Abstract
After pediatric heart transplant, commitment to lifelong immunosuppression is crucial to maintaining graft health. However, a review of the current literature surrounding adherence to immunosuppression in pediatric heart transplant patients is lacking. This systematic review aims to summarize the current landscape of adherence to immunosuppression in pediatric heart transplant patients. We conducted searches in PubMed MEDLINE, Embase, CENTRAL register of Controlled Trials (Wiley), and Scopus, from inception to March 2020. Studies were eligible if they outlined an aspect of adherence to immunosuppression and the measurement of adherence was performed with an objective or otherwise validated measure of adherence (e.g., drug levels, adherence questionnaires). The titles/abstracts of 880 articles were reviewed. After initial screening, 106 articles underwent full text review. As such, 14 articles were included in the final review. Baseline adherence estimates varied greatly, with most values between 40% and 70%. Nonadherence to immunosuppression is associated with worse outcomes (rejection, hospitalization, mortality), impaired quality of life, and mental health concerns in pediatric heart transplant patients. As nonadherence to immunosuppression is common and associated with worse outcomes, there is a need for further development and evaluation of interventions in this space.
1. Introduction
Pediatric heart transplantation is the standard of care for select patients with end-stage heart disease [1,2]. The primary indication for transplant differs by age, with congenital heart disease being most common in infants (57%) and cardiomyopathy being most common in older children (43% in children aged 1–10 years and 53% in children aged 11–17 years) [3].
After pediatric heart transplant (HTx), commitment to lifelong immunosuppression is necessary to maintain graft health. Most post-transplant immunosuppression regimens include a calcineurin inhibitor (CNI) and an antiproliferative agent [2,4]. Adequate adherence to these immunosuppressive medications is essential to preventing poor outcomes [5]. Estimates for rates of nonadherence within the pediatric population are wide ranging, likely due to the lack of standardization in adherence reporting [6]. Additionally, assessing adherence can be difficult due to the subjective nature of self-report. A review of the current literature surrounding adherence to immunosuppression in pediatric heart transplant patients is lacking.
In this manuscript, we evaluated measures of adherence, impact of nonadherence, and interventions to improve adherence outlined in the literature. Our objective was to review and describe the current landscape of immunosuppression adherence in pediatric heart transplant patients by identifying adherence rates and related factors, as well as proposed interventions for improving adherence in this population.
2. Methods
2.1. Study Design
The authors followed all guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [7].
2.2. Search Strategy
We conducted a systematic review of studies on adherence to immunosuppression in adult and pediatric HTx patients. A research librarian (LO) was responsible for a full literature search. Searches were conducted in PubMed MEDLINE, Embase, CENTRAL register of Controlled Trials (Wiley), and Scopus, from inception to March 2020, using search strategies that were collaboratively developed by the authors. The search was employed in PubMed using a combination of MeSH terms for heart transplantation, compliance, and adherence. The same terms were then used with the other databases. Search strategies can be found in Appendix A. No date limits were used. There were only English articles included in the review. TH also hand searched the bibliographies of relevant review articles and the included articles for additional references. Articles were reviewed in Rayyan by two independent reviewers and discussed to reach consensus.
2.3. Eligibility Criteria
Studies were eligible if they outlined an aspect of immunosuppression adherence in HTx patients, including but not limited to measurements of adherence, outcomes associated with nonadherence, and strategies to improve adherence. Studies that included children and adolescent patients were eligible. Studies involving multiple types of solid-organ transplants were included, but the HTx cohort had to contain at least 10 participants and the HTx group data had to be separately reported. Included studies also utilized an objective or otherwise validated measure of adherence (e.g., drug levels or adherence questionnaires). Studies were excluded if adherence was measured with a non-validated measure (e.g., physician report). Studies could be a prospective, observational, cross-sectional survey, or randomized clinical trial.
2.4. Study Selection and Data Extraction
Two authors (TH and SB) independently screened the articles for inclusion and subsequently reviewed the full text of the included articles. Discordant assessments were resolved by discussion between the reviewers to reach consensus. Data extraction was standardized to include population, type of article, study design, immunosuppression used, intervention (if any), measure of adherence, duration, number of HTx participants, participant age, study attrition rate, and main outcomes.
2.5. Assessment of Risk of Bias
Bias was evaluated by two independent reviewers (TH and KN). Reviews utilized the Newcastle–Ottawa for cohort studies [8], a modified version of the Newcastle–Ottawa by Modesti et al. [9] for cross-sectional studies, and Version 2 of the Cochrane risk-of-bias tool (RoB 2) [10] for randomized trials. Disagreements were resolved by discussion between reviewers to reach consensus.
2.6. Data Synthesis
Data were expected to be heterogenous. If sufficient homogeneity was found in outcomes, a meta-analysis or effect size analysis was considered.
3. Results
3.1. Literature Search
The titles/abstracts of 880 articles published before March 2020 were reviewed and 774 articles were excluded in the initial screen, leaving 106 articles for full-text review. Ninety-two articles were excluded, and 14 were included in the final review. The study flowchart and further reasoning for article inclusion/exclusion are outlined in the PRISMA flow diagram (Figure 1).
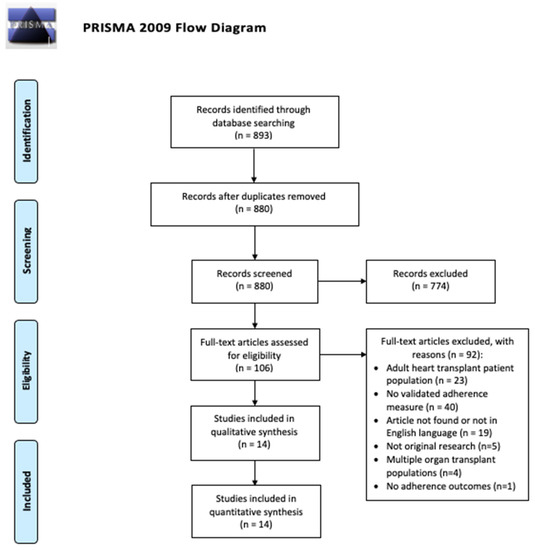
Figure 1.
PRISMA Flow Diagram for the included studies.
3.2. Study Characteristics
Study characteristics, including study design, are outlined in Table 1. Studies were published between 1998 and 2019. Eleven studies were conducted in the USA [6,11,12,13,14,15,16,17,18,19,20] and three studies in Europe [21,22,23]. Study types included cross sectional (seven studies), cohort (six studies), and one randomized trial. The median number of pediatric heart transplant patients was 32, with a range of 12–138. Studies were looked at collectively to determine measures and rates of nonadherence. Studies were then divided into three themes: (1) CNI levels as a marker for nonadherence and correlation to poor outcomes (four studies), (2) impact of nonadherence on quality of life and mental health (seven studies), and (3) the effect of transition programs on adherence (three studies).

Table 1.
Characteristics of all included studies.
3.3. Measures and Rates of Nonadherence
Studies used a variety of subjective (e.g., self-report) and objective (e.g., drug levels) measures. The reported rates of adherence varied greatly. In five studies, the nonadherence rate ranged from 40 to 60% [6,13,17,21,23]. Grady et al. (2018) found that approximately 70% of participants had CNI levels within the target range [16], and Serrano-Ikkos et al. (1998) found 69.8% of patients had good adherence, based on CNI levels and self-reported data [22]. In two studies, approximately 20% of patients reported at least one late or missing dose of immunosuppression medication in the last week [13,18], and 28% of caregivers reported that their adolescent/young adult (AYA) took one or more doses of antirejection medications late in the past week [13]. Figure 2 is a graphical representation of reported nonadherence rates, as described above. Table 2 outlines the different adherence measures used.
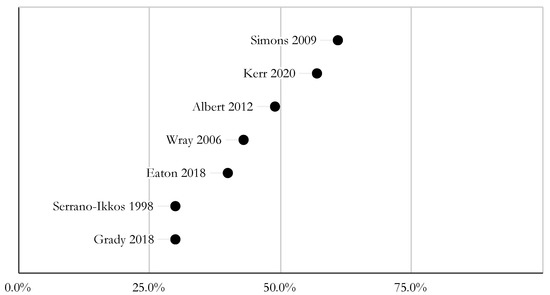
Figure 2.
Reported non-adherence rates.

Table 2.
Measures of Adherence.
3.4. CNI Levels as a Marker for Nonadherence and Poor Outcomes
Table 3 outlines the four studies that included immunosuppressant levels as a marker for nonadherence and poor outcomes. Immunosuppression variability is associated with hospitalization [6,14], rejection [6,14,19], and mortality [6,14]. More specifically, Kerr et al. (2020) looked at rejection risk following subtherapeutic CNI levels. The risk of rejection increased 6.9-fold in the 2 weeks following a subtherapeutic level, and increased 6.1-fold in the 3 months after presenting with a subtherapeutic level (as compared to time period after a therapeutic level) [17]. Further, 22% required treatment for rejection within 3 months of a subtherapeutic level [17]. When looking at self-reported nonadherence, mortality was significantly correlated with adolescent reports of missed doses [6]. In contrast, Ringewald et al. (2001) did not find an association between self-reported nonadherence and abnormal CSA level at admission for rejection. Notably, two-thirds of the patients with late rejection (11 out of 15) admitted to nonadherence [19].

Table 3.
Studies exploring drug levels as a marker for nonadherence and rejection.
3.5. Impact of Nonadherence on Quality of Life and Mental Health
Table 4 outlines the seven studies that included the impact of nonadherence on quality of life and mental health. Some studies found that nonadherence was associated with patient mental health concerns on child and/or caregiver self-report tools [12,23], and poor quality of life scores further correlated with rejection episodes [12]. In two studies, specific psychiatric comorbidities, such as anxiety [18] and depression [21], were found to correlate with nonadherence. In contrast, two studies found that there were no associations between mental illness in the child and nonadherence [13,22], and that caregiver emotional distress did not seem to correlate with missed doses [13]. These findings may be explained by low rates of reported nonadherence, and an overall lower prevalence of adherence problems in the HTx group, as compared to other solid-organ transplant groups in the study [13].

Table 4.
Studies exploring impact of nonadherence on quality of life and mental health.
3.6. The Effect of Transition Programs on Adherence
Table 5 outlines the two studies discussing transition from pediatric to adult health care. Anton et al. (2019) utilized a two-year structured transition program consisting of seven two-hour sessions to improve patients’ overall medical knowledge, medication adherence, readiness to transition, and parental perceptions of child’s readiness to transition [11]. They found a statistically significant decrease in percentage of CNI levels out of range prior to beginning the transition program and after completing the transition program [11]. In addition to improving immunosuppression adherence, the program also enhanced overall patient medical and medication knowledge, which may prevent lapses in medical care [11].

Table 5.
Studies exploring the effect of a transition program on adherence.
Grady et al. (2019) studied a standardized transition program designed to improve outcomes (e.g., adherence to immunosuppression/medical regimen) for young adults who underwent heart transplant as children and transferred to adult care. The program focused on improving heart transplant knowledge, self-care and self-advocacy skills, and enhancing social support. The transition program included computer modules and multiple meetings/telephone calls with dedicated HTx staff. Patients were randomized into the intervention group or to usual care (e.g., standard transfer-of-care meeting). There were no significant between-group or within-group differences in percent of tacrolimus levels within target range from baseline to 6 months (intervention 69–75%, usual care 58–72%) [15]. Additionally, average overall self-reported adherence to the treatment regimen was similarly good in both groups, and no significant group/time interactions were detected. The intervention group actually ended up having significantly more episodes of acute rejection through the 6 months when compared to usual care, though the overall numbers were low (intervention = 5, usual care = 0).
3.7. Studies’ Methodological Quality
Table 6 outlines bias ratings for cohort studies, as scored by the Newcastle–Ottawa scale. Table 7 outlines bias ratings for cross-sectional studies, as scored by the modified Newcastle–Ottawa scale. Table 8 outlines the bias rating for the randomized trial, as scored by Version 2 of the Cochrane risk-of-bias tool for randomized trials. The six cohort studies scored between 7 and 9 out of 9 possible points, with points deducted for lack of controls/adjustments and self-reported outcomes. The seven cross-sectional studies scored between 6 and 8 out of 10 possible points, with points deducted for lack of description of non-respondents, lack of controls/adjustments, only self-reported outcomes, and incomplete presentation of measurement of association. The majority of points were deducted for lack of controls/adjustments for cofounders (20 points amongst 11 studies). The randomized trial scored a low overall risk, acknowledging the nonblinded participants and researchers.

Table 6.
Bias ratings of cohort studies via Newcastle–Ottawa scores.

Table 7.
Bias rating of cross-sectional studies via modified Newcastle–Ottawa scores.

Table 8.
Bias rating of randomized control trails via version 2 of the Cochrane risk-of-bias tool.
4. Discussion
This systematic review outlined measures of adherence and baseline adherence estimates in pediatric heart transplant patients. Baseline adherence rates varied greatly, with most values between 40% and 70%, depending on measurement metrics. Adherence was measured most often with serum immunosuppression levels, though the way in which these levels were reported, interpreted, and assigned clinical significance differed between studies. Some studies also used a validated self-report measure. Variable immunosuppressant drug levels correlated with significant clinical outcomes, including rejection, hospitalizations, and death. Mental health comorbidities were associated with nonadherence in some studies. Finally, findings regarding the utility of transition programs in improving adherence in pediatric patients were mixed.
An agreed upon gold standard for measuring adherence would be beneficial for data analysis and identification of at-risk patients. By doing so, we would be better equipped to compare results between studies, especially in a patient population that is already limited in size. Of note, the lack of standardization for measuring adherence is not an issue unique to pediatric transplant recipients. In the adult literature, there are numerous tools to measure immunosuppression adherence [28]. The majority of these tools are self-report measures [28], which have their limitations (including Hawthorne-type effects and social desirability). While many pediatric studies utilized immunosuppressant drug levels to determine adherence, this was less common in the adult literature [28]. It is worth noting that recent efforts led to the development of the PROMIS Medication Adherence Scale (PMAS), which is a widely available, free self-report measure of adherence. Validation studies for PMAS are ongoing to evaluate its psychometric properties in different pediatric and adult patient populations [29].
Our findings are consistent with the literature, illustrating how intrapatient variability of a drug is associated with poor allograft outcomes (e.g., rejection, death) [30,31]. Cardiac allograft vasculopathy (CAV) is a cause of morbidity and mortality in pediatric heart transplant patients, and is a common indication for re-transplantation. Interestingly, the link between medication nonadherence and CAV has not been clearly elucidated. Per the International Society for Heart and Lung Transplantation (ISHLT) data, rejection within the first year is associated with CAV development [32]. However, in adults, tacrolimus intrapatient variability was not associated with cardiac allograft vasculopathy [33]. Pediatric patients with cardiac allograft vasculopathy have a 50% allograft survival rate at nearly three years [34]. A variety of factors can lead to CAV, including rejection [35].
The relationship between medication nonadherence, quality of life and mental health is likely multifactorial. Symptoms of anxiety and depression may make medication adherence more difficult and lead to intentional nonadherence. It is also possible that medication nonadherence may lead to emotional distress and physical symptoms, which may negatively impact quality of life and mental health. Adult heart transplant patients also struggle with mental health and socioeconomic comorbidities, as well as engaging with support networks [28]. When discussing medication adherence, time should be spent addressing a patient’s mental health concerns to identify those that would benefit from psychiatric/psychological intervention.
Lastly, this review showcased the feasibility of two pediatric to adult heart transplant transition programs and their potential to improve immunosuppression adherence. These publications support the continued development of transition programs for these patients, with an opportunity for further research to improve adherence outcomes. In addition to transition programs, there may be a role for technology-based interventions to improve adherence in heart transplant patients. Digital interventions are already being used for children and adolescents with many chronic health conditions [36,37,38,39,40,41,42,43,44,45,46,47]. Gomis-Pastor et al. published one of the first studies using a mobile health intervention (mHeart) to improve immunosuppression adherence in adult heart transplant patients [48]. Use of the app was significantly associated with improved adherence to immunosuppression, increasing adherence rates from 61% to 87% [48]. A recently published systematic review on mobile health app interventions in transplant recipients showed that medication adherence improved in the majority of studies evaluating m-health as an intervention [49]. The continued development of technology-based interventions to improve medication adherence remains a promising area of research, one which may hopefully lead to improved health outcomes and quality of life.
Strengths and Limitations
The primary strength of this manuscript is its systematic approach to evaluating immunosuppression adherence in pediatric heart transplant patients. All articles were independently screened by at least two authors, and articles were evaluated for bias by two authors. The data were carefully studied and subsequently organized in an attempt to provide clinically relevant information.
One limitation of this paper is the heterogeneous nature of the data. The variety of ways in which immunosuppression levels were described, as well as the combination of different validated self-report measures and qualitative outcomes, precludes a meta-analysis. There is also a lack of controls and adjustments in the data analysis. The included studies have relatively small sample sizes, which is to be expected, given the nature of the pediatric heart transplant cohort. Patient follow-up varied amongst the included studies, limiting the interpretation of timing for outcomes associated with nonadherence. Lastly, only articles published in English were included in our review.
Given that immunosuppressive regimens have changed over the past few decades, comparing data from studies over a 20-year time span has its limitations. The majority of HTx patients are prescribed a three-drug maintenance immunosuppression regimen, including a CNI, antimetabolite, and corticosteroids. Cyclosporine is a CNI that was most popular in clinical practice in the 1980s [50], while tacrolimus was introduced in the early 1990s and has become the preferred CNI due to a more favorable side-effect profile [50]. Additionally, mycophenolate mofetil largely replaced azathioprine as the antimetabolite of choice after the clinical trial by Kobashingawa et al. (1998) showed reduced rates of rejection and improved survival with mycophenolate mofetil [51]. Sirolimus and everolimus are proliferation signal inhibitors that became available in the early 2000s [50]. These drugs may be used as part of a triple-drug regimen or for CNI avoidance, though particular use cases are beyond the scope of this manuscript. There may be a role for simplified medication regimens to improve adherence rates [52,53], which remains a meaningful topic for additional research and review.
5. Conclusions
Nonadherence to immunosuppression in pediatric heart transplant remains a challenge that has an impact on patient outcomes (rejection, hospitalization, mortality), quality of life, and mental health. A gold-standard adherence measure would assist with collective analysis and interpretation of the pediatric heart transplant literature. Transition programs are feasible interventions for young adult patients with the aim to improve adherence, but more research is needed to determine associated outcomes. Lastly, further studies are needed to identify additional strategies to improve adherence in pediatric heart transplant patients.
Author Contributions
Conceptualization, K.N., T.H. and S.M.B.; methodology, K.N, T.H., S.M.B. and L.C.O.; validation, K.N. and T.H.; formal analysis, K.N. and T.H.; investigation, T.H., S.M.B. and L.C.O.; writing—original draft preparation, K.N., T.H. and S.M.B.; writing—review and editing, K.N., T.H., S.M.B., K.G. and K.L.; visualization, K.N., T.H. and S.M.B.; supervision, S.M.B.; funding acquisition, S.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by a grant (K23HL150232, PI: Badawy) from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Search Strategy
PubMed
((“Heart Transplantation” [MeSH Terms] OR “heart transplant*” [Title/Abstract]) OR ((“Organ Transplantation” [MeSH Terms:noexp] OR “transplant *” [Title/Abstract]) AND (“heart” [MeSH Terms] OR “heart *” [Title/Abstract]))) AND (((((((((“Medication Adherence” [MeSH Terms] OR “Patient Compliance” [MeSH Terms]) OR “Adherence” [Title/Abstract]) OR “Nonadherence” [Title/Abstract]) OR “non adherence” [Title/Abstract]) OR “non adherence” [Title/Abstract]) OR “Compliance” [Title/Abstract]) OR “Noncompliance” [Title/Abstract]) OR “non compliance” [Title/Abstract]) OR “non compliance” [Title/Abstract])
Remove review [pt] and animals in title.
Embase
(‘heart transplantation’/exp OR ‘heart transplant*’:ti,ab OR ((‘organ transplantation’/exp OR transplant*:ti,ab) AND (‘heart’/exp OR heart:ti,ab))) AND (‘medication compliance’/exp OR ‘adherence’:ti,ab OR ‘nonadherence’:ti,ab OR ‘non adherence’:ti,ab OR ‘compliance’:ti,ab OR ‘noncompliance’:ti,ab OR ‘non compliance’:ti,ab)
Remove review and meeting abstracts and animals in title.
Central
- #1
- MeSH descriptor: [Heart Transplantation] explode all trees
- #2
- “heart transplant*”:ti,ab
- #3
- #1 OR #2
- #4
- MeSH descriptor: [Organ Transplantation] explode all trees
- #5
- transplant*:ti,ab
- #6
- MeSH descriptor: [Heart] explode all trees
- #7
- heart:ti,ab
- #8
- #4 OR #5
- #9
- #6 OR #7
- #10
- #8 AND #9
- #11
- #3 OR #10
- #12
- MeSH descriptor: [Medication Adherence] explode all trees
- #13
- MeSH descriptor: [Patient Compliance] explode all trees
- #14
- (“adherence” OR “nonadherence” OR “non adherence” OR “compliance” OR “noncompliance” OR “non compliance”).ti,ab
Scopus
TITLE-ABS-KEY (“heart transplant*”) AND TITLE-ABS-KEY (adherence OR nonadherence OR “non adherence” OR compliance OR noncompliance OR “non compliance”) AND (LIMIT-TO (DOCTYPE, “ar”))
References
- Thrush, P.T.; Hoffman, T.M. Pediatric heart transplantation-indications and outcomes in the current era. J. Thorac. Dis. 2014, 6, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- D’Addese, L.; Joong, A.; Burch, M.; Pahl, E. Pediatric heart transplantation in the current era. Curr. Opin. Pediatr. 2019, 31, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Rossano, J.W.; Singh, T.P.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Azzi, J.R.; Sayegh, M.H.; Mallat, S.G. Calcineurin Inhibitors: 40 Years Later, Can’t Live without …. J. Immunol. 2013, 191, 5785–5791. [Google Scholar] [CrossRef] [Green Version]
- Oliva, M.; Singh, T.P.; Gauvreau, K.; Vanderpluym, C.J.; Bastardi, H.J.; Almond, C.S. Impact of medication non-adherence on survival after pediatric heart transplantation in the U.S.A. J. Heart Lung Transplant. 2013, 32, 881–888. [Google Scholar] [CrossRef]
- Simons, L.E.; Gilleland, J.; Blount, R.L.; Amaral, S.; Berg, A.; Mee, L.L. Multidimensional Adherence Classification System: Initial development with adolescent transplant recipients. Pediatr. Transplant. 2009, 13, 590–598. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 September 2021).
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G.; ESH Working Group on CV Risk in Low Resource Settings. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Anton, C.M.; Anton, K.; Butts, R.J. Preparing for transition: The effects of a structured transition program on adolescent heart transplant patients’ adherence and transplant knowledge. Pediatr. Transplant. 2019, 23, e13544. [Google Scholar] [CrossRef]
- Devine, K.A.; Reed-Knight, B.; Loiselle, K.A.; Simons, L.E.; Mee, L.L.; Blount, R.L. Predictors of long-term health-related quality of life in adolescent solid organ transplant recipients. J. Pediatr. Psychol. 2011, 36, 891–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, C.K.; Gutierrez-Colina, A.M.; Quast, L.F.; Liverman, R.; Lee, J.L.; Mee, L.L.; Reed-Knight, B.; Cushman, G.; Chiang, G.; Romero, R.; et al. Multimethod Assessment of Medication Nonadherence and Barriers in Adolescents and Young Adults with Solid Organ Transplants. J. Pediatr. Psychol. 2018, 43, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Flippin, M.S.; Canter, C.E.; Balzer, D.T. Increased morbidity and high variability of cyclosporine levels in pediatric heart transplant recipients. J. Heart Lung Transplant. 2000, 19, 343–349. [Google Scholar] [CrossRef]
- Grady, K.L.; Andrei, A.C.; Shankel, T.; Chinnock, R.; Miyamoto, S.D.; Ambardekar, A.V.; Anderson, A.; Addonizio, L.; Latif, F.; Lefkowitz, D.; et al. Pediatric Heart Transplantation: Transitioning to Adult Care (TRANSIT): Feasibility of a Pilot Randomized Controlled Trial. J. Card. Fail. 2019, 25, 948–958. [Google Scholar] [CrossRef]
- Grady, K.L.; Hof, K.V.; Andrei, A.C.; Shankel, T.; Chinnock, R.; Miyamoto, S.; Ambardekar, A.V.; Anderson, A.; Addonizio, L.; Latif, F.; et al. Pediatric Heart Transplantation: Transitioning to Adult Care (TRANSIT): Baseline Findings. Pediatr. Cardiol. 2018, 39, 354–364. [Google Scholar] [CrossRef]
- Kerr, S.M.; Jorgensen, N.W.; Hong, B.J.; Friedland-Little, J.M.; Albers, E.L.; Newland, D.M.; Law, Y.M.; Kemna, M.S. Assessment of rejection risk following subtherapeutic calcineurin inhibitor levels after pediatric heart transplantation. Pediatr. Transplant. 2020, 24, e13616. [Google Scholar] [CrossRef]
- McCormick, A.D.; Schumacher, K.R.; Zamberlan, M.; Uzark, K.; Yu, S.; Lowery, R.; Rottach, N.; Cousino, M.K. Generalized and specific anxiety in adolescents following heart transplant. Pediatr. Transplant. 2020, 24, e13647. [Google Scholar] [CrossRef]
- Ringewald, J.M.; Gidding, S.S.; Crawford, S.E.; Backer, C.L.; Mavroudis, C.; Pahl, E. Nonadherence is associated with late rejection in pediatric heart transplant recipients. J. Pediatr. 2001, 139, 75–78. [Google Scholar] [CrossRef]
- Wolfe, K.R.; Kelly, S.L.; Steinberg, E.; Pliego, J.; Everitt, M.D. Predictors of neuropsychological functioning and medication adherence in pediatric heart transplant recipients referred for neuropsychological evaluation. Pediatr. Transplant. 2020, 24, e13615. [Google Scholar] [CrossRef]
- Wray, J.; Waters, S.; Radley-Smith, R.; Sensky, T. Adherence in adolescents and young adults following heart or heart-lung transplantation. Pediatr. Transplant. 2006, 10, 694–700. [Google Scholar] [CrossRef]
- Serrano-Ikkos, E.; Lask, B.; Whitehead, B.; Eisler, I. Incomplete adherence after pediatric heart and heart-lung transplantation. J. Heart Lung Transplant. 1998, 17, 1177–1183. [Google Scholar] [PubMed]
- Albert, W.; Hudalla, A.; Traue, K.; Hetzer, R. Impact of heart transplantation in infancy and adolescence on quality of life and compliance. HSR Proc. Intensive Care Cardiovasc. Anesth. 2012, 4, 125–129. [Google Scholar] [PubMed]
- Goetzmann, L.; Klaghofer, R.; Spindler, A.; Wagner-Huber, R.; Scheuer, E.; Buddeberg, C. The “Medication Experience Scale for Immunosuppressants” (MESI): Initial results for a new screening instrument in transplant medicine. Psychother. Psychosom. Med. Psychol. 2006, 56, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zelikovsky, N.; Schast, A.P. Eliciting accurate reports of adherence in a clinical interview: Development of the Medical Adherence Measure. Pediatr. Nurs. 2008, 34, 141–146. [Google Scholar]
- Shemesh, E.; Bucuvalas, J.C.; Anand, R.; Mazariegos, G.V.; Alonso, E.M.; Venick, R.S.; Reyes-Mugica, M.; Annunziato, R.A.; Shneider, B.L. The Medication Level Variability Index (MLVI) Predicts Poor Liver Transplant Outcomes: A Prospective Multi-Site Study. Am. J. Transplant. 2017, 17, 2668–2678. [Google Scholar] [CrossRef]
- Grady, K.L.; Jalowiec, A.; White-Williams, C. Patient compliance at one year and two years after heart transplantation. J. Heart Lung Transplant. 1998, 17, 383–394. [Google Scholar]
- Hussain, T.; Nassetta, K.; O’Dwyer, L.C.; Wilcox, J.E.; Badawy, S.M. Adherence to immunosuppression in adult heart transplant recipients: A systematic review. Transplant. Rev. 2021, 35, 100651. [Google Scholar] [CrossRef]
- Peipert, J.D.; Badawy, S.M.; Baik, S.H.; Oswald, L.B.; Efficace, F.; Garcia, S.F.; Mroczek, D.K.; Wolf, M.; Kaiser, K.; Yanez, B.; et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) Medication Adherence Scale (PMAS). Patient Prefer. Adherence 2020, 14, 971–983. [Google Scholar] [CrossRef]
- Gueta, I.; Markovits, N.; Yarden-Bilavsky, H.; Raichlin, E.; Freimark, D.; Lavee, J.; Loebstein, R.; Peled, Y. High tacrolimus trough level variability is associated with rejections after heart transplant. Am. J. Transplant. 2018, 18, 2571–2578. [Google Scholar] [CrossRef] [Green Version]
- Sirota, M.; Heyrend, C.; Ou, Z.; Masotti, S.; Griffiths, E.; Molina, K. Impact of tacrolimus variability on pediatric heart transplant outcomes. Pediatr. Transplant. 2021, 25, e14043. [Google Scholar] [CrossRef]
- Dipchand, A.I.; Laks, J.A. Pediatric heart transplantation: Long-term outcomes. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Shuker, N.; Bouamar, R.; Hesselink, D.A.; van Gelder, T.; Caliskan, K.; Manintveld, O.C.; Balk, A.H.; Constantinescu, A.A. Intrapatient Variability in Tacrolimus Exposure Does Not Predict The Development of Cardiac Allograft Vasculopathy After Heart Transplant. Exp. Clin. Transplant. 2018, 16, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahl, E.; Naftel, D.C.; Kuhn, M.A.; Shaddy, R.E.; Morrow, W.R.; Canter, C.E.; Kirklin, J.; Pediatric Heart Transplant Study. The impact and outcome of transplant coronary artery disease in a pediatric population: A 9-year multi-institutional study. J. Heart Lung Transplant. 2005, 24, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Schmauss, D.; Weis, M. Cardiac allograft vasculopathy: Recent developments. Circulation 2008, 117, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Thompson, A.A.; Kuhns, L.M. Medication Adherence and Technology-Based Interventions for Adolescents With Chronic Health Conditions: A Few Key Considerations. JMIR Mhealth Uhealth 2017, 5, e202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, S.M.; Kuhns, L.M. Texting and Mobile Phone App Interventions for Improving Adherence to Preventive Behavior in Adolescents: A Systematic Review. JMIR Mhealth Uhealth 2017, 5, e50. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.C.; O’Dwyer, L.C.; Badawy, S.M. Telemedicine in Malignant and Nonmalignant Hematology: Systematic Review of Pediatric and Adult Studies. JMIR Mhealth Uhealth 2021, 9, e29619. [Google Scholar] [CrossRef]
- Badawy, S.M.; Abebe, K.Z.; Reichman, C.A.; Checo, G.; Hamm, M.E.; Stinson, J.; Lalloo, C.; Carroll, P.; Saraf, S.L.; Gordeuk, V.R.; et al. Comparing the Effectiveness of Education Versus Digital Cognitive Behavioral Therapy for Adults with Sickle Cell Disease: Protocol for the Cognitive Behavioral Therapy and Real-time Pain Management Intervention for Sickle Cell via Mobile Applications (CaRISMA) Study. JMIR Res. Protoc. 2021, 10, e29014. [Google Scholar] [CrossRef]
- Shah, A.C.; Badawy, S.M. Telemedicine in Pediatrics: Systematic Review of Randomized Controlled Trials. JMIR Pediatr. Parent. 2021, 4, e22696. [Google Scholar] [CrossRef]
- Badawy, S.M.; Radovic, A. Digital Approaches to Remote Pediatric Health Care Delivery During the COVID-19 Pandemic: Existing Evidence and a Call for Further Research. JMIR Pediatr. Parent. 2020, 3, e20049. [Google Scholar] [CrossRef]
- Alberts, N.M.; Badawy, S.M.; Hodges, J.; Estepp, J.H.; Nwosu, C.; Khan, H.; Smeltzer, M.P.; Homayouni, R.; Norell, S.; Klesges, L.; et al. Development of the InCharge Health Mobile App to Improve Adherence to Hydroxyurea in Patients With Sickle Cell Disease: User-Centered Design Approach. JMIR Mhealth Uhealth 2020, 8, e14884. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, W.A.; Heidelberg, R.E.; Gilbert, A.M.; Heneghan, M.B.; Badawy, S.M.; Alberts, N.M. eHealth and mHealth interventions in pediatric cancer: A systematic review of interventions across the cancer continuum. Psychooncology 2020, 29, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Radovic, A.; Badawy, S.M. Technology Use for Adolescent Health and Wellness. Pediatrics 2020, 145, S186–S194. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Morrone, K.; Thompson, A.; Palermo, T.M. Computer and mobile technology interventions to promote medication adherence and disease management in people with thalassemia. Cochrane Database Syst. Rev. 2019, 6, CD012900. [Google Scholar] [CrossRef]
- Badawy, S.M.; Cronin, R.M.; Hankins, J.; Crosby, L.; DeBaun, M.; Thompson, A.A.; Shah, N. Patient-Centered eHealth Interventions for Children, Adolescents, and Adults With Sickle Cell Disease: Systematic Review. J. Med. Internet Res. 2018, 20, e10940. [Google Scholar] [CrossRef] [Green Version]
- Badawy, S.M.; Barrera, L.; Sinno, M.G.; Kaviany, S.; O’Dwyer, L.C.; Kuhns, L.M. Text Messaging and Mobile Phone Apps as Interventions to Improve Adherence in Adolescents With Chronic Health Conditions: A Systematic Review. JMIR Mhealth Uhealth 2017, 5, e66. [Google Scholar] [CrossRef] [Green Version]
- Gomis-Pastor, M.; Mirabet Perez, S.; Roig Minguell, E.; Brossa Loidi, V.; Lopez Lopez, L.; Ros Abarca, S.; Galvez Tugas, E.; Mas-Malagarriga, N.; Mangues Bafalluy, M.A. Mobile Health to Improve Adherence and Patient Experience in Heart Transplantation Recipients: The mHeart Trial. Healthcare 2021, 9, 463. [Google Scholar] [CrossRef]
- Abasi, S.; Yazdani, A.; Kiani, S.; Mahmoudzadeh-Sagheb, Z. Effectiveness of mobile health-based self-management application for posttransplant cares: A systematic review. Health Sci. Rep. 2021, 4, e434. [Google Scholar] [CrossRef]
- Stehlik, J.; Kobashigawa, J.; Hunt, S.A.; Reichenspurner, H.; Kirklin, J.K. Honoring 50 Years of Clinical Heart Transplantation in Circulation. Circulation 2018, 137, 71–87. [Google Scholar] [CrossRef]
- Kobashigawa, J.; Miller, L.; Renlund, D.; Mentzer, R.; Alderman, E.; Bourge, R.; Costanzo, M.; Eisen, H.; Dureau, G.; Ratkovec, R.; et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation 1998, 66, 507–515. [Google Scholar] [CrossRef]
- Doesch, A.O.; Mueller, S.; Akyol, C.; Erbel, C.; Frankenstein, L.; Ruhparwar, A.; Ehlermann, P.; Dengler, T.J.; Katus, H.A. Increased adherence eight months after switch from twice daily calcineurin inhibitor based treatment to once daily modified released tacrolimus in heart transplantation. Drug Des. Devel. Ther. 2013, 7, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Godinas, L.; Dobbels, F.; Hulst, L.; Verbeeck, I.; De Coninck, I.; Berrevoets, P.; Schaevers, V.; Yserbyt, J.; Dupont, L.J.; Verleden, S.E.; et al. Once daily tacrolimus conversion in lung transplantation: A prospective study on safety and medication adherence. J. Heart Lung Transplant. 2021, 40, 467–477. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).