Reducing Recurrence in Equine Corneolimbal SCC: Outcomes of Adjunctive Cisplatin Biodegradable Bead Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Ophthalmic Examination
2.3. SCC Surgical Excision and CBB Placement
2.4. Postoperative Medical Management
2.5. Statistical Analyses
3. Results
3.1. Animals
3.2. Local SCC Recurrence Rates
3.3. General Adverse Effects After Bead Insertion
3.4. Notable Patient Courses After Bead Insertion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBB | Cisplatin biodegradable bead |
| SCC | Squamous cell carcinoma |
| CHUV | Centre hospitalier universitaire vétérinaire |
| CDVUM | Centre de diagnostic vétérinaire de l’Université de Montréal |
References
- Mosunic, C.B.; Moore, P.A.; Carmicheal, K.P.; Chandler, M.J.; Vidyashankar, A.; Zhao, Y.; Roberts, R.E.; Dietrich, U.M. Effects of treatment with and without adjuvant radiation therapy on recurrence of ocular and adnexal squamous cell carcinoma in horses: 157 cases (1985–2002). J. Am. Vet. Med. Assoc. 2004, 225, 1733–1738. [Google Scholar] [CrossRef]
- Dugan, S.J.; Curtis, C.R.; Roberts, S.M.; Severin, G.A. Epidemiologic study of ocular/adnexal squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 1991, 198, 251–256. [Google Scholar] [CrossRef]
- Schwink, K. Factors influencing morbidity and outcome of equine ocular squamous cell carcinoma. Equine Vet. J. 1987, 19, 198–200. [Google Scholar] [CrossRef]
- Montgomery, K.W. Equine ocular neoplasia: A review. Equine Vet. Educ. 2014, 26, 372–380. [Google Scholar] [CrossRef]
- Chen, L.; Bellone, R.R.; Wang, Y.; Singer-Berk, M.; Sugasawa, K.; Ford, J.M.; Artandi, S.E. A novel DDB2 mutation causes defective recognition of UV-induced DNA damages and prevalent equine squamous cell carcinoma. DNA Repair. 2021, 97, 103022. [Google Scholar] [CrossRef]
- King, T.C.; Priehs, D.R.; Gum, G.G.; Miller, T.R. Therapeutic management of ocular squamous cell carcinoma in the horse: 43 cases (1979–1989). Equine Vet. J. 1991, 23, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Surjan, Y.; Donaldson, D.; Ostwald, P.; Milross, C.; Warren-Forward, H. A Review of Current Treatment Options in the Treatment of Ocular and/or Periocular Squamous Cell Carcinoma in Horses: Is There a Definitive “Best” Practice? J. Equine Vet. Sci. 2014, 34, 1037–1050. [Google Scholar] [CrossRef]
- Clode, A.B.; Miller, C.; McMullen, R.J., Jr.; Gilger, B.C. A retrospective comparison of surgical removal and subsequent CO2 laser ablation versus topical administration of mitomycin C as therapy for equine corneolimbal squamous cell carcinoma. Vet. Ophthalmol. 2012, 15, 254–262. [Google Scholar] [CrossRef]
- Hewes, C.A.; Sullins, K.E. Use of cisplatin-containing biodegradable beads for treatment of cutaneous neoplasia in equidae: 59 cases (2000–2004). J. Am. Vet. Med. Assoc. 2006, 229, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Marble, G.P.; Sullins, K.E.; Powers, B.K. Evaluation of subcutaneously implanted biodegradable matrix with and without cisplatin in horses. Equine Vet. Educ. 2022, 35, 131–137. [Google Scholar] [CrossRef]
- Jones, T.M.; Espitia, C.M.; Ooi, A.; Bauman, J.E.; Carew, J.S.; Nawrocki, S.T. Targeted CUL4A inhibition synergizes with cisplatin to yield long-term survival in models of head and neck squamous cell carcinoma through a DDB2-mediated mechanism. Cell Death Dis. 2022, 13, 350. [Google Scholar] [CrossRef]
- Bao, N.; Han, J.; Zhou, H. A protein with broad functions: Damage-specific DNA-binding protein 2. Mol. Biol. Rep. 2022, 49, 12181–12192. [Google Scholar] [CrossRef]
- Théon, A.P.; Pascoe, J.R.; Madigan, J.E.; Carlson, G.; Metzger, L. Comparison of intratumoral administration of cisplatin versus bleomycin for treatment of periocular squamous cell carcinomas in horses. Am. J. Vet. Res. 1997, 58, 431–436. [Google Scholar] [CrossRef]
- Pfizer Inc. Cisplatin Injection: Material Safety Data Sheet. Pfizer. 30 March 2011. Available online: https://cdn.pfizer.com/pfizercom/products/material_safety_data/PZ01470.pdf (accessed on 1 July 2025).
- Medsafe. DBL Cisplatin Injection: New Zealand Data Sheet. Medsafe. 2020. Available online: https://www.medsafe.govt.nz/profs/datasheet/d/DBLCisplatininj.pdf (accessed on 1 July 2025).
- Sellon, D.C.; Roberts, M.C.; Blikslager, A.T.; Ulibarri, C.; Papich, M.G. Effects of continuous rate intravenous infusion of butorphanol on physiologic and outcome variables in horses after celiotomy. J. Vet. Intern. Med. 2004, 18, 555–563. [Google Scholar] [CrossRef]
- Peraza, J.; Hector, R.C.; Lee, S.; Terhaar, H.M.; Knych, H.K.; Wotman, K.L. Ocular penetration of oral acetaminophen in horses. Equine Vet. J. 2023, 55, 899–904. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 July 2025).
- Salinas Ruíz, J.; Montesinos López, O.A.; Hernández Ramírez, G.; Crossa Hiriart, J. Generalized Linear Models. In Generalized Linear Mixed Models with Applications in Agriculture and Biology; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Cordeiro, G.M. Improved Likelihood Ratio Statistics for Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 1983, 45, 404–413. [Google Scholar] [CrossRef]
- Gelatt, K.N. Ocular neoplasia in horses. In Merck Veterinary Manual; Merck & Co., Inc.: Rahway, NJ, USA, 2019; Revised April 2019; Available online: https://www.merckvetmanual.com/eye-diseases-and-disorders/neoplasia-of-the-eye-and-associated-structures/ocular-neoplasia-in-horses (accessed on 1 July 2025).
- Rebhun, W.C. Treatment of advanced squamous cell carcinomas involving the equine cornea. Vet. Surg. 1990, 19, 297–302. [Google Scholar] [CrossRef] [PubMed]
- University of Tennessee College of Veterinary Medicine, Ophthalmology Service. Equine Ocular Squamous Cell Carcinoma (OSCC) FAQs; University of Tennessee College of Veterinary Medicine: Knoxville, TN, USA, 2024; Revised October 2024; Available online: https://vetmed.tennessee.edu/wp-content/uploads/sites/4/UTCVM_EquineOSCC_FAQs.pdf (accessed on 1 July 2025).
- English, R.V.; Nasisse, M.P.; Davidson, M.G. Carbon dioxide laser ablation for treatment of limbal squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 1990, 196, 439–442. [Google Scholar] [CrossRef]
- Michau, T.M.; Davidson, M.G.; Gilger, B.C. Carbon dioxide laser photoablation adjunctive therapy following superficial lamellar keratectomy and bulbar conjunctivectomy for the treatment of corneolimbal squamous cell carcinoma in horses: A review of 24 cases. Vet. Ophthalmol. 2012, 15, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Bessonnat, A.; Benoit-Biancamano, M.-O.; Vanore, M. Recurrence of conjunctival exuberant granulation tissue in a pony. Equine Vet. Educ. 2023, 35, 351–355. [Google Scholar] [CrossRef]
- Hollis, A.R. Radiotherapy for the treatment of periocular tumours in the horse. Equine Vet. Educ. 2019, 31, 647–652. [Google Scholar] [CrossRef]
- Arturi, F.J.; Arons, D.; Murphy, N.J.; Yu, C.; Panse, D.; Cherry, D.R.; Hsieh, K.; Bloom, J.R.; Nehlsen, A.D.; Resende Salgado, L.; et al. The Effects of Radiation Therapy on the Ocular Apparatus: Implications for Management. Cancers 2025, 17, 2605. [Google Scholar] [CrossRef]
- Gilger, B.C.; Malok, E.; Stewart, T.; Ashton, P.; Smith, T.; Jaffe, G.J.; Allen, J.B. Long-term effect on the equine eye of an intravitreal device used for sustained release of cyclosporine A. Vet. Ophthalmol. 2000, 3, 105–110. [Google Scholar] [CrossRef]
- Huppes, T.; Hermans, H.; Ensink, J.M. A retrospective analysis of the risk factors for surgical site infections and long-term follow-up after transpalpebral enucleation in horses. BMC Vet. Res. 2017, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Zscherpe, P.; Kalbitz, J.; Weber, L.A.; Paschke, R.; Mäder, K.; von Rechenberg, B.; Cavalleri, J.M.; Meißner, J.; Klein, K. Potent drug delivery enhancement of betulinic acid and NVX-207 into equine skin in vitro—A comparison between a novel oxygen flow-assisted transdermal application device and microemulsion gels. BMC Vet. Res. 2024, 20, 202. [Google Scholar] [CrossRef]
- Stewart, A.J. Absorbable Chemotherapeutic Beads Combat Cancer in Horses. dvm360. 1 July 2024. Available online: https://www.dvm360.com/view/absorbable-chemotherapeutic-beads-combat-cancer-in-horses (accessed on 1 July 2025).
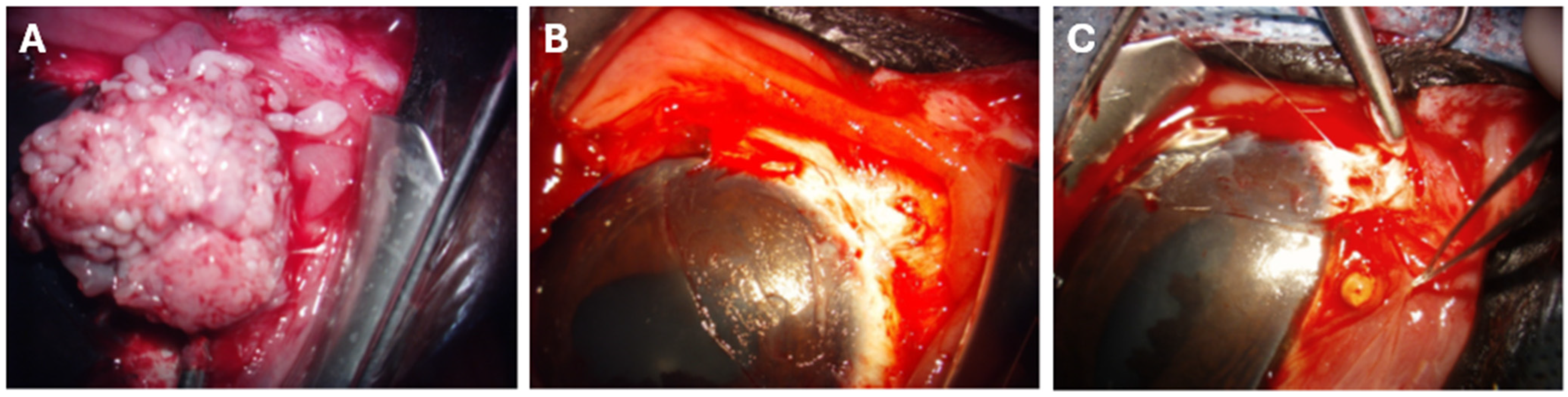
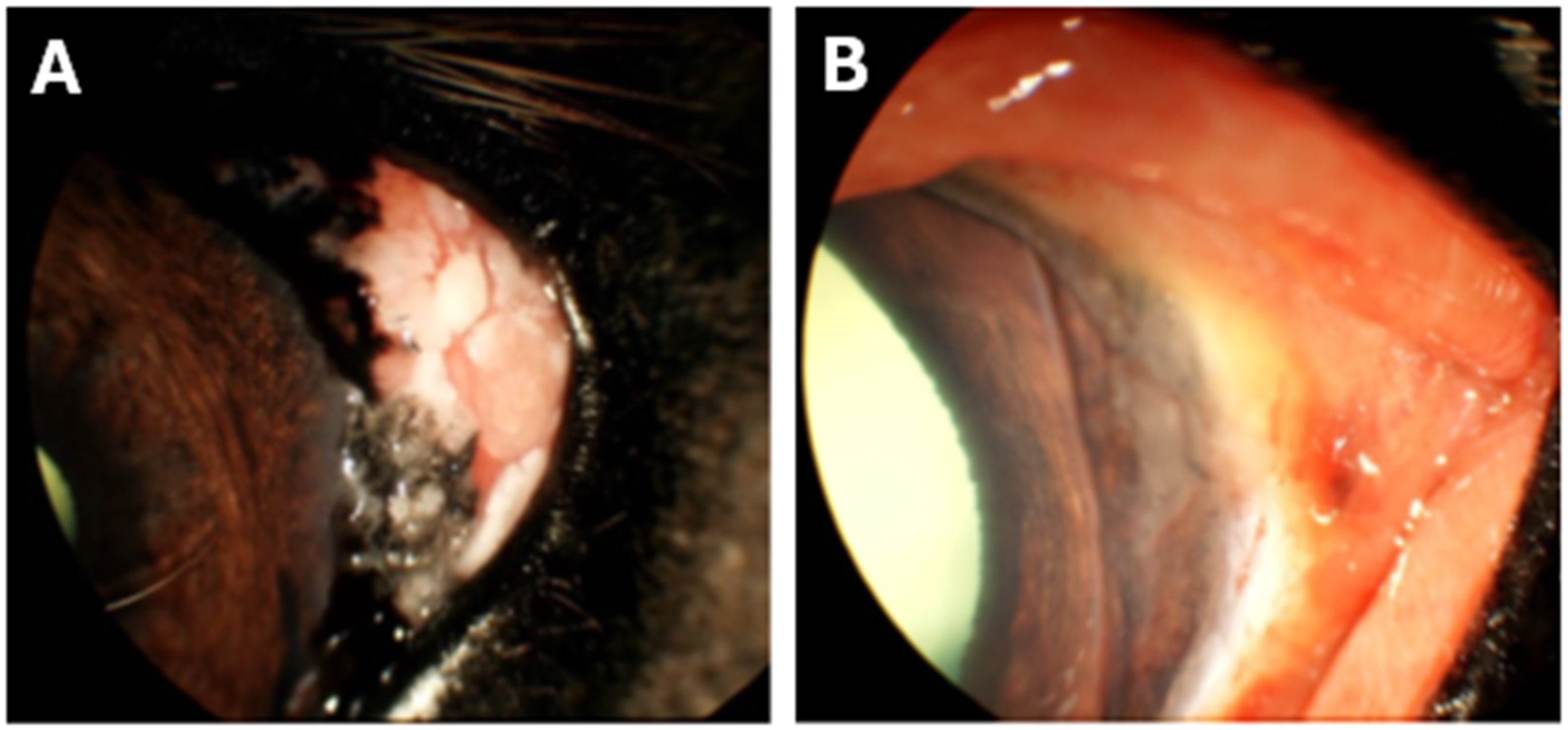
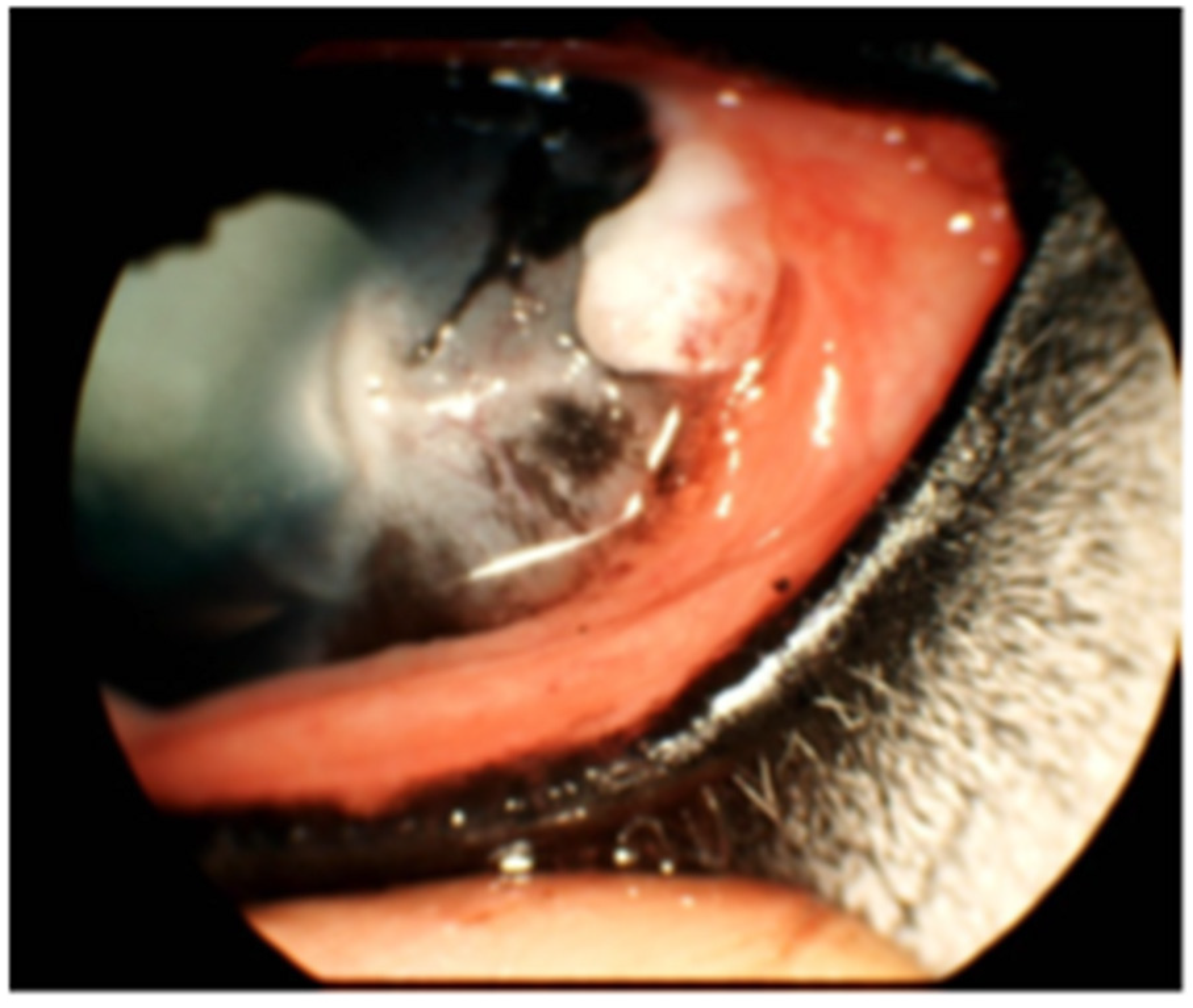
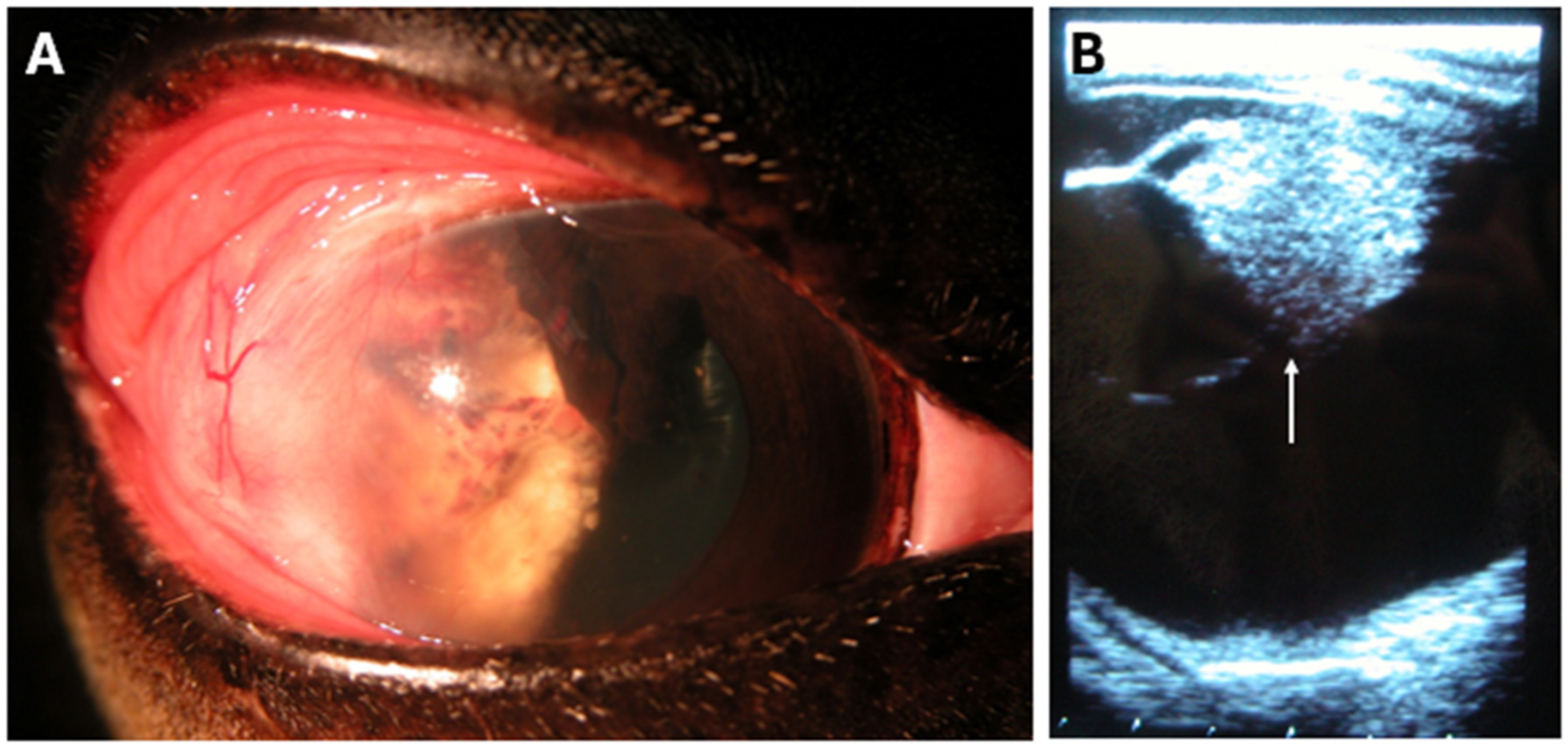
| Horse | Breed | Age (Years) | Sex | Coat Color | Weight (kg) | Tumor Infiltration | Tumor Excision |
|---|---|---|---|---|---|---|---|
| 1 | Appaloosa | 16 | M | Light | 500 | NA | Incomplete |
| 2 | Belgian | 10 | M | Light | 930 | In situ | Complete |
| 3 | Haflinger | 8 | F | Light | 410 | In situ | Complete |
| 4 | Haflinger | 4 | M | Light | 545 | Invasive | NA |
| 5 | Haflinger | 11 | M | Light | 555 | Invasive | Incomplete |
| 6 | Haflinger | 10 | M | Dark | 515 | Invasive | NA |
| 7 | Haflinger | 10 | F | Light | 498 | NA | NA |
| 8 | Haflinger | 20 | M | Dark | 459 | In situ | Complete |
| 9 | Haflinger | 11 | F | Light | 455 | Invasive | Incomplete |
| 10 | Haflinger | 13 | F | Light | 516 | Invasive | Complete |
| 11 | Haflinger | 10 | F | Dark | 508 | Invasive | Complete |
| 12 | Hanoverian | 7 | M | Light | 607 | Invasive | Complete |
| 13 | Quarter horse | 27 | F | NA | 550 | Invasive | NA |
| 14 | Quarter horse | 11 | M | Dark | NA | Invasive | Complete |
| 15 | Quarter horse | 10 | M | NA | 579 | Invasive | Incomplete |
| 16 | Thoroughbred | 9 | M | Light | 517 | In situ | Incomplete |
| Horse | Early Recurrence (<1 Year) | Late Recurrence (>1 Year) | Histopathological Confirmation |
|---|---|---|---|
| 4 | X | No | |
| 5 | X | No | |
| 6 | X | No | |
| 7 | X | Yes |
| Explanatory Variable | Early Recurrence | Late Recurrence | Overall Recurrence |
|---|---|---|---|
| Tumor infiltration | 0.183 | 0.327 | 0.997 |
| Surgical excision | 1.000 | 0.255 | 0.200 |
| Age | 0.076 | 0.626 | 0.127 |
| Sex | 0.937 | 0.355 | 0.615 |
| Coat color | 0.774 | 0.383 | 0.930 |
| Weight | 0.677 | 0.988 | 0.776 |
| Clinical Signs | Number of Presenting Horses (Percentage in Parentheses) |
|---|---|
| Ocular discomfort | 6/11 (55%) |
| Discharge | 7/11 (64%) |
| Conjunctivitis | 4/11 (36%) |
| Chemosis | 4/11 (36%) |
| Hyperemia | 7/11 (64%) |
| Local yellow discoloration | 6/11 (55%) |
| Granuloma | 4/11 (36%) |
| Light uveitis | 1/11 (11%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dagenais, A.; Juette, T.; Benoit-Biancamano, M.-O.; Vanore, M. Reducing Recurrence in Equine Corneolimbal SCC: Outcomes of Adjunctive Cisplatin Biodegradable Bead Therapy. Vet. Sci. 2026, 13, 76. https://doi.org/10.3390/vetsci13010076
Dagenais A, Juette T, Benoit-Biancamano M-O, Vanore M. Reducing Recurrence in Equine Corneolimbal SCC: Outcomes of Adjunctive Cisplatin Biodegradable Bead Therapy. Veterinary Sciences. 2026; 13(1):76. https://doi.org/10.3390/vetsci13010076
Chicago/Turabian StyleDagenais, Amy, Tristan Juette, Marie-Odile Benoit-Biancamano, and Maria Vanore. 2026. "Reducing Recurrence in Equine Corneolimbal SCC: Outcomes of Adjunctive Cisplatin Biodegradable Bead Therapy" Veterinary Sciences 13, no. 1: 76. https://doi.org/10.3390/vetsci13010076
APA StyleDagenais, A., Juette, T., Benoit-Biancamano, M.-O., & Vanore, M. (2026). Reducing Recurrence in Equine Corneolimbal SCC: Outcomes of Adjunctive Cisplatin Biodegradable Bead Therapy. Veterinary Sciences, 13(1), 76. https://doi.org/10.3390/vetsci13010076







