Simple Summary
Microalgae such as Spirulina platensis and essential oils derived from Citrus limon have potential growth-promoting, antimicrobial, antioxidant, and immunostimulant effects for several fish species. This manuscript spotlights Spirulina platensis and Citrus limon essential oil and their roles in improving the performance and health of Nile tilapia. Our results indicate that Spirulina platensis and/or lemon essential oil can be used as natural feed additives during aquafeed formulation to improve fish welfare through nutritional management.
Abstract
The dietary presence of feed additives is crucial for boosting fish growth and immunity. Accordingly, this feeding trial aimed to investigate the effects of the separate and concurrent dietary supplementation of Spirulina platensis (SP) and bitter lemon (Citrus limon) peel essential oil (LEO) on the growth, immunity, antioxidant capacity, and intestinal health of Nile tilapia (Oreochromis niloticus). Four groups of male Nile tilapia were employed. The first group (control) was given the basal diet, while the second and third groups received the basal diet supplemented with LEO extract (1%) and SP (1 g/kg diet), respectively. The fourth group received the basal diet supplemented with a mix of LEO (1%) and SP at 1 g/kg. After two months of feeding, using LEO or/and SP improved the overall growth and immunological parameters, with their combination yielding the best outcomes. The supplementation of LEO or/and SP improved the Nile tilapia’s growth metrics and transcriptomic levels of growth-regulating genes such as (oligo-peptide transporter 1 (Pep1), growth hormone receptors 1 (GHR1), and insulin-like growth factor (IGF1). The improved growth performance was linked to significant increases in the expression levels of mucin and fat metabolism-related genes. Moreover, fish supplemented with LEO, SP, or their combination showed enhanced non-specific immunological measures, including phagocytic and lysozyme activities and the mRNA copies of its regulating genes. Additionally, remarkable increases in the antioxidant enzyme activities and the mRNA levels of their related genes were detected. The complement (C3) gene’s transcriptomic level was also significantly increased. Furthermore, the dietary supplementation of LEO, SP, or their combination improved the histological structures of the spleen, hepatopancreas, and intestine. The enhanced effects of LEO, SP, or their combination on fish immunity and growth are suggested to be due to their contents of bioactive compounds with anti-inflammatory, antioxidant, and antimicrobial properties. Thus, using the LOE and SP blends as feed additives is recommended for better growth and immunity of Nile tilapia.
1. Introduction
Feed additives provide cheap, healthy, eco-friendly alternatives to antibiotics, chemical immune stimulants, and growth promoters [1]. Feed additives include prebiotics, probiotics, phytogenics, or a mix of two types [1]. Most of these additives are rich in bioactive elements, which have a wide range of biological activities such as growth-enhancing, immune-stimulating, antioxidant, and antimicrobial activities [2,3,4]. Recently, the application of several phytogenic products in aquaculture has been recommended as safe and highly effective growth and immune stimulants [5,6,7], with plant-derived essential oils (OS) being used as effective nutritional feed additives in animal and aquaculture sectors [8].
D-limonene is the main component in the citrus that naturally occurs in the peel of citrus fruits, such as oranges or lemons [9]. It is listed, by federal regulations, as generally recognized as safe (GRAS), with a very low toxicity [10]. Limonene has well-known immune-stimulant, anti-inflammatory, and antioxidant impacts in humans and animals [11]. Dietary supplementation of lemon essential oil extracts (LEOs) could improve fish growth performance and intestinal health. Additionally, Nile tilapia fed limonene supplements showed higher weight gains, enhancing hepatic Insulin growth factor-I (igf-1) gene expression [12].
Moreover, it improved intestinal health and nutrient utilization, confirmed by increasing the villi length and goblet cell number and upregulating the expression levels of intestinal health-related genes [12,13]. Additionally, limonene enhances fish’s antioxidant capacity by increasing the mRNA levels of catalase (CAT) and superoxide dismutase (SOD), along with reinforcing the phagocytic and lysozyme activities [14], which protect the cells from toxins and lipid peroxidation (malondialdehyde formation) [15,16]. The limonene in citrus fruit also has strong antibacterial activities against several pathogenic bacterial species, such as Campylobacter jejuni and Staphylococcus aureus [17,18,19].
Microalgae such as Spirulina platensis (SP) are another feed additive that have been recently used in the aquaculture sector due to their highly precious nutritional value [20]. SP has received significant attention in aquaculture as a valuable alternative source of protein instead of those of animal origin [21]. Spirulina protein matches the good-quality reference protein recommended by FAO, as it has a high protein content (70%) in its dry weight and is rich in essential amino acids [22,23,24]. SP dietary supplementation improves the quality of meat fat, as it is richer in polyunsaturated fatty acids than monounsaturated fatty acids [25,26]. Also, dietary supplementation of SP improved many fish species’ growth performance and health status [27,28]. For Nile tilapia, SP improved the growth rate, feed conversion, feed utilization, digestive enzymes, and intestinal health [29,30,31]. It has a strong antioxidant action because of its precious contents of β-carotene, phycocyanin, and tocopherols [32,33,34]. Moreover, it has an optimal level of immune stimulants, minerals, and vitamins, which boost white blood cells (WBCs), phagocytosis, and lysozyme activity [35,36]. SP also improves fish health and water quality by adsorbing heavy metals and nitrites.
The separate dietary supplementation of LEO or SP has been extensively studied, but their synergetic effects, to our knowledge, have yet to be explored. Thus, in this study, we hypothesized that the combined supplementation of both LEO and SP could be more economically effective and induce more effects on Nile tilapia growth performance and immunity. Therefore, this study aimed to evaluate the effects of separate and concurrent dietary supplementation of LEO and SP on Nile tilapia’s growth performance, immune and antioxidant responses, and the transcriptomic profile of growth-, immunity-, antioxidant-, intestinal health-, and fat metabolism-regulating genes.
2. Materials and Methods
2.1. Ethics Committee Approval
This experimental study was approved by the Institutional Animal Care and Use Committee (IACUC), Kafrelsheikh University, Egypt (Approval number KFS-IACUC/112/2023). All methods were carried out according to the relevant guidelines and regulations of the KFS-IACUC. This study was conducted according to ARRIVE guidelines.
2.2. Fish Management
A total of 120 healthy mono-sex (male) Nile tilapia (Oreochromis niloticus) fingerlings (average weight 8.00 ± 0.17 g) were collected from a local farm in Kafrelsheikh governorate, Egypt. The fish were kept in glass aquaria (45 cm width × 55 cm length × 23.5 cm height), previously filled with dechlorinated tap water, and half of the water was exchanged daily. The aquaria were supplied with aeration and mechanical filters to remove waste. The water temperature was maintained at 24.7 ± 2.1 °C and pH 7.7–8.6. The fish were kept for adaptation for two weeks, feeding them a commercial tilapia diet (Table 1). Feeding was performed twice daily at a rate of 3% of the fish’s body weight.

Table 1.
Composition and chemical analysis of the commercial diet used in the experiments (on a dry matter basis).
2.3. Feeding and Experimental Design
The fish were randomly allocated into four groups with three replicates each (10 fish per replicate in each aquarium). The first group was the control group, which was kept on the basal diet (floating feed produced by Aller-Aqua Company, Giza, Egypt) until the end of the experiment. The chemical composition of the basal diet is shown in Table 1. The second group received the basal diet (BD) supplemented with lemon essential oil (LEO) extracts at a 1% concentration, according to Mohamed et al. [13]. The third group was fed BD supplemented with SP at 1 g/kg, according to Al-Deriny et al. [29]. The fourth group was fed BD containing a combination of LEO (1%) and SP at 1 g/kg. The feeding trials continued for two months.
Spirulina and/or LEO were thoroughly mixed with sunflower oil (20 mL/kg diet) and then gently mixed with the feed pellets. The control group’s diet was combined with the same amount of sunflower oil. Finally, the feeds were sealed in vacuum-packed bags and placed into a freezer (−20 °C).
2.4. Growth Performance
At the end of the experimental period, the fish were harvested using a suitable net and anesthetized using clove oil (Merck, Darmstadt, Germany) at 50 μL per liter of water [13]. The fish were weighed individually to obtain the final weight of each fish. The fish growth performance and feed utilization were calculated according to El-Kassas et al. [5]. The body weight gain, specific growth rate, feed conversion ratio, and hepato-somatic index were calculated using the following equations:
Body weight gain (BWG) = final body weight (W1)/g − initial body weight (W0)/g
Specific growth rate (SGR %/day) = 100 × (lnW1 − lnW0)/t
Feed conversion ratio (FCR) = feed intake (g)/BWG (g)
Hepato-somatic index (HSI) = 100 × (liver weight/W1)
2.5. Sampling
At the end of the feeding trial, blood samples were collected from 6 fish/treatment (2 fish/replicate). Two blood samples were collected from the caudal vein of each fish. One sample was collected on heparin as an anticoagulant for hematological analysis. Another blood sample was collected without an anticoagulant for serum separation. Liver and fore-intestine tissue (proximal part) samples were collected on liquid nitrogen and kept at −80 °C for RNA extraction. Other specimens were collected from the intestine (anterior, middle, and posterior segments), liver, and spleen in Bouin’s solution for histomorphological examination.
2.6. Hematological and Immunological Parameter Analysis
Diluted blood samples (with Natt and Herrick’s solution) were used to determine white blood cell (WBC) and red blood cell (RBC) counts. The hemoglobin concentration was measured using the cyanomethemoglobin method using Drabkin’s solution. The microhematocrit method was used to determine the packed cell volume (PCV). For the differential leucocyte count, a blood film for each sample was examined using a computer-assisted light microscope with a 100× oil immersion lens [37].
The phagocytic activity (PA) of the leucocytes to Candida albicans was measured in the heparinized blood samples following the methods of Kawahara et al. [38]. The PA was assessed as the percentage of phagocytic cells that engulfed the yeast cells, while the phagocytic index (PI) was calculated by dividing the total number of phagocytized yeast cells by the number of phagocytic cells. Serum lysozyme activity was analyzed using the method described by Abo-Al-Ela et al. [39]. Measurements of the serum lysozyme activity (LZM) depended on comparing the ability of the lysozyme to digest the bacteria cells (Micrococcus lysodeikticus) present in 1% agarose gel, giving a clear lysed zone against the lysed zone in a standard plate containing a hen egg-white lysozyme solution of 20 mg/mL.
2.7. Serum Biochemical Measurements
The serum total protein and albumin levels were measured using kits from Bio-Diagnostic Co., Dokki, Giza, Egypt, at wavelengths of 550 nm and 630 nm, respectively, according to Doumas et al. [40]. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured at a 540 nm wavelength [41]. Serum cholesterol (CHO) and triglycerides (TGs) were measured using kits from Bio-Diagnostic Co. according to the manufacturer’s instructions. Glucose levels were determined using commercially available kits, according to Ozdemir et al. [42].
2.8. Serum Antioxidant Enzyme Activity and MDA Concentration Assessment
The activities of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPX) and the malondialdehyde (MDA) concentrations were assessed using specific kits (Biodiagnostic, Co., Dokki, Giza, Egypt). The activity of each enzyme was measured using a UV–vis spectrophotometer at a particular wavelength [43,44,45].
2.9. Histomorphological Features of Intestine, Liver, and Spleen
The specimens collected from the intestine (anterior, middle, and posterior segments), liver, and spleen were cut into pieces of approximately 0.5 cm3 and fixed in Bouin’s solution for 18–24 h. Then, the fixed samples were dehydrated in ascending grades of alcohol, cleared with xylene, and embedded in paraffin wax. Then, 5 μm thick sections were obtained using a rotatory microtome (Leica Rotary Microtome, RM 2145, Leica Microsystems, Wetzlar, Germany) and stained with hematoxylin and eosin stain for histological investigation according to Suvarna et al. [46].
2.10. Total RNA Extraction and cDNA Synthesis
The total RNA was extracted from liver and intestinal tissues. Accordingly, a fixed weight of about 50 mg of liver tissue samples was homogenized in phosphate-buffered saline (PBS) and used for total RNA extraction. PBS was used to facilitate the mechanical tissue disruption [47]. For RNA extraction, an intestinal specimen was first ground in a sterile mortar with liquid nitrogen; then, the total RNA was extracted using Trizol (Applied Biotechnology, Giza, Egypt) according to the manufacturer’s manual. The quality of isolated RNA was assessed using 2% ethidium bromide-stained agarose gel electrophoresis. The RNA quantity was analyzed using nanodrop. Two micrograms of the RNA were used for the complementary DNA (cDNA) synthesis using a Thermo-Scientific-Revert-Aid—Frist strand cDNA Synthesis Kit.
2.11. Relative Gene Expression Using qPCR
Real-time PCR (qPCR) was used to evaluate the relative expression levels of growth-related genes [Growth hormone receptors 1 (Ghr1) and Insulin-like growth factor 1 (Igf-1)], fat metabolism-related genes [Fatty acid synthesis (Fas), Lipoprotein lipase (Lpl), Fatty acid transport, fatty acid binding protein 3 (Fabp3)], and cluster of differentiation 36 (CD36). The antioxidants Superoxide dismutase (Sod) and Catalase (Cat) and innate immune response-related genes Lysozyme (Lzm) and Complement (C3) were also evaluated in the liver tissue. Conversely, the relative expression of nutrient absorption and the transporter genes Mucin-like protein (Muc) and Oligo-peptide transporter 1 (Pept1) were analyzed in the intestine. The data were normalized against two housekeeping genes, Beta-actin (β-actin) and Elongation factor-1α (Ef-1α). The preparation of the reaction mixture and the conditions were performed according to Abdo et al. [4]. The specific annealing temperatures and primer sequences for each gene are listed in Table 2. The relative expression levels as fold-changes were calculated based on the 2−ΔΔCt according to the method of Livak and Schmittgen [48].

Table 2.
Primers used in this study.
2.12. Statistical Analysis
All data are presented as means ± SEM. A one-way Analysis of Variance (ANOVA) with Duncan’s multiple comparisons test was used to analyze differences among treatments and the control group. All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad® Software Inc., San Diego, CA, USA). A p < 0.05 was considered significant.
3. Results
3.1. Dietary Supplementation of LEO, SP, and Their Mixture Significantly Modified the Growth Performance of Nile Tilapia
The dietary supplementation of LEO, SP, and their mixture significantly increased the final body weights of Nile tilapia compared to the CG (p < 0.05) (Table 3).

Table 3.
Growth performance of Nile tilapia after two-month dietary supplementation of bitter lemon (Citrus limon) peel essential oil, Spirulina, and their mixture.
The effects of the mixture were non-significantly better than those of the separate administration of LEO and SP. The improved final body weights were linked with apparent increases in the body gains and the feed intake, which decreased the FCR (p < 0.05). Moreover, the fish’s body length and SGR displayed marked increases with the dietary supplementation of LEO, SP, and their mixture compared to the control (p < 0.05). However, the weights of the internal organs, liver, intestine, and HSI were not altered by the dietary administration of LEO, SP, or their mixture.
3.2. LEO, SP, and Their Combination Improved the Antioxidant and Non-Specific Immune Responses
Table 4 shows the effects of the dietary supplementation of LEO, SP, and their mixture on antioxidant enzyme concentration, phagocytic activity, index, and lysozyme activity.

Table 4.
Antioxidant enzyme concentrations, phagocytic response, and lysozyme activity of Nile tilapia after two-month dietary supplementation of bitter lemon (Citrus limon) peel essential oil, Spirulina, and their mixture.
Dietary supplementation of LEO, SP, and their mixture to the Nile tilapia’s diet significantly increased the SOD concentration compared to the basal diet, with the highest concentrations found in the case of the SP and LEO + SP mixture (p < 0.05). Similarly, dietary supplementation of LEO, SP, and their mixture caused marked increases in GPx concentration compared to the basal diet (p < 0.05). The dietary combination of both LEO and SP exhibited the highest GPx concentration compared to the control group and the LEO or SP alone (p < 0.05). Increasing the SOD and GPX levels because of LEO, SP, and their mixture was associated with a significant reduction in MDA concentration (p < 0.05).
Moreover, the dietary addition of LEO, SP, and their mixture distinctly altered PA, with the highest activities reported in the case of both the LEO and SP mixture, followed by LEO alone compared to the basal diet and SP (p < 0.05). LZM activity was also influenced by the dietary addition of LEO, SP, and their combination. The highest activities were noticed in the case of the LEO/SP mixture and LEO alone, followed by SP compared to a non-supplemented basal diet (p < 0.05).
3.3. The Effects of LEO, SP, and Their Combination on Nile Tilapia Hematological Response and the Serum Biochemical Constituents
Dietary supplementation of LEO, SP, and their combination significantly elevated the RBC count, hemoglobin (Hb) %, and packed cell volume (PCV) compared to the basal diet (p < 0.05) (Table 5).

Table 5.
Hematological profile of Nile tilapia after two-month dietary supplementation of bitter lemon (Citrus limon) peel essential oil, Spirulina, and their mixture.
In this context, Nile tilapias supplemented with LEO displayed the highest response compared to the other supplemented groups (p < 0.05). Likewise, the WBC, heterophils, and lymphocyte counts were significantly increased with adding LEO, SP, and their combination to the diet (p < 0.05). Significant increases in WBC and lymphocytes were noticed in the case of LEO, SP, and their combination compared to the control group (p < 0.05). However, a different response of heterophils was reported. LEO, SP, and their combination markedly lowered the heterophil count (p < 0.05). The later response significantly lowered the H/L ratio in tilapias supplemented with LEO, SP, and their combination compared with the un-supplemented tilapias.
The serum biochemical constituents of the Nile tilapia were analyzed following LEO, SP, and their combination (Table 6).

Table 6.
Biochemical analysis of Nile tilapia after two-month dietary supplementation of bitter lemon (Citrus limon) peel essential oil, Spirulina, and their mixture.
Adding LEO and SP, separately or combined, to the Nile tilapia’s diet did not alter the biochemical profile. Only non-significant increases and decreases in TG and cholesterol levels were found, respectively. In addition, non-significant increases in the total protein and albumin concentrations were measured.
3.4. Histological Features of Intestine, Spleen, and Hepatopancreas of Nile Tilapia Supplemented with LEO, SP, and Their Combination
The histological appearance of the control fish intestine showed intact layers of the intestinal wall (mucosa, propria sub-mucosa, muscularis, and serosa) and intestinal villi (Figure 1).
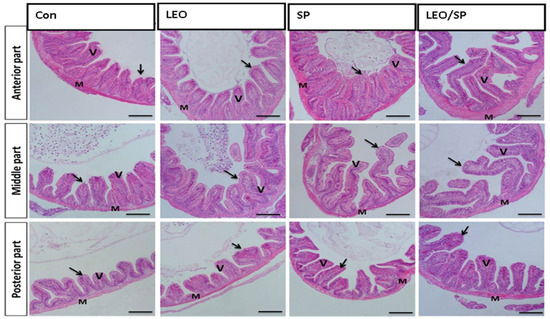
Figure 1.
Histological features of the intestinal wall (mucosa, propria sub-mucosa, muscularis, and serosa) and intestinal villi of Nile tilapia supplemented with LEO, SP, and their combination. LEO: lemon essential oil, SP: Spirulina platensis. Arrow: lining enterocytes with goblet cells. V: intestinal villi. M: intestinal wall.
The intestinal wall demonstrated a normal histomorphological appearance, where the intestinal villi and associated crypt appeared intact without any deterioration. The enterocytes lining the intestinal villi were arranged correctly. Furthermore, there was a noteworthy improvement in the construction of intestinal villi in the groups subjected to LEO and/or SP (Table 7). Accordingly, all treated groups exhibited significant increases in intestinal villi length and width and crept depth compared with the non-supplemented group (p < 0.05). Among the supplemented tilapia, those receiving LEO/SP consistently showed the most extensive length of intestinal villi, width, and crept depth (p < 0.05).

Table 7.
Intestinal morphometry of Nile tilapia after two-month dietary supplementation of bitter lemon (Citrus limon) peel essential oil, Spirulina, and their mixture.
The architecture of the hepatopancreas in the control fish showed a regular appearance of hepatocytes, with large vesicular nuclei that were separated by the blood sinusoid lined by endothelial cells. LEO and/or SP supplementation improved the hepatic condition by increasing glycogen deposition within the hepatocytes, which was more evident in the group supplemented with LEO and SP. The pancreatic acini appeared normal (Figure 2).
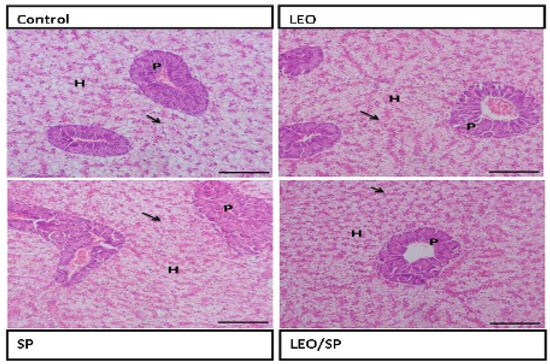
Figure 2.
Histological features of hepatopancreas of Nile tilapia supplemented with LEO, SP, and their combination. LEO: lemon essential oil. SP: Spirulina platensis. H: hepatocytes. P: pancreatic acini. Arrow: glycogen deposition.
The histological structure of the spleen displayed regular white and red pulps (Figure 3).
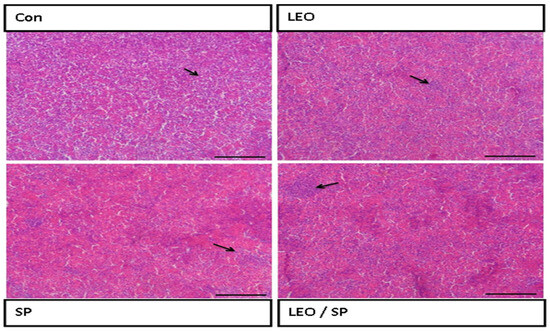
Figure 3.
Histological features of the spleen of Nile tilapia supplemented with LEO, SP, and their combination. LEO: lemon essential oil. SP: Spirulina platensis. Arrow: lymphocytic aggregation in the white pulp.
Adding LEO and/or SP to the treated groups improved the histological structure of the spleen, with increased lymphocyte infiltration (white pulp).
3.5. The Effects of Dietary Supplementation of LEO, SP, and Their Combination on the Expression Levels of Growth, Antioxidant, and Fat Metabolism-Regulating Genes
Including both the SP and LEO/SP mixture in the Nile tilapia diet significantly upregulated mRNA levels of the GHR gene compared to tilapias fed the basal diet (p < 0.05) (Figure 4A). However, LEO supplementation did not alter GHR transcriptomic levels compared to the basal diet. Additionally, no marked differences were observed between the LEO and SP groups when supplemented separately, but there were significant differences between the LEO/SP mixture and LEO alone (p < 0.05). Likewise, adding LEO, SP, and their mixture to the Nile tilapia diet distinctly modified IGF-1 mRNA levels (p < 0.05) (Figure 4B). Significant increases in IGF-1 mRNA copies were measured in cases of LEO, SP, and their combination compared with the basal diet (p < 0.05). Additionally, despite being similar, the effects of either SP alone or the LEO/SP mixture were significantly higher than for LEO alone (p < 0.05).
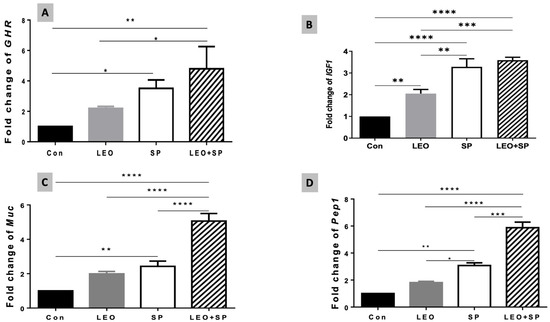
Figure 4.
The effects of dietary supplementation of LEO, SP, and their combination on the expression levels of growth-related genes. (A) Ghr1: growth hormone receptor 1, (B) Igf-1: insulin-like growth factor 1, (C) Muc: mucin-like protein, (D) Pept1: oligo-peptide transporter 1. LEO: lemon essential oil, SP: Spirulina platensis. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, respectively.
The relative expression levels of mucin-like protein (muc) were also altered with the dietary addition of SP, LEO, and their mixture (Figure 4C, p < 0.05). In this regard, both SP only and the LEO/SP mixture, compared to the non-supplemented group and LEO, significantly upregulated the expression levels of the muc gene (p < 0.05). Additionally, the LEO/SP mixture induced the highest levels among all treatments and the control (p < 0.05).
Similarly, Pep1 expression levels were modulated with dietary supplementation of SP, LEO, and their combination (Figure 4D, p < 0.05). Prominent levels of Pep1 were found in the case of SP and the LEO/SP mixture (p < 0.05). Moreover, the effects of SP and the LEO/SP mixture were significantly higher than LEO and the control (p < 0.05).
The expression levels of C3 were also modified following dietary supplementation of SP, LEO, and their combination (Figure 5A). Both SP and the mixture of the two supplements distinctly increased C3 mRNA levels compared to the LEO and the control (p < 0.05). Additionally, LEO alone and its mixture with SP induced significantly higher expression levels of the lysozyme gene (LZM) in comparison to the control group (Figure 5B, p < 0.05). The combination of LEO and SP displayed the highest LZM expression levels compared to the other treatments (p < 0.05).
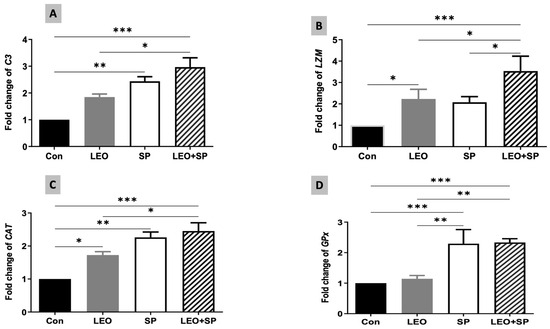
Figure 5.
The effects of dietary supplementation of LEO, SP, and their combination on the expression levels of immune and antioxidant genes. (A) C3: complement, (B) LZM: lysozyme, (C) CAT: catalase, (D) GPX: glutathione peroxidase. LEO: lemon essential oil, SP: Spirulina platensis. * p < 0.05, ** p < 0.01, *** p < 0.001, respectively.
CAT and GPx expression levels were also modified due to the dietary supplementation of SP, LEO, and their combination (Figure 5C,D). For CAT, all the supplements (SP, LEO, and their mixture) increased its expression levels compared to the un-supplemented group (p < 0.05). The effects of the mix were significantly higher than that of LEO alone (p < 0.05). Furthermore, SP and LEO/SP significantly upregulated the expression levels of GPx compared with LEO and the control (p < 0.05).
The relative expression levels of some fat metabolism-regulating genes were modulated by supplementing SP, LEO, and their combination in the Nile tilapia diet (Figure 6). Adding LEO and the LEO/SP mixture to the diet significantly increased the mRNA level of FAS compared to the control and SP (Figure 6). The LEO/SP group displayed the highest FAS mRNA levels among all the groups (p < 0.05). In addition, the LPL expression levels (Figure 6) were upregulated following the dietary supplementation of SP, LEO, and their mixture compared to the control group fed the basal diet (p < 0.05). Yet again, the effects of the LEO and SP mixture (LEO/SP) were the highest among all the groups (p < 0.05). Similarly, the FABP mRNA copies (Figure 6) significantly increased when tilapias were fed the diet supplemented with SP, LEO, and their mixture (p < 0.05). The LEO/SP group had the highest FABP mRNA copies among all the treatments (p < 0.05). Furthermore, mRNA expression levels of CD36 exhibited a similar behavior because of SP, LEO, and their combination (Figure 6). Distinctly higher mRNA copies of CD36 were found in all the supplemented groups compared to the control (p < 0.05). Similarly, LEO/SP had a prominent effect compared to the control and LEO group (p < 0.05).
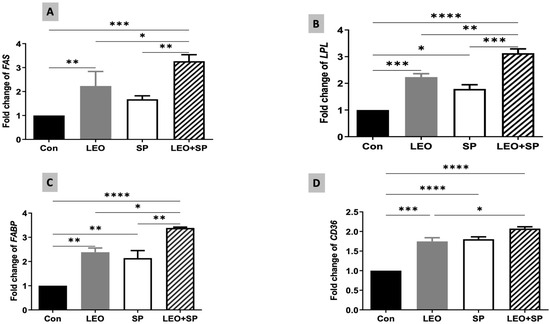
Figure 6.
The effects of dietary supplementation of LEO, SP, and their combination on the expression levels of fat metabolism-regulating genes. (A) FAS: fatty acid synthesis, (B) LPL: lipoprotein lipase, (C) FABP3: fatty acid binding protein 3, (D) CD36: cluster of differentiation 36. LEO: lemon essential oil, SP: Spirulina platensis. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, respectively.
4. Discussion
Using nutritive, non-toxic feed additives in the fish sector has gained global attention recently [54,55]. Most of the studied natural additives modulate fish performance and metabolic pathways [56,57] and enhance the fish’s immune and antioxidant status under normal and stressful conditions because of their bioactive elements [2,58,59]. These additives include phytobiotics, probiotics, and prebiotics. Combining two distinct kinds of these supplements results in more advantageous synergistic effects [4,29]. Accordingly, this study reported the most effective outcomes by combining both LEO and SP as dietary supplements, confirming their synergetic effects on Nile tilapia growth performance, immunity, and antioxidant status.
The dietary supplementation of SP (at 1%) significantly improved final body weights, body gains, SGR, and the FCR. These effects were correlated with increases in hepatic GHR and IGF-1 gene transcriptomic levels. The improved growth performance in the case of SP-supplemented Nile tilapia might be linked to its highly nutritive bioactive components, such as vitamins (vitamin A and vitamin B complex) and minerals including iron, potassium, and magnesium [60]. These results agree with the findings of Rosas et al. [61], who considered SP to be one of the most recommended candidates to replace fish meal. Additionally, Nile tilapia supplemented with SP up to 15% exhibited a significantly enhanced growth rate and Igf-1 gene expression levels [62]. Additionally, several previous studies have suggested that the dietary inclusion of a small amount of SP (1 to 5%) modifies the fish’s growth and health [35,63,64].
Nile tilapia fed LEO-supplemented diet displayed slightly enhanced BWG and SGR compared to the control group, with a decreased FCR. Moreover, LEO supplementation modulated the mRNA levels of growth-related genes; it distinctly up-regulated the Igf-1 mRNA levels. These findings agree with the conclusions from Elsayed, Salem, and Toutou et al. [65,66], who reported that dietary supplementation of limonene from different citrus fruits could improve fish’s growth parameters and feed utilization. The beneficial effects of the essential oils (EOs) extracted from citrus fruits to aqua feed may be because of their rich contents of nutritional components [67,68]. Similarly, LEO dietary inclusion of up to 5% enhanced the growth performance indices in several fish species [19,69]. Furthermore, Nile tilapia supplemented with LEO exhibited enhanced Igf-1 gene expression levels [12,13]. Interestingly, the best growth performance was reported for the Nile tilapias supplemented with LEO and SP, indicating their synergistic effects.
The effects of the separate dietary presence of SP or LEO on growth performance have been explored in other fish species. For example, Zhang et al. demonstrated that Micropterus salmoides fed different concentrations of SP showed significantly enhanced growth performance, body crude protein, muscle amino acid, and protein efficiency [70]. Moreover, common carp (Cyprinus carpio) supplemented with SP (30 g/kg) alone or mixed with 0.5 g/kg citric acid showed enhanced growth and immunity [71]. The supplementation of citrus lemon extract to catfish juveniles (Pangasius hypophthalmus) improved their growth, hematological, and innate immunity parameters and enhanced their bacterial resistance [72]. Additionally, common carp receiving 200 mg/kg of dietary limonene exhibited improved feed efficiency and increased innate immune response and resistance against A. hydrophila [73]. Also, limonene supplementation enhanced the antioxidant and immune response of silver catfish challenged with A. hydrophila as well as their hepatic histological structure and the Igf1 mRNA levels [74]. The improved growth performance and health status due to these LEO and/or SP diets might be correlated with increased digestive enzyme levels (confirmed by increasing the expression of Muc and Pep1 genes) and improved construction of intestinal villi, which facilitates nutrient absorption and subsequently improves growth performance metrics [75,76,77].
Including LEO or SP in the Nile tilapia diet increased the mRNA levels of some fat metabolism-regulatory genes, such as Fas, Lpl, Fabp, and Cd36, with the highest expression levels reported in the case of the LEO/SP mixture. However, the LEO and/or SP combination did not alter the biochemical profile of triglycerides and only induced a slight decrease in cholesterol levels. The triglyceride concentration depends on the level of their biosynthesis and lipolysis, regulated by a series of enzymes. LPL is the main enzyme in triglyceride lipolysis into glycerol and free fatty acids [78]. On the other hand, fatty acid synthesis is regulated by several enzymes, such as FAS, ACC, and acyl-CoA synthetase [79].
Moreover, FA binding proteins (FABPs) and FA translocase (CD36) regulate the fatty acids’ uptake and intracellular transport [80]. In the present study, the separate supplementation of LEO or its mixture with SP did not alter serum TG levels; this could be due to increased Lpl gene expression. Additionally, there was an upregulation in Fas expression, which is unnecessary to increase the body’s triglyceride levels. FAS traditionally catalyzes the de novo synthesis of fatty acids. However, the dietary fat contents affect the regulation of the gene transcription levels and enzyme activities. The de novo synthesis of fatty acids by FAS may contribute to storing energy when the diet is rich in nutrients, especially fats and carbohydrates. However, the secreted triglycerides due to FAS appear negligible compared to other sources of fats under common dietary conditions [79]. These effects may be linked with the improved growth performance in the case of the LEO/SP combination by regulating the energy available from dietary lipids, which is one of the possible growth-stimulatory mechanisms. Accordingly, Nile tilapia supplemented with moringa oleifera leaves and lecithin displayed alteration in fat metabolism, as confirmed by lowered serum cholesterol and triglycerides levels with upregulated FAS and LPL mRNA levels, correlated with improved growth performance [5,50].
Previous studies have reported the essential roles of limonene and SP supplementation in fat metabolism. Accordingly, dietary limonene significantly upregulated the essential enzymes associated with LPL and alkaline phosphatase activities [12]. Additionally, the availability of free fatty acids significantly affects fish growth and innate immunity [81,82,83]. The effects of spirulina are perhaps due to its rich, unique composition of fatty acids and polyunsaturated fatty acids. Similarly, LDL–cholesterol was significantly decreased with SP inclusion (10%) in rainbow trout [84]. SP modulated the harmful effects of hypercholesterolemia, as it lowered the plasma cholesterol and triglyceride levels [85,86].
Intestinal health is another crucial factor regulating fish growth and feed utilization [87]. Enhancing intestinal villi integrity and density improves growth rate because they are the site of absorption and nutrient uptake [88]. Our results demonstrated that LEO and/or SP supplementation maintained the regular appearance of the intestinal wall, where the intestinal villi and associated crypts appeared intact without any deterioration. Furthermore, there was a noteworthy improvement in the construction of intestinal villi. The gastrointestinal tract’s digestive function and health correlate with mucus layer thickness [89], crucial in the intestinal tract’s innate defense and protection [90]. The mucus facilitates nutrient transportation through the gut wall [91]. Also, protein nutrient transporters, such as oligo-peptide transporter I (Pept1), depend on mucus as a medium for active peptide transport [92].
Goblet cell mucus secretion is controlled by mucin-like protein genes such as Muc2 [90]. In the current study, SP or LEO supplementation significantly upregulated Muc and Pep1 gene expression levels, and the highest expressions were reported in the case of the LEO and SP mixture. Several herbals improve gut nutrient digestion and utilization by upregulating mucin expression [91]. For example, orange essential oil (OEO) and LEO improved Nile tilapia growth by improving intestinal villi length, inter-villi space, and the number of goblet cells [13]. Aanyu et al. suggested that the enhanced Nile tilapia weight gain in response to limonene inclusion was due to improved protein absorption and increased mucus secretion, with significant up-regulation of Muc and Pep1 genes [12]. Citrus EOs also motivated the secretion of digestive enzymes such as trypsin, amylase, amino peptidases, and alkaline phosphatase, thus improving feed utilization [93]. They also could increase the levels of beneficial gut microbes compared to pathogenic bacteria, facilitating nutrient absorption [76,94,95]. These improvements may be due to the valuable role of citrus essential oils (CEOs), which consist of some major biologically active compounds like α-/β-pinene, sabinene, d-limonene, β-myrcene, α-humulene, linalool, and α-terpineol belonging to the monoterpenes, aldehyde/alcohol, monoterpene, and sesquiterpenes group. These compounds possess anti-inflammatory, antioxidant, anticancer, and antimicrobial properties, with immense potential for food applications [96].
Similarly, SP could enhance fish growth by improving intestinal health. In this context, rainbow trout fed 5% SP showed higher intestinal villus height, absorption surface area, goblet cell numbers, and intraepithelial lymphocytes than the non-supplemented group [97]. Also, including a selenium-enriched SP diet (10%) significantly increased the number of intestinal goblet cells [98]. Nile tilapia supplemented with SP (1%) exhibited increased villi length, mucosal length, and goblet cell numbers [29]. The diet significantly increased digestive enzyme activities such as protease, amylase, and lipase, thus improving nutrient digestion and absorption [35,99].
Blood parameters could give a reliable indication of fish growth and health [100]. Fish growth, metabolic rate, and immune status affect RBC count and Hb [101]. Our results showed that SP significantly enhanced the measured hematological parameters. Moreover, adding LEO resulted in the highest RBC count, Hb%, and PCV compared to the basal diet and the other groups. Likewise, hematological parameters such as RBC, Hb, and mean corpuscular hemoglobin concentration (MCHC) were significantly increased in rainbow trout supplemented with D-limonene [14].
Moreover, the RBC count was proportionally increased by adding citrus EO up to 5% [19]. Generally, the hematological response to limonene supplementation could differ according to the source of the extract and the fish species [69,102]. However, limonene did not negatively impact fish hematology [103]. SP also improved RBC count and hematological parameters in several fish species [84,104,105].
The antioxidant response of Nile tilapia was also modified by the SP and/or LEO supplementation. Accordingly, the dietary supplementation of SP and/or LEO significantly improved the antioxidant status of Nile tilapia. They exhibited increased serum Gpx content, decreased MDA serum concentrations, and markedly improved hepatic and pancreatic histological features compared to the non-supplemented group. At the molecular level, both SP and/or LEO significantly up-regulated the expression levels of Cat and Gpx genes. Similarly, D-limonene in citrus fruit oil extract enhanced the Nile tilapia serum antioxidant enzymes, up-regulated their gene expression, and improved the liver histological features [53,106]. Also, European sea bass supplemented with Citrus bergamia EO, which is rich in limonene [107], showed significant increases in SOD and GPX serum concentrations [102]. D-limonene in orange peel essential oil markedly improved rainbow trout’s total myeloperoxidase and SOD serum activities and enhanced the fish survival rate against bacterial infection [14].
Additionally, limonene has a high inhibitory activity against MDA formation [108]. Likewise, spirulina dietary inclusion in catfish increased the plasma GPX concentration, lowered the MDA, and up-regulated the expression levels of SOD, CAT, and GPX genes after bacterial exposure [27]. Moreover, grass carp supplemented with 1% SP displayed improved antioxidant activities such as CAT and glutathione and lowered hepatic lipid peroxidation [35]. The strong antioxidant effects of SP are associated with its high contents of minerals, carotenoids, and phenolic compounds [109,110], which significantly improve the vital organ’s antioxidant activities, lower the tissue destruction level, and decrease lipid peroxidation [55,111]. Enhancing the organ’s oxidative capacity improves its histoarchitecture and delimits the destructive effects of free radicals resulting from tissue metabolism and different stressors [112,113].
The enhanced oxidative defense could indirectly activate the innate immune response through positive crosstalk of regulatory transcription factors such as Nrf2 and NF-κB, the main factors regulating the initial protective defense mechanisms [114,115]. The fish’s innate immune response is an essential non-specific defense line against pathogens and toxins via phagocytosis, lysozyme activity, and complement activity [116]. The current feeding trial demonstrated that LEO and SP enhanced PA and serum lysozyme activities. Moreover, the combined dietary supplementation of LEO and SP significantly up-regulated lysozyme (lZM) and complement C3 gene expression levels. The synergetic effects of LEO and SP strengthened the immune response, as confirmed by the highest PA and serum lysozyme activities and the highest expression levels of lysozyme and complement C3 genes. Our results agree with several previous studies that documented the potential immune-stimulating effects of the separate dietary presence of LEO and SP in several fish species. In this regard, spirulina dietary inclusion significantly motivated the plasma lysozyme activity, complement (C3), and IgM concentrations and up-regulated the mRNA levels of the il-1β, il-10, il-8, and LZY genes of the yellow catfish [34]. Spirulina supplementation at 1% also enhanced the immune response of Nile tilapia against Pseudomonas fluorescence infection by increasing the phagocytic and lysozyme activities and the expression levels of IL-1β and TNF-α cytokines [117]. In addition, increasing the dietary inclusion of SP in the sea bass’s diet by up to 5% boosted the fish’s immune response, as it motivated the lysozyme activity and upregulated the gene expression levels of Il-6, Il-8, Tnf-α, and Tgf-β [118].
Moreover, SP supplementation alone or mixed with Bacillus licheniformis enhanced the transcriptional levels of Lysozyme, Il-6, Il-1β, Tgf, and TNF-α, which in turn increased the goldfish resistance to bacterial infection and lowered its mortality rate [119]. Lemon EO and orange EO could efficiently enhance non-specific immunity, as they contain a high percentage of limonene. Nile tilapia diet supplemented with OEO and LEO significantly enhanced the fish’s phagocytic and lysosome activities [13]. Additionally, they showed a dose-dependent effect, where a higher dose of up to 5% dietary inclusion showed more stimulatory effects [19,102]. Enhancing the non-specific immune parameters increases fish resistance to bacterial infection and decreases mortality rates [69,120,121].
The alteration of Nile tilapia’s immune response might be correlated with altering the lipid metabolism following SP and/or LEO dietary supplementation. Fatty acids are important components in the dynamic metabolism of immune cells. They could directly or indirectly contribute to the biological processes of immunocytes, including cell proliferation and differentiation and regulating phagocytic activity [122]. The synthesis of fatty acids within immune cells is regulated by enzymes such as FAS and its related enzymes like acetoacetyl-CoA (ACC) [123]. FAS and ACC promote the cholesterol production required for toll-like receptor (TLR) signal transduction and proinflammatory macrophage activation [124]. Also, CD36 is a scavenger receptor involved in immunity and is present in mononuclear phagocytes [82]. Therefore, the increased expression levels of lipid metabolism-related genes in our study due to the LEO, SP, or LEO/SP diet would reflect the improved immune status of the fish under study. Further studies are recommended to investigate the regulatory relationship between lipid metabolism and immune-regulatory genes.
The total and the differential leucocytic counts are other important indicators of the fish’s health and immune status [125,126]. Leukocytes are the principal constituents of cellular innate immunity [127]. Many well-known dietary immunostimulants efficiently modulated the leucocytes and increased the protective lymphocyte count in the circulation [128]. Likewise, several studies showed that LEO and SP supplementation positively increased lymphocyte count [84,113]. Accordingly, our results demonstrate that the WBCs, heterophils, and lymphocyte counts were significantly modified by adding LEO, SP, and their combination to the diet, where they significantly increased WBCs and lymphocytes. However, they significantly lowered the heterophil count. Consequentially, this resulted in a significant decrease in the H/L ratio in the supplemented groups. The H/L ratio provides information about the fish’s immune status and stress conditions [129].
Based on the foremost results and discussions, this study has some limitations, including the need to study the effects of LEO, SP, and their combination under stressors during a bacterial challenge to reflect their impact on the immunity of Nile tilapia truly. This study also measured the activity of digestive enzymes using a limited sample size. Therefore, more research is advised.
5. Conclusions
Dietary supplementation of LEO and/or SP could improve the growth performance, feed efficiency, health status, and immune-oxidative responses of Nile tilapia. The LEO-SP mixture significantly increased the final body weight, GPX levels, PA, and WBC count. Additionally, the LEO-SP combination significantly increased the expression levels of most genes related to growth, immunity, antioxidants, and lipid metabolism. Therefore, LEO-SP could be used as a natural feed additive during aquafeed formulation to improve fish welfare through dietary management.
Author Contributions
Conceptualization, S.E.A., A.F.E.-N., R.E.A., R.M., M.A.H. and S.E.-K.; methodology, S.E.A. and A.F.E.-N.; software, S.E.-K.; validation, S.E.-K.; formal analysis, S.E.A., A.F.E.-N., R.E.A., R.M., M.A.H. and S.E.-K.; investigation, S.E.A., A.F.E.-N., R.E.A., R.M., M.A.H. and S.E.-K.; resources, M.M.A., A.D.C. and S.E.-K.; data curation, S.E.-K.; writing—original draft preparation, M.M.A., A.D.C. and S.E.-K.; writing—review and editing, M.M.A., A.D.C. and S.E.-K.; project administration, M.M.A., A.D.C. and S.E.-K.; funding acquisition, M.M.A. and A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project (RSPD2024R731), King Saud University (Riyadh, Saudi Arabia).
Institutional Review Board Statement
This experimental study was approved by the Institutional Animal Care and Use Committee (IACUC), Kafrelsheikh University, Egypt (approval number KFS-IACUC/112/2023). All methods were carried out following relevant guidelines and regulations of the KFS-IACUC. This study was conducted following ARRIVE guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding authors upon request.
Acknowledgments
We thank Alfredo Di Cerbo, who kindly helped us revise the text and format the literature using Endnote X9. We also acknowledge the Researchers Supporting Project (RSPD2024R731), King Saud University (Riyadh, Saudi Arabia).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Subramaniam, B.; Antony, C.; Cbt, R.; Arumugam, U.; Ahilan, B.; Aanand, S. Functional feed additives used in fish feeds. Int. J. Fish. Aquat. Stud. 2019, 7, 44–52. [Google Scholar]
- Beltrán, J.M.G.; Esteban, M.Á. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Abdo, S.E.; El-Nahas, A.F.; Abdelmenam, S.; Elmadawy, M.A.; Mohamed, R.; Helal, M.A.; El-Kassas, S. The synergetic effect of Bacillus species and Yucca shidigera extract on water quality, histopathology, antioxidant, and innate immunity in response to acute ammonia exposure in Nile tilapia. Fish Shellfish Immunol. 2022, 128, 123–135. [Google Scholar] [CrossRef]
- El-Kassas, S.; Abdo, S.E.; Abosheashaa, W.; Mohamed, R.; Moustafa, E.M.; Helal, M.A.; El-Naggar, K. Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex Nile tilapia fed Moringa oleifera leaf powder. Aquac. Rep. 2020, 18, 100422. [Google Scholar] [CrossRef]
- El-Kassas, S.; Aljahdali, N.; Abdo, S.E.; Alaryani, F.S.; Moustafa, E.M.; Mohamed, R.; Abosheashaa, W.; Abdulraouf, E.; Helal, M.A.; Shafi, M.E.; et al. Moringa oleifera Leaf Powder Dietary Inclusion Differentially Modulates the Antioxidant, Inflammatory, and Histopathological Responses of Normal and Aeromonas hydrophila-Infected Mono-Sex Nile Tilapia (Oreochromis niloticus). Front. Vet. Sci. 2022, 9, 918933. [Google Scholar] [CrossRef]
- Elangovan, P.; Felix, S.; Nathan, F.; Ahilan, B. An overview on significance of fish nutrition in aquaculture industry. Int. J. Fish. Aquat. Stud. 2017, 5, 349–355. [Google Scholar]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Bozkurt, T.; Gulnaz, O.; Aka Kaçar, Y. Chemical composition of the essential oils from some citrus species and evaluation of the antimicrobial activity. IOSR J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 29–33. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. A J. Clin. Ther. 2007, 12, 259–264. [Google Scholar]
- Kazyoba, P.; Viljoen, A. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.; Monroig, Ó. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 488, 217–226. [Google Scholar] [CrossRef]
- Mohamed, R.; Yousef, M.; El-Tras, W.; Khalafallaa, M. Dietary essential oil extract from sweet orange (Citrus sinensis) and bitter lemon (Citrus limon) peels improved Nile tilapia performance and health status. Aquac. Res. 2020, 52, 1463–1479. [Google Scholar] [CrossRef]
- Gültepe, N. Protective effect of d-limonene derived from orange peel essential oil against Yersinia ruckeri in rainbow trout. Aquac. Rep. 2020, 18, 100417. [Google Scholar] [CrossRef]
- Keinan, E.; Alt, A.; Amir, G.; Bentur, L.; Bibi, H.; Shoseyov, D. Natural ozone scavenger prevents asthma in sensitized rats. Bioorg. Med. Chem. 2005, 13, 557–562. [Google Scholar] [CrossRef]
- Magara, G.; Prearo, M.; Vercelli, C.; Barbero, R.; Micera, M.; Botto, A.; Caimi, C.; Caldaroni, B.; Bertea, C.M.; Mannino, G.; et al. Modulation of Antioxidant Defense in Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with a Diet Supplemented by the Waste Derived from the Supercritical Fluid Extraction of Basil (Ocimum basilicum). Antioxidants 2022, 11, 415. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Ngugi, C.; Oyoo-Okoth, E.; Muchiri, M. Effects of dietary levels of essential oil (EO) extract from bitter lemon (Citrus limon) fruit peels on growth, biochemical, hemato-immunological parameters and disease resistance in Juvenile Labeo victorianus fingerlings challenged with Aeromonas hydrophila. Aquac. Res. 2016, 47, 2253–2265. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Application of Spirulina in aquaculture: A review on wastewater treatment and fish growth. Rev. Aquac. 2020, 12, 582–599. [Google Scholar] [CrossRef]
- Velasquez, S.F.; Chan, M.A.; Abisado, R.G.; Traifalgar, R.F.M.; Tayamen, M.M.; Maliwat, G.C.F.; Ragaza, J.A. Dietary Spirulina (Arthrospira platensis) replacement enhances performance of juvenile Nile tilapia (Oreochromis niloticus). J. Appl. Phycol. 2016, 28, 1023–1030. [Google Scholar] [CrossRef]
- Alagawany, M.; Taha, A.E.; Noreldin, A.; El-Tarabily, K.A.; Abd El-Hack, M.E. Nutritional applications of species of Spirulina and Chlorella in farmed fish: A review. Aquaculture 2021, 542, 736841. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Jiang, S.; Farag, M.R.; Azzam, M.; Al-Abdullatif, A.A.; Alhotan, R.; Dhama, K.; Hassan, F.-u.; Alagawany, M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023, 14, 1072787. [Google Scholar] [CrossRef]
- Teimouri, M.; Yeganeh, S.; Keramat, A. The effects of Spirulina platensis meal on proximate composition, fatty acid profile and lipid peroxidation of rainbow trout (Oncorhynchus mykiss) muscle. Aquac. Nutr. 2015, 22, 559–566. [Google Scholar] [CrossRef]
- Lu, J.; Takeuchi, T.; Ogawa, H. Flesh quality of tilapia Oreochromis niloticus fed solely on raw Spirulina. Fish. Sci. 2003, 69, 529–534. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Han, D.; Xie, S.; Jin, J.; Yang, Y.; Zhu, X. Effects of dietary Arthrospira platensis supplementation on the growth performance, antioxidation and immune related-gene expression in yellow catfish (Pelteobagrus fulvidraco). Aquac. Rep. 2020, 17, 100297. [Google Scholar] [CrossRef]
- Yu, W.; Wen, G.; Lin, H.; Yang, Y.; Huang, X.; Zhou, C.; Zhang, Z.; Duan, Y.; Huang, Z.; Li, T. Effects of dietary Spirulina platensis on growth performance, hematological and serum biochemical parameters, hepatic antioxidant status, immune responses and disease resistance of Coral trout Plectropomus leopardus (Lacepede, 1802). Fish Shellfish Immunol. 2018, 74, 649–655. [Google Scholar] [CrossRef]
- Al-Deriny, S.; Dawood, M.; Abouzaid, A.; El-Tras, W.; Paray, B.; Doan, H.; Mohamed, R. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100390. [Google Scholar] [CrossRef]
- Amer, S. Effect of Spirulina platensis as feed supplement on growth performance, immune response and antioxidant status of mono-sex Nile Tilapia (Oreochromis niloticus). BVMJ 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Shalata, H.A.; Bahattab, O.; Zayed, M.M.; Farrag, F.; Salah, A.S.; Al-Awthan, Y.S.; Ebied, N.A.; Mohamed, R.A. Synergistic effects of dietary sodium butyrate and Spirulina platensis on growth performance, carcass composition, blood health, and intestinal histomorphology of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2021, 19, 100637. [Google Scholar] [CrossRef]
- Awed, E.M.; Sadek, K.M.; Soliman, M.K.; Khalil, R.H.; Younis, E.M.; Abdel-Warith, A.A.; Van Doan, H.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Spirulina platensis Alleviated the Oxidative Damage in the Gills, Liver, and Kidney Organs of Nile Tilapia Intoxicated with Sodium Sulphate. Animals 2020, 10, 2423. [Google Scholar] [CrossRef] [PubMed]
- El-Araby, D.A.; Amer, S.A.; Attia, G.A.; Osman, A.; Fahmy, E.M.; Altohamy, D.E.; Alkafafy, M.; Elakkad, H.A.; Tolba, S.A. Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture 2022, 546, 737413. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Zhu, X.; Han, D.; Jin, J.; Yang, Y.; Xie, S. The Effects of Dietary Arthrospira platensis on Oxidative Stress Response and Pigmentation in Yellow Catfish Pelteobagrus fulvidraco. Antioxidants 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Jamal, R.; Nazeer, N.; Khaliq, S.; Hoseinifar, S.H.; Van Doan, H.; Paolucci, M. Improving Growth, Digestive and Antioxidant Enzymes and Immune Response of Juvenile Grass Carp (Ctenopharyngodon idella) by Using Dietary Spirulina platensis. Fishes 2022, 7, 237. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; El-Ashram, S.; Sayed, A.E.-D.H.; Alagawany, M.; Shukry, M.; Dawood, M.A.O.; Kucharczyk, D. Elucidating the ameliorative effects of the cyanobacterium Spirulina (Arthrospira platensis) and several microalgal species against the negative impacts of contaminants in freshwater fish: A review. Aquaculture 2022, 554, 738155. [Google Scholar] [CrossRef]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kawahara, E.; Ueda, T.; Nomura, S. In Vitro Phagocytic Activity of White-Spotted Char Blood Cells after Injection with Aeromonas salmonicida Extracellular Products. Fish. Pathol. 1991, 26, 213–214. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G.; El-Nahas, A.F.; Mahmoud, S.; Ibrahim, E.M. Vitamin C Modulates the Immunotoxic Effect of 17α-Methyltestosterone in Nile Tilapia. Biochemistry 2017, 56, 2042–2050. [Google Scholar] [CrossRef]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T., Jr.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Ozdemir, C.; Yeni, F.; Odaci, D.; Timur, S. Electrochemical glucose biosensing by pyranose oxidase immobilized in gold nanoparticle-polyaniline/AgCl/gelatin nanocomposite matrix. Food Chem. 2010, 119, 380–385. [Google Scholar] [CrossRef]
- Houston, A. Blood and circulation. In Methods for Fish Biology; American Fisheries Society: Bethesda, MA, USA, 1990; pp. 415–488. [Google Scholar]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Dwivedi, S.; Purohit, P.; Misra, R.; Pareek, P.; Vishnoi, J.R.; Misra, S.; Sharma, P. Methods for Isolation of High Quality and Quantity of miRNA and Single Cell Suspension for Flow-Cytometry from Breast Cancer Tissue: A Comparative Analysis. Indian. J. Clin. Biochem. 2019, 34, 39–44. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Con, P.; Nitzan, T.; Slosman, T.; Harpaz, S.; Cnaani, A. Peptide Transporters in the Primary Gastrointestinal Tract of Pre-Feeding Mozambique Tilapia Larva. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- El-Naggar, K.; Mohamed, R.; El-katcha, M.I.; Abdo, S.E.; Soltan, M.A. Plant Ingredient diet supplemented with lecithin as fish meal and fish oil alternative affects growth performance, serum biochemical, lipid metabolism and growth-related gene expression in Nile tilapia. Aquac. Res. 2021, 52, 6308–6321. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, H.; Han, Z.; Tang, J.; Xiao, Z.; Guo, F.; Wang, Y.; Luo; Zhou, Y. Effects of waterborne exposure to 17β-estradiol on hepatic lipid metabolism genes in tilapia (Oreochromis niloticus). Aquac Rep. 2020b, 17, 100382. [Google Scholar] [CrossRef]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.B.; Monroig, Ó. The effects of combined phytogenics on growth and nutritional physiology of Nile tilapia Oreochromis niloticus. Aquaculture 2020, 519, 734867. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, N.K.M.; Eissa, I.A.M.; Ahmed, E.; Kilany, O.E.; El-Adl, M.; Dawood, M.A.O.; Hassan, A.M.; Abdel-Daim, M.M. Protective role of dietary Spirulina platensis against diazinon-induced Oxidative damage in Nile tilapia; Oreochromis niloticus. Environ. Toxicol. Phar. 2017, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Rombenso, A.; Araújo, B.; Li, E.-C. Recent Advances in Fish Nutrition: Insights on the Nutritional Implications of Modern Formulations. Animals 2022, 12, 1705. [Google Scholar] [CrossRef] [PubMed]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: A Review. Probiotics Antimicrob. Proteins 2022, 14, 130–157. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abo-Al-Ela, H.G.; Hasan, M.T. Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef]
- Gewaily, M.S.; Abdo, S.E.; Moustafa, E.M.; AbdEl-kader, M.F.; Abd El-Razek, I.M.; El-Sharnouby, M.; Alkafafy, M.; Raza, S.H.; El Basuini, M.F.; Van Doan, H.; et al. Dietary Synbiotics Can Help Relieve the Impacts of Deltamethrin Toxicity of Nile Tilapia Reared at Low Temperatures. Animals 2021, 11, 1790. [Google Scholar] [CrossRef]
- Bortolini, D.; Maciel, G.M.; Fernandes, I.; Pedro, A.; Rubio, F.; Brancod, I.; Haminiuk, C. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Rosas, V.T.; Poersch, L.H.; Romano, L.A.; Tesser, M.B. Feasibility of the use of Spirulina in aquaculture diets. Rev. Aquac. 2019, 11, 1367–1378. [Google Scholar] [CrossRef]
- Saleh, H.; Gaber, H.; El-Khayat, H.; Abdel-Motleb, A.; Mohammed, W.; Okasha, H. Influences of Dietary Supplementation of Chlorella vulgaris and Spirulina platensis on Growth-Related Genes Expression and Antioxidant Enzymes in Oreochromis niloticus Fish Exposed to Heavy Metals. Aquac. Studies. 2021, 22, AQUAST793. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dadar, M.; Sakai, M.; Dawood, M. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol. 2016, 56, 436–444. [Google Scholar] [CrossRef]
- Belal, E.; Khalafalla, M.; El-hais, A.M.A. Use of spirulina (Arthrospira fusiformis) for promoting growth of Nile Tilapia fingerlings. Afr. J. Microbiol. Res. 2012, 6, 6423–6431. [Google Scholar] [CrossRef]
- Elsayed, H.A.G.; Salem, M. Effects of dietary orange peel on growth performance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Studies. 2018, 18, 127–134. [Google Scholar] [CrossRef]
- Toutou, M.; Soliman, A.A.; Elokaby, M.; Ahmed, R. Growth performance and biochemical blood parameters of Nile tilapia, Oreochromis niloticus, and thinlip mullet, Liza ramada, fed a diet supplemented with lemon (Citrus aurantifolia) peel in a polyculture system ARTICLE INFO ABSTRACT. Egypt. J. Aquat. Biol. Fish. 2018, 22, 183–192. [Google Scholar] [CrossRef]
- Sutili, F.; Gatlin, D.; Heinzmann, B.; Baldisserotto, B. Plant essential oils as fish diet additives: Benefits on fish health and stability in feed. Rev. Aquac. 2017, 10, 716–726. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Alagawany, M.; Kari, Z.A.; Abdul Razab, M.K.A.; Hamid, N.K.A.; Moonmanee, T.; Van Doan, H. Exploring the Roles of Dietary Herbal Essential Oils in Aquaculture: A Review. Animals 2022, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; Gültepe, N.; Türker, A. Evaluation of the effects of essential oil extracted from sweet orange peel (Citrus sinensis) on growth rate of tilapia (Oreochromis mossambicus) and possible disease resistance against Streptococcus iniae. Aquaculture 2015, 437, 282–286. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, Y.; Yang, Z.; Kong, Q.; Liu, P.; Liao, H.; Tang, H. Effects of partial replacement of fishmeal with Spirulina platensis powder and addition of Spirulina platensis polysaccharide on growth, nutrition, antioxidant capacity and gut microbiota of Micropterus salmoides. Aquaculture 2024, 586, 740802. [Google Scholar] [CrossRef]
- Abbasali, H.S.H.F.L.; Tizkar, Z.B. Influence of Spirulina sp. and citric acid dietary supplements on the growth performance and immune parameters of com-mon carp (Cyprinus carpio). Int. Aquat. Res. 2024, 16, 91–99. [Google Scholar]
- Macedo, J.d.S.; Copatti, C.E.; Costa, E.V.; Da Silva, F.M.A.; Dutra, L.M.; Santos, V.L.d.A.; Almeida, J.R.G.d.S.; Tavares-Dias, M.; Melo, J.F.B. Effects of Citrus limon extract on growth performance and immunity in striped catfish (Pangasius hypophthalmus). Aquac. Int. 2023, 31, 719–738. [Google Scholar] [CrossRef]
- Yousefi, M.; Hoseini, S.M.; Abdel Rahman, A.N.; Vatnikov, Y.A.; Kulikov, E.V.; Kharlitskaya, E.V.; Seleznev, S.B. Effects of Dietary Limonene Supplementation on Growth Performance and Immunological Parameters of Common Carp, Cyprinus carpio, Challenged by Aeromonas hydrophila. Animals 2023, 13, 3197. [Google Scholar] [CrossRef]
- Da Silva, E.G.; Finamor, I.A.; Bressan, C.A.; Schoenau, W.; Vencato, M.D.S.; Pavanato, M.A.; Cargnelutti, J.F.; Da Costa, S.T.; Antoniazzi, A.Q.; Baldisserotto, B. Dietary Supplementation with R-(+)-Limonene Improves Growth, Metabolism, Stress, and Antioxidant Responses of Silver Catfish Uninfected and Infected with Aeromonas hydrophila. Animals 2023, 13, 3307. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology (Reading) 2012, 158, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Fan, G.; Ren, J.N.; Zhang, L.L.; Pan, S.Y. Effects of orange essential oil on intestinal microflora in mice. J. Sci. Food Agric. 2019, 99, 4019–4028. [Google Scholar] [CrossRef]
- Albalat, A.; Saera-Vila, A.; Capilla, E.; Gutierrez, J.; Pérez-Sánchez, J.; Navarro, I. Insulin regulation of lipoprotein lipase (LPL) activity and expression in Gilthead Sea bream (Sparus aurata). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 151–159. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta. 2012, 1821, 747–753. [Google Scholar] [CrossRef]
- Storch, J.; Thumser, A.E. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 2000, 1486, 28–44. [Google Scholar] [CrossRef]
- Nakharuthai, C.; Rodrigues, P.M.; Schrama, D.; Kumkhong, S.; Boonanuntanasarn, S. Effects of Different Dietary Vegetable Lipid Sources on Health Status in Nile Tilapia (Oreochromis niloticus): Haematological Indices, Immune Response Parameters and Plasma Proteome. Animals 2020, 10, 1377. [Google Scholar] [CrossRef]
- Silverstein, R.; Febbraio, M. CD36, a Scavenger Receptor Involved in Immunity, Metabolism, Angiogenesis, and Behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef]
- Zuo, H.; Gao, J.; Yuan, J.; Deng, H.; Yang, L.; Weng, S.; He, J.; Xu, X. Fatty acid synthase plays a positive role in shrimp immune responses against Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2017, 60, 282–288. [Google Scholar] [CrossRef]
- Yeganeh, S.; Teimouri, M.; Amirkolaie, A.K. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci. 2015, 101, 84–88. [Google Scholar] [CrossRef]
- Muga, M.A.; Chao, J.C. Effects of fish oil and spirulina on oxidative stress and inflammation in hypercholesterolemic hamsters. BMC Complement. Altern. Med. 2014, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.-C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2020, 13, 642–663. [Google Scholar] [CrossRef]
- Fu, Y.; Liang, X.; Li, D.; Gao, H.; Wang, Y.; Li, W.; Xu, K.; Hu, F. Effect of Dietary Tryptophan on Growth, Intestinal Microbiota, and Intestinal Gene Expression in an Improved Triploid Crucian Carp. Front. Nutr. 2021, 8, 676035. [Google Scholar] [CrossRef] [PubMed]
- Paola, P.; Patrice, D.C. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232. [Google Scholar] [CrossRef]
- Kim, Y.; Ho, S. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Sangani, A.K.; Masoudi, A.A.; Hosseini, S.A. The effects of herbal plants on Mucin 2 gene expression and performance in ascetic broilers. Iran. J. Vet. Med. 2014, 8, 47–52. [Google Scholar]
- Verri, T.; Terova, G.; Dabrowski, K.; Saroglia, M. Peptide transport and animal growth: The fish paradigm. Biol. Lett. 2011, 7, 597–600. [Google Scholar] [CrossRef]
- Jang, I.; Ko, Y.; Kang, S.; Lee, C. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Bondi, M. Preliminary evaluation of Spirulina maxima and Ascophyllum nodosum effect on 3 different bacterial strains. Minerva Biotecnol. 2015, 27, 131–136. [Google Scholar]
- Farag, M.R.; Alagawany, M.; Abd El-Hack, M.E.; Dhama, K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2016, 12, 36–51. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, N.; Mousavi, S.; Hamidian, G.; Firouzamandi, M.; Khani Oushani, A.; Mardani, K. Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 510, 1–8. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Vatsos, I.N.; Rahman, M.A.; Pham, H.D. Selenium-Enriched Spirulina (SeE-SP) Enhance Antioxidant Response, Immunity, and Disease Resistance in Juvenile Asian Seabass, Lates calcarifer. Antioxidants 2022, 11, 1572. [Google Scholar] [CrossRef]
- Ahmadifar, M.; Esfahani, D.E.; Ahmadifar, E.; Sheikhzadeh, N.; Mood, S.M.; Moradi, S.Z. Combined effects of Spirulina platensis and Pediococcus acidilactici on the growth performance, digestive enzyme activity, antioxidative status, and immune genes in zebrafish. Ann. Anim. Sci. 2023, 23, 1159–1167. [Google Scholar] [CrossRef]
- Esmaeili, M. Blood Performance: A New Formula for Fish Growth and Health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Vazzana, I.; Piccione, G. Influence of Body Size on Blood Hemogram in Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792). Veter Med. Open J. 2017, 2, 91–94. [Google Scholar] [CrossRef]
- Acar, Ü.; Kesbiç, O.S.; İnanan, B.E.; Yılmaz, S. Effects of dietary Bergamot (Citrus bergamia) peel oil on growth, haematology and immune response of European sea bass (Dicentrarchus labrax) juveniles. Aquac. Res. 2019, 50, 3305–3312. [Google Scholar] [CrossRef]
- Vicente, I.; Fleuri, L.; Carvalho, P.; Gardim Guimarães, M.; Naliato, R.; Müller, H.; Sartori, M.M.; Pezzato, L.; Barros, M. Orange peel fragment improves antioxidant capacity and haematological profile of Nile tilapia subjected to heat/dissolved oxygen-induced stress. Aquac. Res. 2018, 50, 80–92. [Google Scholar] [CrossRef]
- Sayed, A.E.H.; Hamed, M.; El-Sayed, A.A.A.; Nunes, B.; Soliman, H.A.M. The mitigating effect of Spirulina (Arthrospira platensis) on the hemotoxicity of gibberellic acid on juvenile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. Int. 2023, 30, 25701–25711. [Google Scholar] [CrossRef] [PubMed]
- Arabi, H.; Gholipourkanani, H.; Shahsavani, D.; Harsij, M. Improving effect of Spirulina platensis on hematological parameters in Cyprinus carpio exposed to sublethal doses of cyanide. Comp. Clin. Path. 2016, 25, 335–342. [Google Scholar] [CrossRef]
- Magouz, F.; El-Din, M.; Amer, A.; Gewaily, M.; El-Dahdoh, W.; Dawood, M. A blend of herbal essential oils enhanced the growth performance, blood bio-immunology traits, and intestinal health of Nile tilapia (Oreochromis niloticus). Ann. Anim. Sci. 2021, 22, 000010247820210066. [Google Scholar] [CrossRef]
- Costa, R.; Dugo, P.; Navarra, M.; Raymo, V.; Dugo, G.; Mondello, L. Study on the chemical composition variability of some processed bergamot (Citrus bergamia) essential oils. Flavour. Fragr. J. 2010, 25, 4–12. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant Activities and Volatile Constituents of Various Essential Oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.A.; El-Haroun, E.R. Comparative study on the effect of dietary β-carotene and phycocyanin extracted from Spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Bhatt, A.; Nishad, D.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis- In Vivo toxicity, Antioxidant and Immunomodulatory Studies. Saudi J. Biol. Sci. 2020, 28, 1853–1859. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Dawood, M.A.O.; AlKahtane, A.A.; Abdeen, A.; Abdel-Latif, H.M.R.; Senousy, H.H.; Aleya, L.; Alkahtani, S. Spirulina platensis mediated the biochemical indices and antioxidative function of Nile tilapia (Oreochromis niloticus) intoxicated with aflatoxin B1. Toxicon 2020, 184, 152–157. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Karim, M.; Natrah, F.M.I. Carotenoids modulate stress tolerance and immune responses in aquatic animals. Rev Aquac. 2023, 15, 872–894. [Google Scholar] [CrossRef]
- Mokhbatly, A.-A.; Assar, D.; Ghazy, E.; Elbialy, Z.; Rizk, S.; Omar, A.; Gaafar, A.; Dawood, M. The protective role of spirulina and β-glucan in African catfish (Clarias gariepinus) against chronic toxicity of chlorpyrifos: Hemato-biochemistry, histopathology, and oxidative stress traits. Environ. Sci. Pollut. Res. 2020, 27, 31636–31651. [Google Scholar] [CrossRef]
- Kumar, S.; Moniruzzaman, M.; Chakraborty, A.; Sarbajna, A.; Chakraborty, S.B. Crosstalk between heat shock proteins, NRF2, NF-κB and different endogenous antioxidants during lead-induced hepatotoxicity in Puntius ticto. Aquat. Toxicol. 2021, 233, 105771. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Mahmoud, M.; Elamie, M.; Kilany, O.; Dessouki, A. Spirulina (Arthrospira platensis) supplementation improves growth performance, feed utilization, immune response, and relieves oxidative stress in Nile tilapia (Oreochromis niloticus) challenged with Pseudomonas fluorescens. Fish Shellfish Immunol. 2017, 72, 291–300. [Google Scholar] [CrossRef]
- Güroy, B.; Güroy, D.; Bilen, S.; Kenanoğlu, O.N.; Şahin, I.; Terzi, E.; Karadal, O.; Mantoğlu, S. Effect of dietary Spirulina (Arthrospira platensis) on the growth performance, immune-related gene expression and resistance to Vibrio anguillarum in European seabass (Dicentrarchus labrax). Aquac. Res. 2022, 53, 2263–2274. [Google Scholar] [CrossRef]
- Yousefi, M.; Ahmadifar, M.; Mohammadzadeh, S.; Kalhor, N.; Esfahani, D.E.; Bagheri, A.; Mashhadizadeh, N.; Moghadam, M.S.; Ahmadifar, E. Individual and combined effects of the dietary Spirulina platensis and Bacillus licheniformis supplementation on growth performance, antioxidant capacity, innate immunity, relative gene expression and resistance of goldfish, Carassius auratus to Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 127, 1070–1078. [Google Scholar] [CrossRef]
- Abdel-Latif, H.; Khafaga, A.; Dawood, M. Dietary oregano essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef]
- Baba, E.; Acar, Ü.; Öntaş, C.; Kesbic, O.; Yilmaz, S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P.C. Fatty acids and immune function: New insights into mechanisms. Br. J. Nutr. 2007, 98 (Suppl. S1), S41–S45. [Google Scholar] [CrossRef]
- Qian, X.; Yang, Z.; Mao, E.; Chen, E. Regulation of fatty acid synthesis in immune cells. Scand. J. Immunol. 2018, 88, e12713. [Google Scholar] [CrossRef]
- Carroll, R.; Zaslona, Z.; Galvan-Pena, S.; Koppe, E.; Sévin, D.; Angiari, S.; Triantafilou, M.; Triantafilou, K.; Modis, L.; O’Neill, L. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018, 293, jbc.RA118.001921. [Google Scholar] [CrossRef] [PubMed]
- Modrá, H.; Svobodová, Z.; Kolářová, J. Comparison of Differential Leukocyte Counts in Fish of Economic and Indicator Importance. Acta Vet. Brno 1998, 67, 215. [Google Scholar] [CrossRef]
- Shlenkina, T.M.; Romanova, E.M.; Lyubomirova, V.N.; Romanov, V.V.; Shadieva, L.A. The effects of the probiotic Subtilis on the peripheral blood system of Clarias gariepinus. BIO Web Conf. 2020, 27, 00133. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-López, F.; Teles, M.; MacKenzie, S. The response of fish to immunostimulant diets. Fish Shellfish Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef] [PubMed]
- Barman, D.; Nen, P.; Mandal, S.; Kumar, V. Immunostimulants for Aquaculture Health Management. JMSRD 2013, 3, 1–11. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).