Abstract
Pleosporales is a highly diverse (and the largest) order in Dothideomycetes, and it is widespread in decaying plants in various environments around the world. During a survey of fungal diversity in Sichuan Province, China, specimens of hyphomycetous and Thyridaria-like fungi were collected from dead branches of pine trees and cherry trees. These taxa were initially identified as members of Massarinaceae and Thyridariaceae through morphological examination. Phylogenetic analyses of the Thyridariaceae, combining ITS, SSU, LSU, RPB2, and TEF1 sequence data, indicated a distinct clade sister to Pseudothyridariella and Thyridariella, distinct from any genus in the family. Thus, a new genus, Vaginospora, is proposed to accommodate the type species Vaginospora sichuanensis, which is characterized by semi-immersed globose to oblong ascomata with an ostiolar neck, cylindrical to clavate asci with an ocular chamber, and hyaline to dark brown, fusiform, 3–5-transversely septate ascospores with an inconspicuous mucilaginous sheath. Based on the morphological comparisons and multi-locus phylogenetic analyses (ITS, SSU, LSU, RPB2, and TEF1) of the Massarinaceae, we have identified three collections belonging to the genus Helminthosporium, leading us to propose H. filamentosa sp. nov., H. pini sp. nov., and H. velutinum as a new host record. According to Phylogenetic analysis, H. pini formed an independent clade sister to H. austriacum and H. yunnanense, and H. filamentosa represents the closest sister clade to H. quercinum. Helminthosporium pini is distinct from H. austriacum by the shorter conidiophores and H. yunnanense by the longer and wider conidia. The H. filamentosa differs from H. quercinum in having longer conidiophores and smaller conidia. This study extends our understanding of diversity within Thyridariaceae and Helminthosporium. Our findings underscore the rich biodiversity and potential for discovering novel fungal taxa within these groups.
1. Introduction
Pleosporales is the largest order in the Dothideomycetes and is primarily characterized by flask-shaped pseudothecia [1,2]. The diversity of species in Pleosporales is high, and most species are saprobes on decaying plant material in freshwater, marine, or terrestrial environments [3,4,5,6]. The members of Pleosporales can also be epiphytes, endophytes, or parasites on living leaves or stems, as well as hyperparasites on fungi or insects [7,8,9].
The family Thyridariaceae was proposed by Hyde et al. [10] to accommodate the genus Thyridaria Sacc. There are eight genera accommodated in Thyridariaceae: Chromolaenomyces Mapook & K.D. Hyde, Cycasicola Wanas., E.B.G. Jones & K.D. Hyde, Liua Phookamsak & K.D. Hyde, Parathyridaria Jaklitsch & Voglmayr, Parathyridariella Prigione, A. Poli, E. Bovio & Varese, Pseudothyridariella Mapook & K.D. Hyde, Thyridaria Sacc., and Thyridariella Devadatha, V.V. Sarma, K.D. Hyde, Wanas. & E.B.G. Jones [1,11]. The members of this family are mostly sexually morphic, characterized by having immersed or semi-immersed, globose, coriaceous, and black ascomata; peridium composed of two layers of textura angularis cells; 8-spored, bitunicate, cylindrical to subclavate, apically rounded with ocular chamber asci; ellipsoid to fusiform, brown, 2–3-septate ascospores [10]. The asexual morphs of Thyridariaceae are coelomycetous [12]. Species of this family saprobes on leaves and branches of woody plants and sometimes infect humans [12,13].
Helminthosporium Link was introduced by Link with H. velutinum Link as the type species [14]. It is an old and species-rich genus in the family Massarinaceae (Pleosporales). Several studies showed that Helminthosporium is polyphyletic, whose members are intermixed with taxa of Byssothecium Fuckel, Haplohelminthosporium Konta & K.D. Hyde, Helminthosporiella (Hern.-Restr., Sarria & Crous) Konta & K.D. Hyde, Pseudosplanchnonema Chethana & K.D. Hyde, and Synhelminthosporium Y.P. Chen & Maharachch. [15,16]. Many species of Helminthosporium have been identified through morphological studies, and the taxonomic history of the genus is complex and in a state of flux [17]. While many species are not congeneric with the generic type, they were reclassified into the other genera such as Bipolaris Shoemaker, Curvularia Boedijn, Drechslera S. Ito, and Exserohilum K.J. Leonard & Suggs [18,19,20]. In addition, some lignicolous species were transferred to Ellismarsporium R.F. Castañeda & X.G. Zhang, Mirohelminthosporium K. Zhang, D.W. Li & R.F. Castañeda, Stanhughesiella R.F. Castañeda & D.W. Li, Varioseptispora L. Qiu, Jian Ma, R.F. Castañeda & X.G. Zhang, and several other genera [21,22,23]. Based on phylogenetic analysis, four Corynespora Güssow species were transferred to the genus Helminthosporium [24]. Corynespora shares overlapping characteristics with Helminthosporium, such as determinate or percurrently extending conidiophores and integrated, terminal conidiogenous cells that produce distoseptate conidia, making it more difficult to distinguish the two genera based on morphology alone [25]. Most Helminthosporium species are established based on their asexual morph, which is characterized by porogenous, distoseptate conidia with a flat, ringed pore scar at the base, conidia that are acropleurogenously borne on septate, and erect conidiophores, which cease growth after the formation of terminal conidia [24,26,27,28].
Sichuan Province is located in a subtropical zone, where tropical and temperate flora coexist [29]. The diverse climatic environment and complex terrain provide highly favorable conditions for the development of biodiversity. Sichuan Province contains many rare plants and a huge fungal diversity that is waiting to be explored [30,31,32,33,34]. We regularly conduct fungal diversity surveys in Sichuan Province and investigate the taxonomy of fungi associated with specific plants, such as gymnosperms and cherries. During the study, a Thyridaria-like fungus and four hyphomycetous fungi were collected from Pinus spp. and Prunus spp. Based on morphological characteristics and multi-locus phylogenetic analysis, we identified these four collections as a new genus, Vaginospora, in the Thyridariaceae, along with two new species and a new host record of Helminthosporium.
2. Materials and Methods
2.1. Sample Collection, Morphological Examination, and Isolation
We surveyed the fungal diversity of Ascomycota on gymnosperms and cherries in Sichuan Province, China, from April 2023 to April 2024. The specimens were taken into the laboratory in paper envelopes for examination. Microscopic characters were observed and recorded using a Nikon SMZ800N stereo microscope equipped with a Nikon DS-Fi3 camera (Nikon Corporation, Tokyo, Japan) and a Nikon ECLIPSE Ni-U microscope (Nikon Corporation, Tokyo, Japan) fitted with a Nikon DS-Ri2 microscope (Nikon Corporation, Tokyo, Japan) camera. Measurements were conducted using the Nikon NIS-Elements Documentation Imaging Version 5.21.00 (Nikon Corporation, Tokyo, Japan). All photographs were processed using Adobe Photoshop version 22.0 (Adobe Inc., San Jose, SA, USA). Single ascospore or conidium isolation was performed following the method described by Senanayake et al. [35]. Germinated ascospore or conidia were individually transferred to potato dextrose agar (PDA) media plates and incubated in the dark at 25 °C. Culture characteristics were examined and recorded regularly after 1–3 weeks.
The holotype specimens were deposited in the Herbarium of Cryptogams Kunming Institute of Botany Academia Sinica (HKAS), Kunming, China, and all specimens were deposited in the Herbarium of the University of Electronic Science and Technology (HUEST), Chengdu, China. The living ex-type cultures were deposited in the China General Microbiological Culture Collection Center (CGMCC) in Beijing, China, and all living cultures were deposited in the University of Electronic Science and Technology Culture Collection (UESTCC), Chengdu, China. The taxonomic descriptions of the new taxa have been deposited in MycoBank.
2.2. DNA Extraction, PCR Amplification, and Sequencing
Fungal genomic DNA was extracted from mycelia using the TreliefTM Plant Genomic DNA Kit (TSINGKE Biotech, Shanghai, China) according to the manufacturer’s protocol. For H. pini specimens, obtaining a culture was not feasible, necessitating the direct extraction of DNA from fruiting structures using the method used by Wanasinghe et al. [36]. Five loci—the nuclear ribosomal internal transcribed spacer (ITS: ITS1-5.8S-ITS2), the nuclear ribosomal small subunit rRNA (SSU), the nuclear ribosomal large subunit rRNA (LSU), the partial translation elongation factor 1-alpha (TEF1), and the partial second largest subunit of RNA polymerase II (RPB2)—were amplified by polymerase chain reaction (PCR). The primers used were ITS9mun/ITS4_KYO1 [37,38] for ITS, LR0R/LR5 [39,40] for LSU, PNS1/NS41 [41] for SSU, EF1-728F/EF1-2218R [42,43] for TEF1, and fRPB2-5F/fRPB2-7cR [44,45] for RPB2. The final reaction volume of the PCR reagent was 25 µL, containing 2 µL of the DNA template, 1 µL each of the forward and reverse primers, 8.5 µL of double-distilled water (ddH2O), and 12.5 µL of 2× Flash PCR MasterMix (mixture of DNA Polymerase, dNTPs, Mg2+, and optimized buffer; CoWin Biosciences, Nanjing, China). The amplification conditions for all five loci consisted of initial denaturation at 94 °C for 3 min, followed by 35 cycles of 30 s at 94 °C, 30 s at 56 °C, and 1 min at 72 °C, and a final extension period of 10 min at 72 °C. The PCR products were visualized by 1% agarose gel electrophoresis. Sanger sequencing was conducted by Tsingke Biological Technology (Beijing, China). Newly generated sequences were deposited in GenBank, and the accession numbers are listed in Table 1 and Table 2.

Table 1.
Species details and their GenBank accession numbers used in phylogenetic analyses of Massarinaceae. Type isolates are in bold, and newly generated sequences are in red.

Table 2.
Species details and their GenBank accession numbers used in phylogenetic analyses of Thyridariaceae. Type isolates are in bold, and newly generated sequences are in red.
2.3. Phylogenetic Analyses
According to the corresponding Sanger sequencing chromatograms, misleading data from the ends of raw sequencing fragments were manually trimmed and assembled into consensus sequences using SeqMan Pro version 7.1.0 (DNASTAR, Inc., Madison, WI, USA). Barcode sequences of all species (Table 1 and Table 2) were downloaded from the NCBI nucleotide database using the R package Analysis of Phylogenetics and Evolution 5.0 (APE, http://ape-package.ird.fr, 26 June 2024) [46].
The multiple sequence alignments were conducted using MAFFT version 7.310 [47] with options “--adjustdirection --auto,” and the alignment files were further trimmed using trimAl version 1.4 [48] with the option “-gapthreshold 0.5”, which only allows 50% of taxa with a gap in each site. The best-fit nucleotide substitution models for each locus were selected using ModelFinder version 2.1.1 [49] under the Corrected Akaike Information Criterion (AICC). All sequence alignments were combined using an in-house Python script.
Maximum Likelihood (ML) and Bayesian analysis (BI) were conducted based on individual and combined datasets. Two phylogenetic trees were constructed by combining the ITS, SSU, LSU, RPB2, and TEF1 gene regions. The first tree represents the phylogenetic analysis of the Massarinaceae, while the second tree represents the phylogenetic analysis of the Thyridariaceae. ML phylogenetic trees were obtained using the IQ-TREE version 2.0.3 [50], and the topology was evaluated using 1000 ultrafast bootstrap replicates. The BI was conducted using parallel MrBayes version 3.2.7a [51]. The ML trees were visualized using ggtree version 2.4.1 [52] and further edited in Adobe Illustrator version 16.0.0.
3. Results
3.1. Phylogenetic Analyses
Sequences of Five loci were successfully obtained for Helminthosporium filamentosa (UESTCC 24.0132), H. pini (HKAS 135177), and H. velutinum (UESTCC 24.0189). A phylogenetic tree of all Helminthosporium species and representative strains from other genera in Massarinaceae was constructed (Figure 1), including 100 taxa, with Periconia pseudodigitata (CBS 139699) as the outgroup. The combined dataset (ITS: 1–564, LSU: 565–1415, SSU: 1416–2440, RPB2: 2441–3555, TEF1: 3556–4781) was composed of 1872 distinct patterns, 1183 parsimony-informative sites, 340 singleton sites, and 3257 constant sites. The best-fit evolution models were GTR+F+I+G4 for the ITS partitions, GTR+F+I+G4 for the LSU partition, K2P+G4 for the SSU partition, HKY+F+I+G4 for the RPB2 partition, and GTR+F+I+G4 for the TEF1 partition. The best-scoring ML tree (lnL = −32012.540) with support values from ML and Bayesian analysis at the node is shown in Figure 1.

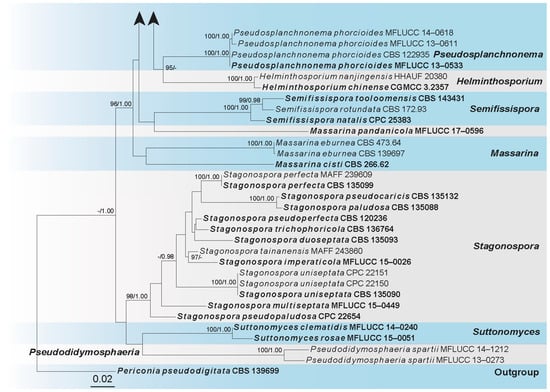
Figure 1.
The phylogram of the family Massarinaceae from ML analysis is based on the concatenated dataset of ITS-LSU-SSU-RPB2-TEF1. The tree is rooted with Periconia pseudodigitata (CBS 139699). Support values of ML-UFBoot ≥ 95 and Bayesian posterior probabilities ≥ 0.95 were displayed at the nodes as ML/PP. Support values below 95 and 0.95 are indicated by a hyphen (-). Newly collected taxa are shown in red. Strains from type materials are in bold.
According to the multi-locus phylogeny (Figure 1), our collection (HKAS 135177) formed an independent clade sister to H. yunnanense (HJAUP C2071) and strains of H. austriacum. Our collection (UESTCC 24.0132) formed a separate branch, sister to H. dalbergiae (MAFF 243853), H. magnisporum (MAFF 239278), and strains of H. quercinum. Our collection (UESTCC 24.0189) nests with H. velutinum strains with 99% ML, 1.00 PP statistical support. Combining the morphological evidence with phylogeny, we propose two new species, H. filamentosa (UESTCC 24.0132) and H. pini (HKAS 135177), isolated from Pinus sp. and Prunus pseudocerasus, respectively. Additionally, we report our collections (UESTCC 24.0189) as a new host record of H. velutinum from Pinus sp.
Sequences of Five loci were successfully obtained for the Vaginospora sichuanensis (UESTCC 24.0191). A phylogenetic tree of representative strains from other genera in Thyridariaceae was constructed (Figure 2), including 36 taxa, with Torula herbarum (CBS 595.96) as the outgroup. The combined dataset (ITS: 1–516, LSU: 517–1372, RPB2: 1373–2402, SSU: 2403–3422, TEF1: 3423–4307) was composed of 1448 distinct patterns, 824 parsimony-informative sites, 518 singleton sites, and 2965 constant sites. The best-fit evolution models were SYM+G4 for the ITS partitions, SYM+I for the LSU partition, SYM+I+G4 for the RPB2 partition, K2P+G4 for the SSU partition, and GTR+F+I+G4 for the TEF1 partition. The best-scoring ML tree (lnL = − 21563.517) with support values from ML and Bayesian analysis at the node is shown in Figure 2.
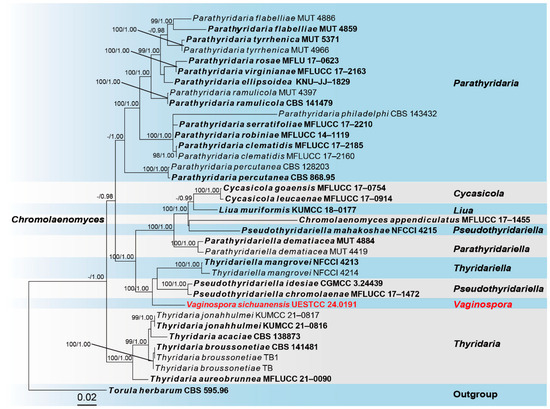
Figure 2.
The phylogram of the family Thyridariaceae from ML analysis is based on the concatenated dataset of ITS-LSU-RPB2-SSU-TEF1. The tree is rooted with Torula herbarum (CBS 595.96). Support values of ML-UFBoot ≥ 95 and Bayesian posterior probabilities ≥ 0.95 were displayed at the nodes as ML/PP. Support values below 95 and 0.95 are indicated by a hyphen (-). Newly collected taxa are shown in red. Strains from type materials are in bold.
According to the multi-locus phylogeny (Figure 1), our collection (UESTCC 24.0191) formed an independent clade sister to Pseudothyridariella chromolaenae (MFLUCC 17–1472), Pseudothyridariella idesiae (CGMCC 3.24439), and strains of Thyridariella mangrovei. Based on the morphological evidence and phylogeny, we propose a new genus, Vaginospora, with the type species Vaginospora sichuanensis.
3.2. Taxonomy
Helminthosporium filamentosa W.H. Tian, Y. Jin & Maharachch., sp. nov. (Figure 3).
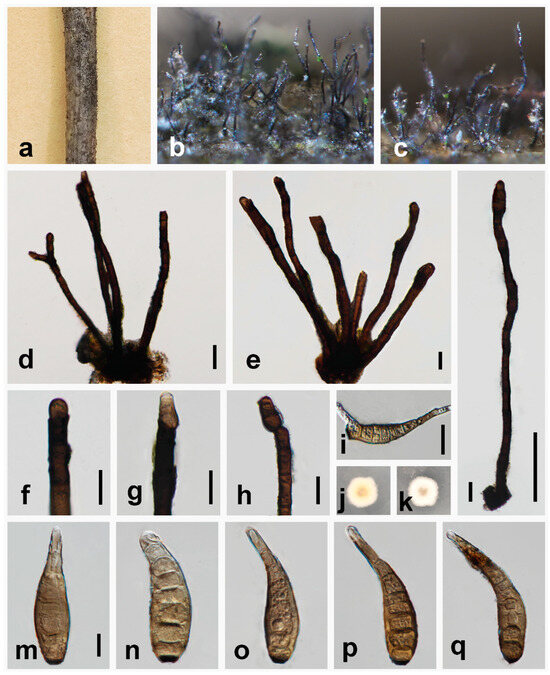
Figure 3.
Helminthosporium filamentosa (HKAS 135176, holotype). (a–c) Colonies on the natural substrate; (d,e) colony and conidiophores; (f–h) conidiophores, conidiogenous cells and apical conidia; (i) germinating conidium; (j,k) culture characteristics on PDA after 10 days (forth and back); (l) solitary conidiophores and apical conidia; (m–q) conidia. Scale bars: 20 μm (d–i); 100 μm (l); 10 μm (m–q); scale bar (m) applies to (m–q).
MycoBank: MB 854338
Etymology: The epithet refers to the filamentous mycelium, from the Latin “filamentum” for filaments.
Saprobic on dead branch of Prunus pseudocerasus. in terrestrial habitats. Asexual morph: Hyphomycetes. Colonies on the natural substratum effuse, scattered, hairy, black. Mycelium superficial, numerous, hairy, black, scattered, solitary or fasciculate. Conidiophores 210–710 × 8–18 μm ( = 350 × 12.5 μm, n = 30), macronematous, mononematous, erect, simple or sometimes branched, straight to curved, verruculose, septate, black, cylindrical, obtuse at apex. Conidiogenous cells enteroblastic, integrated, terminal, cylindrical, black, verruculose. Conidia 43–73 × 10–16 μm ( = 60 × 13 μm, n = 30), cylindrical, obclavate, phragmoconidia, solitary, acrogenous, 7-distoseptate, obclavate, wide at the middle and lower part, straight or curved, uneven width, rounded at apex, truncate at base, brown, thick-walled, verruculose, secession schizolytic. Sexual morph: Undetermined.
Material examined: CHINA, Sichuan Province, Chengdu City, Pujiang Country, Cherry Mountain 30°16′25″ N, 103°50′9″ E, elevation 546 m, 19 October 2023, decaying branches of Prunus pseudocerasus, Y. Jin YTS21_3 (HKAS 135176, holotype), ex-type CGMCC 3.27591 = UESTCC 24.0132.
Culture characteristics: Colonies on PDA reaching 18.5 mm diam after 10 days at 25 °C, colonies from above: irregularly circular, white, margin entire, dense; reverse: cream at the margin, pale yellow in the center.
Notes: Multi-locus phylogenetic analysis indicated that our isolate (UESTCC 24.0132) constitutes an independent clade sister to H. magnisporum (MAFF 239278), H. dalbergiae (MAFF 243853), and strains of H. quercinum (Figure 1). Based on pairwise nucleotide comparisons, the closest hits to our isolate (UESTCC 24.0132) is H. quercinum (CBS 136921), and comparing the ITS, LSU, SSU, RPB2, and TEF1 of their sequence revealed 96.88% (559/577 bp, gaps: 2/577 bp), 99.34% (754/759 bp, without gaps), 99.8% (1004/1006 bp, without gaps), 97.45% (763/783 bp, without gaps), and 97.44% (838/860 bp, gaps: 2/860 bp) similarity, respectively. Morphologically, our isolate (UESTCC 24.0132) differs from H. quercinum (CBS 136921) by color (black vs. brown to dark brown) and longer conidiophores (210–710 × 8–18 μm vs. 74–199 × 11–18 μm), and shorter and narrower conidia (43–73 × 10–16 μm vs. 78–130 × 15.3–18 μm) [24]. Among Helminthosporium species that lack molecular data, only H. davillae, H. obpyriforme, and H. panici have similar conidial sizes to our collection [15,53,54,55]. However, the conidia of our isolate (UESTCC 24.0132) are wider (10–16 μm vs. 4–6 μm) and more distosepta (7-distoseptate vs. 2–4-distoseptate) than H. davillae [55]. Helminthosporium filamentosa (UESTCC 24.0132) differs from H. obpyriforme in the morphology of the conidiophores (sometimes branched vs. simple; black vs. dark brown); it has relatively longer conidiophores (210–710 μm vs. 225–460 μm) and narrow conidia (10–16 μm vs. 14–19 μm) compared to H. obpyriforme [54]. Helminthosporium filamentosa (UESTCC 24.0132) has a different conidial morphology (obclavate vs. ellipsoidal-elongate) and more distoseptate conidia (7-distoseptate vs. 1–4-distoseptate) than H. panici [53]. Therefore, based on morphological characteristics and phylogenetic analysis results, we identified H. filamentosa as a novel species from Prunus pseudocerasus in China.
Helminthosporium pini W.H. Tian & Maharachch., sp. nov. (Figure 4).

Figure 4.
Helminthosporium pini (HKAS 131577, holotype). (a–c) Colonies on the natural substrate; (d) colony and conidiophores; (e) conidiophores with apical conidia; (f–h) conidiophores, conidiogenous cells, and apical conidia; (i,j) immature conidia; (k–p) conidia. Scale bars: 100 μm (d); 20 μm (e–i); 20 μm (j–p). Scale bar (k) applies to (k–p).
MycoBank: MB 854339
Etymology: Named after the host genus where the fungus was collected.
Saprobic on a dead branch of Pinus sp. in terrestrial habitats. Asexual morph: Hyphomycetes. Colonies on the natural substratum effuse, hairy, black. Mycelium superficial, numerous, hairy, black, scattered, solitary or fasciculate. Conidiophores 170–580 × 8–13 μm ( = 305 × 11 μm, n = 30), cylindrical, macronematous, mononematous, simple, erect, straight to slightly curved, verruculose, septate, dark brown to black, obtuse at apex. Conidiogenous cells holoblastic, integrated, terminal and intercalary, cylindrical, dark brown, verruculose. Conidia 60–100 × 12–18 μm ( = 80 × 16 μm, n = 30), cylindric-obclavate, phragmoconidia, solitary, acrogenous, 5–12-distoseptate, elongated, wide at the middle and lower part, straight or flexuous, uneven width, rounded at apex, obconically truncate at base, dark brown, thick-walled, verruculose, secession schizolytic. Sexual morph: Undetermined.
Material examined: CHINA, Sichuan Province, Neijiang City, Songlin Village, 29°32′19″ N, 105°9′28″ E, elevation 373 m, 1 April 2023, decaying branches of Pinus sp., W.H. Tian SLC11_1 (HKAS 131577, holotype).
Notes: Phylogenetic analysis based on the combined dataset of ITS, LSU, SSU, RPB2, and TEF1 loci revealed that our collection (HKAS 131577) forms a separate branch sister to H. yunnanense (HJAUP C2071) and H. austriacum strains (Figure 1). In the NCBI BLASTn search, the closest matches to our isolate (UESTCC 24.0132) is H. austriacum. Comparing the ITS, LSU, SSU, RPB2, and TEF1 of our collection (HKAS 131577) with the type strain of H. austriacum (CBS 139924) sequence revealed 90.87% (378/416 bp, gaps: 5/416 bp), 96.38% (718/745, gaps: 3/745 bp), 99.60% (985/989, without gaps), 90.24% (703/779, without gaps), and 94.81% (676/713, gaps: 2/713 bp) similarity, respectively. The morphological characteristics of our collection (HKAS 131577) overlap with the species of Helminthosporium in having septate and erect conidiophores and acro-pleurogenous and distoseptate conidia (Figure 3) [16,24]. Our collection differs from the H. austriacum by shorter conidiophores (170–580 μm vs. 270–920 μm [56]. Comparing the morphology of 216 Helminthosporium species reported worldwide, except for species with molecular sequences, only H. gossypii, H. guangxiense, H. meliae, and H. pseudotsugae had similar conidial sizes to our collection [15]. However, the conidiophores of our collection (170–580 × 8–13 μm) are longer and wider than H. gossypii (40–185 × 6.5–8.5 μm); shorter and narrower than H. guangxiense (330–850 × 8–20 μm); and longer and narrower than H. meliae (250–350 × 15–22 μm) [54,57,58,59]. The number of distosepta in conidia in our collection (HKAS 131577) (5–12-distoseptate) is more than H. gossypii (1–8-distoseptate), less than H. guangxiense (9–17-distoseptate), and less than H. pseudotsugae (8–14-distoseptate) [54,57,58,59]. Thus, our collection HKAS 131577 is described as a new species based on morphological observation and phylogenetic evidence.
Helminthosporium velutinum W Link [as ‘Helmisporium’], Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 10 (1809) (Figure 5).

Figure 5.
Helminthosporium velutinum (UESTCC 24.0189). (a–c) Colonies on the natural substrate; (d–f) conidiophores with conidia; (g,h) culture characteristics on PDA after 11 days (forth and back); (i–o) conidia; (p) germinating conidium. Scale bars: 100 μm (d); 20 μm (e,f); 10 μm (i–p).
Saprobic on a dead branch of Pinus sp. in terrestrial habitats. Asexual morph: Hyphomycetes. Colonies on the natural substratum effuse, hairy, black. Mycelium superficial, numerous, hairy, black, scattered, solitary. Conidiophores 275–765 × 9–12 μm ( = 525 × 16 μm, n = 30), macronematous, mononematous, unbranched, erect, straight to slightly curved, verruculose, septate, dark brown to black, cylindrical, obtuse at apex. Conidiogenous cells holoblastic, integrated, terminal and intercalary, cylindrical, dark brown, verruculose. Conidia 25.5–70 × 10–14 μm ( = 46.5 × 16 μm, n = 30), cylindric-obclavate, tapering to 4–10 μm ( = 6.5, n = 30) at the distal end, with a blackish-brown 3.5–6.5 μm wide ( = 5, n = 30) scar at the base, phragmoconidia, solitary, acrogenous, 4–10-distoseptate, wide at the middle and lower part, straight or flexuous, uneven width, rounded at apex, obconically truncate at base, brown to dark brown, thick-walled, verruculose, secession schizolytic. Sexual morph: Undetermined.
Material examined: CHINA: Sichuan Province, Chengdu City, Jiudaoguai, 30°30′21″ N, 103°53′47″ E, elevation 502 m, 19 October 2023, within dead branches of Pinus sp., W.H. Tian JDG31 (HUEST 24.0206), living culture UESTCC 24.0189.
Culture characteristics: Colony on PDA reaching 22 mm diam. in 11 days at 25 °C in the dark, colonies from above: irregularly circular, white, uneven entire, raised in center, with denser mycelium at the center; from below: yellowish brown, dark brown in the center, margin undulated.
Notes: Helminthosporium velutinum is the type species of the genus Helminthosporium. It is widely distributed around the world, with 110 known host records, excluding Pinus species [60]. Helminthosporium velutinum has been reported from both freshwater and terrestrial environments in China [16,24]. Multi-locus analyses of combined ITS, LSU, SSU, RPB2, and TEF1 sequence data showed that our collection (UESTCC 24.0189) nests with H. velutinum strains with 99% ML, 1.00 PP statistical support (Figure 1). In addition, the morphological characteristics examined largely overlapped with H. velutinum [16]. Thus, based on morphological comparison and phylogenetic analyses, we report our collections (UESTCC 24.0189) as a new host record of H. velutinum from Pinus sp.
Vaginospora W.H. Tian, Y. Jin & Maharachch., gen. nov.
MycoBank: MB 854941
Etymology: Named after its ascospores that have a sheath, from the Latin words “vagina” for sheath and “spora” for spore.
Saprobic on a dead branch of Prunus sp. in terrestrial habitats. Sexual morph: Pseudothecia solitary scattered, semi-immersed, uni-loculate, sessile, globose to oblong, ampulliform to ovoid, black, ostiolate. Peridium comprising several layers; outer layers dark brown to dark, with compressed cells of textura angularis; inner layers hyaline, with compressed pseudoparenchymatous cells, arranged in textura angularis. Hamathecium comprises dense, branched, septate, cellular pseudoparaphyses. Asci 8-spored, bitunicate, cylindrical to clavate, straight or curved, short pedicelate, apically rounded with an ocular chamber. Ascospores 2-seriate, overlapping and are initially gray-brown, turning dark brown at maturity, fusiform, mostly 3-transversely septate, sometimes 1–2 longitudinal septum appear after maturity, constricted at the septa, smooth-walled, conically rounded at the ends, verruculose, with an inconspicuous mucilaginous sheath. Asexual morph: undetermined.
Type species—Vaginospora sichuanensis W.H. Tian, Y. Jin & Maharachch.
Notes: According to phylogenetic analysis of Thyridariaceae, our collection (UESTCC 24.0191) constitutes a distinct clade, positioned as a sister to Pseudothyridariella and Thyridariella, with 100% ML and 1.00 PP statistical support (Figure 2). However, the sister relationship of Vaginospora to Pseudothyridariella and Thyridariella warrants discussion to clarify the issues of intergeneric affinities. Thyridariella can be distinguished from Vaginospora and Pseudothyridariella by having hyaline ascospores [61,62]. Pseudothyridariella produces ascospores slightly constricted at the central septum, whereas Thyridariella and Vaginospora have ascospores constricted at every septum [61,62]. Furthermore, the size of ascospores and septa in ascospores of Vaginospora is significantly smaller and fewer than in Thyridariella and Pseudothyridariella [61,62]. Therefore, Vaginospora is proposed as a new genus to accommodate Vaginospora sichuanensis and expand the scope of the family Thyridariaceae.
Vaginospora sichuanensis W.H. Tian, Y. Jin & Maharachch., sp. nov. (Figure 6).

Figure 6.
Vaginospora sichuanensis (UESTCC 24.0191, holotype). (a–c) Ascomata immersed in the decaying wood of Prunus sp.; (d,e) longitudinal sections of ascomata; (f) cellular and hyaline pseudoparaphyses; (g) section of peridium comprising cells of textura angularis; (h–l) immature and mature asci; (m,n) culture characteristics on PDA after 11 days (forth and back); (o) germinating ascospore; (p–r) ascospores; (s,t) ascospores with a mucilaginous sheath in India ink. Scale bars: 100 μm (d,e); 20 μm (g,o); 10 μm (f,h–l,p–t).
MycoBank: MB 854942
Etymology: Named after the Sichuan province, China, where the holotype was collected.
Saprobic on a dead branch of Prunus sp. in terrestrial habitats. Sexual morph: Pseudothecia 180–290 μm high × 265–375 μm diam. ( = 225 × 290 μm, n = 10), solitary scattered, semi-immersed, uni-loculate, sessile, globose to oblong, ampulliform to ovoid, black, filled by asci and ascospores and pseudoparaphyses, ostiolate. Peridium 28–48 μm wide, comprising several layers; outer layers dark to dark brown, with compressed cells of textura angularis; inner layers hyaline, with compressed pseudoparenchymatous cells, arranged in textura angularis. Hamathecium comprises dense, 1.3–2.2 μm wide, branched, septate, cellular pseudoparaphyses. Asci 65–140 × 6.5–15 μm ( = 90 × 10 μm, n = 20), 4–8-spored, bitunicate, cylindrical to clavate, straight or curved, short pedicelate, apically rounded with an ocular chamber. Ascospores 13–23 × 4–10 μm ( = 18 × 7 μm, n = 30), 2-seriate, overlapping and are initially gray-brown, turning dark brown at maturity, fusiform, muriform, mostly 3-transversely septate, sometimes 1–2 longitudinal septum appear after maturity, constricted at the septa, smooth-walled, conically rounded at the ends, verruculose, with an inconspicuous mucilaginous sheath. Asexual morph: undetermined.
Material examined: CHINA, Sichuan Province, Chengdu City, Dujiangyan County Yunhuashan, 31°00′62” N, 103°59′14” E, elevation 1535 m, 22 March 2024, decaying branches of Prunus sp., Y. Jin YHS46 (HKAS 136265, holotype), ex-type CGMCC 3.27600 = UESTCC 24.0191.
Culture characteristics: Colony on PDA reaching 24 mm diam. in 11 days at 25 °C in the dark, colonies from above: irregularly circular, white, uneven entire, raised in center, with denser mycelium at the center and becoming sparser at the edge; from below: yellowish brown, brown in the center, margin undulated.
Notes: Phylogenetic analyses of combined ITS, LSU, RPB2, SSU, and TEF1 sequence data showed that our isolate (UESTCC 24.0191) forms a separate branch sister to Pseudothyridariella and Thyridariella with stable support (Figure 2). However, morphologically, our collection (UESTCC 24.0191) differs from the P. chromolaenae and T. mangrovei by narrower asci (6.5–15 μm vs. 14–22 μm vs. 10–30 μm) and smaller ascospores (13–23 × 4–10 μm vs. 23–28 × 9–12.5 μm vs. 23–40 × 8–15 μm), as well as the number of transverse septa in ascospores (1–5 septa vs. 5–8 septa vs. 5–6 septa) [61,62]. The closest matches to our isolate (UESTCC 24.0191) are species of Pseudothyridariella (Figure 2). Sequence comparison for the ITS, LSU, RPB2, SSU, and TEF1 region between our isolate (UESTCC 24.0191) and the type species P. chromolaenae (MFLUCC 17–1472) showed 96.56% (365/378 bp, without gaps), 98.70% (758/768 bp, without gaps), 87.60% (636/726 bp, without gaps), 99.53% (428/430 bp, without gaps), and 95.23% (798/838 bp, without gaps) base pair similarity. Thus, our isolate UESTCC 24.0191 was identified as a new species based on morphological observation and phylogenetic evidence.
4. Discussion
Seven hundred and seventy-nine epithets of Helminthosporium have been listed in Index Fungorum (http://www.indexfungorum.org; 26 June 2024). Konta et al. compiled a list of 216 identified and accepted species of Helminthosporium worldwide based on species records from Index Fungorum [15]. However, most species are characterized solely based on morphology, and only 37 species have sequence data. The lack of extensive molecular data is primarily due to most species being reported and identified before sequencing technologies. This results in the possibility that many species within the Helminthosporium may be conspecific or belong to different genera [18,19,21,22,23]. Therefore, it is essential to re-examine the type specimens of formerly described Helminthosporium-like species to address this matter. Simultaneously, it is necessary to collect fresh specimens, sequence them, and combine multi-locus phylogenetic analysis with morphological examination to designate epi-types or neotypes, which is crucial for clarifying the taxonomic status of many doubtful species.
Helminthosporium is distributed worldwide and is mainly saprophytic on leaves or branches in terrestrial or aquatic environments [15,16,17,24,63]. Silver scurf caused by H. solani has become an important economic disease since the 1990s [64,65,66,67]. Helminthosporium solani mainly causes blemishes in the periderm of potato tubers [68]. In the 20th century, silver scurf disease also emerged in China, resulting in significant potato losses [69].
In recent studies, species of Pseudothyridariella have been grouped into two distinct clades based on phylogenetic analysis [70,71]. The genus Pseudothyridariella was proposed by Mapook et al. [62], with P. chromolaenae as the type species, and Thyridariella mahakoshae was transferred to the Pseudothyridariella genus as P. mahakoshae. However, not many sequence data and species were available for constructing phylogeny at that time. More data are emerging showing that this species is clearly unrelated to the genus Pseudothyridariella, which supports our study [70,71]. Morphologically, P. mahakoshae also differs from P. chromolaenae in that the former has brown or olive-brown to dark brown ascospores with 5–8 transverse septa, whereas the latter has hyaline ascospores with 3–6 transverse septate [61,62]. When the genus Thyridariella was introduced, it was mentioned that one of its characteristic features was the production of hyaline muriform ascospores [61]. Pseudothyridariella idesiae is not comparable here because it is known only for its asexual morph [71]. Our newly introduced genus, Vaginospora sichuanensis, has muriform ascospores that are mostly 3-transversely septate, which are morphologically and phylogenetically distinct from P. mahakoshae. Therefore, more evidence should be collected, and the taxonomic status of P. mahakoshae should be revised in future studies.
The multi-locus phylogenetic analysis in this study revealed that our newly collected taxa of Vaginospora represent a new genus as it constitutes a strongly supported monophyletic clade (Figure 2). Molecular evidence further indicates that Vaginospora belongs to the family Thyridariaceae. Our collection (UESTCC 24.0191) was morphologically similar to the type genus Thyridaria of Thyridariaceae, with immersed or semi-immersed ascomata, composed of textura angularis cells in the peridium, bitunicate, cylindrical to subclavate asci that are apically rounded with an ocular chamber, and fusiform, septate ascospores [12]. They differ in ascospore morphology, with our collection having 1–5 septa and being highly constricted at the septa, 1–2 longitudinal septa at maturity, and a sheath around the ascospores, which is not found in Thyridaria [12]. Morphological character comparison is a traditional classification method that cannot reflect phylogenetic relationships; it could be informative at the generic level in Thyridariaceae. For example, Parathyridaria was introduced into the family for its characteristic features of discoid ostiolar apices always present and pale to greyish-brown ascospores [12], Thyridariella is distinguished from other genera by its hyaline ascospores [61]; a typical feature of Pseudothyridariella is that the ascospores are constricted only at the central septum [62]. Thus, within Thyridariaceae, ascospores morphology is diverse, and when establishing a new genus, it can be combined with molecular data to provide further evidence and support.
Author Contributions
Conceptualization, W.-H.T., Y.J. and S.S.N.M.; methodology, W.-H.T.; data sources, W.-H.T. and Y.J.; formal analysis, W.-H.T., Y.-C.L. and Y.J.; visualization, W.-H.T., Y.J. and Y.-C.L.; writing—original draft preparation, W.-H.T.; writing—review and editing, S.S.N.M., T.K.F. and W.-H.T.; supervision, S.S.N.M.; funding acquisition, X.-Y.G. and S.S.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2023 Xinjiang Production and Construction Corps Agricultural Key Core Technology Research Project, grant number NYHXGG2023AA301; 2023 Seventh Division Huyanghe City “Taking Up the Challenge and Assuming Leadership” Project, grant number QS2023011; 2023 13th Division Xinxing City Science and Technology Plan Project, grant number 2023B4; Talent Introduction and Cultivation Project, University of Electronic Science and Technology of China, grant number A1098531023601245. We extend our appreciation to the Researchers Supporting Project at King Saud University, Riyadh, Saudi Arabia, for funding this research project, fund number RSP2024R487.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequence data are available in NCBI GenBank following the accession numbers in the manuscript.
Acknowledgments
We thank the University of Electronic Science and Technology of China for funding this research. We also thank the Shihezi University and King Saud University for funding this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sanchez-Garcia, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selcuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Tian, X.G.; Bao, D.F.; Karunarathna, S.C.; Jayawardena, R.S.; Hyde, K.D.; Bhat, D.J.; Luo, Z.L.; Elgorban, A.M.; Hongsanan, S.; Maharachchikumbura, S.S.N.; et al. Taxonomy and phylogeny of ascomycetes associated with selected economically important monocotyledons in China and Thailand. Mycosphere 2024, 15, 1–274. [Google Scholar] [CrossRef]
- Câmara, M.P.; Palm, M.E.; van Berkum, P.; O’Neill, N.R. Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 2002, 94, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Kodsueb, R.; Jeewon, R.; Vijaykrishna, D.; McKenzie, E.H.; Lumyong, P.; Lumyong, S.; Hyde, K.D. Systematic revision of Tubeufiaceae based on morphological and molecular data. Fungal Divers. 2006, 21, 105–130. [Google Scholar]
- Zhang, Y.; Jeewon, R.; Fournier, J.; Hyde, K.D. Multi-gene phylogeny and morphotaxonomy of Amniculicola lignicola: A novel freshwater fungus from France and its relationships to the Pleosporales. Mycol. Res. 2008, 112, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fournier, J.; Crous, P.W.; Pointing, S.B.; Hyde, K.D. Phylogenetic and morphological assessment of two new species of Amniculicola and their allies (Pleosporales). Persoonia 2009, 23, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kruys, A.; Eriksson, O.E.; Wedin, M. Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycol. Res. 2006, 110, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Phookamsak, R.; Jeewon, R.; Bhat, D.J.; Mapook, A.; Camporesi, E.; Shang, Q.J.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Molecular taxonomy and morphological characterization reveal new species and new host records of Torula species (Torulaceae, Pleosporales). Mycol. Prog. 2017, 16, 447–461. [Google Scholar] [CrossRef]
- Zhang, Y.; Crous, P.W.; Schoch, C.L.; Hyde, K.D. Pleosporales. Fungal Divers. 2012, 53, 1–221. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Hyde, K.D.; Abdel-Wahab, M.A.; Abdollahzadeh, J.; Abeywickrama, P.D.; Absalan, S.; Afshari, N.; Ainsworth, A.M.; Akulov, O.Y.; Aleoshin, V.V.; Al-Sadi, A.M.; et al. Global consortium for the classification of fungi and fungus-like taxa. Mycosphere 2023, 14, 1960–2012. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Voglmayr, H. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Stud. Mycol. 2016, 85, 35–64. [Google Scholar] [CrossRef] [PubMed]
- de Silva, N.I.; Hyde, K.D.; Lumyong, S.; Phillips, A.J.L.; Bhat, D.J.; Maharachchikumbura, S.S.N.; Thambugala, K.M.; Tennakoon, D.S.; Suwannarach, N.; Karunarathna, S.C. Morphology, phylogeny, host association and geography of fungi associated with plants of Annonaceae, Apocynaceae and Magnoliaceae. Mycosphere 2022, 13, 955–1076. [Google Scholar] [CrossRef]
- Link, H.F. Observationes in Ordines plantarum naturales. Dissertatio prima complectens Anandrarum ordines Epiphytas, Mucedines, Gastromycos et Fungos. Gesellschaft Natur. 1809, 3, 3–42. [Google Scholar]
- Konta, S.; Hyde, K.D.; Karunarathna, S.C.; Mapook, A.; Senwanna, C.; Dauner, L.A.P.; Nanayakkara, C.M.; Xu, J.; Tibpromma, S.; Lumyong, S. Multi-Gene phylogeny and morphology reveal Haplohelminthosporium gen. nov. and Helminthosporiella gen. nov. associated with Palms in Thailand and a checklist for Helminthosporium reported worldwide. Life 2021, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Tian, W.H.; Guo, Y.B.; Madrid, H.; Maharachchikumbura, S.S.N. Synhelminthosporium gen. et sp. nov. and two new species of Helminthosporium (Massarinaceae, Pleosporales) from Sichuan Province, China. J. Fungi 2022, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hirayama, K.; Yonezawa, H.; Sato, G.; Toriyabe, A.; Kudo, H.; Hashimoto, A.; Matsumura, M.; Harada, Y.; Kurihara, Y.; et al. Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud. Mycol. 2015, 82, 75–136. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Ueyama, A.; Nishihara, N. Pseudocochliobolus Nisikadoi, the perfect state of Helminthosporium Coicis. Mycologia 1977, 69, 1109–1120. [Google Scholar] [CrossRef]
- Hu, Y.F.; Liu, J.W.; Xu, Z.H.; Castañeda-Ruíz, R.F.; Zhang, K.; Ma, J. Morphology and multigene phylogeny revealed three new species of Helminthosporium (Massarinaceae, Pleosporales) from China. J. Fungi 2023, 9, 280. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Calabon, M.S.; Jones, E.B.G.; Zhang, Y.X.; Liao, C.F.; Xiong, Y.R.; Chaiwan, N.; Kularathnage, N.D.; Liu, N.G.; Tang, S.M.; et al. Mycosphere notes 345–386. Mycosphere 2022, 13, 454–557. [Google Scholar] [CrossRef]
- Castañeda-Ruiz, R.F.; Li, D.W.; Zhang, X.G.; Kendrick, B.; Ramos-García, B.; Pérez-Martínez, S.; Sosa, D. Ellismarsporium gen. nov. and Stanhughesiella gen. nov. to accommodate atypical Helminthosporium and Corynesporella species. Mycotaxon 2018, 132, 759–766. [Google Scholar] [CrossRef]
- Xu, Z.H.; Qiu, L.; Kuang, W.G.; Shi, X.G.; Zhang, X.G.; Castañeda-Ruíz, R.F.; Ma, J. Varioseptispora chinensis gen. & sp. nov., V. apicalis nom. nov., V. hodgkissii comb. nov., and V. versiseptatis comb. nov. Mycotaxon 2020, 135, 753–759. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Li, D.W.; Castañeda-Ruiz, R.F. Mirohelminthosporium gen. nov. for an atypical Helminthosporium species and H. matsushimae nom. nov. Mycotaxon 2020, 135, 777–783. [Google Scholar] [CrossRef]
- Voglmayr, H.; Jaklitsch, W.M. Corynespora, Exosporium and Helminthosporium revisited—New species and generic reclassification. Stud. Mycol. 2017, 87, 43–76. [Google Scholar] [CrossRef]
- Ellis, M.B. Dematiaceous Hyphomycetes; Cabi: Wallingford, UK, 1971. [Google Scholar]
- Ellis, M.B. Dematiaceous hyphomycetes. III. Mycologia 1961, 53, 629. [Google Scholar] [CrossRef]
- Luttrell, E. Taxonomic criteria in Helminthosporium 11. Mycologia 1963, 55, 643–674. [Google Scholar] [CrossRef]
- Luttrell, E. Systematics of Helminthosporium and related genera. Mycologia 1964, 56, 119–132. [Google Scholar] [CrossRef]
- Li, C.; Adu, B.; Wu, J.; Qin, G.; Li, H.; Han, Y. Spatial and temporal variations of drought in Sichuan Province from 2001 to 2020 based on modified temperature vegetation dryness index (TVDI). Ecol. Indic. 2022, 139, 108883. [Google Scholar] [CrossRef]
- Su, P.W.; Lu, Z.H.; Tian, W.H.; Chen, Y.P.; Maharachchikumbura, S.S.N. Six additions to the genus Periconia (Dothideomycetes: Periconiaceae) from graminaceous plants in China. J. Fungi 2023, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.H.; Chen, Y.P.; Maharachchikumbura, S.S.N. Neodigitodesmium, a novel genus of family Dictyosporiaceae from Sichuan Province, China. Phytotaxa 2022, 559, 176–184. [Google Scholar] [CrossRef]
- Tian, W.H.; Su, P.W.; Chen, Y.P.; Maharachchikumbura, S.S.N. Four new species of Torula (Torulaceae, Pleosporales) from Sichuan, China. J. Fungi 2023, 9, 150. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lu, Z.H.; Faraj, T.K.; Maharachchikumbura, S.S.N. Myxospora poaceicola sp. nov. (Stachybotryaceae, Hypocreales), a novel myrothecium-like fungus from Digitaria sanguinalis (Poaceae). Phytotaxa 2023, 625, 280–288. [Google Scholar] [CrossRef]
- Su, P.W.; Chen, Y.P.; Syed, A.; Bahkali, A.H.; Maharachchikumbura, S.S.N. Taxonomic novelty in Sichuan Province, China: Veronaea polyconidia sp. nov. (Herpotrichiellaceae), a new addition to hyphomycetous fungi. Phytotaxa 2023, 632, 118–130. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Sandamali, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Pem, D.; Dissanayake, L.S.; Wijesinghe, S.N.; Bundhun, D.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Gareth Jones, E.B.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Egger, K.N. Molecular analysis of ectomycorrhizal fungal communities. Can. J. Bot. 1995, 73, 1415–1422. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS primers for the DNA-based identification of Ascomycetes and Basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Cubeta, M.A.; Echandi, E.; Abernethy, T.; Vilgalys, R. Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 1991, 81, 1395–1400. [Google Scholar] [CrossRef]
- Hibbett, D.S. Phylogenetic evidence for horizontal transmission of group I introns in the nuclear ribosomal DNA of mushroom-forming fungi. Mol. Biol. Evol. 1996, 13, 903–917. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. 2007, 44, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic. Acids. Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Yu, G. Using ggtree to Visualize Data on Tree-Like Structures. Curr. Protoco. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef]
- Stevens, F.L. Some meliolicolous parasites and commensals from Porto Rico. Botanical Gazette 1918, 65, 227–249. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.Y. Taxonomic studies of Helminthosporium from China 4. Six new species and a key to Helminthosporium from China. Mycotaxon 2009, 109, 399–413. [Google Scholar] [CrossRef]
- Sydow, P.; Sydow, H. Weitere neue Micromyceten der Philippinen-Inseln. Ann. Mycol. 1920, 18, 98–104. [Google Scholar]
- Voglmayr, H.; Akulov, O.Y.; Jaklitsch, W.M. Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycol. Prog. 2016, 15, 921–937. [Google Scholar] [CrossRef]
- Ciferri, R.; Fragoso, R.G. Hongos Parásitos y Saprófitos de la República Dominicana:(3a. serie); García: Woodstock, IL, USA, 1926. [Google Scholar]
- Tucker, C. A leaf, bract, and boll spot of sea-island cotton caused by Helminthosporium gossyp II n. sp. ¹. J. Agric. Res. 1926, 32, 391. [Google Scholar]
- Cooke, W.B. Western Fungi—II. Mycologia 1952, 44, 245–261. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases; U.S. National Fungus Collections; USDA: Washingtin, DC, USA, 2022. [Google Scholar]
- Devadatha, B.; Sarma, V.V.; Jeewon, R.; Wanasinghe, D.N.; Hyde, K.D.; Gareth Jones, E.B. Thyridariella, a novel marine fungal genus from India: Morphological characterization and phylogeny inferred from multigene DNA sequence analyses. Mycol. Prog. 2018, 17, 791–804. [Google Scholar] [CrossRef]
- Mapook, A.; Hyde, K.D.; McKenzie, E.H.C.; Jones, E.B.G.; Bhat, D.J.; Jeewon, R.; Stadler, M.; Samarakoon, M.C.; Malaithong, M.; Tanunchai, B.; et al. Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed). Fungal Divers. 2020, 101, 1–175. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Luo, X.; Castañeda-Ruíz, R.F.; Ma, J. Three novel species of Helminthosporium (Massarinaceae, Pleosporales) from China. MycoKeys 2022, 94, 73–89. [Google Scholar] [CrossRef]
- Harz, I. Spondylocladium atrovirens. Bull. Soc. Imp. Mosc. 1871, 44, 42. [Google Scholar]
- Ivanyuk, V.; Zezyulina, G. Helminthosporium solani causing silver scab is reported from Belarus. Zashchita Rastenii 1991, 3, 45. [Google Scholar]
- Hermilia-Sanz, B.M. Presence of Helminthosporium solani Dur et Mont, the silver scurf fungus of potatoes, in Chile. Agricultura Tecnica (Santiago) 1976, 36, 5–44. [Google Scholar]
- El Immane-Collet, R.; Elakel, M.; Jouan, B. Comparative study of the agronomical incidence of the silver scurf disease of potato in Morocco and in France. Al Awamia 1995, 91, 1–8. [Google Scholar]
- Errampalli, D.; Saunders, J.M.; Holley, J.D. Emergence of silver scurf (Helminthosporium solani) as an economically important disease of potato. Plant Pathol. 2001, 50, 141–153. [Google Scholar] [CrossRef]
- Tian, S.M.; Chen, Y.C.; Zou, M.Q.; Xue, Q. First Report of Helminthosporium solani causing silver scurf of potato in Hebei Province, North China. Plant Dis. 2007, 91, 460. [Google Scholar] [CrossRef] [PubMed]
- Wanasinghe, D.N.; Mortimer, P.E. Taxonomic and phylogenetic insights into novel Ascomycota from forest woody litter. Biology 2022, 11, 889. [Google Scholar] [CrossRef]
- Li, W.L.; Dissanayake, A.; Liu, J.K. Mycosphere Notes 413–448: Dothideomycetes associated with woody oil plants in China. Mycosphere 2023, 14, 1436–1529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).