Effects of Temperature, pH, and Relative Humidity on Growth of Penicillium crustosum OM1 Isolated from Pears and Its Penitrem A Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Isolation of Fungal Strains from Pears
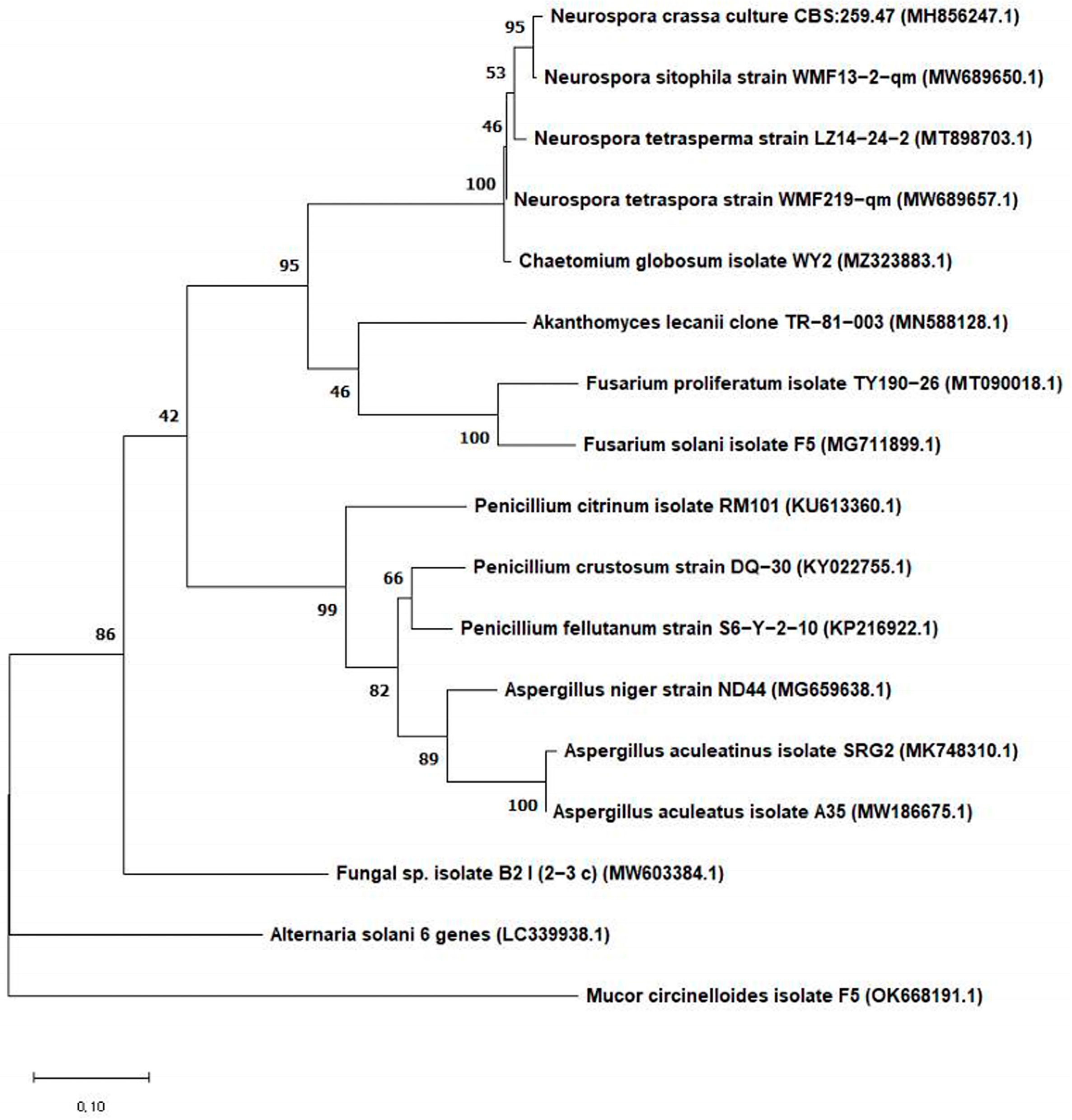
2.3. Morphological and Genetic Identification of Fungal Isolates and Phylogenetic Analysis
2.4. Fungal Culture Conditions
2.5. Measurement of Fungal Growth Rates
2.6. Patulin or Penitrem A Standard Solution
2.7. Patulin or Penitrem A Extraction
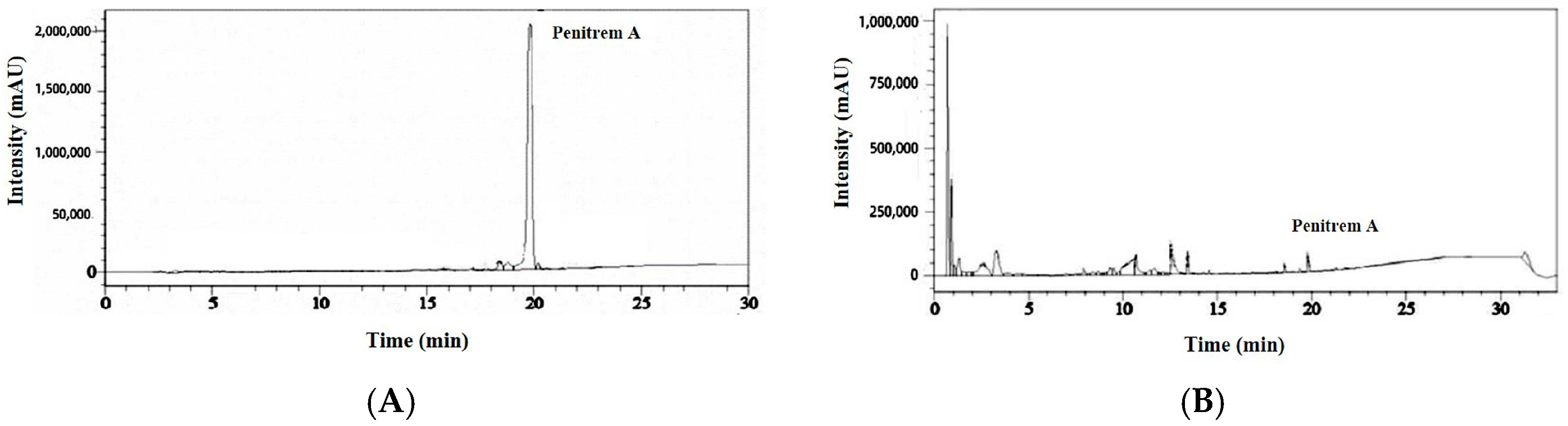
2.8. HPLC Analysis
2.9. LC/MS/MS Analysis
2.10. Relative Gene Expression Analysis by RT-qPCR
2.11. Statistical Analysis
3. Results
3.1. Isolation of Fungi from Pears, Identification of the Isolated Fungi, and Penitrem A Production by the Isolated Fungi
3.2. Effects of Different Sucrose Contents on Penitrem A Production by P. crustosum OM1
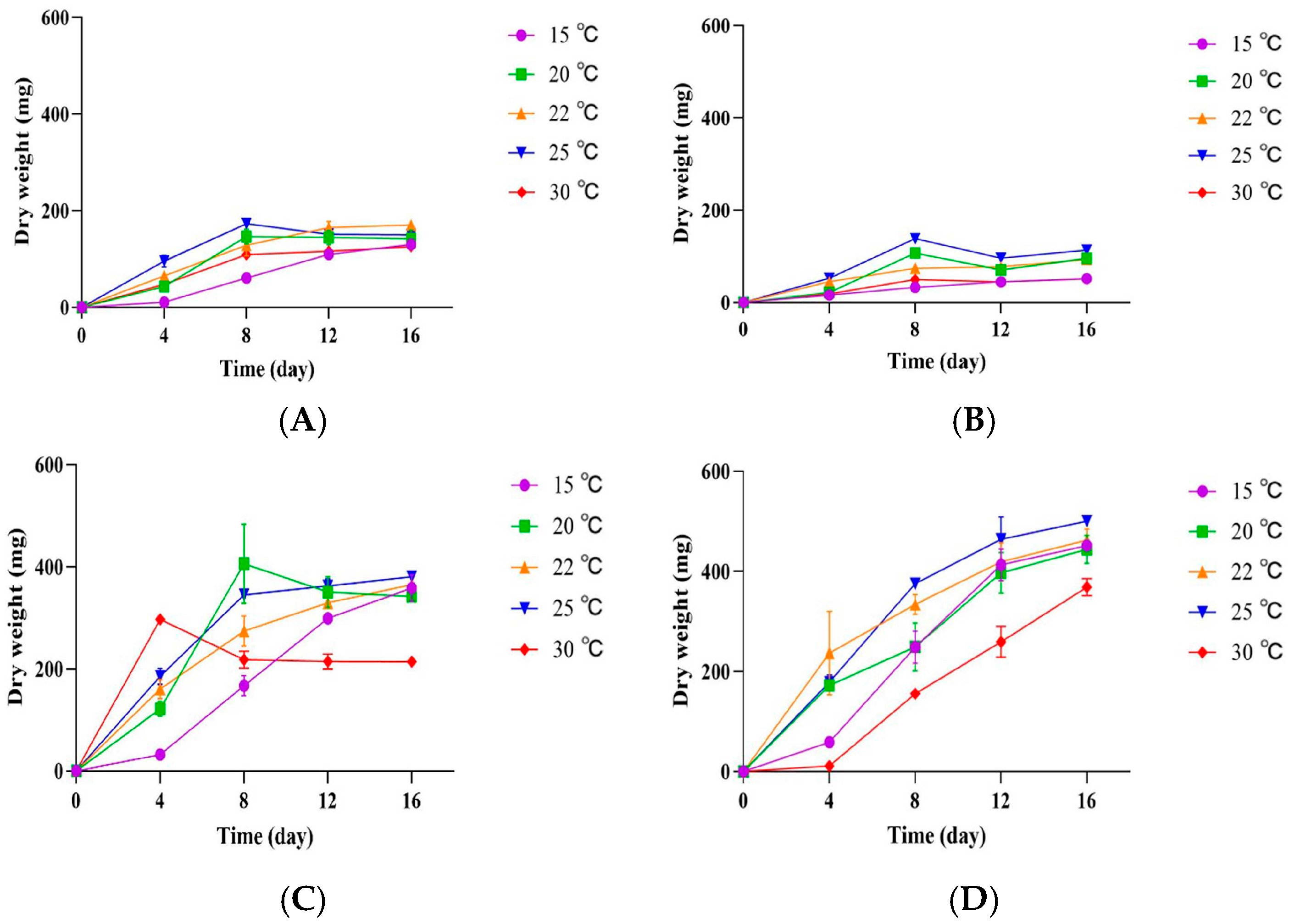
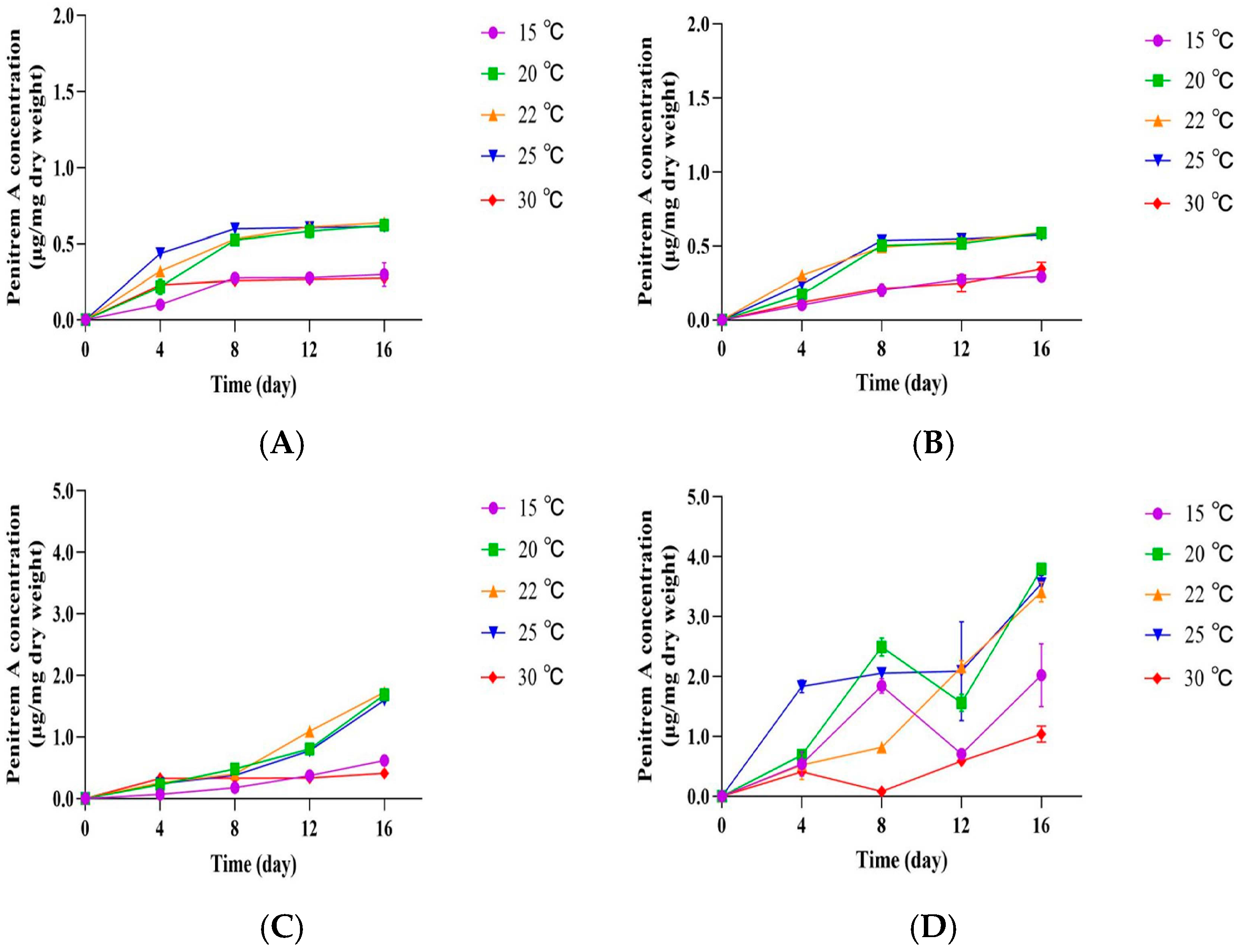
3.3. Influence of Temperature and Culture Media on the Growth of P. crustosum OM1 and Its Penitrem A Production
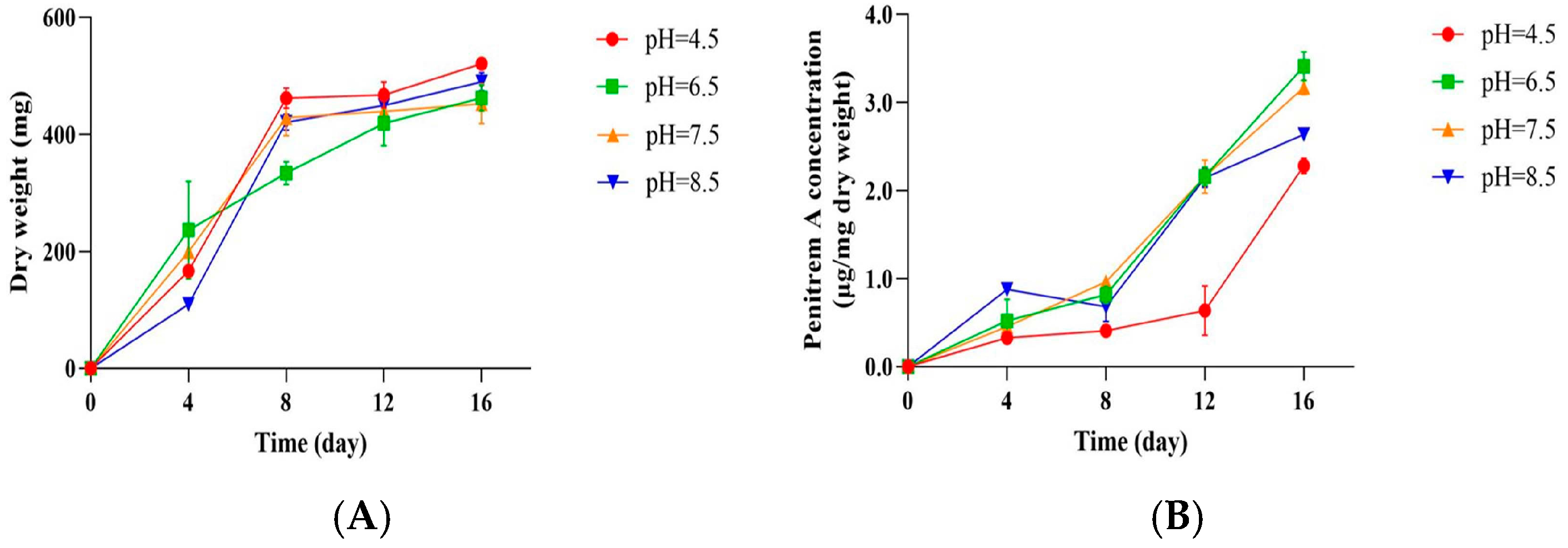
3.4. Influence of pH on the Growth of P. crustosum OM1 and Its Penitrem A Production
3.5. Growth of P. crustosum OM1 and Its Penitrem A Production on PPAM
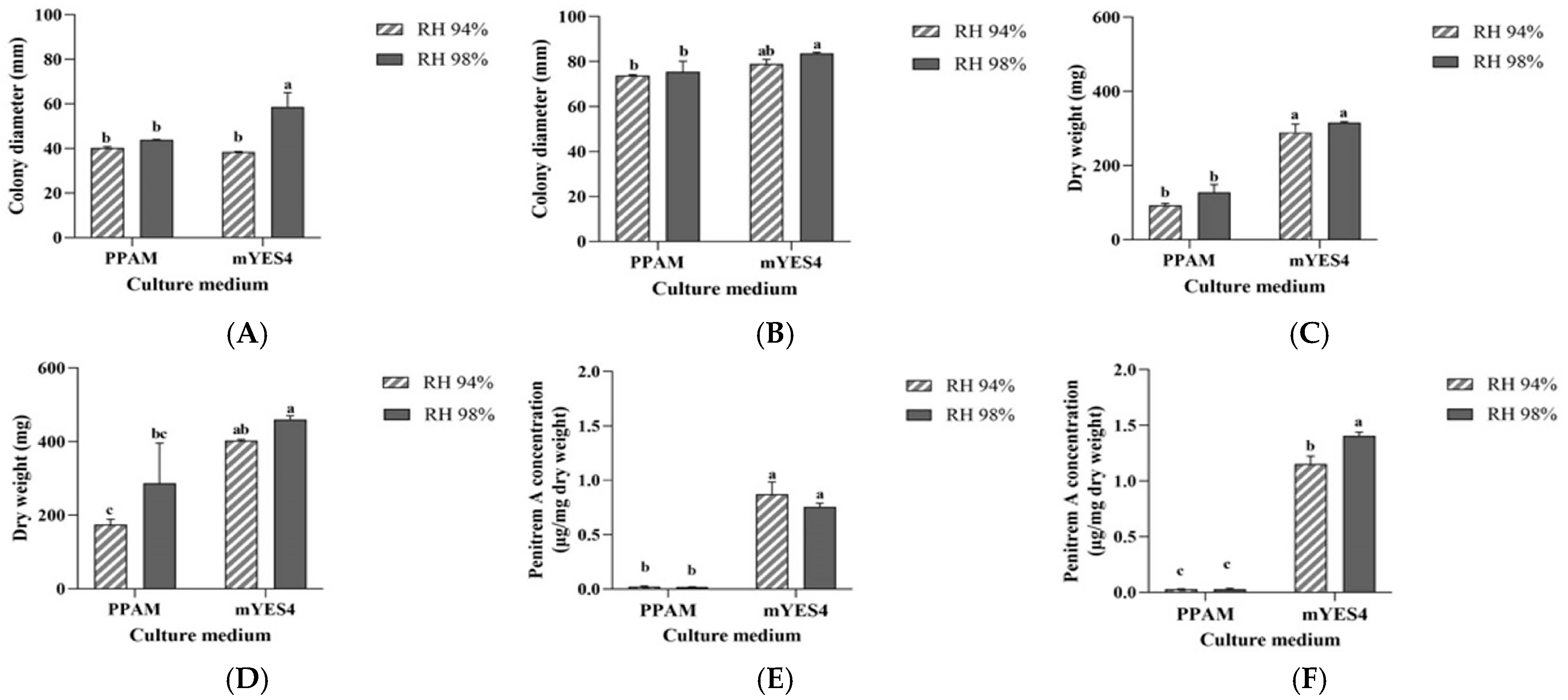
3.6. Influence of RH on the Growth of P. crustosum OM1 and Its Penitrem A Production on PPAM and mYES4
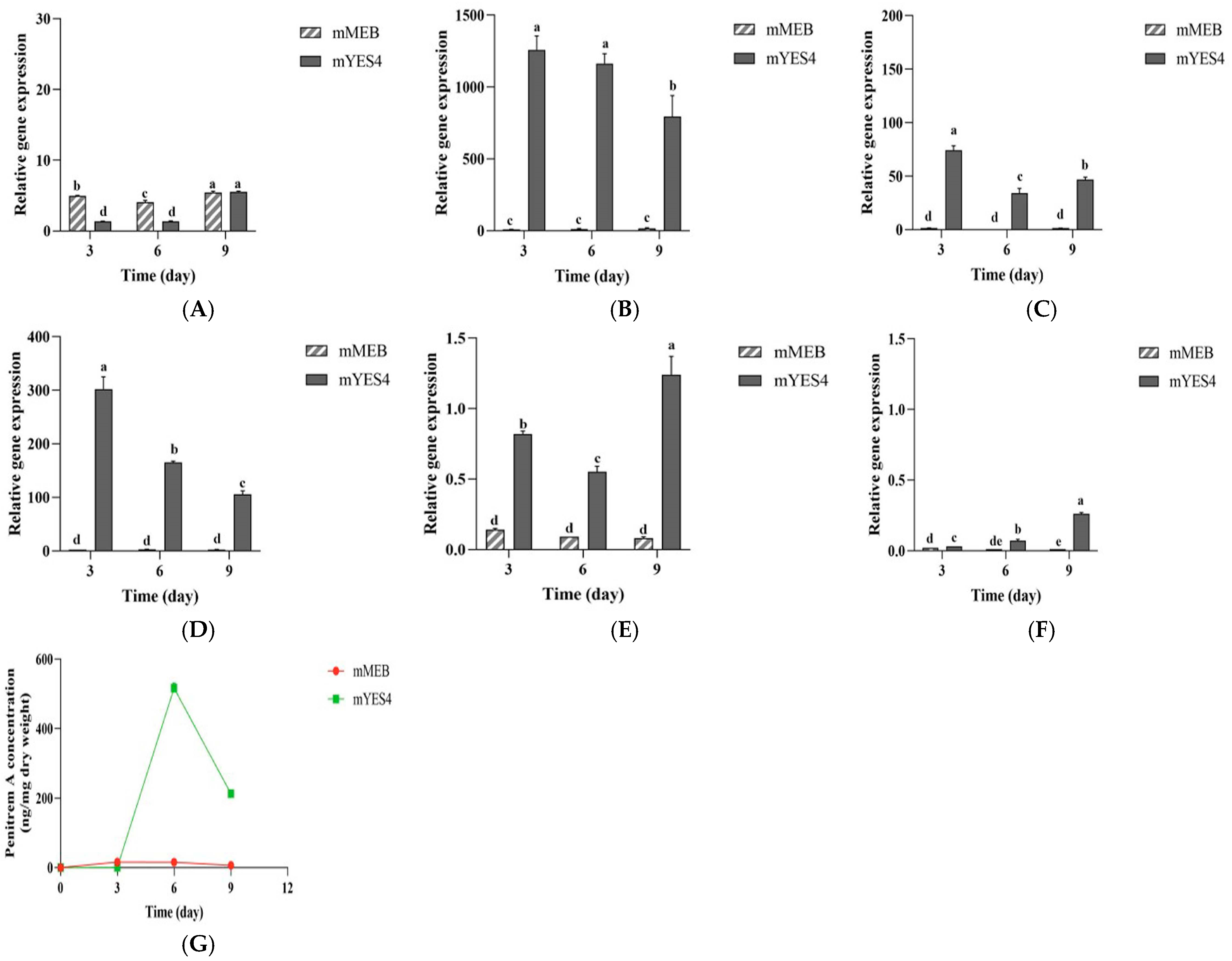
3.7. Growth of P. crustosum OM1 and Its Penitrem A Production in Liquid Media
3.8. Relative Gene Expression Analysis by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GABA | ϒ-aminobutyric acid |
| ITS | Internal transcribed spacer |
| PPAM | Pear puree agar medium |
| RH | Relative humidity |
References
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed. Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Boysen, S.R.; Rozanski, E.A.; Chan, D.L.; Grobe, T.L.; Fallon, M.J.; Rush, J.E. Tremorgenic mycotoxicosis in four dogs from a single household. J. Am. Vet. Med. Assoc. 2002, 221, 1441–1444. [Google Scholar] [CrossRef]
- Prencipe, S.; Siciliano, I.; Gatti, C.; Garibaldi, A.; Gullino, M.L.; Botta, R.; Spadaro, D. Several species of Penicillium isolated from chestnut flour processing are pathogenic on fresh chestnuts and produce mycotoxins. Food Microbiol. 2018, 76, 396–404. [Google Scholar] [CrossRef]
- Richard, J.L.; Arp, L.H. Natural occurrence of the mycotoxin penitrem A in moldy cream cheese. Mycopathologia 1979, 67, 107–109. [Google Scholar] [CrossRef]
- Kalinina, S.A.; Jagels, A.; Cramer, B.; Geisen, R.; Humpf, H.U. Influence of environmental factors on the production of penitrems A-F by Penicillium crustosum. Toxins 2017, 9, 210. [Google Scholar] [CrossRef]
- Saikia, S.; Nicholson, M.J.; Young, C.; Parker, E.J.; Scott, B. The genetic basis for indole-diterpene chemical diversity in filamentous fungi. Mycol. Res. 2008, 112, 184–199. [Google Scholar] [CrossRef]
- Arp, L.H.; Richard, J.L. Intoxication of dogs with the mycotoxin penitrem A. J. Am. Vet. Med. Assoc. 1979, 175, 565–566. [Google Scholar] [CrossRef]
- Hocking, A.D.; Holds, K.; Tobin, N.F. Intoxication by tremorgenic mycotoxin (penitrem A) in a dog. Aust. Vet. J. 1988, 65, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.R.; Donoghue, M.B.; Hocking, A.D.; Cook, L.; Granger, L.V. Tremor syndrome associated with a fungal toxin: Sequelae of food contamination. Med. J. Aust. 2005, 182, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Naudé, T.W.; O’Brien, O.M.; Rundberget, T.; McGregor, A.D.G.; Roux, C.; Flåoyen, A. Tremorgenic neuromycotoxicosis in 2 dogs ascribed to the ingestion of penitrem A and possibly roquefortine in rice contaminated with Penicillium crustosum. J. S. Afr. Vet. Assoc. 2002, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L.; Bacchetti, P.; Arp, L.H. Moldy walnut toxicosis in a dog, caused by the mycotoxin, penitrem A. Mycopathologia 1981, 76, 55–58. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Moldes-Anaya, A.; Fæste, C.K. Penitrem A and analogues: Toxicokinetics, toxicodynamics including mechanism of action and clinical significance. World Mycotoxin J. 2013, 6, 263–272. [Google Scholar] [CrossRef]
- Knaus, H.G.; Mcmanus, O.B.; Lee, S.H.; Schmalhofer, W.A.; Garciacalvo, M.; Helms, L.M.H.; Sanchez, M.; Giangiacomo, K.; Reuben, J.P.; Smith, A.B.; et al. Tremorgenic indole alkaloids potently inhibit smooth-muscle high-conductance calcium-activated potassium channels. Biochemistry 1994, 33, 5819–5828. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.S.; Fonnum, F.; Eriksen, G.S.; Rundberget, T.; Walaas, S.I.; Wigestrand, M.B. In vitro neuropharmacological evaluation of penitrem-induced tremorgenic syndromes: Importance of the GABAergic system. Neurochem. Int. 2011, 59, 1074–1081. [Google Scholar] [CrossRef]
- Norris, P.J.; Smith, C.C.T.; Debelleroche, J.; Bradford, H.F.; Mantle, P.G.; Thomas, A.J.; Penny, R.H.C. Actions of tremorgenic fungal toxins on neurotransmitter release. J. Neurochem. 1980, 34, 33–42. [Google Scholar] [CrossRef]
- Ozaki, T.; Minami, A.; Oikawa, H. Biosynthesis of indole diterpenes: A reconstitution approach in a heterologous host. Nat. Prod. Rep. 2023, 40, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Garello, M.; Piombo, E.; Buonsenso, F.; Prencipe, S.; Valente, S.; Meloni, G.R.; Marcet-Houben, M.; Gabaldón, T.; Spadaro, D. Several secondary metabolite gene clusters in the genomes of ten Penicillium spp. raise the risk of multiple mycotoxin occurrence in chestnuts. Food Microbiol. 2024, 122, 104532. [Google Scholar] [CrossRef]
- Liu, C.W.; Tagami, K.; Minami, A.; Matsumoto, T.; Frisvad, J.C.; Suzuki, H.; Ishikawa, J.; Gomi, K.; Oikawa, H. Reconstitution of biosynthetic machinery for the synthesis of the highly elaborated indole diterpene penitrem. Angew. Chem. Int. Edit 2015, 54, 5748–5752. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- Zheng, X.F.; Wei, W.N.; Zhou, W.Y.; Li, H.X.; Rao, S.Q.; Gao, L.; Yang, Z.Q. Prevention and detoxification of patulin in apple and its products: A review. Food Res. Int. 2021, 140, 110034. [Google Scholar] [CrossRef] [PubMed]
- Boysen, M.; Skouboe, P.; Frisvad, J.; Rossen, L. Reclassification of the Penicillium roqueforti group into three species on the basis of molecular genetic and biochemical profiles. Microbiology 1996, 142, 541–549. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, A.A.; Leistner, L. Production of penitrem A by Penicillium crustosum isolated from foodstuffs. Int. J. Food Microbiol. 1988, 7, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Louw, J.P.; Korsten, L. Pathogenic Penicillium spp. on apple and pear. Plant Dis. 2014, 98, 590–598. [Google Scholar] [CrossRef]
- Sonjak, S.; Frisvad, J.C.; Gunde-Cimerman, N. Comparison of secondary metabolite production by Penicillium crustosum strains, isolated from Arctic and other various ecological niches. FEMS Microbiol. Ecol. 2005, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sumarah, M.W.; Miller, J.D.; Blackwell, B.A. Isolation and metabolite production by Penicilliun roqueforti, Penicillium paneum and Penicillium crustosum isolated in Canada. Mycopathologia 2005, 159, 571–577. [Google Scholar] [CrossRef]
- Rundberget, T.; Skaar, I.; O’Brien, O.; Flåoyen, A. Penitrem and thomitrem formation by Penicillium crustosum. Mycopathologia 2004, 157, 349–357. [Google Scholar] [CrossRef]
- Dantigny, P.; Guilmart, A.; Bensoussan, M. Basis of predictive mycology. Int. J. Food Microbiol. 2005, 100, 187–196. [Google Scholar] [CrossRef]
- Tannous, J.; Atoui, A.; El Khoury, A.; Francis, Z.; Oswald, I.P.; Puel, O.; Lteif, R. A study on the physicochemical parameters for Penicillium expansum growth and patulin production: Effect of temperature, pH, and water activity. Food Sci. Nutr. 2016, 4, 611–622. [Google Scholar] [CrossRef]
- Zhao, W.C.; Hong, S.Y.; Kim, J.Y.; Om, A.S. Effects of temperature, pH, and relative humidity on the growth of Penicillium paneum OM1 isolated from pears and its patulin production. Fungal Biol. 2024, 128, 1885–1897. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Hong, S.B.; Go, S.J.; Shin, H.D.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 2005, 97, 1316–1329. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Lee, S.B.; Taylor, J.W. Isolation of DNA from fungal mycelia and single spores. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 282–287. [Google Scholar]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-joining method—A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Rockland, L.B. Saturated salt solutions for static control of relative humidity between 5-degrees-C and 40-degrees-C. Anal. Chem. 1960, 32, 1375–1376. [Google Scholar] [CrossRef]
- Winston, P.W.; Bates, D.H. Saturated solutions for the control of humidity in biological research. Ecology 1960, 41, 232–237. [Google Scholar] [CrossRef]
- AOACInternational. Official method 995.10. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Moldes-Anaya, A.; Rundberget, T.; Fæste, C.K.; Eriksen, G.S.; Bernhoft, A. Neurotoxicity of Penicillium crustosum secondary metabolites: Tremorgenic activity of orally administered penitrem A and thomitrem A and E in mice. Toxicon 2012, 60, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P.G.; Perera, K.P.W.C.; Maishman, N.J.; Mundy, G.R. Biosynthesis of penitrems and roquefortine by Penicillium crustosum. Appl. Environ. Microbiol. 1983, 45, 1486–1490. [Google Scholar] [CrossRef]
- Wagener, R.E.; Davis, N.D.; Diener, U.L. Penitrem A and roquefortine production by Penicillium commune. Appl. Environ. Microbiol. 1980, 39, 882–887. [Google Scholar] [CrossRef]
- Guo, J.W.; Starr, D.; Guo, H.Z. Classification and review of free PCR primer design software. Bioinformatics 2020, 36, 5263–5268. [Google Scholar] [CrossRef]
- Rodriguez, A.; Rodriguez, M.; Cordoba, J.J.; Andrade, M.J. Design of primers and probes for quantitative real-time PCR methods. Methods Mol. Biol. 2015, 1275, 31–56. [Google Scholar] [CrossRef]
- Singh, J.; Padgett, R.A. Rates of in situ transcription and splicing in large human genes. Nat. Struct. Mol. Biol. 2009, 16, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Gonda, M.; Rufo, C.; Cecchetto, G.; Vero, S. Evaluation of different hurdles on Penicillium crustosum growth in sponge cakes by means of a specific real time PCR. J. Food Sci. Technol. 2019, 56, 2195–2204. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, D.M.; Osborne, S.; Kazan, K.; Manners, J.M. Low pH regulates the production of deoxynivalenol by Fusarium graminearum. Microbiology 2009, 155, 3149–3156. [Google Scholar] [CrossRef] [PubMed]
- Jimdjio, C.K.; Xue, H.L.; Bi, Y.; Nan, M.N.; Li, L.; Zhang, R.; Liu, Q.L.; Pu, L.M. Effect of ambient pH on growth, pathogenicity, and patulin production of Penicillium expansum. Toxins 2021, 13, 550. [Google Scholar] [CrossRef]
- Plumridge, A.; Hesse, S.J.A.; Watson, A.J.; Lowe, K.C.; Stratford, M.; Archer, D.B. The weak acid preservative sorbic acid inhibits conidial germination and mycelial growth of Aspergillus niger through intracellular acidification. Appl. Environ. Microbiol. 2004, 70, 3506–3511. [Google Scholar] [CrossRef]
- Jimenez, M.; Mateo, R.; Mateo, J.J.; Huerta, T.; Hernandez, E. Effect of the incubation conditions on the production of patulin by Penicillium griseofulvum Isolated from wheat. Mycopathologia 1991, 115, 163–168. [Google Scholar] [CrossRef]
- Holmquist, G.U.; Walker, H.W.; Stahr, H.M. Influence of temperature, pH, water activity and anti-fungal agents on growth of Aspergillus flavus and Aspergillus parasiticus. J. Food Sci. 1983, 48, 778–782. [Google Scholar] [CrossRef]
- Morales, H.; Barros, G.; Marín, S.; Chulze, S.; Ramos, A.J.; Sanchis, V. Effects of apple and pear varieties and pH on patulin accumulation by Penicillium expansum. J. Sci. Food Agric. 2008, 88, 2738–2743. [Google Scholar] [CrossRef]
- Oyom, W.; Li, Y.C.; Prusky, D.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Recent advances in postharvest technology of Asia pears fungi disease control: A review. Physiol. Mol. Plant Pathol. 2022, 117, 101771. [Google Scholar] [CrossRef]
- Sardella, D.; Muscat, A.; Brincat, J.P.; Gatt, R.; Decelis, S.; Valdramidis, V. A comprehensive review of the pear fungal diseases. Int. J. Fruit Sci. 2016, 16, 351–377. [Google Scholar] [CrossRef]
- Zambounis, A.; Ganopoulos, I.; Tsaftaris, A.; Valasiadis, D.; Madesis, P. Metagenomics analysis of fungal communities associated with postharvest diseases in pear fruits under the effect of management practices. Arch. Microbiol. 2020, 202, 2391–2400. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, 201–241. [Google Scholar]
- Frisvad, J.C. The connection between the penicillia and aspergilli and mycotoxins with special emphasis on misidentified isolates. Arch. Environ. Con Tox 1989, 18, 452–467. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Filtenborg, O. Terverticillate penicillia—Chemotaxonomy and mycotoxin production. Mycologia 1989, 81, 837–861. [Google Scholar] [CrossRef]
- Kumar, D.; Barad, S.; Chen, Y.; Luo, X.Y.; Tannous, J.; Dubey, A.; Matana, N.G.; Tian, S.P.; Li, B.Q.; Keller, N.; et al. LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose. Mol. Plant Pathol. 2017, 18, 1150–1163. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast extract: Characteristics, production, applications and future perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Yim, S.H.; Nam, S.H. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar] [CrossRef]
- Pitt, J.I. Biology and ecology of toxigenic Penicillium species. Adv. Exp. Med. Biol. 2002, 504, 29–41. [Google Scholar] [PubMed]
- Mislivec, P.B.; Dieter, C.T.; Bruce, V.R. Effect of temperature and relative humidity on spore germination of mycotoxic species of Aspergillus and Penicillium. Mycologia 1975, 67, 1187–1189. [Google Scholar] [CrossRef]
- Barberel, S.I.; Walker, J.R.L. The effect of aeration upon the secondary metabolism of microorganisms. Biotechnol. Genet. Eng. Rev. 2000, 17, 281–323. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.N.; Marth, E.H. Aflatoxin formation, lipid-synthesis, and glucose metabolism by Aspergillus parasiticus during incubation with and without agitation. Biochim. Biophys. Acta 1974, 338, 286–296. [Google Scholar] [CrossRef]

| Gene | Orientation | Primer Sequence (5′ → 3′) |
|---|---|---|
| ptmB | Forward | GTCCTGCACTGTGGCTTTTG |
| Reverse | CTCCACAAAACCAGAGGGCT | |
| ptmJ | Forward | ACTCCAGTCTGCACCCATTG |
| Reverse | TCACGCAGAGACTCAGGAGA | |
| ptmK | Forward | CTGACCCAGCAGATCAGACG |
| Reverse | CGTGGTGCCCAATAGACCAT | |
| ptmO | Forward | CCGCAAAACACGAGAAGAGC |
| Reverse | TGGTTCAATGTCGGCGTGTA | |
| ptmS | Forward | CCCAAGGGCACTACTTTGGT |
| Reverse | AAAGAGGACGATTGCTGCGA | |
| ptmT | Forward | GGAATCATGTCGGGCATTGC |
| Reverse | TGAAGACCACAGTGCCGATC | |
| β-tubulin | Forward | GTCCAACGACAGGAAACCGA |
| Reverse | CAGGCTCCAAATCGACGAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Hong, S.-Y.; Jung, G.-M.; Om, A.-S. Effects of Temperature, pH, and Relative Humidity on Growth of Penicillium crustosum OM1 Isolated from Pears and Its Penitrem A Production. J. Fungi 2025, 11, 741. https://doi.org/10.3390/jof11100741
Gao S, Hong S-Y, Jung G-M, Om A-S. Effects of Temperature, pH, and Relative Humidity on Growth of Penicillium crustosum OM1 Isolated from Pears and Its Penitrem A Production. Journal of Fungi. 2025; 11(10):741. https://doi.org/10.3390/jof11100741
Chicago/Turabian StyleGao, Shengming, Sung-Yong Hong, Gyu-Mi Jung, and Ae-Son Om. 2025. "Effects of Temperature, pH, and Relative Humidity on Growth of Penicillium crustosum OM1 Isolated from Pears and Its Penitrem A Production" Journal of Fungi 11, no. 10: 741. https://doi.org/10.3390/jof11100741
APA StyleGao, S., Hong, S.-Y., Jung, G.-M., & Om, A.-S. (2025). Effects of Temperature, pH, and Relative Humidity on Growth of Penicillium crustosum OM1 Isolated from Pears and Its Penitrem A Production. Journal of Fungi, 11(10), 741. https://doi.org/10.3390/jof11100741






