Abstract
Species belonging to the Pezizales are mainly saprobes in nature. They are most commonly observed in woodlands and humid environments. As a result of recent research conducted on the distribution of species in sandy areas and some National Forests Parks, five new species belonging to three genera were identified. A total of five species of disk fungi from Northeast China were identified and described based on morphological classification and molecular phylogenetics. These included Pulvinula (Pulvinula elsenensis, Pulvinula sublaeterubra), Microstoma (Microstoma jilinense, Microstoma changchunense), and Sarcoscypha (Sarcoscypha hongshiensis). Maximum likelihood and Bayesian analyses were performed using a combined nuc rDNA internal transcribed spacer region (ITS) and nuc 28S rDNA (nrLSU) dataset for the construction of phylogenetic trees. Morphological descriptions, line illustrations, and photographs of the ascocarps of these new species are provided, along with lists of the salient attributes exhibited by the species in the three genera under consideration.
1. Introduction
In 1885, Boundier discovered a novel genus, Pulvinula Boundier, within the family Humaries. It was characterized by a smooth hymenium, spherical ascospores, and paraphyses that were branched and curved at the apex. The genus initially included Peziza convexella Karst., Peziza sanguinaria Cooke, and Peziza constellatio (Berk. and Br.) [1]. Boundier (1907) later revised the diagnosis of the genus and the nomenclature of two of these species [2]. Pulvinula is composed of five species: Pulvimila cinnabarina (Fuck.) Bond., P. carhonaria (Fuck.) Bond., P. constellatio (Berk. and Br.) Bond., P. hacmastigma (Iledw. ex Fr.) Bond, and P. snhanrantia (Bounn. and Rouss.) Bond. However, this genus was not recognized until 1953, when it was discovered by Le Gal, who used Peziza convexella as the species type [3]. Species of this genus are distributed among Barlaea Sacc., Barlaeina Sacc., Crouania Fuckel, Detonia Sacc., and Lamprospora De Not. [4]. Dennis (1968) stated that one of the characteristics of the species within the genus Pulvinula is that the base of the ascus is forked [5]. Pfister (1976) challenged the conclusions of Boudier and Le Gal regarding the synonymy of Pulvinula haemastigma (Hedw.) Boud. and Pulvinula convexella. This was because no specimens of Pulvinula haemastigma then existed and the microscopic structure of the species was not sufficiently characterized to allow a definitive determination of synonymy based on a limited number of key features in its line drawing. Furthermore, the dimensions of the ascus and ascospores had not been documented. Concurrently, Pfister examined five varieties and one subspecies from five distinct genera and determined that they all belonged to Pulvinula convexella, representing the diversity within a complex species. Pfister elucidated the rationale behind this categorization, citing the color and dimensions of the ascocarp, the size of the asci and ascospores, and the presence or absence of croziers as determining factors [4]. In 1984, Krof and Zhuang Wenying proposed that the ascospores of species within Pulvinula should be classified as either spherical or elliptical and suggested the establishment of a new genus to address this discrepancy [6]. Yao Yijian and Spooner posited that, although Pulvinula and Boubovia Svrček, are characterized by shared taxonomic traits, including apically curved lateral filaments and forked asci at the base of most species, Pulvinula is distinguished by its spherical ascospores and thin-walled asci throughout development, whereas the asci of Boubovia species are thick-walled at a specific stage of development [7,8]. Hansen (2013) corroborated the paraphyletic status of the family Pyronemataceae Corda by constructing a phylogenetic tree using multigene fragments. Additionally, he posited that Boubovia, Geopyxis (Pers.) Sacc., Pseudombrophila Boud.m and Pulvinula are closely related to the Ascodesmidaceae J. Schröt., and Glaziellaceae J.L. Gibson families. It has been proposed that these four genera can be divided into two or more families [9,10,11]. Subsequently, Ekanayaka et al. (2018) conducted further research and classified three genera (Pulvinula, Lazuardia Rifai, and Pseudoboubovia: U. Lindem., M. Vega, B. Perić and R. Tena) that formed a monophyletic and independent branch on the phylogenetic tree as part of Pezizales J. Schröt. incertae sedis with Pyronemataceae. As a result, the family Pulvinulaceae was established, and Pulvinula was designated as the genus type [12,13].
Sarcoscyphaceae Le Gal ex Eckblad is a type of saprophytic fungus that grows on plant matter, including branches, stumps, fallen trees, and twigs. These fungi are distributed throughout tropical and temperate regions. Their main characteristic features are as follows: ascocarps consist of apothecia, which are solitary, scattered, or aggregated. These include disks, cups, goblet shapes, and auricles. Some species within this family have hair on the excipulum, either with or without stipes. They are mostly fleshy or corky in texture, with variable colors ranging from white to orange and to gray [14]. One of the common characteristics of this family is that the asci have thick walls and a suboperculum [15].
The ascus apex structure of the family Sarcoscyphaceae exhibits characteristics that are intermediate between those of the operculum and the inoperculum. In 1946, Le Gal designated this operculum type as a suboperculum and, based on this feature, introduced the name of Sarcoscyphacea for the first time. They further subdivided the species into two tribes, although no Latin diagnosis of the family name was provided at that time [16]. In 1953, Krof identified that this family warranted a special taxonomic status within the order Peziales based on his findings regarding morphological and cytological characteristics [17]. Eckblad (1968) provided a Latin diagnosis of Le Gal’s terminology, conferring legitimacy to the nomenclature [18]. In the same year, Rifai (1968) postulated that the ascospores and subascospores in Pezizales should be in the same position, thus establishing Sarcoscyphineae. At that time, Sarcoscyphineae was only thought to comprise Sarcoscyphaceae, which was further divided into two tribes: Sarcoscypheae, with brightly colred apothecia, and Urnuleae, with dark apothecia [19]. Following a detailed examination of cytology, pigment composition, ultrastructure, asci, and ascospore development (Korf, 1970, 1972, 1973), it was concluded that the classification proposed by Rifai (1968) was valid. This classification elevated the two previously identified tribes to the family level (Sarcoscyphaceae and Sarcosomataceae Kobayasi) and provided a clear delineation of the characteristics associated with these two families [20,21,22]. Subsequent scholars concurred with Korf’s classification [23,24,25]. Cabello (1988) employed a numerical classification method to analyze 22 genera and 34 species of Sarcoscyphineae to investigate the genus relationships within the family Sarcoscyphaceae. His findings supported the view that Sarcoscyphaceae can be distinguished from Sarcosomataceae [24]. Phylogenetic trees of Pezizales and Sarcoscyphaceae were established using molecular techniques, including the analysis of the ITS, SSU, and LSU, as well as other genetic data. These results demonstrate that Sarcoscyphaceae is a monophyletic group [26,27]. In their respective studies, researchers concluded that the family comprises 13 genera and approximately 83 species [12,28].
Microstoma Bernstein is mainly characterized by deeply cup-shaped or triangular cup-shaped ascocarps, solitary white hairs on the excipulum, gelatinous ectal excipulum, and smooth ascospores. The most marked classification characteristics of the species within this genus are the presence or absence of a connection between the base of the stipe, the presence or absence of rhizomes, and the shape of the top of the hairs, which may be blunt or sharp [29]. The macroscopic morphology of the species in this genus is similar to that of Cookeina Kuntze. Consequently, many researchers have concluded that the two genera are closely related. However, the distinguishing characteristic between the two is that the hairs of Cookeina are composed of bundles of hyphae that are typically needle-shaped [30,31]. To date, nine species have been identified in this genus. The species type is Microstoma protractum (Fr.) Kanouse (https://indexfungorum.org/Names/Names.asp, accessed on 20 November 2024).
The species of Sarcoscypha (Fr.) Boud. are characterized by a disk- or cup-shaped apothecium, with hymenia that may be dirty white, yellow, or scarlet, and ascospores that are elliptical to oblong in shape. The notable features of the ascospores include blunt ends, which may be flat or dented, and surface textures, which range from smooth to nearly smooth, or are at times irregularly wrinkled. The number of guttulates within ascospores is an important diagnostic characteristic for species identification within this genus [32,33,34]. Currently, 35 species are known in this genus. The species type is Sarcoscypha coccinea (Jacq.) Lambotte (https://indexfungorum.org/Names/Names.asp, accessed on 20 November 2024).
In 1991, Wenying Zhuang collected 14 species from the genus Sarcoscypha in Jiaohe City, which were classified into three distinct taxa: Sarcoscypha occidentalis f. occidentalis (Schwein.) Sacc., Sarcoscypha occidentalis f. citrina W.Y. Zhuang, and Sarcoscypha vassiljevae Raitv. This study revealed that the ascospores of these species displayed three distinct surface morphologies: smooth, subsmooth, and wrinkled [35]. In a separate effort, Tolgor Bau (2005) cataloged the ascomycetes of Jilin Province, thereby expanding the known distribution of the Sarcoscypha genus in Northeast China [36]. Guiying Chang (2006) documented Sarcoscypha coccinea during an investigation of the biological resources in the Zuojia Nature Reserve [37]. In a subsequent study, Chuhan Shi (2016) identified specimens collected between 2013 and 2016, along with those already housed in collections, confirming the presence of 60 species of Pezizales in Jilin Province, representing 20 genera across 7 families. This research also led to the discovery of one new genus and four new species for China, and ten new species were recorded in Jilin Province [38]. Tolgor Bau (2017) observed that the species distribution of Sarcoscyphaceae exhibited distinct patterns across different vegetation zones of Changbai Mountain [39].
Northeast China is situated in the heart of Eurasia, and is characterized by a temperate continental climate. Precipitation is primarily concentrated in the summer and autumn months. This region boasts rich vegetation resources, creating an ideal environment for fungal growth. An investigation of the resources of Northeast China’s sandy land and some National Forests Parks was conducted from 2022 to 2024. This revealed that the species richness of Pezizales in Northeast China is high, including five new species. This study provides a comprehensive account of the newly identified species, accompanied by line illustrations and electron microscope images of ascospores. Additionally, it presents a detailed account of the main characteristics of the three genera and species in tabular form.
2. Materials and Methods
2.1. Morphological Studies
Specimens were collected in Northeast China between June 2022 and September 2024. The ascocarps were documented with an Olympus Tg-6 camera during the field collection process, with the objective of recording the macroscopic characteristics of the ascocarps. The color of fresh ascocarp was described using the color-coding system developed by the German Institute for Quality Assurance and Certification (Reichs-Ausschuss fur Lieferbedingungen und Guetesicherung, https://www.ral-guetezeichen.de/, accessed on 5 October 2024). Then, specimens were dried using silica gel, and the specimens are currently stored in the herbarium Fungarium of Jilin Agricultural University (FJAU). The line illustrations were based on photos of the ascocarps in the field collection. Light microscopy (LM: Olympus CX33) was used to observe the microstructure, the samples were rehydrated in water, and OPLENIC Pro v1.92 was utilized to measure the tissue structures of asci, ascospores, paraphyses, and the excipulum. Additionally, {a/b/c} represent data for the length, width, and Q value of ascospore, which are derived from a ascospores within b ascocarps across c specimens. d − e × f − i represents the minimum–maximum value of the length × width of the ascospores, and Q = j − k represents the minimum–maximum value of the length/width of the ascospores.
In addition, Melzer’s reagent was employed to determine whether the asci and paraphyses at the apex wall were amyloid or not.
2.2. Phylogenetic Studies
DNA was extracted from dried specimens using the NuClean PlantGen DNA kit (CWBIO, Beijing, China). In PCR amplification, the primer pairs ITS1F/ITS and LR0R/LR were utilized [40,41,42,43]. The PCR program followed pre-denaturation at 94 °C for 5 min, followed by 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 1.5 min. This took 33 cycles. The PCR products were purified and sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). The newly generated sequences were deposited in GenBank.
The phylogenetic analysis included the available sequences of Sarcoscyphaceae and Pulvinula. Working according to a study by Zeng Ming et al. (2023), Chorioactis geaster (Peck) Kupfer ex Eckblad, Neournula pouchetii (Berthet and Riousset) Paden, Boubovia luteola Svrček, Boubovia vermiphila Brumm., and R. Kristiansen were selected as the outgroups [44].
Finally, the analyzed matrix contained 179 ITS sequences and 70 nrLSU sequences, which are listed in Table 1. The alignment was performed using the online Mafft version 7 (https://mafft.cbrc.jp/alignment/server/, accessed on 10 November 2024) and then manually refined and trimmed using MEGA7. ModelFinder was used to select the best-fit model using the AIC criterion [45].
The best-fit model partition model (edge-unlinked) was selected using the AIC criterion with ModelFinder. For Sarcoscyphaceae, according to the AIC criterion, the best-fit model was GTR + F + R4 for ITS and TIM2 + F + I + G4 (ITS) and TIM2 + F + R3 (nrLSU) for ITS-nrLSU. For Pulvinula, according to the AIC criterion, the best-fit model was GTR + F + I + G4 (ITS) and TIM2 + F + G4 (nrLSU) for ITS-nrLSU. Maximum likelihood phylogenies were inferred using the IQTREE under the edge-unlinked partition model for 10,000 ultrafast bootstraps and using the Shimodaira–Hasegawa-like approximate likelihood-ratio test. For Sarcoscyphaceae, Bayesian inference phylogenies were inferred using MrBayes 3.2.6 under the GTR+I+G+F model (2 parallel runs, 2,085,100 generations) for ITS and under the GTR+I+G model (2 parallel runs, 1,488,000 generations) for ITS-nrLSU. For Pulvinula, they were inferred under the GTR+I+G model (2 parallel runs, 2,114,700 generations), in which the initial 25% of sampled data were discarded as burn-in [46,47,48,49].

Table 1.
Sequence in formation from phylogenetic trees.
Table 1.
Sequence in formation from phylogenetic trees.
| Species | Country | Voucher/Strain Number | GenBank No. | References | |
|---|---|---|---|---|---|
| ITS | nrLSU | ||||
| Boubovia luteola | Germany | R.K. 94/05 | KX592793 | KX592805 | [50] |
| Boubovia vermiphila | Germany | R.K. 89/18 | KX592804 | [50] | |
| Chorioactis geaster | USA | ZZ2 FH | AY307935 | AY307943 | [51] |
| Cookeina colensoi | Mexico | CUP 62500 | AF394040 | [31] | |
| Cookeina colensoi | Australia | DAR 63642 | AF394038 | [31] | |
| Cookeina colensoi | New Zealand | PDD 55306 | AF394037 | [31] | |
| Cookeina cremeirosea | American Samoa | UTC000275474 | KU306964 | [52] | |
| Cookeina cremeirosea | American Samoa | UTC000275475 | KU306963 | [52] | |
| Cookeina garethjonesii | China | HKAS90509 | KY094617 | MG871315 | [30] |
| Cookeina garethjonesii | China | HKAS90513 | KY094622 | MG871316 | [30] |
| Cookeina indica | Thailand | MFLU 20-0548 | MT941004 | [53] | |
| Cookeina indica | China | HKAS 121171 | OK170053 | OK398387 | [44] |
| Cookeina insititia | China | FH Wang sp 2 | AF394033 | [31] | |
| Cookeina insititia | China | HMAS 71942 | AF394031 | [31] | |
| Cookeina korfii | Philippines | CUP-SA-1797 | KT893782 | [54] | |
| Cookeina korfii | Philippines | CUP-SA-2454 | KT893781 | [54] | |
| Cookeina sinensis | China | HKAS 121175 | OK170056 | OK398385 | [44] |
| Cookeina sinensis | Thailand | MFLU 21-0155 | OK413269 | OK398383 | [44] |
| Cookeina speciosa | Malaysia | C TL 6035 | AF394018 | [31] | |
| Cookeina speciosa | Venezuela | FH Iturriaga 1C-D4 | AF394011 | [31] | |
| Cookeina speciosa | Thailand | MFLU 21-0156 | OK413270 | OK398390 | [31] |
| Cookeina sulcipes | Thailand | MFLU 15-2358 | KY094620 | [30] | |
| Cookeina tricholoma | China | MFLU 15-2359 | KY094619 | MG871317 | [12,30] |
| Cookeina tricholoma | Thailand | MFLU 21-0165 | OK413279 | OK398394 | [44] |
| Cookeina venezuelae | Puerto Rico | FH00432502 | AF394041 | [31] | |
| Cookeina venezuelae | Venezuela | FH Iturriaga 6065 | AF394044 | [31] | |
| Cookeina venezuelae | Guadeloupe | FH00432503 | AF394042 | [31] | |
| Geodina guanacastensis | Bahamas | FH | MN096939 | MN096940 | [55] |
| Geodina guanacastensis | Costa Rica | CUP CA84 | MN096938 | [55] | |
| Kompsoscypha chudei | China | HKAS 107663A | MT907443 | MT907444 | [53] |
| Microstoma aggregatum | Japan | TNS: F-88858 | LC584238 | [56] | |
| Microstoma aggregatum | Japan | TNS: F-80795 | LC584235 | [56] | |
| Microstoma apiculosporum | Japan | KPM:NC-28117 | LC584241 | [56] | |
| Microstoma apiculosporum | Japan | TNS: F-37021 | LC584239 | [56] | |
| Microstoma changchunense | China | FJAU71961 | PQ497118 | PQ498790 | This study |
| Microstoma changchunense | China | FJAU71962 | PQ498903 | PQ498797 | This study |
| Microstoma floccosum | Mexico | FH K. Griffith (Micro45) | AF394046 | [31] | |
| Microstoma floccosum | Mexico | FH K. Griffith (Micro46) | AF394045 | [31] | |
| Microstoma macrosporum | Japan | TNS: F:91415 | LC671644 | [56] | |
| Microstoma macrosporum | Japan | TNS: F-80822 | LC584258 | [56] | |
| Microstoma jilinense | China | FJAU71958 | PQ496899 | PQ510812 | This study |
| Microstoma jilinense | China | FJAU71959 | PQ496900 | PQ510814 | This study |
| Microstoma jilinense | China | FJAU71960 | PQ497023 | PQ498789 | This study |
| Microstoma protracta | Poland | AW001_Pn | MG920536 | [57] | |
| Microstoma protracta | Poland | AW010_Kr | MG920535 | [57] | |
| Microstoma longipilum | Japan | TNS: F-60530 | LC584252 | [56] | |
| Microstoma longipilum | Japan | TNS: F-60527 | LC584251 | [56] | |
| Microstoma ningshanicum | China | SXIM20200016 | MW718686 | MW718687 | [58] |
| Microstoma radicatum | China | CFSZ 10,833 I5I4-3 | MG845232 | [29] | |
| Microstoma radicatum | China | CFSZ 10,833 I5I4-1 | MG845230 | [29] | |
| Microstoma sp. | China | FJAU71963 | PQ507629 | PQ498798 | This study |
| Nanoscypha striatispora | China | HMAS 61133 | U66016 | [26] | |
| Nanoscypha tetraspora | Puerto Rico | FH 00464570 | AF117352 | DQ220374 | [26,59] |
| Nanoscypha aequispora | Thailand | MFLU 21-0170 | OK413284 | OK398399 | [44] |
| Nanoscypha aequispora | Thailand | MFLU 21-0171 | OK413285 | OK398400 | [44] |
| Neournula pouchetii | USA | MO 205345 | KT968605 | [60] | |
| Neournula pouchetii | TUR-A195798 | JX669837 | [61] | ||
| Phillipsia carnicolor | Thailand | DHP-7126 (FH | AF117353 | JQ260811 | [59] |
| Phillipsia carnicolor | Thailand | MFLU 18-0713 | MH602282 | [62] | |
| Phillipsia chinensis | China | HMAS 76094 | AY254710 | [63] | |
| Phillipsia crispata | Ecuador | T. Læssøe AAU-44801 | AF117354 | [59] | |
| Phillipsia crispata | Ecuador | T. Læssøe AAU-44895a | AF117355 | AY945845 | [51] |
| Phillipsia domingensis | USA | CO-1864 (NO) | AF117363 | [59] | |
| Phillipsia domingensis | China | HKAS 121192 | OK170062 | [44] | |
| Phillipsia gelatinosa | Thailand | MFLU 15-2360 | KY498595 | KY498589 | [64] |
| Phillipsia gelatinosa | Thailand | MFLU 16-2956 | KY498593 | [64] | |
| Phillipsia hydei | Thailand | MFLU 18-0714 | MH602283 | [62] | |
| Phillipsia hydei | Thailand | MFLU 18-1329 | MH602284 | [62] | |
| Phillipsia lutea | French Guiana | NY-4113 (NY) | AF117374 | JQ260816 | [59] |
| Phillipsia olivacea | Costa Rica | Franco-M 1360 (NY) | AF117375 | [59] | |
| Phillipsia olivacea | Venezuela | Halling-5456 (NY) | AF117376 | JQ260814 | [59] |
| Phillipsia subpurpurea | China | MFLU 16-0612 | KY498596 | [64] | |
| Pithya cupressina | USA | mh 208 | U66009 | JQ260818 | [55,65] |
| Pithya sp. | China | DWS8m3 | KJ188703 | [66] | |
| Pithya sp. | USA | T5N32c | AY465469 | [67] | |
| Pithya vulgaris | RK 90.01 | U66008 | [65] | ||
| Pithya villosa | China | HKAS 104653 | OK170069 | OK398401 | [44] |
| Pithya villosa | China | HKAS 121194 | OK170068 | OK398402 | [44] |
| Pulvinula archeri | USA | FDS-CA-03585 | PQ211251 | [60] | |
| Pulvinula archeri | USA | HAY-F-003851 | PP789523 | [60] | |
| Pulvinula archeri | USA | FLAS-F-66445 | OR372796 | OR360871 | [60] |
| Pulvinula archeri | USA | FLAS-F-68939 | OR149300 | OR134550 | [60] |
| Pulvinula constellatio | Italy | AF289074 | [68] | ||
| Pulvinula constellatio | Spain | Ectomycorrhiza | OP847398 | [69] | |
| Pulvinula convexella | UK | OTU_740s | MT095859 | [70] | |
| Pulvinula convexella | UK | OTU_739s | MT095858 | [70] | |
| Pulvinula elsenensis | China | FJAU71964 | PQ507630 | This study | |
| Pulvinula elsenensis | China | FJAU71966 | PQ507632 | PQ498805 | This study |
| Pulvinula elsenensis | China | FJAU71967 | PQ507631 | PQ498806 | This study |
| Pulvinula elsenensis | China | FJAU71968 | PQ507634 | PQ498807 | This study |
| Pulvinula elsenensis | China | FJAU71969 | PQ507633 | PQ498808 | This study |
| Pulvinula elsenensis | China | FJAU71970 | PQ507635 | PQ517230 | This study |
| Pulvinula laeterubra | USA | FLAS-F-68187 | OR149263 | [60] | |
| Pulvinula miltina | New Zealand | PDD: 106213 | OL653010 | [60] | |
| Pulvinula niveoalba | Spain | ARAN-Fungi 13804 | MW248488 | MW248512 | [71] |
| Pulvinula niveoalba | Germany | M.A.R. 290,809 27 | KX592796 | KX592808 | [50] |
| Pulvinula orichalcea | Spain | ERD-8008 | MT432167 | MT425197 | [60] |
| Pulvinula sp. | Germany | PP461743 | [60] | ||
| Pulvinula sp. | USA | FLAS: F-6890 | PP210638 | [60] | |
| Pulvinula sp. | USA | MES-1034 | KY462405 | [72] | |
| Pulvinula sp. | USA | FLAS-F-70784 | OQ150525 | [60] | |
| Pulvinula sp. | USA | FLAS-F-69785 | OQ150476 | OP870119 | [60] |
| Pulvinula sp. | USA | FLAS-F-69718 | OQ150419 | [60] | |
| Pulvinula sp. | USA | TREC2_d5_C | OL348350 | [60] | |
| Pulvinula sp. | USA | MICH:352306 | OL756006 | OL742450 | [60] |
| Pulvinula sp. | USA | DBG: F-030576 | OP178120 | [73] | |
| Pulvinula sp. | USA | AK2160 | MZ091960 | MZ019040 | [74] |
| Pulvinula sp. | USA | AEA10751 | MZ091959 | MZ019039 | [74] |
| Pulvinula sp. | USA | FLAS-F-68819 | OM672974 | OM523302 | [60] |
| Pulvinula sp. | USA | FLAS-F-68775 | OM672946 | OM523282 | [60] |
| Pulvinula sp. | USA | FLAS-F-68214 | OM672686 | OM523218 | [60] |
| Pulvinula sp. | Canada | ITS2_OTU_93 | MW424606 | [75] | |
| Pulvinula sp. | UK | OTU_746s | MT095865 | [70] | |
| Pulvinula sp. | UK | OTU_745s | MT095864 | [70] | |
| Pulvinula sp. | UK | OTU_744s | MT095863 | [70] | |
| Pulvinula sp. | UK | OTU_743s | MT095862 | [70] | |
| Pulvinula sp. | UK | OTU_742s | MT095861 | [70] | |
| Pulvinula sp. | UK | OTU_741s | MT095860 | [70] | |
| Pulvinula sp. | UK | OTU_738s | MT095857 | [70] | |
| Pulvinula sp. | UK | OTU_737s | MT095856 | [70] | |
| Pulvinula sp. | USA | FLAS-F-61412 | MT374021 | MT350474 | [60] |
| Pulvinula sp. | UK | OTU082s | MK838257 | [76] | |
| Pulvinula sp. | USA | FLAS-F-63841 | MT156532 | [60] | |
| Pulvinula sp. | USA | OTU1272 | MK019168 | [60] | |
| Pulvinula sp. | USA | OTU992 | MK018732 | [60] | |
| Pulvinula sp. | USA | OTU361 | MK018616 | [60] | |
| Pulvinula sp. | USA | OTU631 | MK018575 | [60] | |
| Pulvinula sp. | USA | OTU336 | MK018540 | [60] | |
| Pulvinula sp. | Mexico | 2774 | MK397149 | [60] | |
| Pulvinula sp. 1 | China | FJAU71975 | PQ507765 | PQ510821 | This study |
| Pulvinula sp. 2 | China | FJAU71976 | PQ507766 | PQ510823 | This study |
| Pulvinula sublaeterubra | China | FJAU71972 | PQ507726 | PQ498809 | This study |
| Pulvinula sublaeterubra | China | FJAU71973 | PQ507727 | PQ510830 | This study |
| Pulvinula sublaeterubra | China | FJAU71974 | PQ507764 | PQ498812 | This study |
| Pulvinula sublaeterubra | China | FJAU71965 | PQ513528 | PQ510816 | This study |
| Rickiella edulis | Argentina | BAFC 51697 | JQ260808 | [27] | |
| Sarcoscypha austriaca | Norway | CUP 62771 | U66010 | [65] | |
| Sarcoscypha austriaca | USA | CUP 63162 | U66011 | [65] | |
| Sarcoscypha coccinea | AFTOL-ID 50 | DQ491486 | AY544647 | [77] | |
| Sarcoscypha coccinea | USA | CUP 63160 | U66015 | [65] | |
| Sarcoscypha dudleyi | USA | CUP 62775 | U66018 | [65] | |
| Sarcoscypha dudleyi | China | HMJAU36044 | KU234218 | [78] | |
| Sarcoscypha emarginata | Luxembourg | CUP 62723 | U66020 | [65] | |
| Sarcoscypha emarginata | China | HB2861 | U66021 | [65] | |
| Sarcoscypha hongshiensis | China | FJAU71952 | PQ496884 | PQ498771 | This study |
| Sarcoscypha hongshiensis | China | FJAU71953 | PQ496886 | PQ498777 | This study |
| Sarcoscypha hongshiensis | China | FJAU71954 | PQ496887 | PQ498785 | This study |
| Sarcoscypha hongshiensis | China | FJAU71955 | PQ496892 | PQ498784 | This study |
| Sarcoscypha hongshiensis | China | FJAU71956 | PQ496893 | PQ498787 | This study |
| Sarcoscypha hongshiensis | China | FJAU71957 | PQ496894 | PQ498788 | This study |
| Sarcoscypha hosoyae | TRL 456 | U66031 | [65] | ||
| Sarcoscypha humberiana | China | TNM F28630 | KT716833 | [79] | |
| Sarcoscypha humberiana | China | CUP 63489 | U66028 | [65] | |
| Sarcoscypha javensis | China | HMAS 61198 | U66026 | [65] | |
| Sarcoscypha knixoniana | TRL 1006 | U66030 | [65] | ||
| Sarcoscypha korfiana | mh 705 | AF026308 | [26] | ||
| Sarcoscypha longitudinalis | China | HKAS 121195 | OK170051 | OK398403 | [44] |
| Sarcoscypha longitudinalis | China | HKAS 121196 | OK170052 | OK398404 | [44] |
| Sarcoscypha macaronesica | Canary Islands | CUP-MM 2628 | U66022 | [65] | |
| Sarcoscypha macaronesica | TFC-MIC 6460 | U66023 | [65] | ||
| Sarcpscypha mesocyatha | China | TNM F3688 | KT936558 | [79] | |
| Sarcpscypha mesocyatha | China | TNM F5134 | KT936559 | [79] | |
| Sarcoscypha mesocyatha | USA | CUP 62699 | U66029 | [65] | |
| Sarcoscypha minuta | China | TNM F28831 | KT716834 | [79] | |
| Sarcoscypha occidentalis | USA | CUP 62777 | U66024 | [65] | |
| Sarcoscypha occidentalis | USA | CUP 63484 | U66025 | [65] | |
| Sarcoscypha sp. | China | HMAS 61202 | U66027 | [65] | |
| Sarcoscypha tatakensis | China | TNM F0754 | KT716835 | [79] | |
| Sarcoscypha tatakensis | China | TNM F0993 | KT716836 | [79] | |
| Sarcoscypha vassiljevae | China | HKAS 89817 | MG871302 | MG871337 | [12] |
| Sarcoscypha vassiljevae | China | HMAS 61210 | U66017 | [65] | |
| Wynnea americana | USA | FH 00445978 | MK599141 | MK599148 | [55] |
| Wynnea americana | USA | HKAS 75484 | MG871308 | [12] | |
| Wynnea gigantea | Brazil | FH s. n. | MK335781 | MK335801 | [80] |
| Wynnea gigantea | Brazil | FH ACM624 | MK335782 | MK335802 | [80] |
| Wynnea macrospora | China | FH 00445975 | MK335784 | MK335803 | [80] |
| Wynnea macrospora | China | FH 00940720 | MK335785 | [80] | |
| Wynnea macrotis | USA | CUP 2684 | MK335786 | MK335804 | [80] |
| Wynnea sparassoides | USA | NYBG02480090 | MK335787 | MK335805 | [80] |
New sequences generated for this study are shown in bold.
3. Results
3.1. Phylogenetic Analyses
This study generated a total of 47 new sequences, including 24 ITS sequences and 23 nrLSU sequences. These new sequences were uploaded to GenBank. The multilocus dataset (ITS + nrLSU) of Pulvinula had an aligned length of 1581 total characters and Sarcoscyphaceae had an aligned length of 1103 (ITS) and 1919 (ITS + nrLSU) total characters, including gaps. Only the topological structures of Bayesian inference are displayed, as the topological structures of ML and BI are very similar. Bayesian posterior probability (PP) values ≥ 0.75 and bootstrap support (BS) values ≥ 95% are indicated on branches (PP/BS) (Figure 1 and Figure 2).
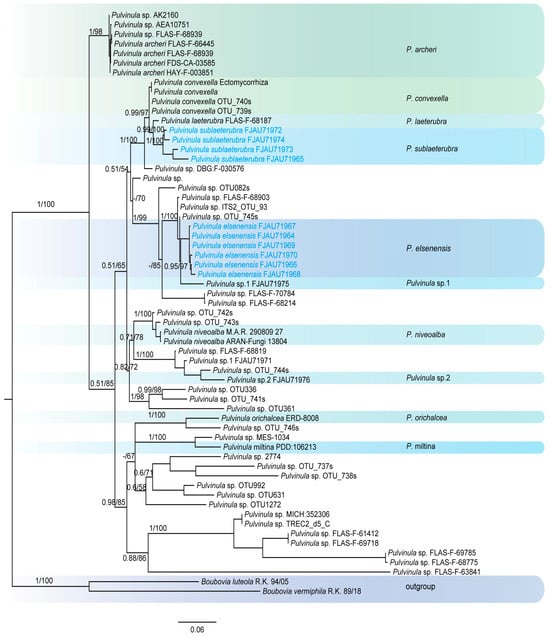
Figure 1.
The phylogeny of Pulvinula by Bayesian inference based on the ITS and LSU dataset.
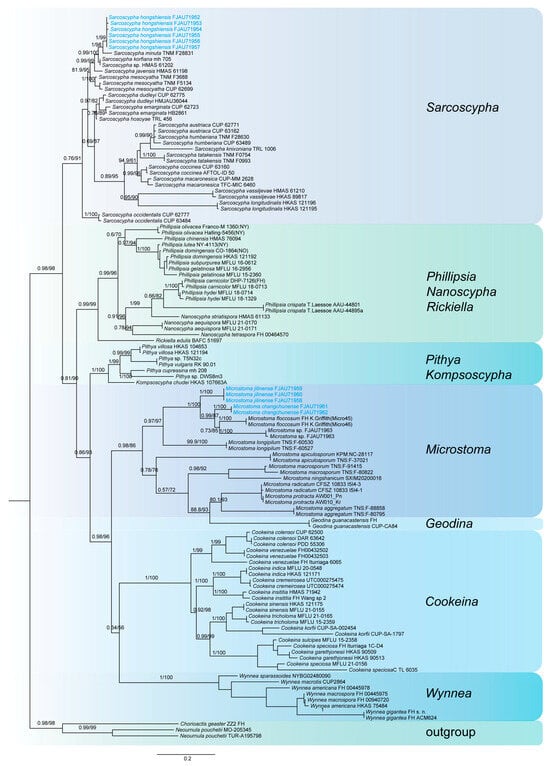
Figure 2.
The phylogeny of Sarcoscyphaceae as assessed by Bayesian inference based on the ITS dataset.
The phylogenetic tree of Pulvinula (Figure 1) shows that this genus belongs to a polyphyletic group. Pulvinula elsenensis and Pulvinula sp. 1 (FJAU71975) form a sister-group relationship with high support (PP/BS = 0.95/97), while Pulvinula sublaeterubra and Pulvinula laeterubra form a sister-group relationship with very high support (PP/BS = 0.99/100).
The phylogenetic tree of Sarcoscyphaceae (Figure 2 and Figure 3) shows that Microstoma jilinense forms a distinct branch with full support (PP/BS = 1/100) in both Figure 2 and Figure 3. In Figure 2, it can be seen that Microstoma changchunense is in a sister-group relationship with Microstoma floccosum and Microstoma sp. (FJAU71963), with strong support (PP/BS = 1/100). In Figure 3, Microstoma changchunense forms a sister-group relationship with Microstoma sp. (FJAU71963), but with lower support (PP/BS = 0.64/76). Additionally, in Figure 2, Sarcoscypha hongshiensis and Sarcoscypha minuta form a sister-group relationship with high support (PP/BS = 1/99), which is also strongly supported in Figure 3 (PP/BS = 1/100).
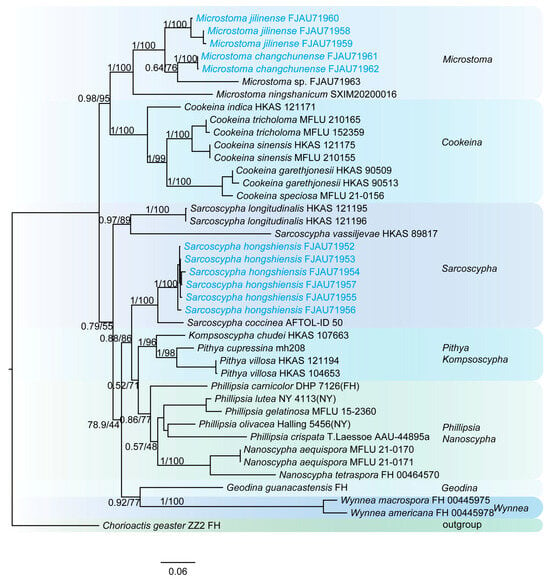
Figure 3.
The phylogeny of Sarcoscyphaceae as assessed by Bayesian inference based on the ITS and LSU dataset.
3.2. Taxonomy
Pulvinula elsenensis T. Bau et Z. Q. Chen, sp. nov.


Figure 4.
Ascocarps of Pulvinula elsenensis (A,B); Pulvinula sublaeterubra; (C,D) Microstoma jilinense (E,F); Microstoma changchunense (G,H); Sarcoscypha hongshiensis (I,J). Scale bars: (A–D) = 0.3 cm; (E–J) = 1 cm. Collection site and collection time: (A,B): Jilin Province, 2023; (C): Inner Mongolia Autonomous Region, 2022, (D): Inner Mongolia Autonomous Region, 2023; (E): Liaoning Province, 2024, (F): Jilin Province, 2024; (G, H): Jilin Province, 2024; (I, J): Jilin Province, 2023.
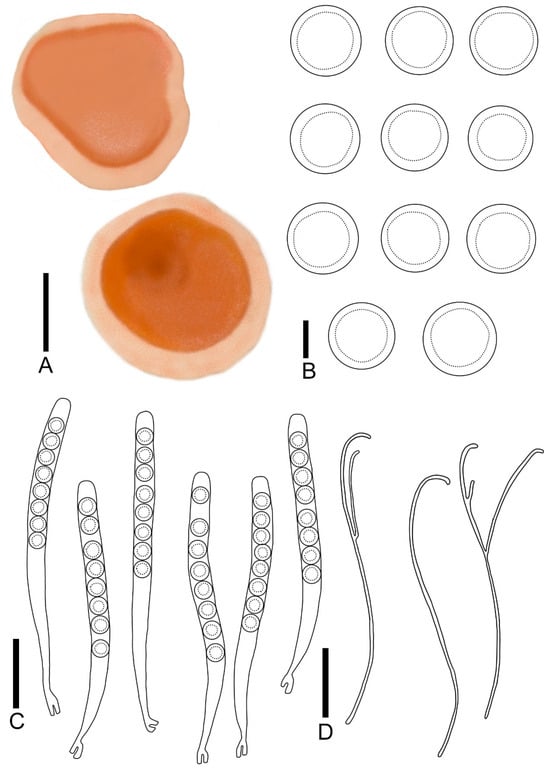
Figure 5.
Pulvinula elsenensis: (A) ascocarps; (B) ascospores; (C) asci; (D) paraphyses. Scale bars: (A) = 0.3 cm; (B) = 8 μm; (C,D) = 55 μm.
MycoBank number: 856999
Diagnosis: Pulvinula elsenensis is characterized by sessile ascocarp; a yellow orange hymenium; and a conspicuous margin. It forms a crozier at the base of asci, has ascospores of 13.0–16.4 μm, and is curved at the apex without enlarged paraphyses.
Etymology: elsenensis is a Mongolian word for “sandy land”, which means that type specimens are collected in sandy area
Type: CHINA. Inner Mongolia Autonomous Region, Tongliao City, 43°01′ N, 122°44′ E, 363 m, 3 June 2023, Weinan Hou and Tolgor Bau (FJAU71966, H230615, holotypus!). Jilin Province Songyuan City, 44°17′ N, 123°35′ E, 133 m, 25 June 2023, Zhengqing Chen (FJAU71964, CZQ2362503, paratypus!).
Description: Apothecia concave–discoid when young, discoid when mature, conspicuous margin, sessile, 0.2–0.7 cm broad. Hymenium smooth, yellow-orange (RAL2000) when fresh. Receptacle surface cream (RAL9001). Ectal excipulum 52.6–78.3 μm broad, composed of textura angularis, hyaline, outer most cell 6.8–12.5 × 4.2–8.5 μm. Medullary excitulum 30.7–42.0 μm broad, composed of textura intricate, hyphae 2.9–4.5 μm, hyaline. Asci cylindrical, 216–268 × 14.2–16.5 μm, 8-spored, operculate, becoming narrow towards the base, distinctly forked base formed by a crozier, J− in Melzer’s reagent, hyaline. Ascospores {6/2/20}, 13.0–16.4 μm, Q = 1.00 − 1.04, spherical, smooth, a large guttulate, hyaline, uniseriate. Paraphyses filiform, slender, septate, branched, curved at apex without enlarged, 2.2–2.9 μm broad.
Habitat: in summer, it grows in clusters on sandy ground covered with mosses.
Distribution: currently, it is only distributed in China’s Inner Mongolia Autonomous Region and Jilin Province.
Additional specimens examined: CHINA. Inner Mongolia Autonomous Region, Tongliao City, 43°01′ N, 122°44′ E, 363 m, 22 August 2022, Weinan Hou and Tolgor Bau (FJAU71970, H2208133). Same location, 17 July 2023, Weinan Hou and Tolgor Bau (FJAU71968, H2307151); 7 September 2023 Weinan Hou and Tolgor Bau (FJAU71967, H230967); Jilin Province Songyuan City, 25 June 2023, Mu Liu (FJAU71969, lm23062).
Notes: In terms of macroscopic morphology, Pulvinula elsenensis is easily confused with P. anthracobia T. Schumach., P. multiguttula (L.R. Batra) S.C. Kaushal, and P. orichalcea (Cooke) Rifai in the wild. However, several distinguishing characteristics set it apart. The asci of P. anthracobia (140–170 × 11–13 μm) and its ascospores (10.1–12.2 μm) are both smaller, and this species lacks croziers [81]. In contrast, the asci of P. multiguttula are wider (14–19 μm) and contain 5–9 guttulates, while the paraphyses are unbranched [82]. P. orichalcea can be differentiated by its receptacle surface, which is covered in white pubescence, and its ascospores, which contain three or more small guttulates [83]. Although Pulvinula elsenensis shares similarities with P. tetraspora (Hansf.) Rifai in its younger stage, the latter may exhibit an orange coloration when fresh and, in contrast, typically has mature asci containing four spores, with a few containing two or five spores [84]. This distinction makes the two species easily identifiable.
Pulvinula sublaeterubra T. Bau et Z. Q. Chen, sp. nov.
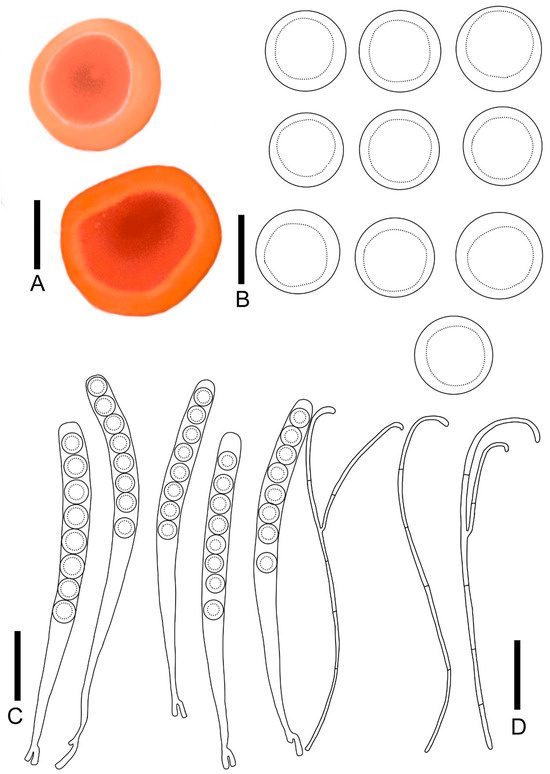
Figure 6.
Pulvinula sublaeterubra: (A) ascocarps; (B) ascospores; (C) asci; (D) paraphyses. Scale bars: (A) = 0.3 cm; (B) = 15 μm; (C,D) = 50 μm.
MycoBank number: 857000
Diagnosis: Pulvinula sublaeterubra is characterized by a sessile ascocarp, a pastel orange hymenium, a yellow-orange receptacle surface, and a conspicuous margin. It forms a crozier at the base of asci, displays ascospores at 14.9–17.1 μm, and is curved at the apex without enlarged paraphyses.
Etymology: the specific epithet “sublaeterubra” refers to the similarity of the type species to Pulvinula laeterubra.
Type: CHINA. Inner Mongolia Autonomous Region, Tongliao City, 43°01′ N, 122°44′ E, 363 m, 14 July 2022, Weinan Hou and Tolgor Bau (FJAU71974, H220762, holotypus!). Same location, 2 June 2023, Hong Cheng and Tolgor Bau (FJAU71973, C2023060204, paratypus!).
Description: Apothecia concave initially, matting and discoiding to spreasing with maturation, distinct margins, not elevated, 0.2–0.5 cm broad, sessile. Hymenium smooth, pastel orange (RAL2003) when fresh, red-orange (RAL2001) when dry. Receptacle surface yellow-orange (RAL2000) when fresh. Ectal excipulum 152.1–182.4 μm broad, composed of textura angularis, hyaline, outer most cell 8.5–12.0 × 6.1–9.1 μm. medullary excitulum 56.5–75.6 μm broad, composed of textura intricate, hyphae 1.8–3.2 μm, hyaline. Asci cylindrical, 254–302 × 15.7–20.9 μm, 8-spored, operculate, becoming narrow towards the base, distinctly forked base formed by a crozier, J− in Melzer’s reagent, hyaline. Ascospores {4/2/20}, 14.8–17.1 μm, Q = 1.00 − 1.03, spherical, smooth, a large guttulate, hyaline, uniseriate. Paraphyses filiform, slender, septate, branched, curved at apex without enlarged, 1.8–3.2 μm broad.
Habitat: in summer, it grows in clusters on sandy grasslands in broad-leaved forests and shrubs.
Distribution: it is only distributed in China’s Inner Mongolia Autonomous Region.
Additional specimens examined: CHINA. Inner Mongolia Autonomous Region, Tongliao City, 43°01′ N, 122°44′ E, 363 m, 2 June 2023 Weinan Hou and Tolgor Bau (FJAU71965, H230604). Same location, 19 July 2023, Hong Cheng and Tolgor Bau (FJAU71972, C2371915).
Notes: Pulvinula sublaeterubra is morphologically similar to P. miltina, although the latter can be distinguished by several key characteristics. P. miltina has shorter asci (200–250 × 16–20 μm) with either a single large guttulate or multiple smaller guttulates in the ascospores. Additionally, the apexes of its paraphyses are enlarged and unbranched, and typically grow on calcareous substrates [85]. In contrast, the species in question can be differentiated based on their distinct habitat and morphological traits. When compared to P. nepalensis, the latter lacks regular croziers, has enlarged paraphysis apexs, and is predominantly found in burned bamboo forests [86]. P. pyrophila (Snyder) Donadini, G. Riousset and Riousset, and P. salmonicolor (Seaver) Pfister may also be confused with P. sublaeterubra in the field. However, P. pyrophila is easily distinguishable by its smaller asci (150–200 × 10–12 μm) and ascospores (7–9 μm), as well as its occurrence on burned land in early spring [87]. In contrast, P. salmonicolor has larger, wider asci (20–24 μm), larger ascospores (up to 20 μm), and clavate paraphyses, features which facilitate identification [88].
Microstoma jilinense T. Bau et Z. Q. Chen, sp. nov.
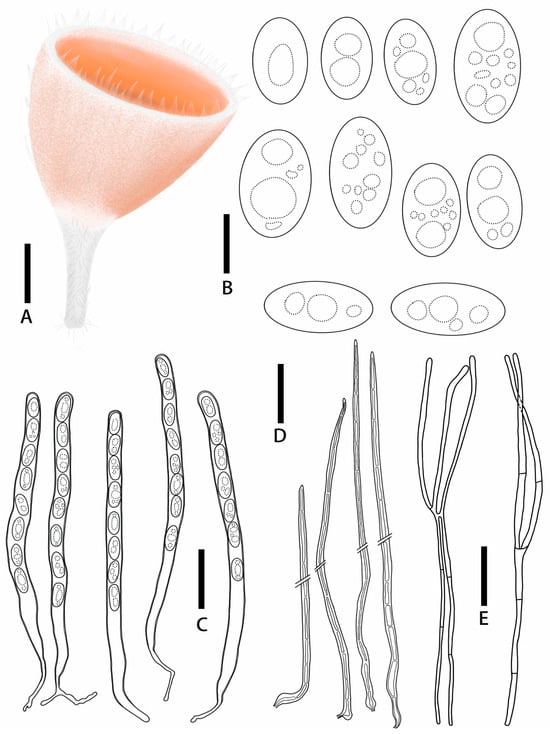
Figure 7.
Microstoma jilinense: (A) ascocarps; (B) ascospores; (C) asci; (D) paraphyses. Scale bars: (A) = 0.5 cm; (B) = 12 μm; (C–E) = 55 μm.
MycoBank number: 857001
Diagnosis: Microstoma jilinense is characterized by salmon orange hymenium; longer hairs at the edges, which are acute toward the apices; asci of 247–325 × 10.6–14.2 μm; elliptical to cylindrical ascospores (15.9–24.3 × 9.0–14.9 μm) without appurtenance on the ends; and fewer paraphyses.
Etymology: the specific epithet “jilinense” refers to the discovery of a type specimen in Jilin Province, China.
Type: CHINA. Jilin Province, Huadian City, Hongshi National Forest Park, 24 June 2024, 127°08′12″ E, 42°49′57″ N, alt. 498 m, Zhengqing Chen (FJAU71958, Q2462405, holotypus!). Liaoning Province, Benxi City, Guanyin Mountain Park, 4 July 2024, 124°6′ E, 41°17′ N, alt. 488, Weinan Hou (FJAU71960, H24070402, paratypus!)
Description: Apothecia deeply cupulate, 0.3–0.65 cm broad, 0.3–0.7 cm deep, sessile or stipitate. Hymenium salmon orange (RAL2012) when fresh, carmine red (RAL 3002) when dry. Receptacle surface salmon orange (RAL2012) when fresh, covered with hairs which longer and gathered into pointed bundles. Stipe 0.5–1.2 × 0.1–0.4 cm, pure white (RAL9010), densely covered with hairs. Ectal excipulum21.5–30.5 μm broad, tissue gelatinous, composed of textura porrecta to textura prismatica, hyaline. Medullary excitulum 84.3–121.3 μm broad, composed of textura intricate, hyphae 2.4–4.1 μm, hyaline. Hairs arising from outer and inner ectal excipular cells, cylindrical, 627–267 μm high, setaceous, septate, acute toward the apices, with thick walls, 1.6–4.6 μm thick. Asci cylindrical, 247–325 × 10.6–14.2 μm, 8-spored, J− in Melzer’s reagent, suboperculate, with slightly thick walls, becoming narrow towards the base. Ascospores {3/1/20}, 15.9–24.3 × 9.0–14.9 μm, Q = 1.43–2.19, elliptical to cylindrical, with a large guttulate when mature, without appurtenance on the ends, smooth, hyaline, uniseriate. Paraphyses filiform, septate, branched, 2.3–4.3 μm broad.
Habitat: in summer, it grows scattered on rotten wood in mixed coniferous and broad-leaved forests.
Distribution: currently, it is only distributed in China’s Jilin Province and Liaoning Province.
Additional specimens examined: CHINA. Jilin Province, Changchun City, Jilin Agricultural University, 22 July 2024, 125°24′19″ E, 43°48′37″ N, alt. 222 m, Tolgor Bau and Zhengqing Chen (FJAU71959, Q247222).
Notes: The ascospores of Microstoma jilinense are devoid of appurtenance at both ends, distinguishing it from M. apiculosporum (Yei Z. Wan) and M. camerunense (Douanla-Meli) [89,90]. Additionally, its more dispersed ascocarp stalk base and the absence of pseudorhizoid differentiate it from M. protractum (Fr.) Kanouse and M. radicatum, as outlined by T.Z. Liu, Wulantuya, and W.Y. Zhuang [29,91]. Morphologically, Microstoma jilinense is often confused with M. floccosum (Sacc.) Raitv., which is characterized by a scarlet hymenium and larger ascospores (22–40 × 10–16.8 μm) [15]. Both this species and M. longipilum Tochihara, T. Hirao, and Hosoya exhibit dense white hairs along the edge of the hymenium and receptacle that form pointed tufts. However, M. longipilum has a dull pink to pale orange hymenium, longer asci (275–350 × 10–17.5 μm), shorter ascospores (11–12.5 μm), and anenlarged apex in some paraphyses [56].
Microstoma changchunense T. Bau et Z. Q. Chen, sp. nov.
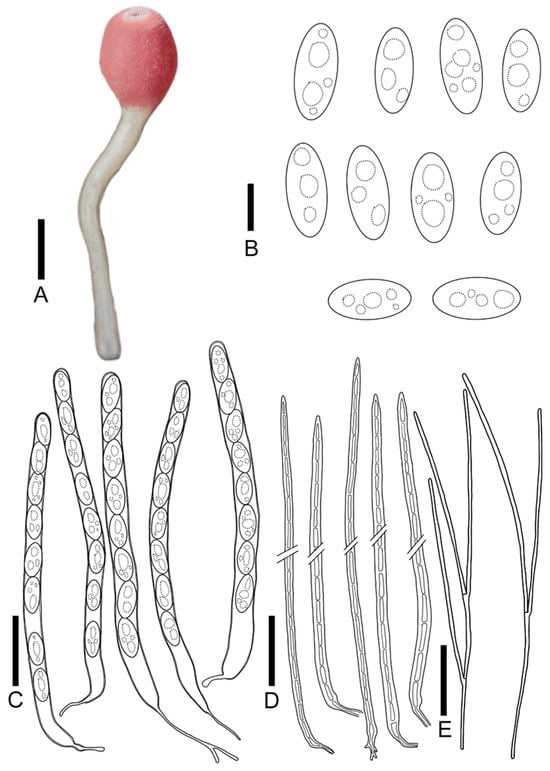
Figure 8.
Microstoma changchunense: (A) ascocarps; (B) ascospores; (C) asci; (D) hairs; (E) paraphyses. Scale bars: (A) = 0.8 cm; (B) = 15 μm; (C–E) = 55 μm)
MycoBank number: 857002
Diagnosis: Microstoma changchunense is characterized by light pink hymenium, a cream long stipe, shorter hairs (360–600 μm), asci of 295–345 × 13.5–19.5 μm, and long elliptical ascospores (25.0–36.2 × 11.5–14.5 μm) without appurtenance on the ends.
Etymology: the specific epithet “changchunense” refers to the discovery of a type specimen in Changchun City Jilin Province, China.
Type: CHINA, Jilin Province, Changchun City, Jingyuetan National Forest Park, 9 June 2024, 125°28′01″ E, 43°46′09″ N, alt. 264 m, Tolgor Bau and Yu Wang (FJAU71961, WY24060905, holotypus!); Same location, 9 June 2024, 125°28′01″ E, 43°46′09″ N, alt. 264 m, Tolgor Bau and Tianyu Zhang (FJAU71962, ZTY24695, paratypus!)
Description: Apothecia cupulate, 0.2–0.4 cm broad, 0.4–0.7 cm deep, stipitate. Hymenium light pink (RAL3015) when fresh, red-orange (RAL2001) when dry. Receptacle surface light pink (RAL3015) when fresh, carmine (red RAL3002) when dry, densely covered with hairs at the margins. Stipe 1.9–3.5 × 0.1–0.2 cm, cream (RAL9001). Ectal excipulum 39.8–64.3 μm broad, tissue gelatinous, composed of textura prismatica, hyaline. Medullary excitulum 54.6–85.8 μm broad, composed of textura intricate, hyphae 2.1–3.7 μm, hyaline. Hairs arising from outer and inner ectal excipular cells, cylindrical, 360–600 μm high, setaceous, septate, acute toward the apices, with thickwalls, 3.2–5.7 μm thick. Asci cylindrical, 295–345 × 13.5–19.5 μm, 8-spored, J− in Melzer’s reagent, suboperculate, with slightly thick walls, becoming narrow towards the base. Ascospores {2/2/20}, 25.0–36.2 × 11.5–14.5 μm, Q = 1.90–2.62, long elliptical, with a or more guttulate when mature, without appurtenance on the ends, smooth, hyaline, uniseriate. Paraphyses filiform, septate, branched, 2.1–4.2 μm broad.
Habitat: in summer, it grows scattered on rotten wood in broad-leaved forests.
Distribution: currently, it is only distributed in China’s Jilin Province.
Notes: Microstoma changchunense is characterized by a deep, cup-shaped ascocarp and relatively short hairs. It bears resemblance to M. floccosum, but the latter is distinguishable by its red hymenium and receptacle [29]. Additionally, while M. changchunense shares a similar hymenium and receptacle coloration with M. macrosporum (Y. Otani), as outlined by Y. Harada and S. Kudo, M. macrosporum differs in having an inverted triangular cup-shaped ascocarp and larger ascospores (42–60 × 16–21 μm) [92]. Misidentification with M. radicatum is also possible; however, M. radicatum exhibits clustered ascocarps, a dentate margin, and a rhizoid at the base of the stalk [29]. Morphologically, Microstoma changchunense resembles M. ningshanicum, as outlined by W.Y. Huo, Yu Liu, L.G. Zhang, and J.Z. Li, but its ascospores lack terminal appendages. Furthermore, M. ningshanicum has longer asci (449–517 × 16–20.5 μm) and ascospores (31–45 × 13–17 μm), with distinct longitudinal shallow striations, aiding in differentiation [58].
Sarcoscypha hongshiensis T. Bau et Z. Q. Chen, sp. nov.
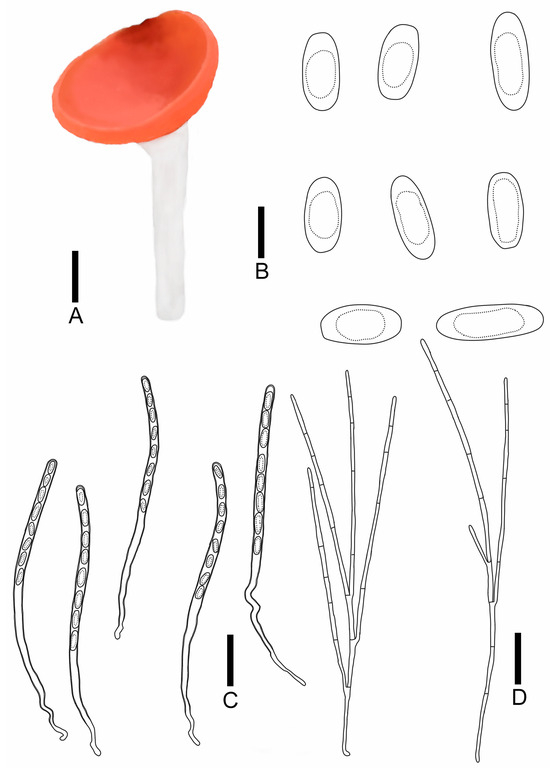
Figure 9.
Sarcoscypha hongshiensis: (A) ascocarps; (B) ascospores; (C) asci; (D) paraphyses. Scale bars: (A) = 0.5 cm; (B) = 15 μm; (C,D) = 60 μm.
MycoBank number: 857003
Diagnosis: Sarcoscypha hongshiensis is characterized by pure orange hymenium, a pure white receptacle surface, a pure white stipe, asci of 284–323 × 9.0–9.9 μm, cylindrical to rod-shaped ascospores (19.2–27.0 × 8.2–9.7 μm) with blunt ends, and both ends being partly truncated.
Etymology: the specific epithet “hongshiensis” refers to the locality from where the type species was collected.
Type: CHINA. Jilin Province, Jiaohe City, Qianjin Forest Farm, 25 August 2023, 127°41′54″ E, 43°57′03″ N, alt. 444 m, Tolgor Bau and Xia Wang, (FJAU71956 W23082515, holotypus!). Jilin Province, Huadian City, Hongshi National Forest Park, 28 August 2023, 127°08′12″ E, 42°49′57″ N, alt. 498 m, Tolgor Bau and Chen zhengqing (FJAU71953, Q2382819, paratypus!).
Description: Apothecia 0.3–2.0 cm broad, discoid, fleshy, stipitate. Hymenium pure orange (RAL2004) when fresh, pastel yellow (RAL1034) when dry, margin integrity. Receptacle surface pure white (RAL9010), with orange-red hue, the edge is similar to hymenium’s color, and longitudinal striations. Stipe subcylindrical, 0.3–4.5 × 0.3–0.5 cm, pure white (RAL9010). Ectal excipulum 66.8–80.6 μm broad, composed of textura porrecta to textura prismatica, cells 17.7–33.9 × 3.7–6.0 μm, hyaline to pale brown. Medullary excitulum 48.9–82.0 μm broad, composed of textura intricate, hyphae 3.5–4.8 μm, hyaline. Asci cylindrical, 284–323 × 9.0–9.9 μm, 8-spored, J− in Melzer’s reagent, suboperculate, becoming narrow towards the base, slim, curvaceous. Ascospores {6/2/20}, 19.2–27.0 × 8.2–9.7 μm, Q = 2.04–3.36, cylindrical to rod-shaped, with blunt ends, partly both ends truncated, smooth, with a large guttulate, hyaline, uniseriate. Paraphyses filiform, septate, branched, 1.6–2.1 μm broad.
Habitat: in summer, it grows in a scattered way on rotten wood in mixed coniferous and broad-leaved forests.
Distribution: currently, it is only distributed in China’s Jilin Province.
Additional specimens examined: CHINA, Jilin Province, Huadian City, Hongshi National Forest Park, 27 August 2023, Tolgor Bau and Chen zhengqing (FJAU71957, Q2382709). Same location, 28 August 2023, Tolgor Bau and Mu Liu (FJAU71955, lm230849); 28 August 2023, T. Bau and Zhengqing Chen (FJAU71952, Q2382809); 28 August 2023, Tolgor Bau and Zhengqing Chen (FJAU71954, Q2382823)
Notes: Sarcoscypha hongshiensis shares a similar ascocarp color with S. macaronesica Baral and Korf. However, it is distinguishable by the latter’s dentate margin, larger asci (350 × 15.0 μm), and larger ascospores (22–33 × 9.0–11.5 μm) [32]. When comparing it with S. dudleyi (Peck) Baral, Sarcoscypha hongshiensis stands apart in that S. dudleyi is sessile, has longer asci (290–415 × 7–10 μm), and shorter ascospores (18–24 μm) [79]. Sarcoscypha hongshiensis may be confused with S. javensis Höhn., S. knixoniana F.A. Harr., and S. occidentalis (Schwein.) Sacc. in the wild. However, S. occidentalis is characterized by a receptacle covered in white pubescence and shorter ascospores (18 × 9 μm), whereas S. javensis features smaller asci (230 × 10 μm) and ascospores (23 × 8 μm) [93,94]. S. knixoniana has longer asci (ranging from 270 to 350 μm, extending up to 420 μm) and ascospores with deep depressions at both ends, which aids in distinguishing it from Sarcoscypha hongshiensis [95].
4. Discussion
Comprehensive molecular data for many species of Pulvinula are lacking, and the molecular information available for the species type, P. convexella, is often contradictory. The majority of species are only represented by data from the internal transcribed spacer (ITS) region, with just a few having additional sequences from other genetic markers, such as the large subunit (LSU), RNA polymerase II (RPB2), and translation elongation factor 1-alpha (TEF1-α). This necessitates more detailed and comprehensive data acquisitions to facilitate further studies on Pulvinula. The macroscopic morphology and microscopic structure of numerous species of Pulvinula are similar. For example, the ascocarps of species in this genus are relatively small, and the color of the hymenium is mainly divided into two categories: white to milky white or light yellow to orange-red (Table 2). It is common for these species to be confused based on their macroscopic morphology alone. Consequently, the utilization of molecular data and phylogenetic analysis is imperative for the accurate identification of Pulvinula species that exhibit similar morphological characteristics. P. elsenensis and P. sublaeterubra form separate branches in the phylogenetic tree; however, the support for P. elsenensis is not very high, suggesting that the phylogenetic position of this branch is unstable. P. sublaeterubra and P. laeterubra are sister groups. However, notable differences are observed between them. The hymenium of P. laeterubra is orange-pink, the asci (180 − 200 × 12 μm) and ascospores (10 μm) are smaller, and the habitats of the two are also different. P. laeterubra grows exclusively on burnt humus layers [92].
Zeng Ming’s phylogenetic framework was integrated with sequence data from specimens collected in northeastern China, resulting in the construction of a robust phylogenetic tree based on reliable ITS and LSU sequences [44]. The newly described species forms S. hongshiensis, a distinct evolutionary lineage within this tree, with S. minuta as its sister group. This is outlined by Yei Z. Wang, Cheng L. Huang, and J.L. Wei. However, differences were observed between the two species’ collection sites and habitats. The former is distributed on decaying wood in mixed coniferous and broadleaf forests in a temperate monsoon climate, whereas the latter thrives in a subtropical monsoon climate, where it grows on husks. S. minuta can be differentiated by its orange to cadmium yellow ascocarp color, crenulate margins, smaller asci (180–250 × 7–10 μm), and ascospores (16–20 × 8–10 μm) [79]. Additionally, during the species description within this genus, it was noted that the paraphyses of S. aestiva Velen., S. albovillosa Rehm, and S. jurana (Boud.) Baral exhibited a color change when exposed to iodine, turning blue or green (Table 4). No such discoloration was observed in other species, although this phenomenon was recorded in collections of S. javensis and S. hongshiensis. Given the limited number of species collected so far, it remains difficult to confirm this phenomenon. Additional specimens of these species are required to validate and elucidate these characteristics.
The species of Microstoma are primarily distributed in temperate regions where they either grow in aggregates or as solitary specimens. The receptacle surface is covered with white hairs composed of individual hyphae. This is a key distinguishing feature of the closely related genus Cookeina, which is typically solitary and found predominantly in tropical and subtropical regions [30,31,96]. The key morphological traits for species differentiation within Microstoma include the color of the ascospore disks, the presence of pseudorhizomes, the shape of the hairs at the edges, the shape of the ascospores, the presence or absence of appurtenance on at ends, and the number of guttulates (Table 3). In the present study, M. jilinense and M. changchunense were clearly differentiated from other species within the genus (Table 3). This differentiation was supported by the absence of pseudorhizomes in both species, the lack of appurtenance at the ends of the ascospores, and the observation that M. jilinense exhibited longer hairs (627–1267 μm) than M. changchunense, which had shorter hairs (360–600 μm).
Furthermore, Microstoma sp. (FJAU71963), Pulvinula sp. 1 (FJAU71975), and Pulvinula sp. 2 (FJAU71976) each produced a distinct and separate evolutionary lineage within the phylogenetic tree. However, this particular lineage has not been characterized or described at present, owing to only one specimen being available for observation.
The northeastern region of China has a substantial diversity of Pezizales species, with Pulvinula, Microstoma, and Sarcoscypha being predominant. In the present study, five novel species were identified and characterized based on morphological and phylogenetic analyses, thereby augmenting the species richness of Pezizales in this region. Concurrently, these results imply the potential existence of hitherto undetected species in northeastern China, which merit further in-depth investigation.

Table 2.
Current list of Pulvinula species and their diagnostic characteristics. Non-English descriptions from references are not translated to avoid misunderstanding.
Table 2.
Current list of Pulvinula species and their diagnostic characteristics. Non-English descriptions from references are not translated to avoid misunderstanding.
| Species | Apothecia Color When Fresh | Asci | Crozier | Ascospores | Paraphyses | Habitat | References |
|---|---|---|---|---|---|---|---|
| P. alba | White | Cylindric, 225–290 × 20–26(–30) μm, 8-spored | Base forked | Unicellular, globose, 17.0–19.0(20.5) μm, one large guttule, smooth under the ligth microscope | Filiform, septate, apically curved, not enlarged at the apex | On damp ground | [8] |
| P. albida | Albidis | 180 μm longis, 16 μm latis | Globosis, 15 μm, | filiformes | At terram | [97] | |
| P. anthracobia | Orange to crimson | Cylindrical, 140–170 × 11–13 μm, 8-spored | Unforked base | Globose, smooth, a single large oil globule, 10.1–12.2 μm | Filiform, 0.9–1.6 μm, moderately curved and branched from the middle of their length, not enlarged or branched towards the apex, slightly longer than the asci | On soil and charred wood in fire bed | [81] |
| P. archeri | Cinnabarina, bright-crimson | Cylindrical | Globes, 1/3500 inch across, with a large uncleus | On dead leaves of some succulent plant | [98] | ||
| P. carbonaria | aurantio-sanguineis | Cylindraceis, 8sporis, 116 Mik., long, 16 Mik. crass | Golbosis, episporio reticulato, 16Mik., diamater | Filiformibus, aurantiacia,copiosis | Auf verlassenen brandstellen kohlenmeilern und dergl., sehr selten, im Herbst. | [99] | |
| P. cinnabarina | Cinnabarinis | Cylindraceis, 8sporis, 144 Mik. long, 18 Mik. crass | Golbosis, episporio reticulato, 18Mik., diamater | Filiformibus, copiosis, aurantiacia | Auf dem sande des Rheinbettes bei Ragaz, in der Schweiz, nachst der Eisenbahnbrucke, hier haufig, im Herbst | [99] | |
| P. convexella | Sangvineo-rubra | Sphaeroideae, 15–16 mmm | Graciles, flexuoxae, intus granulosae | supra terram nudam in rivuli margine | [100] | ||
| P. discoidea | Albo-cinereis | Cylindreeeo-clavatia, apice rotundatis, 8sporis, 100–110 × 14–16 μm | Globosis,lacvibus, hyaline-subtlavesentibus, 12–14 μm | Filiformilms,guttulatis, vel septatis | Auf lehuigem erdboden wachsenden | [101] | |
| P. elsenensis | Yellow-orange | cylindrical, 216–268 × 14.2–16.5 μm, 8-spored, operculate, becoming narrow towards the base | distinctly forked base formed by a crozier | spherical, 13.0–16.4 μm, smooth, | filiform, slender, septate, branched, curved at apex without enlarged | grows in clusters on sandy ground covered with mosses | This study |
| P. etiolata | Alba | cylindraceis | Globosis, laevibus | filiformibus | On the ground | [102] | |
| P. globifera | Globosis, 0.002 inch | On rutten logs in wood | [103] | ||||
| P. johannis | Pale or more or less deep pink samon | Cylindrical or cylindrical–clavate, 170–200 × 12–14.5 μm, 8-spored | A distinctly forked base formed by a crozier | Spherical, 9–11 μm, generally with a large and sometimes eccentric oil-drop, smooth, uniseriate in the ascus | Very slender, thread-shape, curved or hooked in the upper part simple (not forked), some weakly visible septa | on bare, humid ground | [104] |
| P. lacteoalba | Tota lacteoalba | Cylindracei, tetraspori, 180–230 × 15 μm | Globosae, 10–12.5(–13.5) μm, guttulis 2–4 instructae laeves | Filiformes apice curvatae | Ad terram nudam in olla | [105] | |
| P. laeterubra | Laete rubro, | Cylindracei, apice rotundati, 180–200 × 8 μm, 8 sporae | Globosae, guttam 1 magnam oleosam includentes, 10 μm | Filiformes, septatae,ad apicem | [106] | ||
| P. miltina | Crimson | linear | Globose, 1/1750 of an inch in diameter | On the bare ground, amongst moss, on haills | [107] | ||
| P. multiguttula | Orange Chrome and cadmium orange | Cylindracei, octospordei, 210–240(–290) × 14–19 μm | Periecte globosae,5–9-guttulatae, 12–13(–14)μm | Non ramosae, septatae, ad apicem crassatae | super terram | [82] | |
| P. mussooriensis | Cream-colored to yellow | Cylindric, apex rounded to subtruncate or truncate, 180–213 × 9–13.5 μm | Overlapping in some asci, globose, smooth, 9.7–11.2 μm | Filiform, nonseptate, simple or branched, apex not swollen but strongly curved, 178–218 × 0.7–2 μm | On soil amid mosses | [108] | |
| P. neotropica | Pale yellowish-greenish | 165–177 × 11–14 μm, 8-spored, not broad at base | Arising from prominent croziers | Globose, with a single large oil globule, smooth-walled, (12–)13–14(–15) μm | Thin, apex swollen, mostly curved in the upper portion but not strongly | On burned wood | [4] |
| P. nepalensis | Yellow | 180–218 × 14–18 μm, 8-spored, | Lack regular croziers | 12.8–15.5 μm | Expanding, up to 3 μm at their bent to curved apices | On charcoal and burnt soil around a fireplace in a bamboo grove | [86] |
| P. orichalcea | Lutea | Cylindraceis | Globosis, laevibus | Leniter incrassates, flavidia, septatis | On the ground | [83] | |
| P. pyrophila | Salmon-pink | Cylindrical, 150–200 × 10–12 μm, 8-spored | Globose, 7–9 μm, 1-seriate, smooth | Filiform, hooked at their apices, often forked | In the spring, on burnt ground | [87] | |
| P. salmonicolor | Pale slmon-colored | Cylindric or subcylindric, 275 × 20–24 μm | Not described | Globose, 20 μm | Clavate, reaching a diameter at their apices | On bare ground | [88] |
| P. subaurantia | Orange | Cylindriques | Globuleuses, lisses, uni-ou pluriguttulees, 12–15 × 12–15 μm | Filiformes, largement reeourbees au Sommet | Espece cespiteuse croissant parmi la mousse | [109] | |
| P. sublaeterubra | pastel orange | Cylindrical, 254–302 × 15.7–20.9 μm, 8-spored, operculate, becoming narrow towards the base, | distinctly forked base formed by a crozier | spherical, 14.8–17.1 μm, smooth | filiform, slender, septate, branched, curved at apex without enlarged | grows in clusters on sandy grasslands in broad-leaved forests and shrubs | This study |
| P. tetraspora | Possibly orange when fresh | Cylindracei, narrowed below into a short foot, 180 × 17–19 μm, mostly 4-spored when mature, a few with 2 or 5 spores,but forming 8 spores ay first | Globosae to slightly ellipsoid, globule,quite smooth, 15–17 μm,1-guttulatae | Filiformes, numerosae, doubtfully septatae, not enlarged at apex | in terra | [84] |

Table 3.
Current list of Microstoma species and their diagnostic characteristics. Non-English descriptions from references are not translated to avoid misunderstanding.
Table 3.
Current list of Microstoma species and their diagnostic characteristics. Non-English descriptions from references are not translated to avoid misunderstanding.
| Species | Apothecia Color When Fresh | Asci | Crozier | Ascospores | Paraphyses | Habitat | References |
| M. aggregatum | Roseus | Cylindracei rotundati ad apicem crassitunicati operculati cum operculo laterali stipitati octospori, 214.2–272.0 × 12.8–14.4 μm | Subcrassitunicatae cllipsoideae laeve 24.0–32.0 × 9.6–12.8 μm | Filiformes septatae, simplices vel inferne ramosae superne subincrassatae usque 3 μm | In ligno putrescentis | [110] | |
| M. apiculosporum | Orange red | Clavate, suboperculate, 325–335 × 12–14 μm, 8-spored | Ellipsoid, smooth, 25–30 × 9–10 μm, filled with oil droplets, apiculate at both ends of spores, apiculi hemispherical, surrounded with a gelatinous sheath when freshly released | Branched, connected as a net around the asci, filled with many orange granules | On dead sticks of broadleaf tree | [89] | |
| M. camerunense | Pinkish | Cylindrical, suboperculate, 245–335 × (6–)7–12 μm, tapering towards the base to a flexuous stalk with thickened walls in the subhymenium | Narrow ellipsoid to fusiform, (13–)14–16(–17) × 3–4(–4.5) μm, smooth, apiculate, at ends, apicual conical | Branched, connected as a net, with subclavate apices | On dead wood | [90] | |
| M. changchunense | Light pink | cylindrical, 295–345 × 13.5–19.5 μm, suboperculate, with slightly thick walls | long elliptical, 25.0–36.2 × 11.5–14.5 μm, with a or more guttulate when mature, without appurtenance on the ends, smooth | filiform, septate, branched | scattered on rotten wood in broad-leaved forests | This study | |
| M. floccosum | Eleganter coccineo | Cylindraceis | Ellipticis, 20 × 11 μm | filiformibus | Ramos dejectos et terram | [93] | |
| M. jilinense | Salmon orange | cylindrical, 247–325 × 10.6–14.2 μm, 8-spored, suboperculate, with slightly thick walls | elliptical to cylindrical, 15.9–24.3 × 9.0–14.9 μm, with a large guttulate when mature, without appurtenance on the ends, smooth | filiform, septate, branched | scattered on rotten wood in mixed coniferous and broad-leaved forests | This study | |
| M. macrosporum | Aurantio-cinnabarnae | Cylindro-clavati, 500–560 × 23–26 μm, octospori | Ellipsoideae vel fusiformes, 42–60 × 16–21 μm, smooth, crassituicatae, multi-guttulatae | Filiformes, septatae, ramosae et anastomosantes inter se | Dead stem on the ground in early winter tjrought early spring | [92] | |
| M. protractum | Externally bright orange-red, interior, vivid rose-red | Cylindrical, 200–275 × 20–23 μm, 8-spored, operculum lateral | Ellipsoid, to fusoid, 24–45 × 10–14 μm, usually, containing conspicuous globules which vary in size and number | Not flexuous, dichotomously branched several time | On buried sticks and root | [91] | |
| M. longipilum | Dull pink to pale orange | Clavate, 275–350 × 10–17.5 μm, operculate with eccentric opercula, thick-walled | Ovoid to ellipsoid without apiculi, (20–)21.9–26.1(–27.5) × 11–12.5 μm, smooth, | Filiform, with apices sometimes swelling or branched irregularly | On rotten wood | [56] | |
| M. ningshanicum | Red, orange-red, or light orange-red | Subcylindrical, 8-spored, 449–517 × 16–20.5 μm | Long ellipsoidal to cylindrical, 31–45 × 13–17 μm, non-smooth, without appurtenance on both end | On rotten wood hodden in the ground in a broad-leaved forest | [58] | ||
| M. radicatum | Hymenuium surface rosy red to red, receptacle surface red, orange-red to light orange-red | Subcylindrical, 8-spored, 390–575 × 15–22 μm | Ellipsoidal, to fusoidal-ellipsoidal, (25–)35–50(–60) × (11–)12.58–20(–22.5) μm, smooth, uniguttulate | Filiform, with apex slightly enlarged | on rotten wood under Larix gmelinii (Rupr.) Rupr. forest | [29] |

Table 4.
Current list of part Sarcoscypha species and their diagnostic characteristics. Non-English descriptions from references are not translated to avoid misunderstanding.
Table 4.
Current list of part Sarcoscypha species and their diagnostic characteristics. Non-English descriptions from references are not translated to avoid misunderstanding.
| Species | Apothecia Color When Fresh | Asci | Crozier | Ascospores | Paraphyses | Habitat | References |
|---|---|---|---|---|---|---|---|
| S. aestiva | Ohnive cervine (trochu do oranzova) | Verc. Valcor., uftat. 12–15 μm tl. s 8 jednor. | zaoblene, ellipt., hladke, s 2 velikymi tel., 25–30 μm | velmi hojne, na konci kyjovite ztlustele a oranzove, jodem zelene. | [111] | ||
| S. albovillosa | Coccineo | Cylindrico, apice truncatis, 300 × 15 μm | Ellipsoideae, guttuam 1 magnam centralem incudentes, episporio sexangulariter retuiculato, 18–21 × 10–12 μm | Filiformes, apice vix hamatae, apice sensim usque 5 μm crassae, jodii ope coerulee decolorates repletae | Ad terram | [112] | |
| S. austriaca | Laete cinnabarino, serius coccineo | Cylindraceis, versus apicem rotundatum paullo ampliatis, 437–500 × 22 μm | Ellipsoideis vel orculaeformibus, levibus, 34–38 × 11–14 μm | Filiformibus apice incrassates roseis | In ramis humo tectis | [93] | |
| S. cerebriforimis | Bright yellow | Subcylindrical, 290–350 × 12–13 μm, | Rectangular ellipse, 23–28 × 9.5–11.5 μm, slightly rough, with two guttulates | Filiform, 2–3 μm wide | On hardwood | [15] | |
| S. concatenata | Extus albis, sericeis, venosis, intus avellaneo-roseis | Cylindricis, 10–12 μm, latis | 18–30 μm, longis, 10 μm latis, plerumque ellipticis | Filiformes | In ramo pini | [97] | |
| S. coccinea | Scarlet | Cylindrical, 15–17 μm wide | 23–33 × 10–14, ellipsoid, Both ends are blunt and round, without depression, smooth, two or more guttulates | Filiform, slightly thicker at the top, branched, septate | On rotten wood | [15] | |
| S. dawsonensis | Red or orange | Cylindric, 200–280 μm long | Elliptic, 20 μmlong, 10 μm broad | Filiform, slender, slightly thicker at the top | Among mosses | [113] | |
| S. dudleyi | Bright yellow inclining to saffron or orange | cylindrical | Oblong, 0.001 to 0.0012 in. long, 0.0005 to 0.0006 broad, | Filiform, slightly thickened at the tips | Ground and decayed wood | [79] | |
| S. excelsa | Carneo-coccineo | Cylindracei, ad apicem obtuse, 250–400 μm longi, 16–20 μm crassi | Anguste ellipsoideae vel potius ellipsoideo-fusiformes, leves, 26–33 × 8–9 μm | Ramosae, ad apicem dilatatae | Ad terram arenosam, ad litora marina | [114] | |
| S. groenlandica | Warzchen im unreifen zustande gelbgranlich | auf blattern von saxifraga cernua L. f. ramose | [115] | ||||
| S. hongshiensis | Pure orange | cylindrical, 284–323 × 9.0–9.9 μm, 8-spored, J- in Melzer’s reagent, suboperculate, becoming narrow towards the base, slim, curvaceous. | Cylindrical to rod-shaped, partly both ends truncated, 19.2–27.0 × 8.2–9.7 μm, smooth, with a large guttulate | filiform, septate, branched | scattered on rotten wood in mixed coniferous and broad-leaved forests | This study | |
| S. hosoyae | “Grenadine Red” (Ridgway) to “Scarlet” or rarely white | (320–)350–430(–460) × 13–18 μm | 22–38(–45) × 9–12 μm, some truncate | Filiform, branching most in lower and upper 1/3, and anastomosing throughout length, do not exceed mature asci, slightly enlarged at tip to spearshaped, rounded, or irregularly lobed | Wet areas of deciduous woods on partially buried twigs/branchlets of angiosperms | [95] | |
| S. humberiana | “Grenadine Red” (Ridgway) to “Scarlet” | (230–)270–310(–330) × 10–12.5 μm, tapering, then expanding at base | (16.5–)18–23(–27) × 8.5–10(–12) μm, shallow depression at truncate ends | Generally, evenly filiform with apex slightly enlarged clavate, branching | Wet area on partially buried teigs/branchlets | [95] | |
| S. javensis | Hellkarminrot, heller rötlich | Zylindrisch, 230 × 10 μm, achtsporig | Zylindrisch-elliptisch, beidendig meist zbgestumpft, ohne öltropfen, 23 × 8 μm | Fadig unten ein bis zweimal verzweigt, Jod gibt keine Blaufarbunfg | An morschem holze | [94] | |
| S. jurana | pulchre-coccineo | Longissimae, operculatae, octosporae, 350–450 μm longae, 15 μmcrassae | Oblong, aut oblong-truncatae, leaves, 24–29 μm longae. 13–14 μm latae | Tenues, dichotomo-ramosae, ramis acutis, iodo caerulescentibus aut virentibus | Ad ramos infossos tiliae | [116] | |
| S. kecskemetiensis | pallide sulphurea | Cylindraceis, 120–140 × 8–9 μm, octosporis | Ovatis, levibus, biduttulatis, 12 × 6 μm | Filiformibus, non septatis, apice increassatis | ad terram inter folia decidua et lignula putrida | [117] | |
| S. knixoniana | “Spectrum Red” to rose red | (250–)270–350(–420) × 9–13 μm | 18–25 × 8–12 μm, with shallow or slightly deeper depressions | Generally simple and filiform, with slightly inflated to clavate, some slightly hooked, branching | Wet areas of deciduous wood on partially buried twigs/branchlets of angiosperms | [95] | |
| S. korfiana | “Picric Yellow”, “Lemon Chrome” | 220–265(–340) × 9–12 μm, | All shallowly truncate with slight depression at the poles, (14–)16–21 × (7–)8–11 μm | Filiform, gradually tapering at the slightly enlarged, clavate-shaped | on wood | [95] | |
| S. longitudinalis | Broen | Subcylindrical, terminally operculate, apex obtuse, 8-spored, 197–359 × 12–14 μm | Broadly fusiform, (18.3–)19.3–21.4(–22.4) × (9.5–)10.7–12.1(–12.8) μm, ornamentation with several longitudinal striae | Filiform, branched, septate, with a rounded end | On unidentified dead branch under broadleaved forest | [44] | |
| S. macaronesica | lucido-sanguineo, margine ochraceo-albido | cira 350/13.5 μm (turgescente) | Ellipsoidales, 22–29 × 9–12 μm, lisas, | Multis anastomosis instructae, apice leniter incrassatae | in ramulis dejectis crescentia | [32] | |
| S. mesocyatha | “Grenadine Red” to “Scarlet” | (260–)280–330–360) × 10–11(–12.5) μm usually eight ascospores but sometimes only six or senve | (16.5–)21–27(–30) × 9–10.5(–12.5) | Filifirm, with apex slightly enlarged, clavate, banching | Wet area on partially buried teigs/branchlets | [95] | |
| S. minuta | Orange to Cadmium Yellow | Cylindrical, 8-spored, 180–250 × 7–10 μm | Without | Broadly ellipsoid, (15–)16–20(–22) × (7–)8–10 μm, smooth, containing a large guttule | Filiform, slightly exceeding the asci, sparsely septate, aoex simple | On fruit shell | [79] |
| S. occidentalis | Luteo-coccineo | Cylindraceis | Obtuse ellipticis, 18 × 9 μm | filiformibus | Ad ramos dejectos | [93] | |
| S. pseudomelastoma | schwarzlich-olivenfarbig | Cylindrisch, 180–200 μm | ellipsoid, glatter, arbloser membrane, 12–16 × 6–8 μm | Fadenforming, nacho ben kaum verdickt,gelblich | zwishchen Torfmoospolstern wachsende | [118] | |
| S. racovitzae | Disco luteo-aurantio, extus albida | Cylindraceis, 270 × 12–15 μm | Ellipsoideis, 18–20 × 8–9 μm | linearibus rarioribus | In ligno putrescente | [115] | |
| S. rubrans | Rouge brillant | Ellipsoide lanceolee, bi-triocellee | Sur les branches moortes | [119] | |||
| S. shennongjiana | Orange red to red | Suboperculate, 8–spored, 240–292 × (9.9–)10–13.5 μm | Ellipsoid with shallow depression at truncate ends, with two large guttulesand many small ones | subcylindrical | On twigs of a broadleaf tree | [34] | |
| S. sherriffii | Levis, aurantioflvum | Cylindracei, 350–400 × 13–15 μm | Ellipsoideae vel oblongae, 25–35 × 10–12 μm, leves | filiformae | Ad ramos putridos dejectos in silva densa | [120] | |
| S. tatakensis | Scarlet red | Cylindrical 260–325 × 10–13 μm | Without | Ellipsoid, with shallow depressions at both ends, smooth, (16–)21–27(–30) × 9–11(–14) μm, usually with 2–3 large guttules | Filiform, slightly exceeding the asci, sparsely septate | On dead twigs | [79] |
| S. vassiljevae | Dirty white | Subcylindrical, 290–360 × 9–13 μm | Ellipsoid, 17–25 × 9–13 μm, with a large guttules | Filiform, septate, branched, sometimes connected to a net | On dead twigs | [15] |
Author Contributions
Conceptualization, T.B. and Z.C.; methodology, Z.C.; software, Z.C.; vali dation, Z.C. and T.B.; formal analysis, Z.C.; investigation, Z.C. and T.B.; resources, Z.C. and T.B.; data curation, Z.C. and T.B.; writing—original draft preparation, Z.C.; writing—review and editing, Z.C. and T.B.; visualization, Z.C. and T.B.; supervision, T.B.; project administration, T.B.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Education Innovation Team (No. IRT1134, IRT-15R25).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the sequences have been deposited in GenBank (https://www. ncbi.nlm.nih.gov, accessed on 20 November 2024) and MycoBank (https://www.mycobank.org, accessed on 1 December 2024).
Acknowledgments
The authors sincerely thank the teacher and the team for their help. The authors thank the reviewers and responsible editors for their corrections and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boudier, J.L.É. Nouvelle classification naturelle des Discomycetes charnus connus generalement sous le nom de Pezizes. Bull. Soc. Mycol. Fr. 1885, 1, 91–120. [Google Scholar]
- Boudier, É. Histoire et Classification des Discomycètes d’Europe; Librairie des Sciences Naturelles Paul Klincksieck, éditeur: Corneille, France, 1907; pp. 1–468. [Google Scholar]
- Le Gal, M. Les Discomycètes de Madagascar; Laboratoire de Cryptogamie du Museum National d’Histoire Naturelle: Paris, France, 1953; Volume 4, pp. 1–465. [Google Scholar]
- Pfister, D.H. A synopsis of the genus Pulvinula. Occ. Pap. Farlow Herb. Crypt. Bot. 1976, 9, 1–19. [Google Scholar]
- Dennis, R.W.G. British Ascomycetes; J. Cramer: London, UK, 1968; pp. 1–455. [Google Scholar]
- Korf, R.; Zhuang, W.-Y. The ellipsoid-spored species of Pulvinula (Pezizales). Mycotaxon 1984, 20, 607–616. [Google Scholar]
- Yao, Y.-J.; Spooner, B. Delimitation of Boubovia and Pulvinula. Mycol. Res. 1996, 100, 193–194. [Google Scholar] [CrossRef]
- Yao, Y.-J.; Spooner, B.M. Notes on British species of Pulvinula, with two newly recorded species. Fungal Biol. 1996, 100, 883–884. [Google Scholar] [CrossRef]
- Hansen, K.; Perry, B.A.; Dranginis, A.W.; Pfister, D.H. A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected life history traits. Mol. Phylogenet. Evol. 2013, 67, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Pfister, D.H. Systematics of the Pezizomycetes—The operculate discomycetes. Mycologia 2006, 98, 1029–1040. [Google Scholar] [PubMed]
- Perry, B.A.; Hansen, K.; Pfister, D.H. A phylogenetic overview of the family Pyronemataceae (Ascomycota, Pezizales). Mycol. Res. 2007, 111 Pt 5, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, A.H.; Hyde, K.D.; Jones, E.B.G.; Zhao, Q. Taxonomy and phylogeny of operculate discomycetes: Pezizomycetes. Fungal Divers. 2018, 90, 161–243. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Lumbsch, H.T.; Liu, J.K.; Maharachchikumbura, S.S.N.; Ekanayaka, A.H.; Tian, Q.; Phookamsak, R. Outline of Ascomycota: 2017. Fungal Divers. 2018, 88, 167–263. [Google Scholar] [CrossRef]
- Tedersoo, L.; Hansen, K.; Perry, B.A.; Kjøller, R. Molecular and morphological diversity of pezizalean ectomycorrhiza. New Phytol. 2006, 170, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W. Hyaloscyphaceae, Sarcoscyphaceae et Sarcosomataceae; Science Press: Beijing, China, 2004; Volume 21, pp. 1–208. [Google Scholar]
- Le Gal, M. Les Discomycetes Subopercules; Mémoires de la SoMémoires de la Société des Sciences Naturelles de l’Ouest de la: Nantes, France, 1946; pp. 1–90. [Google Scholar]
- Korf, R.P. The new rules of typification as they affect Sarcoscypha and Velutaria. Mycologia 1953, 45, 296–301. [Google Scholar] [CrossRef]
- Eckbiad, F. The genera of the operculate Discomycetes. A re-evaluation of their taxonomy, phylogeny, and nomenclature. Nor. J. Bot. 1968, 15, 1–30. [Google Scholar]
- Rifai, M.A. The Australasian Pezizales in the Herbarium of the Royal Botanic Gardens, Kew; N.V. Noord-Hollandsche Uitgevers Maatschappij: Amsterdam, The Netherlands, 1968; Volume 57, pp. 1–296. [Google Scholar]
- Korf, R.P. Nomenclatural notes. VII. Family and tribe names in the Sarcoscyphineae (Discomycetes) and a new taxonomic disposition of the genera. Taxon 1970, 19, 782–786. [Google Scholar] [CrossRef]
- Korf, R.P. Synoptic key to the genera of the Pezizales. Mycologia 1972, 64, 937–994. [Google Scholar] [CrossRef]
- Korf, R.P. Discomycetes and tuberales. Fungi 1973, Vol. IrA, 249–319. [Google Scholar]
- Samuelson, D.A.; Benny, G.L.; Kimbrough, J.W. Asci of the Pezizales. VII. The apical apparatus of Galiella rufa and Sarcosoma globosum: Reevaluation of the suboperculate ascus. Botany 1980, 58, 1235–1243. [Google Scholar] [CrossRef]
- Cabello, M. Estudio sistematico del suborden Sarcoscyphineae (Pezizales, Ascomycotina) empleando tecnicas numericas. Bol. Soc. Argent. Bot. 1988, 25, 395–413. [Google Scholar]
- Ainsworth, G.C. Ainsworth & Bisby’s Dictionary of the Fungi, 8th ed.; CAB International: Wallingford, UK, 1995; pp. 1–616. [Google Scholar]
- Harrington, F.; Pfister, D.H.; Potter, D.; Donoghue, M.J. Phylogenetic studies within the Pezizales. I. 18S rRNA sequence data and classification. Mycologia 1999, 91, 41–50. [Google Scholar] [CrossRef]
- Romero, A.I.; Robledo, G.; LoBuglio, K.F.; Pfister, D.H. Rickiella edulis and its phylogenetic relationships within Sarcoscyphaceae. Kurtziana 2012, 37, 79–89. [Google Scholar]
- Pfister, D.H. Chapter 2. Pezizomycotina: Pezizomycetes, Orbiliomycetes; The Mycota VII (B); Springer: Berlin/Heidelberg, Germany, 2015; pp. 35–55. [Google Scholar]
- Tiezhi, L.; Wenying, Z. A new species of Microstoma (Sarcoscyphaceae) from China with a key to known species of the genus. Mycosystema 2018, 37, 555–558. [Google Scholar]
- Ekanayaka, A.H.; Hyde, K.; Zhao, Q. The genus Cookeina. Mycosphere 2016, 7, 1399–1413. [Google Scholar] [CrossRef]
- Weinstein, R.N.; Pfister, D.H.; Iturriaga, T. A phylogenetic study of the genus Cookeina. Mycologia 2002, 94, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Baral, H.O. Taxonomische und okologische Studien uber Sarcoscypha coccinea agg., Zinnoberrote Kelchbecherlinge. Z. Mykol. 1984, 50, 117–145. [Google Scholar]
- Butterfill, G.; Spooner, B. Sarcoscypha (Pezizales) in Britain. Mycologist 1995, 9, 20–26. [Google Scholar] [CrossRef]
- Wenying, Z. Additional notes on Sarcoscypha in China. Mycotaxon 2000, 76, 1–8. [Google Scholar]
- Wenying, Z. The Genus Sarcoscypha in Jiaohe, JIlin Province, with Notes on Surface Morphology of Ascospores. Mycosystema 1992, 11, 65–72. [Google Scholar]
- Bau, T. Species Diversity of Higher Ascomycetes in Jilin Province. J. Fungal Res. 2005, 1, 1–6. [Google Scholar]
- Guiying , C.; Cang, S.; Jianrui, W.; Tolgor. Investigation of Large-scale Fungal Resources in Zuojia Area (III). J. Jilin Agric. Univ. 2006, 5, 505–509. [Google Scholar]
- Chuhan, S. Inventory of Pezizales and Taxonomy of Pezizaceae and Pyronemataceae from Jilin Province. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2016. [Google Scholar]
- Bau, T.; Longguo, P.; Yuguang, F. Mushrooms and Their Natural Habitat; Shanghai Popular Science Press: Shanghai, China, 2017; pp. 1–64. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Zeng, M.; Gentekaki, E.; Hyde, K.D.; Zhao, Q.; Matočec, N.; Kušan, I. Phylogeny and morphology of novel species and new collections related to Sarcoscyphaceae (Pezizales, Ascomycota) from Southwestern China and Thailand. Biology 2023, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Mark, P.v.d.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, U.; Alvarado, P. Revision der Gattung Kotlabaea, Teil 2: K. aurantiaca, K. carestiae, K. danuviana und K. trondii nebst taxonomischen Bemerkungen zu Boubovia vermiphila, Cheilymenia stercoraria und zur Gattung Pseudombrophila. Z. Mykol. 2017, 83, 103–126. [Google Scholar]
- Pfister, D.H.; Slater, C.; Hansen, K. Chorioactidaceae: A new family in the Pezizales (Ascomycota) with four genera. Mycol. Res. 2008, 112, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Kropp, B.R. Cookeina cremeirosea, a new species of cup fungus from the South Pacific. Mycoscience 2016, 58, 40–44. [Google Scholar] [CrossRef]
- Chethana, K.; Niranjan, M.; Dong, W.; Samarakoon, M.; Bao, D.; Calabon, M.; Chaiwan, N.; Chuankid, B.; Dayarathne, M.; De Silva, N. AJOM new records and collections of fungi: 101–150. Asian J. Mycol. 2021, 4, 113–260. [Google Scholar]
- Iturriaga, T.; Xu, F.; Pfister, D.H. Cookeina korfii, a new species hidden in Cookeina tricholoma. Ascomycete.org 2015, 7, 331–335. [Google Scholar]
- Pfister, D.; Quijada, L.; LoBuglio, K. Geodina (Pezizomycetes: Wynneaceae) has a single widespread species in tropical America. Fungal Syst. Evol. 2020, 5, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tochihara, Y.; Hirao, T.; Ohmae, M.; Hosaka, K.; Hosoya, T. Microstoma longipilum sp. nov.(Sarcoscyphaceae, Pezizales) from Japan. Mycoscience 2021, 62, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, A.; Bujakiewicz, A.; Winiecki, A.; Dabert, M.; Kubinski, R. The occurrence of Microstoma protracta (Fr.) Kanouse in Poland and assessment of its threat status. Acta Mycol. 2018, 53, 1114. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, L.; Liu, Y.; He, X.; Qi, P.; Dai, L.; Qiao, T.; Lu, P.; Li, J. Microstoma Ningshanica, a new species of Microstoma based on molecular, light and scanning electron microscopy analyses from Shaanxi Province, China. All Life 2022, 15, 901–907. [Google Scholar] [CrossRef]
- Hansen, K.; Pfister, D.H.; Hibbett, D.S. Phylogenetic relationships among species of Phillipsia inferred from molecular and morphological data. Mycologia 1999, 91, 299–314. [Google Scholar] [CrossRef]
- Nucleotide. Available online: https://www.ncbi.nlm.nih.gov/nucleotide (accessed on 20 November 2024).
- Carbone, M.; Agnello, C.; Alvarado, P. Phylogenetic studies in the family Sarcosomataceae (Ascomycota, Pezizales). Ascomycete.org 2013, 5, 1–12. [Google Scholar]
- Zeng, M.; Ekanayaka, A.H.; Zhao, Q. Phylogeny and morphology of Phillipsia hydei sp. nov.(Sarcoscyphaceae) from Thailand. Phytotaxa 2019, 395, 277–286. [Google Scholar] [CrossRef]
- Wenying, Z. Re-dispositions of Phillipsia (Pezizales) collections from China. Mycotaxon 2003, 86, 291–301. [Google Scholar]
- Ekanayaka, A.H.; Bhat, D.; Hyde, K.D.; Jones, E.G.; Zhao, Q. The genus Phillipsia from China and Thailand. Phytotaxa 2017, 316, 138–148. [Google Scholar] [CrossRef]
- Harrington, F.A.; Potter, D. Phylogenetic relationships within Sarcoscypha based upon nucleotide sequences of the internal transcribed spacer of nuclear ribosomal DNA. Mycologia 1997, 89, 258–267. [Google Scholar] [CrossRef]
- Luo, J.; Walsh, E.; Naik, A.; Zhuang, W.; Zhang, K.; Cai, L.; Zhang, N. Temperate pine barrens and tropical rain forests are both rich in undescribed fungi. PLoS ONE 2014, 9, e103753. [Google Scholar] [CrossRef]
- Ganley, R.J.; Newcombe, G. Fungal endophytes in seeds and needles of Pinus monticola. Mycol. Res. 2006, 110, 318–327. [Google Scholar] [CrossRef]
- Amicucci, A.; Zambonelli, A.; Guidi, C.; Stocchi, V. Morphological and molecular characterisation of Pulvinula constellatio ectomycorrhizae. FEMS Microbiol. Lett. 2001, 194, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Gómez Molina, E.; Sánchez Durán, S.; Puig Pey, M.; García Barreda, S. Sequential application of inoculation methods for the mycorrhization of Quercus ilex seedlings with Tuber melanosporum. Fungal Biol. 2023, 127, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Arraiano-Castilho, R.; Bidartondo, M.I.; Niskanen, T.; Clarkson, J.J.; Brunner, I.; Zimmermann, S.; Senn-Irle, B.; Frey, B.; Peintner, U.; Mrak, T.; et al. Habitat specialisation controls ectomycorrhizal fungi above the treeline in the European Alps. New Phytol. 2021, 229, 2901–2916. [Google Scholar] [CrossRef] [PubMed]
- Olariaga, I.; Ballester, L.; Fernandes, U.; López-Amiano, J.I.; Magaña, V.; Martínez-Gil, R.; Martín, J.; Pancorbo, F.; Daniëls, P.P.; Pérez-del-Amo, C.; et al. II Encuentro de la Sociedad Ibérica de Micología (Cerler, 26–30 de septiembre de 2018): Hallazgo de 103 especies nuevas para Aragón, 15 para la Península Ibérica y 11 especies nuevas para la ciencia. Fung. Iber. 2020, 1, 7–80. [Google Scholar] [CrossRef]
- Truong, C.; Mujic, A.B.; Healy, R.; Kuhar, F.; Furci, G.; Torres, D.; Niskanen, T.; Sandoval-Leiva, P.A.; Fernández, N.; Escobar, J.M. How to know the fungi: Combining field inventories and DNA-barcoding to document fungal diversity. New Phytol. 2017, 214, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Olds, C.G.; Berta-Thompson, J.W.; Loucks, J.J.; Levy, R.A.; Wilson, A.W. Applying a modified metabarcoding approach for the sequencing of macrofungal specimens from fungarium collections. Apps. Plant Sci. 2023, 11, e11508. [Google Scholar] [CrossRef] [PubMed]
- Healy, R.A.; Arnold, A.E.; Bonito, G.; Huang, Y.L.; Lemmond, B.; Pfister, D.H.; Smith, M.E. Endophytism and endolichenism in Pezizomycetes: The exception or the rule? New Phytol. 2022, 233, 1974–1983. [Google Scholar] [CrossRef]
- Poelman, A.; Weerasuriya, N.; Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F.; Thorn, R.G. Fungi associated with aeroponic roots in caves and mines of New Brunswick. Fungal Ecol. 2021, 52, 101074. [Google Scholar] [CrossRef]
- Arraiano-Castilho, R.; Bidartondo, M.I.; Niskanen, T.; Zimmermann, S.; Frey, B.; Brunner, I.; Senn-Irlet, B.; Hörand, E.; Gramlich, S.; Suz, L.M. Plant-fungal interactions in hybrid zones: Ectomycorrhizal communities of willows (Salix) in an alpine glacier forefield. Fungal Ecol. 2020, 45, 100936. [Google Scholar] [CrossRef]
- Celio, G.; Padamsee, M.; Dentinger, B.; Bauer, R.; McLaughlin, D. Assembling the fungal tree of life: Constructing the structural and biochemical database. Mycologia 2006, 98, 850–859. [Google Scholar] [CrossRef]
- Shi, C.; Tolgor, B.; Yu, L. Newly recorded genus and species of Pezizales in China. Mycosystema 2016, 35, 1348–1356. [Google Scholar]
- Yeizeng, W.; Chunlin, H.; Jialing, W. Two new species of Sarcoscypha (Sarcosyphaceae, Pezizales) from Taiwan. Phytotaxa 2016, 245, 169–177. [Google Scholar]
- Xu, F.; LoBuglio, K.; Pfister, D. On the co-occurrence of species of Wynnea (Ascomycota, Pezizales, Sarcoscyphaceae) and armillaria (basidiomycota, agaricales, physalacriaceae). Fungal Syst. Evol. 2019, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T. Ascomycetes from northern Thailand. Nord. J. Bot. 1982, 2, 257–263. [Google Scholar] [CrossRef]
- Batra, L.R. New Species of Discomycetes from India: II. Mycologia 1960, 52, 665–667. [Google Scholar] [CrossRef]
- Cooke, M.C. Mycographia, Seu Icones Fungorum: Figures of Fungi from All Parts of the World; William & Norgate: London, UK, 1877; Volume 1, pp. 1–526. [Google Scholar]
- Hansford, C. Australian fungi. II. New records and revisions. Proc. Linn. Soc. NSW. 1954, 79, 97–141. [Google Scholar]
- Meihua, L. Two new species of Pulvinula from China. Acta Mycol. Sinica 1991, 10, 185–189. [Google Scholar]
- Kaushal, R. A reinvestigation of the northwest Himalayan Pulvinulas [Fungi; India]. Mycotaxon 1982, 16, 117–122. [Google Scholar]
- Snyder, L.C. New and unusual discomycetes of western Washington. Mycologia 1936, 28, 483–488. [Google Scholar] [CrossRef]
- Seaver, F.J. Studies in Tropical Ascomycetes—III: Porto Rican Cup-Fungi. Mycologia 1925, 17, 45–50. [Google Scholar] [CrossRef]
- Yeizeng, W. A new species of Microstoma from Taiwan. Mycotaxon 2004, 89, 119–122. [Google Scholar]
- Douanla Meli, C.; Langer, E. Notes on Discomycetes (Helotiales, Pezizales): New species and new records from Cameroon. Mycotaxon 2005, 92, 223–237. [Google Scholar]
- Kanouse, B.B. The genus Plectania and its segregates in North America. Mycologia 1948, 40, 482–497. [Google Scholar] [CrossRef]
- Harada, Y.; Kudo, S.-I. Microstoma macrosporum stat. nov., a new taxonomic treatment of a vernal discomycete (Sarcoscyphaceae, Pezizales). Mycoscience 2000, 41, 275–278. [Google Scholar] [CrossRef]
- Saccardo, P.A. Discomyceteae et Phymatosphoraeriaceae. Syll. Fung. 1889, 8, 1–1143. [Google Scholar]
- Höhnel, F. Fragmente zur Mykologie. (VI. Mitteilung, Nr. 182 bis 288). Sitzungsber. Kais. Akad. Wiss. Wien Math.-Naturwiss. K1asse 1909, 118, 275–452. [Google Scholar]
- Harrington, F. New species of Sarcoscypha (Sarcoscyphaceae, Pezizales). Harv. Pap. Bot. 1997, 1, 53–64. [Google Scholar]
- Iturriaga, T.; Pfister, D.H. A monograph of the genus Cookeina (Ascomycota, Pezizales, Sarcoscyphaceae). Mycotaxon 2006, 95, e180. [Google Scholar]
- Rick, J. Pilze aus Rio Grande do Sul (Brasilien). Brotéria 1906, 5, 5–53. [Google Scholar]
- Hooker, J.D. The Botany of the Antarctic Voyage of H.M. Discovery, III. Flora Tasmaniae; Reeve and Company: London, UK, 1860; Volume 3, pp. 1–422. [Google Scholar]
- Fuckel, K.W.G.L. Symbolae Mycologicae: Beiträge zur Kenntniss der Rheinischen Pilze; J. Niedner: Rhine, Germany, 1873; Volume 27–28. [Google Scholar]
- Karsten, P.A. Monographia pezizarum fennicarum. Not. Fauna Flora Fenn. 1869, 10, 101–206. [Google Scholar]
- Hennings, P. Fungi monsunenses. Monsunia 1900, 1, 137–174. [Google Scholar]
- Cooke, M.C. Mycographia, Seu Icones Fungorum: Figures of Fungi from All Parts of the World; William & Norgate: London, UK, 1879; Volume 1, pp. 215–267. [Google Scholar]
- Berkeley, M.J.; Curtis, M.A. Fungi Cubenses (Hymenomycetes). Bot. J. Linn. Soc. 1869, 10, 280–320. [Google Scholar] [CrossRef]
- Lantieri, A. Pulvinula johannis, a new species from Sicily, Italy. Sydowia 2008, 60, 247–252. [Google Scholar]
- Moravec, J. Nektere operkulatni diskomycety nalezene v okresech Mlada Boleslav a Jicin. Cesk. Mykol. 1969, 23, 222–235. [Google Scholar]
- Rehm, H. Ascomycetes americae borealis. Annal. Mycol. 1904, 3, 516–520. [Google Scholar]
- Hooker, J.D. The Botany of the Antarctic Voyage. II. Flora Novae-Zelandiae. Part II. Flowerless Plants; Reeve and Company: London, UK, 1955; Volume 2, pp. 1–378. [Google Scholar]
- Thind, K.; Cash, E.K.; Singh, P. The Pezizaceae of the Mussoorie Hills (India)—VII. Mycologia 1959, 51, 457–464. [Google Scholar] [CrossRef]
- Bommer, É.C.; Rousseau, M. Florule mycologique des environs de Bruxelles. Bull. Soc. Roy. Bot. Belg. 1884, 23, 15–365. [Google Scholar]
- Otani, Y. Miscellaneous notes on Japanese discomycetes. Rept. Tottori Mycol. Inst. 1990, 28, 251–265. [Google Scholar]
- Velenovský, J. České Houby; České Botanické Společnosti: Praha, Czech, 1922; Volume 4–5, pp. 633–950. [Google Scholar]
- Rehm, H. Ascomycetes americae borealis. Annal. Mycol. 1904, 2, 32–37. [Google Scholar]
- Peck, C.H. New species of fungi. Bull. Torrey Bot. Club 1906, 33, 213–221. [Google Scholar] [CrossRef]
- Sydow, H. Beiträge zur Kenntnis der Pilzflora Neu-Seelands-I. Annal. Mycol. 1924, 22, 293–317. [Google Scholar]
- Allescher, A.; Hennings, P. Pilze aus dem Umanakdistrikt. Biblioth. Bot. 1897, 8, 40–54. [Google Scholar]
- Boudier, E. Note sur quelques Ascomycètes nouveaux du Jura. Bull. Soc. Mycol. Fr. 1903, 19, 193–199. [Google Scholar]
- Saccardo, P.A.; Sydow, P. Sylloge Fungorum; University Library, University of Illinois Urbana Champaign: Urbana, IL, USA, 2010; Volume 16, pp. 1–1291. [Google Scholar]
- Hennings, P. Einige neue deutsche Pezizaceen. Hedwigia 1902, 41, 164–166. [Google Scholar]
- Quélet, L. Description des champignons nouveaux. Rev. Mycol. 1892, 14, 64–67. [Google Scholar]
- Balfour-Browne, F.L. Some Himalayan fungi. Bull. Br. Mus. Nat. Hist. 1955, 1, 189–218. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).