Optimization of Gamma Image Quality Through Experimental Evaluation Using 3D-Printed Phantoms Across Energy Window Levels
Abstract
1. Introduction
2. Materials and Methods
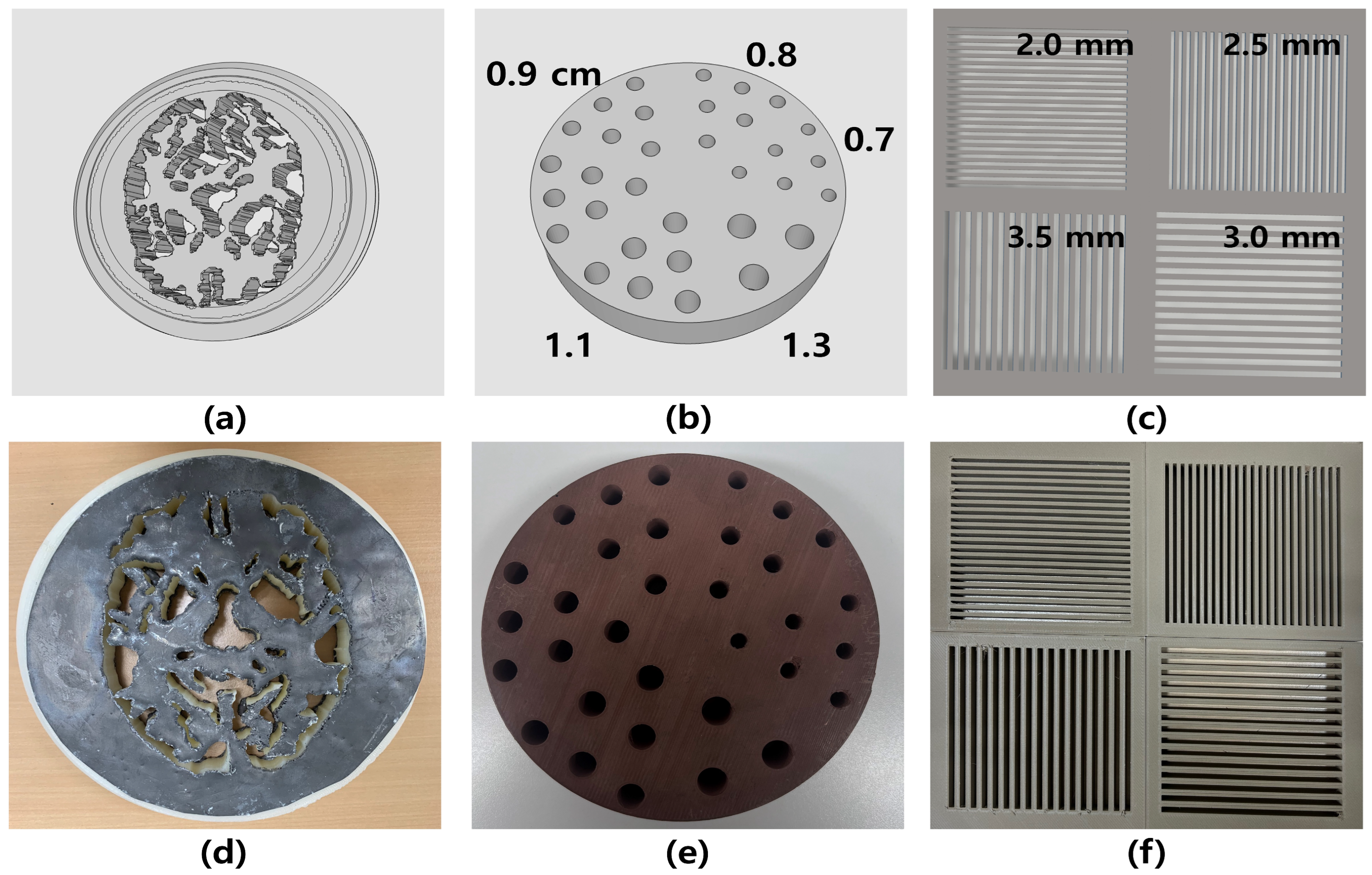
2.1. Dedicated 3D Printing Nuclear Medicine Phantom
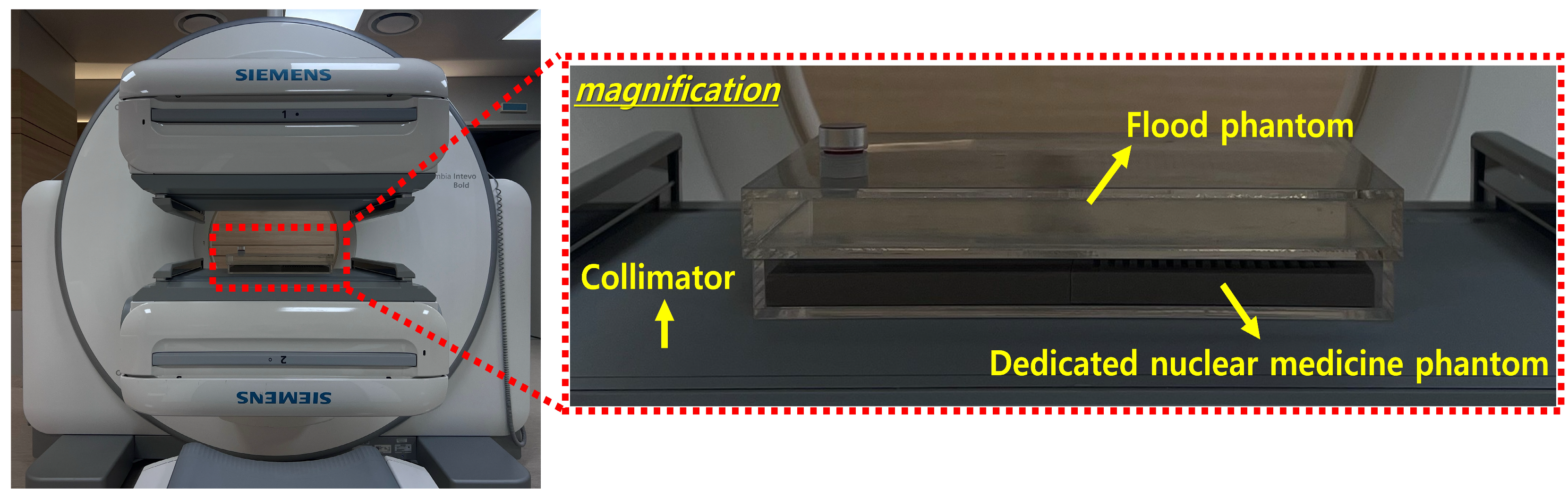
2.2. Image Acquisition Parameters
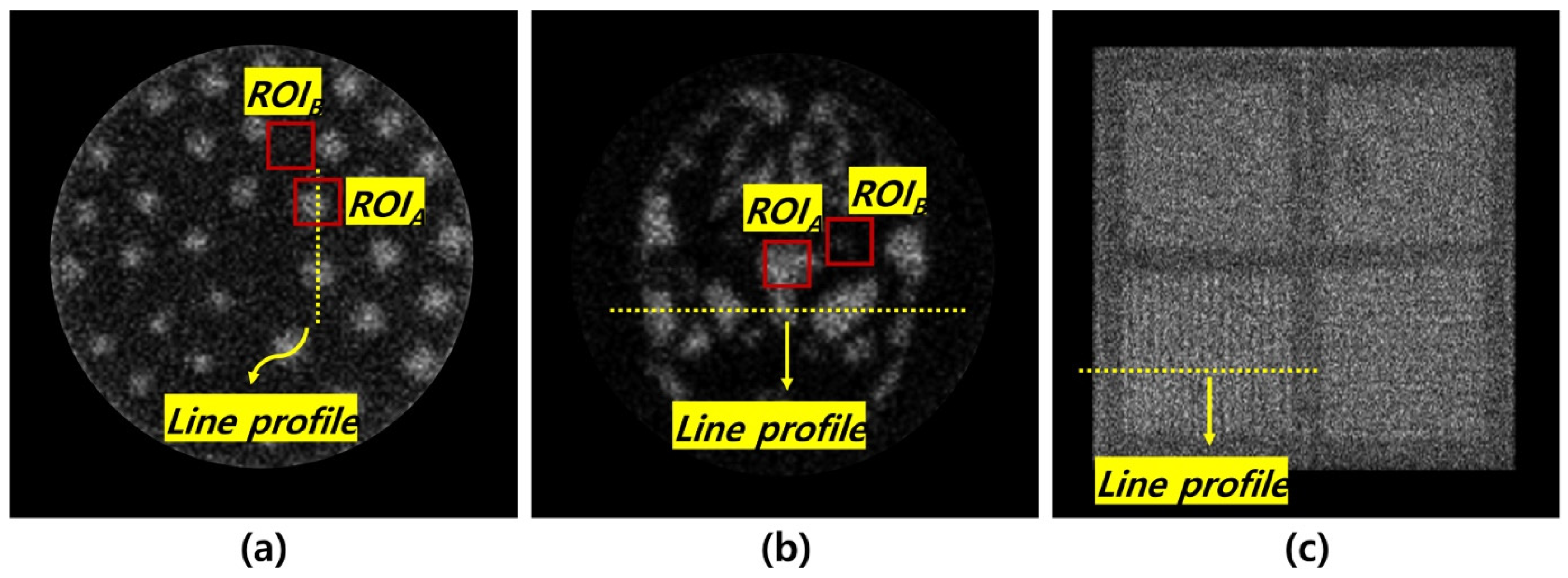
2.3. Quantitative Analysis
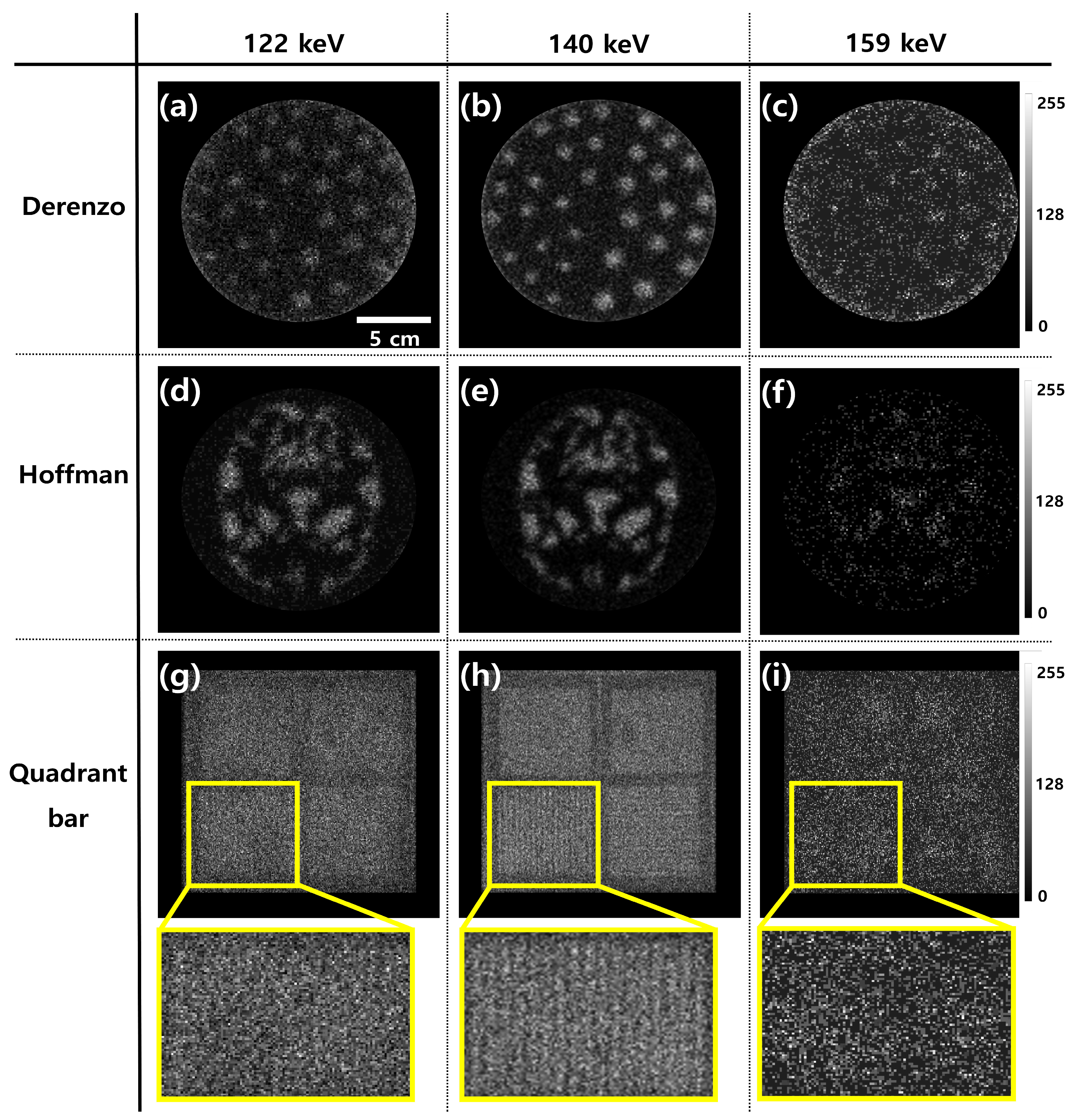
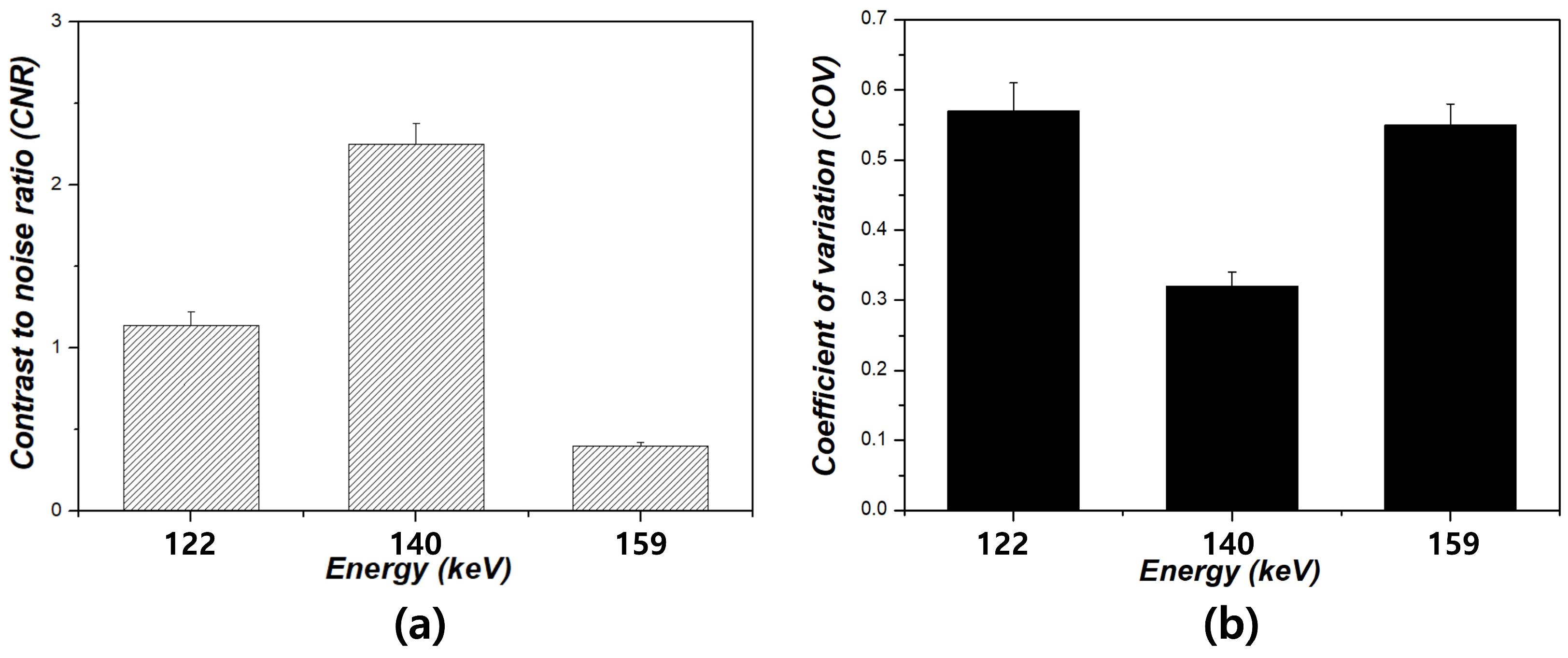
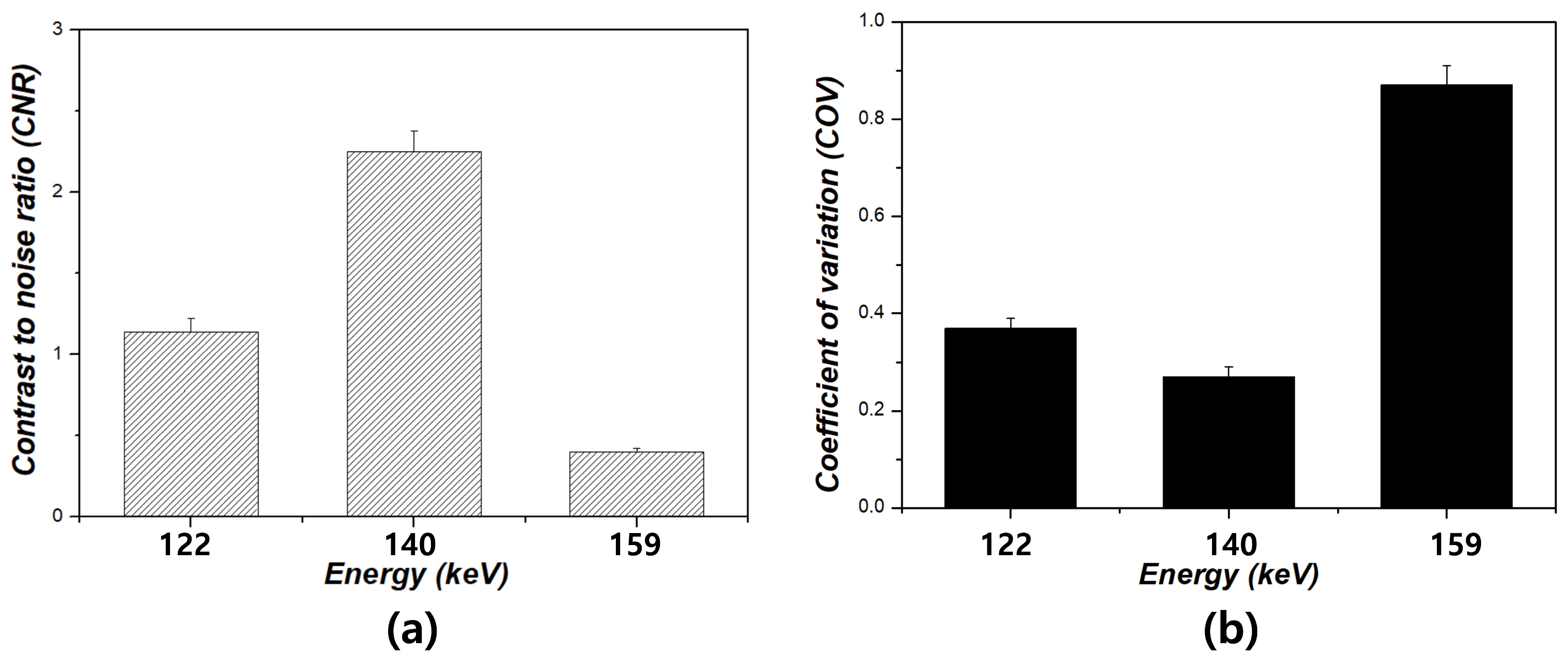
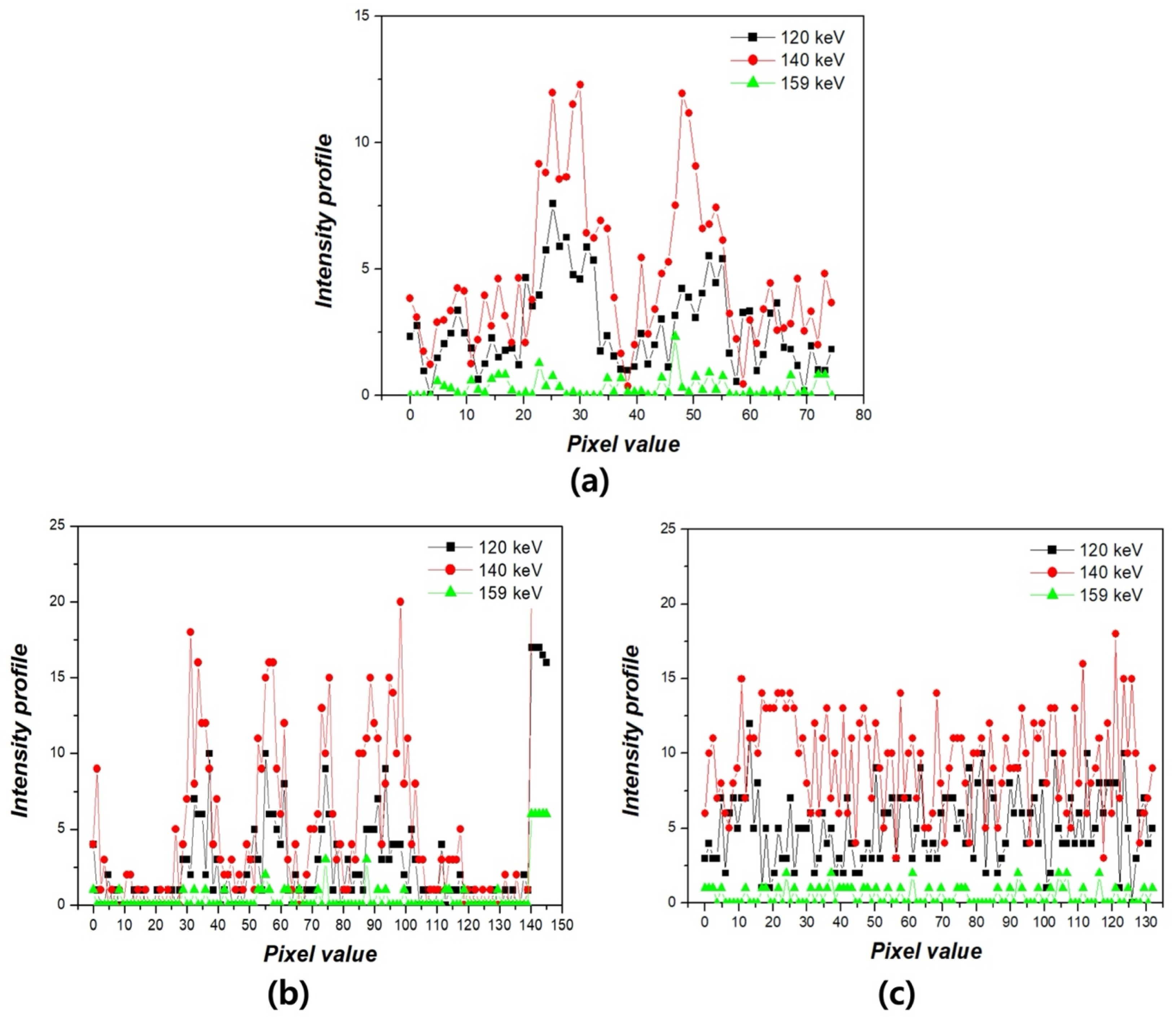
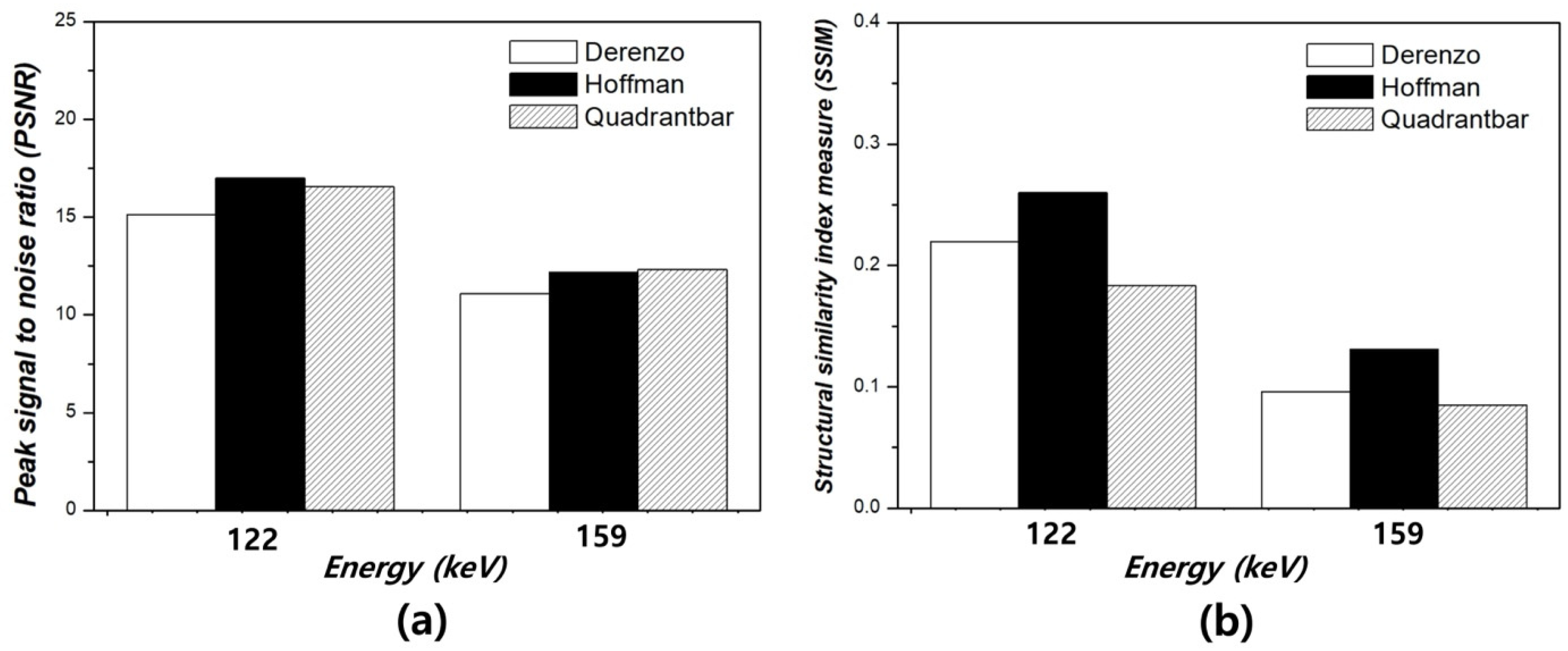
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelhalim, M.A.K.; Rizk, R.A.M.; Farag, H.I.; Reda, S.M. Effect of energy window width on planer and SPECT image uniformity. J. King Saud Univ. Sci. 2009, 21, 145–150. [Google Scholar] [CrossRef]
- Vaz, S.C.; Oliveira, F.; Herrmann, K.; Veit-Haibach, P. Nuclear medicine and molecular imaging advances in the 21st century. Br. J. Radiol. 2020, 93, 20200095. [Google Scholar] [CrossRef]
- O’Connor, M.K.; Kemp, B.J. Single-Photon Emission Computed Tomography/Computed Tomography: Basic Instrumentation and Innovations. Semin. Nucl. Med. 2006, 36, 258–266. [Google Scholar] [CrossRef]
- Al-Muqbel, K.M. Utility of 99mTechnetium Pertechnetate Thyroid Scan and Uptake in Thyrotoxic Patients: Jordanian Experience. World J. Nucl. Med. 2023, 22, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Asgari, A.; Ashoor, M.; Sohrabpour, M.; Shokrani, P.; Rezaei, A. Evaluation of various energy windows at different radionuclides for scatter and attenuation correction in nuclear medicine. Ann. Nucl. Med. 2015, 29, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Bouzekraoui, Y.; Bentayeb, F.; Asmi, H.; Bonutti, F. Energy Window and Contrast Optimization for Single-photon Emission Computed Tomography Bremsstrahlung Imaging with Yttrium-90. Indian J. Nucl. Med. 2019, 34, 125–128. [Google Scholar] [CrossRef]
- Kojima, A.; Matsumoto, M.; Takahashi, M. Experimental analysis of scattered photons in Tc-99m imaging with a gamma camera. Ann. Nucl. Med. 1991, 5, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Can, M.; Karadeniz, Ö.; Kaya, G.Ç.; Ertay, T. A study on energy window-based scatter correction methods in 99mTc and 123I imaging. J. Instrum. 2023, 18, P10009. [Google Scholar] [CrossRef]
- Ínce, C.; Karadeniz, Ö.; Ertay, T.; Durak, H. Collimator and energy window optimization for YTTRIUM-90 bremsstrahlung SPECT imaging. Appl. Radiat. Isot. 2021, 167, 109453. [Google Scholar] [CrossRef]
- Demirtaş, C.K.; Can, M.; Karadeniz, Ö.; Çilengiroğlu, Ö.V.; Ertay, T.; Kaya, G.Ç. Energy window optimization in bremsstrahlung imaging after Yttrium-90 microsphere therapy. Biomed. Phys. Eng. Express 2024, 10, 025028. [Google Scholar] [CrossRef]
- Edam, A.N.; Sulieman, A.; Sam, A.K.; Salih, I.; Alkhorayef, M.; Bradley, D.A. Quality control of radiopharmaceuticals and diagnostic nuclear medicine equipment. Radiat. Phys. Chem. 2020, 167, 108247. [Google Scholar] [CrossRef]
- Bunka, M.; Müller, C.; Bermeulen, C.; Haller, S.; Türler, A.; Schibli, R.; Meulen, N.P.V.D. Imaging quality of 44Sc in comparison with five other PET radionuclides using Derenzo phantoms and preclinical PET. Appl. Radiat. Isot. 2016, 110, 129–133. [Google Scholar] [CrossRef]
- Harrison, R.L.; Elston, B.F.; Byrd, D.W.; Alessio, A.M.; Swanson, K.R.; Kinahan, P.E. Technical Note: A digital reference object representing Hoffman’s 3D brain phantom for PET scanner simulations. Med. Phys. 2020, 47, 1174–1180. [Google Scholar] [CrossRef]
- Tazegul, T.E.; Polemi, A.M.; Snyder, A.; Snyder, C.; Collins, P.G. Automated phantom analysis for gamma cameras and SPECT: A methodology for use in a clinical setting. J. Appl. Clin. Med. Phys. 2020, 21, 205–214. [Google Scholar] [CrossRef]
- Berthon, B.; Marshall, C.; Holmes, R.; Spezi, E. A novel phantom technique for evaluating the performance of PET auto-segmentation methods in delineating heterogeneous and irregular lesions. EJNMMI Phys. 2015, 2, 13. [Google Scholar] [CrossRef]
- Markiewicz, P.J.; Angelis, G.I.; Kotasidis, F.; Green, M.; Lionheart, W.R.; Reader, A.J.; Matthews, J.C. A custom-built PET phantom design for quantitative imaging of printed distributions. Phys. Med. Biol. 2011, 56, N247. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Dhal, K.; Gupta, R.; Tappa, K.; Rybicki, F.J.; Ravi, P. Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges. Bioengineering 2023, 10, 782. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Desai, S. The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects. Bioengineering 2024, 11, 32. [Google Scholar] [CrossRef]
- Strangis, G.; Labardi, M.; Gallone, G.; Milazzo, M.; Capaccioli, S.; Forli, F.; Cinelli, P.; Berrettini, S.; Seggiani, M.; Danti, S.; et al. 3D Printed Piezoelectric BaTiO3/Polyhydroxybutyrate Nanocomposite Scaffolds for Bone Tissue Engineering. Bioengineering 2024, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.; Balkay, L.; Kukuts, K.; Miko, M.; Forgacs, A.; Trencsenyi, G.; Krizsan, A.K. 3D printed anthropomorphic left ventricular myocardial phantom for nuclear medicine imaging applications. EJNMMI Phys. 2022, 9, 34. [Google Scholar] [CrossRef]
- Läppchen, T.; Meier, L.P.; Fürstner, M.; Prenosil, G.A.; Krause, T.; Rominger, A.; Klaeser, B.; Hentschel, M. 3D printing of radioactive phantoms for nuclear medicine imaging. EJNMMI Phys. 2020, 7, 22. [Google Scholar] [CrossRef]
- Bieniosek, M.F.; Lee, B.J.; Levin, C.S. Technical Note: Characterization of custom 3D printed multimodality imaging phantoms. Med. Phys. 2015, 42, 5913–5918. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, J.; Choi, J.; Yoon, M.S.; Park, C. Design and analysis of a dedicated quality control phantom in nuclear medicine imaging based on the 3D printing technique using various printing materials. Optik 2022, 270, 169909. [Google Scholar] [CrossRef]
- Ferreira, F.C.L.; Souza, D.D.N. Liver phantom for quality control and training in nuclear medicine. Nucl. Instrum. Methods Phys. Res. A 2011, 652, 791–793. [Google Scholar] [CrossRef]
- Green, S.; Grice, J. Technical note: 3D-printed phantom for dedicated cardiac protocols and geometries in nuclear medicine. Med. Phys. 2022, 49, 943–951. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Hong, J.; Lee, M.-G. Optimization of Gamma Image Quality Through Experimental Evaluation Using 3D-Printed Phantoms Across Energy Window Levels. Bioengineering 2025, 12, 1211. https://doi.org/10.3390/bioengineering12111211
Park C, Hong J, Lee M-G. Optimization of Gamma Image Quality Through Experimental Evaluation Using 3D-Printed Phantoms Across Energy Window Levels. Bioengineering. 2025; 12(11):1211. https://doi.org/10.3390/bioengineering12111211
Chicago/Turabian StylePark, Chanrok, Joowan Hong, and Min-Gwan Lee. 2025. "Optimization of Gamma Image Quality Through Experimental Evaluation Using 3D-Printed Phantoms Across Energy Window Levels" Bioengineering 12, no. 11: 1211. https://doi.org/10.3390/bioengineering12111211
APA StylePark, C., Hong, J., & Lee, M.-G. (2025). Optimization of Gamma Image Quality Through Experimental Evaluation Using 3D-Printed Phantoms Across Energy Window Levels. Bioengineering, 12(11), 1211. https://doi.org/10.3390/bioengineering12111211





