Serotyping and Antibiotic Resistance Profiles of Salmonella spp. and Listeria monocytogenes Strains Isolated from Pet Food and Feed Samples: A One Health Perspective †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, Pathogen Isolation, and Confirmation of the Isolates
2.2. Antimicrobial Susceptibility Testing (AST) and Antibiotic Resistance Profiles of Salmonella spp. and L. monocytogenes Strains
2.3. Serotyping of Salmonella spp. and L. monocytogenes Isolates
3. Results
3.1. Salmonella spp. and L. monocytogenes Isolates from Pet Food and Feed Samples
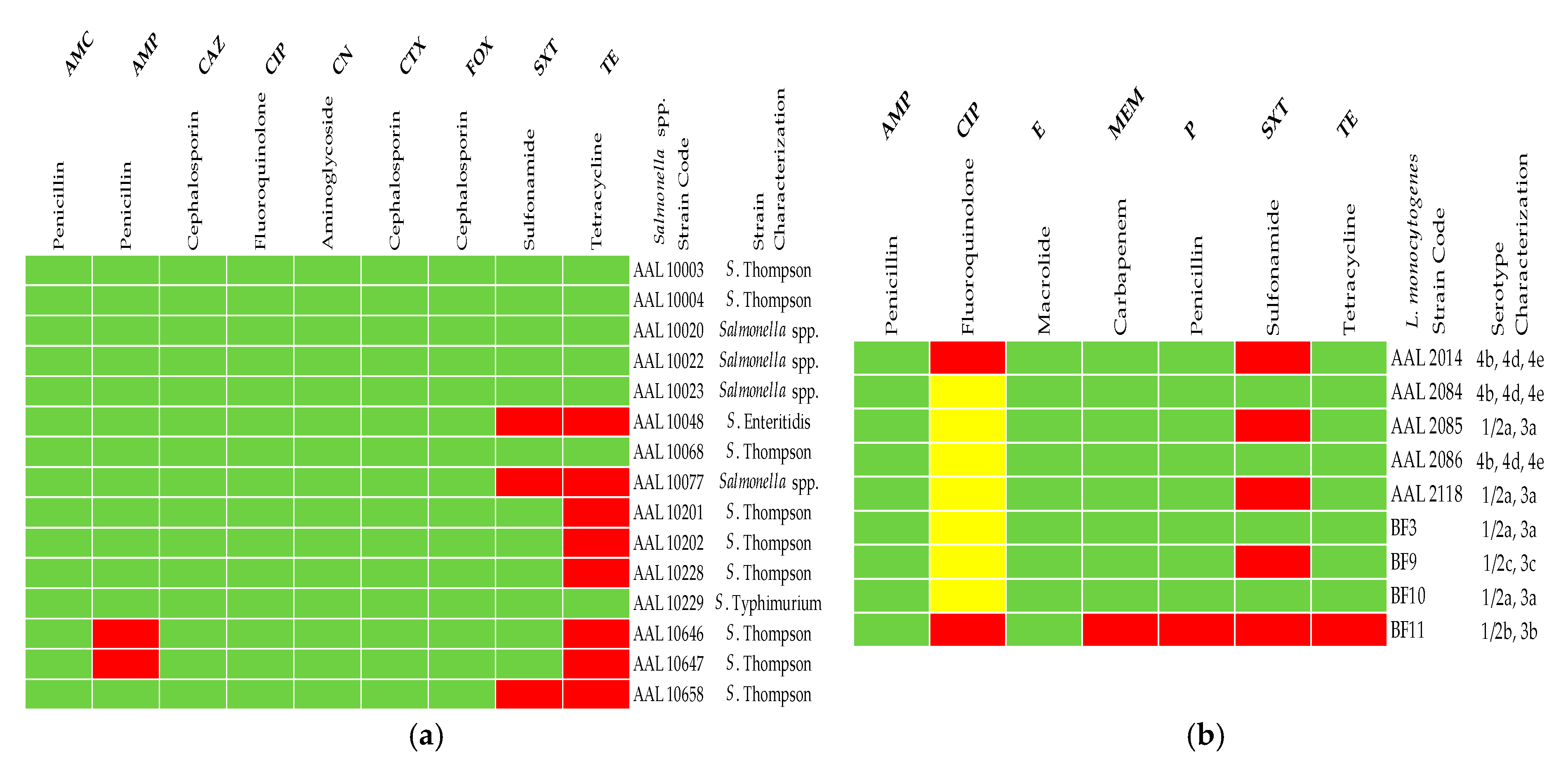
3.2. AST of Microbial Isolates
3.3. Antibiotic Resistance Profiles of the Pet Food and Feed Bacterial Isolates
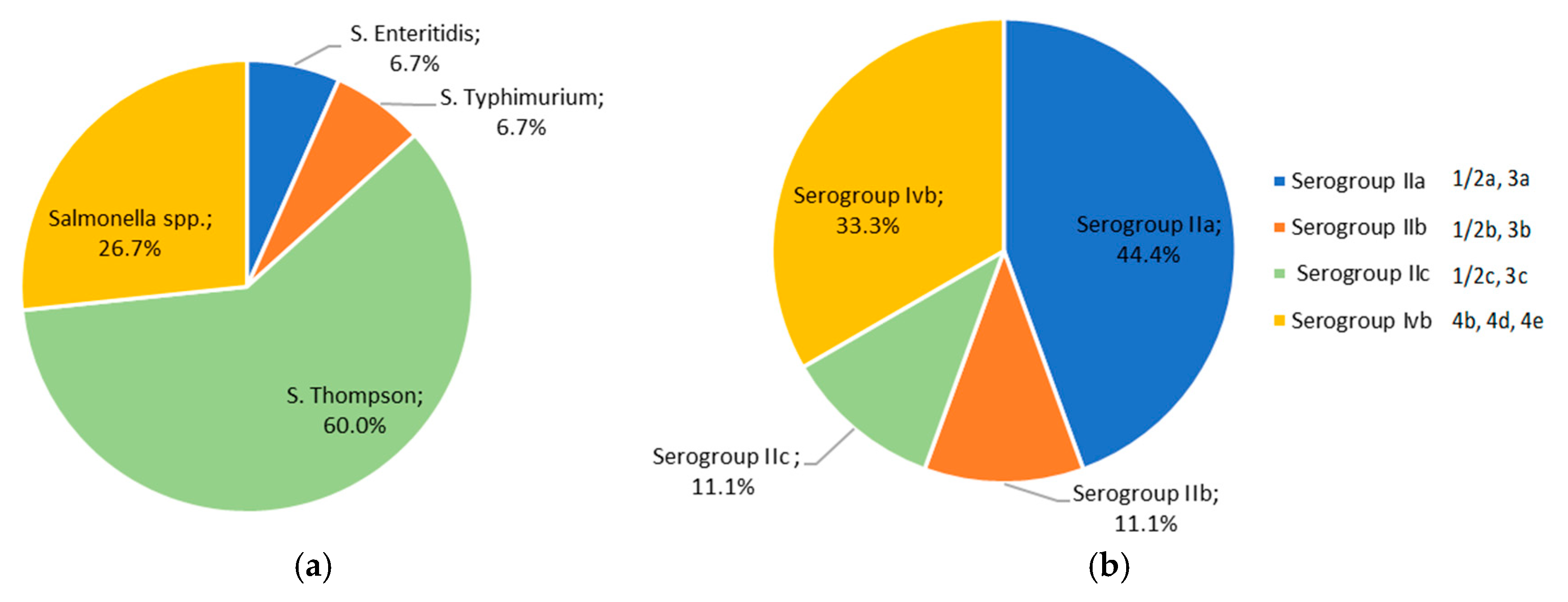
3.4. Serotyping of Salmonella spp. and L. monocytogenes Strains Isolated from Pet Food and Feed Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMC | amoxycillin-clavulanate |
| AMP | ampicillin |
| AMR | antimicrobial resistance |
| AST | antimicrobial susceptibility testing |
| BARF | biologically appropriate raw food |
| CAZ | ceftazidime |
| COVID-19 | Coronavirus disease 2019 |
| CN | gentamicin |
| CTX | cefotaxime |
| E | erythromycin |
| ECDC | European Centre for Disease Control and Prevention |
| EFSA | European Food Safety Authority |
| EU | European Union |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| FOX | cefoxitin |
| ISO | International Organization for Standardization |
| LDC | L-lysine decarboxylation medium |
| MDR | multidrug resistance |
| MH | Mueller–Hinton |
| MH-F | Mueller–Hinton with 5% defibrinated horse blood and 20 mg/L β-NAD |
| mPCR | multiplex polymerase chain reaction |
| P | benzylpenicillin |
| SXT | trimethoprim-sulfamethoxazole 1:19 |
| TE | tetracycline |
| TSI | triple sugar iron |
References
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2018 zoonoses report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Available online: https://www.who.int/health-topics/one-health#tab=tab_1 (accessed on 28 June 2025).
- USGS (United States Geological Survey). Available online: https://www.usgs.gov/media/images/one-health-conceptual-diagram (accessed on 28 June 2025).
- Mackenzie, J.S.; Jeggo, M. The One Health approach–Why is it so important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- Nemser, S.M.; Doran, T.; Grabenstein, M.; McConnell, T.; McGrath, T.; Pamboukian, R.; Smith, A.C.; Achen, M.; Danzeisen, G.; Kim, S.; et al. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog. Dis. 2014, 11, 706–709. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2022–2023. EFSA J. 2025, 23, e9237. [Google Scholar] [CrossRef]
- Alabi, E.D.; Rabiu, A.G.; Adesoji, A.T. A review of antimicrobial resistance challenges in Nigeria: The need for one health approach. One Health 2025, 20, 101053. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Kumar, S.; Varela, M.F. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms 2017, 5, 11. [Google Scholar] [CrossRef]
- Bland, R.; Waite-Cusic, J.; Weisberg, A.J.; Riutta, E.R.; Chang, J.H.; Kovacevic, J. Adaptation to a commercial quaternary ammonium compound sanitizer leads to cross-resistance to select antibiotics in Listeria monocytogenes isolated from fresh produce environments. Front. Microbiol. 2022, 12, 782920. [Google Scholar] [CrossRef] [PubMed]
- Braoudaki, M.; Hilton, A.C. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J. Clin. Microbiol. 2004, 42, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Guérin, A.; Bridier, A.; Le Grandois, P.; Sévellec, Y.; Palma, F.; Félix, B.; LISTADAPT Study Group; Roussel, S.; Soumet, C. Exposure to quaternary ammonium compounds selects resistance to ciprofloxacin in Listeria monocytogenes. Pathogens 2021, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Kode, D.; Nannapaneni, R.; Bansal, M.; Chang, S.; Cheng, W.-H.; Sharma, C.S.; Kiess, A. Low-level tolerance to fluoroquinolone antibiotic ciprofloxacin in QAC-adapted subpopulations of Listeria monocytogenes. Microorganisms 2021, 9, 1052. [Google Scholar] [CrossRef]
- Xiao, X.; Bai, L.; Wang, S.; Liu, L.; Qu, X.; Zhang, J.; Xiao, Y.; Tang, B.; Li, Y.; Yang, H.; et al. Chlorine tolerance and cross-resistance to antibiotics in poultry-associated Salmonella isolates in China. Front. Microbiol. 2022, 12, 833743. [Google Scholar] [CrossRef]
- Qamar, M.U.; Aatika; Chughtai, M.I.; Ejaz, H.; Mazhari, B.B.Z.; Maqbool, U.; Alanazi, A.; Alruwaili, Y.; Junaid, K. Antibiotic-resistant bacteria, antimicrobial resistance genes, and antibiotic residue in food from animal sources: One health food safety concern. Microorganisms 2023, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Carraturo, F.; Gargiulo, G.; Giorgio, A.; Aliberti, F.; Guida, M. Prevalence, distribution, and diversity of Salmonella spp. in meat samples collected from Italian slaughterhouses. J. Food Sci. 2016, 81, M2545–M2551. [Google Scholar] [CrossRef] [PubMed]
- DeBeer, J.; Finke, M.; Maxfield, A.; Osgood, A.-M.; Baumgartel, D.M.; Blickem, E.R. A review of pet food recalls from 2003 through 2022. J. Food Prot. 2024, 87, 100199. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.L.; Davanzo, E.F.A.; Palma, J.M.; Castro, V.H.dL.; da Costa, H.M.B.; Dallago, B.S.L.; Perecmanis, S.; Santana, Â.P. Molecular characterization and biofilm-formation analysis of Listeria monocytogenes, Salmonella spp., and Escherichia coli isolated from Brazilian swine slaughterhouses. PLoS ONE 2022, 17, e0274636. [Google Scholar] [CrossRef]
- Guidi, F.; Centorotola, G.; Chiaverini, A.; Iannetti, L.; Schirone, M.; Visciano, P.; Cornacchia, A.; Scattolini, S.; Pomilio, F.; D’Alterio, N.; et al. The slaughterhouse as hotspot of CC1 and CC6 Listeria monocytogenes strains with hypervirulent profiles in an integrated poultry chain of Italy. Microorganisms 2023, 11, 1543. [Google Scholar] [CrossRef]
- Hailu, W.; Helmy, Y.A.; Carney-Knisely, G.; Kauffman, M.; Fraga, D.; Rajashekara, G. Prevalence and antimicrobial resistance profiles of foodborne pathogens isolated from dairy cattle and poultry manure amended farms in northeastern Ohio, the United States. Antibiotics 2021, 10, 1450. [Google Scholar] [CrossRef]
- Horlbog, J.A.; Stephan, R.; Stevens, M.J.A.; Overesch, G.; Kittl, S.; Napoleoni, M.; Silenzi, V.; Nüesch-Inderbinen, M.; Albini, S. Feedborne Salmonella enterica serovar Jerusalem outbreak in different organic poultry flocks in Switzerland and Italy linked to soya expeller. Microorganisms 2021, 9, 1367. [Google Scholar] [CrossRef]
- Kousta, M.; Mataragas, M.; Skandamis, P.; Drosinos, E.H. Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control 2010, 21, 805–815. [Google Scholar] [CrossRef]
- Varsaki, A.; Ortiz, S.; Santorum, P.; López, P.; López-Alonso, V.; Hernández, M.; Abad, D.; Rodríguez-Grande, J.; Ocampo-Sosa, A.A.; Martínez-Suárez, J.V. Prevalence and population diversity of Listeria monocytogenes isolated from dairy cattle farms in the Cantabria region of Spain. Animals 2022, 12, 2477. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2023 zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- ISO 6579:2002; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella spp. ISO: Geneva, Switzerland, 2002.
- ISO 6579-1:2017; Microbiology of the Food Chain–Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- ISO 11290-1:2017; Microbiology of the Food Chain–Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. ISO: Geneva, Switzerland, 2017.
- Andritsos, N.D.; Stasinou, V.; Tserolas, D.; Giaouris, E. Temperature distribution and hygienic status of domestic refrigerators in Lemnos island, Greece. Food Control 2021, 127, 108121. [Google Scholar] [CrossRef]
- Kitch, T.T.; Jacobs, M.R.; Appelbaum, P.C. Evaluation of RapID onE system for identification of 379 strains in the family Enterobacteriaceae and oxidase-negative, Gram-negative nonfermenters. J. Clin. Microbiol. 1994, 32, 931–934. [Google Scholar] [CrossRef]
- Andritsos, N.D.; Mataragas, M. Characterization and antibiotic resistance of Listeria monocytogenes strains isolated from Greek Myzithra soft whey cheese and related food processing surfaces over two-and-a-half years of safety monitoring in a cheese processing facility. Foods 2023, 12, 1200. [Google Scholar] [CrossRef]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef]
- Doumith, M.; Jacquet, C.; Gerner-Smidt, P.; Graves, L.M.; Loncarevic, S.; Mathisen, T.; Morvan, A.; Salcedo, C.; Torpdahl, M.; Vazquez, J.A.; et al. Multicenter validation of a multiplex PCR assay for differentiating the major Listeria monocytogenes serovars 1/2a, 1/2b, 1/2c, and 4b: Toward an international standard. J. Food Prot. 2005, 68, 2648–2650. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2009, 15, 23. Available online: https://asm.org/protocols/kirby-bauer-disk-diffusion-susceptibility-test-pro (accessed on 28 August 2025).
- Brown, D.F.; Brown, L. Evaluation of the E test, a novel method of quantifying antimicrobial activity. J. Antimicrob. Chemother. 1991, 27, 185–190. [Google Scholar] [CrossRef] [PubMed]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. 2025. Available online: https:///www.eucast.org (accessed on 28 June 2025).
- Tzimotoudis, N.; Mataragka, A.; Andritsos, N.D.; Ikonomopoulos, J. Antibiotic susceptibility testing of Escherichia coli and coliform isolates detected in samples of drinking water from central Greece. Appl. Sci. 2025, 15, 2664. [Google Scholar] [CrossRef]
- Shimizu, R.; Osawa, K.; Shigemura, K.; Yoshida, H.; Fujiwara, M.; Iijima, Y.; Fujisawa, M.; Shirakawa, T. Development of multiplex PCR for rapid identification of four Salmonella serovars most commonly isolated in Japan. Southeast Asian J. Trop. Med. Public Health 2014, 45, 654–661. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbath, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Anturaniemi, J.; Barrouin-Melo, S.M.; Zaldivar-López, S.; Sinkko, A.; Hielm-Björkman, A. Owners’ perception of acquiring infections through raw pet food: A comprehensive internet-based survey. Vet. Rec. 2019, 185, 658. [Google Scholar] [CrossRef]
- Bulochova, V.; Evans, E.W. Raw meat-based pet feeding and food safety: Netnography study of pet owner comments and review of manufacturers’ information provision. J. Food Prot. 2021, 84, 2099–2108. [Google Scholar] [CrossRef]
- Conrad, C.C.; Stanford, K.; Narvaez-Bravo, C.; Callaway, T.; McAllister, T. Farm fairs and petting zoos: A review of animal contact as a source of zoonotic enteric disease. Foodborne Pathog. Dis. 2017, 14, 59–73. [Google Scholar] [CrossRef]
- Parker, E.M.; Mollenkopf, D.F.; Li, C.; Ballash, G.A.; Wittum, T.E. Pet treats, Salmonella, and antimicrobial resistance; a One Health problem. Prev. Vet. Med. 2025, 244, 106622. [Google Scholar] [CrossRef]
- Ma, J.; Almanza, B.A.; Ge, L.; Her, E.; Liu, Y.; Lando, A.; Wu, F.; Verrill, L. Pet ownership and pet type influence food safety in the home: Evidence from a national survey. J. Food Prot. 2020, 83, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.H.; Lawes, J.R.; Wales, A.D. Raw diets for dogs and cats: A review, with particular reference to microbiological hazards. J. Small Anim. Pract. 2019, 60, 329–339. [Google Scholar] [CrossRef]
- Lambertini, E.; Buchanan, R.L.; Narrod, C.; Pradhan, A.K. Transmission of bacterial zoonotic pathogens between pets and humans: The role of pet food. Crit. Rev. Food Sci. Nutr. 2016, 56, 364–418. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Menchetti, L.; Panseri, S.; Cami, M.; Balzaretti, C.M. When pet snacks look like children’s toys! The potential role of pet snacks in transmission of bacterial zoonotic pathogens in the household. Foodborne Pathog. Dis. 2021, 18, 56–62. [Google Scholar] [CrossRef]
- Menini, A.; Mascarello, G.; Giaretta, M.; Brombin, A.; Marcolin, S.; Personeni, F.; Pinto, A.; Crovato, S. The critical role of consumers in the prevention of foodborne diseases: An ethnographic study of Italian families. Foods 2022, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
- Morelli, G.; Stefanutti, D.; Ricci, R. A survey among dog and cat owners on pet food storage and preservation in the households. Animals 2021, 11, 273. [Google Scholar] [CrossRef]
- Ovca, A.; Bulochova, V.; Pirnat, T.; Evans, E.W. Risk perception and food safety practices among Slovenian pet owners: Does raw meat feeding of pets make a difference? J. Consum. Prot. Food Saf. 2024, 19, 293–302. [Google Scholar] [CrossRef]
- Leiva, A.; Molina, A.; Redondo-Solano, M.; Artavia, G.; Rojas-Bogantes, R.; Granados-Chinchilla, F. Pet food quality assurance and safety and quality assurance survey within the Costa Rican pet food industry. Animals 2019, 9, 980. [Google Scholar] [CrossRef]
- Pigłowski, M. Pathogenic and non-pathogenic microorganisms in the Rapid Alert System for Food and Feed. Int. J. Environ. Res. Public Health 2019, 16, 477. [Google Scholar] [CrossRef] [PubMed]
- Kananub, S.; Pinniam, N.; Phothitheerabut, S.; Krajanglikit, P. Contamination factors associated with surviving bacteria in Thai commercial raw pet foods. Vet. World 2020, 13, 1988–1991. [Google Scholar] [CrossRef] [PubMed]
- Hellström, S.; Laukkanen, R.; Siekkinen, K.-M.; Ranta, J.; Maijala, R.; Korkeala, H. Listeria monocytogenes contamination in pork can originate from farms. J. Food Prot. 2010, 73, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, S.J.; Daly, E.R.; Seiferth, J.; Nadeau, A.M.; Mahoney, J.; Finnigan, J.; Wikoff, P.; Kiebler, C.A.; Simmons, L. Human outbreak of Salmonella Typhimurium associated with exposure to locally made chicken jerky pet treats, New Hampshire, 2013. Foodborne Path. Dis. 2015, 12, 441–446. [Google Scholar] [CrossRef]
- Dhakal, J.; Cancio, L.P.M.; Deliephan, A.; Chaves, B.D.; Tubene, S. Salmonella presence and risk mitigation in pet foods; a growing challenge with implications for human health. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70060. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Stapleton, G.S.; Rotstein, D.S.; Gollarza, L.; Adams, J.; Caidi, H.; Chen, J.; Hodges, A.; Glover, M.; Peloquin, S.; et al. Outbreak of multidrug-resistant Salmonella infections in people linked to pig ear pet treats, United States, 2015-2019: Results of a multistate investigation. Lancet Reg. Health Am. 2024, 34, 100769. [Google Scholar] [CrossRef] [PubMed]
- Medić, H.; Kušec, I.D.; Pleadin, J.; Kozačinski, L.; Njari, B.; Hengl, B.; Kušec, G. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto-Shinohara, Y.; Sukenobe, J.; Imaizumi, T.; Nakahara, T. Survival of freeze-dried bacteria. J. Gen. Appl. Microbiol. 2008, 54, 9–24. [Google Scholar] [CrossRef]
- Mahmoud, S.; Aboul-Ella, H.; Marouf, S.; Armanious, W. Low temperature-survivability behavior of Salmonella enterica subsp. enterica serovar Typhimurium and Salmonella enterica subsp. enterica serovar Enteritidis in a minced beef meat model as an evaluation of the cold chain’s preserving-effectiveness. Int. J. Vet. Sci. 2023, 12, 853–859. [Google Scholar] [CrossRef]
- Taormina, P.J. Survival rate of Salmonella on cooked pig ear pet treats at refrigerated and ambient temperature storage. J. Food Prot. 2014, 77, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B.; Govindan, B.N.; Gänzle, M.; Roopesh, M.S. Influence of water activity on the heat resistance of Salmonella enterica in selected low-moisture foods. Int. J. Food Microbiol. 2020, 334, 108813. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, J.; Tadapanemi, R.K.; Yang, R.; Zhu, M.-J. Exponentially increased thermal resistance of Salmonella spp. and Enterococcus faecium at reduced water activity. Appl. Environ. Microbiol. 2018, 84, e02742-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Yin, H.-B.; Upadhayay, A.; Brown, S.; Venkitanarayanan, K. Efficacy of plant-derived antimicrobials for controlling Salmonella Schwarzengrund on dry pet food. Int. J. Food Microbiol. 2019, 296, 1–7. [Google Scholar] [CrossRef]
- Kiprotich, S.S.; Aldrich, C.G. A review of food additives to control the proliferation and transmission of pathogenic microorganisms with emphasis on applications to raw meat-based diets for companion animals. Front. Vet. Sci. 2022, 9, 1049731. [Google Scholar] [CrossRef]
- Lee, A.; Marks-Warren, N.; Aguilar, V.; Piszczor, K.; Swicegood, B.; Ye, M.; Warren, J.; O’Neill, E.; Fleck, M.; Tejayadi, S. Inactivation of Salmonella, Shiga toxin-producing E. coli, and Listeria monocytogenes in raw diet pet foods using high-pressure processing. J. Food Prot. 2023, 86, 100124. [Google Scholar] [CrossRef]
- Owens, T.G.; King, B.A.; Radford, D.R.; Strange, P.; Arvaj, L.; Pezzali, J.G.; Edwards, A.M.; Ganesh, D.; DeVries, T.J.; McBride, B.W.; et al. Use of 2-hydroxy-4-(methylthio)-butanoic acid to inhibit Salmonella and Listeria in raw meat for feline diets and palatability in domestic cats. J. Anim. Sci. 2021, 99, skab253. [Google Scholar] [CrossRef]
- Fisher, C.D.; Call, D.R.; Omulo, S. Detection of antibiotic resistant Enterobacterales in commercial raw pet food: A preliminary study. Front. Vet. Sci. 2024, 11, 1294575. [Google Scholar] [CrossRef]
- Bacci, C.; Vismarra, A.; Dander, S.; Barilli, E.; Superchi, P. Occurrence and antimicrobial profile of bacterial pathogens in former foodstuff meat products used for pet diets. J. Food Prot. 2019, 82, 316–324. [Google Scholar] [CrossRef]
- Bernaquez, I.; Dumaresq, J.; Picard, I.; Gaulin, C.; Dion, R.; Weaver, K.; Walker, M.; Kearney, A.; Bharat, A.; Fafard, J.; et al. Dogs fed raw meat-based diets are vectors of drug-resistant Salmonella infection in humans. Commun. Med. 2025, 5, 214. [Google Scholar] [CrossRef]
- Indra, R.; Sroithongkham, P.; Leelapsawas, C.; Yindee, J.; Wetchasirigul, S.; Chuanchuen, R.; Chanchaithong, P. Contamination with antimicrobial-resistant Escherichia coli, Salmonella, and Enterococcus in raw meat-based diets for pets. J. Anim. Physiol. Anim. Nutr. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Almeida, M.; Mourão, J.; Magalhães, M.; Freitas, A.R.; Novais, C.; Peixe, L.; Antunes, P. Raw meat-based diet for pets: A neglected source of human exposure to Salmonella and pathogenic Escherichia coli clones carrying mcr, Portugal, September 2019 to January 2020. Euro Surveill. 2024, 29, 2300561. [Google Scholar] [CrossRef]
- Fathi, M.M.; Samir, A.; Marouf, S.; Ali, A.R.; Al-Amry, K. Phenotypic and Genotypic Characteristics of Antimicrobial Resistance of Gram-Negative Bacteria Isolated from Pet Animal. J. Adv. Vet. Sci. 2022, 12, 597–604. Available online: https://www.advetresearch.com/index.php/AVR/article/view/1073 (accessed on 28 August 2025).
- Sivanandy, P.; Yuk, L.S.; Yi, C.S.; Kaur, I.; Ern, F.H.S.; Manirajan, P. A systematic review of recent outbreaks and the efficacy and safety of drugs approved for the treatment of Salmonella infections. IJID Reg. 2024, 14, 100516. [Google Scholar] [CrossRef] [PubMed]
- Morasi, R.M.; da Silva, A.Z.; Nuñez, K.V.M.; Dantas, S.T.A.; Faganello, C.; Juliano, L.C.B.; Tiba-Casas, M.R.; Pantoja, J.C.F.; Amarante, A.F.; Júnior, A.F.; et al. Overview of antimicrobial resistance and virulence factors in Salmonella spp. isolated in the last two decades from chicken in Brazil. Food Res. Int. 2022, 162, 111955. [Google Scholar] [CrossRef] [PubMed]
- Peruzy, M.F.; Capuano, F.; Proroga, Y.T.R.; Cristiano, D.; Carullo, M.R.; Murru, N. Antimicrobial susceptibility testing for Salmonella serovars isolated from food samples: Five-year monitoring (2015–2019). Antibiotics 2020, 9, 365. [Google Scholar] [CrossRef]
- Tayeb, B.A.; Mohamed-Sharif, Y.H.; Choli, F.R.; Haji, S.S.; Ibrahim, M.M.; Haji, S.K.; Rasheed, M.J.; Mustafa, N.A. Antimicrobial susceptibility profile of Listeria monocytogenes isolated from meat products: A systematic review and meta-analysis. Foodborne Pathog. Dis. 2023, 20, 315–333. [Google Scholar] [CrossRef]
- Tîrziu, E.; Bărbălan, G.; Morar, A.; Herman, V.; Cristina, R.T.; Imre, K. Occurrence and antimicrobial susceptibility profile of Salmonella spp. in raw and ready-to-eat foods and Campylobacter spp. in retail raw chicken meat in Transylvania, Romania. Foodborne Pathog. Dis. 2020, 17, 479–484. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, Y.; Xu, B.; Fang, Z.; Chen, W.; Feng, J.; Zhu, Y.; Liu, Y.; Gu, Q.; Luo, J.; et al. Serotype and antimicrobial resistance of Salmonella from poultry meats in 2021 Shanghai, China. Food Agric. Immunol. 2023, 34, 2220568. [Google Scholar] [CrossRef]
- Gutierrez, A.; De, J.; Schneider, K.R. Prevalence, concentration, and antimicrobial resistance profiles of Salmonella isolated from Florida poultry litter. J. Food Prot. 2020, 83, 2179–2186. [Google Scholar] [CrossRef]
- Romero-Barrios, P.; Deckert, A.; Parmley, E.J.; Leclair, D. Antimicrobial resistance profiles of Escherichia coli and Salmonella isolates in Canadian broiler chickens and their products. Foodborne Pathog. Dis. 2020, 17, 672–678. [Google Scholar] [CrossRef]
- Bishnoi, K.; Moudgil, P.; Soni, D.; Jadhav, V.J. Occurrence and risk assessment of tetracycline residues in layer eggs in Haryana, India. J. Food Prot. 2025, 88, 100449. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Kania, S.A.; Abouelkhair, M.A.; Whitlock, B.; Okafor, C.C. Residues of tetracycline, erythromycin, and sulfonamides in beef, eggs, and honey from grocery stores in Knoxville, Tennessee, USA: Failure of cooking to decrease drug concentrations. Vet. Sci. 2024, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Karthik, K.; Tiwari, R.; Shabbir, M.Z.; Barbuddhe, S.; Malik, S.V.S.; Singh, R.K. Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: A comprehensive review. Vet. Q. 2015, 35, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Matano, S.; Satoh, S.; Harada, Y.; Nagata, H.; Sugimoto, T. Antibiotic treatment for bacterial meningitis caused by Listeria monocytogenes in a patient with multiple myeloma. J. Infect. Chemother. 2010, 16, 123–125. [Google Scholar] [CrossRef]
- Mataragka, A.; Tzimotoudis, N.; Mavrommatis, A.; Tsiplakou, E.; Symeonidou, A.; Kotsikori, M.; Zervas, G.; Ikonomopoulos, J. Investigating the spread of antimicrobial drug-resistant microorganisms in dairy sheep farms: A follow-up study. Appl. Sci. 2023, 13, 8165. [Google Scholar] [CrossRef]
- Scallan Walter, E.J.; Cui, Z.; Tierney, R.; Griffin, P.M.; Hoekstra, R.M.; Payne, D.C.; Rose, E.B.; Devine, C.; Namwase, A.S.; Mirza, S.A.; et al. Foodborne illness acquired in the United States – Major pathogens, 2019. Emerg. Inf. Dis. 2025, 31, 669–677. [Google Scholar] [CrossRef]
- Luvsansharav, U.O.; Vieira, A.; Bennett, S.; Huang, J.; Healy, J.M.; Hoekstra, R.M.; Bruce, B.B.; Cole, D. Salmonella serotypes: A novel measure of association with foodborne transmission. Foodborne Pathog. Dis. 2020, 17, 151–155. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Kim, M.; Barnett-Neefs, C.; Chavez, R.A.; Kealey, E.; Wiedmann, M.; Stasiewicz, M. Risk assessment predicts most of the salmonellosis risk in raw chicken parts is concentrated in those few products with high levels of high-virulence serotypes of Salmonella. J. Food Prot. 2024, 87, 100304. [Google Scholar] [CrossRef] [PubMed]
- Fenske, G.J.; Pouzou, J.G.; Pouillot, R.; Taylor, D.D.; Costard, S.; Zagmutt, F.J. The genomic and epidemiological virulence patterns of Salmonella enterica serovars in the United States. PLoS ONE 2023, 18, e0294624. [Google Scholar] [CrossRef]
- McMillan, E.A.; Weinroth, M.D.; Frye, J.G. Increased prevalence of Salmonella Infantis isolated from raw chicken and turkey products in the United States is due to a single clonal lineage carrying the pESI plasmid. Microorganisms 2022, 10, 1478. [Google Scholar] [CrossRef]
- Lim, Y.C.; Ong, K.H.; Khor, W.C.; Chua, F.Y.X.; Lim, J.Q.; Tan, L.K.; Chen, S.L.; Wong, W.K.; Maiwald, M.; Barkham, T.; et al. Sequence types and antimicrobial resistance profiles of Salmonella Typhimurium in the food chain in Singapore. Microorganisms 2024, 12, 1912. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, M.; Zhao, L.; Du, P.; Cui, S.; Liu, Y.; Tan, D.; Zeng, X.; Yang, B.; Li, R.; et al. Tracing the evolution: The rise of Salmonella Thompson co-resistant to clinically important antibiotics in China, 1997–2020. mSystems 2025, 10, e0101824. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Frye, J.G.; Hu, J.; Fedorka-Gray, P.J.; Gautom, R.; Boyle, D.S. Multiplex PCR-based method for identification of common clinical serotypes of Salmonella enterica subsp. enterica. J. Clin. Microbiol. 2006, 44, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Andritsos, N.D.; Paramithiotis, S.; Mataragas, M.; Drosinos, E.H. Listeria monocytogenes serogroup 1/2 strains have a competitive growth advantage over serotype 4b during refrigerated storage of an artificially contaminated ready-to-eat pork meat product. Appl. Sci. 2021, 11, 6096. [Google Scholar] [CrossRef]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EC) No 178/2002 on Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Off. J. Eur. Union 2002, L31, 1–24. Available online: https://eur-lex.europa.eu/eli/reg/2002/178/oj/eng (accessed on 12 August 2025).
| (A) | |||
| Target Gene | Sequence (5′-3′) | Product Size | Serovar Designation |
| invA | F: GCCATGGTATGGATTTGTCC | 118 bp | Salmonella spp. |
| R: GTCACGATAAAACCGGCACT | |||
| sdf | F: TGTGTTTTATCTGATGCAAGAGG | 333 bp | S. Enteritidis |
| R: CGTTCTTCTGGTACTTACGATGAC | |||
| fliC-r | F: AACAACGACAGCTTATGCCG | 413 bp | S. Infantis |
| R: CCACCTGCGCCAACGCT | |||
| fliC-i | F: ACTCAGGCTTCCCGTAACGC | 551 bp | S. Typhimurium |
| R: ATAGCCATTTACCAGTTCC | |||
| fliC-k | F: AACGACGGTATCTCCATTGC | 658 bp | S. Thompson |
| R: CAGCCGAACTCGGTGTATTT | |||
| (B) | |||
| Target Gene | Sequence (5′-3′) | Product Size | Serotype Designation |
| prs | F: GCTGAAGAGATTGCGAAAGAAG | 370 bp | Listeria spp. |
| R: CAAAGAAACCTTGGATTTGCGG | |||
| ORF2819 | F: AGCAAAATGCCAAAACTCGT | 471 bp | 1/2b, 3b, 4b, 4d, and 4e |
| R: CATCACTAAAGCCTCCCATTG | |||
| ORF2110 | F: AGTGGACAATTGATTGGTGAA | 597 bp | 4b, 4d, and 4e |
| R: CATCCATCCCTTACTTTGGAC | |||
| lmo0737 | F: AGGGCTTCAAGGACTTACCC | 691 bp | 1/2a, 1/2c, 3a, and 3c |
| R: ACGATTTCTGCTTGCCATTC | |||
| lmo1118 | F: AGGGGTCTTAAATCCTGGAA | 906 bp | 1/2c and 3c |
| R: CGGCTTGTTCGGCATACTTA | |||
| (A) | |||||||||||
| Strain | AMC (%) | AMP (%) | CAZ (%) | CIP (%) | CN (%) | CTX (%) | FOX (%) | SXT (%) | TE (%) | ||
| Resistant | 0 (0.0) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (20.0) | 8 (53.3) | ||
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Susceptible | 15 (100) | 13 (86.7) | 15 (100) | 15 (100) | 15 (100) | 15 (100) | 15 (100) | 12 (80.0) | 7 (46.7) | ||
| Total | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | ||
| (B) | |||||||||||
| Strain | AMP (%) | CIP (%) | E (%) | MEM (%) | P (%) | SXT (%) | TE (%) | ||||
| Resistant | 0 (0.0) | 2 (22.2) | 0 (0.0) | 1 (11.1) | 1 (11.1) | 5 (55.6) | 1 (11.1) | ||||
| Intermediate | 0 (0.0) | 7 (77.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Susceptible | 9 (100) | 0 (0.0) | 9 (100) | 8 (88.9) | 8 (88.9) | 4 (44.4) | 8 (88.9) | ||||
| Total | 9 | 9 | 9 | 9 | 9 | 9 | 9 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andritsos, N.D.; Mataragka, A.; Tzimotoudis, N.; Chatzopoulou, A.-S.; Kotsikori, M.; Ikonomopoulos, J. Serotyping and Antibiotic Resistance Profiles of Salmonella spp. and Listeria monocytogenes Strains Isolated from Pet Food and Feed Samples: A One Health Perspective. Vet. Sci. 2025, 12, 844. https://doi.org/10.3390/vetsci12090844
Andritsos ND, Mataragka A, Tzimotoudis N, Chatzopoulou A-S, Kotsikori M, Ikonomopoulos J. Serotyping and Antibiotic Resistance Profiles of Salmonella spp. and Listeria monocytogenes Strains Isolated from Pet Food and Feed Samples: A One Health Perspective. Veterinary Sciences. 2025; 12(9):844. https://doi.org/10.3390/vetsci12090844
Chicago/Turabian StyleAndritsos, Nikolaos D., Antonia Mataragka, Nikolaos Tzimotoudis, Anastasia-Spyridoula Chatzopoulou, Maria Kotsikori, and John Ikonomopoulos. 2025. "Serotyping and Antibiotic Resistance Profiles of Salmonella spp. and Listeria monocytogenes Strains Isolated from Pet Food and Feed Samples: A One Health Perspective" Veterinary Sciences 12, no. 9: 844. https://doi.org/10.3390/vetsci12090844
APA StyleAndritsos, N. D., Mataragka, A., Tzimotoudis, N., Chatzopoulou, A.-S., Kotsikori, M., & Ikonomopoulos, J. (2025). Serotyping and Antibiotic Resistance Profiles of Salmonella spp. and Listeria monocytogenes Strains Isolated from Pet Food and Feed Samples: A One Health Perspective. Veterinary Sciences, 12(9), 844. https://doi.org/10.3390/vetsci12090844









