Abstract
Over the past two decades, numerous novel species have been identified within Dictyosporiaceae, primarily in Dictyocheirospora and Dictyosporium. A recent monograph has revealed that these two genera exhibit a distinct preference for freshwater habitats, particularly in southern China. However, further investigation into the distribution and diversity of the two genera in Guangdong and Guizhou Provinces remains insufficient. In this study, we conducted an analysis of four intriguing cheiroid hyphomycetes collected from flowing rivers in these two regions. Through morphological and phylogenetic analyses incorporating combined LSU, SSU, ITS, and tef1-α sequence data, we have identified them as a novel species in Dictyocheirospora (Dictyoc. submersa sp. nov.), two novel species in Dictyosporium (Dictyos. guangdongense sp. nov. and Dictyos. variabilisporum sp. nov.), and one previously documented species (Dictyos. digitatum). Specifically, the identification of Dictyos. guangdongense is primarily based on its distinct morphology, characterized by complanate, cheiroid, and brown to dark brown conidia, with a hyaline, short, and atrophied appendage arising from the apical cell of the outer row. In addition, the morphological distinctions between Dictyocheirospora and Dictyosporium are further clarified based on our new data. This study also highlights a few phylogenetic matters regarding Dictyosporiaceae.
1. Introduction
Dictyosporiaceae was established by Boonmee et al. [1] to accommodate a group of cheiroid species, and it is classified within Pleosporales, Dothideomycetes. The species within Dictyosporiaceae exhibit the ability to colonize diverse decaying plant materials in both terrestrial and aquatic habitats, displaying a global distribution [2]. The introduction of novel genera has been significantly increased in the past two decades through extensive sampling and molecular phylogenetic analyses [1,3,4,5,6,7]. Currently, 20 genera are accepted within the family [8], with the majority being recognized as asexual morphs and only 5 genera having been documented to possess sexual morphs, viz., Dictyosporium, Gregarithecium, Immotthia, Pseudocoleophoma, and Verrucoccum [9,10,11,12,13].
The type genus Dictyosporium, typified by Dictyos. elegans, was introduced to accommodate the species with sporodochia and cheiroid conidia produced on micronematous conidiophores [1,14]. The generic concept has been amended by Goh et al. [3], and the literature provides a continuously updated compilation of morphological characteristics or keys for all accepted species [3,15,16,17,18]. Subsequently, two sexual morphs from Thailand, viz., Dictyos. meiosporum and Dictyos. sexualis, and a sexual morph from China, Dictyos. karsti, were added to the genus [1,9,19]. The morphological traits of Dictyosporium can now be comprehensively characterized, including perithecial, superficial ascomata with an apical ostiole, a membranaceous peridium, cylindric–clavate asci, and fusiform, hyaline, 1-septate ascospores with or without a mucilaginous sheath in its sexual morph, as well as micronematous conidiophores producing cheiroid, olive to brown, complanate conidia composed of 4–7 rows of cells in its asexual morph [1,19]. Currently, 86 species epithets are documented in the genus, and some have been transferred to the similar genus Dictyocheirospora based on molecular phylogeny or morphological characteristics [1,20]. Many species have been sequenced, and they cluster in a well-supported monophyletic clade [1,19,21,22,23].
Dictyocheirospora, typified by Dictyoc. rotunda, is another cheiroid genus that produces pale brown, complanate or non-complanate, and euseptate or distoseptate conidia [1]. The genus contains a total of 28 species, with 8 being transferred from Dictyosporium, primarily accomplished by Boonmee et al. [1] and Yang et al. [20]. The new combinations were mainly based on the findings of their phylogenetic analyses. The majority of species have sequence data, and they also cluster in a well-supported monophyletic clade [2,22,24,25]. Four species, viz., Dictyoc. himachalensis, Dictyoc. hydei, Dictyoc. indica, and Dictyoc. musae, have not been sequenced yet [20,26].
During our ongoing study of the taxonomy and diversity of freshwater fungi in southern China and along a north–south gradient [27], four interesting collections that morphologically belong to Dictyosporiaceae were encountered. Their phylogenetic placements were inferred using a combined dataset of the nuclear ribosomal large subunit 28S rRNA gene (LSU), ribosomal small subunit 18S rRNA gene (SSU), nuclear ribosomal internal transcribed spacers (ITSs), and fragments of the translation elongation factor 1-alpha (tef1-α). Three new species are proposed and a new fresh collection is described.
2. Materials and Methods
2.1. Sampling, Isolation, and Morphological Examination
The specimens were derived from freshwater habitats in Guangdong and Guizhou Provinces, China. The decaying plant materials, including wood, branches, and twigs, submerged in freshwater were collected and placed in zip-lock plastic bags. Essential information such as the collecting site, date, and collector details were recorded. All specimens were transported to the laboratory within three days. If the surfaces of the specimens were heavily covered with mud, they were gently rinsed with tap water. Subsequently, around 4–5 specimens were transferred to a new zip-lock plastic bag or plastic box containing moistened tissue paper/cotton and incubated at room temperature (24–27 °C) for 1–2 weeks. Sampling and specimen incubation followed the method described by Senanayake et al. [28]. A stereomicroscope (Chongqing Optec Instrument Co., Ltd., Chongqing, China) was employed to examine the sporodochia developed on the natural substratum. A compound microscope (Nikon Eclipse Ni-U, Tokyo, Japan) equipped with a digital camera (Canon 750D, Tokyo, Japan) was utilized to capture the fungal structures. Single-spore isolations were made from conidium on potato dextrose agar (PDA, Shanghai Bio-way technology Co., Ltd., Shanghai, China) at room temperature. After aseptic transfer, the germinated spores were incubated at room temperature.
Herbarium specimens (dry wood with fungal colonies) were deposited in the herbaria at Zhongkai University of Agriculture and Engineering (MHZU), Guangzhou, China. Living cultures were deposited in the Zhongkai University of Agriculture and Engineering Culture Collection (ZHKUCC), Guangzhou, China. The novel taxa were registered in the databases Facesoffungi (http://www.facesoffungi.org, accessed on 12 February 2024) [29] and Index Fungorum (http://www.indexfungorum.org/names/names.asp, accessed on 12 February 2024).
2.2. DNA Extraction, PCR Amplification, and Sequencing
After a month of cultivation, fungal mycelia were carefully scraped from the colonies for subsequent DNA extraction. The entire genomic DNA was extracted from 100–200 mg of axenic mycelia. The cell fragmentation was accomplished using a homogenizer (Allsheng, Hangzhou, China). The Biospin fungi DNA isolation kit (Bioer, Hangzhou, China) was utilized for genomic DNA extraction in accordance with the manufacturer’s instructions. In this study, primer pairs LR0R/LR5, NS1/NS4, ITS5/ITS4, and EF1-983F/EF1-2218R were used to amplify the LSU, SSU, ITSs, and tef1-α, respectively. The amplifications were carried out in a 25 μL reaction volume containing 9.5 μL of double-distilled sterilized water (ddH2O), 12.5 μL of 2 × FastTaq PCR Master Mix (Vazyme Co., Nanjing, China), 1 μL of DNA template, and 1 μL of each forward and reverse primer (10 μM). The PCR thermal cycle program for the amplification of the LSU, SSU, ITSs, and tef1-α was started with an initial denaturation step at 97 °C for 3 min, followed by 38 cycles consisting of denaturation at 94 °C for 30 s, annealing at 53 °C for 50 s, elongation at 72 °C for 1 min, and a final extension step at 72 °C for 10 min [30,31,32]. PCR products were checked on 1% agarose electrophoresis gels stained with ethidium bromide (EB). The sequencing reactions were carried out by Tianyi Huiyuan Biotechnology Co., Ltd. (Guangzhou, China).
2.3. Phylogenetic Analyses
The newly obtained sequences were analyzed using FinchTV v. 1.4.0. The consensus sequences were generated using BioEdit v. 7.2 [33]. The Blast analysis was performed on the NCBI platform (https://blast.ncbi.nlm.nih.gov (accessed on 10 February 2024)) to identify taxonomic matches. The taxa selected for constructing the phylogenetic tree were based on a recent published paper [25] and Blast results obtained from the NCBI. The dataset of each gene region was initially aligned independently using the ‘auto’ strategy (based on data size) by MAFFT v. 7 [34]. Subsequently, manual editing was performed to eliminate uninformative gaps or ambiguous regions in BioEdit v. 7.2 [33]. Each dataset (LSU, SSU, ITS, and tef1-α) was concatenated in Mesquite v. 3.81 [35]. The fasta file was converted to PHYLIP (for ML) and NEXUS (for BI) format in the Alignment Transformation Environment (ALTER) online program (http://www.sing-group.org/ALTER/ (accessed on 10 February 2024)). Phylogenetic analyses were conducted with maximum likelihood (ML) and Bayesian inference (BI) algorithms in the CIPRES Science Gateway (http://www.phylo.org/portal2 (accessed on 10 February 2024)) [36]. The ML analysis was performed with RAxML-HPC2 v. 8.2.12 on XSEDE with 1000 rapid bootstrap replicates [37]. The model selected for ML analysis was GTR + GAMMA. The BI analysis was performed in MrBayes v. 3.2.7a [38] and the best-fitting model was estimated via MrModeltest v. 2.2 [39].
The Markov Chain Monte Carlo (MCMC) was run for 10,000,000 generations, and the trees were sampled every 100th generation. The first 25% of the trees that represented the burn-in phase were discarded, and the remaining 75% of the trees were used for calculating the posterior probabilities (PPs) for the majority-rule consensus tree [40]. Phylogenetic trees were visualized using FigTree v. 1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 10 February 2024). The editing and typesetting were accomplished using Microsoft Office PowerPoint 2007 (Microsoft Corporation, Redmond, WA, USA).
3. Results
3.1. Phylogenetic Analyses
The dataset consisted of the combined LSU, SSU, ITS, and tef1-α sequence data of 102 taxa in Dictyosporiaceae, with Periconia igniaria (CBS 379.86 and CBS 845.96) as the outgroup taxon (Figure 1). The topologies obtained from both the ML and BI analyses exhibited similar patterns across the major clades. The best RAxML tree had a final likelihood value of −24,403.726506. The matrix had 1540 distinct alignment patterns, with 42.54% undetermined characters or gaps. The Bayesian analysis resulted in 24,485 trees after 2,000,000 generations. Phylogenetic analyses indicated that ZHKUCC 24-0001 was classified within the genus Dictyocheirospora, exhibiting close relationships with Dictyoc. gigantica, Dictyoc. pandanicola, Dictyoc. pseudomusae, and Dictyoc. vinaya. The collection ZHKUCC 24-0004 formed a clade with four strains of Dictyosporium digitatum (KUMCC 17-0269, KT 2660, SDBR-CMU459, and yone 280). The collection ZHKUCC 24-0002 clustered with five species of Dictyosporium, viz., Dictyos. alatum, Dictyos. appendiculatum, Dictyos. pandanicola, Dictyos. strelitziae, and Dictyos. thailandicum, while the collection ZHKUCC 24-0003 clustered as a distinct branch within Dictyosporium (Figure 1).
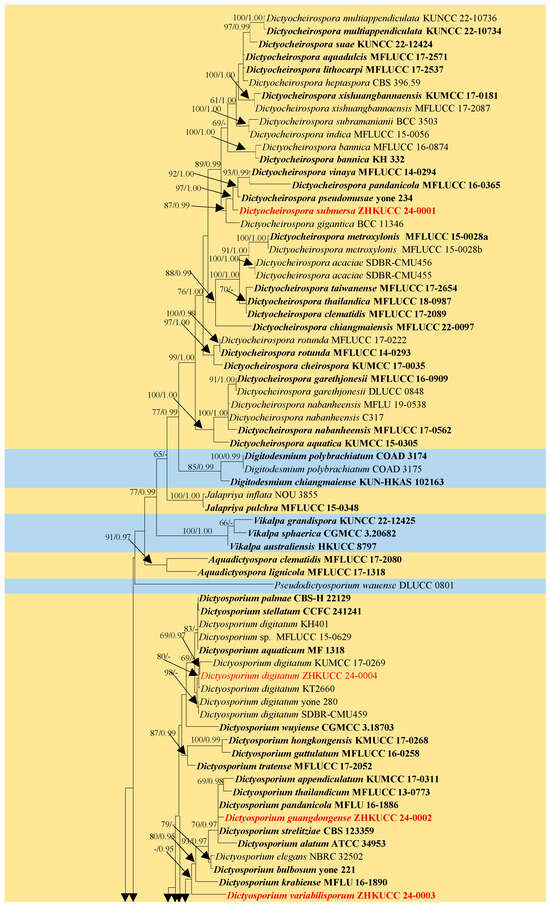
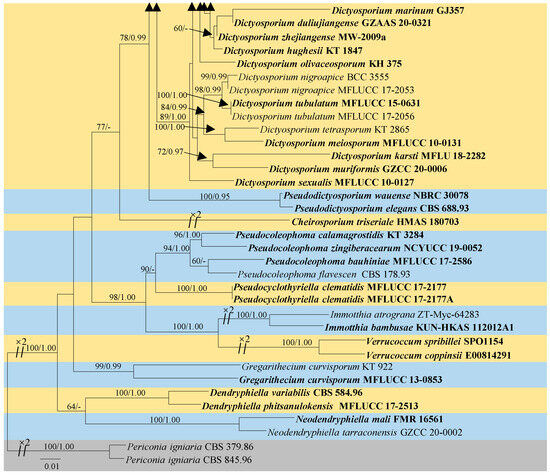
Figure 1.
The maximum likelihood (ML) tree is constructed using combined LSU, SSU, ITS, and tef1-α sequence data. Bootstrap support values with an ML greater than 60% and Bayesian posterior probabilities (PPs) greater than 0.95 are indicated above the nodes as “ML/PP”. The tree is rooted to Periconia igniaria (CBS 379.86 and CBS 845.96). Newly generated sequences are highlighted in red, and the type strains are indicated in bold.
3.2. Taxonomy
Dictyocheirospora M.J. D’souza, Boonmee & K.D. Hyde, Fungal Diversity 80: 465 (2016).
Dictyocheirospora submersa Y.X. Shu & W. Dong, sp. nov. (Figure 2).
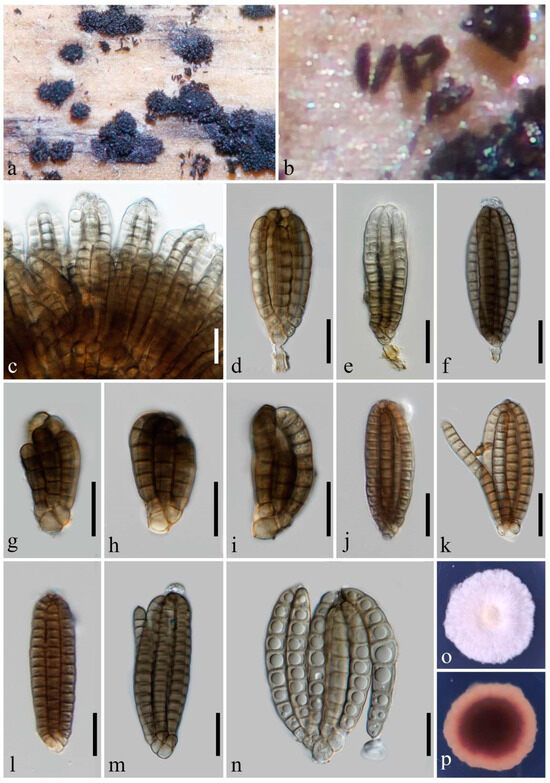
Figure 2.
Dictyocheirospora submersa (MHZU 24-0001, holotype). (a,b) Sporodochia on natural substratum. (c) Conidia heap. (d) Conidiophores with conidia. (e–i) Young conidia. (j–n) Conidia. (o,p) A 20-day-old colony on PDA at room temperature (o: obverse, p: reverse). Scale bars: (c–n) = 20 µm.
Index Fungorum number: IF901680; Facesofungi number: FoF 15509.
Etymology: referring to submerged wood from which the fungus was isolated.
Holotype: MHZU 24-0001.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: undetermined. Asexual morph: hyphomycetes. Sporodochia on natural substratum, punctiform, scattered, and black. Conidiophores reduced to conidiogenous cells. Conidiogenous cells: 3.5–4 × 3–3.5 μm ( = 3.8 × 3.3 μm, n = 5), holoblastic, integrated, terminal, subcylindrical, and pale brown. Conidia: (20–)35–85 × 12–30 μm ( = 57.5 × 22.5 μm, n = 30), non-complanate, cheiroid, clavate, ellipsoidal, subcylindrical, consisting of 30–78 cells arranged in 6–7 tightly appressed rows, 4–16 euseptate in each row, slightly constricted at septa, each row inwardly curved, conidial arms appressed, occasionally becoming divergent, with a cuneiform or rounded basal cell, pale olivaceous, brown to dark brown, acrogenous, guttulate, smooth-walled, and without appendages or mucilaginous sheaths. Conidial secession: schizolytic.
Cultural characteristics: Conidia germinating on PDA within 24 h and germ tubes produced from the basal cell. Colonies on PDA reaching 35 mm diam. at room temperature (24–27 °C) in natural light after 20 days, irregular, rough, dry, fluffy, and dense; margins undulate with sparse mycelia, yellow in the middle and white at the margin; and the reverse brown in the middle and pale brown at the margin.
Material examined: China, Guizhou Province, Yuping City, on submerged wood in a river, 7 February 2023, YX Shu, YP2-1.3 (MHZU 24-0001, holotype), ex-type culture ZHKUCC 24-0001. GenBank accession numbers: LSU: PP326216, SSU: PP335106, ITS: PP326193, tef1-α: PP333113.
Notes: In the phylogenetic analysis, Dictyocheirospora submersa clustered as a distinct branch within Dictyocheirospora, exhibiting close affinities with Dictyoc. gigantica, Dictyoc. pandanicola, Dictyoc. pseudomusae, and Dictyoc. vinaya (Figure 1). Dictyocheirospora submersa can be easily distinguished from Dictyoc. pseudomusae by the absence of globose to subglobose, hyaline appendages growing from the apical cells or side of the outer rows [10]. The conidia of Dictyoc. gigantica differ from those of Dictyoc. submersa by their cylindrical and longer conidia measuring 105–121 × 25–32 μm, whereas Dictyoc. submersa has shorter and mostly clavate, ellipsoidal, or occasionally subcylindrical conidia measuring (20–)35–85 × 12–30 μm [3]. Dictyocheirospora submersa is quite similar to Dictyoc. pandanicola and Dictyoc. vinaya in terms of their conidial morphology and dimensions. However, they can be easily distinguished based on the color of their conidia. Dictyocheirospora submersa exhibits pale olivaceous, brown to dark brown conidia, while Dictyoc. vinaya has reddish-brown conidia (from their photo plate), and Dictyoc. pandanicola has pale brown conidia [1,41]. In addition, the conidial arms of Dictyoc. submersa are tightly appressed, with minimal divergence observed upon squashing. The conidial arms of Dictyoc. pandanicola and Dictyoc. vinaya, in contrast, easily become divergent, with the conidial arms of Dictyoc. pandanicola even detaching from the conidial body. After conducting a full morphological comparison, we found that Dictyoc. submersa does not correspond to any existing species within the genus. Therefore, it is introduced as a novel species of Dictyocheirospora.
Dictyosporium Corda, Weitenweber’s Beitr. Nat. 1: 87 (1837).
Dictyosporium digitatum J.L. Chen, C.H. Hwang and Tzean, Mycological Research 95: 1145 (1991) (Figure 3).

Figure 3.
Dictyosporium digitatum (MHZU 24-0004). (a,b) Sporodochia on natural substratum. (c–e) Conidiogenous cells and young conidia. (f–h) Conidiophores with mature conidia. (i–k) Mature conidia. (l) Germinating conidium. (m,n) A 15-day-old colony on PDA at room temperature (m: obverse, n: reverse). Scale bars: (c–l) = 20 µm.
Index Fungorum number: IF355284; Facesofungi number: FoF 04487.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: undetermined. Asexual morph: hyphomycetes. Sporodochia on natural substratum: punctiform, scattered, black, and granular. Conidiophores: micronematous, reduced to conidiogenous cells. Conidiogenous cells: 3–5 × 3–3.5 μm ( = 3.5 × 3 μm, n = 5), holoblastic, monoblastic, integrated, determinate, terminal, subcylindrical, and pale brown. Conidia: (20–)30–90 × 20–37 μm ( = 57.5 × 28 μm, n = 30), complanate, cheiroid, consisting of (30–)50–110 cells arranged in 7–8 tightly appressed rows, two types of shapes: (1) cheirosporous with concave apex, (1–)3–16 euseptate in each row, (2) ovoid or subcylindrical, 10–21 euseptate in each row, slightly constricted and strongly pigmented at septa, yellowish-brown to reddish-brown, dark brown, becoming paler to subhyaline in the terminal top 2–4 cells, terminal cells digitate, straight or incurved, or even curled, hyaline, thin-walled, with a cuneiform basal cell, acrogenous, guttulate, smooth-walled, and without an appendage. Conidial secession: schizolytic.
Cultural characteristics: Conidia germinating on PDA within 24 h, and all the cells can produce germ tubes. Colonies on PDA reaching 30 mm diam. at room temperature (24–27 °C) in natural light after 15 days, irregular, rough, dry, fluffy, and dense in the middle; margin sparse and wavy, white and orange-brown; and the reverse orange-brown in the middle and pale orange-brown at the margin.
Material examined: China, Guangdong Province, Guangzhou City, on submerged wood in a lake, 26 February 2023, YX Shu SDGY4 (MHZU 24-0004), living culture ZHKUCC 24-0004. GenBank accession numbers: LSU: PP326214, SSU: PP335104, ITS: PP326191, tef1-α: PP333111.
Notes: In the phylogenetic analysis, the collection ZHKUCC 24-0004 forms a clade with four strains of Dictyosporium digitatum (KUMCC 17-0269, KT 2660, SDBR CMU459, and yone 280). The morphological characteristics of ZHKUCC 24-0004, such as possessing cheiroid, reddish-brown conidia with distinctly thin-walled, hyaline, digitate, incurved terminal cells, are in accordance with those of Dictyos. digitatum [3,10,41,42]. Therefore, ZHKUCC 24-0004 is identified as Dictyos. digitatum based on morphology and phylogenetic analysis. Except for cheirosporous conidia that have been frequently documented in the literature, we have observed the presence of a concave apex in some conidia (Figure 3c–e) from our collection.
Dictyosporium guangdongense Y.X. Shu & W. Dong, sp. nov. (Figure 4).

Figure 4.
Dictyosporium guangdongense (MHZU 24-0002, holotype). (a,b) Sporodochia on natural substratum. (c) Conidiogenous cell with conidium. (d–g) Conidia with apical appendages. (h–k) Conidia. (l,m) Appendages. (n) Germinating conidium. (o,p) A 20-day-old colony on PDA at room temperature (o: obverse, p: reverse). Scale bars: (c–k,n) = 20 µm; (l,m) = 10 µm.
Index Fungorum number: IF901681; Facesofungi number: FoF 15510.
Etymology: referring to Guangdong Province, from where the holotype was collected.
Holotype: MHZU 24-0002.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: undetermined. Asexual morph: hyphomycetes. Sporodochia on natural substratum: punctiform, scattered, black, and granular. Conidiophores reduced to conidiogenous cells. Conidiogenous cells: 4.5–10 × 3–4 μm ( = 6 × 3.5 μm, n = 5), holoblastic, monoblastic, integrated, determinate, terminal, subcylindrical, and hyaline to pale brown. Conidia: 35–55 × 18–32 μm ( = 44 × 23 μm, n = 40), complanate, cheiroid, consisting of 50–60 cells arranged in (4–)5(–6) tightly appressed rows, 7–11 euseptate in each outer row, 9–11 euseptate in each inner row, constricted and strongly pigmented at septa, with a cuneiform basal cell, brown to dark brown, acrogenous, guttulate, smooth-walled, with a hyaline, short, wizened appendage arising from the apical cell of the outer row, and without mucilaginous sheaths. Conidial secession: schizolytic.
Cultural characteristics: Conidia germinating on PDA within 36 h and germ tubes produced from the basal cell. Colonies on PDA reaching 50 mm diam. at room temperature (24–27 °C) in natural light after 20 days, irregular, rough, dry, fluffy, and dense; margins undulate with sparse mycelia, white in the middle, with masses of black sporodochia produced at the margin; and the reverse yellowish-brown in the middle and pale yellowish-brown at the margin.
Material examined: China, Guangdong Province, Yangjiang City, on submerged wood in a river, 9 April 2023, YX Shu YJ3-14 (MHZU 24-0002, holotype), ex-type culture ZHKUCC 24-0002. GenBank accession numbers: LSU: PP326213, SSU: PP335103, ITS: PP326190.
Notes: In the phylogenetic analysis, Dictyosporium guangdongense forms a clade with Dictyos. alatum, Dictyos. appendiculatum, Dictyos. pandanicola, Dictyos. strelitziae, and Dictyos. thailandicum (Figure 1). The distinct and well-defined shape of the appendages in Dictyos. alatum, Dictyos. appendiculatum, Dictyos. strelitziae, and Dictyos. thailandicum can be identified to distinguish them easily from Dictyos. guangdongense [3,9,15,41]. In contrast, the appendages of Dictyos. guangdongense are hyaline, short, and atrophied structures arising from the apical cell of the outer row. Dictyosporium pandanicola is quite similar to Dictyos. guangdongense in terms of their conidial morphology and dimensions. However, Dictyos. pandanicola can be distinguished by the absence of any appendages [41]. According to a comprehensive morphological comparison of all species mentioned/not mentioned in the referenced key [3,17,18], Dictyos. guangdongense does not match to any known species within the genus. The phylogenetic analyses cannot distinguish between Dictyos. guangdongense and its related species mentioned above due to the limited sequence data available for them, which only include LSU and SSU for Dictyos. appendiculatum, Dictyos. Strelitziae, and Dictyos. thailandicum, as well as LSU, SSU, and ITS for Dictyos. alatum. The tef1-α sequence data are available for Dictyos. pandanicola, but not for Dictyos. guangdongense.
Dictyosporium variabilisporum Y.X. Shu & W. Dong, sp. nov. (Figure 5).

Figure 5.
Dictyosporium variabilisporum (MHZU 24-0003, holotype). (a,b) Sporodochia on natural substratum. (c,d) Conidiophores with conidia. (e–h) Young conidia. (i–n) Conidia with appendages. (o) Germinating conidium. (p,q) A 15-day-old colony on PDA at room temperature (p: obverse, q: reverse). Scale bars: (c–o) = 10 µm.
Index Fungorum number: IF901682; Facesofungi number: FoF 15511.
Etymology: referring to the variable color of conidia of the holotype.
Holotype: MHZU 24-0003.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: undetermined. Asexual morph: hyphomycetes. Sporodochia on natural substratum: punctiform, scattered, black, olivaceous, and granular. Conidiophores: 8–22 × 2–4 μm ( = 17.5 × 3.5 μm, n = 5), semi-macronematous, mononematous, subcylindrical, septate, not constricted at septa, and pale yellowish-brown or pale olivaceous-brown. Conidiogenous cells: 6–9.5 × 2–4 μm ( = 7 × 3.5 μm, n = 5), holoblastic, monoblastic, integrated, determinate, terminal, subcylindrical, and pale yellowish-brown or pale olivaceous-brown. Conidia: 15–35 × 11–20 μm ( = 25 × 16 μm, n = 40), complanate, cheiroid, consisting of 12–32 cells arranged in 4–5 tightly appressed rows, (2–)3–7 euseptate in each row, slightly constricted at septa, usually each row has a similar length of cells, with a cuneiform or swollen basal cell, yellowish-brown, producing olivaceous pigmentation at a later stage and becoming olivaceous-brown, acrogenous, guttulate, smooth-walled, and with 2–5 digitate or hypha-like, subcylindrical, hyaline, curved, thin-walled, apical appendages. Conidial secession: schizolytic.
Cultural characteristics: Conidia germinating on PDA within 24 h and germ tubes produced from the basal cell. Colonies on PDA reaching 50 mm diam. at room temperature (24–27 °C) in natural light after 15 days, circular, rough, dry, fluffy, and dense; margin entirely covered with white mycelia; and the reverse pale brown in the middle and white at the margin.
Material examined: China, Guangdong Province, Yangjiang City, on submerged wood in a river, 9 April 2023, YX Shu YJ1-22 (MHZU 24-0003, holotype), ex-type culture ZHKUCC 24-0003. GenBank accession numbers: LSU: PP326215, SSU: PP335105, ITS: PP326192, tef1-α: PP333112.
Notes: In the phylogenetic analysis, Dictyosporium variabilisporum clustered as a distinct branch within Dictyosporium (Figure 1). The complanate, cheiroid conidia with apical appendages of Dictyos. variabilisporum correspond to the generic concept of Dictyosporium. However, it is unusual within the genus for the variable color of its conidia that are initially yellowish-brown and become olivaceous-brown. In addition, although several phylogenetically related species, viz., Dictyos. alatum, Dictyos. appendiculatum, Dictyos. bulbosum, Dictyos. krabiense, Dictyos. strelitziae, and Dictyos. thailandicum, have apical appendages that are similar to those of Dictyos. variabilisporum, they can be distinguished based on the morphology or quantity of their appendages [9,15,41,42,43,44,45]. The earlier stage of Dictyos. variabilisporum is also similar to Dictyos. digitatum in having digitate, curved, hyaline, thin-walled terminal cells [10,20,41,42]. However, they can be easily distinguished by the conidial morphology, color, and appendages at maturity as shown in Figure 3 and Figure 5. After conducting a full morphological comparison based on the literature and key provided by Goh et al. [3], we found that Dictyos. variabilisporum does not correspond to any existing species within the genus. Therefore, it is introduced as a novel species of Dictyosporium.
4. Discussion
Numerous studies in the early 2,000s on lignicolous fungi in freshwater streams revealed Dictyosporium species [46,47,48]; however, the taxa were identified based on morphology, and the names should be questioned. The taxonomy of freshwater fungi and Dictyosporaceae has been extensively investigated over the past decades by incorporating barcoding of nuclear ribosomal regions (LSU, SSU, and ITS) and protein-coding genes (tef1-α and rpb2). The unresolved fungal groups and intriguing taxonomic issues have been effectively addressed using the extensive collections obtained from China and Thailand [49,50,51,52,53]. Furthermore, the continuous generation of a wealth of information has resulted in the publication of numerous reviews, books, and monographs aimed at compiling an updated account of freshwater fungi [24,51,52,53,54,55]. A significant milestone has been achieved by Calabon and his co-authors who provide a comprehensive overview of the different facets of freshwater fungal biology [56].
Within Dictyosporaceae, Dictyocheirospora and Dictyosporium are the two most prominent genera that accommodate the majority of freshwater species, with 11 and 9 reported freshwater species, respectively [24,25]. A geographical distribution review showed that numerous species within the family were reported from China and Thailand [2]. In this study, we describe an additional four hyphomycetes collected from freshwater habitats in China. The findings of our study further validate the role of freshwater habitats as important reservoirs for species of Dictyosporiaceae.
Dictyocheirospora and Dictyosporium are phylogenetically related and share quite similar morphological characteristics. Yang et al. [20] highlighted that Dictyocheirospora can be distinguished from the latter genus by its non-complanate or cylindrical conidia, which are predominantly characterized by closely clustered terminal cells at the apex. Based on this classification scheme, Dictyosporium hydei, Dictyos. indicum, Dictyos. musae, and Dictyos. tetraploides have been transferred to Dictyocheirospora [20]. The confirmation of the transfer through sequence data is still pending; however, we concur with this conclusion based on our newly acquired data. The newly discovered species Dictyoc. submersa has non-complanate and subcylindrical conidia with closely clustered terminal cells at the apex (Figure 2), while Dictyos. digitatum, Dictyos. guangdongense, and Dictyos. variabilisporum possess complanate conidia in which the terminal cells are not significantly clustered (Figure 3, Figure 4 and Figure 5).
The phylogenetic relationships between genera in Dictyosporiaceae have been well investigated using DNA sequence data [1,2,7,12,24,25]. However, the interspecific phylogenetic relationships in some cases remain ambiguous due to the limitation of insufficient sequence data included in the phylogenetic tree. In this study, the establishment of Dictyosporium guangdongense as a novel species is primarily based on its morphological characteristics, as the limited molecular data available cannot distinguish between Dictyos. guangdongense and its related species (see notes on Dictyos. guangdongense) (Table S1, Figure 1). In addition, Dictyosporium aquaticum, Dictyos. digitatum, Dictyos. palmae, and Dictyos. stellatum exhibit indistinguishable genetic relationships despite their distinct morphologies (Figure 1) [9,15,42,57]. In the phylogenetic analysis, Dictyocheirospora clematidis and Dictyoc. thailandica also cannot be distinguished due to the absence of tef1-α sequence data for Dictyoc. thailandica [25]. The protein-coding genes are quite necessary in species identification within this fungal group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10040259/s1, Table S1: Taxa used for phylogenetic analyses and their corresponding GenBank accession numbers. Newly generated sequences are indicated in red, ex-type strains are indicated in bold, and missing sequences are indicated with “–”.
Author Contributions
Conceptualization, W.D. and M.D.; writing—original draft preparation, Y.-X.S.; writing—review and editing, W.D., M.D. and S.B.; supervision, W.D. and B.X.; funding acquisition, W.D. and B.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 32200015) and the Foundation of Guangzhou Bureau of Science and Technology (Grant No. 2023A04J1425).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated from this study can be found in the Index Fungorum (http://www.indexfungorum.org/names/names.asp (accessed on 15 February 2024)) and GenBank (https://www.ncbi.nlm.nih.gov/nuccore (accessed on 25 February 2024)).
Acknowledgments
Wei Dong thanks the foundations of Guangdong Provincial Department of Education (2022KCXTD015; 2022ZDJS020), and the Talent Program of Zhongkai University of Agriculture and Engineering (KA22016B787). Mingkwan Doilom acknowledges the Foundation of Guangzhou Bureau of Science and Technology (Grant No. 2023A04J1426), the Guangdong Provincial College Key Laboratory of Green Prevention and Control of Fruit and Vegetable Pests and Diseases (Grant No. KA21031C502), and the Talent Program of Zhongkai University of Agriculture and Engineering (KA22016B746). Biao Xu thanks the foundations of Guangdong Provincial Department of Education (2022KCXTD015; 2022ZDJS020). We would like to acknowledge Shaun Pennycook, Nomenclature Editor, Mycotaxon for helping with new species names.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boonmee, S.; D’souza, M.J.; Luo, Z.L.; Pinruan, U.; Tanaka, K.; Su, H.Y.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Taylor, J.E.; et al. Dictyosporiaceae fam. nov. Fungal Divers. 2016, 80, 457–482. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; de Silva, N.I.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Kumla, J.; Suwannarach, N.; Lumyong, S. Exploring more on Dictyosporiaceae: The species geographical distribution and intriguing novel additions from plant litter. Diversity 2023, 15, 410. [Google Scholar] [CrossRef]
- Goh, T.K.; Hyde, K.D.; Ho, W.H. A revision of the genus Dictyosporium, with descriptions of three new species. Fungal Divers. 1999, 2, 65–100. [Google Scholar]
- Kodsueb, R.; Lumyong, S.; Ho, W.H.; Hyde, K.D.; Mckenzie, E.H.; Jeewon, R. Morphological and molecular characterization of Aquaticheirospora and phylogenetics of Massarinaceae (Pleosporales). Bot. J. Linn. Soc. 2007, 155, 283–296. [Google Scholar] [CrossRef]
- Cai, L.; Guo, X.Y.; Hyde, K.D. Morphological and molecular characterisation of a new anamorphic genus Cheirosporium from freshwater in China. Persoonia 2008, 20, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Luo, Z.L.; Liu, J.K.; Bhat, D.J.; Bao, D.F.; Su, H.Y.; Hyde, K.D. Lignicolous freshwater fungi from China I: Aquadictyospora lignicola gen. et sp. nov. and new record of Pseudodictyosporium wauense from northwestern Yunnan Province. Mycosphere 2017, 8, 1587–1597. [Google Scholar] [CrossRef]
- Tian, W.H.; Chen, Y.P.; Maharachchikumbura, S.S.N. Neodigitodesmium, a novel genus of family Dictyosporiaceae from Sichuan Province, China. Phytotaxa 2022, 559, 176–184. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Ariyawansa, H.A.; Bhat, D.J.; Boonmee, S.; Maharachchikumbura, S.S.N.; McKenzie, E.H.C.; Phookamsak, R.; Phukhamsakda, C.; et al. Fungal diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015, 72, 1–197. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirayama, K.; Yonezawa, H.; Sato, G.; Toriyabe, A.; Kudo, H.; Hashimoto, A.; Matsumura, M.; Harada, Y.; Kurihara, Y.; et al. Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud. Mycol. 2015, 82, 75–136. [Google Scholar] [CrossRef]
- Atienza, V.; Hawksworth, D.L.; Perez-Ortega, S. Verrucoccum (Dothideomycetes, Dictyosporiaceae), a new genus of lichenicolous fungi on Lobaria s. lat. for the Dothidea hymeniicola species complex. Mycologia 2021, 113, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.B.; Jeewon, R.; Karunarathna, S.C.; Phukhamsakda, C.; Doilom, M.; Kakumyan, P.; Suwannarach, N.; Phookamsak, R.; Lumyong, S. Reappraisal of Immotthia in Dictyosporiaceae, Pleosporales: Introducing Immotthia bambusae sp. nov. and Pseudocyclothyriella clematidis comb. et gen. nov. based on morphology and phylogeny. Front. Microbiol. 2021, 12, 656235. [Google Scholar] [CrossRef]
- Dong, W.; Hyde, K.D.; Jeewon, R.; Liao, C.F.; Zhao, H.J.; Kularathnage, N.D.; Li, H.; Yang, Y.H.; Pem, D.; Shu, Y.X.; et al. Mycosphere notes 449–468: Saprobic and endophytic fungi in China, Thailand, and Uzbekistan. Mycosphere 2023, 14, 2208–2262. [Google Scholar] [CrossRef]
- Corda, A.J.C. Mykologische Beobachtungen. In Beitrage zur Gesammtem Natur-und Heilwissenschaften, 1st ed.; Weitenweber, W.R., Ed.; Commission bei Kronberger und Weber: Prague, Czech Republic, 1836; Volume 1, pp. 80–88. [Google Scholar]
- Crous, P.W.; Braun, U.; Wingfield, M.J.; Wood, A.R.; Shin, H.D.; Summerell, B.A.; Alfenas, A.C.; Cumagun, C.J.R.; Groenewald, J.Z. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 2009, 22, 139–161. [Google Scholar] [CrossRef]
- Whitton, S.R.; McKenzie, E.H.C.; Hyde, K.D. Fungi associated with Pandanaceae. Fungal Divers. Res. Ser. 2012, 21, 278–280. [Google Scholar]
- Silva, C.R.; Gusmão, L.F.P.; Castañeda-Ruiz, R.F. Dictyosporium amoenum sp. nov. from Chapada Diamantina, Bahia, Brazil. Mycotaxon 2016, 130, 1125–1133. [Google Scholar] [CrossRef]
- Dubey, R. Dictyosporium matherense sp. nov.: A new-fangled cheirosporous fungal species described from the Western Ghats of India. Asian J. For. 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liu, J.K.; Hyde, K.D.; Chen, Y.Y.; Ran, H.Y.; Liu, Z.Y. Ascomycetes from karst landscapes of Guizhou Province, China. Fungal Divers. 2023, 122, 1–160. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Liu, Z.-Y. New species in Dictyosporium, new combinations in Dictyocheirospora and an updated backbone tree for Dictyosporiaceae. MycoKeys 2018, 36, 83–105. [Google Scholar] [CrossRef]
- Hyde, K.D.; Dong, Y.; Phookamsak, R.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Liu, N.-G.; Abeywickrama, P.D.; Mapook, A.; Wei, D.; et al. Fungal diversity notes 1151–1276: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2020, 100, 5–277. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; McKenzie, E.H.C.; Phillips, A.J.L.; Gareth Jones, E.B.; Jayarama Bhat, D.; Stadler, M.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Kularathnage, N.D.; Wanasinghe, D.N.; Senanayake, I.C.; Yang, Y.H.; Manawasinghe, I.S.; Phillips, A.J.L.; Hyde, K.D.; Dong, W.; Song, J.G. Microfungi associated with ornamental palms: Byssosphaeria phoenicis sp. nov. (Melanommataceae) and Pseudocoleophoma rhapidis sp. nov. (Dictyosporiaceae) from south China. Phytotaxa 2022, 568, 149–169. [Google Scholar] [CrossRef]
- Dong, W.; Wang, B.; Hyde, K.D.; McKenzie, E.H.C.; Raja, H.A.; Tanaka, K.; Abdel-Wahab, M.A.; Abdel-Aziz, F.A.; Doilom, M.; Phookamsak, R.; et al. Freshwater Dothideomycetes. Fungal Divers. 2020, 105, 319–575. [Google Scholar] [CrossRef]
- Shen, H.W.; Bao, D.F.; Wanasinghe, D.N.; Boonmee, S.; Liu, J.K.; Luo, Z.L. Novel species and records of Dictyosporiaceae from freshwater habitats in China and Thailand. J. Fungi 2022, 8, 1200. [Google Scholar] [CrossRef] [PubMed]
- Sushma; Verma, R.K.; Prasher, I.B.; Gautam, A.K.; Rajeshkumar, K.C.; Castaneda-Ruiz, R.F. Dictyocheirospora himachalensis sp. nov. from Himachal Pradesh, India. Mycotaxon 2022, 137, 455–463. [Google Scholar] [CrossRef]
- Hyde, K.D.; Fryar, S.; Tian, Q.; Bahkali, A.H.; Xu, J.C. Lignicolous freshwater fungi along a north–south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecol. 2016, 19, 190–200. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.; Sandamali, D.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Pem, D.; Dissanayake, L.; Wijesinghe, S.N.; Bundhun, D.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Ariyawansa, H.A.; Bhat, J.; Buyck, B.; Cai, L.; Dai, Y.C.; Abd-Elsalam, K.A.; Ertz, D.; Hidayat, I.; et al. The faces of fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015, 74, 3–18. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Rehner, S.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Slatkin, M.; Maddison, W.P. A cladistic measure of gene flow inferred from the phylogenies of allel. Genetics 1989, 123, 603–613. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Tibpromma, S.; Hyde, K.D.; McKenzie, E.H.C.; Bhat, D.J.; Phillips, A.J.L.; Wanasinghe, D.N.; Samarakoon, M.C.; Jayawardena, R.S.; Dissanayake, A.J.; Tennakoon, D.S.; et al. Fungal diversity notes 840–928: Micro-fungi associated with Pandanaceae. Fungal Divers. 2018, 92, 1–160. [Google Scholar] [CrossRef]
- Chen, L.J.; Hwang, H.C.; Tzean, S.S. Dictyosporium digitatum, a new hyphomycete from Taiwan. Mycol. Res. 1991, 95, 1145–1149. [Google Scholar] [CrossRef]
- Tzean, S.S.; Chen, J.L. Two new species of Dictyosporium from Taiwan. Mycol. Res. 1989, 92, 497–502. [Google Scholar] [CrossRef]
- Gómez, E.F. Fungi of the Caribbean. In An Annotated Checklist; Minter, D.W., Rodriguez Hernandez, M., Portales, M., Eds.; PDMS Publishing: Isleworth, UK, 2001; p. 94. [Google Scholar]
- Barbosa, F.R.; Gusmao, L.F.P.; Ruiz, R.F.C.; Marques, M.F.O.; Maia, L.C. Conidial fungi from the semi-arid Caatinga biome of Brazil. New species Deightoniella rugosa and Diplocladiella cornitumida with new records for the neotropics. Mycotaxon 2007, 102, 39–49. [Google Scholar]
- Tsui, C.K.M.; Hyde, K.D.; Hodgkiss, I.J. Biodiversity of fungi on submerged wood in Hong Kong streams. Aquat. Microb. Ecol. 2020, 21, 289–298. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, K.Q.; McKenzie, E.H.C.; Hyde, K.D. Freshwater fungi from bamboo and wood submerged in the Liput River in the Philippines. Fungal Divers. 2004, 13, 1–12. [Google Scholar]
- Luo, J.; Yin, J.F.; Cai, L.; Zhang, K.Q.; Hyde, K.D. Freshwater fungi in Lake Dianchi, a heavily polluted lake in Yunnan, China. Fungal Divers. 2004, 16, 93–112. [Google Scholar]
- Zhang, H.; Dong, W.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Hongsanan, S.; Jayarama Bhat, D.; Al-Sadi, A.M.; Zhang, D. Towards a natural classification of annulatascaceae-like taxa: Introducing Atractosporales ord. nov. and six new families. Fungal Divers. 2017, 85, 75–110. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Liu, J.K.; Hyde, K.D.; Jeewon, R.; Kang, J.C.; Fan, C.; Boonmee, S.; Bhat, D.J.; Luo, Z.L.; Lin, C.G.; et al. A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers. 2018, 92, 131–344. [Google Scholar] [CrossRef]
- Dong, W.; Hyde, K.D.; Jeewon, R.; Doilom, M.; Yu, X.D.; Wang, G.N.; Liu, N.G.; Hu, D.M.; Nalumpang, S.; Zhang, H. Towards a natural classification of annulatascaceae-like taxa II: Introducing five new genera and eighteen new species from freshwater. Mycosphere 2021, 12, 1–88. [Google Scholar] [CrossRef]
- Hyde, K.D.; Bao, D.F.; Hongsanan, S.; Chethana, K.W.T.; Yang, J.; Suwannarach, N. Evolution of freshwater Diaporthomycetidae (Sordariomycetes) provides evidence for five new orders and six new families. Fungal Divers. 2021, 107, 71–105. [Google Scholar] [CrossRef]
- Bao, D.F.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Perera, R.H.; Thiyagaraja, V.; Hongsanan, S.; Wanasinghe, D.N.; Shen, H.W.; Tian, X.G.; Yang, L.Q.; et al. Taxonomy, phylogeny and evolution of freshwater Hypocreomycetidae (Sordariomycetes). Fungal Divers. 2023, 121, 1–94. [Google Scholar] [CrossRef]
- Luo, Z.L.; Hyde, K.D.; Liu, J.K.; Maharachchikumbura, S.S.N.; Jeewon, R.; Bao, D.F.; Bhat, D.J.; Lin, C.G.; Li, W.-L.; Yang, J.; et al. Freshwater Sordariomycetes. Fungal Divers. 2019, 99, 451–660. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.L.; Jones, E.B.G.; Hyde, K.D.; Liu, Z.Y.; Bao, D.F.; Liu, N.G.; Li, W.L.; Shen, H.W.; Yu, X.D.; et al. Freshwater fungi from karst landscapes in China and Thailand. Fungal Divers. 2023, 119, 1–212. [Google Scholar] [CrossRef]
- Calabon, M.S.; Hyde, K.D.; Jones, E.B.G.; Bao, D.F.; Bhunjun, C.S.; Phukhamsakda, C.; Shen, H.W.; Gentekaki, E.; Al Sharie, A.H.; Barros, J.; et al. Freshwater fungal biology. Mycosphere 2023, 14, 195–413. [Google Scholar] [CrossRef]
- Abdel-Aziz, F.A. Two new cheirosporous asexual taxa (Dictyosporiaceae, Pleosporales, Dothideomycetes) from freshwater habitats in Egypt. Mycosphere 2016, 7, 448–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).