Abstract
The role of calcium, an essential secondary messenger, in cell division remains an outstanding question in cell biology despite several significant findings over the past few decades. Among them is the landmark discovery of intracellular calcium waves during cytokinesis, the last stage of cell division, in fish cells. Nevertheless, subsequent studies have been largely unable to determine the underlying molecular mechanism of these cytokinetic transients. At the center of this stalemate stands two challenging questions, how these calcium transients rise and what they do during cytokinesis. Yeast, despite its proven prowess as a model organism to study cell cycle, has not drawn much interest in addressing these questions. However, the recent discovery of cytokinetic calcium spikes in the fission yeast Schizosaccharomyces pombe has provided novel insights into how calcium regulates cytokinesis. In this review, I will primarily focus on our current understanding of the molecular mechanism of cytokinetic calcium transients in yeast cells. First, I will briefly recount the discovery of cytokinetic calcium transients in animal cells. This will be followed by an introduction to the intracellular calcium homeostasis. Next, I will discuss yeast cytokinetic calcium spikes, the ion channel Pkd2 that promotes these spikes, and the potential molecular targets of these spikes. I will also compare the calcium regulation of cytokinesis between yeast and animal cells. I will conclude by presenting a few critical questions in our continued quest to understand how calcium regulates cytokinesis.
1. Introduction
Almost thirty years have passed since the landmark discovery of cytokinetic calcium (Ca2+) waves in Japanese rice fish (Oryzias latipes) cells [1], but the underlying molecular mechanism remains very much unknown. It has long been known that Ca2+ is essential to cytokinesis [2]. However, the question of why was never answered. By injecting a fluorescent Ca2+ reporter protein aequorin into the fish eggs [1], the group of Lionel Jaffe revealed for the first time that the Ca2+ level increases dramatically in a few seconds at the ingressing cleavage furrow. Such Ca2+ transients then quickly spread towards the poles of the dividing eggs. Therefore, the authors named them Ca2+ waves. This is the first demonstration that the last stage of cell division cytokinesis is concurrent with the rise and fall of intracellular Ca2+ transients. Subsequent studies by many other groups confirmed the existence of similar cytokinetic Ca2+ transients in either the oocytes or eggs of animals including zebrafish (Danio rerio) and African clawed frog (Xenopus laevis) [3,4] (for a review of calcium transients in animal cells, see [5]). The discovery of these Ca2+ transients firmly established a link between Ca2+ and cytokinesis in animal eggs and embryos.
These earlier discoveries generated both excitement and many unanswered questions. The chief ones are what role these Ca2+ waves play in cytokinesis and how they are regulated. Between the two, the first question appears easier to answer. Two groups, including that of Lionel Jaffe, worked independently by injecting Ca2+ chelators into dividing cells [3,6]. They found that both cleavage furrow ingression and the following cell separation are inhibited by the chelators. This would have settled the first question. However, another research group, led by Issei Mabuchi, made a starkly opposite finding that the Ca2+ chelators had no effect on either the cleavage furrow ingression or the daughter cell separation of frog eggs [7]. They concluded that cytokinetic Ca2+ waves have no significant role in cytokinesis. These contradicting results raised the question of whether there is a causal relationship between Ca2+ waves and cytokinesis. This controversy likely dented enthusiasm towards the study of these cytokinetic Ca2+ waves, partially contributing to a prolonged stalemate in determining their molecular mechanisms. Therefore, three decades after the discovery of these Ca2+ transients, their origin and cellular function have remained largely unknown.
The interest in this decades-old question is rekindled by the recent surprising discovery of cytokinetic Ca2+ spikes in the unicellular model organism S. pombe. In this review, I will focus on what we have learned about the regulation of fission yeast cytokinesis by Ca2+. I will start by summarizing our current understanding of the molecular mechanism of cytokinesis and Ca2+ homeostasis. This will be followed by a discussion of the newly discovered cytokinetic Ca2+ spikes. I will then review the findings demonstrating how a putative mechanosensitive cation channel Pkd2 promotes Ca2+ spikes in cytokinesis. Then, I will discuss the potential targets of these spikes including the essential Ca2+-binding molecules calmodulin and calcineurin. Lastly, I will discuss the challenges and the future directions for us to fully understand the role of Ca2+ in cytokinesis.
2. Molecular Mechanism of Cytokinesis
Cytokinesis is the last stage of cell division. During this stage, the cytoplasm of daughter cells separate from each other, following the separation of chromosomes during mitosis. Cytokinesis failure can lead to polyploidy and is associated with many human diseases including cancers [8]. As an essential cellular process, cytokinesis has been extensively studied since the early days of cell biology. In particular, over the last three decades, we have made substantial advances in understanding the molecular mechanism of cytokinesis. These studies have revealed that cytokinesis is highly conserved in almost all eukaryotic organisms including yeast, social amoebae, and animals (for a review, see [9]).
Like many eukaryotic cells, fission yeast cytokinesis can be divided into four steps (for a review, see [10]). The first is the selection of the equatorial division plane, a process largely depending on the nucleus [11]. This is followed by the assembly of the actomyosin contractile ring at the division plane. This actin-centric process requires many actin cytoskeletal proteins including the motor protein type II myosins, the actin nucleator formin, and the actin filament-severing protein cofilin [12,13,14]. The third step is the ingression of the cleavage furrow, driven by mechanical force, jointly produced by the contractile ring, the septum, and turgor pressure [15]. The last step is the separation of daughter cells. Fission yeast cell separation relies on the assembly of the septum, a process regulated by the septation initiation network (SIN) pathway (for a review, see [16]).
The primary advantages of using fission yeast to study the molecular mechanism of cytokinesis are two. First, yeast genetics is a powerful tool to study cytokinesis. It has helped to discover many essential genes required for cytokinesis (for a review, see [16]). Most of these genes are conserved in animal cells. The second advantage is the application of quantitative microscopy. The method, not yet available in most other model organisms, has allowed precise measurement of molecular number of proteins in live fission yeast cells [17]. Better yet, it can be combined with computer-assisted image analysis to quantify thousands of cells all at once [18]. However, compared to other model organisms such as C. elegans (round worm) and D. melanogaster (fruit fly), the method of Ca2+ imaging remains under-developed in yeast. Despite our extensive understanding of the mechanism of cytokinesis, many questions regarding this essential cellular process remain open [19]. Among them, one of the most important ones is the role of Ca2+ in cytokinesis.
3. Ca2+ Homeostasis of Eukaryotic Cells
Ca2+ is one of the most important secondary messengers in living organisms. Due to the importance of Ca2+ in signaling, the intracellular [Ca2+] of eukaryotic cells is tightly regulated. In the cytoplasm, [Ca2+] remains relatively low, in the nanomolar range. In comparison, several intracellular organelles including the endoplasmic reticulum (ER) and lysosomes maintain much higher [Ca2+]. Extracellular [Ca2+] is also higher, in the micromolar range. As a result of this asymmetric distribution of Ca2+, a transient increase in cytoplasmic Ca2+ level will trigger downstream signaling pathways in diverse cellular processes, including exocytosis, muscle contractility, and neurotransmission.
Like animal cells, yeast cells keep their cytoplasmic [Ca2+] much lower than that of the extracellular environment. The [Ca2+] in the fission yeast cytoplasm is about 100 nM [20]. Like most eukaryotic cells, yeast maintains much higher [Ca2+] in two intracellular organelles, the ER and the lysosome equivalent of vacuoles. In budding yeast cells, the ER [Ca2+] is ~10 µM, and the vacuolar [Ca2+] is ~2 mM [21].
A large number of Ca2+ channels, transporters, and pumps help maintain the homeostasis of Ca2+ in a eukaryotic cell (for a review, see [22]). The channels permeate Ca2+ in the direction of the ion gradient. It does not require any energy. A well-studied example are the voltage-gated Ca2+ channels (VGCCs) found in animal cells (for a review, see [23]). Fission yeast possesses only one putative VGCC [24]. Cch1 is the presumed α-subunit of this channel and Yam8 is projected to be the β-subunit [25]. Compared to a channel, a transporter couples the flow of Ca2+ to that of another ion. For example, Ca2+-sodium exchangers (NCXs) remove Ca2+ from the cytoplasm by coupling it to the flow of Na+. Budding yeast possesses one putative NCX in Ycx1 [26], but no such transporter has been identified yet in fission yeast. Lastly, a Ca2+-ATPase moves Ca2+ against the ion gradient by utilizing the energy produced by ATP hydrolysis. Fission yeast possesses several Ca2+-ATPases in intracellular organelles including the ER-specific Pmr1 [27] and Cta4 [28], the vacuolar pump Pmc1 [27], and the Golgi-localized Cta5 [29]. Overall, yeast cells maintain similar intracellular Ca2+ homeostasis to that of animal cells.
4. Cytokinetic Ca2+ Spikes in Yeast
Similar to animal cells, yeast requires Ca2+ for cell division [30], but the existence of cytokinetic Ca2+ transients in yeast was not known until recently. In the past, organic Ca2+-sensitive dyes, such as Fura-2 developed by the group of Roger Tsien, were commonly used to detect Ca2+ in live cells [31]. However, these dyes do not usually penetrate either the cell wall or the plasma membrane of yeast efficiently. Injection, a commonly used strategy in large animal cells, unfortunately is not applicable to yeast. The technical advance that helped uncover cytokinetic Ca2+ transients in fission yeast came from an unexpected source, the study of neurons. Over the last decade, genetically encoded Ca2+ indicators, in particular GCaMP [32], have revolutionized the study of Ca2+ transients by neuroscientists. This reporter consists of the permuted Green Fluorescence Protein (GFP), calmodulin, and the M13 peptide from myosin light chain kinase (MLCK) [33]. It has been widely used to detect Ca2+ transients among excitable neuronal cells. The newest edition jGCaMP8 has even achieved temporal precision and sensitivity, comparable to the traditional electrophysical recording in neuronal cells [34]. However, its application in the detection of Ca2+ transients in non-excitable cells, particularly in yeast, remains limited.
To investigate Ca2+ transients in fission yeast cells, our lab has developed a novel Ca2+ imaging approach by combining GCaMP with quantitative microscopy. Poddar et al. first integrated the GCaMP coding sequence into the genome of fission yeast [35]. This reporter detects the Ca2+ transients triggered by sudden downshift of extracellular osmolarity. To our knowledge, this is the first demonstration of intracellular Ca2+ transients in this widely used unicellular model organism. To determine whether intracellular [Ca2+] rises during cytokinesis, Poddar et al. took two complementary approaches. First, they examined the correlation between intracellular Ca2+ and cell-cycle stages in an asynchronized population. Secondly, they employed time-lapse fluorescence microscopy to measure [Ca2+] in the dividing cells. Both methods detect a dramatic increase in intracellular [Ca2+] during cell division.
Two Ca2+ spikes rise during fission yeast cytokinesis (Figure 1). They are named “Constriction spikes” and “Separation spikes”, respectively. As the name implies, the former accompanies cleavage furrow ingression and the concurrent contractile ring constriction. Interestingly, this wave of Ca2+ spikes remains active throughout the duration of cleavage furrow ingression for ~30 min. This is followed by the second Ca2+ spikes during daughter cell separation (Figure 1). Surprisingly, in ~40% of dividing cells, the spikes rise asymmetrically, appearing in two daughter cells sequentially. The asymmetry of fission yeast cytokinesis [36] may have played a role in this asymmetry of Separation spikes.
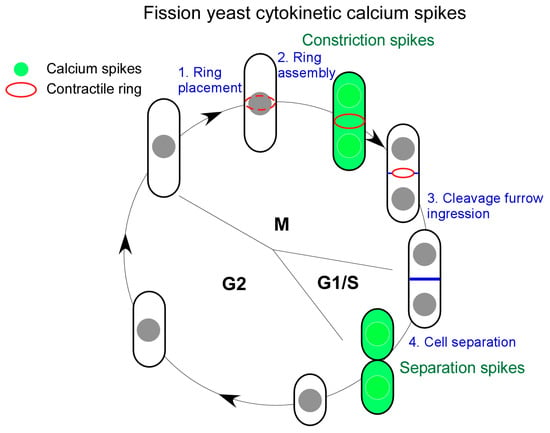
Figure 1.
Cytokinetic Ca2+ spikes of fission yeast cells. Fission yeast cytokinesis, in four consecutive steps (blue), is accompanied by two distinct Ca2+ spikes (green) during both M and G1/S phases of cell cycle. “Constriction spikes” rise at the start of the contractile ring constriction. In contrast, “Separation spikes” appear at the end of cytokinesis when two daughter cells separate.
5. Regulatory Mechanism of Cytokinetic Ca2+ Spikes
The discovery of cytokinetic Ca2+ spikes in fission yeast cells leads to a similar question raised by the study of Fluck et al. [1] more than three decades ago, where Ca2+ comes from. There are two likely sources, one through the influx of Ca2+ from the extracellular environment and the other through efflux from the intracellular organelles. Both may have contributed to the cytokinetic Ca2+ waves of animal cells [5,37].
Surprisingly, fission yeast cells employ a putative force-sensitive ion channel Pkd2 to promote Ca2+ influx during cytokinesis (Figure 2). Pkd2 is the ancient homologue of the animal polycystin channel [38]. Two such channels, PC1 and PC2, can be found in animal cells, including humans. Mutations of human polycystin genes, Pkd1 or Pkd2, lead to one of the most common human genetic disorders, Autosomal Polycystic Kidney Disorder (ADPKD) (for reviews, see [39,40]). Fission yeast Pkd2 is an essential protein localized on the plasma membrane throughout the cell cycle [41,42,43]. During interphase, Pkd2 is enriched at the cell tips and the plasma membrane invagination sites called eisosomes [44]. It is frequently internalized from the plasma membrane through the endocytic pathway, depending on its C-terminal cytoplasmic tail, like many G-protein-coupled receptors [44]. Pkd2 regulates the fission yeast turgor pressure required for the cell size expansion during interphase [45]. During cytokinesis, this putative channel is recruited to the equatorial division plane at the time of cleavage furrow ingression.
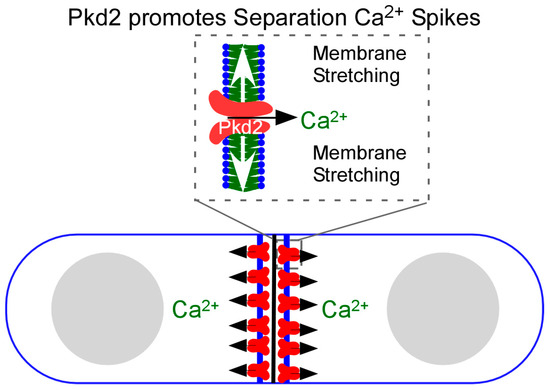
Figure 2.
Pkd2 promotes Ca2+ influx during the daughter cell separation. Pkd2 is recruited to the plasma membrane at the equatorial division plane during cytokinesis. The membrane expansion during the cell separation is presumed to activate this mechanosensitive channel through stretching the plasma membrane at the division plane. This channel then promotes Ca2+ influx, leading to the Separation spikes.
Fission yeast Pkd2 is essential for the last step of cytokinesis, daughter cell separation. Its depletion leads to much slower separation of daughter cells or a complete failure. Fittingly, Pkd2 contributes significantly to the Separation Ca2+ spikes [46]. Without it, the second wave of cytokinetic Ca2+ transients largely disappears. This ion channel is likely activated through the plasma membrane stretching produced by the cytokinetic machinery (Figure 2). Membrane stretching activates the in vitro reconstituted Pkd2 in giant unilamellar vesicles (GUVs) to allow the passage of Ca2+ through this channel. To our knowledge, Pkd2 is the only known yeast Ca2+ channel that contributes to cytokinetic Ca2+ spikes thus far.
In addition to Pkd2, fission yeast possesses other mechanosensitive ion channels that may contribute to cytokinetic Ca2+ spikes. Msy1 and Msy2 are the homologues of the bacterial MscS channel [47]. They provide the essential Ca2+ spikes required for adaption to hypo-osmotic shocks of yeast cells [48]. These transients promote lipid transfer from the ER to the plasma membrane, preventing potential membrane rupture [49]. Trp1322 and Trp633 are another two potential mechanosensitive channels [50]. Their predicted tertiary structures are similar to that of Pkd2, but they localize to the plasma membrane and the intracellular membrane structures, respectively [44]. The function of these mechanosensitive channels in cytokinesis remains to be elucidated.
6. The Effectors of Cytokinetic Ca2+ Spikes
The function of cytokinetic Ca2+ transients has long been debated. On one side, many researchers [1] argued that these intracellular Ca2+ transients promote cleavage furrow ingression. The transients could activate contraction of the actomyosin contractile ring and promote membrane trafficking at the cleavage furrow [5]. This hypothesis is based on the observations that chelation of intracellular Ca2+ during cytokinesis inhibits cleavage furrow ingression [3,6,51]. Such a model fits the long-standing theory that the constriction of cytokinetic contractile ring, like muscle contraction, is triggered by Ca2+. In skeletal muscle cells, Ca2+ activates the type II muscle myosin through myosin light chain kinase (MLCK) and troponin to initiate actomyosin contractility (for a review, see [52]). However, this model has been controversial [7]. To identify the effects of cytokinetic Ca2+ transients, an approach combining quantitative cell biology and genetics is direly needed.
Fission yeast provides a strong platform for analyzing the importance of Ca2+ transients in cytokinesis quantitatively. Both the temporal and spatial regulation of fission yeast cytokinesis have been carefully measured [17]. The molecular numbers of many components of the cytokinetic machinery, including actin, myosin II, and formins, have been measured through the pioneering method of quantitative microscopy [18,53]. Its cytokinesis has even been simulated in silico using a computation model, the first among all model organisms [54]. Taking advantage of these quantitative tools, Poddar et al. identified two critical roles of cytokinetic Ca2+ spikes. One is to promote cleavage furrow ingression and the other is to safeguard daughter integrity during cell separation [35]. The study of fission yeast thus demonstrates clearly that these transients are crucial for both temporal regulation and the robustness of cytokinesis.
Yeast genetics provide another powerful tool in determining the potential targets of cytokinetic Ca2+ spikes. Decades of genetic studies have generated hundreds of either deletion or conditional mutants of the genes that are important to cytokinesis. A deletion library of more than 3000 non-essential fission yeast genes is also available commercially [55]. All these mutants can be leveraged to identify the molecules activated by a sudden increase in [Ca2+] either directly or indirectly during cytokinesis. The former includes calmodulin (CaM), calcineurin (CaN), and Ca2+/CaM-sensitive kinases (CaMK). The latter includes many other CaM-binding proteins including motor protein myosins and ion channels. Here, I will discuss some of the most likely targets of the cytokinetic Ca2+ spikes (Figure 3).
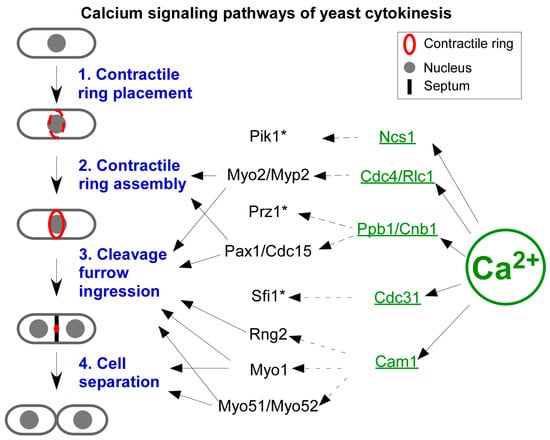
Figure 3.
A model of the Ca2+ signaling pathways in fission yeast cytokinesis. Ca2+ directly binds and activates five EF-hand proteins (green) including Ncs1, the type II myosin light chains Cdc4 and Rlc1, the calcineurin Ppb1 and Cnb1, the centrin Cdc31, and the calmodulin Cam1. By interacting with a wide range of targets, these Ca2+ sensors promote the last three steps (blue) of cytokinesis, including contractile ring assembly, cleavage furrow ingression, which is coupled to contractile ring constriction, and cell separation, which is concurrent with septation. *: These proteins have no clear role in cytokinesis.
CaM is an evolutionarily conserved Ca2+-binding protein, found in almost all eukaryotes including fungi, amoeba, plants, and animals (for a review of budding yeast calmodulin, see [56]). Fission yeast CaM Cam1 is an essential protein [57] (Figure 3). Like other calmodulins, it contains four EF-hand motifs, each of which can bind one Ca2+ [58]. Cam1 requires Ca2+ for its essential functions [59]. This small protein distributes between two subcellular structures. First, Cam1 is a constitutive component of spindle pole bodies (SPBs) [59], the yeast equivalent of centrosomes, where it interacts with the pericentrin homologue Pcp1 [60,61]. Secondly, Cam1 is in endocytic actin patches as the regulatory light chain for the type I myosin Myo1 [62,63]. However, it remains unknown whether the interaction between Cam1 and Myo1 depends on Ca2+ [64]. In addition to Cam1, fission yeast also possesses a second camodulin-like protein. However, Cam2 does not bind Ca2+ directly [65]. Cam2 localizes to endocytic actin patches, but it is absent from SPBs. It remains unclear what the Ca2+-dependent functions of Cam1 are during cytokinesis.
The second likely target of Ca2+ spikes is calcineurin (CaN), the evolutionarily conserved Ca2+-, and CaM-sensitive phosphatase (for a review, see [66]). CaN consists of one regulatory and one catalytic subunit (Figure 3). The former is a CaM-like protein, whose four EF-hand motifs can each bind one Ca2+. The latter contains the catalytic core of this phosphatase, whose activity is auto-inhibited by its own C-terminal inhibitory domain. CaN only becomes active when it binds both Ca2+ and CaM. The fission yeast homologues of CaN regulatory and catalytic subunits are Cnb1 and Ppb1, respectively [67]. Neither is an essential gene, but both play critical roles in cell proliferation. During cytokinesis, Ppb1 highly concentrates at the contractile ring [68,69]. There, it interacts with two essential components of the ring, the paxillin Pxl1 and the Fes/Cip4 homology Bin/amphiphysin/Rvs (F-BAR) protein Cdc15 [68]. As a part of the contractile ring, Ppb1 promotes the proper placement of the contractile ring, the ring constriction, and the daughter cell separation [67]. One way that Ppb1 carries out its function is through dephosphorylating Cdc15 to stabilize this membrane-sculpturing protein [68]. In addition to Cdc15, Ppb1 also dephosphorylates the transcription factor Prz1 [70], whose function during cytokinesis remains unclear (Figure 3). Future work will need to determine whether any of the above-described functions of CaN depend on cytokinetic Ca2+ spikes.
Other potential targets of cytokinetic Ca2+ spikes include the PKA pathway, the centrin-like protein Cdc31, myosin light chains, the Ca2+ sensor Ncs1, and Ca2+/CaM-dependent kinases (Figure 3). Ca2+ regulates the association between the PKA pathway kinase Pka1 and its regulatory subunit Cgs1 [71]. Cdc31 is a CaM-like protein with four EF-hands. It is an essential component of SPBs [72]. Similarly, both the regulatory and essential light chains of yeast myosin II, Rlc1, and Cdc4, respectively, are CaM-like proteins. Both possess four EF-hands that are capable of binding Ca2+ directly [73,74]. Ncs1 is another CaM-like protein that binds Ca2+ through its EF-hands [75]. It promotes the activity of phosphatidylinositol-4 kinase Pik1 at the Golgi apparatus, similar to its budding yeast homologue Frq1 [76,77]. Like budding yeast, fission yeast also possesses two putative Ca2+/CaM-sensitive kinases Cmk1 and Cmk2 [78,79,80,81]. Further studies will need to determine what roles of these potential Ca2+ spikes’ targets play during cytokinesis.
7. Comparison of Ca2+ Homeostasis and Signaling Between Two Yeasts
Although budding yeast S. cerevisiae and fission yeast S. pombe are separated by more than 300 million years of evolution [82], these two model organisms share many similarities in their regulation of Ca2+ homeostasis and signaling. Both utilized a small number of ion channels and Ca2+-ATPases (Table 1) to maintain comparably low [Ca2+] in the cytoplasm. For example, both possess a single VGCC in Cch1 and its regulatory subunit Mid1 (budding yeast)/Yam8 (fission yeast). Both yeasts use a vacuolar Ca2+ pump Pmc1 and an ER/Golgi pump in Pmr1. In response to hypo-osmotic shocks, both yeasts trigger intracellular Ca2+ spikes and the CaN-Prz1 pathway [35,50,83]. CaM of both yeasts targets the pericentrin-like protein in SPBs during mitosis [60,84]. In both, CaN activates a Zinc finger transcription factor Crz1 (budding yeast)/Prz1 (fission yeast) through dephosphorylation under stress conditions [70,85].
One of the most striking differences between budding and fission yeast is in CaM. In budding yeast, Cmd1 can only bind three Ca2+ ions maximally. Its capacity for Ca2+-binding is not essential for budding yeast [86]. In contrast, fission yeast CaM can bind four Ca2+ ions simultaneously. Its ability to bind Ca2+ is indispensable for fission yeast viability [58]. Therefore, only fission yeast Cam1 needs Ca2+ for its essential functions.
There are other differences between these two yeasts (Table 1). Fission yeast possesses two homologues of the bacterial mechanosensitive ion channel MscS, Msy1 and Msy2, which are absent in budding yeast (Table 1). Although budding yeast uses a Transient Receptor Potential (TRP) channel Yvc1 to promote Ca2+ efflux at the vacuoles [87,88], no such vacuolar channel has been found in fission yeast yet. Budding yeast VGCCs, consisting of Mid1, Cch1, and Ecm7, are essential for Ca2+ signaling during mating [89,90]. The ER-localized putative Ca2+ channel Csg2 promotes autophagy in budding yeast cells [91], but no such ER Ca2+ channel has been identified yet in fission yeast. Budding yeast Ca2+ homeostasis has been examined using the genetically encoded Ca2+ reporters of either aequorin [92] or GCaMP [93]. Furthermore, a computation model has been constructed to simulate Ca2+ homeostasis in S. cerevisiae cells [94]. However, no cytokinetic Ca2+ spikes have yet been identified in this yeast.

Table 1.
Comparison of Ca2+ homeostasis and signaling between two yeasts.
Table 1.
Comparison of Ca2+ homeostasis and signaling between two yeasts.
| S. cerevisiae | S. pombe | ||
|---|---|---|---|
| Ca2+ Channels | Voltage-gated | Cch1+Mid1+Ecm7 [24,95] | Cch1+Yam8 [50,96] |
| TRP family | Yvc1 [87] | None identified | |
| Mechanosensitive Pkd2 family | Flc1, Flc2, Flc3, and YOR365C [83,97] | Pkd2, Trp1322, and Trp663 [44] | |
| Mechanosensitive MscS-like | None identified | Msy1 and Msy2 [47] | |
| Others | Csg2 [91] | None identified | |
| Ca2+-ATPases | ER | Spf1 [98] | Pmr1 and Cta4 [27,28] |
| Golgi | Pmr1 [21] | None identified | |
| Vacuolar | Pmc1 [99] | Pmc1 [27] | |
| Ca2+-binding EF-hand proteins | Calmodulin | Cmd1 [100] | Cam1 [57] |
| Calcineurin | Cna1/Cna2+Cnb1 [101] | Ppb1+Cnb1 [67,102] | |
| Centrin | Cdc31 [103] | Cdc31 [72] | |
| Myosin light chains | Mlc1 and Mlc2 [104,105] | Cdc4 and Rlc1 [73,74] | |
| Neuronal Ca2+ sensor | Frq1 [77] | Ncs1 [75] |
8. Comparison Between Regulation of Cytokinesis by Ca2+ in Yeast and Animal Cells
The discovery of cytokinetic spikes in fission yeast cells naturally invites a comparison to the Ca2+ waves discovered earlier in animal cells. In both, cytokinesis is accompanied by two waves of Ca2+ transients (Table 2). In addition to amphibian or fish embryonic cells, mammalian embryonic cells also trigger Ca2+ waves during their cytokinesis [106]. In animal cells, the first wave of transients coincides with the start of cleavage furrow ingression. This so-called “Furrowing wave” originates at the equatorial division plane, exactly where the membrane starts to ingress [1,6]. It then spreads towards the cell pole at a rate of ~4 µm/min, a comparably slow one among Ca2+ waves [107]. The second wave of the transients accompanies the separation of daughter cells, the final step of cytokinesis [1,35]. It was thus aptly named the “Zipping wave” due to the zipping motion of the two separated cells. There are striking similarities between the temporal regulation of cytokinetic Ca2+ transients in yeast and that in animal cells.
Nevertheless, there are some critical differences between Ca2+ transients of yeast and those of animal cells (Table 2). First is their respective timescale. Yeast Ca2+ spikes, lasting up to three minutes, are much longer-lived than the Ca2+ transients discovered in embryonic cells, which have a lifetime of just a few seconds [1,4]. Second is their vastly different spatial distribution. Yeast Ca2+ spikes are global in their intracellular distribution [35]. In contrast, Ca2+ transients of the much bigger animal cells rise locally at the equatorial division plane, before spreading throughout the division cells. The spatial distribution of Ca2+ transients may be due to the different sizes of these two systems.
Animal and yeast cells also differ in their respective regulatory mechanism of these cytokinetic Ca2+ transients (Table 2). The Ca2+ influx of animal cells contributes only partially to the cytokinetic Ca2+ waves [108]. This is mediated through the store-operated Ca2+ entry (SOCE) system consisting of the plasma membrane-localized ORAI channel and its interacting partner STIM at the ER (for reviews, see [109,110]). Thus far, no SOCE has been found in yeast [111]. In comparison, yeast cells depend on the mechanosensitive channel Pkd2 to mediate Ca2+ influx during cytokinesis. Animal cells possess two polycystin homologues in PC1 and PC2. Like yeast Pkd2, the animal polycystin PC2 is a mechanosensitive channel [112,113]. However, the role of polycystins in animal cell cytokinesis remains unknown. The animal cells also mobilize intracellular stored Ca2+ from the ER for the transients. The ER Ca2+ channel inositol-1,4,5-triphosphate (IP3) receptor promotes cytokinetic Ca2+ transients [4,114]. In contrast, the role of Ca2+ efflux during yeast cytokinesis remains unknown.

Table 2.
Comparison of cytokinetic Ca2+ transients between animal and yeast cells.
Table 2.
Comparison of cytokinetic Ca2+ transients between animal and yeast cells.
| Animal | Yeast | |
|---|---|---|
| Cytokinetic Ca2+ transients | Furrowing wave Zipping wave [1] | Constriction spike Separation spike [35] |
| Efflux channel promoting Ca2+ transients | IP3 receptor [37] | None identified |
| Influx channel promoting Ca2+ transients | ORAI [108] | Pkd2 [46] |
| Potential targets of Ca2+ transients | Calmodulin [115] | Cam1 [58] |
| Calcineurin [116] | Ppb1+Cnb1 [67,117] | |
| MLCK [118] | None identified | |
| CaMKII | Cmk1 and Cmk2 | |
| Annexin [119] | None identified | |
| VAMP2 [120] | None identified |
Animal and yeast cells also differ significantly on the targets of their Ca2+ cytokinetic transients (Table 2), although exocytosis is likely targeted by Ca2+ transients in both. Most animal cells including fruit flies utilize the Ca2+/camodulin-sensitive myosin light chain kinase (MLCK) to trigger actomyosin contractility during cytokinesis [118]. In comparison, yeast cells do not possess any MLCK homologue. In animal cells, CaM promotes the assembly of the central spindle, which is essential for animal cell cytokinesis [121]. CaMKII (Ca2+/calmodulin-dependent kinase II), a potential target of Ca2+ transients, activates the small GTPases RhoA and Cdc42 to re-organize the actin cytoskeleton during animal cell cytokinesis [115]. In comparison, yeast CaMKII homologues, Cmk1 and Cmk2, have no clear role in cytokinesis. In animal cells, Ca2+-sensitive proteins such as VAMP2 and annexin also contribute to cytokinesis [119,122], but their homologues in yeast cytokinesis have not been determined. Overall, many potential targets of cytokinetic Ca2+ transients remain to be identified in either fission yeast or animal cells.
9. Conclusions and Open Questions
Many exciting questions remain open in our understanding of the Ca2+ transients accompanying cytokinesis. Some of them require improved technology, while others demand more conceptual advances. Here, I will list a few of the most important ones.
The main challenge in determining the role of Ca2+ in yeast cytokinesis is our lack of understanding of Ca2+ homeostasis in fission yeast. To start, we do not know how fission yeast cells maintain cytoplasmic Ca2+ concentration. We have yet to determine whether Ca2+ efflux from either the ER or vacuoles contributes to such homeostasis. Adding to the mystery is that no ER- or vacuole-specific efflux channels have been identified in fission yeast. Organelle-specific Ca2+ reporters will help us to measure Ca2+ efflux in yeast cells.
Another challenge is to identify the ion channels required for cytokinetic Ca2+ spikes. To our knowledge, Pkd2 is the only known channel that plays a clear role in promoting cytokinetic Ca2+ transients. It remains to be determined whether any other yeast channels, such as the putative voltage-gated channel Cch1 and the putative mechanosensitive channels Msy1 and Msy2 [47,48], contribute to the spikes. More work is also needed to identify novel fission yeast ion channels through a combination of yeast genetics, Ca2+ imaging, and electrophysiology.
Several questions remain unanswered about the molecular role of Pkd2 in cytokinesis. First, it remains unknown whether Pkd2 is a non-selective cation channel. The human homologue PC2 is permissive to both K+ and Na+, in addition to Ca2+ [123]. Secondly, the atomic structure of the Pkd2 channel, likely in an oligomeric form, remains largely unknown, despite the monomeric structure predicted by AlphaFold [44]. The human Pkd2 homologues PC-1 and PC-2 can form either a homo-tetramer or a hetero-tetramer channel [124,125]. Lastly, it remains to be determined whether Pkd2 does more than promote Ca2+ spikes during cell separation. The cytokinetic defects of pkd2 mutant cells differ significantly from those cells whose Ca2+ spikes have been largely blocked. In particular, pkd2 mutant cells take much longer than wild-type cells to separate. In comparison, cells treated with the Ca2+ chelator EGTA separate normally, but often lyse following the separation. Further studies would be needed to identify the signaling pathways that Pkd2 interacts with during cytokinesis.
The interplay between Ca2+ spikes and the re-organization of actin cytoskeletal structures during cytokinesis has yet to be explored. This is an exciting direction in light of the recent finding that Pkd2 modulates the assembly of the actomyosin contractile ring [126]. Hypermorphic pkd2 mutation increases the number of both actin filaments and type II myosins in the ring. Of course, there is also the question of whether Ca2+ spikes can activate myosins during cytokinesis. In animal cells, Ca2+ can activate the myosin light chain kinase (MLCK) to phosphorylate the type II myosin regulatory light chain. This activates myosin II and triggers contraction of the ring. However, fission yeast does not possess an MLCK homologue. This makes it unlikely that Ca2+ spikes can directly activate the type II myosins Myo2 and Myp2. Moving forward, more studies will be needed to determine whether Ca2+ spikes can activate other myosins such as type I and V myosins during cytokinesis.
Another interesting question to answer is whether there are any connections between cytokinetic Ca2+ transients and intracellular bioelectricity. Bioelectricity refers to signaling due to a change in the plasma membrane potential (for a review, see [127]). The resting potential of the plasma membrane usually remains negative. Either hyper- or hypo-polarization of the membrane can change the potential, resulting in activation of voltage-sensitive ion channels and ion pumps. Cytokinesis could trigger intracellular bioelectricity, leading to cytokinetic Ca2+ transients. Future studies will be needed to measure plasma membrane potential throughout cytokinesis of fission yeast cells.
The discovery of cytokinetic Ca2+ transients in the unicellular model organism fission yeast presents a new opportunity to determine the role of Ca2+ during cytokinesis. With a combination of quantitative fluorescence microscopy, yeast genetics, and in vitro biochemistry, we may finally be able to tackle the decades-old questions of where Ca2+ transients come from and what their roles are during cytokinesis.
Funding
The author is supported by the NSF grant CAREER 2144701 and the NIH grant R01GM144652.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The author would like to thank the Chen lab members at the University of Toledo for their helpful comments.
Conflicts of Interest
The author declares no conflict of interest.
References
- Fluck, R.A.; Miller, A.L.; Jaffe, L.F. Slow calcium waves accompany cytokinesis in medaka fish eggs. J. Cell Biol. 1991, 115, 1259–1265. [Google Scholar] [PubMed]
- Arnold, J.M. Effect of calcium in cytokinesis as demonstrated with ionophore A231ß7. Cytobiologie 1975, 11, 1–9. [Google Scholar]
- Snow, P.; Nuccitelli, R. Calcium buffer injections delay cleavage in Xenopus laevis blastomeres. J. Cell Biol. 1993, 122, 387–394. [Google Scholar] [CrossRef]
- Chang, D.C.; Meng, C. A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J. Cell Biol. 1995, 131, 1539–1545. [Google Scholar]
- Webb, S.E.; Miller, A.L. Ca2+ Signalling and Membrane Dynamics During Cytokinesis in Animal Cells. Adv. Exp. Med. Biol. 2017, 981, 389–412. [Google Scholar] [CrossRef]
- Miller, A.L.; Fluck, R.A.; McLaughlin, J.A.; Jaffe, L.F. Calcium buffer injections inhibit cytokinesis in Xenopus eggs. J. Cell Sci. 1993, 106 Pt 2, 523–534. [Google Scholar]
- Noguchi, T.; Mabuchi, I. Localized calcium signals along the cleavage furrow of the Xenopus egg are not involved in cytokinesis. Mol. Biol. Cell 2002, 13, 1263–1273. [Google Scholar] [CrossRef]
- Fujiwara, T.; Bandi, M.; Nitta, M.; Ivanova, E.V.; Bronson, R.T.; Pellman, D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 2005, 437, 1043–1047. [Google Scholar] [CrossRef]
- Pollard, T.D.; O’Shaughnessy, B. Molecular Mechanism of Cytokinesis. Annu. Rev. Biochem. 2019, 88, 661–689. [Google Scholar] [CrossRef]
- Pollard, T.D.; Wu, J.Q. Understanding cytokinesis: Lessons from fission yeast. Nat. Rev. 2010, 11, 149–155. [Google Scholar]
- Daga, R.R.; Chang, F. Dynamic positioning of the fission yeast cell division plane. Proc. Natl. Acad. Sci. USA 2005, 102, 8228–8232. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, C.; Sugimoto, A.; Yamamoto, M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 1997, 137, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Drubin, D.; Nurse, P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 1997, 137, 169–182. [Google Scholar] [PubMed]
- Chen, Q.; Pollard, T.D. Actin filament severing by cofilin is more important for assembly than constriction of the cytokinetic contractile ring. J. Cell Biol. 2011, 195, 485–498. [Google Scholar] [CrossRef]
- Proctor, S.A.; Minc, N.; Boudaoud, A.; Chang, F. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr. Biol. 2012, 22, 1601–1608. [Google Scholar] [CrossRef]
- Johnson, A.E.; McCollum, D.; Gould, K.L. Polar opposites: Fine-tuning cytokinesis through SIN asymmetry. Cytoskeleton 2012, 69, 686–699. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kuhn, J.R.; Kovar, D.R.; Pollard, T.D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 2003, 5, 723–734. [Google Scholar] [CrossRef]
- Courtemanche, N.; Pollard, T.D.; Chen, Q. Avoiding artefacts when counting polymerized actin in live cells with LifeAct fused to fluorescent proteins. Nat. Cell Biol. 2016, 18, 676–683. [Google Scholar] [CrossRef]
- Pollard, T.D. Nine unanswered questions about cytokinesis. J. Cell Biol. 2017, 216, 3007–3016. [Google Scholar] [CrossRef]
- Dunn, T.; Gable, K.; Beeler, T. Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem. 1994, 269, 7273–7278. [Google Scholar] [CrossRef]
- Strayle, J.; Pozzan, T.; Rudolph, H.K. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 microM and is mainly controlled by the secretory pathway pump pmr1. EMBO J. 1999, 18, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef]
- Paidhungat, M.; Garrett, S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell Biol. 1997, 17, 6339–6347. [Google Scholar] [CrossRef]
- Carnero, E.; Ribas, J.C.; Garcia, B.; Duran, A.; Sanchez, Y. Schizosaccharomyces pombe ehs1p is involved in maintaining cell wall integrity and in calcium uptake. Mol. Gen. Genet. 2000, 264, 173–183. [Google Scholar] [CrossRef]
- Lee, G.M.; Weng, F.; Cranley, J.; Rajasekhar, A.; Stoeckel, M.; Kane, T.; Tisi, R.; Wang, Y. The Ycx1 protein encoded by the yeast YDL206W gene plays a role in calcium and calcineurin signaling. J. Biol. Chem. 2023, 299, 104647. [Google Scholar] [CrossRef]
- Cortes, J.C.; Katoh-Fukui, R.; Moto, K.; Ribas, J.C.; Ishiguro, J. Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Eukaryot. Cell 2004, 3, 1124–1135. [Google Scholar] [CrossRef]
- Facanha, A.L.; Appelgren, H.; Tabish, M.; Okorokov, L.; Ekwall, K. The endoplasmic reticulum cation P-type ATPase Cta4p is required for control of cell shape and microtubule dynamics. J. Cell Biol. 2002, 157, 1029–1039. [Google Scholar] [CrossRef]
- Furune, T.; Hashimoto, K.; Ishiguro, J. Characterization of a fission yeast P(5)-type ATPase homologue that is essential for Ca2+/Mn2+ homeostasis in the absence of P(2)-type ATPases. Genes. Genet. Syst. 2008, 83, 373–381. [Google Scholar] [CrossRef]
- Duffus, J.H.; Patterson, L.J. Control of cell division in yeast using the ionophore, A23187 with calcium and magnesium. Nature 1974, 251, 626–627. [Google Scholar] [CrossRef]
- Williams, D.A.; Fogarty, K.E.; Tsien, R.Y.; Fay, F.S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature 1985, 318, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rozsa, M.; Liang, Y.; Bushey, D.; Wei, Z.; Zheng, J.; Reep, D.; Broussard, G.J.; Tsang, A.; Tsegaye, G.; et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature 2023, 615, 884–891. [Google Scholar] [CrossRef]
- Poddar, A.; Sidibe, O.; Ray, A.; Chen, Q. Calcium spikes accompany cleavage furrow ingression and cell separation during fission yeast cytokinesis. Mol. Biol. Cell 2021, 32, 15–27. [Google Scholar] [CrossRef]
- Cerutti, L.; Simanis, V. Asymmetry of the spindle pole bodies and spg1p GAP segregation during mitosis in fission yeast. J. Cell Sci. 1999, 112 Pt 14, 2313–2321. [Google Scholar] [CrossRef]
- Lee, K.W.; Webb, S.E.; Miller, A.L. Ca2+ released via IP3 receptors is required for furrow deepening during cytokinesis in zebrafish embryos. Int. J. Dev. Biol. 2003, 47, 411–421. [Google Scholar]
- Palmer, C.P.; Aydar, E.; Djamgoz, M.B. A microbial TRP-like polycystic-kidney-disease-related ion channel gene. Biochem. J. 2005, 387, 211–219. [Google Scholar] [CrossRef]
- Hu, J.; Harris, P.C. Regulation of polycystin expression, maturation and trafficking. Cell. Signal. 2020, 72, 109630. [Google Scholar] [CrossRef]
- Chapin, H.C.; Caplan, M.J. The cell biology of polycystic kidney disease. J. Cell Biol. 2010, 191, 701–710. [Google Scholar] [CrossRef]
- Morris, Z.; Sinha, D.; Poddar, A.; Morris, B.; Chen, Q. Fission yeast TRP channel Pkd2p localizes to the cleavage furrow and regulates cell separation during cytokinesis. Mol. Biol. Cell 2019, 30, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Aydar, E.; Palmer, C.P. Polycystic kidney disease channel and synaptotagmin homologues play roles in schizosaccharomyces pombe cell wall synthesis/repair and membrane protein trafficking. J. Membr. Biol. 2009, 229, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Koyano, T.; Fujimoto, T.; Onishi, K.; Matsuyama, M.; Fukushima, M.; Kume, K. Pkd2, mutations linking to autosomal dominant polycystic kidney disease, localizes to the endoplasmic reticulum and regulates calcium signaling in fission yeast. Genes Cells 2023, 28, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.; Sinha, D.; Chowdhury, P.; Bisesi, B.T.; Chen, Q. The cytoplasmic tail of the mechanosensitive channel Pkd2 regulates its internalization and clustering in eisosomes. J. Cell Sci. 2023, 136, jcs.260598. [Google Scholar] [CrossRef]
- Sinha, D.; Ivan, D.; Gibbs, E.; Chetluru, M.; Goss, J.; Chen, Q. Fission yeast polycystin Pkd2p promotes cell size expansion and antagonizes the Hippo-related SIN pathway. J. Cell Sci. 2022, 135, jcs.259046. [Google Scholar] [CrossRef]
- Poddar, A.; Hsu, Y.Y.; Zhang, F.; Shamma, A.; Kreais, Z.; Muller, C.; Malla, M.; Ray, A.; Liu, A.; Chen, Q. Membrane stretching activates calcium-permeability of a putative channel Pkd2 during fission yeast cytokinesis. Mol. Biol. Cell 2022, 33, ar134. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yoshimura, K.; Iida, H. Organellar mechanosensitive channels in fission yeast regulate the hypo-osmotic shock response. Nat. Commun. 2012, 3, 1020. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hirata, A.; Iida, H. Mechanosensitive channels Msy1 and Msy2 are required for maintaining organelle integrity upon hypoosmotic shock in Schizosaccharomyces pombe. FEMS Yeast Res. 2014, 14, 992–994. [Google Scholar] [CrossRef]
- Mu, B.; Rutkowski, D.M.; Grenci, G.; Vavylonis, D.; Zhang, D. Ca2+-dependent vesicular and non-vesicular lipid transfer controls hypoosmotic plasma membrane expansion. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ma, Y.; Sugiura, R.; Koike, A.; Ebina, H.; Sio, S.O.; Kuno, T. Transient receptor potential (TRP) and Cch1-Yam8 channels play key roles in the regulation of cytoplasmic Ca2+ in fission yeast. PLoS ONE 2011, 6, e22421. [Google Scholar] [CrossRef]
- Fluck, R.A.; Abraham, V.C.; Miller, A.L.; Jaffe, L.F. Calcium Buffer Injections Block Ooplasmic Segregation in Oryzias latipes (Medaka) Eggs. Biol. Bull. 1992, 183, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Wu, J.Q.; Pollard, T.D. Counting cytokinesis proteins globally and locally in fission yeast. Science 2005, 310, 310–314. [Google Scholar] [PubMed]
- Vavylonis, D.; Kovar, D.R.; O’Shaughnessy, B.; Pollard, T.D. Model of formin-associated actin filament elongation. Mol. Cell 2006, 21, 455–466. [Google Scholar]
- Kim, D.U.; Hayles, J.; Kim, D.; Wood, V.; Park, H.O.; Won, M.; Yoo, H.S.; Duhig, T.; Nam, M.; Palmer, G.; et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 2010, 28, 617–623. [Google Scholar] [CrossRef]
- Cyert, M.S. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu. Rev. Genet. 2001, 35, 647–672. [Google Scholar] [CrossRef]
- Takeda, T.; Yamamoto, M. Analysis and in vivo disruption of the gene coding for calmodulin in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 1987, 84, 3580–3584. [Google Scholar] [CrossRef]
- Moser, M.J.; Lee, S.Y.; Klevit, R.E.; Davis, T.N. Ca2+ binding to calmodulin and its role in Schizosaccharomyces pombe as revealed by mutagenesis and NMR spectroscopy. J. Biol. Chem. 1995, 270, 20643–20652. [Google Scholar] [CrossRef]
- Moser, M.J.; Flory, M.R.; Davis, T.N. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J. Cell Sci. 1997, 110 Pt 15, 1805–1812. [Google Scholar]
- Flory, M.R.; Morphew, M.; Joseph, J.D.; Means, A.R.; Davis, T.N. Pcp1p, an Spc110p-related calmodulin target at the centrosome of the fission yeast Schizosaccharomyces pombe. Cell Growth Differ. 2002, 13, 47–58. [Google Scholar]
- Fong, C.S.; Sato, M.; Toda, T. Fission yeast Pcp1 links polo kinase-mediated mitotic entry to gamma-tubulin-dependent spindle formation. EMBO J. 2010, 29, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Bezanilla, M.; Pollard, T.D. Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J. Cell Biol. 2000, 151, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Win, T.Z.; Gachet, Y.; Mulvihill, D.P.; May, K.M.; Hyams, J.S. Two type V myosins with non-overlapping functions in the fission yeast Schizosaccharomyces pombe: Myo52 is concerned with growth polarity and cytokinesis, Myo51 is a component of the cytokinetic actin ring. J. Cell Sci. 2001, 114, 69–79. [Google Scholar] [PubMed]
- Tang, Q.; Billington, N.; Krementsova, E.B.; Bookwalter, C.S.; Lord, M.; Trybus, K.M. A single-headed fission yeast myosin V transports actin in a tropomyosin-dependent manner. J. Cell Biol. 2016, 214, 167–179. [Google Scholar] [CrossRef]
- Itadani, A.; Nakamura, T.; Shimoda, C. Localization of type I myosin and F-actin to the leading edge region of the forespore membrane in Schizosaccharomyces pombe. Cell Struct. Funct. 2006, 31, 181–195. [Google Scholar] [CrossRef]
- Li, H.; Rao, A.; Hogan, P.G. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011, 21, 91–103. [Google Scholar] [CrossRef]
- Yoshida, T.; Toda, T.; Yanagida, M. A calcineurin-like gene ppb1+ in fission yeast: Mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 1994, 107 Pt 7, 1725–1735. [Google Scholar] [CrossRef]
- Martin-Garcia, R.; Arribas, V.; Coll, P.M.; Pinar, M.; Viana, R.A.; Rincon, S.A.; Correa-Bordes, J.; Ribas, J.C.; Perez, P. Paxillin-Mediated Recruitment of Calcineurin to the Contractile Ring Is Required for the Correct Progression of Cytokinesis in Fission Yeast. Cell Rep. 2018, 25, 772–783.e774. [Google Scholar] [CrossRef]
- Mangione, M.C.; Snider, C.E.; Gould, K.L. The intrinsically disordered region of the cytokinetic F-BAR protein Cdc15 performs a unique essential function in maintenance of cytokinetic ring integrity. Mol. Biol. Cell 2019, 30, 2790–2801. [Google Scholar] [CrossRef]
- Hirayama, S.; Sugiura, R.; Lu, Y.; Maeda, T.; Kawagishi, K.; Yokoyama, M.; Tohda, H.; Giga-Hama, Y.; Shuntoh, H.; Kuno, T. Zinc finger protein Prz1 regulates Ca2+ but not Cl- homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J. Biol. Chem. 2003, 278, 18078–18084. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kawamukai, M. cAMP-dependent protein kinase involves calcium tolerance through the regulation of Prz1 in Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2017, 81, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, A.; Bordes, N.; Haddad, R.; Schwartz, C.L.; Chang, F.; Bornens, M. Fission yeast cdc31p is a component of the half-bridge and controls SPB duplication. Mol. Biol. Cell 2003, 14, 2793–2808. [Google Scholar] [CrossRef] [PubMed]
- McCollum, D.; Balasubramanian, M.K.; Pelcher, L.E.; Hemmingsen, S.M.; Gould, K.L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 1995, 130, 651–660. [Google Scholar]
- Le Goff, X.; Motegi, F.; Salimova, E.; Mabuchi, I.; Simanis, V. The S. pombe rlc1 gene encodes a putative myosin regulatory light chain that binds the type II myosins myo3p and myo2p. J. Cell Sci. 2000, 113 Pt 23, 4157–4163. [Google Scholar]
- Hamasaki-Katagiri, N.; Molchanova, T.; Takeda, K.; Ames, J.B. Fission yeast homolog of neuronal calcium sensor-1 (Ncs1p) regulates sporulation and confers calcium tolerance. J. Biol. Chem. 2004, 279, 12744–12754. [Google Scholar] [CrossRef]
- Willet, A.H.; Turner, L.A.; Park, J.S.; Ren, L.; Snider, C.E.; Gould, K.L. Characterization of Pik1 function in fission yeast reveals its conserved role in lipid synthesis and not cytokinesis. J. Cell Sci. 2023, 136, jcs261415. [Google Scholar] [CrossRef]
- Hendricks, K.B.; Wang, B.Q.; Schnieders, E.A.; Thorner, J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat. Cell Biol. 1999, 1, 234–241. [Google Scholar] [CrossRef]
- Ohya, Y.; Kawasaki, H.; Suzuki, K.; Londesborough, J.; Anraku, Y. Two yeast genes encoding calmodulin-dependent protein kinases. Isolation, sequencing and bacterial expressions of CMK1 and CMK2. J. Biol. Chem. 1991, 266, 12784–12794. [Google Scholar]
- Rasmussen, C.D. Cloning of a calmodulin kinase I homologue from Schizosaccharomyces pombe. J. Biol. Chem. 2000, 275, 685–690. [Google Scholar] [CrossRef]
- Cisneros-Barroso, E.; Yance-Chavez, T.; Kito, A.; Sugiura, R.; Gomez-Hierro, A.; Gimenez-Zaragoza, D.; Aligue, R. Negative feedback regulation of calcineurin-dependent Prz1 transcription factor by the CaMKK-CaMK1 axis in fission yeast. Nucleic Acids Res. 2014, 42, 9573–9587. [Google Scholar] [CrossRef]
- Sanchez-Piris, M.; Posas, F.; Alemany, V.; Winge, I.; Hidalgo, E.; Bachs, O.; Aligue, R. The serine/threonine kinase Cmk2 is required for oxidative stress response in fission yeast. J. Biol. Chem. 2002, 277, 17722–17727. [Google Scholar] [CrossRef] [PubMed]
- Rhind, N.; Chen, Z.; Yassour, M.; Thompson, D.A.; Haas, B.J.; Habib, N.; Wapinski, I.; Roy, S.; Lin, M.F.; Heiman, D.I.; et al. Comparative functional genomics of the fission yeasts. Science 2011, 332, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, M.; Groppi, S.; Belotti, F.; Ambrosini, R.; Filippi, G.; Martegani, E.; Tisi, R. Hypotonic stress-induced calcium signaling in Saccharomyces cerevisiae involves TRP-like transporters on the endoplasmic reticulum membrane. Cell Calcium 2015, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Geiser, J.R.; Sundberg, H.A.; Chang, B.H.; Muller, E.G.; Davis, T.N. The essential mitotic target of calmodulin is the 110-kilodalton component of the spindle pole body in Saccharomyces cerevisiae. Mol. Cell Biol. 1993, 13, 7913–7924. [Google Scholar] [CrossRef]
- Stathopoulos, A.M.; Cyert, M.S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997, 11, 3432–3444. [Google Scholar] [CrossRef]
- Geiser, J.R.; van Tuinen, D.; Brockerhoff, S.E.; Neff, M.M.; Davis, T.N. Can calmodulin function without binding calcium? Cell 1991, 65, 949–959. [Google Scholar]
- Palmer, C.P.; Zhou, X.L.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef]
- Denis, V.; Cyert, M.S. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 2002, 156, 29–34. [Google Scholar] [CrossRef]
- Fischer, M.; Schnell, N.; Chattaway, J.; Davies, P.; Dixon, G.; Sanders, D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 1997, 419, 259–262. [Google Scholar] [CrossRef]
- Iida, H.; Nakamura, H.; Ono, T.; Okumura, M.S.; Anraku, Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell Biol. 1994, 14, 8259–8271. [Google Scholar] [CrossRef]
- Liu, S.; Chen, M.; Wang, Y.; Lei, Y.; Huang, T.; Zhang, Y.; Lam, S.M.; Li, H.; Qi, S.; Geng, J.; et al. The ER calcium channel Csg2 integrates sphingolipid metabolism with autophagy. Nat. Commun. 2023, 14, 3725. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, P.; Coun, C.; Van Eyck, V.; Faes, L.; Ghillebert, R.; Marien, L.; Winderickx, J.; Callewaert, G. Ca2+ homeostasis in the budding yeast Saccharomyces cerevisiae: Impact of ER/Golgi Ca2+ storage. Cell Calcium 2015, 58, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Carbo, N.; Tarkowski, N.; Ipina, E.P.; Dawson, S.P.; Aguilar, P.S. Sexual pheromone modulates the frequency of cytosolic Ca2+ bursts in Saccharomyces cerevisiae. Mol. Biol. Cell 2017, 28, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Spolaor, S.; Rovetta, M.; Nobile, M.S.; Cazzaniga, P.; Tisi, R.; Besozzi, D. Modeling Calcium Signaling in S. cerevisiae Highlights the Role and Regulation of the Calmodulin-Calcineurin Pathway in Response to Hypotonic Shock. Front. Mol. Biosci. 2022, 9, 856030. [Google Scholar] [CrossRef]
- Martin, D.C.; Kim, H.; Mackin, N.A.; Maldonado-Baez, L.; Evangelista, C.C., Jr.; Beaudry, V.G.; Dudgeon, D.D.; Naiman, D.Q.; Erdman, S.E.; Cunningham, K.W. New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast. J. Biol. Chem. 2011, 286, 10744–10754. [Google Scholar] [CrossRef]
- Deng, L.; Sugiura, R.; Takeuchi, M.; Suzuki, M.; Ebina, H.; Takami, T.; Koike, A.; Iba, S.; Kuno, T. Real-time monitoring of calcineurin activity in living cells: Evidence for two distinct Ca2+-dependent pathways in fission yeast. Mol. Biol. Cell 2006, 17, 4790–4800. [Google Scholar] [CrossRef]
- Protchenko, O.; Rodriguez-Suarez, R.; Androphy, R.; Bussey, H.; Philpott, C.C. A screen for genes of heme uptake identifies the FLC family required for import of FAD into the endoplasmic reticulum. J. Biol. Chem. 2006, 281, 21445–21457. [Google Scholar] [CrossRef]
- Suzuki, C.; Shimma, Y.I. P-type ATPase spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol. Microbiol. 1999, 32, 813–823. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef]
- Ohya, Y.; Anraku, Y. A galactose-dependent cmd1 mutant of Saccharomyces cerevisiae: Involvement of calmodulin in nuclear division. Curr. Genet. 1989, 15, 113–120. [Google Scholar] [CrossRef]
- Cyert, M.S.; Kunisawa, R.; Kaim, D.; Thorner, J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 1991, 88, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Sio, S.O.; Suehiro, T.; Sugiura, R.; Takeuchi, M.; Mukai, H.; Kuno, T. The role of the regulatory subunit of fission yeast calcineurin for in vivo activity and its relevance to FK506 sensitivity. J. Biol. Chem. 2005, 280, 12231–12238. [Google Scholar] [CrossRef] [PubMed]
- Spang, A.; Courtney, I.; Fackler, U.; Matzner, M.; Schiebel, E. The calcium-binding protein cell division cycle 31 of Saccharomyces cerevisiae is a component of the half bridge of the spindle pole body. J. Cell Biol. 1993, 123, 405–416. [Google Scholar] [CrossRef]
- Stevens, R.C.; Davis, T.N. Mlc1p is a light chain for the unconventional myosin Myo2p in Saccharomyces cerevisiae. J. Cell Biol. 1998, 142, 711–722. [Google Scholar] [CrossRef]
- Luo, J.; Vallen, E.A.; Dravis, C.; Tcheperegine, S.E.; Drees, B.; Bi, E. Identification and functional analysis of the essential and regulatory light chains of the only type II myosin Myo1p in Saccharomyces cerevisiae. J. Cell Biol. 2004, 165, 843–855. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Fu, J.; Lv, D.; Chen, D.; Li, Y.; Ma, W. Two-photon fluorescence real-time imaging on the development of early mouse embryo by stages. J. Microsc. 2011, 241, 212–218. [Google Scholar] [CrossRef]
- Jaffe, L.F.; Creton, R. On the conservation of calcium wave speeds. Cell Calcium 1998, 24, 1–8. [Google Scholar] [CrossRef]
- Chan, C.M.; Chen, Y.; Hung, T.S.; Miller, A.L.; Shipley, A.M.; Webb, S.E. Inhibition of SOCE disrupts cytokinesis in zebrafish embryos via inhibition of cleavage furrow deepening. Int. J. Dev. Biol. 2015, 59, 289–301. [Google Scholar] [CrossRef]
- Trebak, M.; Putney, J.W., Jr. ORAI Calcium Channels. Physiology 2017, 32, 332–342. [Google Scholar] [CrossRef]
- Carrasco, S.; Meyer, T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu. Rev. Biochem. 2011, 80, 973–1000. [Google Scholar] [CrossRef]
- Collins, S.R.; Meyer, T. Evolutionary origins of STIM1 and STIM2 within ancient Ca2+ signaling systems. Trends Cell Biol. 2011, 21, 202–211. [Google Scholar] [CrossRef]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.; Lu, W.; Brown, E.M.; Quinn, S.J.; et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef]
- Djenoune, L.; Mahamdeh, M.; Truong, T.V.; Nguyen, C.T.; Fraser, S.E.; Brueckner, M.; Howard, J.; Yuan, S. Cilia function as calcium-mediated mechanosensors that instruct left-right asymmetry. Science 2023, 379, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.E.; Lee, K.W.; Karplus, E.; Miller, A.L. Localized calcium transients accompany furrow positioning, propagation, and deepening during the early cleavage period of zebrafish embryos. Dev. Biol. 1997, 192, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Chen, Y.; Dai, G.; Chen, J.; Sun, X.M.; Wen, C.J.; Zhao, D.H.; Chang, D.C.; Li, C.J. The association of calmodulin with central spindle regulates the initiation of cytokinesis in HeLa cells. Int. J. Biochem. Cell Biol. 2004, 36, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Chircop, M.; Malladi, C.S.; Lian, A.T.; Page, S.L.; Zavortink, M.; Gordon, C.P.; McCluskey, A.; Robinson, P.J. Calcineurin activity is required for the completion of cytokinesis. Cell. Mol. Life Sci. CMLS 2010, 67, 3725–3737. [Google Scholar] [CrossRef]
- Fan, J.Q.; Deng, X.L.; Feng, B.W.; Wang, J.F.; Yu, Y.; Lv, H. Cnb1 involved in cytokinesis in Schizosaccharomyces pombe. Yi Chuan Hered. 2013, 35, 1030–1039. [Google Scholar] [CrossRef]
- Smith, J.L.; Silveira, L.A.; Spudich, J.A. Myosin light chain kinase (MLCK) gene disruption in Dictyostelium: A role for MLCK-A in cytokinesis and evidence for multiple MLCKs. Proc. Natl. Acad. Sci. USA 1996, 93, 12321–12326. [Google Scholar] [CrossRef]
- Benaud, C.; Le Dez, G.; Mironov, S.; Galli, F.; Reboutier, D.; Prigent, C. Annexin A2 is required for the early steps of cytokinesis. EMBO Rep. 2015, 16, 481–489. [Google Scholar] [CrossRef]
- Li, W.M.; Webb, S.E.; Lee, K.W.; Miller, A.L. Recruitment and SNARE-mediated fusion of vesicles in furrow membrane remodeling during cytokinesis in zebrafish embryos. Exp. Cell Res. 2006, 312, 3260–3275. [Google Scholar] [CrossRef]
- Li, C.J.; Heim, R.; Lu, P.; Pu, Y.; Tsien, R.Y.; Chang, D.C. Dynamic redistribution of calmodulin in HeLa cells during cell division as revealed by a GFP-calmodulin fusion protein technique. J. Cell Sci. 1999, 112 Pt 10, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Webb, S.E.; Chan, C.M.; Miller, A.L. Multiple roles of the furrow deepening Ca2+ transient during cytokinesis in zebrafish embryos. Dev. Biol. 2008, 316, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vien, T.; Duan, J.; Sheu, S.H.; DeCaen, P.G.; Clapham, D.E. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 2018, 7, e33183. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Hu, F.; Ge, X.; Lei, J.; Yu, S.; Wang, T.; Zhou, Q.; Mei, C.; Shi, Y. Structure of the human PKD1-PKD2 complex. Science 2018, 361, eaat9819. [Google Scholar] [CrossRef]
- Shen, P.S.; Yang, X.; DeCaen, P.G.; Liu, X.; Bulkley, D.; Clapham, D.E.; Cao, E. The Structure of the Polycystic Kidney Disease Channel PKD2 in Lipid Nanodiscs. Cell 2016, 167, 763–773.e711. [Google Scholar] [CrossRef]
- Chowdhury, P.; Sinha, D.; Poddar, A.; Chetluru, M.; Chen, Q. The Mechanosensitive Pkd2 Channel Modulates the Recruitment of Myosin II and Actin to the Cytokinetic Contractile Ring. J. Fungi 2024, 10, 455. [Google Scholar] [CrossRef]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).