Assessment of Various Tissues in Broilers Reared Under Different Lighting Programs with Respect to Rearing Disorders
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Gross Examination and Morphometric Measurements
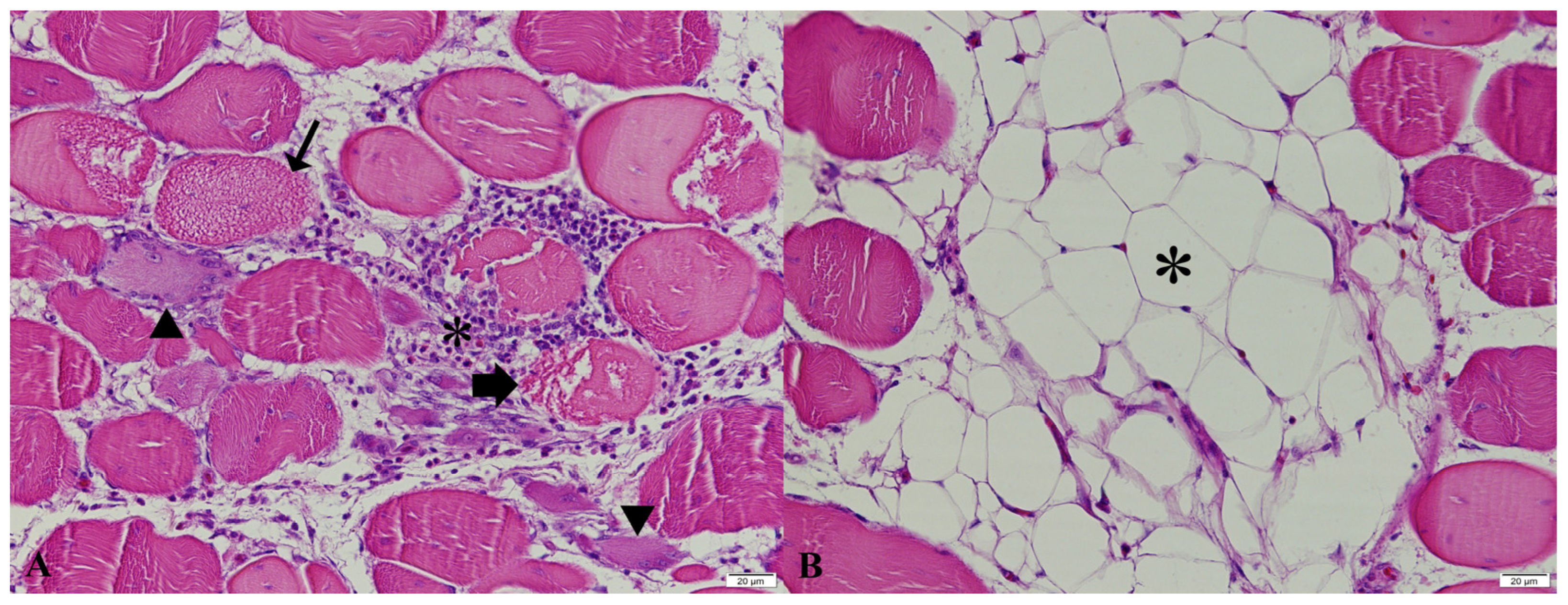
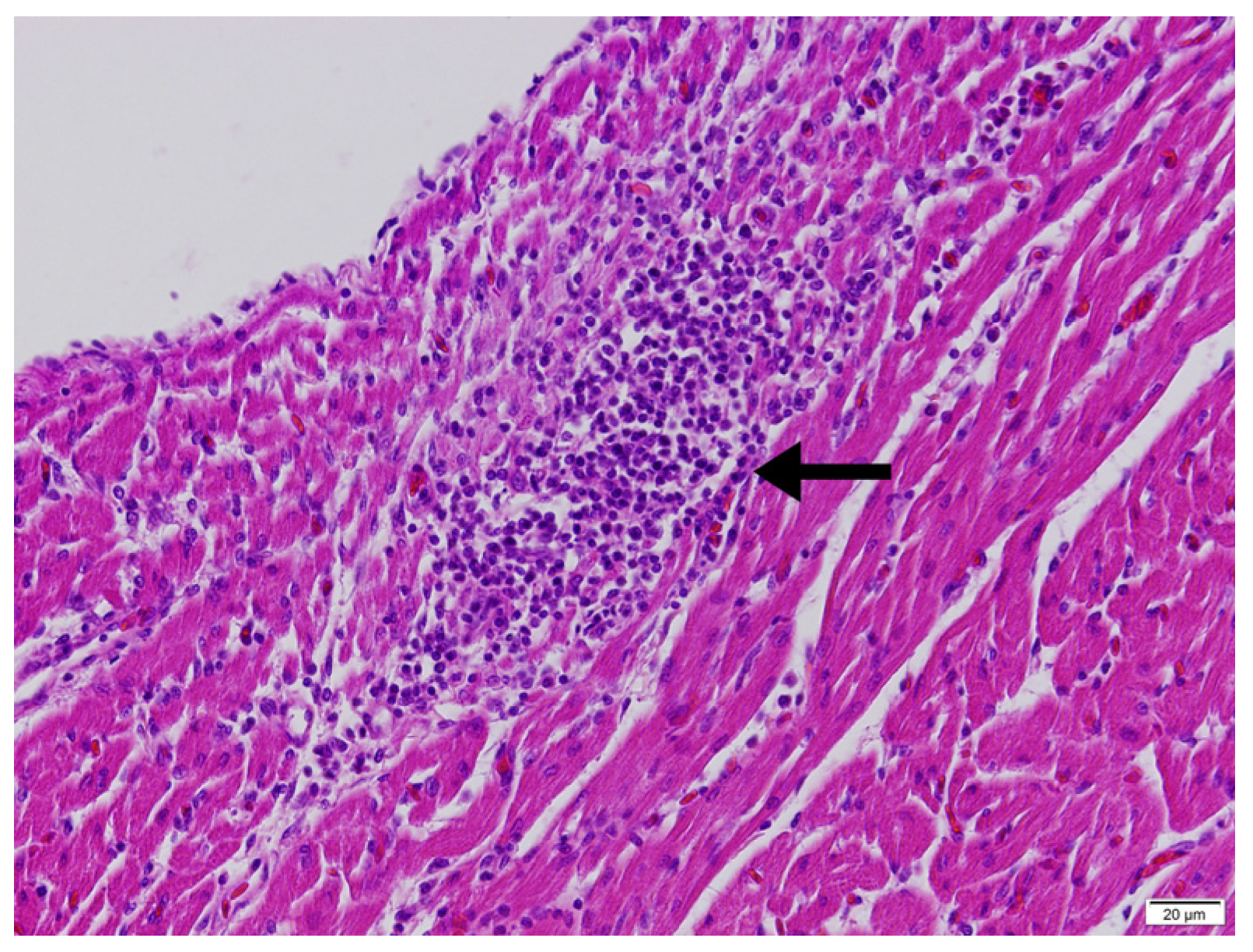
2.3. Histopathological Examination
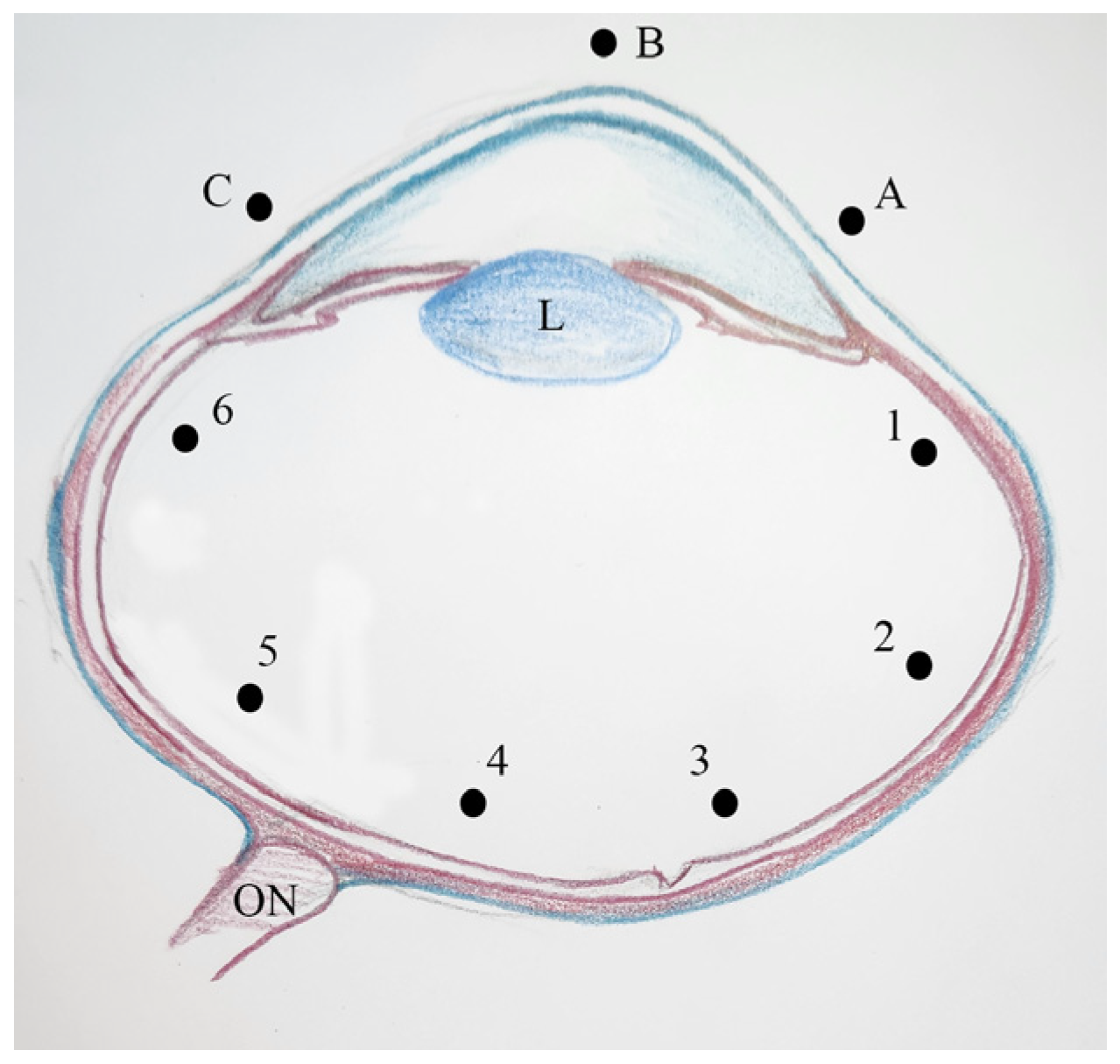
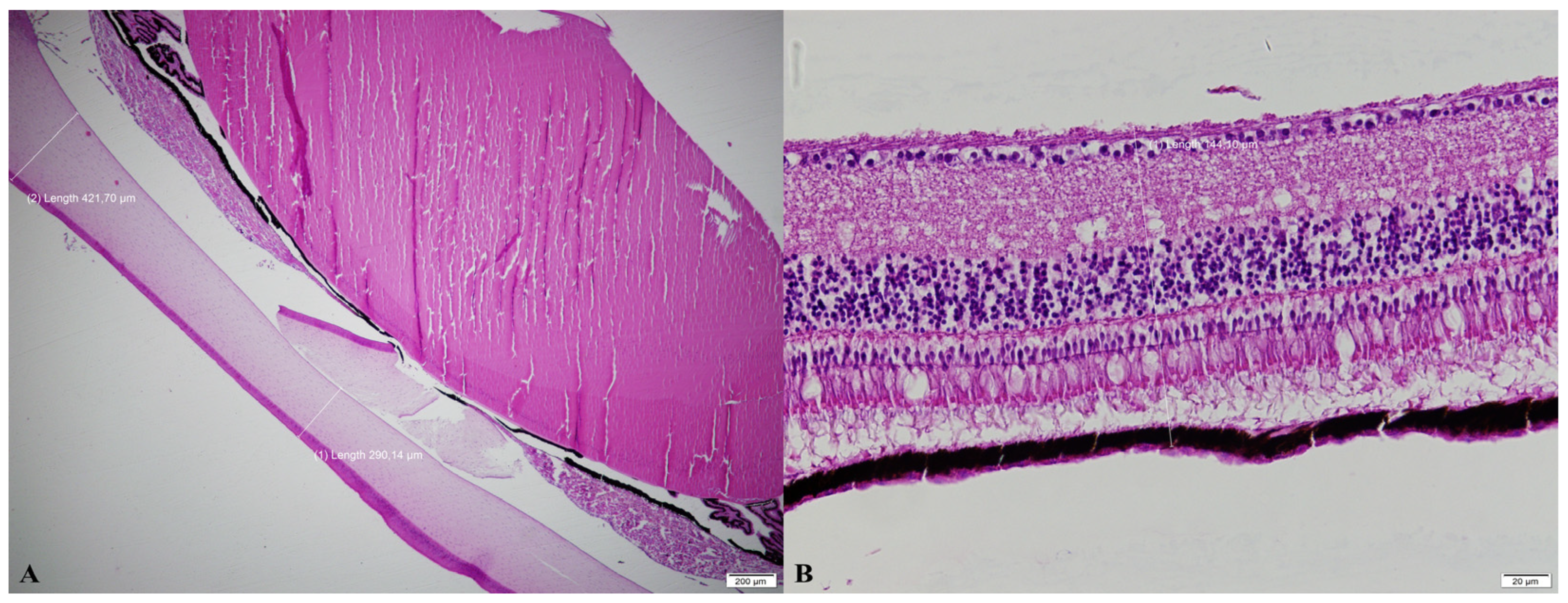
2.4. Histomorphometric Analyses of the Eyes
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Crude protein |
| HE | Hematoxylin and eosin |
| ME | Metabolizable energy |
| RV | Right ventricle |
| TD | Tibial dyschondroplasia |
| TV | Total ventricle |
References
- Ahmed, I.; Riaz, R.; Sızmaz, Ö. Sustainable livestock farming with oil seed crops and their by-products. Ank. Univ. Vet. Fak. Derg. 2024, 71, 371–383. [Google Scholar] [CrossRef]
- Peker, A.; Orkan, Ş.; Aral, Y.; Aral, G.İ. A comprehensive outlook on cultured meat and conventional meat production. Ank. Univ. Vet. Fak. Derg. 2024, 71, 511–522. [Google Scholar] [CrossRef]
- Decuypere, E.; Buyse, J.; Buyse, N. Ascites in broiler chickens: Exogenous and endogenous structural and functional causal factors. World’s Poult. Sci. J. 2000, 56, 367–377. [Google Scholar] [CrossRef]
- Pakdel, A.; Van Arendonk, J.A.; Vereijken, A.L.; Bovenhuis, H. Direct and maternal genetic effects for ascites-related traits in broilers. Poult. Sci. 2002, 81, 1273–1279. [Google Scholar] [CrossRef]
- Nabati, A.; Chamani, M.; Foroudi, F.; Sadeghi, A.; Aminafshar, M. Effect of feeding processed soybean meal on broiler performance, intestinal morphology, cecal microbial population, and immune response. Ankara Univ. Vet. Fak. Derg. 2025, 72, 67–75. [Google Scholar] [CrossRef]
- Hoving-Bolink, A.H.; Kranen, R.W.; Klont, R.E.; Gerritsen, C.L.; de Greef, K.H. Fibre area and capillary supply in broiler breast muscle in relation to productivity and ascites. Meat Sci. 2000, 56, 397–402. [Google Scholar] [CrossRef]
- Decuypere, E.; Hassanzadeh, M.; Buys, N.; Buyse, J. Further insights into the susceptibility of broilers to ascites. Vet. J. 2005, 169, 319–320. [Google Scholar] [PubMed]
- Lewis, P.D.; Morris, T.R. Responses of domestic poultry to various light sources. World’s Poult. Sci. J. 1998, 54, 7–25. [Google Scholar] [CrossRef]
- Wineland, M.J. Fundamentals of managing light for poultry. In Commercial Chicken Meat and Egg Production, 5th ed.; Bell, D.D., Weaver, W.D., Jr., Eds.; Springer: Boston, MA, USA, 2002; pp. 129–148. [Google Scholar]
- Manser, C.E. Effects of lighting on the welfare of domestic poultry: A review. Anim. Welf. 1996, 5, 341–360. [Google Scholar] [CrossRef]
- Lewis, P.D.; Morris, T.R. Poultry and coloured light. World’s Poult. Sci. J. 2000, 56, 189–207. [Google Scholar]
- Prescott, N.B.; Wathes, C.M.; Jarvis, J.R. Light, vision and the welfare of poultry. Anim. Welf. 2003, 12, 269–288. [Google Scholar] [CrossRef]
- Tekin, M.; Ünal, N.; Onbaşılar, E.E. The effects of LED lights in different colors on fattening performance, litter characteristics, meat properties, and some welfare parameters in broilers. Ank. Univ. Vet. Fak. Derg. 2025, 72, 77–82. [Google Scholar]
- Gündoğar, U.C.; Onbaşılar, E.E.; Ahlat, O. Effects of abrupt and gradual light/dark switching on growth performance, behavior, villus development, meat characteristics, and immunity of broilers. Anim. Sci. J. 2024, 95, e13962. [Google Scholar] [CrossRef]
- Özkan, S.; Plavnik, I.; Yahav, S. Effects of early feed restriction on performance and ascites development in broiler chickens subsequently raised at low ambient temperature. J. Appl. Poult. Res. 2006, 15, 9–19. [Google Scholar] [CrossRef]
- Vitorovic, D.; Nikolic, Z. Longitudinal growth of leg and wing bones of chickens reared in cages and on the floor. Anat. Histol. Embryol. 1995, 24, 81–83. [Google Scholar] [CrossRef]
- Onbaşılar, E.E.; Erol, H.; Cantekin, Z.; Kaya, U. Influence of intermittent lighting on broiler performance, incidence of tibial dyschondroplasia, tonic immobility, some blood parameters and antibody production. Asian-Australas. J. Anim. Sci. 2007, 20, 550–555. [Google Scholar] [CrossRef]
- Nickla, D.L. Ocular diurnal rhythms and eye growth regulation: Where we are 50 years after Lauber. Exp. Eye Res. 2013, 114, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Raginski, C.; Schwean-Lardner, K.; Fancher, B.I.; Classen, H.L. Minimum light intensity threshold to prevent negative effects on broiler production and welfare. Br. Poult. Sci. 2013, 54, 686–694. [Google Scholar] [CrossRef]
- Onbaşılar, İ.; Yalçın, S.; Eser, H.; Ramay, M.S.; Yalçın, S.; Özsoy, B.; Erbay Elibol, F.K.; Taban, S.; Koçoğlu, S.T.; Torlak, E. Combined use of essential oils with organic acids in modifying performance, intestinal health, cecal microflora, and selected blood and bone parameters in broilers. Ank. Univ. Vet. Fak. Derg. 2025, 72, 377–386. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Shivaprasad, H.L.; Shaw, D.P.; Valentine, B.A.; Hargis, B.M.; Clark, F.D.; McKee, S.R.; Owens, C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013, 92, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.J. The effect of increased sodium in the drinking water on right ventricular hypertrophy, right ventricular failure and ascites in broiler chickens. Avian Pathol. 1987, 16, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kocabağlı, N. The effect of dietary phytase supplementation at different levels on tibial bone characteristics and strength in broilers. Turk. J. Vet. Anim. Sci. 2001, 25, 797–802. [Google Scholar]
- Seedor, J.G.; Quartuccio, H.A.; Thompson, D.D. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J. Bone Miner. Res. 1991, 6, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.E. Determination of Effect of Smoke Inhalation on the Skeleton During Growth Period in the Rat by Using Morphometric Methods. Ph.D. Thesis, İstanbul University, İstanbul, Türkiye, 2002. [Google Scholar]
- Huff, W.E. Evaluation of tibial dyschondroplasia during aflatoxicosis and feed restriction in young broiler chickens. Poult. Sci. 1980, 59, 991–995. [Google Scholar] [CrossRef]
- Ross, C.F.; Hall, M.I.; Heesy, C.P. Were basal primates nocturnal? Evidence from eye and orbit shape. In Primate Origins: Adaptations and Evolution; Ravosa, M.J., Dagosto, M., Eds.; Springer: Boston, MA, USA, 2007; pp. 233–256. [Google Scholar]
- Khurana, A.K. Anatomy and physiology of eye. In Comprehensive Ophthalmology; Jaypee Brothers Medical Publishers: New Delhi, India, 2019; pp. 3–22. [Google Scholar]
- Werther, K.; Candioto, C.G.; Korbel, R. Ocular histomorphometry of free-living common kestrels (Falco tinnunculus). J. Avian Med. Surg. 2017, 31, 319–326. [Google Scholar] [CrossRef]
- Bailey, R.A.; Watson, K.A.; Bilgili, S.F.; Avendano, S. The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult. Sci. 2015, 94, 2870–2879. [Google Scholar] [CrossRef]
- Nawaz, A.H.; Zheng, J.H.; Zhang, W.L.; Wang, F.J.; Jiao, Z.H.; Amoah, K.; Zhang, L. Breast muscle myopathies in broiler: Mechanism, status and their impact on meat quality—A review. Ann. Anim. Sci. 2022, 22, 551–560. [Google Scholar]
- Julian, R.J. Rapid growth problems: Ascites and skeletal deformities in broilers. Poult. Sci. 1998, 77, 1773–1780. [Google Scholar] [CrossRef]
- Vitorović, D.; Božičković, I.; Lukić, M.; Relić, R.; Škrbić, Z.; Petričević, V.; Krstić, N. Tibia growth and development in broiler chicks reared under continuous light and melatonin dietary supplementation during the first two weeks of life. Acta Vet. 2023, 73, 262–270. [Google Scholar] [CrossRef]
- Yang, H.; Xing, H.; Wang, Z.; Xia, J.; Wan, Y.; Hou, B.; Zhang, J. Effects of intermittent lighting on broiler growth performance, slaughter performance, serum biochemical parameters and tibia parameters. Ital. J. Anim. Sci. 2015, 14, 4143. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; Slater, J.P.; Johnson, N.A.; Bondi, C.D.; Dodda, B.R.; Kotlarczyk, M.P.; Clafshenkel, W.P.; Sethi, S.; Higginbotham, S.; Rutkowski, J.L.; et al. Effects on bone by the light/dark cycle and chronic treatment with melatonin and/or hormone replacement therapy in intact female mice. J. Pineal Res. 2012, 53, 374–384. [Google Scholar] [CrossRef]
- Schwean-Lardner, K.; Vermette, C.; Leis, M.; Classen, H.L. Basing turkey lighting programs on broiler research: A good idea? A comparison of 18 daylength effects on broiler and turkey welfare. Animals 2016, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Leis, M.L.; Dodd, M.U.; Starrak, G.; Vermette, C.J.; Gomis, S.; Bauer, B.S.; Sandmeyer, L.S.; Schwean-Lardner, K.; Classen, H.L.; Grahn, B.H. Effect of prolonged photoperiod on ocular tissues of domestic turkeys. Vet. Ophthalmol. 2017, 20, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Schwean-Lardner, K.; Fancher, B.I.; Gomis, S.; Van Kessel, A.; Dalal, S.; Classen, H.L. Effect of day length on cause of mortality, leg health, and ocular health in broilers. Poult. Sci. 2013, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Light and Dark Transition Periods | Significance | ||
|---|---|---|---|---|
| Abrupt Transition | 30-min Transition | 1-h Transition | ||
| White Striping Score *, % | ||||
| Score 0 | 30 | 10 | 40 | χ2 = 2.83, p = 0.586, Cramér’s V = 0.22 |
| Score 1 | 40 | 60 | 30 | |
| Score 2 | 30 | 30 | 30 | |
| Histopathological Findings | Light and Dark Transition Periods | p | ||
|---|---|---|---|---|
| Abrupt Transition | 30-min Transition | 1-h Transition | ||
| Degeneration | 1.0 (0–1) | 1.0 (1–1.5) | 2.0 (1.0–2.0) | 0.064 |
| Necrosis | 1.0 (0–1) | 1.0 (0.5–1.0) | 1.5 (1.0–2.0) | 0.101 |
| Regeneration | 0 (0–1.0) | 0 (0–1) | 1.0 (0.0–1.25) | 0.481 |
| Fibrosis | 0 (0–1.0) | 0 (0–0.5) | 0.5 (0–1.0) | 0.530 |
| Adipose Tissue Infiltration | 1.0 (1.0–1.0) | 1.0 (1.0–1.5) | 1.0 (1.0–2.0) | 0.726 |
| Mononuclear Cell Infiltration | 1.0 (1.0–1.0) | 1.0 (0.5–1.0) | 1.0 (1.0–1.0) | 0.219 |
| Characteristic | Light and Dark Transition Periods | p | ||
|---|---|---|---|---|
| Abrupt Transition | 30-min Transition | 1-h Transition | ||
| Relative heart weight, % | 0.53 ± 0.02 b | 0.60 ± 0.02 a | 0.56 ± 0.01 ab | 0.016 |
| RV/TV | 0.21 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.900 |
| Characteristic | Light and Dark Transition Periods | p | ||
|---|---|---|---|---|
| Abrupt Transition | 30-min Transition | 1-h Transition | ||
| Tibia weight, g | 22.2 ± 0.7 | 21.5 ± 1.0 | 22.2 ± 0.6 | 0.720 |
| Tibia length, mm | 111.7 ± 1.3 | 110.6 ± 1.4 | 110.9 ± 0.61 | 0.801 |
| Diaphysis diameter, mm | 10.1 ± 0.2 | 9.8 ± 0.3 | 10.0 ± 0.2 | 0.576 |
| Cortical index, mm | 29.48 ± 1.08 | 28.37 ± 0.89 | 31.44 ± 0.95 | 0.098 |
| Strength index | 3982 ± 37 | 3990 ± 49 | 3952 ± 39 | 0.794 |
| Characteristic | Light and Dark Transition Periods | Significance | ||
|---|---|---|---|---|
| Abrupt Transition | 30-min Transition | 1-h Transition | ||
| Tibial Dyschondroplasia Score *, % | ||||
| Score 1 | 50 | 50 | 50 | χ2 = 0.67, p = 0.95, Cramér’s V = 0.10 |
| Score 2 | 30 | 40 | 40 | |
| Score 3 | 20 | 10 | 10 | |
| Characteristic | Light and Dark Transition Periods | p | ||
|---|---|---|---|---|
| Abrupt Transition | 30-min Transition | 1-h Transition | ||
| Weight, g | 2.24 ± 0.07 b | 2.20 ± 0.05 b | 2.46 ± 0.06 a | 0.009 |
| Relative weight, X10−6 | 7.41 ± 0.17 b | 7.28 ± 0.23 b | 8.27 ± 0.23 a | 0.006 |
| Corneal diameter, mm | 7.66 ± 0.09 | 7.80 ± 0.16 | 8.03 ± 0.10 | 0.109 |
| Mediolateral diameter, mm | 17.36 ± 0.22 | 17.07 ± 0.23 | 17.80 ± 0.19 | 0.071 |
| Dorsoventral diameter, mm | 15.99 ± 0.17 | 16.40 ± 0.25 | 15.98 ± 0.20 | 0.280 |
| Anteroposterior diameter, mm | 13.04 ± 0.63 | 13.75 ± 0.20 | 14.44 ± 0.18 | 0.061 |
| Thickness (µm) | Light and Dark Transition Periods | p | ||
|---|---|---|---|---|
| Abrupt Transition | 30-min Transition | 1-h Transition | ||
| Cornea Point A | 505.3 ± 240.5 | 405.2 ± 97.7 | 436.8 ± 60.0 | 0.280 |
| Cornea Point B | 445.2 ± 117.3 | 365.1 ± 208.4 | 355.6 ± 116.1 | 0.460 |
| Cornea Point C | 418.7 ± 78.8 | 383.5 ± 84.4 | 452.3 ± 120.6 | 0.310 |
| Retina Point 1 | 111.9 ± 25.7 | 109.2 ± 14.5 | 110.7 ± 27.3 | 0.970 |
| Retina Point 2 | 158.9 ± 46.6 | 145.3 ± 34.1 | 157.1 ± 38.7 | 0.630 |
| Retina Point 3 | 206.4 ± 45.5 | 175.7 ± 33.4 | 192.8 ± 42.4 | 0.210 |
| Retina Point 4 | 208.7 ± 44.5 | 204.5 ± 52.1 | 192.3 ± 30.3 | 0.680 |
| Retina Point 5 | 175.6 ± 19.6 | 206.0 ± 73.2 | 185.9 ± 56.2 | 0.370 |
| Retina Point 6 | 135.4 ± 21.8 | 120.6 ± 18.4 | 113.4 ± 9.2 | 0.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gündoğar, U.C.; Ahlat, O.; Onbaşılar, E.E. Assessment of Various Tissues in Broilers Reared Under Different Lighting Programs with Respect to Rearing Disorders. Vet. Sci. 2026, 13, 75. https://doi.org/10.3390/vetsci13010075
Gündoğar UC, Ahlat O, Onbaşılar EE. Assessment of Various Tissues in Broilers Reared Under Different Lighting Programs with Respect to Rearing Disorders. Veterinary Sciences. 2026; 13(1):75. https://doi.org/10.3390/vetsci13010075
Chicago/Turabian StyleGündoğar, Umut Can, Ozan Ahlat, and Esin Ebru Onbaşılar. 2026. "Assessment of Various Tissues in Broilers Reared Under Different Lighting Programs with Respect to Rearing Disorders" Veterinary Sciences 13, no. 1: 75. https://doi.org/10.3390/vetsci13010075
APA StyleGündoğar, U. C., Ahlat, O., & Onbaşılar, E. E. (2026). Assessment of Various Tissues in Broilers Reared Under Different Lighting Programs with Respect to Rearing Disorders. Veterinary Sciences, 13(1), 75. https://doi.org/10.3390/vetsci13010075






