Untargeted Metabolomics-Based Characterization of the Metabolic Profile and Antioxidant Activity of Ophiocordyceps sinensis and Its Substitutes
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Sample Materials
2.2. In Vitro Antioxidant Assay
2.3. Untargeted Metabolomics Profiling
2.3.1. QC Sample Preparation and Sample Extraction
2.3.2. LC-MS/MS Analysis
2.3.3. Mass Spectrometry Conditions
2.3.4. Data Processing
3. Analysis of Results
3.1. Comparative Analysis of In Vitro Antioxidant Activity
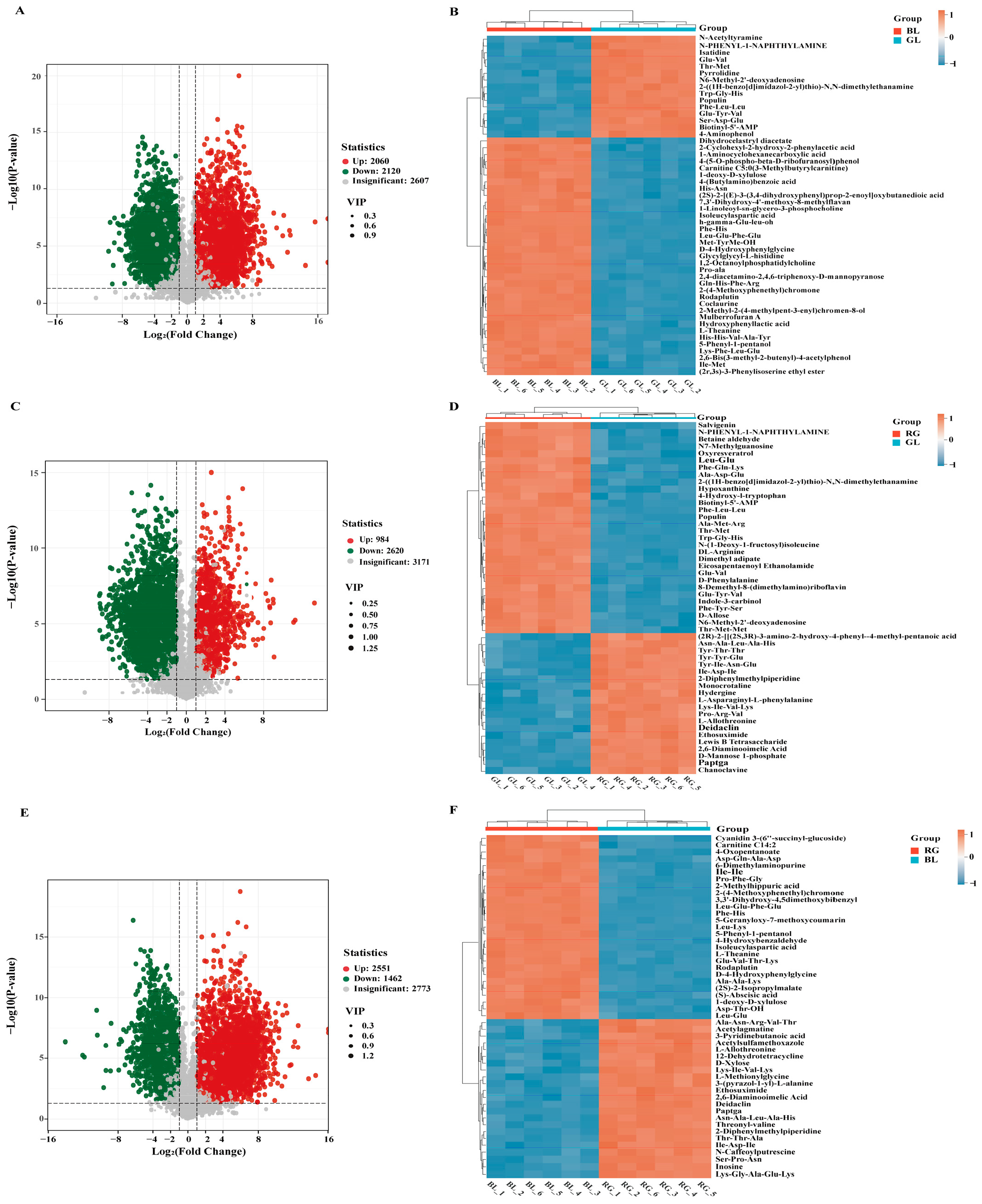
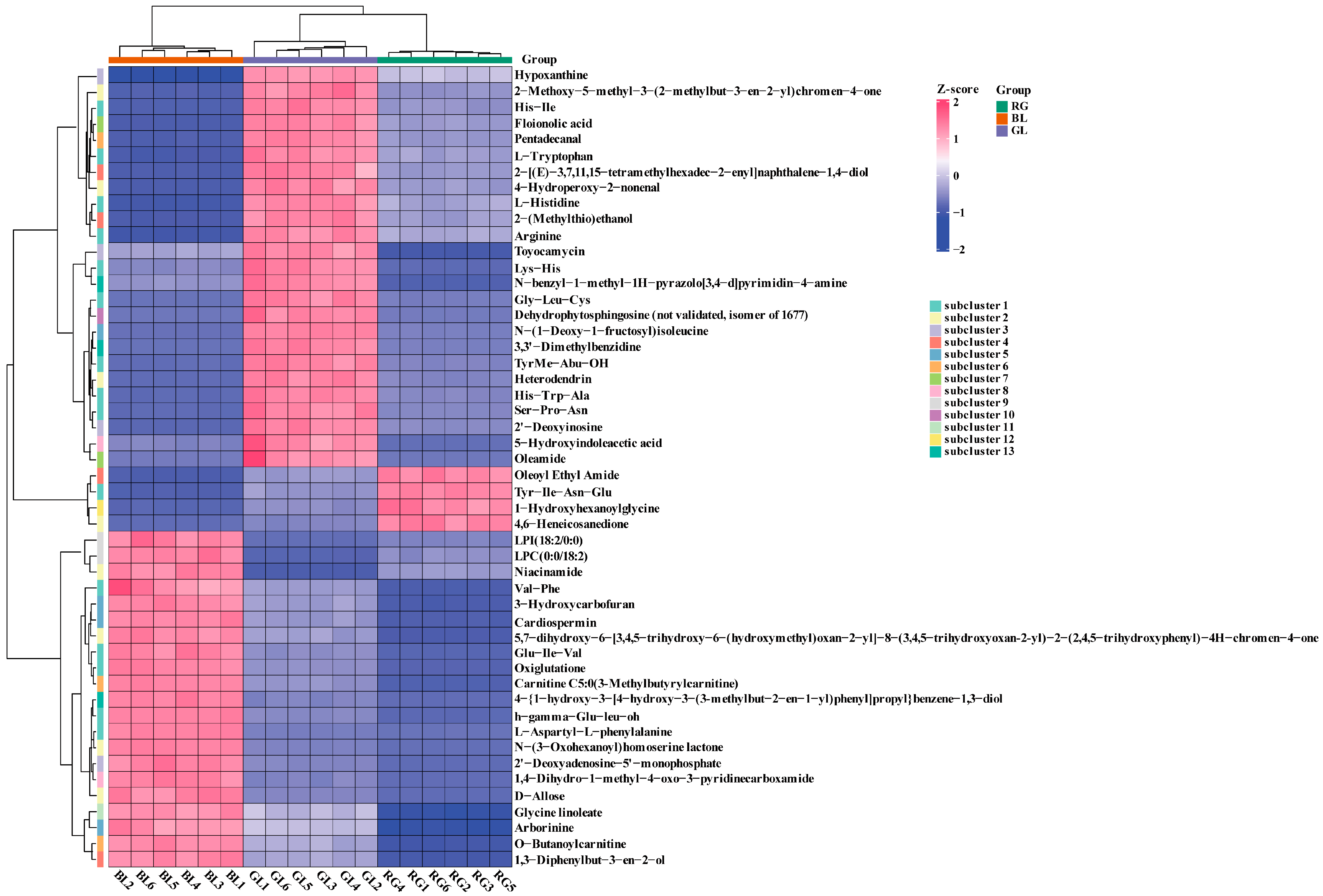
3.2. Analysis of Metabolite Profiles
3.3. Screening of Differentially Accumulated Metabolites (DAM)
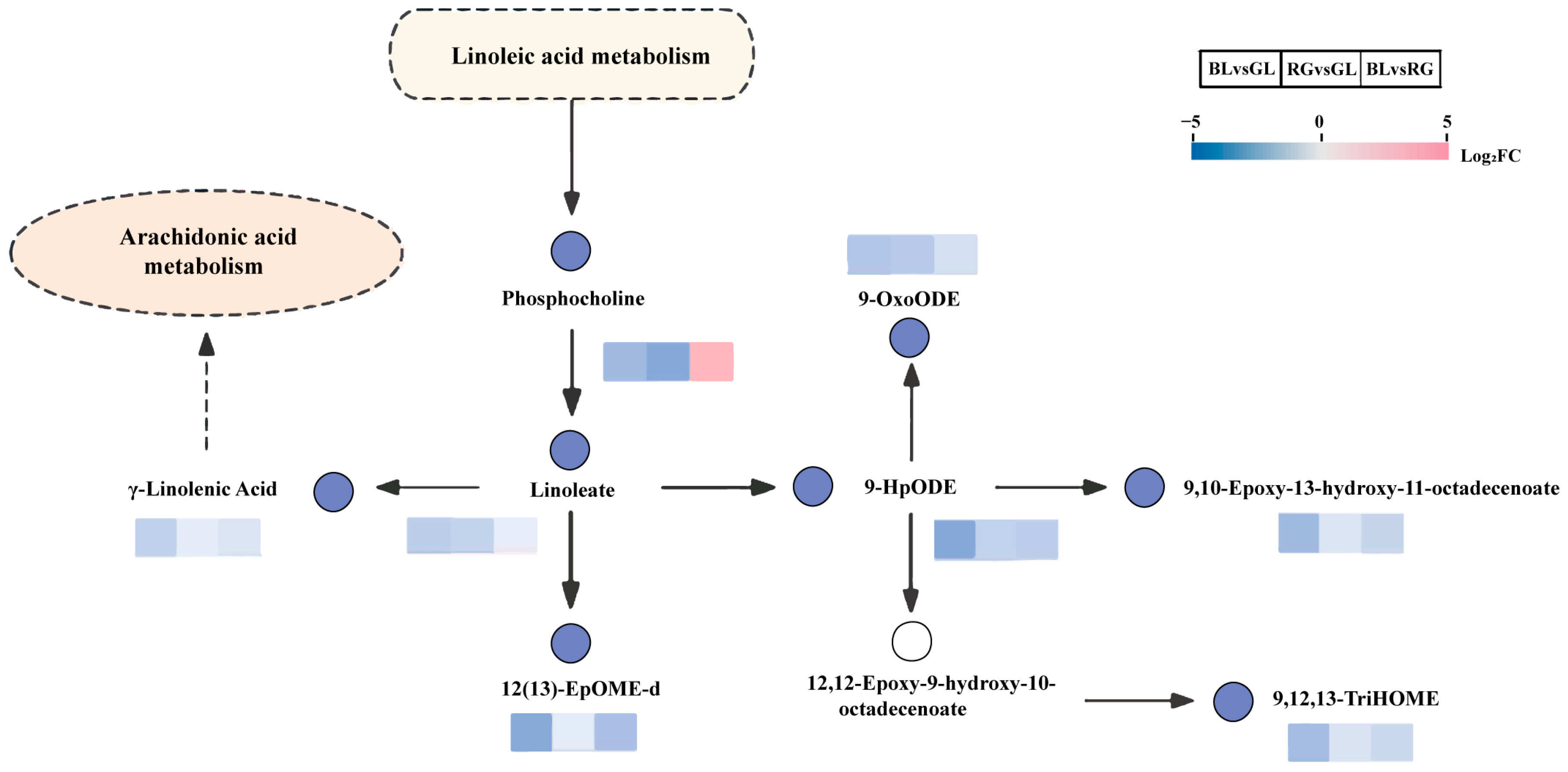
3.4. KEGG Enrichment Pathway Analysis
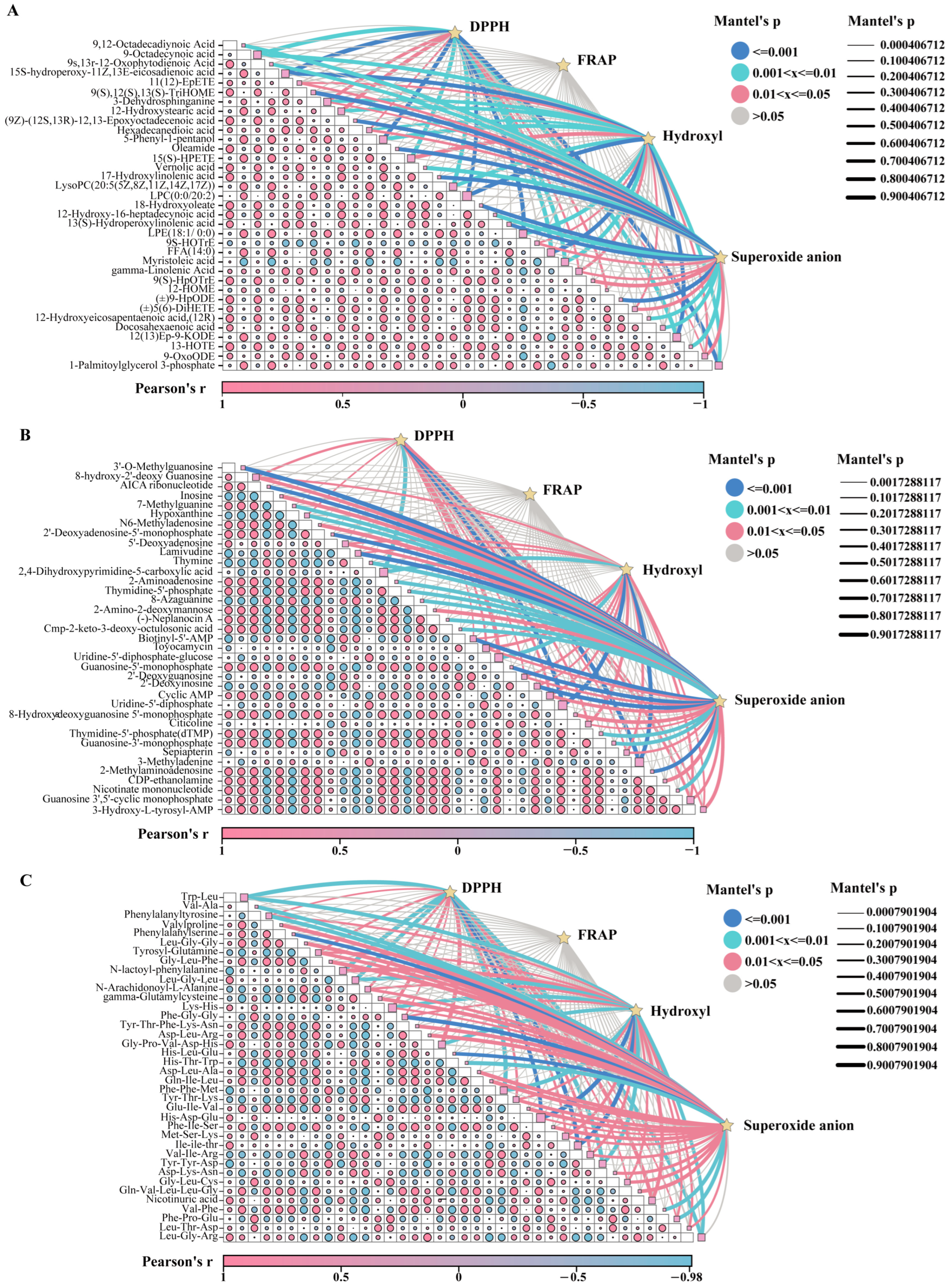
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.Q.; Kan, L.J.; Nie, S.P.; Chen, H.H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Li, Y.J.; Xie, M.Y. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT-Food Sci. Technol. 2015, 63, 2–7. [Google Scholar] [CrossRef]
- Committee National Pharmacopoeia. Pharmacopoeia of People’s Republic of China, Part 1; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Zhao, J.; Xie, J.; Wang, L.Y.; Li, S.P. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef]
- Xu, J.; Huang, Y.; Chen, X.X.; Zheng, S.C.; Chen, P.; Mo, M.H. The Mechanisms of Pharmacological Activities of Ophiocordyceps sinensis Fungi. Phytother. Res. 2016, 30, 1572–1583. [Google Scholar] [CrossRef]
- Lo, H.C.; Hsieh, C.; Lin, F.Y.; Hsu, T.H. A Systematic Review of the Mysterious Caterpillar Fungus Ophiocordyceps sinensis in Dong-ChongXiaCao (Dōng Chóng Xià Cǎo) and Related Bioactive Ingredients. J. Tradit. Complement. Med. 2013, 3, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.C.; Chang, C.Y.; Cheng, T.L.; Wu, M.J. Immunomodulatory effect of exo-polysaccharides from submerged cultured Cordyceps sinensis: Enhancement of cytokine synthesis, CD11b expression, and phagocytosis. Appl. Microbiol. Biotechnol. 2007, 75, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.; Lo, C.K.; Cheung, J.K.; Zhu, S.Q.; Tsim, K.W. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003, 73, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.H.; Xie, F.; Tan, J.; Yuan, Y.; Mei, H.; Zheng, Y.; Sheng, R. Extraction, structure and pharmacological effects of the polysaccharides from Cordyceps sinensis: A review. J. Funct. Foods 2022, 89, 104909. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, J.; Zhu, Q.; Li, S.P. Macrophage biospecific extraction and high performance liquid chromatography for hypothesis of immunological active components in Cordyceps sinensis. J. Pharm. Biomed. Anal. 2007, 44, 439–443. [Google Scholar] [CrossRef]
- Guo, L.-X.; Xu, X.-M.; Wu, C.-F.; Lin, L.; Zou, S.-C.; Luan, T.-G.; Yuan, J.-P.; Wang, J.-H. Fatty acid composition of lipids in wild Cordyceps sinensis from major habitats in China. Biomed. Prevent. Nutr. 2012, 2, 42–50. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.L.; Jiao, L.; Jiang, Y.; Li, H.; Jiang, S.P.; Lhosumtseiring, N.; Fu, S.Z.; Dong, C.H.; Zhan, Y.; et al. A survey of the geographic distribution of Ophiocordyceps sinensis. J. Microbiol. 2011, 49, 913–919. [Google Scholar] [CrossRef]
- Quan, Q.M.; Chen, L.L.; Wang, X.; Li, S.; Yang, X.L.; Zhu, Y.G.; Wang, M.; Cheng, Z. Genetic diversity and distribution patterns of host insects of Caterpillar Fungus Ophiocordyceps sinensis in the Qinghai-Tibet Plateau. PLoS ONE 2014, 9, e92293. [Google Scholar] [CrossRef]
- Zhou, X.W.; Li, L.J.; Tian, E.W. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit. Rev. Biotechnol. 2014, 34, 233–243. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Li, W.J.; Li, Q.P.; Qian, Z.M.; Liu, X.Z.; Dong, C.H. A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2019, 39, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Wang, N.; Chen, W.X.; Zhang, W.P.; Zhang, H.S.; Yu, H.; Yi, Y. Integrated metabolomics and transcriptomics reveal metabolites difference between wild and cultivated Ophiocordyceps sinensis. Food Res. Int. 2023, 163, 112275. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.F.; Liau, J.C.; Lee, C.S.; Chiu, C.Y.; Martel, J.; Lin, C.S.; Tseng, S.F.; Ojcius, D.M.; Lu, C.C.; Lai, H.C.; et al. Isolation, Culture and Characterization of Hirsutella sinensis Mycelium from Caterpillar Fungus Fruiting Body. PLoS ONE 2017, 12, e0168734. [Google Scholar] [CrossRef]
- Zhao, X.M.; Zhang, H.; Jiang, H.X.; Zhang, X.Z.; Su, Y.Y.; Wang, Y. Molecular identification for the asexual stage of Taishan cordyceps. Zhong Yao Cai 2010, 33, 1373–1375. [Google Scholar] [PubMed]
- Liu, Z.Y.; Yao, Y.J.; Liang, Z.Q.; Liu, A.Y.; Pegler, D.N.; Chase, M.W. Molecular evidence for the anamorph-teleomorph connection in Cordyceps sinensis. Mycol. Res. 2001, 105, 827–832. [Google Scholar] [CrossRef]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H.N. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef]
- Xu, J.; Shen, R.; Jiao, Z.; Chen, W.; Peng, D.; Wang, L.; Yu, N.; Peng, C.; Cai, B.; Song, H.; et al. Current Advancements in Antitumor Properties and Mechanisms of Medicinal Components in Edible Mushrooms. Nutrients 2022, 14, 2622. [Google Scholar] [CrossRef]
- Pandya, C.D.; Vekaria, H.; Joseph, B.; Slone, S.A.; Gensel, J.C.; Sullivan, P.G.; Miller, B.A. Hemoglobin induces oxidative stress and mitochondrial dysfunction in oligodendrocyte progenitor cells. Transl. Res. 2021, 231, 13–23. [Google Scholar] [CrossRef]
- Dong, C.; Guo, S.; Wang, W.; Liu, X. Cordyceps industry in China. Mycology 2015, 6, 121–129. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, T.; Zhu, W.; Chen, L.; Guan, Y.; Jin, C. Quality control method of sterols in fermented Cordyceps sinensis based on combined fingerprint and quantitative analysis of multicomponents by single marker. J. Food Sci. 2020, 85, 2994–3002. [Google Scholar] [CrossRef]
- Cong, B. Perspectives in Food & Medicine Homology. Food Med. Homol. 2024, 1, 9420018. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Karpinska, B.; Foyer, C.H. Superoxide signalling and antioxidant processing in the plant nucleus. J. Exp. Bot. 2024, 75, 4599–4610. [Google Scholar] [CrossRef]
- Wang, D.; Zhai, Y.K.; Wang, Y.; Fu, X.; Ji, Y.B.; Li, R.J. Dual-color reversible fluorescent carbon dots designed for dynamic monitoring of cellular superoxide anion radicals. J. Mater. Chem. B 2025, 13, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.; Abee, T. Generation of Variants in Listeria monocytogenes Continuous-Flow Biofilms Is Dependent on Radical-Induced DNA Damage and RecA-Mediated Repair. PLoS ONE 2011, 6, e28590. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood-Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xia, M.X.; Chen, J.; Yuan, R.; Deng, F.N.; Shen, F.F. Gene Expression Characteristics and Regulation Mechanisms of Superoxide Dismutase and Its Physiological Roles in Plants under Stress. Biochemistry 2016, 81, 465–480. [Google Scholar]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
- Han, X.X.; Jeong, Y.; Irudayaraj, J. Nanocatalase-Based Oxygen-Generating Nanocarriers for Active Oxygen Delivery to Relieve Hypoxia in Pancreatic Cancer. ACS Appl. Nano Mater. 2022, 5, 17248–17257. [Google Scholar] [CrossRef]
- Borchani, C.; Besbes, S.; Masmoudi, M.; Blecker, C.; Paquot, M.; Attia, H. Effect of drying methods on physico-chemical and antioxidant properties of date fibre concentrates. Food Chem. 2011, 125, 1194–1201. [Google Scholar] [CrossRef]
- Ubando-Rivera, J.; Navarro-Ocaña, A.; Valdivia-López, M.A. Mexican lime peel: Comparative study on contents of dietary fibre and associated antioxidant activity. Food Chem. 2005, 89, 57–61. [Google Scholar] [CrossRef]
- Jabeen, K.; Malik, U.; Mansoor, S.; Shahzad, S.; Zahid, S.; Javed, A. Effect of oxidative stress and calcium deregulation on FAM26F (CALHM6) expression during hepatitis B virus infection. BMC Infect. Dis. 2021, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Wang, S.Q.; Lu, L.; Song, T.Y.; Xu, X.X.; Yu, J.; Liu, T.X. Optimization of Cordyceps sinensis fermentation Marsdenia tenacissima process and the differences of metabolites before and after fermentation. Heliyon 2022, 8, e12586. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xie, F.; Zhou, G.; Chen, Z.H.; Wang, J.Y.; Wang, C.G. Transcriptome and metabonomics combined analysis revealed the energy supply mechanism involved in fruiting body initiation in Chinese cordyceps. Sci. Rep. 2023, 13, 9500. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Tang, C.Y.; Wang, T.; Xiao, M.J.; Li, Y.L.; Li, X.Z. Analysis of Metabolic Profiles and Antioxidant Activity of Chinese Cordyceps, Ophiocordyceps sinensis, and Paecilomyces hepiali Based on Untargeted Metabolomics. Biology 2024, 13, 683. [Google Scholar] [CrossRef]
- Sun, H.Q.; Zhu, Z.Y.; Tang, Y.L.; Ren, Y.Y.; Song, Q.Y.; Tang, Y.; Zhang, Y.M. Structural characterization and antitumor activity of a novel Se-polysaccharide from seleniumenriched Cordyceps gunnii. Food Funct. 2018, 9, 2744–2754. [Google Scholar] [CrossRef]
- Law, S.K.; Au, D.C.T. A review of medicine and food homology on traditional Chinese medicine as functional food. Food Med. Homol. 2025, 2026, 9420091. [Google Scholar] [CrossRef]
- Ma, S.; Dai, Y. Principal component analysis based methods in bioinformatics studies. Brief. Bioinform. 2011, 12, 714–722. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Nguyen, T.T.; Liu, J.; Wu, T.; Lee, E.; Tu, X.M. Partial least squares regression and principal component analysis: Similarity and differences between two popular variable reduction approaches. Gen. Psychiatr. 2022, 35, e100662. [Google Scholar] [CrossRef]
- Pal, M.; Bhardwaj, A.; Manickam, M.; Tulsawani, R.; Srivastava, M.; Sugadev, R.; Misra, K. Protective Efficacy of the Caterpillar Mushroom, Ophiocordyceps sinensis (Ascomycetes), from India in Neuronal Hippocampal Cells against Hypoxia. Int. J. Med. Mushrooms 2015, 17, 829–840. [Google Scholar] [CrossRef]
- Kong, B.H.; Yap, C.A.; Razif, M.F.M.; Ng, S.T.; Tan, C.S.; Fung, S.Y. Antioxidant and Cytotoxic Effects and Identification of Ophiocordyceps sinensis Bioactive Proteins Using Shotgun Proteomic Analysis. Food Technol. Biotech. 2021, 59, 201–208. [Google Scholar] [CrossRef]
- Mishra, J.; Rajput, R.; Singh, K.; Bansal, A.; Misra, K. Antioxidant-Rich Peptide Fractions Derived from High-Altitude Chinese Caterpillar Medicinal Mushroom Ophiocordyceps sinensis (Ascomycetes) Inhibit Bacterial Pathogens. Int. J. Med. Mushrooms 2019, 21, 155–168. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kagota, S.; Nakamura, K.; Shinozuka, K.; Kunitomo, M. Antioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensis. Phytother. Res. 2000, 14, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Sheri, V.; Kumar, M.; Jaconis, S.; Zhang, B. Antioxidant defense in cotton under environmental stresses: Unraveling the crucial role of a universal defense regulator for enhanced cotton sustainability. Plant Physiol. Bioch 2023, 204, 108141. [Google Scholar]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Lunina, J.N.; Samoilenko, V.A.; Morgunov, I.G. Effect of Nitrogen Concentration on the Biosynthesis of Citric Acid, Protein, and Lipids in the Yeast Yarrowia lipolytica. Biomolecules 2022, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Li, H.; Huang, H.; Xie, J.; Wang, Z.; Chen, W.; Tao, Y. Development of a mass spectrometry-based metabolomics workflow for traceability of wild and cultivated Cordyceps sinensis. Food Addit. Contam. A 2022, 39, 1773–1784. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Zhang, H.; Huang, Z.; Ma, J. Comparative study of the composition of cultivated, naturally grown Cordyceps sinensis, and stiff worms across different sampling years. PLoS ONE 2019, 14, e0225750. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, C.; Xiao, M.; Cao, Z.; He, M.; Qi, J.; Li, Y.; Li, X. Effect of Air Drying on the Metabolic Profile of Fresh Wild and Artificial Cordyceps sinensis. Foods 2023, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, P.; Tong, X.; Gao, T.; Peng, T.; Guo, J. Comparative analysis of fatty acid metabolism based on transcriptome sequencing of wild and cultivated Ophiocordyceps sinensis. PeerJ 2021, 9, e11681. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, C.; Xiao, M.; Cao, Z.; He, H.; He, M.; Li, Y.; Li, X. Analysis of metabolic spectrum characteristics of naturally and cultivated Ophiocordyceps sinensis based on non-targeted metabolomics. Sci. Rep. 2024, 14, 17425. [Google Scholar] [CrossRef]
- Lin, M.T.; Guo, S.; Xie, D.; Li, S.; Hu, H.K. Lipidomic profiling of wild cordyceps and its substituents by liquid chromatography-electrospray ionization-tandem mass spectrometry. LWT-Food Sci. Technol. 2022, 163, 113497. [Google Scholar] [CrossRef]
- Yu, H.M.; Wang, B.S.; Huang, S.C.; Duh, P.D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006, 54, 3132–3138. [Google Scholar] [CrossRef]
- Tang, C.Y.; Fan, Y.J.; Wang, T.; Wang, J.; Xiao, M.J.; He, M.; Chang, X.Y.; Li, Y.L.; Li, X.Z. Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants 2024, 13, 620. [Google Scholar] [CrossRef]
- Yuan, T.J.; Wang, J.; Chen, L.T.; Shan, J.J.; Di, L.Q. Glycyrrhizic acid improving the liver protective effect by restoring the composition of Lactobacillus. J. Funct. Foods 2019, 52, 219–227. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ishida, K.; Otsu, W.; Muramatsu, A.; Nakamura, S.; Yamada, W.; Tsusaki, H.; Shimoda, H.; Hara, H.; Shimazawa, M. Delphinidins from Maqui Berry (Aristotelia chilensis) ameliorate the subcellular organelle damage induced by blue light exposure in murine photoreceptor-derived cells. BMC Complement. Med. Ther. 2024, 24, 3. [Google Scholar] [CrossRef]
- Oliva, M.A.; Staffieri, S.; Sanchez, M.; Arcella, A. Isoginkgetin-A Natural Compound to Control U87MG Glioblastoma Cell Growth and Migration Activating Apoptosis and Autophagy. Molecules 2022, 27, 8335. [Google Scholar]
- Lee, J.; Choi, J.W.; Sohng, J.K.; Pandey, R.P.; Park, Y.I. The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-κB signaling pathway. Int. Immunopharmacol. 2016, 31, 88–97. [Google Scholar] [CrossRef]
- Huang, L.; Kuang, J.H.; Yu, J.Y.; Yu, Q.; Xu, W.Q.; Liu, M.Z.; Wei, Y.Y.; Han, S.Y.; Huang, Y.H.; Li, P.F. Antiviral activity of epicatechin against Singapore grouper iridovirus in vitro and in vivo. Fish. Shellfish. Immun. 2025, 162, 110331. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Yang, C.J.; Jiang, Z.; Wang, Y.; Zhu, F.; Li, T.; Wan, X.C.; Xu, Y.H.; Xie, Z.J.; Li, D.X.; et al. Epicatechin-3-Gallate Signaling and Protection against Cardiac Ischemia/Reperfusion Injury. J. Pharmacol. Exp. Ther. 2019, 371, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, C.; Xiao, M.; He, M.; Li, Y.; Li, X. Characteristics of lipid accumulation induced by high-altitude environment improve the total antioxidant capacity of Ophiocordyceps sinensis. Food Chem. 2025, 480, 143812. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Falanga, D.; Ricci, L.; Colantuono, A.; Greco, G.; Angelillo, M.; Nugnes, F.; Di Serio, T.; Costa, D.; Tito, A.; et al. NAD-Driven Sirtuin Activation by Cordyceps sinensis Extract: Exploring the Adaptogenic Potential to Promote Skin Longevity. Int. J. Mol. Sci. 2024, 25, 4282. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Sun, H.L.; Lii, C.K.; Chen, H.W.; Chen, P.Y.; Liu, K.L. Gamma-linolenic acid inhibits inflammatory responses by regulating NF-kappaB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation 2010, 33, 46–57. [Google Scholar]
- Zhang, Y.; Cong, B. The development road of Chinese characteristic food & medicine homology. Food Med. Homol. 2025, 2026, 9420104. [Google Scholar]



| Measurement Indicators | Formulas | Note | Absorbance Value |
|---|---|---|---|
| Protein | y = 4.59x + 0.176, R2 = 0.9998 | BCA method | 562 nm |
| Total polyphenols | total phenol content (mg/g) = 0.356 × (A − 0.0012) ÷ W | A = A0 − A1, A0 is the measured sample, A1 is the control sample, and W is the sample quality | 760 nm |
| Flavonoids | flavonoid content (mg/g) = 0.398 × (A − 0.0007) ÷ W | A = A0 − A1, A0 is the measured sample, A1 is the blank sample, and W is the sample quality | 510 nm |
| SOD | SOD activit (U/g) = 20 × A ÷ (100 − A) ÷ W | A = (A0 − A1) ÷ A0 × 100%, A0 is the control sample, A1 is the measured sample, and W is the sample quality | 450 nm |
| POD | POD activit (U/g) = 2000 × A ÷ W | A = A1 − A0, A0 is the absorbance value at one minute, A1 is the absorbance value at 2 min, and W is the sample quality | 470 nm |
| DPPH free radical scavenging rate | DPPH free radical scavenging rate (%) = (A1 − A0) ÷ A1 × 100% | A0 is the determination sample and A1 is the blank sample | 515 nm |
| FRAP antioxidant capacity | y = 2.4832x + 0.0134, R2 = 0.9996 | X is the concentration of Trolox (μmol/mL) | 593 nm |
| Hydroxyl radical scavenging rate | hydroxyl radical scavenging rate (%) = (A1 − A0) ÷ (A1 − A2) × 100% | A2, A1, and A0 are blank tube, control tube and measuring tube, respectively | 510 nm |
| Superoxide anion radical scavenging rate | superoxide anion scavenging rate (%) = (A1 − A0) ÷ A1 × 100% | A0 is the determination sample and A1 is the blank sample | 530 nm |
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0.0 | 95 | 5 |
| 5.0 | 35 | 65 |
| 6.0 | 1 | 99 |
| 7.5 | 1 | 99 |
| 7.6 | 95 | 5 |
| 1.0 | 95 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, B.; Tang, H.; Tang, C.; Feng, C.; Li, Y.; Li, X. Untargeted Metabolomics-Based Characterization of the Metabolic Profile and Antioxidant Activity of Ophiocordyceps sinensis and Its Substitutes. J. Fungi 2025, 11, 740. https://doi.org/10.3390/jof11100740
Jia B, Tang H, Tang C, Feng C, Li Y, Li X. Untargeted Metabolomics-Based Characterization of the Metabolic Profile and Antioxidant Activity of Ophiocordyceps sinensis and Its Substitutes. Journal of Fungi. 2025; 11(10):740. https://doi.org/10.3390/jof11100740
Chicago/Turabian StyleJia, Bing, Haoxu Tang, Chuyu Tang, Chao Feng, Yuling Li, and Xiuzhang Li. 2025. "Untargeted Metabolomics-Based Characterization of the Metabolic Profile and Antioxidant Activity of Ophiocordyceps sinensis and Its Substitutes" Journal of Fungi 11, no. 10: 740. https://doi.org/10.3390/jof11100740
APA StyleJia, B., Tang, H., Tang, C., Feng, C., Li, Y., & Li, X. (2025). Untargeted Metabolomics-Based Characterization of the Metabolic Profile and Antioxidant Activity of Ophiocordyceps sinensis and Its Substitutes. Journal of Fungi, 11(10), 740. https://doi.org/10.3390/jof11100740







