Abstract
As spontaneous ascending aortic thrombi (AATs) are uncommon in modern clinical practice, despite the application of new technology and the widespread use of contrast-enhanced computer tomography during primary assessments in patients without underlying predisposing conditions, a thrombus floating in the ascending aorta is rarely discovered in a timely manner; moreover, the ascending tract represents an unusual site for thrombus formation. The clinical presentation of AATs is also often in the form of peripheral arterial embolization, which can cause a wide variety of symptoms, from stroke to limb ischemia, and thus delay correct diagnosis. Medical management is a risky strategy, while surgical treatment is usually challenging due to the risk of thrombus dislodgement and difficulties related to prior embolization complication management. In this study, faced with a peculiar case of embolic stroke in an otherwise healthy 71-year-old woman, we analyzed the status of knowledge on spontaneous ascending aortic thrombus treatments and outcomes. A multidisciplinary approach represents the best choice for defining a patient’s timing of surgery and ensuring the management of complications. Sequential multistage treatment minimizes further complications and prevents worsening patient outcomes, leading to the best management for every possible clinical presentation. A less invasive surgical approach could lead to complete resolution of the pathology, avoiding further potentially lethal complications, facilitating postoperative management, avoiding delayed treatments, and resulting in better outcomes.
1. Introduction
Ascending aortic thrombi (AATs) are rare findings usually caused by preexisting aortic conditions such as atherosclerosis or acute events such as aortic dissection (AD) or traumatic aortic injuries (TAIs). The incidence rate for an aortic mural thrombus without a definite underlying cause, which can lead to AATs, is about 0.45% [1].
Both structural pathologies and medical conditions, such as hypercoagulability, tobacco use, malignancies, medication use, autoimmune diseases, acute or chronic inflammation, primary endothelial disorders, and vasculitis, could promote clotting, thus leading to aortic thrombus formation [2]. Additionally, aortic thrombi are commonly detected in the thoracic descending aorta or the aortic arch, making the ascending aorta the most uncommon location for thrombi to develop due to its high-blood-flow environment and sheer wall stress [3].
Currently, no unique treatment exists for this pathology. However, the fact that embolization could lead to catastrophic effects underlines the importance of a rapid, punctual treatment strategy that could prevent further complications and solve the acute problem.
2. Case Presentation
A 71-year-old woman was admitted to our hospital with sudden left hemisyndrome and dysarthria. The patient was affected by empty sella syndrome and bipolar disorder under medication and had a recent hospital admission for dyspnea, where her chest computed tomography angiography (CTA) was negative for pulmonary embolism or pneumonia. Her nucleic acid test for COVID-19 was also negative. After further neurological evaluation, clinical suspicion of a stroke was confirmed, also presenting neglect and anosognosia, with a National Institutes of Health Stroke Scale score of 10. An urgent CTA was performed, showing complete occlusion of the right middle cerebral artery, which caused a massive ischemic stroke (Figure 1).
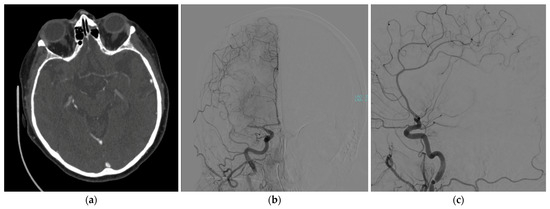
Figure 1.
Brain CTA showing a complete occlusion of the right middle cerebral artery in (a) axial view; confirmed during a cerebral angiography: (b) coronal and (c) sagittal views.
Due to a suspicion of aortic dissection, a subsequent chest contrast-enhanced CTA was performed, revealing the presence of a 20 mm thrombus in the ascending aorta, 4 cm above the aortic valve and close to the common origin of the brachiocephalic artery and the left common carotid artery, without evidence of atherosclerosis or intimal tear (Figure 2).
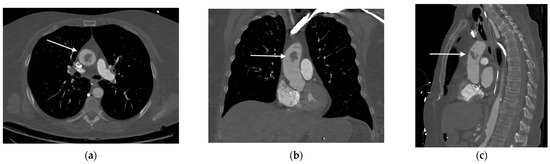
Figure 2.
Contrast-enhanced chest CT revealing the presence of a mass (as indicated by the arrows) in the ascending aorta: (a) axial; (b) coronal; and (c) sagittal views.
Due to these clinical conditions, a multistage approach was planned after a multidisciplinary evaluation. Prompt mechanical thrombectomy for the MCA occlusion was performed, with optimal results at the following CT scan. The next day, after complete neurological recovery with no residual impairment, the patient underwent a surgical thrombectomy via standard median sternotomy. The femoral artery and right atrium were the selected sites of cannulation; an epiaortic echography confirmed the presence of a floating mass close to the origin of the brachiocephalic artery (Figure 3).
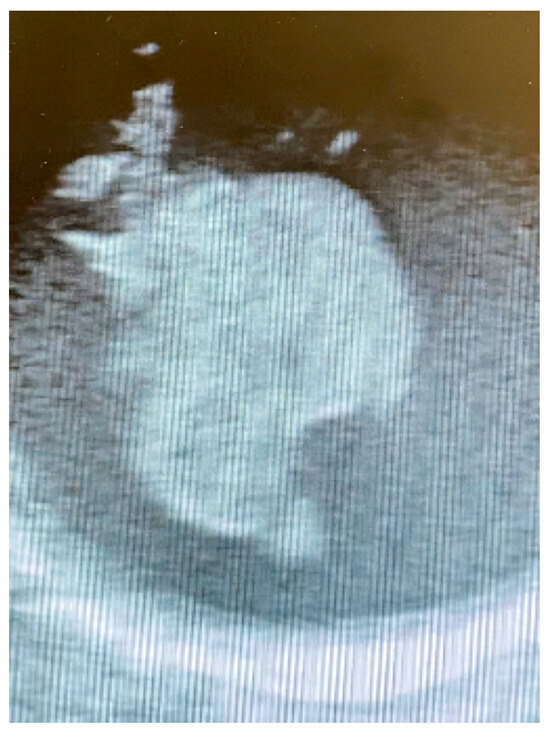
Figure 3.
Intraoperative epiaortic echography showing the floating thrombus.
Due to the high risk of embolization connected to the aortic cross-clamp, the surgical strategy was to remove the mass during moderate hypothermic circulatory arrest at 26 °C through a longitudinal aortotomy. The 28 mm × 24 mm thrombus was linked to the aortic wall through a thin peduncle; the attachment site was in the ascending aorta, leading to the mass floating just in front of the innominate artery origin. After a meticulous inspection, a minimal ulcerated aortic plaque was found in the aortic wall (Figure 4).
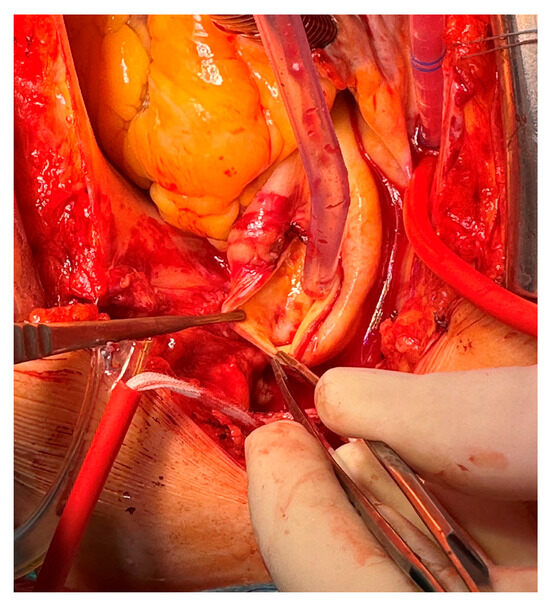
Figure 4.
Minimal ulcerated plaque; thrombus attachment site to the aortic wall.
To minimize the circulatory arrest time and the risk of further brain ischemic insult, the thrombus was rapidly excised and the aortotomy was closed without ascending aorta replacement. The cardiopulmonary bypass was promptly restarted, with only 8 min of complete hypothermic circulatory arrest. Both the cerebral embolus and aortic mass turned out to be thrombotic formations according to a histological analysis, with classical Zahn lines found in the aortic sample (Figure 5).
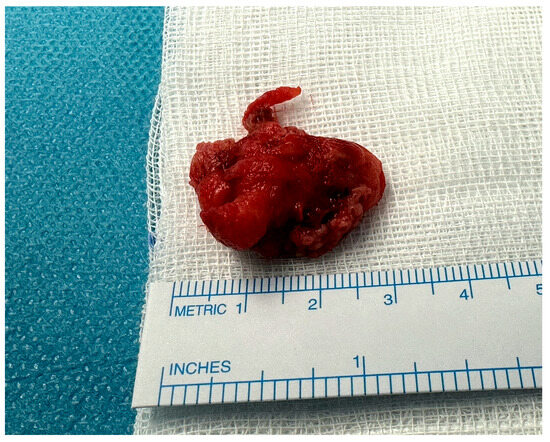
Figure 5.
Excised aortic floating thrombus.
A further investigation into genetic coagulation disorders turned out to be negative, with no mutations such as in Leiden V factor, or altered prothrombin; no alterations in APC resistance; and heterozygosity for an A1298C mutation on methylenetetrahydrofolate reductase (MTHFR), with no C677T mutation. Antiphospholipid antibody syndrome (APS) was also determined to be negative, with just a slight alteration in anti-cardiolipin IgM (64.8 U/mL) and normal IgG (15.8 U/mL). Neither malignancies nor infectious diseases were detected during further investigations. After 14 days, the patient was discharged home, with complete neurological recovery, and administered anticoagulation therapy with warfarin after a hematological evaluation. A postoperative CTA confirmed a standard aortic wall; no recurrences were found on a 2-month follow-up CTA scan.
3. Methods
The MEDLINE/PubMed database was searched for publications about ascending aortic floating thrombi, including surgical treatment and anticoagulation strategy (Table 1). We restricted the case report research to the last 5 years due to the recent evolution of surgical approaches and new technological developments that have changed aortic pathology treatment. We managed to include all articles with the following keywords: aortic, ascending, thrombus, embolization, and anticoagulation. A manual search of all relevant publications supplemented the article collection. We have excluded publications about severe atherosclerotic aorta, aortic root pathology, and thrombi caused by a well-determined preexisting prothrombotic disease.

Table 1.
Literature review.
4. Discussion
Although a rare condition, floating aortic thrombi are associated with early complications such as peripheral embolization and stroke, making its occurrence a potentially life-threatening situation. Typically, its clinical presentation is linked to embolism, with stroke and myocardial infarction being the most common initial signs of aortic thrombus [41].
Effective management of these early complications is crucial as the right approach can help minimize adverse outcomes. In cases of peripheral embolism, while cardiac sources are the most frequent causes, it is important to investigate aortic thrombus even in the absence of any underlying coagulation disorders or structural issues. A floating aortic thrombus is recognized as the source of arterial embolism in 5% to 9% of patients [42,43].
The etiopathogenesis of aortic thrombi is heterogeneous and still not fully understood, although the recent literature has depicted some predisposing factors. Endothelial lesions represent the most common sub-stratum for thrombosis; additionally, in mild atherosclerotic aortas, wall microlesioned plaques are usually the attachment site of a floating mobile mass due to the subsequent deposition of adherent debris [20,44]. Clinical conditions, including inflammation and hypercoagulation, whether genetically determined or acquired, can promote thrombotic manifestations. Notably, the recent COVID-19 pandemic has led to a significant increase in thromboembolic events involving the ascending aorta. This phenomenon is linked to the hyperinflammatory process associated with a hyper-coagulable state as observed in several case reports [45,46]. Smoking habits, including the use of electronic cigarettes, are strongly associated with the formation of blood clots, likely due to the inflammatory response they induce [2,30]. Additionally, the use of certain medications and drugs, such as cocaine, may pose significant risk factors. Thrombotic promotion has also been shown to occur with the use of steroids or chemotherapy agents. Cisplatin, for example, causes endothelial damage and is related to vascular events in about 45% of patients; it is also linked to thrombus formation in the ascending aorta [47,48,49,50,51].
Malignancies pose a risk for systemic thrombosis, including the development of primary aortic tumors. Among these tumors, angiosarcoma is the most common histotype and is closely associated with the formation of aortic thrombus in the ascending aorta [52]. When a primary wall neoplasm is suspected, aortic resection is often necessary to prevent cancer recurrence, and a biopsy is essential for making a definitive diagnosis [11,47].
During a patient’s first assessment, an extensive investigation of possible hypercoagulability and autoimmune disorders should always be conducted. A systemic prothrombotic state could be determined based on numerous medical conditions, including antiphospholipid syndrome, lupus anticoagulant, hyperhomocysteinemia, and endocrine disorders such as hyperprolactinemia [13,22,53].
Structural abnormalities, such as a bovine arch or abnormalities in the supra-aortic vessels, may contribute to the development of a unique flow pattern in the ascending aorta. This altered flow can potentially lead to the formation of thrombi. However, to date, this phenomenon has only been documented in a limited number of cases and requires further research for validation [5,54,55].
As a rare clinical finding, only a few AATs are described in the literature, and still no consensus exists about the best treatment strategy (Table 2). Guidelines about aortic pathology describe the possibility of medical management and surgical treatment of the condition, but a case-by-case evaluation is mandatory to determine the best choice between the two [56].

Table 2.
Comparison of medical and surgical treatment.
The decision between a conservative and a surgical approach should depend on the characteristics of the thrombus, with the surgical option being preferred in the presence of significant embolic risk. Thrombus characterization primarily involves assessing its location, with the ascending aorta being the least common site, accounting for only 12% of cases as indicated in a meta-analysis by Fayad and colleagues [57].
The classification proposed by Verma et al. divides the pathological findings by location. Type I includes the ascending aorta up to the origin of the left subclavian artery, including the most dangerous type of thrombi, which can lead to cerebral embolism [58]. This classification could be used as a reference to determine the best treatment: usually, a surgical approach is preferred for type I thrombi to prevent potentially lethal cerebral and cardiac embolism. A previous study has demonstrated that ascending aorta location and prior cerebral embolization are two stronger predictors of embolization recurrence [57].
Morphological features are also important when deciding on AAT management. Based on their relationship with the aortic wall and vessel lumen, aortic thrombi can be classified as sessile, pedunculated, or occlusive [59]. Smaller attachment sites, such as in pedunculated thrombi, have been proven to be linked to severe peripheral embolism compared with mural thrombi (73% vs. 12%) [60]. Yang and colleagues proposed a new index called the break-off risk ratio (boRR), which is calculated as the ratio of the length of the floating portion of a lesion to the length of its attachment point. This index aims to highlight the significance of thrombus morphology and hemodynamics [61]. However, it remains largely theoretical, and further studies are needed to demonstrate its effectiveness. Other researchers have emphasized the importance of evaluating hemodynamics in an aortic floating thrombus to predict the risk of embolization. They encourage the use of advanced imaging techniques, such as cardiac computed tomography, four-dimensional computed tomography angiography (4DTCA), or intra-vascular ultrasound (IVUS), to study floating masses more comprehensively [6,24]. A significant challenge in this area is that clinical presentations are typically acute and often related to embolic complications. As a result, patients are frequently assessed using CTA along with transthoracic and transesophageal echocardiography; additional investigations are usually limited to rare anecdotal instances. Furthermore, monitoring for thrombus via transesophageal echocardiography or epiaortic ultrasonography is recommended during surgical preparation for cardiopulmonary bypass and cannulation to detect any potential adverse embolisms [12,62,63]. Magnetic resonance imaging has rarely been reported as a useful tool in excluding malignancy in particularly challenging cases [64].
Multiparameter evaluation and risk stratification should guide clinicians in choosing the best treatment option between conservative and operative management. Medical therapy, consisting of administering anticoagulant or antiplatelet drugs, is still widely used, especially when surgical risk is prohibitive or embolic risk is low [14,65,66]. In a systematic review by Fayad et al., the mortality rate was similar between conservative and surgical management. However, the complication rate, the recurrence of thrombus formation, and embolic episodes were considerably more frequent after medical treatment alone [57]. A recent meta-analysis described thrombus persistence in approximately one-third of patients after a primarily pharmacological treatment, with a 21% rate of recurrent embolization [41]. Other studies have shown that even one out of five presented the need for a subsequent surgical operation for illness recurrence [67]. A probable explanation could be that usually aortic thrombi are mostly sub-acute or chronic by the time they are detected, making thrombolysis ineffective [17]. In contrast, anti-coagulation treatment appears to be effective for thrombi in the descending aorta and aortic arch, with complete dissolution achieved in 71.4% to 100% of cases [68].
When considering surgical options, choosing a less invasive method whenever possible is best. In some cases, simply removing the thrombus may be sufficient to treat the acute condition without leading to recurrence. Avoiding unnecessary aortic substitution with a prosthetic graft, particularly in cases involving a non-atherosclerotic or lesioned aorta, can help prevent complications related to surgery. Graft replacement involves longer surgery times, and proximal and distal anastomotic lines could lead to endothelial injury. This injury is one of the three factors in Virchow’s triad that promote thrombogenesis, thereby increasing the risk of thrombus recurrence [69].
Recent advancements in technology have expanded the options available for operative approaches. Endovascular and interventional techniques, which are commonly used for treating descending aortic issues, are now being applied, albeit less frequently, to cases involving thrombi in the ascending aorta. Several articles, including a literature review by Meyermann and colleagues, have highlighted the effectiveness of the endovascular approach in excluding thrombi from the descending aorta [70]. However, still, only a limited number of documented cases utilize endovascular stent-grafting or hybrid procedures to treat AATs. Notably, the percutaneous approach using the An-gioVac system has also proven to be feasible for these cases [71,72,73,74]. Furthermore, endovascular surgery procedures have increased remarkably within the last 20 years, to the point that in addition to aortic thrombi, we may face a new problem of in-stent thrombi formation [61,75].
Postoperative management plays a fundamental role in determining patients’ long-term outcomes. Anticoagulation should be used preoperatively and continued after an eventual operation. However, clear evidence on what specific anticoagulant to use remains lacking. Preoperative treatment could vary from warfarin to direct oral anticoagulants. Moreover, regarding secondary prevention, the use of dual antiplatelet therapy or moderate-intensity anticoagulation with warfarin seems to lower the risk of recurrent stroke. In addition, retrospective studies have suggested that anticoagulation is beneficial in patients with mobile mural thrombi [76]. Specific medical conditions could benefit from reasoned medical therapy; for example, prophylactic anticoagulation during cancer chemotherapy may help prevent thromboembolic complications [50].
A regular CTA or at least echocardiographic follow-up is mandatory. This allows for monitoring potential recurrences and designing further treatments to prevent subsequent complications.
5. Conclusions
Rare and often spontaneous, aortic thrombosis has a poor prognosis if not identified and treated promptly. Currently, no established guidelines exist for its management, and each treatment option has its advantages and disadvantages. Effective management begins with a thorough preoperative assessment and strategic surgical planning. Thanks to advancements in technology, we can now approach aortic pathologies from innovative perspectives. Catheter-directed thrombolysis can help alleviate the thrombosis to some extent; however, an open aortic thrombectomy is often required. Embracing minimally invasive techniques can significantly lower the risk of complications. Additional research is, therefore, needed to develop recommendations for the best treatment approaches and future monitoring of aortic thrombosis. Postoperative care must not be overlooked; implementing a comprehensive follow-up strategy that includes tailored medical therapy and consistent monitoring is crucial for preventing future embolism and disease recurrences. Prioritizing these steps can lead to improved patient outcomes and a better quality of life.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAT | Ascending Aortic Thrombus |
| AD | Aortic Dissection |
| TAI | Traumatic Aortic Injury |
| CTA | Computed Tomography Angiography |
References
- Machleder, H.I.; Takiff, H.; Lois, J.F.; Holburt, E. Aortic Mural Thrombus: An Occult Source of Arterial Thromboembolism. J. Vasc. Surg. 1986, 4, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Bojko, M.; Clothier, J.S.; Starnes, V.A.; Baker, C.J. Surgical Resection of a Symptomatic As-cending Aortic Mural Thrombus. Ann. Thorac. Surg. 2022, 114, e279–e282. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Kermani-Alghoraishi, M.; Pourmoghadas, M. Ascending Aorta Thrombose: A Rare Cause of Simultaneous Acute Myocardial Infarction and Upper Limb Ischemia. Acta Cardiol. Sin. 2020, 36, 382–385. [Google Scholar] [CrossRef]
- Campanile, A.; Sardone, M.; Pasquino, S.; Cagini, A.; Di Manici, G.; Cavallini, C. Surgical Management of a Free-Floating Thrombus in the Ascending Aorta. Asian Cardiovasc. Thorac. Ann. 2019, 27, 221–223. [Google Scholar] [CrossRef]
- Wang, B.; Ma, D.; Cao, D.; Man, X. Huge Thrombus in the Ascending Aorta: A Case Report and Literature Review. J. Cardiothorac. Surg. 2019, 14, 157. [Google Scholar] [CrossRef]
- Asahara, D.; Kuno, T.; Koizumi, K.; Itoh, T.; Numasawa, Y.; Kodaira, M.; Isozumi, K.; Ko-matsumoto, S. Utility of Four-Dimensional Computed Tomography Angiography for Evaluating the Mobility of a Thrombus in the Ascending Aorta. Radiol. Case Rep. 2020, 15, 246–249. [Google Scholar] [CrossRef]
- Yang, P.; Li, Y.; Huang, Y.; Lu, C.; Liang, W.; Hu, J. A Giant Floating Thrombus in the As-cending Aorta: A Case Report. BMC Surg. 2020, 20, 321. [Google Scholar] [CrossRef]
- Frisoli, T.M.; So, C.-Y.; Guruswamy, J.G.; Chebl, A.B.; Lee, J.C.; Eng, M.H. Vacuuming in Crowded Dangerous Spaces: Aspiration of Large Ascending Aortic Thrombus. JACC Case Rep. 2020, 2, 1979–1983. [Google Scholar] [CrossRef]
- Ivanov, B.; Djordjevic, I.; Eghbalzadeh, K.; Weber, C.; Mader, N.; Kuhn-Regnier, F.; Wahlers, T. Multiple Emboli Caused by Ascending Aorta Thrombus—Surgical Approach. J. Card. Surg. 2020, 35, 435–436. [Google Scholar] [CrossRef]
- Furuta, A.; Morimoto, H.; Mukai, S.; Futagami, D.; Kitaura, J. Missing Floating Mass in the Ascending Aorta; Report of a Case. Kyobu Geka 2020, 73, 690–693. [Google Scholar]
- Kawai, Y.; Koizumi, K.; Itoh, T.; Iio, M.; Shimizu, H. Mobile Thrombus in the Ascending Aorta. Ann. Vasc. Dis. 2020, 13, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Gueldich, M.; Piscitelli, M.; Derbel, H.; Boughanmi, K.; Bergoend, E.; Chanai, N.; Folliguet, T.; Fiore, A. Floating Thrombus in the Ascending Aorta Revealed by Peripheral Arterial Embolism. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Issa, R.; Gallissot, F.; Cochet, A.; Cottin, Y. Hyperacute Simultaneous Cardiocerebral Infarc-tion Related to Floating Thrombus in the Ascending Aorta: A Case Report. Eur. Heart J. Case Rep. 2021, 5, ytab450. [Google Scholar] [CrossRef] [PubMed]
- Koutroulou, I.; Tsivgoulis, G.; Rafailidis, V.; Psoma, E.; Kouskouras, K.; Fotiadis, P.; Grigori-adis, N.; Karapanayiotides, T. Off-Label Intravenous Thrombolysis for Early Recurrent Brain Embolism Associated with Aortic Arch Thrombus. Neurol. Res. Pract. 2021, 3, 4. [Google Scholar] [CrossRef]
- Quach, N.; Medina, M.; Burton, É. Incidentally Found Ascending Aortic Thrombus: Presentation and Management. JACC Case Rep. 2021, 3, 1489–1493. [Google Scholar] [CrossRef]
- Dai, X.; Ni, C.; Luo, W.; Miao, S.; Ma, L. Large Mural Thrombus in the Non-Aneurysmal and Non-Atherosclerotic Ascending Aorta: A Case Report. J. Cardiothorac. Surg. 2021, 16, 200. [Google Scholar] [CrossRef]
- Oki, N.; Inoue, Y.; Kotani, S. Free-Floating Thrombus of the Aorta: 3 Case Reports. Surg. Case Rep. 2021, 7, 141. [Google Scholar] [CrossRef]
- Karkos, C.D.; Papadopoulos, C.E. A Large Floating Thrombus in the Ascending Aorta: To Treat or Not to Treat? Eur. J. Vasc. Endovasc. Surg. 2021, 62, 63. [Google Scholar] [CrossRef]
- Christou, N.; Gourgiotis, I.; Dakis, K.; Liasidis, C. Embolic Strokes in a Patient with a Large Floating Thrombus in the Ascending Aorta. Hippokratia 2021, 25, 172–174. [Google Scholar]
- Prasad, S.N.; Singh, V.; Selvamurugan, V.; Phadke, R.V. Complete Resolution of Long Pedunculated Thrombus of the Proximal Ascending Aorta Following Conservative Management: An Interesting Case. BMJ Case Rep. 2021, 14, e236777. [Google Scholar] [CrossRef]
- Abe, N.; Yasumori, K.; Shimabukuro, N.; Yamazato, T.; Munakata, H. Two Cases of Protruding Thrombus in the Ascending Aorta. Ann. Vasc. Dis. 2021, 14, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Shoda, M.; Yamamoto, H.; Kawashima, M.; Kondo, T.; Murakami, H.; Kawai, H.; Takaya, T. Acute Coronary and Cerebral Emboli From a Pedunculated Ascending Aorta Thrombus. JACC Case Rep. 2021, 3, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, H.; Bulescu, C.; Sibișan, A.-M.; Țigănașu, R.; Cacoveanu, C.; Nica, C.; Rachieru, A.; Gheorghiță, D.; Zaharia, O.; Bălănescu, Ș; et al. A Large Ascending Aorta Thrombus in a Patient with Acute Myocardial Infarction-Case Report. Medicina 2021, 57, 1176. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, H.; Nakama, T.; Asano, K.; Obunai, K. Acute Coronary Syndrome of the Left Main Coronary Artery Caused by a Huge Floating Thrombus in the Ascending Aorta: A Case Report of Intravascular Ultrasound Effectiveness. Eur. Heart J. Case Rep. 2021, 5, ytab279. [Google Scholar] [CrossRef]
- Noda, R.; Tamai, Y.; Inoue, M.; Hara, T. Cerebral Infarction Due to Aortic Mural Thrombus in a Non-Atherosclerotic Ascending Aorta, Detected by Cardiac CT. NMC Case Rep. J. 2021, 8, 325–330. [Google Scholar] [CrossRef]
- Hirata, R.; Tago, M.; Nakashima, T.; Hirakawa, Y. A Floating Mural Thrombus in the Ascending Aorta Can Cause Multiorgan Infarction. BMJ Case Rep. 2022, 15, e250147. [Google Scholar] [CrossRef]
- Neves, N.M.; Coelho, S.C.; Marto, N.F.; Horta, A.B. Ascending Aortic Thrombus With Peripheral Embolization. Cureus 2022, 14, e28766. [Google Scholar] [CrossRef]
- Rathnayake, A.; Chang, O.; Narita, T.; Mejia, R. Aortic Mural Thrombus in a Normal Ascending Aorta. ANZ J. Surg. 2022, 92, 3082–3083. [Google Scholar] [CrossRef]
- Ewers, A.; Muegge, A.; Patsalis, P.C.; Aweimer, A. Complete Primary Aortic Ascending Thrombosis Resolution after Dabigatran Therapy. Eur. Heart J. 2022, 43, 2530. [Google Scholar] [CrossRef]
- Akcelik, A.; Minakata, K.; Sunagawa, G.; Mangukia, C.; Boova, R.; Toyoda, Y. Surgical Management of Primary Aortic Thrombus in Thoracic Aorta. JTCVS Open 2023, 16, 84–92. [Google Scholar] [CrossRef]
- Wakami, T.; Fukunaga, N.; Shimoji, A.; Maeda, T.; Mori, O.; Yoshizawa, K.; Okada, T.; Tamura, N. Idiopathic Ascending Aortic Thrombosis with Multiple Embolism: Report of a Case. Kyobu Geka 2023, 76, 477–480. [Google Scholar] [PubMed]
- Sattar, Y.; Rashid, R.; Sultana Shaik, A.; Sigh, R.; Sattar, A.; Das, P.; Bennett, B.; Aiya, U.; Deb, A.; Patel, N. The diagnostic challenge of acute aortic thrombus concealed by asthma exacerbation: A case report. Chest 2023, 164, A234–A235. [Google Scholar] [CrossRef]
- Thurston, A.J.F.; Chapman, A.R.; Bing, R. Case Report: Concurrent Myocardial and Cerebral Infarction Due to Aortic Thrombus. Eur. Heart J. Case Rep. 2023, 7, ytad492. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Y.; Wang, H.; Liu, Y.; Liu, H.; Sun, H.; Wang, Z. Case Report: Surgical Strategies of a Giant Thrombus from the Ascending Aorta to the Arch. Front. Cardiovasc. Med. 2023, 10, 1091303. [Google Scholar] [CrossRef]
- Xiong, J.; Xie, D.; Yu, W.; Yu, J. A Rare Floating Thrombus in the Ascending Aorta: A Case Report. Asian J. Surg. 2024, 47, 2210–2211. [Google Scholar] [CrossRef]
- Barbarossa, A.; Finori, L.; Berretta, P.; Dello Russo, A.; Di Eusanio, M. An Idiopathic Floating Ascending Aorta Thrombus in a Triple Rule-in CT. Eur. Heart J. Cardiovasc. Imaging 2024, 25, e204. [Google Scholar] [CrossRef]
- Johno, T.; Kawano, H.; Nagahama, K.; Kubota, H.; Hirano, T. Acute Large Vessel Occlusion Caused by Giant Floating Aortic Thrombus. Stroke 2024, 55, e117–e118. [Google Scholar] [CrossRef]
- Egbe, A.; Tadesse, E.; Ruffino, C.; Arshad, K.; Sandio, A.; Mozaffari, A.; Subahi, A.; Jamil, M.; Tynes, D.; Delafontaine, P. An Ascending Aortic Thrombus. Cardiol. Cardiovasc. Med. 2024, 8, 202–205. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Z.; Xu, Y.; Zhou, Y.; Yang, J.; Qiu, Y.; Xing, Y.; Peng, F.; Tang, W. Repeated Acute Coronary Syndrome Caused by a Mind-Bending Mural Thrombus in Ascending Aorta: A Case Report and Review of the Literature. BMC Cardiovasc. Disord. 2024, 24, 281. [Google Scholar] [CrossRef]
- Inoue, K.; Ogata, T.; Mishima, T.; Ishibashi, H.; Hirai, F.; Tsuboi, Y. Embolic Stroke Due to Ascending Aortic Thrombus in a Patient with Treatment-Resistant Ulcerative Colitis. Rinsho Shinkeigaku 2024, 64, 93–98. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yen, H.-T.; Wu, C.-C.; Huang, K.-R.; Sheu, J.-J.; Lee, F.-Y. Aortic Thrombus in a Nonaneurysmal Ascending Aorta. Ann. Vasc. Surg. 2021, 72, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, M.E.; Weaver, F.A.; Liebman, H.A.; Rowe, V.L.; Hood, D.B. Anticoagulation Is an Effective Treatment for Aortic Mural Thrombi. J. Vasc. Surg. 2002, 36, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, V.; Vilacosta, I.; Luaces, M.; Bustos, A.; Ferreirós, J.; Aragoncillo, P.; Pérez de Isla, L.; Rodríguez, E. Thrombosis of an Apparently Normal Thoracic Aorta and Arterial Embolism. Rev. Esp. Cardiol. 2008, 61, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Ishii, H.; Tsuchiya, H.; Takahashi, Y.; Shimoyamada, H.; Isomura, A.; Nakajima, M.; Hirano, T.; Ohkura, Y.; Kubota, H. Pathologic Features of Lone Aortic Mobile Thrombus in the Ascending Aorta. Ann. Thorac. Surg. 2016, 102, e313–e315. [Google Scholar] [CrossRef][Green Version]
- Soumer, K.; Mallouki, M.; Azabou, N.; Horchani, H.; Nsiri, S.; Bousnina, M.; Jemel, A. Aortic Floating Thrombus in Patients with COVID-19: A Report of Eight Cases. Gen. Thorac. Cardiovasc. Surg. 2024, 73, 164–170. [Google Scholar] [CrossRef]
- Bouratzis, V.; Katsouras, C.; Lakkas, L.; Bechlioulis, A.; Michalis, L.K. Ascending Aorta Thrombosis in a Patient with COVID-19 Who Was Already Receiving Anticoagulation Therapy. Eur. Heart J. Case Rep. 2023, 7, ytad268. [Google Scholar] [CrossRef]
- Nishimura, T.; Sueyoshi, E.; Tasaki, Y.; Uetani, M. Asymptomatic Floating Thrombus in the Ascending Aorta Depicted on Four-Dimensional Computed Tomography. SAGE Open Med. Case Rep. 2020, 8, 2050313X20971894. [Google Scholar] [CrossRef]
- Sato, N.; Mishima, T.; Okubo, Y.; Okamoto, T.; Shiraishi, S.; Tsuchida, M. Acute Aortic Thrombosis in the Ascending Aorta after Cisplatin-Based Chemotherapy for Esophageal Cancer: A Case Report. Surg. Case Rep. 2022, 8, 75. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Koop, A.H.; Diaz-Gomez, J.L.; Guru, P.K. Non-Atherosclerotic Aortic Mural Thrombus: A Rare Source of Embolism. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Iosifescu, A.G.; Radu, C.; Marin, S.L.; Enache, R. Floating Thrombus of the Ascending Aorta after Treatment of Ureteral Carcinoma: A Case Report. Turk. Gogus Kalp Damar Cerrahisi Derg. 2022, 30, 444–447. [Google Scholar] [CrossRef]
- Vu, P.Q.; Patel, S.; Pathak, P.R.; Basu, A.K. Cocaine-Induced Ascending Aortic Thrombus. Cureus 2023, 15, e47539. [Google Scholar] [CrossRef] [PubMed]
- Deiana, G.; Genadiev, G.; Giordano, A.N.; Moro, M.; Spanu, F.; Urru, F.; Camparini, S. Infiltrating Angiosarcoma of the Ascending, Arch and Descending Aorta Manifested as Acute Mesenteric Ischemia. Vasc. Spec. Int. 2021, 37, 46–49. [Google Scholar] [CrossRef]
- Dalal, A.R.; Kabirpour, A.; MacArthur, J.W. Surgical Excision of a Free Floating Ascending Aortic Thrombus. J. Card. Surg. 2020, 35, 429–430. [Google Scholar] [CrossRef]
- Khedija, S.; Nadia, A.; Houcine, H.; Mouna, B.; Amine, J. Incidental Finding of Arteria Luso-ria in COVID-19 Patient with Aortic Thrombus Complicated by Recurrent Limb Ischemia. Int. J. Angiol. 2023, 32, 296–298. [Google Scholar] [CrossRef]
- Suárez González, L.Á.; Busto Suárez, S.; Fernández-Samos Gutiérrez, R.; Ballesteros Pomar, M. Symptomatic Aortic Arch Floating Thrombus In A Patient With “Bovine Arch”. Portuguese J. Card. Thorac. Vasc. Surg. 2022, 29, 79–81. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Fayad, Z.Y.; Semaan, E.; Fahoum, B.; Briggs, M.; Tortolani, A.; D’Ayala, M. Aortic Mural Thrombus in the Normal or Minimally Atherosclerotic Aorta. Ann. Vasc. Surg. 2013, 27, 282–290. [Google Scholar] [CrossRef]
- Verma, H.; Meda, N.; Vora, S.; George, R.K.; Tripathi, R.K. Contemporary Management of Symptomatic Primary Aortic Mural Thrombus. J. Vasc. Surg. 2014, 60, 1524–1534. [Google Scholar] [CrossRef]
- Karaolanis, G.; Moris, D.; Bakoyiannis, C.; Tsilimigras, D.I.; Palla, V.-V.; Spartalis, E.; Schi-zas, D.; Georgopoulos, S. A Critical Reappraisal of the Treatment Modalities of Normal Ap-pearing Thoracic Aorta Mural Thrombi. Ann. Transl. Med. 2017, 5, 306. [Google Scholar] [CrossRef]
- Karalis, D.G.; Chandrasekaran, K.; Victor, M.F.; Ross, J.J.; Mintz, G.S. Recognition and Em-bolic Potential of Intraaortic Atherosclerotic Debris. J. Am. Coll. Cardiol. 1991, 17, 73–78. [Google Scholar] [CrossRef]
- Yang, S.; Yu, J.; Zeng, W.; Yang, L.; Teng, L.; Cui, Y.; Shi, H. Aortic Floating Thrombus Detected by Computed Tomography Angiography Incidentally: Five Cases and a Literature Re-view. J. Thorac. Cardiovasc. Surg. 2017, 153, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, N.; Yuji, D.; Sato, M.; Inoue, K.; Wakita, N. A Floating Thrombus in the Ascending Aorta Complicated by Acute Myocardial Infarction. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 213–215. [Google Scholar] [CrossRef] [PubMed]
- De Maat, G.E.; Vigano, G.; Mariani, M.A.; Natour, E. Catching a Floating Thrombus; a Case Report on the Treatment of a Large Thrombus in the Ascending Aorta. J. Cardiothorac. Surg. 2017, 12, 34. [Google Scholar] [CrossRef]
- Scott, D.J.; White, J.M.; Arthurs, Z.M. Endovascular Management of a Mobile Thoracic Aortic Thrombus Following Recurrent Distal Thromboembolism: A Case Report and Literature Review. Vasc. Endovasc. Surg. 2014, 48, 246–250. [Google Scholar] [CrossRef]
- Toyama, M.; Nakayama, M.; Hasegawa, M.; Yuasa, T.; Sato, B.; Ohno, O. Direct Oral Anti-coagulant Therapy as an Alternative to Surgery for the Treatment of a Patient with a Floating Thrombus in the Ascending Aorta and Pulmonary Embolism. J. Vasc. Surg. Cases Innov. Tech. 2018, 4, 170–172. [Google Scholar] [CrossRef]
- Houmsse, M.; McDavid, A.; Kilic, A. Large de Novo Ascending Aortic Thrombus Success-fully Treated with Anticoagulation. J. Cardiovasc. Thorac. Res. 2018, 10, 113–114. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Hanack, U.; Pisimisis, G.; Yousefi, S.; Wintzer, C.; Rückert, R.I. Thrombus in the Non-Aneurysmal, Non-Atherosclerotic Descending Thoracic Aorta—An Unusual Source of Arterial Embolism. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 450–457. [Google Scholar] [CrossRef]
- Reyes Valdivia, A.; Duque Santos, A.; Garnica Ureña, M.; Romero Lozano, A.; Aracil Sanus, E.; Ocaña Guaita, J.; Gandaria, C. Anticoagulation Alone for Aortic Segment Treatment in Symptomatic Primary Aortic Mural Thrombus Patients. Ann. Vasc. Surg. 2017, 43, 121–126. [Google Scholar] [CrossRef]
- Tajima, S.; Kudo, T.; Mori, D.; Kitabayashi, K. Floating Ascending Aortic Thrombus with An-tiphospholipid Syndrome: A Case Report. General. Thorac. Cardiovasc. Surg. Cases 2024, 3, 49. [Google Scholar] [CrossRef]
- Meyermann, K.; Trani, J.; Caputo, F.J.; Lombardi, J. V Descending Thoracic Aortic Mural Thrombus Presentation and Treatment Strategies. J. Vasc. Surg. 2017, 66, 931–936. [Google Scholar] [CrossRef]
- Kahlberg, A.; Montorfano, M.; Cambiaghi, T.; Bertoglio, L.; Melissano, G.; Chiesa, R. Endovascular Stent-Grafting of the Ascending Aorta for Symptomatic Parietal Thrombus. J. Endovasc. Ther. 2016, 23, 969–972. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Di Sebastiano, P.; Palazzo, V. Endovascular Solutions for Symptomatic Free-Floating Thrombus in Thoracic Aorta in Rheumatoid Arthritis Patients: Two Clinical Cases. Vascular 2024, 17085381241269748. [Google Scholar] [CrossRef]
- Wilson, W.R.; McCusker, K.H.; Peeran, S.M.; Dourdoufis, P.J. Endovascular Removal of a Large Free-Floating Thrombus of the Descending Thoracic Aorta Using the AngioVac System. J. Vasc. Surg. Cases Innov. Tech. 2024, 10, 101460. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Spanos, K.; Debus, E.S.; Rohlffs, F.; Kölbel, T. Technical Aspects of Using the AngioVac System for Thrombus Aspiration From the Ascending Aorta. J. Endovasc. Ther. 2018, 25, 550–553. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Zhang, X.; Liu, X.; Chen, L.; Liang, B.; Sun, W.; Shi, H. Computed Tomography Angiography-Confirmed Aortic in-Stents Floating Thrombus after Endovascular Stenting: A Retrospective Study. Quant. Imaging Med. Surg. 2024, 14, 2556–2567. [Google Scholar] [CrossRef]
- Caron, F.; Anand, S.S. Antithrombotic Therapy in Aortic Diseases: A Narrative Review. Vasc. Med. 2017, 22, 57–65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).