) 2023, 49, 813–824. (In Chinese) [Google Scholar]
- Dang, J.; Chen, C.; Ma, J.; Dawa, Y.; Wang, Q.; Tao, Y.; Wang, Q.; Ji, T. Preparative Isolation of Highly Polar Free Radical Inhibitor from Floccularia luteovirens Using Hydrophilic Interaction Chromatography Directed by On-Line HPLC-DPPH Assay. J. Chromatogr. B 2020, 1142, 122043. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, J.; Hou, L.; Gao, Y.; Sun, J.; Zhang, N.; Fan, B.; Wang, F. The Small Molecule Fractions of Floccularia luteovirens Induce Apoptosis of NSCLC Cells through Activating Caspase-3 Activity. Int. J. Mol. Sci. 2021, 22, 10609. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.M.; Chen, C.B.; Li, Y. Anti-inflammatory and analgetic effects of the aqueous extract of Floccularia luteovirens on NTG-induced migraine in rats. Mycosystema 2020, 39, 917–922. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.E. GC-MS analysis of supercritical carbon dioxide extraction products from Floccularia luteovirens. Mycosystema 2015, 34, 321–327. (In Chinese) [Google Scholar] [CrossRef]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Anti-Inflammatory Potential of Mushroom Extracts and Isolated Metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef]
- Yu, G.; Ge, X.; Wang, Y.; Mo, X.; Yu, H.; Tan, L.; Yang, S. Discovery of Novel Terpenoids from the Basidiomycete Pleurotus ostreatus through Genome Mining and Coculture Optimization. J. Agric. Food Chem. 2023, 71, 11110–11123. [Google Scholar] [CrossRef]
- Ziemert, N.; Alanjary, M.; Weber, T. The Evolution of Genome Mining in Microbes—A Review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef]
- Luo, P.; Huang, J.-H.; Lv, J.-M.; Wang, G.-Q.; Hu, D.; Gao, H. Biosynthesis of Fungal Terpenoids. Nat. Prod. Rep. 2024, 41, 748–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, Y.H.; Mao, Y.J.; Meng, L.J.; Liu, H.L. Analysis on Isolation, Purification, Component and Structure of Armillaria luteo-virens. J. Chang. Univ. Sci. Technol. (Nat. Sci. Ed.) 2007, 02, 102–105. (In Chinese) [Google Scholar] [CrossRef]
- Brosnan, J.T. Interorgan Amino Acid Transport and Its Regulation. J. Nutr. 2003, 133, 2068S–2072S. [Google Scholar] [CrossRef]
- Adil, B.; Xiang, Q.; He, M.; Wu, Y.; Asghar, M.A.; Arshad, M.; Qin, P.; Gu, Y.; Yu, X.; Zhao, K.; et al. Effect of Sodium and Calcium on Polysaccharide Production and the Activities of Enzymes Involved in the Polysaccharide Synthesis of Lentinus Edodes. AMB Expr. 2020, 10, 47. [Google Scholar] [CrossRef]
- Zan, X.-Y.; Wu, X.-H.; Cui, F.-J.; Zhu, H.-A.; Sun, W.-J.; Jiang, L.-H.; Tao, T.-L.; Zhao, X. UDP-Glucose Pyrophosphorylase Gene Affects Mycelia Growth and Polysaccharide Synthesis of Grifola frondosa. Int. J. Biol. Macromol. 2020, 161, 1161–1170. [Google Scholar] [CrossRef]
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the Fungal Terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, H.; Zhong, J. Biosynthesis of a Ganoderic Acid in Saccharomyces cerevisiae by Expressing a Cytochrome P450 Gene from Ganoderma lucidum. Biotech. Bioeng. 2018, 115, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Macías-Rubalcava, M.L.; Trujillo-Roldán, M.A. Overview of Fungal Terpene Synthases and Their Regulation. World J. Microbiol. Biotechnol. 2023, 39, 194. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic Gene Clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Godio, R.P.; Fouces, R.; Martín, J.F. A Squalene Epoxidase Is Involved in Biosynthesis of Both the Antitumor Compound Clavaric Acid and Sterols in the Basidiomycete H. sublateritium. Chem. Biol. 2007, 14, 1334–1346. [Google Scholar] [CrossRef]
- Cui, M.; Yang, H.; He, G. Submerged Fermentation Production and Characterization of Intracellular Triterpenoids from Ganoderma lucidum Using HPLC-ESI-MS. J. Zhejiang Univ. Sci. B 2015, 16, 998–1010. [Google Scholar] [CrossRef]
- Hu, G.; Zhai, M.; Niu, R.; Xu, X.; Liu, Q.; Jia, J. Optimization of Culture Condition for Ganoderic Acid Production in Ganoderma lucidum Liquid Static Culture and Design of a Suitable Bioreactor. Molecules 2018, 23, 2563. [Google Scholar] [CrossRef]
- Li, H.-J.; Zhang, D.-H.; Han, L.-L.; Yu, X.; Zhao, P.; Li, T.; Zhong, J.-J.; Xu, J.-W. Further Improvement in Ganoderic Acid Production in Static Liquid Culture of Ganoderma lucidum by Integrating Nitrogen Limitation and Calcium Ion Addition. Bioprocess. Biosyst. Eng. 2016, 39, 75–80. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, Q.; Tamrakar, S.; Amen, Y.; Mori, Y.; Suhara, H.; Kaneko, S.; Kawashima, H.; Okuzono, K.; Inoue, Y.; et al. Changes in Content of Triterpenoids and Polysaccharides in Ganoderma Lingzhi at Different Growth Stages. J. Nat. Med. 2018, 72, 734–744. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.-J. A Novel Three-Stage Light Irradiation Strategy in the Submerged Fermentation of Medicinal Mushroom Ganoderma lucidum for the Efficient Production of Ganoderic Acid and Ganoderma Polysaccharides. Biotechnol. Prog. 2008, 24, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; Mu, D.S.; Li, Y.X.; Zhao, M.W. Methyl Jasmonate Induces Ganoderic Acid Biosynthesis in the Basidiomycetous Fungus Ganoderma lucidum. Bioresour. Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef]
- Jiang, A.-L.; Liu, Y.-N.; Liu, R.; Ren, A.; Ma, H.-Y.; Shu, L.-B.; Shi, L.; Zhu, J.; Zhao, M.-W. Integrated Proteomics and Metabolomics Analysis Provides Insights into Ganoderic Acid Biosynthesis in Response to Methyl Jasmonate in Ganoderma lucidum. Int. J. Mol. Sci. 2019, 20, 6116. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.-L.; Huang, W.-F.; Ren, Y.; Onac, E.; Zhou, G.-F.; Peng, S.; Wang, X.-J.; Li, H.-H. LED Lights Increase Bioactive Substances at Low Energy Costs in Culturing Fruiting Bodies of Cordyceps militaris. Sci. Hortic. 2014, 175, 139–143. [Google Scholar] [CrossRef]
- Baltz, R.H. Natural Product Drug Discovery in the Genomic Era: Realities, Conjectures, Misconceptions, and Opportunities. J. Ind. Microbiol. Biotechnol. 2019, 46, 281–299. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Dhakal, D.; Pham, V.T.T.; Nguyen, H.T.; Sohng, J.-K. Recent Advances in Strategies for Activation and Discovery/Characterization of Cryptic Biosynthetic Gene Clusters in Streptomyces. Microorganisms 2020, 8, 616. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Ishikawa, J.; Hara, H.; Suzuki, H.; Ikenoya, M.; Ikeda, H.; Yamashita, A.; Hattori, M.; Horinouchi, S. Genome Sequence of the Streptomycin-Producing Microorganism Streptomyces griseus IFO 13350. J. Bacteriol. 2008, 190, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, M.; Samborskyy, M.; Lester, J.B.; Mironenko, T.; Scott, N.; Dickens, S.; Haydock, S.F.; Leadlay, P.F. Complete Genome Sequence of the Erythromycin-Producing Bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol. 2007, 25, 447–453. [Google Scholar] [CrossRef]
- Kalkreuter, E.; Pan, G.; Cepeda, A.J.; Shen, B. Targeting Bacterial Genomes for Natural Product Discovery. Trends Pharmacol. Sci. 2020, 41, 13–26. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into Secondary Metabolism from a Global Analysis of Prokaryotic Biosynthetic Gene Clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, J.; Meng, Q.; Zhang, H.; Zhou, G.; Li, M.; Wu, P.; Zhao, Y.; Chen, C.; Qin, Q. A New High-Quality Draft Genome Assembly of the Chinese Cordyceps Ophiocordyceps sinensis. Genome Biol. Evol. 2020, 12, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, Q.; Baiyintala; Wuhanqimuge. The Whole-Genome Sequence Analysis of Morchella Sextelata. Sci. Rep. 2019, 9, 15376. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Cao, D.; Zhang, Z.; Cheng, S.; Wei, L.; Li, S.; Liu, B. Draft Genome Assembly of Floccularia luteovirens, an Edible and Symbiotic Mushroom on Qinghai-Tibet Plateau. G3 2020, 10, 1167–1173. [Google Scholar] [CrossRef]
- Li, W.; Zou, G.; Bao, D.; Wu, Y. Current Advances in the Functional Genes of Edible and Medicinal Fungi: Research Techniques, Functional Analysis, and Prospects. J. Fungi 2024, 10, 311. [Google Scholar] [CrossRef]
- Ren, A.; Li, M.-J.; Shi, L.; Mu, D.-S.; Jiang, A.-L.; Han, Q.; Zhao, M.-W. Profiling and Quantifying Differential Gene Transcription Provide Insights into Ganoderic Acid Biosynthesis in Ganoderma lucidum in Response to Methyl Jasmonate. PLoS ONE 2013, 8, e65027. [Google Scholar] [CrossRef]
- Cao, P.-F.; Wu, C.-G.; Dang, Z.-H.; Shi, L.; Jiang, A.-L.; Ren, A.; Zhao, M.-W. Effects of Exogenous Salicylic Acid on Ganoderic Acid Biosynthesis and the Expression of Key Genes in the Ganoderic Acid Biosynthesis Pathway in the Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Li, X.-B.; Miao, Z.-G.; Shi, L.; Jaing, A.-L.; Zhao, M.-W. Transcript and Metabolite Alterations Increase Ganoderic Acid Content in Ganoderma lucidum Using Acetic Acid as an Inducer. Biotechnol. Lett. 2014, 36, 2529–2536. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Xia, X.-X.; Zhong, J.-J. Induced Effect of Na+ on Ganoderic Acid Biosynthesis in Static Liquid Culture of Ganoderma lucidum via Calcineurin Signal Transduction. Biotechnol. Bioeng. 2013, 110, 1913–1923. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, A.; Li, M.-J.; Cao, P.-F.; Chen, T.-X.; Zhang, G.; Shi, L.; Jiang, A.-L.; Zhao, M.-W. Heat Stress Modulates Mycelium Growth, Heat Shock Protein Expression, Ganoderic Acid Biosynthesis, and Hyphal Branching of Ganoderma lucidum via Cytosolic Ca2+. Appl. Environ. Microbiol. 2016, 82, 4112–4125. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-L.; Zhang, G.; Ren, A.; Dang, Z.-H.; Shi, L.; Jiang, A.-L.; Zhao, M.-W. The pH-Responsive Transcription Factor PacC Regulates Mycelial Growth, Fruiting Body Development, and Ganoderic Acid Biosynthesis in Ganoderma lucidum. Mycologia 2016, 108, 1104–1113. [Google Scholar] [CrossRef]
- Hu, Y.; Ahmed, S.; Li, J.; Luo, B.; Gao, Z.; Zhang, Q.; Li, X.; Hu, X. Improved Ganoderic Acids Production in Ganoderma lucidum by Wood Decaying Components. Sci. Rep. 2017, 7, 46623. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, J.-J.; Geng, A. Improvement of Ganoderic Acid Production by Fermentation of Ganoderma lucidum with Cellulase as an Elicitor. Process Biochem. 2014, 49, 1580–1586. [Google Scholar] [CrossRef]
- Cai, Y.; Deng, P.; Liu, J.; Luo, Y.; Sangzhu, T.; Li, H.; Zhao, Y.; Tang, C.; Yang, M. Metabolomics-Based Discrimination of Geographical Variability in Quality and Antioxidant Activity of Golden Mushroom (Floccularia luteovirens) from the Qinghai-Tibet Plateau. Food Biosci. 2025, 68, 106536. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhang, T.-J.; Lu, X.-X.; Ma, B.-L.; Ren, A.; Shi, L.; Jiang, A.-L.; Yu, H.-S.; Zhao, M.-W. Membrane Fluidity Is Involved in the Regulation of Heat Stress Induced Secondary Metabolism in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 1653–1668. [Google Scholar] [CrossRef]
- Mei, X.L.; Chen, R.Y.; Li, B.M.; Chen, X.D.; Zhao, Z.; Lan, J. Effect of light quality on growth and triterpenic acid content of Ganoderma lucidum mycelium. Chin. Tradit. Herb. Drugs 2013, 44, 3546–3550. (In Chinese) [Google Scholar]
- Liu, R.; Shi, L.; Zhu, T.; Yang, T.; Ren, A.; Zhu, J.; Zhao, M.-W. Cross Talk between Nitric Oxide and Calcium-Calmodulin Regulates Ganoderic Acid Biosynthesis in Ganoderma lucidum under Heat Stress. Appl. Environ. Microbiol. 2018, 84, e00043-18. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Lu, X.-X.; Ren, A.; Shi, L.; Zhu, J.; Jiang, A.-L.; Yu, H.-S.; Zhao, M.-W. Conversion of Phosphatidylinositol (PI) to PI4-Phosphate (PI4P) and Then to PI(4,5)P2 Is Essential for the Cytosolic Ca2+ Concentration under Heat Stress in Ganoderma lucidum. Environ. Microbiol. 2018, 20, 2456–2468. [Google Scholar] [CrossRef]
- Gao, T.; Shi, L.; Zhang, T.; Ren, A.; Jiang, A.; Yu, H.; Zhao, M. Cross Talk between Calcium and Reactive Oxygen Species Regulates Hyphal Branching and Ganoderic Acid Biosynthesis in Ganoderma lucidum under Copper Stress. Appl. Environ. Microbiol. 2018, 84, e00438-18. [Google Scholar] [CrossRef]
- Wu, S.; Lu, H.-Y.; Chen, Q.-H.; Xie, H.-C.; Jiao, Y.-C. Anthocyanin Extract from Lycium ruthenicum Enhanced Production of Biomass and Polysaccharides during Submerged Fermentation of Agaricus bitorquis (Quél.) Sacc. Chaidam. Bioprocess. Biosyst. Eng. 2021, 44, 2303–2313. [Google Scholar] [CrossRef]
- Wang, H.; You, X.Q.; Yu, M. Studies on the fermentation culture of extracellular polysaccharide production by Armillaria luteo-virens. North. Hortic. 2010, 22, 174–176. (In Chinese) [Google Scholar]
- Diao, Z.M. Preliminary studies on the nutritional and physiological properties of the mycelium of Armillaria luteo-virens. J. Microbiol. 1997, 01, 14–17. (In Chinese) [Google Scholar]
- Cai, X.; Zhang, Y.; Wang, X.L. Study on Medium Optimization of Yellow-green Armillaria luteo virens and Condition of Artificial Culture. Anhui Agric. Sci. Bull. 2013, 19, 33–34+50. (In Chinese) [Google Scholar] [CrossRef]
- Liu, H.Z.; Wang, H.X.; Liu, Q.H.; Feng, K. Preliminary Study on Mycelium Growing Conditions of Armillaria luteovirens. Edible Fungi China 2007, 04, 16–19. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, J.S.; Jiao, Y.C.; Sheng, H.Y.; Xiong, H.Y.; Yang, C.J. Effects of Triacontanol, Culture Medium pH, and Incubation Temperature on the Mycelial Growth of Armillaria luteovirens. Edible Fungi 2011, 33, 8–9. (In Chinese) [Google Scholar]
- Liu, Z.J. Study on Physiological Characteristics, Importantbioactive Compounds, the Multi-Omics Elucidation Andgenetic Characterization of Floccularia luteovirens. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2022. (In Chinese). [Google Scholar]
- Xiong, X.; Liu, Y.; Zhang, J.; Wang, S.; Li, L.; Gao, M. Mutational Analysis of MpPhy Reveals Magnetoreception and Photosensitivity Involvement in Secondary Metabolites Biosynthesis in Monascus Purpureus. J. Photochem. Photobiol. B 2021, 217, 112164. [Google Scholar] [CrossRef]
- Zhu, L.; Su, Y.; Ma, Z.; Guo, L.; Yang, S.; Yu, H. Comparative Proteomic Analysis Reveals Differential Protein Expression of Hypsizygus marmoreus in Response to Different Light Qualities. Int. J. Biol. Macromol. 2022, 223, 1320–1334. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.B.; Zhang, B.; Tong, X.D.; Wang, S.M.; Li, Y. Antioxidant activities and extraction technique optimization of crude polysaccharides from the fruiting body of Floccularia luteovirens. Mycosystema 2019, 38, 1681–1688. (In Chinese) [Google Scholar] [CrossRef]
- Xiao, M.; Wang, T.; Tang, C.; He, M.; Pu, X.; Zhao, T.; Li, Y. Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis. J. Fungi 2025, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Yang, W.; Shi, Y.; Sun, Y.; Mariga, A.M.; Zhao, L.; Fang, Y.; Ma, N.; An, X.; Hu, Q. Comparison of Freeze-Drying with Three Different Combinations of Drying Methods and Their Influence on Colour, Texture, Microstructure and Nutrient Retention of Button Mushroom (Agaricus bisporus) Slices. Food Bioprocess. Technol. 2014, 7, 702–710. [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi-a Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Yu, M. Modelling of mycelial dynamics in the batch fermentation of Armillaria luteo-virens. Sci. Technol. Food Ind. 2010, 31, 135–137. (In Chinese) [Google Scholar] [CrossRef]
- Ni, Y.; Cao, L.; Li, W.; Zhang, Q.; Feng, R.; Zhao, Z.; Zhao, X. The Research Status and Prospects of Floccularia luteovirens: A Mycorrhizal Fungus with Edible Fruiting Bodies. J. Fungi 2023, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).