The Catalase Gene MrCat1 Contributes to Oxidative Stress Tolerance, Microsclerotia Formation, and Virulence in the Entomopathogenic Fungus Metarhizium rileyi
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Cloning the MrCat1 Gene of M. rileyi
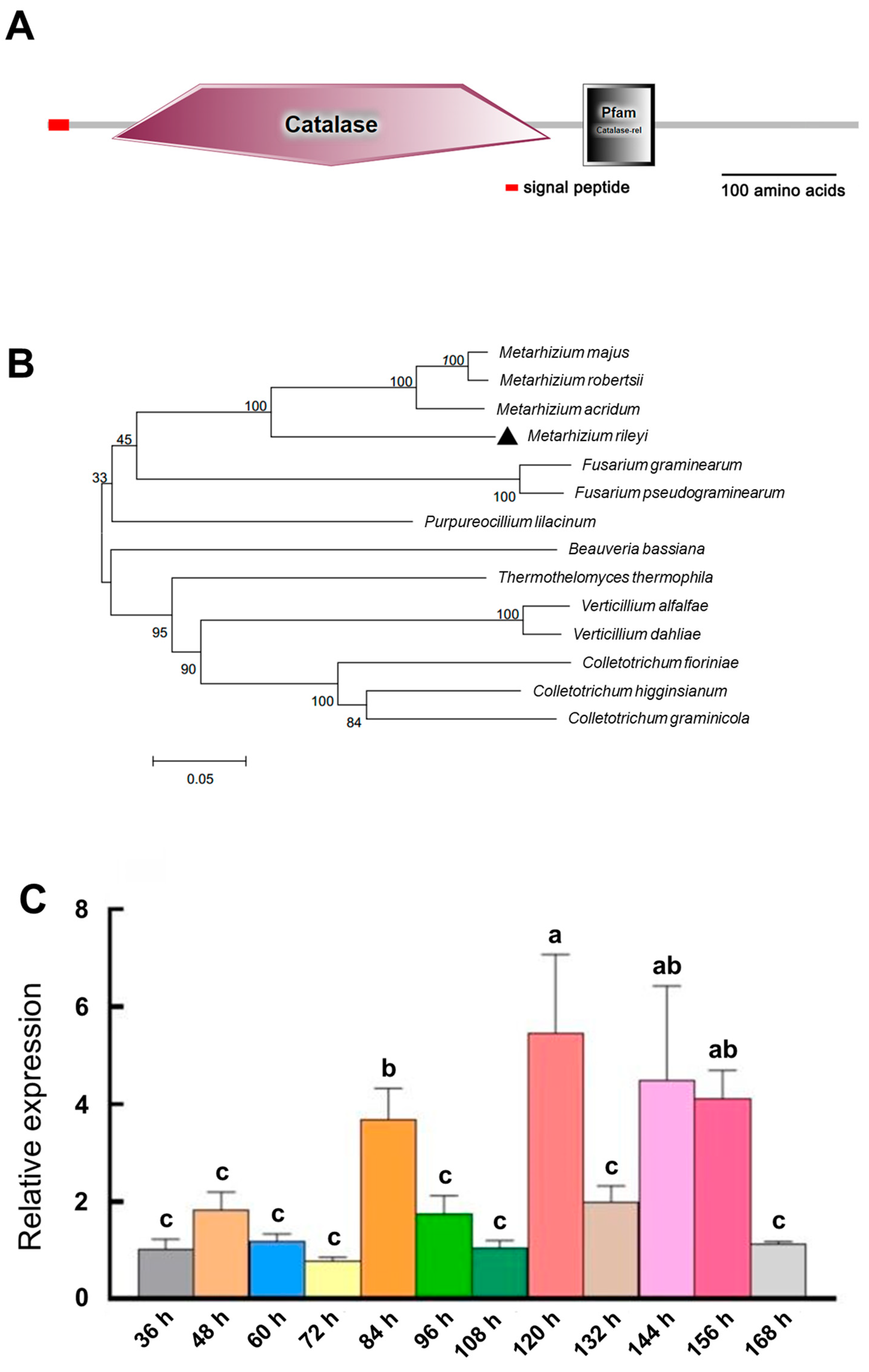
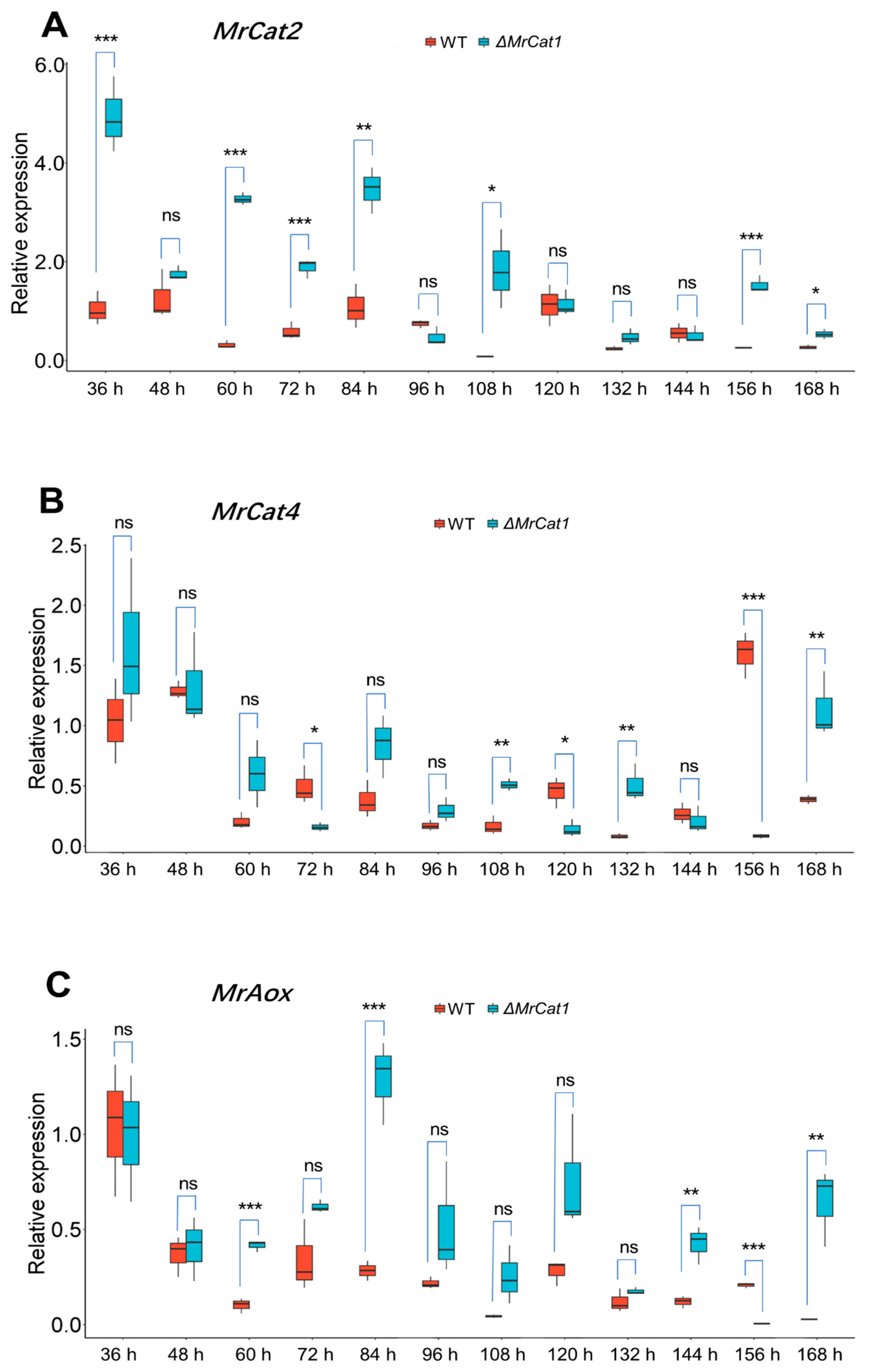
2.3. Gene Expression Patterns of M. rileyi WT and ΔMrCat1 Strains during MS Development
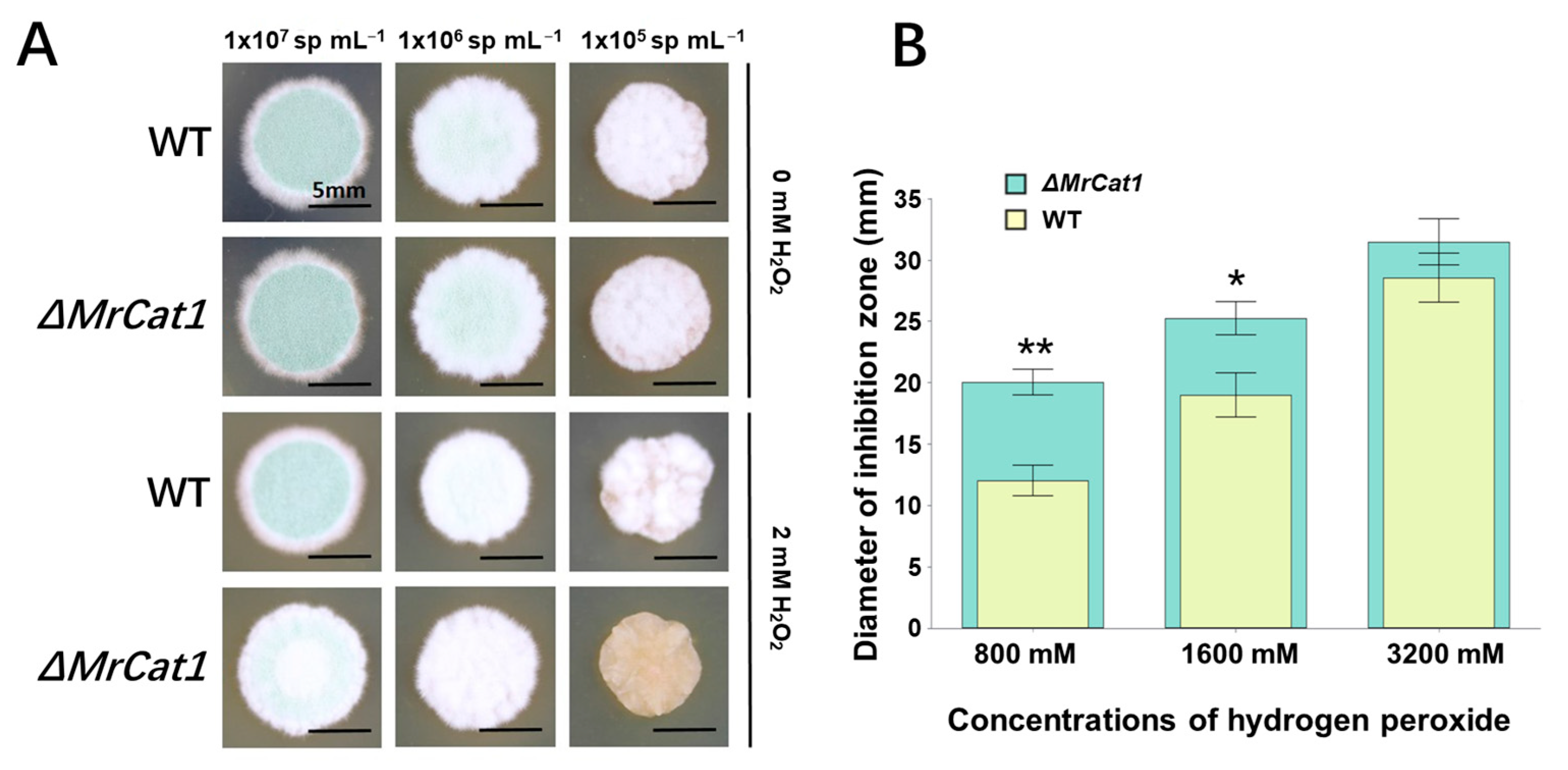
2.4. H2O2 Sensitivity of WT and ΔMrCat1 Strains
2.5. Hyperosmolarity Tolerance of WT and ΔMrCat1 Strains
2.6. MS Formation of WT and ΔMrCat1 Strains
2.7. Virulence Assays
2.8. Data Statistical Analysis
3. Results
3.1. Features of MrCat1 in M. rileyi
3.2. Deletion of MrCat1 Impaired the Tolerance to H2O2 in M. rileyi
3.3. Deletion of MrCat1 Did Not Affect the Hyperosmotic Tolerance of M. rileyi
3.4. Deletion of MrCat1 Affected MS Development of M. rileyi
3.5. Deletion of MrCat1 Resulted in Enhancing the Expression of MrCat2, MrCat4, and MrAox during MS Development of M. rileyi
3.6. Deletion of MrCat1 Reduced the Virulence of M. rileyi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Primers | Sequence 5′-3′ |
|---|---|
| RT-tub-F | GGCAAGGTCGCTATGAAG |
| RT-tub-R | CTGGATGGAGGTAGAGTTAC |
| RT-tef-F | GTCATCGTCCTCAACCATC |
| RT-tef-R | CAGTCTCAACAGCCTTACC |
| RT-Cat1-F | TTCGTCAACGAAGAGGGTGA |
| RT-Cat1-R | GTGGAAGTCGGAGTTCTTGC |
| RT-Cat2-F | ACTGGAAGCTCAACAACCCT |
| RT-Cat2-R | GCGCATCTGGACATTCTTTA |
| RT-Cat4-F | GGCGTCCCTGATATTCCTGA |
| RT-Cat4-R | GGCGATGATATCCGTGTGTG |
| RT-Aox-F | AATCCCACTACATCCGTGGT |
| RT-Aox-R | GCTCTCGAGAAAGACGAACC |
| Strains | Concentrations of Conidial Suspensions | LT50 (d) |
|---|---|---|
| WT | 2.5 × 107 sp mL−1 | 7.29 ± 0.57 |
| 5.0 × 107 sp mL−1 | 6.56 ± 0.33 | |
| 1.0 × 108 sp mL−1 | 5.57 ± 0.27 | |
| ΔMrCat1 | 2.5 × 107 sp mL−1 | >9 * |
| 5.0 × 107 sp mL−1 | >9 * | |
| 1.0 × 108 sp mL−1 | 6.81 ± 0.52 |
References
- Boucias, D.G.; Tigano, M.S.; Sosa-Gomez, D.R.; Glare, T.R.; Inglis, P.W. Genotypic properties of the entomopathogenic fungus Nomuraea rileyi. Biol. Control 2000, 19, 124–138. [Google Scholar] [CrossRef]
- Chen, H.; Yin, Y.P.; Li, Y.; Mahmud, M.S.; Wang, Z.K. Identification and analysis of gene differentially expressed in the Spodoptera litura fat body in response to the biocontrol fungus, Nomuraea rileyi. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 163, 203–210. [Google Scholar] [CrossRef]
- Devi, P.S.; Prasad, Y.G.; Chowdary, D.A.; Rao, L.M.; Balakrishnan, K. Identification of virulent isolates of the entomopathogenic fungus Nomuraea rileyi (F) Samson for the management of Helicoverpa armigera and Spodoptera litura (identification of virulent isolates of N. rileyi). Mycopathologia 2003, 156, 356–373. [Google Scholar]
- Supakdamrongkul, P.; Bhumiratana, A.; Wiwat, C. Characterization of an extracellular lipase from the biocontrol fungus, Nomuraea rileyi MJ, and its toxicity toward Spodoptera litura. J. Invertebr. Pathol. 2010, 105, 228–235. [Google Scholar] [CrossRef]
- Vega-Aquino, P.; Sanchez-Peña, S.; Blanco, C.A. Activity of oil-formulated conidia of the fungal entomopathogens Nomuraea rileyi and Isaria tenuipes against lepidopterous larvae. J. Invertebr. Pathol. 2010, 103, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.P.; Shan, H.; Song, Z.Y.; Wang, Z.K. Microsclerotia artificial inductions of Nomuraea rileyi CQNr01. China Agric. Sci. 2012, 4523, 4801–4807. (In Chinese) [Google Scholar]
- Jackson, M.A.; Jaronski, S.T. Development of pilot-scale fermentation and stabilisation processes for the production of microsclerotia of the entomopathogenic fungus Metarhizium brunneum strain F52. Biocontrol Sci. Technol. 2012, 22, 915–930. [Google Scholar] [CrossRef]
- Jaronski, S.T.; Jackson, M.A. Efficacy of Metarhizium anisopliae microsclerotial granules. Biocontrol Sci. Technol. 2008, 18, 849–863. [Google Scholar] [CrossRef]
- Kawasaki, L.; Aguirre, J. Multiple catalase genes are differentially regulated in Aspergillus nidulans. J. Bacteriol. 2001, 183, 1434–1440. [Google Scholar] [CrossRef]
- Song, Z.Y.; Yin, Y.P.; Jiang, S.S.; Liu, J.J.; Chen, H.; Wang, Z.K. Comparative transcriptome analysis of microsclerotia development in Nomuraea rileyi. BMC Genom. 2013, 141, 411. [Google Scholar] [CrossRef]
- Jiang, S.S.; Yin, Y.P.; Song, Z.Y.; Zhou, G.L.; Wang, Z.K. Raca and cdc42 regulate polarized growth and microsclerotium formation in the dimorphic fungus Nomuraea rileyi. Res. Microbiol. 2014, 1653, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Wei, J.; Yin, Y.P.; Wang, Z.K. Polarity Proteins Mrcdc24 and Mrbem1 Required for Hypha Growth and Microsclerotia Formation in Metarhizium Rileyi. Biocontrol Sci. Techn. 2016, 26, 733–745. [Google Scholar] [CrossRef]
- Liu, J.J.; Yin, Y.P.; Song, Z.Y.; Li, Y.; Jiang, S.S.; Shao, C.W.; Wang, Z.K. NADH: Flavin oxidoreductase/NADH oxidase and ROS regulate microsclerotium development in Nomuraea rileyi. World J. Microbiol. Biotechnol. 2014, 30, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.L.; Song, Z.Y.; Yin, Y.P.; Jiang, W.; Wang, Z.K. Involvement of alternative oxidase in the regulation of hypha growth and microsclerotia formation in Nomuraea rileyi CQNr01. World J. Microbiol. Biotechnol. 2015, 31, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.Q.; Li, B.J.; Wang, J.H.; Li, X.X.; Ma, F.L.; Du, F.; Li, H.L.; Lin, Y.L. Multifunctional regulation of NADPH oxidase in growth, microsclerotia formation and virulence in Metarhizium rileyi. Biotechnol. Lett. 2023, 45, 1441–1455. [Google Scholar] [CrossRef]
- Song, Z.Y.; Shen, L.; Yin, Y.P.; Tan, W.Y.; Shao, C.W.; Xu, J.; Wang, Z.K. Role of two Nomuraea rileyi transmembrane sensors sho1p and sln1p in adaptation to stress due to changing culture conditions during microsclerotia development. World J. Microbiol. Biotechnol. 2015, 31, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Zhong, Q.; Yin, Y.P.; Shen, L.; Li, Y.; Wang, Z.K. The high osmotic response and cell wall integrity pathways cooperate to regulate morphology, microsclerotia development, and virulence in Metarhizium rileyi. Sci. Rep. 2016, 6, 38765. [Google Scholar] [CrossRef] [PubMed]
- Polidoros, A.N.; Mylona, P.V.; Scandalios, J.G. Transgenic tobacco plants expressing the maize Cat2 gene have altered catalase levels that affect plant–pathogen interactions and resistance to oxidative stress. Transgenic Res. 2001, 10, 555–569. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases SODs in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Schouten, A.; Tenberge, K.B.; Vermeer, J.; Stewart, J.; Wagemakers, L.; Williamson, B.; van Kan, J.A. Functional analysis of an extracellular catalase of Botrytis cinerea. Mol. Plant Pathol. 2002, 3, 227–238. [Google Scholar] [CrossRef]
- Kwok, L.Y.; Schlüter, D.; Clayton, C.; Clayton, C.; Soldati, D. The antioxidant system in Toxoplasma gondii and the role of cytosolic catalase in defense against oxidative injury. Mol. Microbiol. 2004, 51, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Zhang, L.B.; Ying, S.H.; Feng, M.G. Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ. Microbiol. 2013, 152, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Morales Hernandez, C.E.; Padilla Guerrero, I.E.; Gonzalez Hernandez, G.A.; Salazar Solis, E.; Torres Guzman, J.C. Catalase overexpression reduces the germination time and increases the pathogenicity of the fungus Metarhizium anisopliae. Appl. Microbiol. Biotechnol. 2010, 87, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Y.; Yoshida, Y.; Hasunuma, K. Loss of Catalase-1 (Cat-1) results in decreased conidial viability enhanced by exposure to light in Neurospora crassa. Mol. Genet. Genom. 2007, 277, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Michán, S.; Lledías, F.; Hansberg, W. Asexual development is increased in Neurospora crassa cat-3-null mutant strains. Eukaryot. Cell 2003, 2, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Hisada, H.; Hata, Y.; Kawato, A.; Abe, Y.; Akita, O. Cloning and expression analysis of two catalase genes from Aspergillus oryzae. J. Biosci. Bioeng. 2005, 99, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Garre, V.; Tenberge, K.B.; Eising, R. Secretion of a fungal extracellular catalase by Claviceps purpurea during infection of rye: Putative role in pathogenicity and suppression of host defense. Phytopathology 1998, 88, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, N.; Juárez, M.P.; Crespo, R.; de Alaniz, M.J. Clues on the role of Beauveria bassiana catalases in alkane degradation events. Mycologia 2006, 98, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, Z.K.; Shao, C.W.; Luo, Y.L.; Wang, L.; Yin, Y.P. An efficient gene disruption method using a positive-negative split-selection marker and Agrobacterium tumefaciens-mediated transformation for Nomuraea rileyi. World J. Microbiol. Biotechnol. 2018, 34, 26. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, S.F.; Li, J.J.; Zheng, B.; Bao, M.Z.; Ning, G.G. Fusion primer and nested integrated PCR FPNI-PCR: A new high-efficiency strategy for rapid chromosome walking or flanking sequence cloning. BMC Biotechnol. 2011, 11, 109. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Jin, K.; Ying, S.H.; Zhang, Y.J.; Xiao, G.H.; Shang, Y.F.; Duan, Z.B.; Hu, X.; Xie, X.Q.; Zhou, G.; et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Z.G.; Lin, R.M.; Li, E.F.; Mao, Z.C.; Ling, J.; Yang, Y.H.; Yin, W.B.; Xie, B.Y. Biosynthesis of Antibiotic Leucinostatins in Bio-control Fungus Purpureocillium lilacinum and Their Inhibition on Phytophthora Revealed by Genome Mining. PLoS Pathog. 2016, 12, e1005685. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.S.; Stajich, J.E.; Nichols, C.; Gerrald, Q.D.; Alspaugh, J.A.; Dietrich, F.; Perfect, J.R. The Cryptococcus neoformans catalase gene family and its role in antioxidant defense. Eukaryot. Cell 2006, 5, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Skamnioti, P.; Henderson, C.; Zhang, Z.; Robinson, Z.; Gurr, S.J. A novel role for catalase B in the maintenance of fungal cell-wall integrity during host invasion in the rice blast fungus Magnaporthe grisea. Mol. Plant-Microbe Interact. 2007, 20, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.D.; Petropoulou, K.P. The role of ascorbic acid role in the differentiation of sclerotia in sclerotinia minor. Mycopathologia 2002, 154, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.D.; Zees, A. Lipofuscins and sclerotial differentiation in phytopathogenic fungi. Mycopathologia 2002, 153, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.D.; Patsoukis, N.; Papapostolou, I.; Zervoudakis, G. Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr. Comp. Biol. 2006, 46, 691–712. [Google Scholar] [CrossRef]
- Patsoukis, N.; Georgiou, C.D. Thiol redox state and oxidative stress affect sclerotial differentiation of the phytopathogenic fungi sclerotium rolfsii and sclerotinia sclerotiorum. J. Appl. Microbiol. 2008, 104, 42–50. [Google Scholar] [CrossRef]
- Hattori, T.; Honda, Y.; Kino, K.; Kirimura, K. Expression of alternative oxidase gene (aox1) at the stage of single-cell conidium in citric acid-producing Aspergillus niger. J. Biosci. Bioeng. 2008, 105, 55–57. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Stan, M.; Murphy, M.P.; Sherman, F.; Oppenheim, F.G. The concomitant expression and availability of conventional and alternative, cyanide-insensitive, respiratory pathways in Candida albicans. Mitochondrion 2005, 5, 200–211. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Wang, X.; Luo, Y.; Jiang, H.; Tang, G.; Liu, H. The Catalase Gene MrCat1 Contributes to Oxidative Stress Tolerance, Microsclerotia Formation, and Virulence in the Entomopathogenic Fungus Metarhizium rileyi. J. Fungi 2024, 10, 543. https://doi.org/10.3390/jof10080543
Su Y, Wang X, Luo Y, Jiang H, Tang G, Liu H. The Catalase Gene MrCat1 Contributes to Oxidative Stress Tolerance, Microsclerotia Formation, and Virulence in the Entomopathogenic Fungus Metarhizium rileyi. Journal of Fungi. 2024; 10(8):543. https://doi.org/10.3390/jof10080543
Chicago/Turabian StyleSu, Yu, Xuyi Wang, Yuanli Luo, Huan Jiang, Guiting Tang, and Huai Liu. 2024. "The Catalase Gene MrCat1 Contributes to Oxidative Stress Tolerance, Microsclerotia Formation, and Virulence in the Entomopathogenic Fungus Metarhizium rileyi" Journal of Fungi 10, no. 8: 543. https://doi.org/10.3390/jof10080543
APA StyleSu, Y., Wang, X., Luo, Y., Jiang, H., Tang, G., & Liu, H. (2024). The Catalase Gene MrCat1 Contributes to Oxidative Stress Tolerance, Microsclerotia Formation, and Virulence in the Entomopathogenic Fungus Metarhizium rileyi. Journal of Fungi, 10(8), 543. https://doi.org/10.3390/jof10080543






