Evaluation of the Preoperative Antiseptic Efficacy of Ozone on Dog Skin in Comparison with Traditional Methods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Preparation of Ozonated Bidistilled Water and Other Antiseptic Supplies
2.3. Sampling Procedure
- -
- Area 1: soap-based chlorhexidine + alcoholic chlorhexidine
- -
- Area 2: soap-based chlorhexidine + ozonated water (20 µg/mL)
- -
- Area 3: ethyl alcohol + ozonated water (20 µg/mL)
- -
- Area 4: ozonated water (20 µg/mL)
2.4. Mesophilic Bacterial Count Determination
- represents the total number of colonies counted for each dilution.
- V is the volume in millilitres (mL) of sterile saline solution in which the bacterial swab was immersed.
- denotes the number of dilutions performed.
- indicates the dilution factor applied in the first dilution.
- is the surface area analyzed, expressed in cm2.
- A correction factor of 40% was incorporated by the authors, grounded in scientific evidence suggesting that only approximately 60% of the microbial sample is recovered from the swab surface [26].
2.5. Statistical Analysis
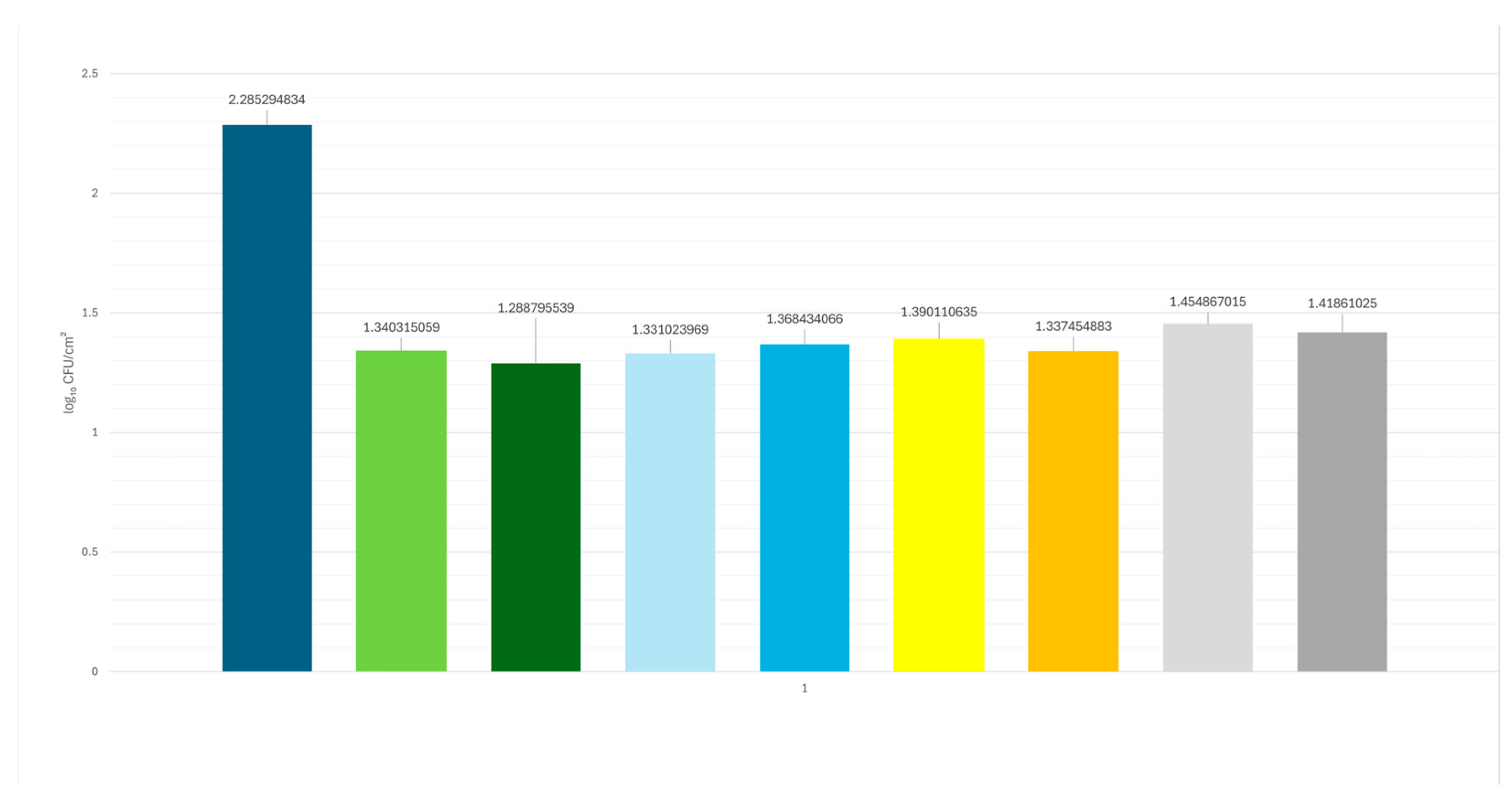
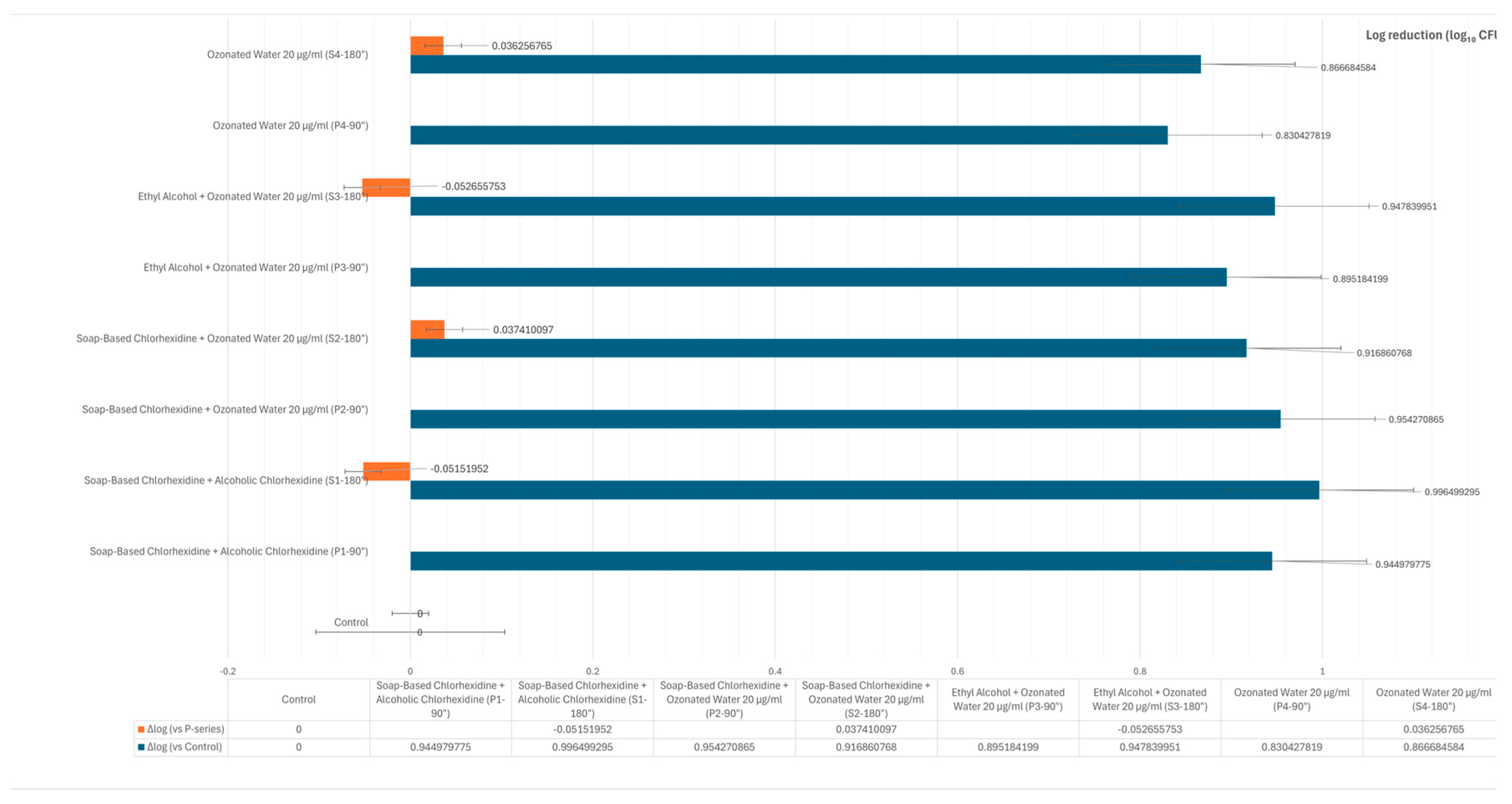
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baudouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, B.T.; Kim, H.J. Effect of indoor air pollution on atopic dermatitis in dogs. Allergy 2023, 78, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.; Costin, M.; Granick, J. AAFP/AAHA Antimicrobial Stewardship Guidelines. J. Am. Anim. Hosp. Assoc. 2022, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, T.M.; Scahill, K.; Ruperez, J.E. Antimicrobial prophylaxis in companion animal surgery: A scoping review for European Network for Optimization of Antimicrobial Therapy (ENOVAT) guidelines. Vet. J. 2024, 304, 106101. [Google Scholar] [CrossRef]
- Thongrueang, N.; Liu, S.S.; Hsu, H.Y.; Lee, H.H. An in vitro comparison of antimicrobial efficacy and cytotoxicity between povidone-iodine and chlorhexidine for treating clinical endometritis in dairy cows. PLoS ONE 2022, 17, e0271274. [Google Scholar] [CrossRef]
- Crosse, K.R. Pre-surgical hand preparation in veterinary practice. N. Z. Vet. J. 2022, 70, 69–78. [Google Scholar] [CrossRef]
- Stawarz-Janeczek, M.; Kryczyk-Poprawa, A.; Muszyńska, B. Disinfectants used in stomatology and SARS-CoV-2 infection. Eur. J. Dent. 2021, 15, 388–400. [Google Scholar] [CrossRef]
- Hoang, T.P.N.; Ghori, M.U.; Conway, B.R. Topical Antiseptic Formulations for Skin and Soft Tissue Infections. Pharmaceutics 2021, 13, 558. [Google Scholar] [CrossRef]
- Belo, L.; Serrano, I.; Cunha, E. Skin asepsis protocols as a preventive measure of surgical site infections in dogs: Chlorhexidine-alcohol versus povidone-iodine. BMC Vet. Res. 2018, 14, 95. [Google Scholar] [CrossRef]
- Wade, R.G.; Burr, N.E.; McCauley, G. The Comparative Efficacy of Chlorhexidine Gluconate and Povidone-iodine Antiseptics for the Prevention of Infection in Clean Surgery: A Systematic Review and Network Meta-analysis. Ann. Surg. 2021, 274, e481–e488. [Google Scholar] [CrossRef]
- Privitera, G.P.; Costa, A.L.; Brusaferro, S. Skin antisepsis with chlorhexidine versus iodine for the prevention of surgical site infection: A systematic review and meta-analysis. Am. J. Infect. Control. 2017, 45, 180–189. [Google Scholar] [CrossRef]
- WHO, World Health Organization. Global Guidelines on the Prevention of Surgical Site Infection. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/250680/9789241549882-eng.pdf?sequence=8 (accessed on 30 April 2022).
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance [published correction appears in Clin Microbiol Rev 2001 Jan;14(1):227]. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Auda, S.H.; Mahrous, G.M.; Ibrahim, M.A. Novel chlorhexidine dermal patches, preparation characterization and antimicrobial evaluation. Polymer Bull. 2017, 74, 3995–4007. [Google Scholar] [CrossRef]
- Epelle, E.I.; Cojuhari, N.; Mohamedsalih, A. The synergistic antibacterial activity of ozone and surfactant mists. RSC Adv. 2023, 13, 22593–22605. [Google Scholar] [CrossRef] [PubMed]
- Viebahn-Haensler, R.; León Fernández, O.S. Ozone in Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C. Potent Activity of a High Concentration of Chemical Ozone against Antibiotic-Resistant Bacteria. Molecules. 2022, 27, 3998. [Google Scholar] [CrossRef]
- Burgassi, S.; Zanardi, I.; Travagli, V. How much ozone bactericidal activity is compromised by plasma components? J. Appl. Microbiol. 2009, 106, 1715–1721. [Google Scholar] [CrossRef]
- Song, M.; Zeng, Q.; Xiang, Y. The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol. Med. Rep. 2018, 17, 2449–2455. [Google Scholar] [CrossRef]
- Roth, A.; Maruthamuthu, M.K.; Nejati, S. Wearable adjunct ozone and antibiotic therapy system for treatment of Gram-negative dermal bacterial infection. Sci. Rep. 2022, 12, 13927. [Google Scholar] [CrossRef]
- Wu, T.; Li, Z.; Wei, Y. Advances in understanding mechanisms underlying mitochondrial structure and function damage by ozone. Sci. Total Environ. 2023, 861, 160589. [Google Scholar] [CrossRef]
- Fontes, B.; Cattani Heimbecker, A.M.; de Souza Brito, G. Effect of low-dose gaseous ozone on pathogenic bacteria. BMC Infect. Dis. 2012, 12, 358. [Google Scholar] [CrossRef]
- Bocci, V. Ossigeno Ozono Terapia: Comprensione dei Meccanismi di Azione e Possibilità Terapeutiche; CEA: Pove del Grappa VI, Italy, 2000. [Google Scholar]
- Bocci, V.; E Borrelli, V. Travagli, and I. Zanardi. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef] [PubMed]
- ISO 7932: 2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus cereus—Colony-Count Technique at 30 Degrees C. ISO: Geneva, Switzerland, 2004.
- Dalmaso, G.; Bini, M. Qualification of high recovery, flocked swabs as compared to traditional rayon swabs for microbiological environmental monitoring of surfaces. PDA J. Pharm. Sci. Technol. 2008, 62, 191–199. [Google Scholar] [PubMed]
- Štempelová, L.; Micenková, L.; Andrla, P.; Strompfová, V. The skin microbiome on healthy and inflammatory altered canine skin determined by next generation sequencing. Front. Microbiol. 2025, 16, 1528747. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef]
- Hiragaki, K.; Tomiya, I.; Masaru, N. Generation of ozone foam and its application for disinfection. Eur. Phys. J. Appl. Phys. 2015, 71, 20810. [Google Scholar] [CrossRef]
- Matsuda, A.; Ano, T.; Nakamura, Y.; Itoi, T.; Arai, K.; Kutara, K.; Sugimoto, K.; Maeta, N. Ozone water has antibacterial properties in dogs without skin barrier impairment. Vet. Dermatol. 2025, 36, 283–290. [Google Scholar] [CrossRef]
- Zeng, J.; Lu, J. Mechanisms of action involved in ozone-therapy in skin diseases. Int. Immunopharmacol. 2018, 56, 235–241. [Google Scholar] [CrossRef]
- Breidablik, H.J.; Lysebo, D.E.; Johannessen, L. Effects of hand disinfection with alcohol hand rub, ozonated water, or soap and water: Time for reconsideration? J. Hosp. Infect. 2020, 105, 213–215. [Google Scholar] [CrossRef]
- Bialoszewski, D.; Pietruczuk-Padzik, A.; Kalicinska, A. Activity of ozonated water and ozone against Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Med. Sci. Monit. 2011, 17, BR339–BR344. [Google Scholar] [CrossRef]
- Wang, X.; Liao, D.; Ji, Q.M. Analysis of Bactericidal Effect of Three Medical Ozonation Dosage Forms on Multidrug-Resistant Bacteria from Burn Patients. Infect. Drug Resist. 2022, 15, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.K.; Held, E.; Johansen, J.D. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br. J. Dermatol. 2005, 153, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Asimus, E.; Palierne, S.; Blondel, M. Comparison of hydroalcoholic rubbing and conventional chlorhexidine scrubbing for aseptic skin preparation in dogs. Vet. Surg. 2019, 48, 1466–1472. [Google Scholar] [CrossRef]
- Epstein, N.E. Review: Perspective on ocular toxicity of presurgical skin preparations utilizing Chlorhexidine Gluconate/Hibiclens/Chloraprep. Surg. Neurol. Int. 2021, 12, 335. [Google Scholar] [CrossRef]
- Boyce, J.M. Alcohols as Surface Antiseptics in Healthcare Settings. Infect. Control Hosp. Epidemiol. 2018, 39, 323–328. [Google Scholar] [CrossRef]
- Bever, G.J.; Brodie, F.L.; Hwang, D.G. Corneal Injury from Presurgical Chlorhexidine Skin Preparation. World Neurosurg. 2016, 96, e1–e610. [Google Scholar] [CrossRef]
- Sharp, G.; Green, S.; Rose, M. Chlorhexidine-induced anaphylaxis in surgical patients: A review of the literature. ANZ J. Surg. 2016, 86, 237–243. [Google Scholar] [CrossRef]
- Opstrup, M.S.; Jemec, G.B.E.; Garvey, L.H. Chlorhexidine Allergy: On the Rise and Often Overlooked. Curr. Allergy Asthma Rep. 2019, 19, 23. [Google Scholar] [CrossRef]
- Rutkowski, K.; Wagner, A. Chlorhexidine: A new latex? Eur. Urol. 2015, 68, 345–347. [Google Scholar] [CrossRef]
- Swales, N.; Cogan, T. Failure to achieve asepsis following surgical skin preparation is influenced by bacterial resistance to chlorhexidine, but not skin preparation technique. Vet. Nurs. J. 2017, 32, 224–227. [Google Scholar] [CrossRef][Green Version]
- Melanie, P.; Niola, C.; Plataroti, I.; Mancini, S.; Fratini, F. Use of Ozone in Veterinary Dentistry as an Alternative to Conventional Antibiotics and Antiseptics. Vet. Sci. 2024, 11, 163. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Ziegler, E.; Palmer, K.L. Chlorhexidine induces VanA-type Vancomycin resistance genes in enterococci. Antimicrob. Agents Chemother. 2016, 60, 2209–2221. [Google Scholar] [CrossRef]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of Increased Resistance to Chlorhexidine and Cross-Resistance to Colistin following Exposure of Klebsiella pneumoniae Clinical Isolates to Chlorhexidine. Antimicrob. Agents Chemother. 2016, 61, e01162-16. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kawamura, K.; Matsui, M.; Suzuki, M.; Suzuki, S.; Shibayama, K.; Arakawa, Y. Reduction in chlorhexidine efficacy against multi-drug-resistant Acinetobacter baumannii international clone II. J. Hosp. Infect. 2017, 95, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.; Farajzadeh, D.; Dehghanzadeh, R. Optimizing ozone dose and contact time for removal of antibiotic-resistant P. aeruginosa, A. baumannii, E. coli, and associated resistant genes in effluent of an activated sludge process in a municipal WWTP. Environ. Sci. Pollut. Res. Int. 2023, 30, 55569–55581. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Usui, M.; Hayashi, T. Inactivation of Antibiotic-Resistant Bacteria in Wastewater by Ozone-Based Advanced Water Treatment Processes. Antibiotics 2022, 11, 210. [Google Scholar] [CrossRef]
- Botondi, R.; Lembo, M.; Eramo, V. The Use of Ozone Technology: An Eco-Friendly Method for the Sanitization of the Dairy Supply Chain. Foods 2023, 12, 987. [Google Scholar] [CrossRef]
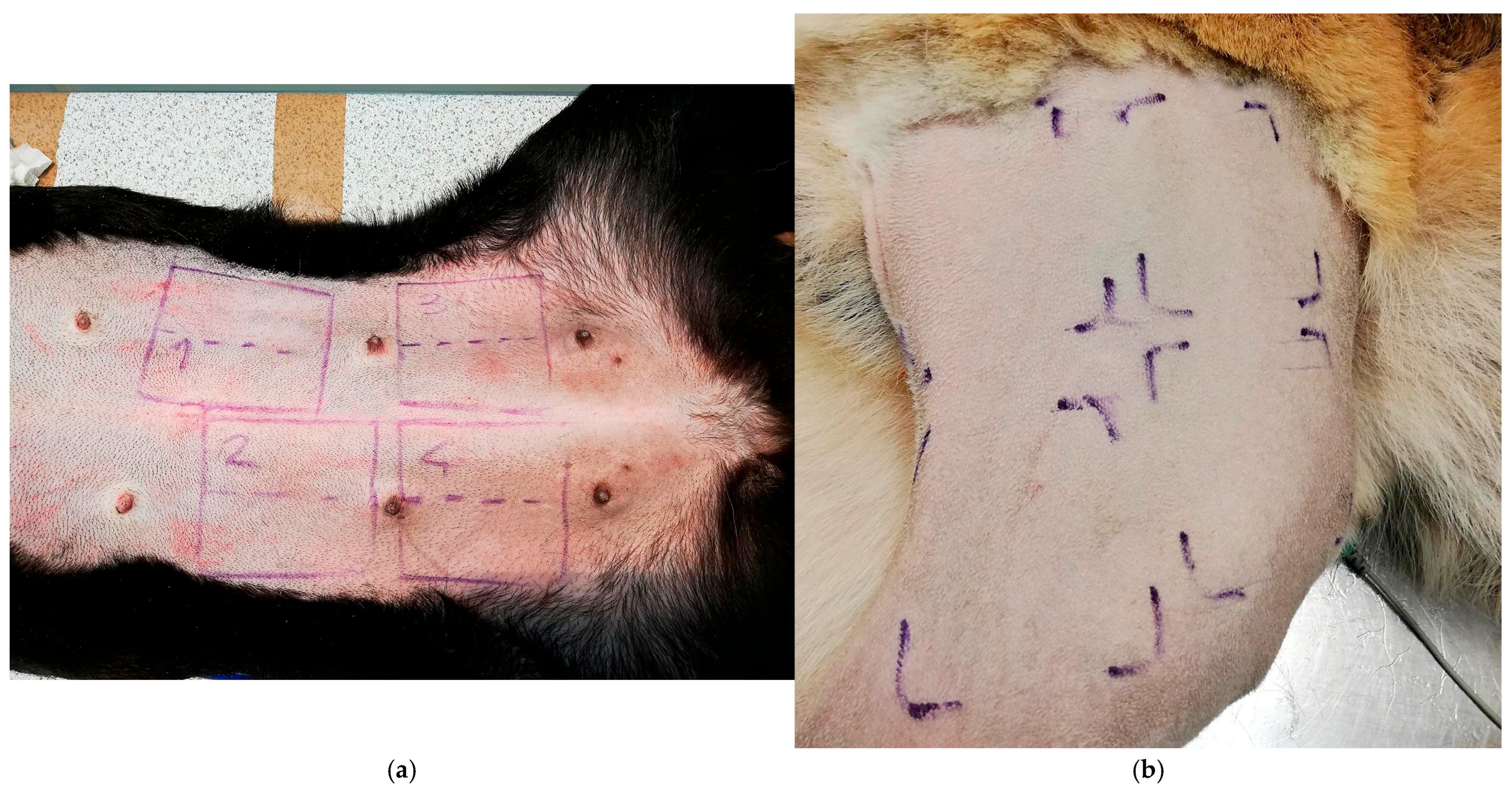
| Primary Sampling Area | Antiseptic Treatment | First Sampling (90”) | Second Sampling (180”) |
|---|---|---|---|
| Control | - | - | - |
| Area 1 6 × 6 cm | Soap-based chlorhexidine + alcoholic chlorhexidine | Subzone 1 “P1” 3 × 6 cm | Subzone 1 “S1” 3 × 6 cm |
| Area 2 6 × 6 cm | Soap-based chlorhexidine + ozonated water (20 µg/mL) | Subzone 2 “P2” 3 × 6 cm | Subzone 2 “S2” 3 × 6 cm |
| Area 3 6 × 6 cm | Ethyl alcohol + ozonated water (20 µg/mL) | Subzone 3 “P3” 3 × 6 cm | Subzone 3 “S3” 3 × 6 cm |
| Area 4 6 × 6 cm | Ozonated water (20 µg/mL) | Subzone 4 “P4” 3 × 6 cm | Subzone 4 “S4” 3 × 6 cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melanie, P.; Niola, C.; Guerrini, F.; Pareto, N.; Mancini, S.; Fratini, F. Evaluation of the Preoperative Antiseptic Efficacy of Ozone on Dog Skin in Comparison with Traditional Methods. Vet. Sci. 2025, 12, 843. https://doi.org/10.3390/vetsci12090843
Melanie P, Niola C, Guerrini F, Pareto N, Mancini S, Fratini F. Evaluation of the Preoperative Antiseptic Efficacy of Ozone on Dog Skin in Comparison with Traditional Methods. Veterinary Sciences. 2025; 12(9):843. https://doi.org/10.3390/vetsci12090843
Chicago/Turabian StyleMelanie, Pierre, Carlotta Niola, Federico Guerrini, Nicolò Pareto, Simone Mancini, and Filippo Fratini. 2025. "Evaluation of the Preoperative Antiseptic Efficacy of Ozone on Dog Skin in Comparison with Traditional Methods" Veterinary Sciences 12, no. 9: 843. https://doi.org/10.3390/vetsci12090843
APA StyleMelanie, P., Niola, C., Guerrini, F., Pareto, N., Mancini, S., & Fratini, F. (2025). Evaluation of the Preoperative Antiseptic Efficacy of Ozone on Dog Skin in Comparison with Traditional Methods. Veterinary Sciences, 12(9), 843. https://doi.org/10.3390/vetsci12090843











