Molecular Identification, Mycelial Growth Kinetics, and Antimicrobial Potential of Newly Isolated Medicinal Mushroom Fomitopsis pinicola from Bulgaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation, In Vitro Cultivation, and Morphological Characterization
2.2. Molecular Identification by ITS1-5.8S-ITS2 rRNA Gene Sequence Analysis
2.3. Phylogenetic Analysis
2.4. Cultivation Procedure and Kinetic Modeling of the Process
2.5. Determination of Antimicrobial Activity
2.5.1. Submerged Cultivation of Fomitopsis pinicola
2.5.2. Preparation of Extracts from Mycelial Biomass
2.5.3. Determination of the Minimum Inhibitory Concentration (MIC) of Fomitopsis pinicola extracts
2.6. Statistical Analysis
3. Results and Discussion
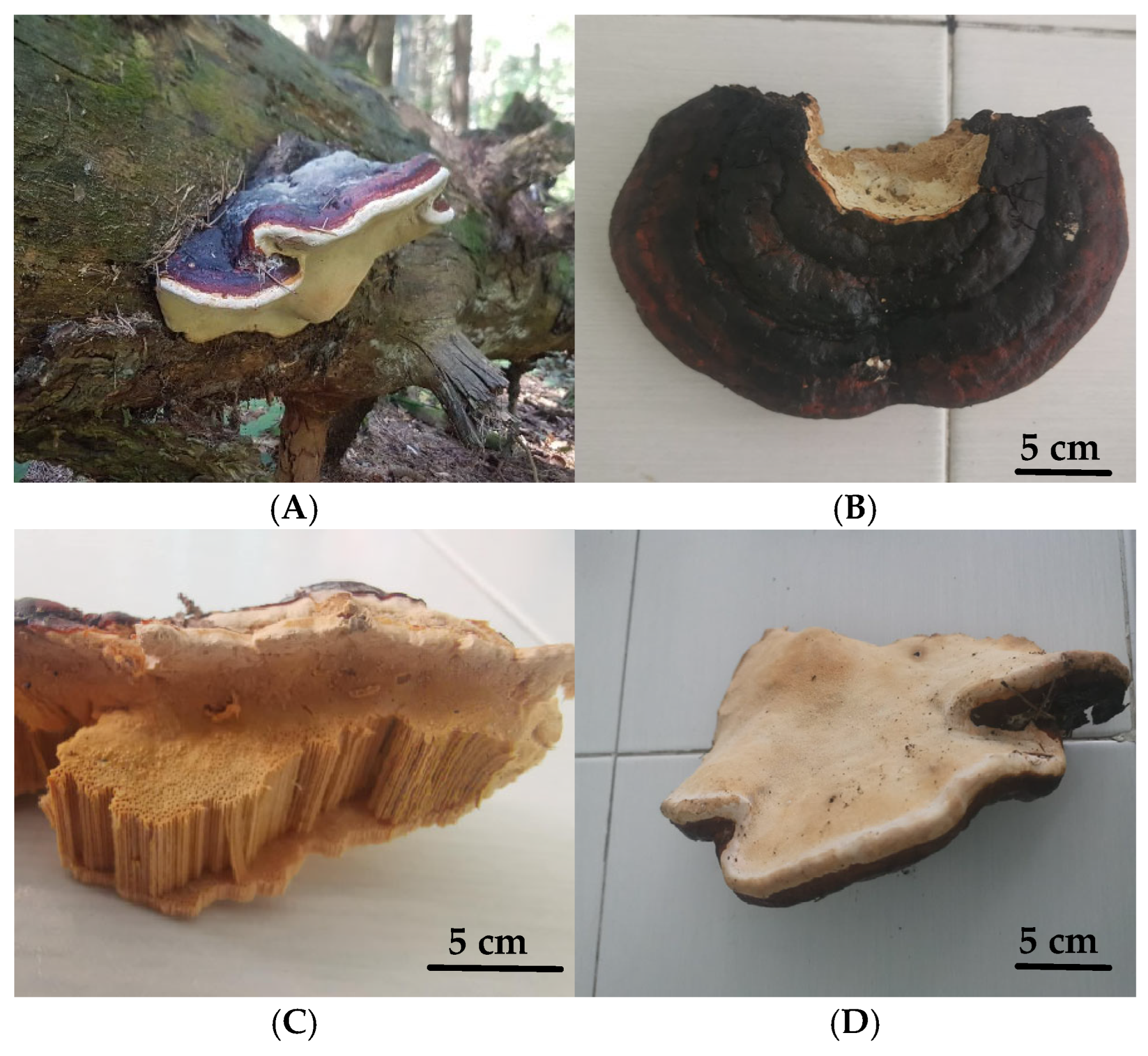
3.1. Morphological Characterization
3.2. Molecular Identification and Phylogenetic Analysis
3.3. Kinetic Modeling of the Process
3.4. Antimicrobial Activity of F. pinicola Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RBCA | Rose Bengal Chloramphenicol Agar |
| MCM | Mushroom Complete Medium |
| UPGMA | Unweighted Pair Group Method using the Arithmetic Average |
| CD | Czapek Dox |
| GP | Glucose-peptone |
| MEA | Malt extract agar |
| MYE | Malt yeast extract |
| PDA | Potato dextrose agar |
| YEA | Yeast extract agar |
| YGC | Yeast glucose chloramphenicol |
| YMA | Yeast extract malt extract agar |
| DW | Dry weight |
| MIC | Minimum Inhibitory Concentration |
| SD | Standard deviation |
| ANOVA | Analysis of Variance |
References
- Fu, C.; Song, Y.; Zhang, D.; Wang, H.; Chen, X.; Li, J. Whole genome sequencing and analysis of Inonotus hispidus isolated from the Chengde Mountain Resort, China. Res. Sq. 2024, preprint. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.; Duan, Y.; Wang, P.; Qi, J.; Gao, J.-M.; Liu, C. Biosynthesis of sesquiterpenes in basidiomycetes: A review. J. Fungi 2022, 8, 913. [Google Scholar] [CrossRef] [PubMed]
- Halbwachs, H.; Harper, C.J.; Krings, M. Fossil Ascomycota and Basidiomycota, with notes on fossil lichens and nematophytes. Encycl. Mycol. 2021, 1, 378–395. [Google Scholar] [CrossRef]
- Ghobad-Nejhad, M.; Dima, B.; Cui, B.-K.; Si, J. Editorial: Basidiomycete fungi: From biosystematics and biodiversity to biotechnology. Front. Microbiol. 2023, 14, 1128319. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska-Ziaja, K.; Szewczyk, A.; Galanty, A.; Gdula-Argasińska, J.; Muszyńska, B. Chemical composition and biological activity of extracts from fruiting bodies and mycelial cultures of Fomitopsis betulina. Mol. Biol. Rep. 2018, 45, 2535–2544. [Google Scholar] [CrossRef]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Mowna Sundari, T.; Alwin Prem Anand, A.; Jenifer, P.; Shenbagarathai, R. Bioprospection of Basidiomycetes and molecular phylogenetic analysis using internal transcribed spacer (ITS) and 5.8S rRNA gene sequence. Sci. Rep. 2018, 8, 29046. [Google Scholar] [CrossRef]
- Hillis, D.M. Phylogenetic analysis. Curr. Biol. 1997, 7, R129–R131. [Google Scholar] [CrossRef]
- Bishop, K.S. Characterisation of extracts and anti-cancer activities of Fomitopsis pinicola. Nutrients 2020, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, C.G.; Xu, T.M.; Ji, X.; Wu, D.M.; Cui, B.K. Species diversity, molecular phylogeny, and ecological habits of Fomitopsis (Polyporales, Basidiomycota). Front. Microbiol. 2022, 13, 859411. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Chen, Y.Y.; Shen, L.L.; Song, J.; Vlasák, J.; Dai, Y.C.; Cui, B.K. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 2016, 80, 343–373. [Google Scholar] [CrossRef]
- Liu, S.; Han, M.L.; Xu, T.M.; Wang, Y.; Wu, D.M.; Cui, B.K. Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from East Asia. Front. Microbiol. 2021, 12, 644979. [Google Scholar] [CrossRef]
- Sigoillot, J.C.; Berrin, J.G.; Bey, M.; Lesage-Meessen, L.; Levasseur, A.; Lomascolo, A.; Record, E.; Uzan-Boukhris, E. Fungal Strategies for Lignin Degradation. Adv. Bot. Res. 2012, 61, 263–308. [Google Scholar] [CrossRef]
- Varghese, R.; Dalvi, Y.B.; Lamrood, P.Y.; Shinde, B.P.; Nair, C.K.K. Historical and current perspectives on therapeutic potential of higher basidiomycetes: An overview. 3 Biotech 2019, 9, 1886. [Google Scholar] [CrossRef]
- Wainwright, M. Some highlights in the history of fungi in medicine—A personal journey. Fungal Biol. Rev. 2008, 22, 97–102. [Google Scholar] [CrossRef]
- Kurchenko, V.P.; Sushinskaya, N.V.; Kiseleva, I.S.; Ermoshin, A.A. Biologically active substances in fruit bodies of wood decomposing fungi. In Proceedings of the Actual Problems of Organic Chemistry and Biotechnology (OCBT2020): Proceedings of the International Scientific Conference, Ekaterinburg, Russia, 18–21 November 2022; American Institute of Physics Inc.: College Park, MD, USA, 2022; p. 2390. [Google Scholar] [CrossRef]
- Zahid, T.M.; Idrees, M.; Ying, W.; Zaki, H.A.; Abdullah, I.; Haiying, B. Review of chemical constituents and pharmacology of brown-rot fungus Fomitopsis pinicola. J. Nat. Sci. Res. 2020, 10, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Wang, P.; Wang, L.; Fan, J.; Wang, X.; Liu, Q. Investigating migration inhibition and apoptotic effects of Fomitopsis pinicola chloroform extract on human colorectal cancer SW-480 cells. PLoS ONE 2014, 9, e101303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Lin, C.Y.; Lur, H.S.; Chen, H.P.; Lu, M.K. Properties and biological functions of polysaccharides and ethanolic extracts isolated from medicinal fungus, Fomitopsis pinicola. Process Biochem. 2008, 43, 829–834. [Google Scholar] [CrossRef]
- Ravikumar, K.S.; Ramya, H.; Ajith, T.A.; Shah, M.A.; Janardhanan, K.K. Bioactive extract of Fomitopsis pinicola rich in 11-α-acetoxykhivorin mediates anticancer activity by cytotoxicity, induction of apoptosis, inhibition of tumor growth, angiogenesis and cell cycle progression. J. Funct. Foods 2021, 78, 104372. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Ye, L.; Wu, L.; Su, C.; Iyu, X.; Fu, J. The protective effect and potential mechanism analysis of Fomitopsis pinicola mycelia polysaccharides (FPMPS) on acute alcoholic liver injury in mice. 2023, preprint. Authorea 2023. preprint. [Google Scholar]
- Taylor, T.N.; Krings, M.; Taylor, E.L. Basidiomycota. Foss. Fungi 2015, 173–199. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Krupodorova, T.; Barshteyn, V.; Sekan, A. Review of the basic cultivation conditions influence on the growth of basidiomycetes. Curr. Res. Environ. Appl. Mycol. 2021, 11, 494–531. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhu, L.W.; Li, H.M.; Li, D.S. Submerged culture of mushrooms in bioreactors—Challenges, current state-of-the-art, and future prospects. Food Technol. Biotechnol. 2007, 45, 221–229. [Google Scholar]
- Angelova, G.; Stefanova, P.; Brazkova, M.; Krastanov, A. Molecular and morphological characterization of Xylaria karsticola (Ascomycota) isolated from the fruiting body of Macrolepiota procera (Basidiomycota) from Bulgaria. PLoS ONE 2023, 18, e0287679. [Google Scholar] [CrossRef]
- Stefanova, P.; Brazkova, M.; Angelova, G. Comparative study of DNA extraction methods for identification of medicinal mushrooms. BIO Web Conf. 2022, 45, 02007. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Gene Bank Database. Available online: https://www.ncbi.nlm.nih.gov (accessed on 5 August 2025).
- QIAGEN Digital Insights. Available online: https://digitalinsights.qiagen.com (accessed on 25 August 2025).
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Jo, W.S.; Kim, D.G.; Seok, S.J.; Jung, H.Y.; Park, S.C. The culture conditions for the mycelial growth of Auricularia auricula-judae. J. Mushrooms 2014, 12, 88–95. [Google Scholar] [CrossRef]
- Bouguettoucha, A.; Balannec, B.; Amrane, A. Unstructured models for lactic acid fermentation—A review. Food Technol. Biotechnol. 2011, 49, 3–12. [Google Scholar]
- Ibarz, A.; Augusto, P.E. An autocatalytic kinetic model for describing microbial growth during fermentation. Bioprocess Biosyst. Eng. 2015, 38, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Al-Zahrani, S.M.; Lee, S.Y. Kinetic model-based feed-forward controlled fed-batch fermentation of Lactobacillus rhamnosus for the production of lactic acid from Arabic date juice. Bioprocess Biosyst. Eng. 2014, 37, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Kemmer, G.; Keller, S. Nonlinear least-squares data fitting in Excel spreadsheets. Nat. Protoc. 2010, 5, 267–281. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed.; CLSI Document M02-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Bower, J.A. Statistics for food science V: ANOVA and multiple comparisons (Part B). Nutr. Food Sci. 1998, 98, 41–48. [Google Scholar] [CrossRef]
- Gáper, J.; Gáperová, S.; Pristaš, P.; Šebesta, M.; Kollárová, P.; Gallay, I.; Slobodník, B. The geographical distribution, trophic modes, and host preferences of Fomitopsis pinicola in Central Europe: A comprehensive review. Cent. Eur. For. J. 2025, 71, 73–82. [Google Scholar] [CrossRef]
- Dresch, P.; D’Aguanno, M.N.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. AMB Express 2015, 5, 4. [Google Scholar] [CrossRef]
- Gaude, N.; Bortfeld, S.; Erban, A.; Kopka, J.; Krajinski, F. Symbiosis-dependent accumulation of primary metabolites in arbuscule-containing cells. BMC Plant Biol. 2015, 15, 234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pekşen, A.; Kibar, B. Effects of various carbon and nitrogen sources on mycelial biomass production of Macrolepiota procera and Polyporus squamosus in submerged culture. Anadolu Tarım Bilim. Derg. 2016, 31, 16–24. [Google Scholar] [CrossRef]
- Wiriya, J.; Kavinlertvatana, P.; Lumyong, S. Effects of different culture media, carbon and nitrogen sources and solid substrates on growth of Termitomyces mushrooms. Chiang Mai J. Sci. 2014, 41, 542–546. [Google Scholar]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Abdel Aziz, N.H.; Yousef, N.S.; El-Haddad, M.E.; El-Tayeb, T.S. Influence of nutritional and climatic conditions on mycelial growth of three oyster mushroom strains. Arab Univ. J. Agric. Sci. 2018, 26, 1165–1173. [Google Scholar] [CrossRef]
- Arana-Gabriel, Y.; Burrola-Aguilar, C.; Alcala-Adan, A.; Zepeda-Gomez, C.; Estrada Zuniga, M.E. Mycelial growth of the edible wild mushroom Floccularia luteovirens in different culture mediums and pH. Agro Prod. 2020, 13, 33–38. [Google Scholar] [CrossRef]
- Muthu, N.; Shanmugasundaram, K. Effect of five different culture media on mycelial growth of Agrocybe aegerita. Int. J. Pharm. Sci. Res. 2015, 6, 5193–5197. [Google Scholar] [CrossRef]
- Gbolagade, J.S.; Fasidi, I.O.; Ajayi, E.J.; Sobowale, A.A. Effect of physico-chemical factors and semi-synthetic media on vegetative growth of Lentinus subnudus (Berk.), an edible mushroom from Nigeria. Food Chem. 2006, 99, 742–747. [Google Scholar] [CrossRef]
- Alam, N.; Shim, M.J.; Lee, M.W.; Shin, P.G.; Yoo, Y.B.; Lee, T.S. Vegetative growth and phylogenetic relationship of commercially cultivated strains of Pleurotus eryngii based on ITS sequence and RAPD. Mycobiology 2009, 37, 258–266. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Park, J.P.; Cho, Y.J.; Song, C.H.; Yun, J.W. Mycelial growth and exo-biopolymer production by submerged culture of various edible mushrooms under different media. Lett. Appl. Microbiol. 2002, 34, 56–61. [Google Scholar] [CrossRef]
- Fletcher, I.; Freer, A.; Ahmed, A.; Fitzgerald, P. Effect of temperature and growth media on mycelium growth of Pleurotus ostreatus and Ganoderma lucidum strains. Cohes. J. Microbiol. Infect. Dis. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Jo, W.S.; Kang, M.G.; Choi, S.Y.; Yoo, Y.B.; Seok, S.J.; Jung, H.Y. Culture conditions for mycelial growth of Coriolus versicolor. Mycobiology 2010, 38, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.S.; Cho, Y.J.; Cho, D.H.; Park, S.D.; Yoo, Y.B.; Seok, S.J. Culture conditions for the mycelial growth of Ganoderma applanatum. Mycobiology 2009, 37, 94–102. [Google Scholar] [CrossRef]
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.; Monte, A. Antibacterial Compounds from Mushrooms II: Lanostane Triterpenoids and an Ergostane Steroid With Activity Against Bacillus cereus Isolated from Fomitopsis Pinicola. Planta Medica 2010, 76, 464–466. [Google Scholar] [CrossRef]
- Keller, A.C.; Maillard, M.P.; Hostettmann, K. Antimicrobial Steroids from the Fungus Fomitopsis Pinicola. Phytochemistry 1996, 41, 1041–1046. [Google Scholar] [CrossRef]
- Karaca, B.; Kyalo Kilonzo, N.; Korkmaz, Ş.; Onar, O.; Yıldırım, Ö.; Çöleri Cihan, A. The Dual Role of the Medicinal Mushroom Fomitopsis pinicola in Inhibiting Biofilm and Reducing Antibiotic Resistance of Methicillin-Resistant Staphylococcus aureus. Food Sci. Nutr. 2025, 13, e70355. [Google Scholar] [CrossRef]
- Pala, S.A.; Wani, A.H.; Ganai, B.A. Antimicrobial Potential of Some Wild Macromycetes Collected from Kashmir Himalayas. Plant Sci. Today 2019, 6, 137–146. [Google Scholar] [CrossRef]
- Huguet, C.; Bourjot, M.; Bellanger, J.M.; Prévost, G.; Urbain, A. Screening for Antibacterial Activity of French Mushrooms Against Pathogenic and Multidrug Resistant Bacteria. Appl. Sci. 2022, 12, 5229. [Google Scholar] [CrossRef]
- Bragina, O.; Kuhtinskaja, M.; Elisashvili, V.; Asatiani, M.; Kulp, M. Antibacterial Properties of Submerged Cultivated Fomitopsis pinicola, Targeting Gram-Negative Pathogens, Including Borrelia burgdorferi. Sci. 2025, 7, 104. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Martins, A.; Pintado, M. Antimicrobial Activity of Wild Mushroom Extracts Against Clinical Isolates Resistant To Different Antibiotics. J. Appl. Microbiol. 2012, 113, 466–475. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Berhe Sbhatu, D. Determination of Antimicrobial Activity of Extracts of Indigenous Wild Mushrooms against Pathogenic Organisms. eCAM 2019, 18, 6212673. [Google Scholar] [CrossRef] [PubMed]




| Component, g/L | Culture Media | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | GP | Hennerberg | Hopkins | Leonian | MCM | MEA | MYE | PDA | YEA | YGC | YMA | |
| Glucose | - | 10.0 | 50.0 | 10.0 | 25.0 | 20.0 | - | 10.0 | 20.0 | 10.0 | 20.0 | 10.0 |
| Sucrose | 30 | - | - | - | - | - | - | - | - | - | - | - |
| Peptone | - | 10.0 | - | - | - | 2.0 | 3.0 | - | - | - | - | 5.0 |
| Yeast extract | - | 10.0 | - | - | - | 2.0 | - | 5.0 | - | 5.0 | 5.0 | 3.0 |
| Malt extract | - | 15.0 | - | - | - | - | 30.0 | 3.0 | - | - | - | 3.0 |
| Potato extract | - | - | - | - | - | - | - | - | 4.0 | - | - | - |
| NaNO3 | 3.0 | - | 2.0 | - | - | - | - | - | - | - | - | - |
| MgSO4.7H2O | 0.5 | - | 0.5 | 0.5 | 0.5 | 0.5 | - | - | - | - | - | - |
| KCl | 0.5 | - | - | - | - | - | - | - | - | - | - | - |
| FeSO4.7H2O | 0.01 | - | - | - | 0.02 | - | - | - | - | - | - | - |
| CaCl2.2H2O | - | - | 0.1 | - | - | - | - | - | - | - | - | - |
| ZnSO4.7H2O | - | - | - | - | - | - | - | - | - | - | - | - |
| MnSO4.5H2O | - | - | - | - | 0.01 | - | - | - | - | - | - | - |
| K2HPO4 | 1.0 | - | - | - | - | 1.0 | - | - | - | - | - | - |
| KH2PO4 | 1.0 | - | 1.0 | 0.1 | 1.0 | 0.5 | - | - | - | - | - | - |
| KNO3 | - | - | 2.0 | 2.0 | - | - | - | - | - | - | - | - |
| Chloramphenicol | - | - | - | - | - | - | - | - | - | - | 0.1 | - |
| Agar | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Medium | Logistic Curve Model | Reversible Autocatalytic Growth | |||||
|---|---|---|---|---|---|---|---|
| µmax, d−1 | δ, mm.d−1 | R2 | K1, d−1 | K/1 + K | R2 | ||
| CD | 0.605 ± 0.075 | 0.0079 ± 0.0012 | 0.9946 | 0.0045 ± 0.0005 | 86 ± 0.2 | 0.6733 ± 0.0211 | 0.9001 |
| GP | 0.879 ± 0.035 | 0.0097 ± 0.0044 | 0.9963 | 0.0065 ± 0.0021 | 88 ± 2.0 | 0.9055 ± 0.0011 | 0.9639 |
| Hennerberg | 0.710 ± 0.054 | 0.0082 ± 0.0017 | 0.9988 | 0.0064 ± 0.0003 | 101 ± 1.0 | 0.7658 ± 0.0229 | 0.9983 |
| Hopkins | 0.802 ± 0.067 | 0.0106 ± 0.0010 | 0.9974 | 0.0060 ± 0.0017 | 104 ± 2.0 | 0.8298 ± 0.0142 | 0.9974 |
| Leonian | 0.777 ± 0.061 | 0.0106 ± 0.0011 | 0.9952 | 0.0074 ± 0.0004 | 90 ± 0.3 | 0.8444 ± 0.0021 | 0.9971 |
| MCM | 0.925 ± 0.031 | 0.0134 ± 0.0024 | 0.9972 | 0.0093 ± 0.0002 | 96 ± 0.3 | 0.7967 ± 0.0093 | 0.9874 |
| MEA | 0.903 ± 0.010 | 0.0127 ± 0.0013 | 0.9975 | 0.0095 ± 0.0003 | 92 ± 0.4 | 0.8193 ± 0.0044 | 0.9975 |
| MYE | 0.900 ± 0.010 | 0.0123 ± 0.0011 | 0.9949 | 0.0094 ± 0.0002 | 91 ± 0.1 | 0.8321 ± 0.0108 | 0.9951 |
| PDA | 0.845 ± 0.020 | 0.0112 ± 0.0003 | 0.9981 | 0.0088 ± 0.0002 | 94 ± 3.0 | 0.8034 ± 0.0213 | 0.9982 |
| YEA | 0.870 ± 0.016 | 0.0126 ± 0.0021 | 0.9956 | 0.0086 ± 0.0006 | 101 ± 0.6 | 0.7506 ± 0.0298 | 0.9957 |
| YGC | 0.649 ± 0.043 | 0.0085 ± 0.0007 | 0.9982 | 0.0086 ± 0.0002 | 102 ± 2.0 | 0.7273 ± 0.0067 | 0.9951 |
| YMA | 0.901±0.032 | 0.0126 ± 0.0016 | 0.9959 | 0.0088 ± 0.0001 | 87 ± 1.0 | 0.7731 ± 0.0006 | 0.9962 |
| Test Microorganism | Water | Methanol | Ethanol | Butanol | Ethyl Acetate | Methylene Chloride | Hexane | Hot Water |
|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | 1250 | 1250 | 1250 | 1250 | 2500 | 2500 | 1250 | 1250 |
| Listeria monocytogenes ATCC 8787 | 1250 | 2500 | 2500 | 1250 | 2500 | 625 | 1250 | 1250 |
| Bacillus subtilis ATCC 6633 | 1250 | 625 | 625 | 625 | 625 | 625 | 625 | 1250 |
| Bacillus cereus ATCC 11778 | 1250 | 625 | 1250 | 1250 | 625 | 1250 | 1250 | 1250 |
| Pseudomonas aeruginosa ATCC 9027 | 625 | 625 | 625 | 625 | 2500 | 625 | 1250 | 1250 |
| Klebsiella pneumoniae ATCC 13883 | 625 | 625 | 625 | 625 | 625 | 625 | 625 | 1250 |
| Escherichia coli ATCC 8739 | 312.5 | 625 | 625 | 625 | 2500 | 625 | 1250 | 312.5 |
| Salmonella enterica ssp. enterica ser. Enetritidis ATCC 13076 | 625 | 625 | 625 | 1250 | 2500 | 625 | 312.5 | 625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanova, P.; Georgieva, A.; Brazkova, M.; Baldzhieva, R.; Goranov, B.; Blazheva, D.; Slavov, A.; Angelova, G. Molecular Identification, Mycelial Growth Kinetics, and Antimicrobial Potential of Newly Isolated Medicinal Mushroom Fomitopsis pinicola from Bulgaria. J. Fungi 2025, 11, 727. https://doi.org/10.3390/jof11100727
Stefanova P, Georgieva A, Brazkova M, Baldzhieva R, Goranov B, Blazheva D, Slavov A, Angelova G. Molecular Identification, Mycelial Growth Kinetics, and Antimicrobial Potential of Newly Isolated Medicinal Mushroom Fomitopsis pinicola from Bulgaria. Journal of Fungi. 2025; 11(10):727. https://doi.org/10.3390/jof11100727
Chicago/Turabian StyleStefanova, Petya, Anateya Georgieva, Mariya Brazkova, Radka Baldzhieva, Bogdan Goranov, Denica Blazheva, Anton Slavov, and Galena Angelova. 2025. "Molecular Identification, Mycelial Growth Kinetics, and Antimicrobial Potential of Newly Isolated Medicinal Mushroom Fomitopsis pinicola from Bulgaria" Journal of Fungi 11, no. 10: 727. https://doi.org/10.3390/jof11100727
APA StyleStefanova, P., Georgieva, A., Brazkova, M., Baldzhieva, R., Goranov, B., Blazheva, D., Slavov, A., & Angelova, G. (2025). Molecular Identification, Mycelial Growth Kinetics, and Antimicrobial Potential of Newly Isolated Medicinal Mushroom Fomitopsis pinicola from Bulgaria. Journal of Fungi, 11(10), 727. https://doi.org/10.3390/jof11100727








