Composition and Biodiversity of Culturable Endophytic Fungi in the Roots of Alpine Medicinal Plants in Xinjiang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Root Sample Processing, Morphological and Anatomical Observation
2.3. Isolation and Cultivation of Endophytic Fungi
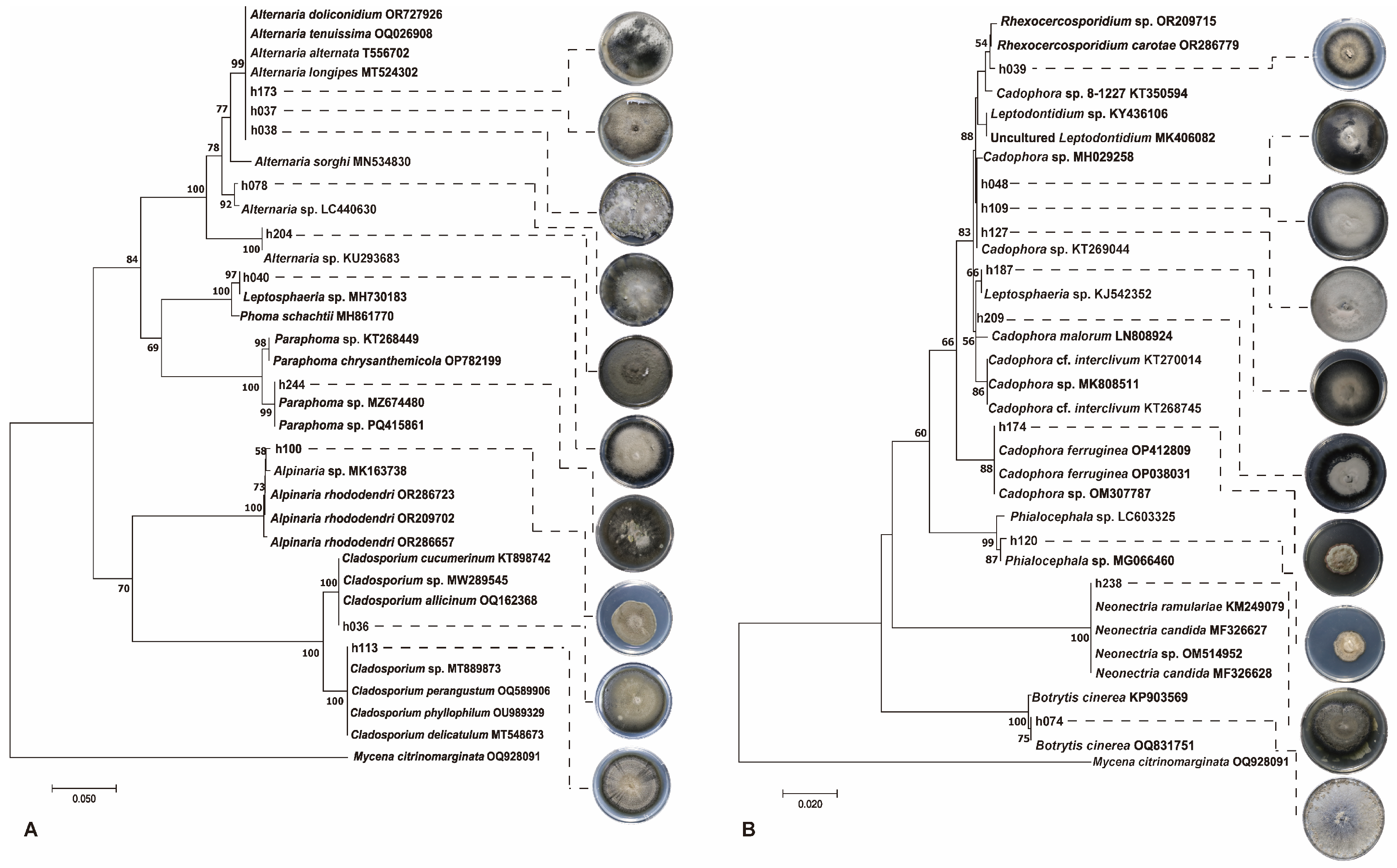
2.4. Identification of Culturable Endophytic Fungi
2.5. Data Analysis
3. Results
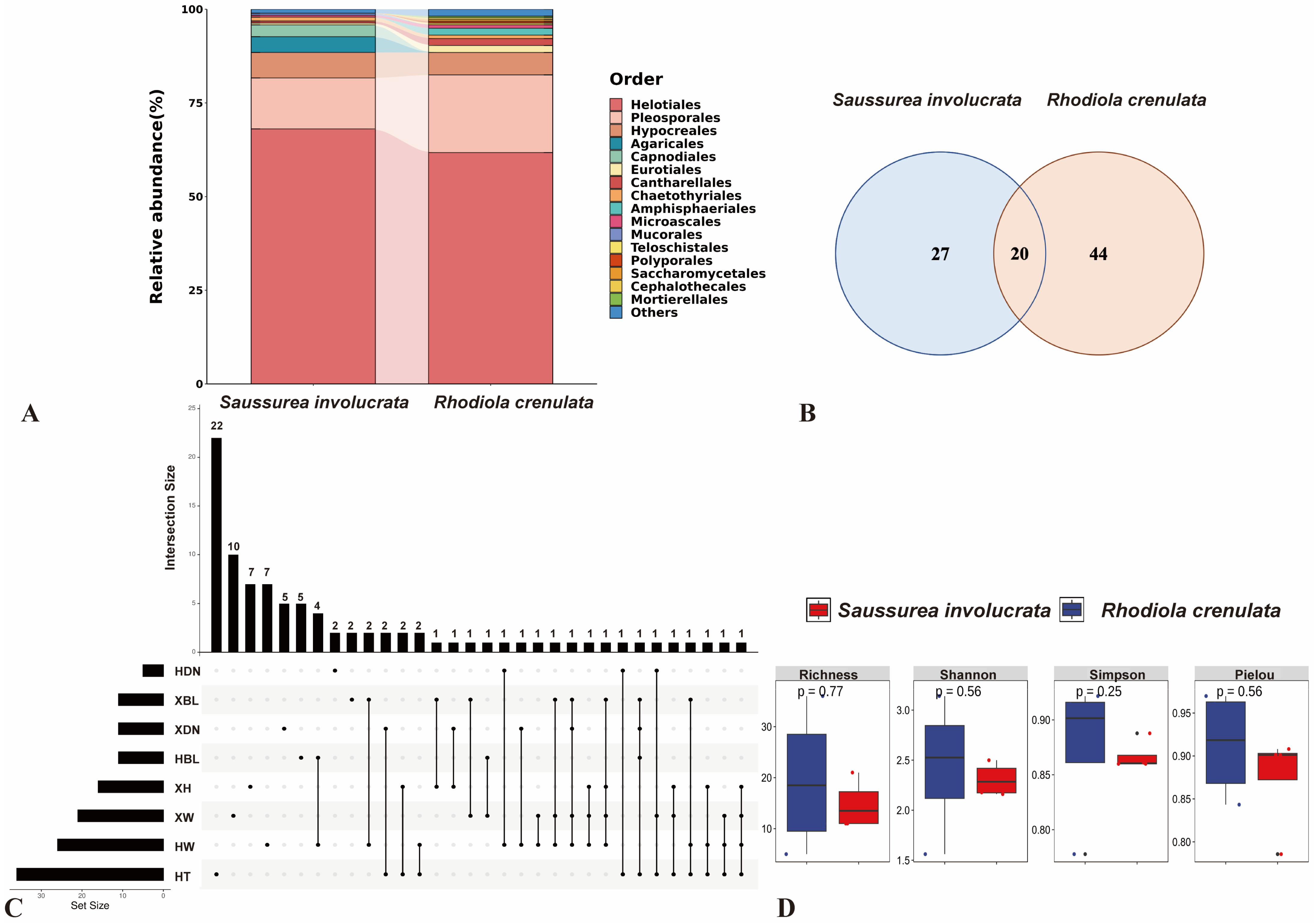
3.1. Comparison of Cultural Endophytic Fungal Community Structure and Diversity in Two Alpine Plants
3.2. Analysis of the Community Composition and Diversity of Endophytic Fungi in Two Alpine Medicinal Plants from Different Sampling Sites
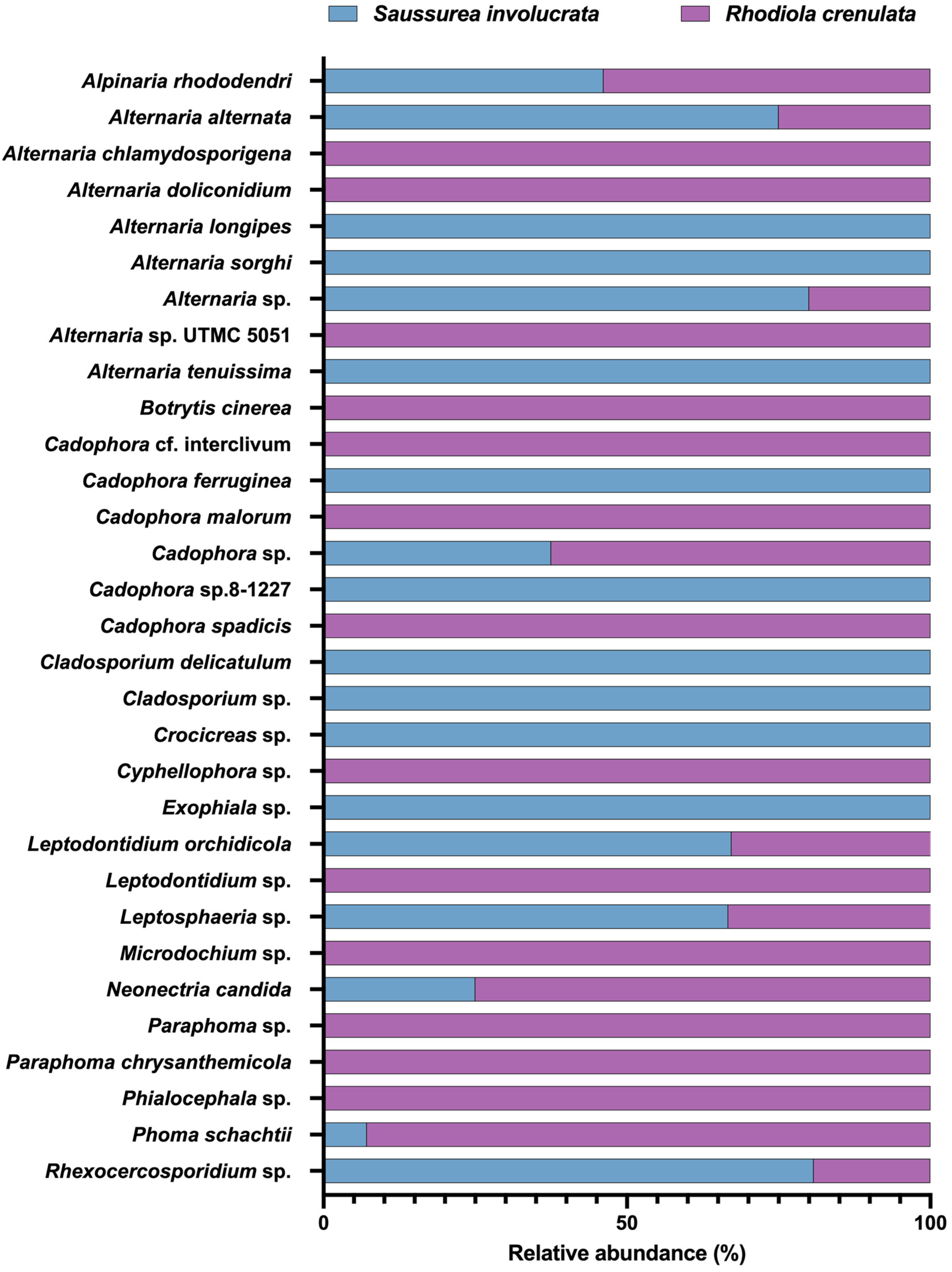
3.3. The Dominant DSE in the Culturable Endophytic Fungal Communities of Alpine Medicinal Plants
3.4. DSE Community Composition of Two Alpine Medicinal Plants in Different Sites
3.5. Analysis of DSE Diversity of the Two Species in Different Ways
4. Discussion
4.1. Common Characteristics of the Culturable Endophytic Fungal Communities in the Roots of Both Plant Species
4.2. Effects of Plant Species on Endophytic Fungal Community
4.3. Effects of Sampling Sites on Endophytic Fungal Community
4.4. Potential Ecological Roles of Culturable DSE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, A.A.D.; Snell, H.S.K.; Michas, A.; Pritchard, W.J.; Newbold, L.; Cordero, I.; Goodall, T.; Schallhart, N.; Kaufmann, R.; Griffiths, R.I.; et al. Climate Change Alters Temporal Dynamics of Alpine Soil Microbial Functioning and Biogeochemical Cycling via Earlier Snowmelt. ISME J. 2021, 15, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Legge, S.; Rumpff, L.; Garnett, S.T.; Woinarski, J.C.Z. Loss of Terrestrial Biodiversity in Australia: Magnitude, Causation, and Response. Science 2023, 381, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrío-Dillon, G.; de Ruffray, P.; Vidal, C.; Pierrat, J.-C.; Gégout, J.-C. Changes in Plant Community Composition Lag behind Climate Warming in Lowland Forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef]
- Parada, R.; Mendoza, L.; Cotoras, M.; Ortiz, C. Endophytic Fungi Isolated from Plants Present in a Mine Tailing Facility Show a Differential Growth Response to Lead. Lett. Appl. Microbiol. 2022, 75, 345–354. [Google Scholar] [CrossRef]
- Ding, W.-N.; Ree, R.H.; Spicer, R.A.; Xing, Y.-W. Ancient Orogenic and Monsoon-Driven Assembly of the World’s Richest Temperate Alpine Flora. Science 2020, 369, 578–581. [Google Scholar] [CrossRef]
- Wang, W.; Ding, M.; Gardner, J.D.; Wang, Y.; Miao, B.; Guo, W.; Wu, X.; Ruan, Q.; Yu, J.; Hu, X.; et al. Ancient Xinjiang Mitogenomes Reveal Intense Admixture with High Genetic Diversity. Sci. Adv. 2021, 7, eabd6690. [Google Scholar] [CrossRef]
- Wu, C.-L.; Lin, L.-F.; Hsu, H.-C.; Huang, L.-F.; Hsiao, C.-D.; Chou, M.-L. Saussurea involucrata (Snow Lotus) ICE1 and ICE2 Orthologues Involved in Regulating Cold Stress Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10850. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Yan, B.; Li, J.M.; Guo, S.X. Preliminary Identification of Endophytic Fungi Colonized in the Root of Saussurea involucrata and Rhodiola rosea from Xinjiang Region. Mycosystema 2018, 37, 110–119. (In Chinese) [Google Scholar] [CrossRef]
- Gong, G.; Xie, F.; Zheng, Y.; Hu, W.; Qi, B.; He, H.; Dong, T.T.; Tsim, K.W. The Effect of Methanol Extract from Saussurea involucrata in the Lipopolysaccharide-Stimulated Inflammation in Cultured RAW 264.7 Cells. J. Ethnopharmacol. 2020, 251, 112532. [Google Scholar] [CrossRef]
- Cong, S.; Wang, L.; Meng, Y.; Cai, X.; Zhang, C.; Gu, Y.; Ma, X.; Luo, L. Saussurea involucrata Oral Liquid Regulates Gut Microbiota and Serum Metabolism During Alleviation of Collagen-Induced Arthritis in Rats. Phytother. Res. 2023, 37, 1242–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, K.; Jing, R.; Zhao, W.; Guo, K.; Hu, Z.; Liu, G.; Xu, N.; Zhao, J.; Lin, L.; et al. Protective Effect of Saussurea involucrata Polysaccharide Against Skin Dryness Induced by Ultraviolet Radiation. Front. Pharmacol. 2023, 14, 1089537. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, A.; Landis, J.B.; Shi, W.; Zhang, X.; Sun, H.; Wang, H. Genome Assembly of the Snow Lotus Species Saussurea involucrata Provides Insights into Acacetin and Rutin Biosynthesis and Tolerance to an Alpine Environment. Hortic. Res. 2023, 10, uhad180. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, L.; Hao, S.; Guan, R.; Fan, G.; Shi, C.; Wan, H.; Chen, W.; Zhang, H.; Liu, G.; et al. Draft Genome Sequence of the Tibetan Medicinal Herb Rhodiola crenulata. Gigascience 2017, 6, gix033. [Google Scholar] [CrossRef]
- Pu, W.-L.; Zhang, M.-Y.; Bai, R.-Y.; Sun, L.-K.; Li, W.-H.; Yu, Y.-L.; Zhang, Y.; Song, L.; Wang, Z.-X.; Peng, Y.-F.; et al. Anti-Inflammatory Effects of Rhodiola rosea L.: A Review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, F.; Chen, S.; Chi, X. Checklist of National Key Protected Wild Plants on the Qinghai-Tibetan Plateau. Biodivers. Data J. 2023, 11, e103289. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and Host Range of Foliar Fungal Endophytes: Are Tropical Leaves Biodiversity Hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Pan, Y.; Zheng, X.; Liang, X.; Sheng, L.; Zhang, D.; Sun, Q.; Wang, Q. Research Advances on Endophytic Fungi and Their Bioactive Metabolites. Bioprocess Biosyst. Eng. 2023, 46, 165–170. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark Septate Endophytes: A Review of Facultative Biotrophic Root-colonizing Fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Mateu, M.; Baldwin, A.H.; Maul, J.E.; Yarwood, S.A. Dark Septate Endophyte Improves Salt Tolerance of Native and Invasive Lineages of Phragmites australis. ISME J. 2020, 14, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Netherway, T.; Bengtsson, J.; Buegger, F.; Fritscher, J.; Oja, J.; Pritsch, K.; Hildebrand, F.; Krab, E.J.; Bahram, M. Pervasive Associations Between Dark Septate Endophytic Fungi with Tree Root and Soil Microbiomes Across Europe. Nat. Commun. 2024, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Chik, W.-I.; Zhu, L.; Fan, L.-L.; Yi, T.; Zhu, G.-Y.; Gou, X.-J.; Tang, Y.-N.; Xu, J.; Yeung, W.-P.; Zhao, Z.-Z.; et al. Saussurea involucrata: A Review of the Botany, Phytochemistry and Ethnopharmacology of a Rare Traditional Herbal Medicine. J. Ethnopharmacol. 2015, 172, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Fan, F.; Xie, N.; Zhang, Y.; Wang, X.; Meng, X. Rhodiola crenulata Alleviates Hypobaric Hypoxia-Induced Brain Injury by Maintaining BBB Integrity and Balancing Energy Metabolism Dysfunction. Phytomedicine 2024, 128, 155529. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhou, D.-Y.; Dai, J.; Li, Y.; Xing, Y.-M.; Guo, S.-X.; Chen, J. Potential Specificity Between Mycorrhizal Fungi Isolated from Widespread Dendrobium spp. and Rare D. huoshanense Seeds. Curr. Microbiol. 2022, 79, 264. [Google Scholar] [CrossRef]
- Hughes, C.E.; Atchison, G.W. The Ubiquity of Alpine Plant Radiations: From the Andes to the Hengduan Mountains. New Phytol. 2015, 207, 275–282. [Google Scholar] [CrossRef]
- Shannon, C.E. The Mathematical Theory of Communication. 1963. MD Comput 1997, 14, 306–317. [Google Scholar]
- Johnston, E.L.; Roberts, D.A. Contaminants Reduce the Richness and Evenness of Marine Communities: A Review and Meta-Analysis. Environ. Pollut. 2009, 157, 1745–1752. [Google Scholar] [CrossRef]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-96239-7. [Google Scholar]
- Vasconcelos Rissi, D.; Ijaz, M.; Baschien, C. Comparative Genome Analysis of the Freshwater Fungus Filosporella fistucella Indicates Potential for Plant-Litter Degradation at Cold Temperatures. G3 Genes Genomes Genet. (Bethesda) 2023, 13, jkad190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, X.; Li, G.; Wang, Q.-M.; Wang, M. Cadophora Species from Marine Glaciers in the Qinghai-Tibet Plateau: An Example of Unsuspected Hidden Biodiversity. IMA Fungus 2022, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.; Pradhan, A.; Pascoal, C.; Cássio, F. Proteomic Responses to Silver Nanoparticles Vary with the Fungal Ecotype. Sci. Total Environ. 2020, 704, 135385. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Carvalho, A.; Bärlocher, F.; Canhoto, C. Are Fungal Strains from Salinized Streams Adapted to Salt-Rich Conditions? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 374, 20180018. [Google Scholar] [CrossRef]
- Probst, M.; Telagathoti, A.; Mandolini, E.; Peintner, U. Fungal and Bacterial Communities and Their Associations in Snow-Free and Snow Covered (Sub-)Alpine Pinus cembra Forest Soils. Environ. Microbiome 2024, 19, 20. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, Y.-L.; Li, S.-X.; Wang, Y.-Q.; Wang, Y.-L. The Characteristics of Soil Fungal Community in Degraded Alpine Meadow in the Three Rivers Source Region, China. Ying Yong Sheng Tai Xue Bao 2021, 32, 869–877. [Google Scholar] [CrossRef]
- Gupta, P.; Vakhlu, J.; Sharma, Y.P.; Imchen, M.; Kumavath, R. Metagenomic Insights into the Fungal Assemblages of the Northwest Himalayan Cold Desert. Extremophiles 2020, 24, 749–758. [Google Scholar] [CrossRef]
- Liao, H.; Huang, L.; Li, N.; Ke, W.; Xiang, Y.; Ma, Y. Auxiliary Rapid Identification of Pathogenic and Antagonistic Microorganisms Associated with Coptis chinensis Root Rot by High-Throughput Sequencing. Sci. Rep. 2021, 11, 11141. [Google Scholar] [CrossRef]
- Hou, L.W.; Groenewald, J.Z.; Pfenning, L.H.; Yarden, O.; Crous, P.W.; Cai, L. The Phoma-like Dilemma. Stud. Mycol. 2020, 96, 309–396. [Google Scholar] [CrossRef]
- Kernaghan, G.; Patriquin, G. Host Associations Between Fungal Root Endophytes and Boreal Trees. Microb. Ecol. 2011, 62, 460–473. [Google Scholar] [CrossRef]
- Zeng, Q.; Lebreton, A.; Auer, L.; Man, X.; Jia, L.; Wang, G.; Gong, S.; Lombard, V.; Buée, M.; Wu, G.; et al. Stable Functional Structure despite High Taxonomic Variability across Fungal Communities in Soils of Old-Growth Montane Forests. Microbiome 2023, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Linares, D.R.; Grosch, R.; Restrepo, S.; Krumbein, A.; Franken, P. Effects of Dark Septate Endophytes on Tomato Plant Performance. Mycorrhiza 2011, 21, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, C.; Blaudez, D.; Beguiristain, T.; Chalot, M.; Leyval, C. Co-Inoculation of Lolium perenne with Funneliformis mosseae and the Dark Septate Endophyte Cadophora sp. in a Trace Element-Polluted Soil. Mycorrhiza 2018, 28, 301–314. [Google Scholar] [CrossRef]
- Venkateswarulu, N.; Shameer, S.; Bramhachari, P.V.; Basha, S.K.T.; Nagaraju, C.; Vijaya, T. Isolation and Characterization of Plumbagin (5-Hydroxyl-2-Methylnaptalene-1,4-Dione) Producing Endophytic Fungi Cladosporium delicatulum from Endemic Medicinal Plants: Isolation and Characterization of Plumbagin Producing Endophytic Fungi from Endemic Medicinal Plants. Biotechnol. Rep. 2018, 20, e00282. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Lan, F.; Qiao, Y.M.; Wei, J.G.; Huang, R.S.; Li, L.B. Endophytic Fungi Harbored in the Root of Sophora tonkinensis Gapnep: Diversity and Biocontrol Potential against Phytopathogens. Microbiologyopen 2017, 6, e00437. [Google Scholar] [CrossRef]
- Currah, R.S.; Hambleton, S.; Smreciu, A. Mycorrhizae and Mmcorrhizal Fungi of Calypso bulbosa. Am. J. Bot. 1988, 75, 739–752. [Google Scholar] [CrossRef]
- Kohout, P.; Sýkorová, Z.; Ctvrtlíková, M.; Rydlová, J.; Suda, J.; Vohník, M.; Sudová, R. Surprising Spectra of Root-Associated Fungi in Submerged Aquatic Plants. FEMS Microbiol. Ecol. 2012, 80, 216–235. [Google Scholar] [CrossRef]
- Jabiol, J.; Lecerf, A.; Lamothe, S.; Gessner, M.O.; Chauvet, E. Litter Quality Modulates Effects of Dissolved Nitrogen on Leaf Decomposition by Stream Microbial Communities. Microb. Ecol. 2019, 77, 959–966. [Google Scholar] [CrossRef]
- Freestone, M.; Reiter, N.; Swarts, N.D.; Linde, C.C. Temporal Turnover of Ceratobasidiaceae Orchid Mycorrhizal Fungal Communities with Ontogenetic and Phenological Development in Prasophyllum (Orchidaceae). Ann. Bot. 2024, 134, mcae089. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal Fungi Affect Orchid Distribution and Population Dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J.S.; Seo, C.W.; Lee, J.W.; Kim, S.H.; Cho, Y.; Lim, Y.W. Diversity of Cladosporium (Cladosporiales, Cladosporiaceae) Species in Marine Environments and Report on Five New Species. MycoKeys 2023, 98, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped Potential of Marine-Associated Cladosporium Species: An Overview on Secondary Metabolites, Biotechnological Relevance, and Biological Activities. Mar. Drugs 2021, 19, 645. [Google Scholar] [CrossRef] [PubMed]
- Prasannath, K.; Shivas, R.G.; Galea, V.J.; Akinsanmi, O.A. Novel Botrytis and Cladosporium Species Associated with Flower Diseases of Macadamia in Australia. J. Fungi 2021, 7, 898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Chen, X.; Kulyar, M.F.-E.-A.; Duan, K.; Li, H.; Bhutta, Z.A.; Wu, Y.; Li, K. Gut Fungal Microbiome Responses to Natural Cryptosporidium Infection in Horses. Front. Microbiol. 2022, 13, 877280. [Google Scholar] [CrossRef]
- Tanney, J.B.; Seifert, K.A. Mollisiaceae: An Overlooked Lineage of Diverse Endophytes. Stud. Mycol. 2020, 95, 293–380. [Google Scholar] [CrossRef]
- Badet, T.; Peyraud, R.; Raffaele, S. Common Protein Sequence Signatures Associate with Sclerotinia borealis Lifestyle and Secretion in Fungal Pathogens of the Sclerotiniaceae. Front. Plant Sci. 2015, 6, 776. [Google Scholar] [CrossRef]
- Wang, K.; Lei, J.; Wei, J.; Yao, N. Bioactive Natural Compounds from the Plant Endophytic Fungi Pestalotiopsis spp. Mini Rev. Med. Chem. 2012, 12, 1382–1393. [Google Scholar] [CrossRef]
- Telagathoti, A.; Probst, M.; Peintner, U. Habitat, Snow-Cover and Soil pH, Affect the Distribution and Diversity of Mortierellaceae Species and Their Associations to Bacteria. Front. Microbiol. 2021, 12, 669784. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Khashi U Rahman, M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific Plant Interaction via Root Exudates Structures the Disease Suppressiveness of Rhizosphere Microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for Help with Root Exudates: Adaptive Mechanisms by Which Stressed Plants Assemble Health-Promoting Soil Microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef]
- Adams, A.E.; Besozzi, E.M.; Shahrokhi, G.; Patten, M.A. A Case for Associational Resistance: Apparent Support for the Stress Gradient Hypothesis Varies with Study System. Ecol. Lett. 2022, 25, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Wen, D.; Bates, C.T.; Wu, L.; Guo, X.; Liu, S.; Su, Y.; Lei, J.; Zhou, J.; Yang, Y. Nutrient Supply Controls the Linkage Between Species Abundance and Ecological Interactions in Marine Bacterial Communities. Nat. Commun. 2022, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial Effects of Endophytic Fungi Colonization on Plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Deng, Z.-S.; Liu, X.-D.; Zhang, B.-C.; Jiao, S.; Qi, X.-Y.; Sun, Z.-H.; He, X.-L.; Liu, Y.-Z.; Li, J.; Chen, K.-K.; et al. The Root Endophytic Fungi Community Structure of Pennisetum sinese from Four Representative Provinces in China. Microorganisms 2019, 7, 332. [Google Scholar] [CrossRef]
- Glynou, K.; Nam, B.; Thines, M.; Maciá-Vicente, J.G. Facultative Root-Colonizing Fungi Dominate Endophytic Assemblages in Roots of Nonmycorrhizal microthlaspi Species. New Phytol. 2018, 217, 1190–1202. [Google Scholar] [CrossRef]
- Toju, H.; Kurokawa, H.; Kenta, T. Factors Influencing Leaf- and Root-Associated Communities of Bacteria and Fungi Across 33 Plant Orders in a Grassland. Front. Microbiol. 2019, 10, 241. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Liu, J.; Qu, Y.; Gao, Y.; Ren, A. Tripartite Interactions Between Endophytic Fungi, Arbuscular Mycorrhizal Fungi, and Leymus chinensis. Microb. Ecol. 2020, 79, 98–109. [Google Scholar] [CrossRef]
- Mahdi, L.K.; Miyauchi, S.; Uhlmann, C.; Garrido-Oter, R.; Langen, G.; Wawra, S.; Niu, Y.; Guan, R.; Robertson-Albertyn, S.; Bulgarelli, D.; et al. The Fungal Root Endophyte Serendipita vermifera Displays Inter-Kingdom Synergistic Beneficial Effects with the Microbiota in Arabidopsis thaliana and Barley. ISME J. 2022, 16, 876–889. [Google Scholar] [CrossRef]
- Redkar, A.; Sabale, M.; Zuccaro, A.; Di Pietro, A. Determinants of Endophytic and Pathogenic Lifestyle in Root Colonizing Fungi. Curr. Opin. Plant Biol. 2022, 67, 102226. [Google Scholar] [CrossRef]
- Sun, R.; Yi, Z.; Fu, Y.; Liu, H. Dynamic Changes in Rhizosphere Fungi in Different Developmental Stages of Wheat in a Confined and Isolated Environment. Appl. Microbiol. Biotechnol. 2022, 106, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Glynou, K.; Ali, T.; Buch, A.-K.; Haghi Kia, S.; Ploch, S.; Xia, X.; Çelik, A.; Thines, M.; Maciá-Vicente, J.G. The Local Environment Determines the Assembly of Root Endophytic Fungi at a Continental Scale. Environ. Microbiol. 2016, 18, 2418–2434. [Google Scholar] [CrossRef]
- Mandyam, K.G.; Jumpponen, A. Mutualism-Parasitism Paradigm Synthesized from Results of Root-Endophyte Models. Front. Microbiol. 2014, 5, 776. [Google Scholar] [CrossRef]
- Du, W.-B.; Jia, P.; Du, G.-Z. Current Patterns of Plant Diversity and Phylogenetic Structure on the Kunlun Mountains. Plant Divers. 2022, 44, 30–38. [Google Scholar] [CrossRef]
- Fan, M.; Xu, J.; Yu, W.; Chen, Y.; Wang, M.; Dai, W.; Wang, Y. Recent Tianshan Warming in Relation to Large-Scale Climate Teleconnections. Sci. Total Environ. 2023, 856, 159201. [Google Scholar] [CrossRef]
- Bueno de Mesquita, C.P.; Martinez Del Río, C.M.; Suding, K.N.; Schmidt, S.K. Rapid Temporal Changes in Root Colonization by Arbuscular Mycorrhizal Fungi and Fine Root Endophytes, Not Dark Septate Endophytes, Track Plant Activity and Environment in an Alpine Ecosystem. Mycorrhiza 2018, 28, 717–726. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, M.; Ye, Q.; Gao, H.; He, X. Improved Tolerance of Artemisia ordosica to Drought Stress via Dark Septate Endophyte (DSE) Symbiosis. J. Fungi 2022, 8, 730. [Google Scholar] [CrossRef]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary Delivery of siRNA Against Acute Lung Injury/Acute Respiratory Distress Syndrome. Acta Pharm. Sin. B 2022, 12, 600–620. [Google Scholar] [CrossRef]
- Chau, T.P.; Saravanan, M.; Al-Ansari, M.M.; Al-Dahmash, N.D.; Kuriakose, L.L.; Sindhu, R. Antimicrobial and Biocompatibility Nature of Methanol Extract of Lannea coromandelica Bark and Edible Coating Film Preparation for Fruit Preservation. Environ. Res. 2024, 243, 117861. [Google Scholar] [CrossRef]
- Li, X.; He, X.-L.; Zhou, Y.; Hou, Y.-T.; Zuo, Y.-L. Effects of Dark Septate Endophytes on the Performance of Hedysarum scoparium Under Water Deficit Stress. Front. Plant Sci. 2019, 10, 903. [Google Scholar] [CrossRef]
- Yakti, W.; Kovács, G.M.; Franken, P. Differential Interaction of the Dark Septate Endophyte Cadophora sp. and Fungal Pathogens In Vitro and in Planta. FEMS Microbiol. Ecol. 2019, 95, fiz164. [Google Scholar] [CrossRef] [PubMed]
- Parvandi, M.; Rezadoost, H.; Farzaneh, M. Introducing Alternaria tenuissima SBUp1, as an Endophytic Fungus of Ferula assa-Foetida from Iran, Which Is a Rich Source of Rosmarinic Acid. Lett. Appl. Microbiol. 2021, 73, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, A.L.; Kauppinen, M.; Wäli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark Septate Endophytes: Mutualism from by-Products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, M.; Shi, X.; Zhao, Z. Dark Septate Endophyte (DSE) Fungi Isolated from Metal Polluted Soils: Their Taxonomic Position, Tolerance, and Accumulation of Heavy Metals In Vitro. J. Microbiol. 2008, 46, 624–632. [Google Scholar] [CrossRef]
- Fracchia, S.; Krapovickas, L.; Aranda-Rickert, A.; Valentinuzzi, V.S. Dispersal of Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes by Ctenomys cf. Knighti (Rodentia) in the Northern Monte Desert of Argentina. J. Arid. Environ. 2011, 75, 1016–1023. [Google Scholar] [CrossRef]
- Kauppinen, M.; Raveala, K.; Wäli, P.R.; Ruotsalainen, A.L. Contrasting Preferences of Arbuscular Mycorrhizal and Dark Septate Fungi Colonizing Boreal and Subarctic Avenella flexuosa. Mycorrhiza 2014, 24, 171–177. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Piotrowska-Seget, Z. Plant Association with Dark Septate Endophytes: When the Going Gets Tough (and Stressful), the Tough Fungi Get Going. Chemosphere 2022, 302, 134830. [Google Scholar] [CrossRef]
- Li, Z.; Meng, L.; Ma, Q.; Wang, Z.; Zhao, Y.; Luo, D. Polyketides with IDH1 R132h and PTP1B Inhibitory Activities from the Desert-Plant-Derived Fungus Alternaria sp. HM 134. Front. Microbiol. 2022, 13, 975579. [Google Scholar] [CrossRef]
- Gao, R.; Liu, R.; Sun, C. A Marine Fungus Alternaria alternata FB1 Efficiently Degrades Polyethylene. J. Hazard. Mater. 2022, 431, 128617. [Google Scholar] [CrossRef]
- Berthelot, C.; Leyval, C.; Chalot, M.; Blaudez, D. Interactions between Dark Septate Endophytes, Ectomycorrhizal Fungi and Root Pathogens In Vitro. FEMS Microbiol. Lett. 2019, 366, fnz158. [Google Scholar] [CrossRef]
- Xie, F.; Li, H.-T.; Wang, M.; Chen, J.-Y.; Duan, H.-J.; Xia, D.-D.; Xie, T.-P.; Gao, Y.-H.; Zhou, H.; Ding, Z.-T. Phialocetones A-J, C12 Lactones from the Rhizospheric Soil-Derived Fungus Phialocephala sp. YUD18001 Associated with Gastrodia elata. Phytochemistry 2022, 202, 113359. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress Tolerance in Plants via Habitat-Adapted Symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Salmi, D.; Riou, C.; Issawi, M.; Titouche, Y.; Ambrosini, V.; Smail-Saadoun, N.; Abbaci, H.; Houali, K. Antibacterial and Antioxidant Activities of Endophytic Fungi and Nettle (Urtica dioica L.) Leaves as Their Host. Cell. Mol. Biol. 2021, 67, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, H.; Shi, T.; Wang, B. The Genus Cladosporium: A Prospective Producer of Natural Products. Int. J. Mol. Sci. 2024, 25, 1652. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, J.; Zhang, K.; Wei, S.; Lin, R.; Polyak, S.W.; Yang, N.; Song, F. New Isocoumarin Analogues from the Marine-Derived Fungus Paraphoma sp. CUGBMF180003. Mar. Drugs 2021, 19, 313. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, T.-K.; Shi, Q.; Yang, X.-L. Sesquiterpenoids and Diterpenes with Antimicrobial Activity from Leptosphaeria sp. XL026, an Endophytic Fungus in Panax notoginseng. Fitoterapia 2019, 137, 104243. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J.; Li, X. Dark Septate Endophytes Isolated from Wild Licorice Roots Grown in the Desert Regions of Northwest China Enhance the Growth of Host Plants Under Water Deficit Stress. Front. Microbiol. 2021, 12, 522449. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Moreno, L.F.; Stielow, B.J.; Muszewska, A.; Hainaut, M.; Gonzaga, L.; Abouelleil, A.; Patané, J.S.L.; Priest, M.; Souza, R.; et al. Exploring the Genomic Diversity of Black Yeasts and Relatives (Chaetothyriales, Ascomycota). Stud. Mycol. 2017, 86, 1–28. [Google Scholar] [CrossRef]
- Donalle, G.C.; Martorell, M.M.; Siless, G.E.; Ruberto, L.; Cabrera, G.M. Cyclic Heptapeptides with Metal Binding Properties Isolated from the Fungus Cadophora malorum from Antarctic Soil. Nat. Prod. Bioprospect. 2022, 12, 26. [Google Scholar] [CrossRef]
- Alves, I.M.S.; Gonçalves, V.N.; Oliveira, F.S.; Schaefer, C.E.G.R.; Rosa, C.A.; Rosa, L.H. The Diversity, Distribution, and Pathogenic Potential of Cultivable Fungi Present in Rocks from the South Shetlands Archipelago, Maritime Antarctica. Extremophiles 2019, 23, 327–336. [Google Scholar] [CrossRef]
- Li, D.-H.; Cai, S.-X.; Zhu, T.-J.; Wang, F.-P.; Xiao, X.; Gu, Q.-Q. New Cytotoxic Metabolites from a Deep-Sea-Derived Fungus, Phialocephala sp., Strain FL30r. Chem. Biodivers. 2011, 8, 895–901. [Google Scholar] [CrossRef]
- Mohammadian, E.; Babai Ahari, A.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to Heavy Metals in Filamentous Fungi Isolated from Contaminated Mining Soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]


| Mountains | Geographical Symbol | Host Plant’s Species and Numbers | Sampling Site | Geographical Location | Altitude |
|---|---|---|---|---|---|
| Tianshan Mountains | XW | 6 S. involucrata | Tianshan Mountain No.1 glacier | 42°59′23″ N 86°24′7″ E | 3259.2 |
| HW | 11 R. crenulata | Tianshan Mountain No.1 glacier | 42°59′23″ N 86°24′7″ E | 3259.2 | |
| XBL | 6 S. involucrata | Bayinbuluke, Hejing County | 43°4′5″ N 86°44′9″ E | 3502.1 | |
| HBL | 12 R. crenulata | Bayinbuluke, Hejing County | 43°4′5″ N 86°44′9″ E | 3502.1 | |
| XDN | 14 S. involucrata | Danangou Uzbek Township | 43°31′6″ N 90°17′19″ E | 3236.3 | |
| HDN | 3 R. crenulata | Danangou Uzbek Township | 43°31′6″ N 90°17′19″ E | 3236.3 | |
| XH | 8 S. involucrata | Houxia, Urumqi | 43°9′51″ N 87°11′18″ E | 3317.1 | |
| Kunlun Mountains | HT | 12 R. crenulata | Taxkorgan | 42°35′24″ N 86°32′47″ E | 3374.4 |
| Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|
| Ascomycota | Dothideomycetes | Capnodiales | Cladosporiaceae | Cladosporium | Cladosporium sp. |
| Cladosporium delicatulum | |||||
| Pleosporales | Didymellaceae | Phoma | Phoma schachtii | ||
| Incertae sedis, Pleosporales | Paraphoma | Paraphoma chrysanthemicola | |||
| Paraphoma sp. | |||||
| Melanommataceae | Alpinaria | Alpinaria rhododendri | |||
| Pleosporaceae | Alternaria | Alternaria alternata | |||
| Alternaria chlamydosporigena | |||||
| Alternaria doliconidium | |||||
| Alternaria longipes | |||||
| Alternaria sorghi | |||||
| Alternaria sp. | |||||
| Alternaria sp. UTMC 5051 | |||||
| Alternaria tenuissima | |||||
| Eurotiomycetes | Chaetothyriales | Cyphellophoraceae | Cyphellophora | Cyphellophora sp. | |
| Herpotrichiellaceae | Exophiala | Exophiala sp. | |||
| Leotiomycetes | Helotiales | Incertae sedis, Helotiales | Crocicreas | Crocicreas sp. | |
| Rhexocercosporidium | Rhexocercosporidium sp. | ||||
| Leptodontidiaceae | Leptodontidium | Leptodontidium orchidicola | |||
| Leptodontidium sp. | |||||
| Leptosphaeria | Leptosphaeria sp. | ||||
| Mollisiaceae | Phialocephala | Phialocephala sp. | |||
| Ploettnerulaceae | Cadophora | Cadophora cf. interclivum | |||
| Cadophora ferruginea | |||||
| Cadophora malorum | |||||
| Cadophora sp. | |||||
| Cadophora spadicis | |||||
| Cadophora sp. 8-1227 | |||||
| Sclerotiniaceae | Botrytis | Botrytis cinerea | |||
| Sordariomycetes | Amphisphaeriales | Amphisphaeriaceae | Microdochium | Microdochium sp. | |
| Hypocreales | Nectriceae | Neonectria | Neonectria candida |
| Sample Sites | Sorenson’s Similarity Coefficient | Diversity Index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XW | XBL | XDN | XH | HW | HBL | HDN | HT | Richness | Shannon–Wiener | Simpson | Pielou | |
| XW | 1.0000 | 21.0000 | 2.3922 | 0.8600 | 0.7857 | |||||||
| XBL | 0.2500 | 1.0000 | 11.0000 | 2.1609 | 0.8611 | 0.9012 | ||||||
| XDN | 0.0625 | 0.1818 | 1.0000 | 11.0000 | 2.1775 | 0.8600 | 0.9081 | |||||
| XH | 0.1622 | 0.0741 | 0.0741 | 1.0000 | 16.0000 | 2.4992 | 0.8878 | 0.9014 | ||||
| HW | 0.2979 | 0.3243 | 0.0541 | 0.1429 | 1.0000 | 26.0000 | 2.7477 | 0.9142 | 0.8433 | |||
| HBL | 0.0625 | 0.0909 | 0.0909 | 0.0000 | 0.2162 | 1.0000 | 11.0000 | 2.3035 | 0.8889 | 0.9606 | ||
| HDN | 0.0769 | 0.0000 | 0.0000 | 0.0000 | 0.0645 | 0.0000 | 1.0000 | 5.0000 | 1.5607 | 0.7778 | 0.9697 | |
| HT | 0.1404 | 0.0851 | 0.1277 | 0.1923 | 0.1935 | 0.0426 | 0.0976 | 1.0000 | 36.0000 | 3.1403 | 0.9217 | 0.8763 |
| Type of Morphology | Corresponding Lications | Fungal Species | No. of Strains | HOST Plants | Color of Colonies | Characteristics of Colonies |
|---|---|---|---|---|---|---|
| Type 1 | XW | Cladosporium sp. | h036 | S. involucrata | Olive green | The colony was loose and felt-like in texture, with a surface covered in villi. Some hyphae were basal, and margins were radial. |
| Type 2 | XH, XDN, HT | A. alternata | h038 | S. involucrata, R. crenulata | Gray | The colony was dense and villous, with well-developed aerial structures and irregular margins. |
| Type 3 | XW, XH, HT | Cadophora sp. | h048 | S. involucrata, R. crenulata | Olive green, middle gray | The colony was loose with a felt-like surface, covered in villi, partially basal, with a radial margin and a slight central elevation. |
| Type 4 | XDN, HT | Alternaria sp. | h079 | S. involucrata, R. crenulata | Gray | The colony was loose, felt-like, covered with white villi on the surface, with irregular margins. |
| Type 5 | HW | P. schachtii | h092 | S. involucrata, R. crenulata | Black, middle brown | The colony was relatively loose and felt-like in texture, slightly raised in the middle, part of the basal mycelium, with radial margin. |
| Type 6 | XW, HW | A. rhododendri | h100 | S. involucrata, R. crenulata | Green, middle black | The colony was loose, intrabasal, with irregular wavy edges. |
| Type 7 | XW, HW | L. orchidicola | h109 | S. involucrata, R. crenulata | Dark green | The colony was loose, felt-like, covered with villi, and some were intrabasal hyphae with irregular margins. |
| Type 8 | XW | Cladosporium delicatulum | h113 | S. involucrata | Olive green, middle brown | The colony was loose, felt-like, with a villous surface; colonies were flat and wrinkled, with a neat, radial edge. |
| Type 9 | HW | Phialocephala sp. | h120 | R. crenulata | Brown | The colony was loose and villous, with well-developed aerial hyphae, some basal hyphae, and a neat colony edge. |
| Type 10 | HW | Leptodontidium sp. | h127 | R. crenulata | Gray | The colony was dense and felt-like, with well-developed aerial mycelium, slightly raised overall. |
| Type 11 | HT | Paraphoma sp. | h244 | R. crenulata | Olive green, middle white | The colony was loose and felt-like, with a raised white central part and irregular margins. |
| Type 12 | HT | A. doliconidium | h240 | R. crenulata | Olive green | The colony was loose and felt-like, with a predominantly intrabasal structure. |
| Type 13 | HT | Cadophora cf. interclivum | h209 | R. crenulata | Black, middle white | The colony was dense, covered with white villi on the surface, slightly raised in the middle, with radial margins, and some were basal hyphae. |
| Type 14 | HT | Alternaria sp. | h208 | R. crenulata | Olive green | The colony was loose, producing red pigment, felt-like, slightly raised in the middle, with irregular margins. |
| Type 15 | XH, HT | Leptosphaeria sp. | h236 | S. involucrata, R. crenulata | Olive green | The colony was dense, the aerial mycelium was well developed, slightly raised overall, with an irregular edge, and some were basal mycelium. |
| Type 16 | XH, HT | Leptosphaeria sp. | h188 | S. involucrata, R. crenulata | Dark green, middle white | The colony was loose, flat, slightly raised in the middle, primarily intrabasal with neat margins. |
| Type 17 | XH | Cadophora sp. | h176 | S. involucrata, R. crenulata | Olive green, middle brown | The colony was loose, felt-like, and concentrically round, covered with brown villi on the surface, raised in the middle, with neat edges and exudates. |
| Type 18 | HW, HBL | Cadophora spadicis | h091 | R. crenulata | Red brown | The colony was loose, flat, basal hyphae with red aerial hyphae in the middle, with radial margins. |
| Type 19 | XH, HT | Leptosphaeria sp. | h188 | S. involucrata, R. crenulata | Green | The colony was loose, felt, covered with white villi on the surface, raised in the middle, with neat edges and exudates. |
| Type 20 | HT | Cadophora malorum | h230 | R. crenulata | Dark green | The colony was loose and villous, with white surface and slightly raised brown surface and wavy margins. |
| Sampling Sites | Richness Index | Shannon–Wiener Index | Simpson Index | Pielou Index |
|---|---|---|---|---|
| XW | 8.0000 | 1.6130 | 0.7189 | 0.7757 |
| XBL | 6.0000 | 1.6112 | 0.7778 | 0.8992 |
| XDN | 4.0000 | 1.1622 | 0.6446 | 0.8384 |
| XH | 6.0000 | 1.6094 | 0.7600 | 0.8982 |
| HW | 11.0000 | 1.9307 | 0.8018 | 0.8052 |
| HBL | 3.0000 | 1.0397 | 0.6250 | 0.9464 |
| HDN | 1.0000 | 0.0000 | 0.0000 | NA |
| HT | 13.0000 | 2.1080 | 0.8186 | 0.8219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Zhu, J.; Leng, C.; Huang, X.; Yang, M.; Yin, Y.; Xing, Y.; Chen, J. Composition and Biodiversity of Culturable Endophytic Fungi in the Roots of Alpine Medicinal Plants in Xinjiang, China. J. Fungi 2025, 11, 113. https://doi.org/10.3390/jof11020113
Hou M, Zhu J, Leng C, Huang X, Yang M, Yin Y, Xing Y, Chen J. Composition and Biodiversity of Culturable Endophytic Fungi in the Roots of Alpine Medicinal Plants in Xinjiang, China. Journal of Fungi. 2025; 11(2):113. https://doi.org/10.3390/jof11020113
Chicago/Turabian StyleHou, Mengyan, Jun Zhu, Chunyan Leng, Xinjie Huang, Mingshu Yang, Yifei Yin, Yongmei Xing, and Juan Chen. 2025. "Composition and Biodiversity of Culturable Endophytic Fungi in the Roots of Alpine Medicinal Plants in Xinjiang, China" Journal of Fungi 11, no. 2: 113. https://doi.org/10.3390/jof11020113
APA StyleHou, M., Zhu, J., Leng, C., Huang, X., Yang, M., Yin, Y., Xing, Y., & Chen, J. (2025). Composition and Biodiversity of Culturable Endophytic Fungi in the Roots of Alpine Medicinal Plants in Xinjiang, China. Journal of Fungi, 11(2), 113. https://doi.org/10.3390/jof11020113





